3.1. A brief story and typification of Frankenia nodiflora
Lamarck (1788) included four species in his treatment of
Frankenia for the
Encyclopaedia. Among them, he described
Frankenia nodiflora (numbered 3) as new, after
F. hirsuta L. (numbered 2) and before
F. pulverulenta L. (numbered 4). No syntypes or illustrations were cited in the protologue, though the new species was described from dried material as follows: “3. FRANQUENNE nodiflore,
Frankenia nodiflora, Frankenia caulibus simplicibus filiformibus subglabris, fasciculis florum lateralibus axillaribus &
oppositis. N[obis].
/ Ses tiges sont longues de six à huit pouces, simples ou presque simples, filiformes, feuillées, & presque glabres. Leurs feuilles sont opposées, pétiolées, ovales, glabres, à bords réfléchis en dessous, & longues de deux lignes & demie. Dans leurs aisselles, on observe sur toute la longueur des tiges, des rameaux non développés, opposes, plus courts que les entre-noeuds, feuillés & fleuris, & qui sont paroître les tiges entrecoupées dans leur longueur par des touffes ou paquets de feuilles & de fleurs biens sépares les uns des autres. Les fleurs ont leur calice oblong, légèrement anguleux, glabre, & naissent comme en faisceau entre les feuilles, aux noeuds des tiges. / Cette plante croît naturellement au Cap de Bonne-Espérance, & a l’aspect d’une espèce de Salicaire. (
v. s.).” Furthermore, in the comment for
F. hirsuta he clearly indicated the origin and collector of the material used for the description of
F. nodiflora: “La plante β [of the intended “
F. hirsuta”, probably corresponding to
F. repens] a ses tiges presqu’entièrement glabres, ainsi que ses calices; elle croît au Cap de Bonne-Espérance, & nous a été communiquée avec la suivante [
F. nodiflora] par M. Sonnerat.” In fact, the French naturalist and explorer Pierre Sonnerat (1748−1814) gathered that material most probably in the surroundings of Cape Town during his travels to the East Indies and China in 1774−1781. The results of those expeditions were published later in a two-volume work [
35], which includes observations on the Cape area in the second volume that covers his visits to Cape Town, Madagascar, the Maldives, Mauritius, Ceylon (Sri Lanka), Indonesia, Burma, China, and the Philippines.
Frankenia nodiflora was later depicted in Tab. 262 of Lamarck’s
Tableau [
36], though the details are poor (
Figure 1). He also added a brief diagnosis in French and Latin languages, with slight changes with regard to the protologue in the branching pattern of the stem: “4. FRANQUENNE nodiflore. Dict. n° 3. / F. tiges simples, filiformes, presque glabres; fascicules des fleurs latéraux, axillaires et opposés. [FRANKENIA nodiflora. / F. caulibus simplicibus, filiformibus, subglabris; fasciculis florum lateralibus, axillaribus et oppositis.].”
Details in the protologue of
F. nodiflora on the length and branching pattern of the type material (“Ses tiges sont longues de six à huit pouces, simples ou presque simples…”) point out to the existence of various vouchers on which the description was prepared. In the herbarium P, we have traced two specimens matching the protologue, which are relevant for typification. First, the voucher P00287094 in Lamarck’s herbarium is labelled “Frankenia nodiflora Lam. / dict. / e cap[ut]. b[onae]. Sp[ei].” by Lamarck himself and includes a single unbranched fragment of ca. 15 cm long (ca. 6 inches). Secondly, the voucher P05038792, which is marked as part of Maire’s herbarium, bears a label reading “Frankenia nodiflora Lam. / (ego.) / Cap de B[onne]. Espér[ance]” in a calligraphy not incompatible with Lamarck’s handwriting and includes two unequal fragments: one of ca. 19 cm long (ca. 7.6 inches) with a short lateral branch in the upper part, similar to that illustrated in Lamarck [
36], and the other of ca. 4 cm long (ca. 1.6 inches). Both specimens bear fragments very similar to each other, more likely coming from a single collection, and therefore they might be regarded as duplicates belonging to original material of that name. Because the specimen P00287094 is in Lamarck’s collection, it is designated below as lectotype for
F. nodiflora; the specimen P05038792, despite some doubts about the handwriting on its label being Lamarck’s (C. Aupic pers. comm.) and why it was placed among Maire’s material, is here regarded as isolectotype.
Among the diagnostic characters in the protologue summarised by Lamarck [
13,
36], the long internodes, the condensed glomerular inflorescences, the ovate petiolate and entirely glabrous leaves, and the angulose and glabrous calyces are differential for
F. nodiflora. The revision of herbarium material at K and P revealed the existence of plants occurring in the surroundings of Cape Town, which perfectly match Lamarck’s type material. They are perennial shrublets producing suffruticose procumbent, non-rooting stems with long internodes; leaves entirely glabrous, mostly concolorous (bright green on both sides), broadly elliptic to oblong, flattened and only folded downwards on margins (at least on the upper third), mostly falcate upwards, subacute to minutely mucronate at apex, fleshy, with conspicuous petiole 0.5–0.8 mm long, sometimes glabrous; bracteoles broad and flat, about half to two thirds the length of the calyx; flowers mostly disposed in crowded dichasial glomerules on lateral short branches; calyxes often glabrous, with teeth cucullate bearing a notable subapical mucro ca. 0.5 mm long (diagnostic character not present in other African taxa of the genus); and seeds ca. 1 mm long, covered with unequal medium-sized papillae 12–33 μm long, globose to conical-obtuse, more densely disposed on the distal part, among other characters.
Frankenia nodiflora has been treated in quite different ways. Candolle [
37] accepted it as a distinct species, but Harvey [
38], who did not know the Lamarck’s specimens, suggested its probable inclusion in
F. pulverulenta perhaps as a variety; in the same work he surprisingly also regarded the South African “
F. pulverulenta &
F. nodiflora, of Drège’s Coll.”, which included gatherings of the true
F. nodiflora (conserved in different European herbaria; see below), to belong to
F. pulverulenta. Similarly,
F. nodiflora was often considered to be merely a synonym of
F. pulverulenta [
1,
4,
39], and subsequently recent works did not accept the Lamarck’s species in the Southern African floras [
10,
11,
40,
41], probably assuming implicit synonymisation with
F. pulverulenta. That synonymic treatment is currently accepted in POWO [
12] under the name
F. pulverulenta subsp.
pulverulenta (
https://powo.science.kew.org/taxon/urn:lsid:ipni.org:names:77229962-1#synonyms).
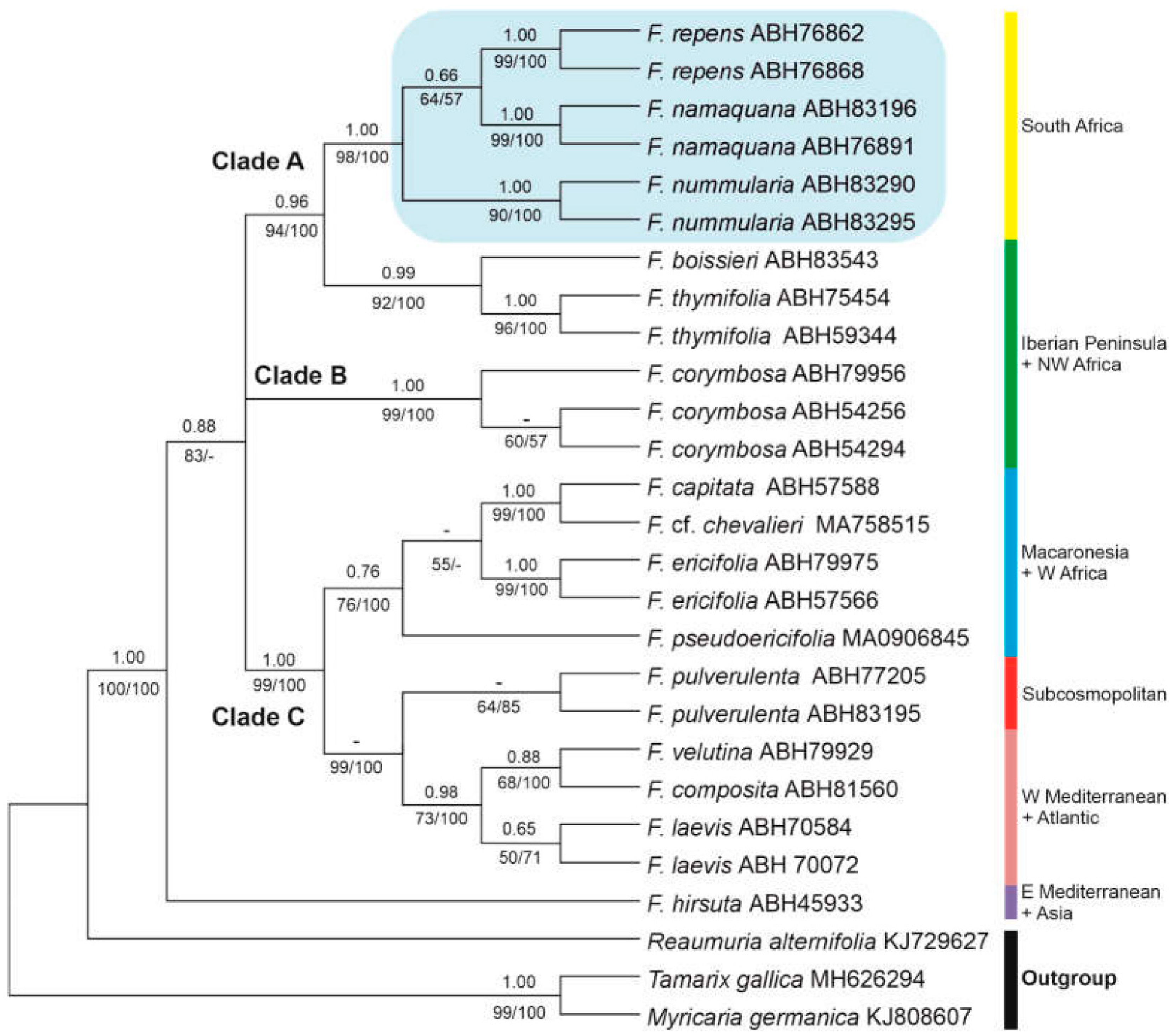
Figure 1.
Relevant material of
F. nodiflora Lam. (
a) Lectotype here designated from Lamarck’s collection (P00287094) with a close-up detail of inflorescences (scale bar: 5 mm); reproduced with permission (© Muséum National d’Histoire Naturelle, Herbarium, Paris); (
b) Comparative illustrations of
F. pulverulenta (left; num. 3) and
F. nodiflora (right; num. 4), according to
Tableau encyclopédique et méthodique of Lamarck [
13], plate 262 [partially modified]).
Figure 1.
Relevant material of
F. nodiflora Lam. (
a) Lectotype here designated from Lamarck’s collection (P00287094) with a close-up detail of inflorescences (scale bar: 5 mm); reproduced with permission (© Muséum National d’Histoire Naturelle, Herbarium, Paris); (
b) Comparative illustrations of
F. pulverulenta (left; num. 3) and
F. nodiflora (right; num. 4), according to
Tableau encyclopédique et méthodique of Lamarck [
13], plate 262 [partially modified]).
Nevertheless, in our view the Cape plants matching the Lamarckian concept of F. nodiflora are morphologically quite distinct from the Linnaean F. pulverulenta, showing a unique combination of characters unknown in the other Southern African species of Frankenia, this supporting acceptance at specific rank. Other similar populations growing in the northern and western inland areas of the Nama-Karoo and Succulent-Karoo biomes that show some resemblances to F. nodiflora and were often misidentified as F. pulverulenta, F. capitata or F. repens (incl. F. krebsii), are here re-evaluated in the light of new morphological and molecular data.
3.3. Taxonomic treatment of Frankenia nodiflora and description of new related species
3.3.1. Frankenia nodiflora Lam., Encycl. 2(2): 543. 1788 ≡ Franca nodiflora (Lam.) Vis. in Mem. Reale Ist. Veneto Sci. 16: 158. 1871.
Type: [South Africa. Western Cape]. E Cap[ut]. B[onae]. Sp[ei]. [Sonnerat s.n.] (lecto. designated here: P00287094!; isolectotype: P05038792! (Cap de B[onne]. Espér[ance]. [Sonnerat s.n.]).
−F. pulverulenta auct. pl. atque F. krebsii auct. pl.
Description: Shrublet densely branched, tap-rooted, woody at the base with grey bark, mostly glabrous. Stems rooting at base, diffuse, usually creeping, suffruticose, up to 40 cm long, often divaricate branches 1–3 cm long; young branchlets with internodes up to 35 mm long, yellowish to reddish, glabrous or sometimes shortly and loosely puberulous. Leaves opposite-decussate, patent to erect-patent, bright-green or sometimes reddish, glabrous on both sides; petiole 0.6–1.2 × 0.2–0.3 mm, flattened, tapering distally; sheath extending along margins of petiole to blade, laxly ciliate (occasionally subglabrous), with 2–5 pairs of lateral cilia 0.2–0.8 mm long, unequal, whitish, flattened, obtuse or acute; leaf blade 2.5–3.5 × 1–2 mm, broadly ovate-elliptic to oblong, mostly falcate upwards, subacute to minutely mucronate at apex, fleshy, concolorous, sometimes slightly paler abaxially with minute glands bearing salt depositions, often cochleariform (spoon-like convex) to flattened, longitudinally folded downwards, with margins strongly to loosely and gradually revolute (at least on the upper third), somewhat thickened; midrib narrow, linear, tapering slightly towards apex, continuous with petiole below, somewhat raised abaxially, extending all along the blade length; young leaves on short shoots fasciculate, similar but smaller than those on long shoots and sometimes narrower. Flowers pentamerous, perfect, borne commonly in dichasial groups, usually condensed at stem nodes, glomerular or with short axillary branches up to 20 mm long (usually shorter); the central flower sessile, the lateral ones with pedicels up to 1 mm long. Floral bracts 2, 2–3 mm long, leaf-like, subpatent to erect-patent, connate at base, enveloping basal part of calyx for 0.5–0.7 mm. Bracteoles 2, 1–2 mm long, bract-like but smaller, about half to two thirds the length of calyx, adnate to the calyx base and alternating with bracts. Calyx 4–4.5 × 1–1.5 mm, tubular at anthesis, untwisted, straight, indurate, with 5 prominent thickened ribs, entirely glabrous; teeth 5, 0.9–1.2 mm long, triangular, narrowly membranous and shortly papillate on margins, often reddish, slightly recurved after anthesis, cucullate with a notable subapical acumen ca. 0.7 mm long, obtuse and slightly divergent. Petals 5, 5–7 × 1–2 mm, obovate-cuneate, pinkish-mauve but whitish below, overlapping only in the basal part; claw 2–3 × 0.5–0.7 mm, narrowly cuneate, imperceptibly tapering to blade, yellowish, hidden into the calyx tube; ligule 1.5–2.5 × 0.3–0.4 mm, narrowly oblanceolate, longitudinally adnate to claw, the free apex ca. 0.5 mm long, oblong-obtuse, entire; blade 2.3–3 × 1.5–2 mm, broadly obovate with apex rounded to truncate, slightly emarginate and irregularly sinuate (not erose-denticulate). Stamens 6, in 2 unequal whorls, usually long exserted, overtopping 1.5–2 mm the calyx teeth at anthesis; filaments 3–6 mm long, expanded ca. 0.5 mm wide in the lower half, but gradually tapering and filiform in the distal half, whitish; anthers 0.5–0.7 mm long, oblong-ellipsoid, versatile, yellowish. Ovary ellipsoid, subtrigonous, with 3 carpels; placentae 3, parietal-basal, extending up to the lower half to two thirds of carpel wall length, ventral traces moderately to highly branched; ovules 4–6 per placenta, attached along most of placenta by erect funiculi 0.1–0.2 mm long. Style 3–4.5 mm long, terete, somewhat sigmoid at base, exserted and elongating up to 8 mm after pollination, whitish; style branches 3, filiform, 0.5–0.7 mm long, whitish; stigmas slightly clavate. Capsule 2.5–3.5 × 0.6–2 mm, ovoid-ellipsoid, hidden into calyx tube, dark reddish-brown, early dehiscent. Seeds 9–12, 0.7–1.0 × 0.3–0.5 mm, sulcate on one side, ellipsoid, pale brown, darker at the funicular part, developing rapidly even before flower has completely withered; testa thin, not mucilaginous, with surface weakly and irregularly ornamented with a subrectangular-reticulate pattern, finely striate, covered with medium-sized papillae 12–33 μm long, heterogeneous, globose to conical-obtuse, more densely disposed on the distal part.
Etymology: The specific epithet (nodiflorus, –a, –um = with flowers at nodes) refers to the disposition of flowers and inflorescences, mostly crowded at stem nodes.
Phenology: Flowering in late October–early January (occasionally in July–August), fruiting in November–February (occasionally in August–September).
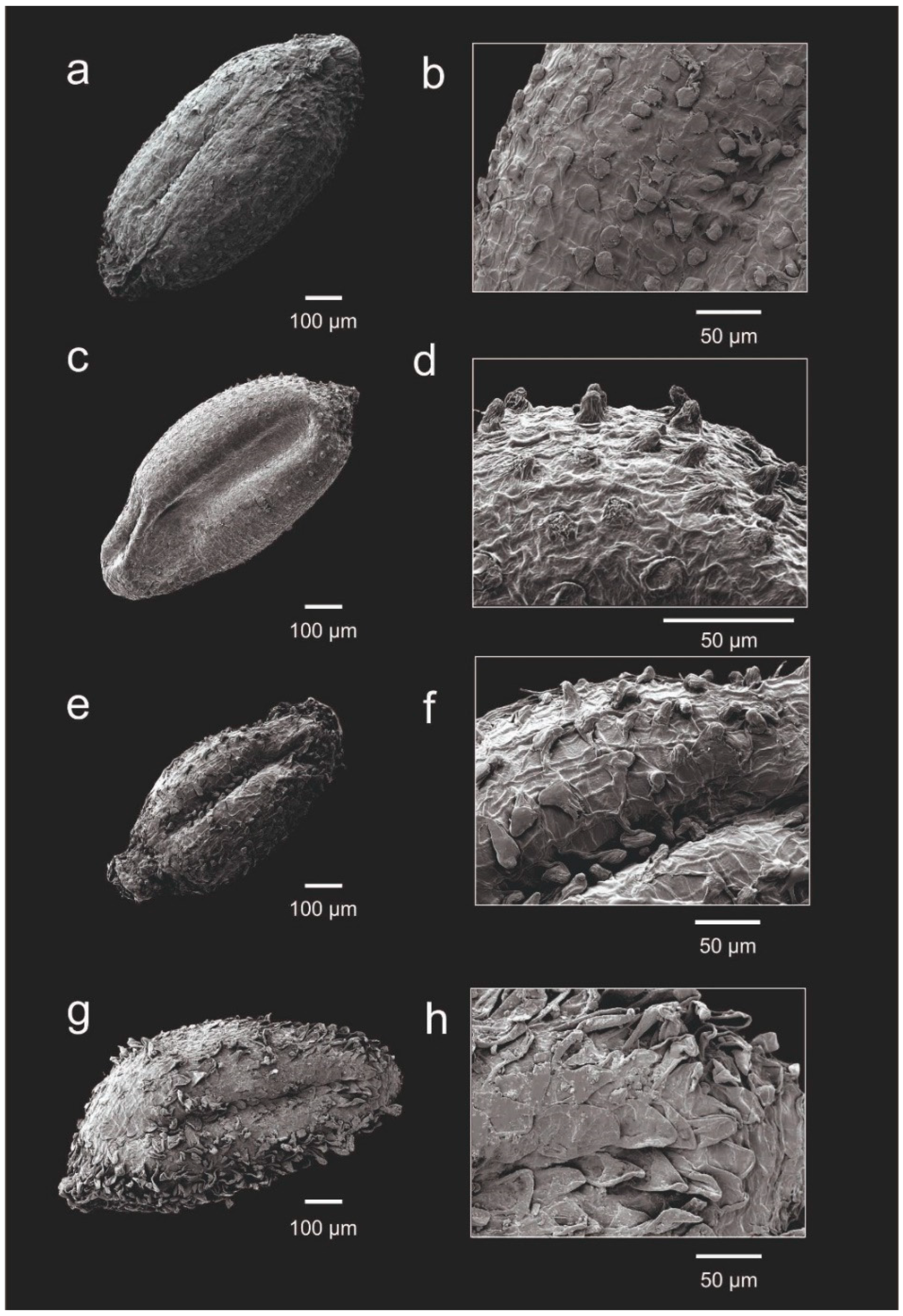
Figure 4.
Frankenia nummularia sp. nov.
(a) Western Cape: Beukesfontein,
H.H.W.Pearson 5005; Isotype: P05038725); available at:
https://science.mnhn.fr/institution/mnhn/collection/p/item/p05038725 (© Muséum National d’Histoire Naturelle, Herbarium, Paris);
(b) Northern Cape: Kookfontein river; living plants in habitat, with details of leaves and withered inflorescences.
Figure 4.
Frankenia nummularia sp. nov.
(a) Western Cape: Beukesfontein,
H.H.W.Pearson 5005; Isotype: P05038725); available at:
https://science.mnhn.fr/institution/mnhn/collection/p/item/p05038725 (© Muséum National d’Histoire Naturelle, Herbarium, Paris);
(b) Northern Cape: Kookfontein river; living plants in habitat, with details of leaves and withered inflorescences.
Habitat and distribution:
Frankenia nodiflora occurs on saline, azonal soils of salt pans and saline riverbeds, in the coastal lowlands at elevations of 0–150 m above sea level. The known distribution of the species extends across the Cape plains into neighbouring areas northeast from Cape Town in the Western Cape Province (
Figure 3), a territory broadly included in the Fynbos (F) biome (mostly the F07 Bioregion)
sensu Mucina and Rutherford [
14], where it specifically inhabits the so-called “Cape Inland Salt Pans” (code AZi 9). In the coastal lowlands of the Fynbos biome, the climate is mild and oceanic (ameliorated by the ocean influences), with average temperatures ranging about 7°C in winter to 30°C in summer (average annual temperature ca 17°C) and frosts being rare and occasional. The average annual precipitation amounts to ca 500–540 mm, though rather differently distributed, the rainfall occurring mostly during winter (May to August) with a peak in July [
14].
Notes: In the past,
F. nodiflora surely was much commoner than today, just before the severe changes of landscape caused by extensive agriculture and urbanisation. Nowadays, the known extant populations of this species are concealed to nature reserves where meadows and patches of halophytic vegetation are conserved, in a few sites between Durbanville to Paarl. The most important threats are related to invasion of the natural habitats by alien species such as
Cynodon dactylon L. and
Cenchrus clandestinus (Hochst. ex Chiov.) Morrone (
Pennisetum cladestinum Hochst. ex Chiov.) (P. Winter pers. comm.). In this context, urgent field prospections are required to locate new populations of this rare endemism that likely remain unnoticed, and also active management of the natural sites is required to avoid decline and extinction of the scarce wild populations. Therefore, new data are necessary for an accurate conservational labelling of the species, including counts of the number of populations and individuals, as well as their evolution in time. In the meantime, the conservation status of
F. nodiflora is suggested here as Data Deficient (DD), though very likely it might be assessed at least as Vulnerable (VU) according to IUCN [
42].
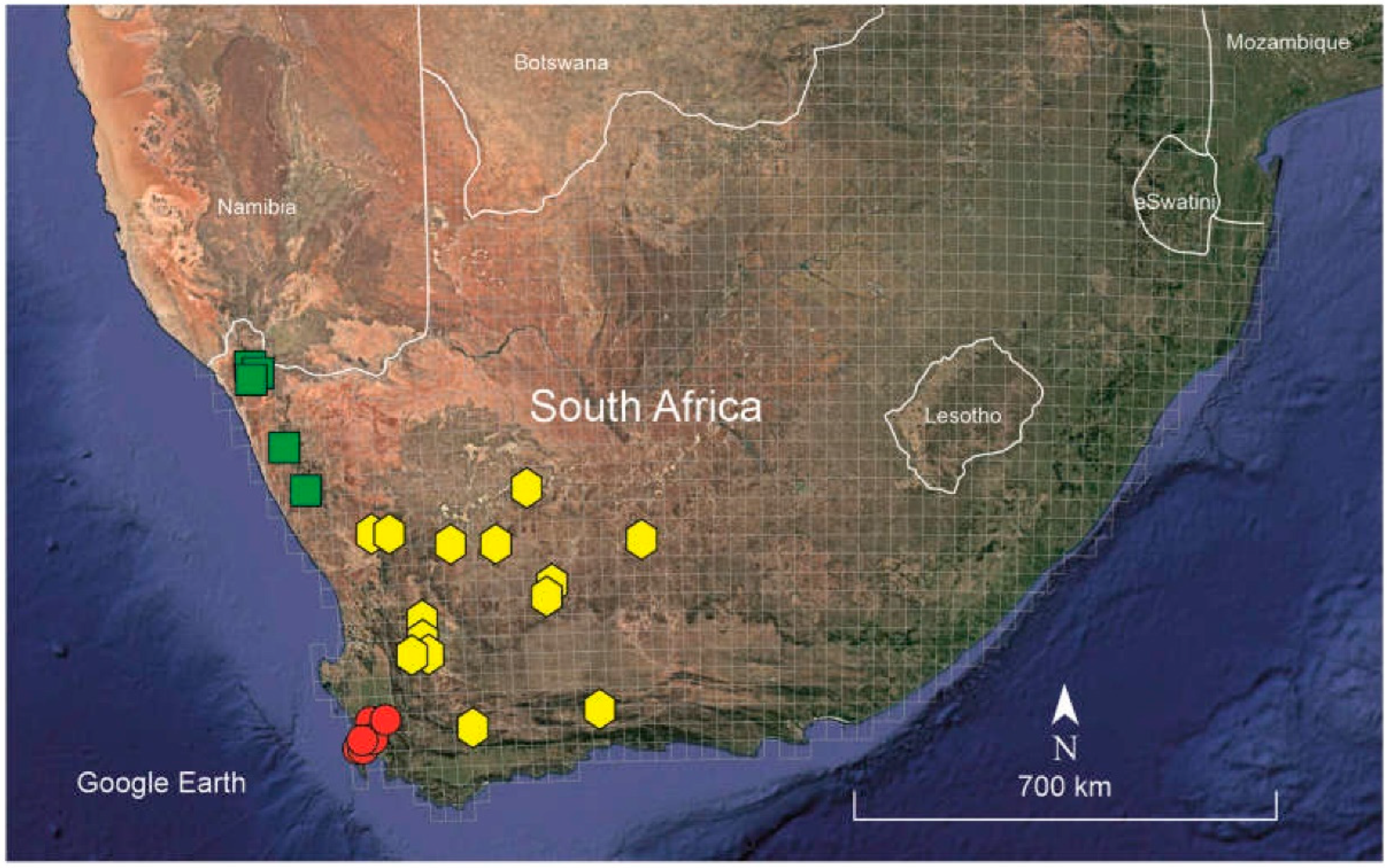
Figure 3.
Distribution map of the studied material (both herbarium vouchers and iNaturalist data) of Frankenia nodiflora (red circles), F. nummularia (yellow hexagons) and F. namaquana (green squares).
Figure 3.
Distribution map of the studied material (both herbarium vouchers and iNaturalist data) of Frankenia nodiflora (red circles), F. nummularia (yellow hexagons) and F. namaquana (green squares).
Other studied materials: South Africa. Western Cape Province: 3318 (Cape Town): Cape of Good Hope, Table Mountain (-CD), December 1832, J.Mac Gillivray 584 (K!); 3318 (Cape Town): Cape Town, Green Point (-CD), November 1846, A.Prior s.n. (K!); 3318 (Cape Town): Cape Town, Mowbray (-CD), shores of vlei, August 1912, W.C.Worsdell s.n. (K!); 3318 (Cape Town): Cape Peninsula, Raapenberg Vley (-CD), 26 November 1896, A.H.Wooley 2110 (BOL !, K!, excl. fragment in the central part); 3318 (Cape Town): Cape Town, about Salt River near the Windmills (-CD), 14 November 1811, [Pl. Africae Australis Extratropicae,] Burchell 513 (G-DC G00211140!, K!, P05038790!). 3318 (Cape Town): Cape Town, Uitkamp Wetlands Nature Reserve (-DC), 33°48’59.7”S 18°38’26.0”E, 137 m elev., 7 November 2014, H. Stummer s.n. (ABH83529!).
Unidentified sites: South Africa. Habitat ad C[apum] B[onae] Spei (B-W06993). Cape, Dr. Pappe s.n. (K000232047 !). Cap de Bonne-Espérance, 1842 (MPU693060 !). Cap de Bonne-Espérance (P05038793!). C[aput] B[onae] S[pei], Mrs. Gilavray s.n. (P05038816!). Cap de Bone Espérance, ex herb. Pet. Thouare s.n. (P05038804!). [Cape] Pl. Capenses, Ecklon (P05038764!).
Digital iNaturalist images:
South Africa.
Western Cape Province. 3318 (Cape Town): Cape Town, Durbanville, Belleville, Uitkamp Wetlands Nature Reserve (-DC), 33°48’59.7”S 18°38’26.0”E, 137 m elev., 7 November 2014,
M.Goets (v.v.):
https://www.inaturalist.org/observations/11060535; 3318DA (Cape Town): Cape Town, Malmesbury Farms, Teleport Rd, 33°41’18.0”S 18°42’25.0”E, 88 m elev., 26 April 2019,
I. Ebrahim (v.v.):
https://www.inaturalist.org/observations/24159910; 3318 (Cape Town): Paarl, Cape Winelands District, Windmeul Farm, Langerug Private Nature Reserve (-BD), 33°39’37.14"S 18°54’1.62"E, 146 m elev., 10 November 2017,
J. Wicht (v.v.):
https://www.inaturalist.org/observations/148456049
3.3.2. Frankenia nummularia M.B.Crespo, M.Á.Alonso, Mart.-Azorín, J.L.Villar & Mucina, sp. nov.
Type:
South Africa. Western Cape: Karoo, Beukesfontein, sandy river bed, 1420 ft elev., 1 December 1908,
H.H.W.Pearson 5005 (holo.: BOL!; iso.: K!, P05038725!).
Figure 4a.
−F. pulverulenta auct. pl. atque F. repens auct. pl.
Diagnosis: Planta speciosa a F. pulverulenta et F. nodiflora foliis latis, subplanis (vel ad margines parve revolutis), longe petiolatis, et caulibus prostratis vel ascendentibus accedenti, sed ab eas distinctissima et bene distinguenda. A priore insuper differt caulibus suffruticosis, perennibus (non herbaceis annuis); foliis glaberrimis (non subtus pilosis); bracteolis calycem multo brevioribus (non aequantibus); et seminibus minus numerosis (ad 20, non 45) minoribusque (0.7–1.1 mm long., non 0.5-0.7 mm long.). A posteriore insuper discrepat foliis discoloribus, subtus valde pallidioribus (non subconcoloribus); calycibus majoribus 4.5–6 mm long., dentibus acutis vel mucrone inconspicuo ad 0.2 mm long. (non calycibus 4–4.5 mm long., dentibus mucrone magno ad 0.7 mm long.); ; et praesertim seminum testa papillis brevioribus, 3.5–9 μm long., subhomogeneis, omnibus conico-obtusis (non papillis longioribus 12–33 μm long., heterogeneis, aliis globosis aliis conico-obtusis).
Description: Shrublet densely branched, tap-rooted, woody at the base with grey bark, glabrous to long hairy. Stems rooting at base, procumbent to ascending, suffruticose, up to 40 cm long, usually with erect, often divaricate branches 5–35 cm long; young branchlets with internodes up to 30 mm long, yellowish to reddish, glabrous to ± densely hairy covered on one side with heterogeneous indumentum of both curled minute trichomes (ca. 0.2 mm) and flexuous long pluricellular complanate trichomes (0.7–1 mm long), denser below nodes. Leaves opposite-decussate, patent to erect-patent, greyish- to bright-green, somewhat glaucescent, glabrous on both sides, sometimes with salt depositions; petiole 0.7–1.5 × 0.3–0.5 mm, flattened, tapering distally; sheath extending along margins of petiole almost to blade, densely ciliate (occasionally almost glabrous), with 2–5 pairs of lateral cilia 0.3–0.8 mm long, unequal, whitish; leaf blade 2–7 × 1.5–6 mm, mostly suborbicular or broadly elliptic, rounded to slightly pointed at apex, somewhat fleshy, often discolorous, abaxially paler with minute glands usually bearing salt depositions, often longitudinally folded, with margins loosely and gradually revolute, somewhat thickened; midrib narrow, linear, tapering slightly towards apex, continuous with petiole below, somewhat raised abaxially, extending about half the blade length; young leaves on short shoots fasciculate, similar but smaller than those on long shoots and sometimes narrower and incurved on margins at the upper part. Flowers pentamerous, perfect, borne commonly in loose dichasial groups, usually widely branched, with erect-patent branchlets up to 20 mm long; the lowermost flowers solitary, sessile, the uppermost dichasia often denser and subcorymbose (the central flower always sessile, the lateral ones with pedicels 1–2 mm long). Floral bracts 2, 2–7 mm long, leaf-like, subpatent to erect-patent, connate at base, enveloping basal part of calyx for 0.5–0.7 mm. Bracteoles 2, 1–2 mm long, bract-like but smaller, up to half the length of calyx, adnate to the calyx base and alternating with bracts. Calyx 4.5–6 × 1–1.5 mm, fusiform-tubular to gradually fusiform after anthesis, untwisted, straight to slightly curved, indurate, with 5 prominent thickened ribs, entirely glabrous or sparsely puberulous to hirtellous at base and on ribs, hairs up to 0.5 mm long, whitish, occasionally with scattered whitish depositions on the upper part; teeth 5, 0.7–0.9 mm long, triangular, acute to minutely mucronate (mucro up to 0.2 mm long), minutely papillate on margins, often yellowish, erect to slightly incurved after anthesis. Petals 5, 6–9 × 1–2 mm, long obovate cuneate, pinkish-mauve, overlapping in most of their length; claw 4–5 × 0.8–1 mm, narrowly cuneate, imperceptibly tapering to blade, whitish, hidden into the calyx tube; ligule 2–3 × 0.5–1 mm, narrowly oblanceolate, longitudinally adnate to claw, the free apex ca. 1 mm long, triangular-acute, entire; blade 2.3–4 × 1.5–2 mm, broadly obovate to suborbicular with apex rounded to truncate, irregularly erose-denticulate. Stamens 6, in 2 unequal whorls, exserted, overtopping 1.5–2 mm the calyx teeth at anthesis; filaments 6–8 mm long, expanded ca. 0.5 mm wide in the lower half, but gradually tapering and filiform in the distal half, pinkish to bluish-pink; anthers 0.8–1.2 mm long, ellipsoid, versatile, yellowish to reddish. Ovary ellipsoid, subtrigonous, with 3 carpels; placentae 3, parietal-basal, extending up to the lower half to two thirds of carpel wall length, ventral traces moderately to highly branched; ovules 6–10 per placenta, attached along most of placenta by erect funiculi 0.3–0.4 mm long. Style 6–9 mm long, terete, somewhat sigmoid at the base, exserted and elongating up to 11 mm after pollination, whitish; style branches 3, filiform, 0.5–0.7 mm long, pinkish-white to reddish; stigmas slightly clavate. Capsule 2.5–3.5 × 1–2 mm, ovoid-ellipsoid, hidden into calyx tube, dark reddish-brown, early dehiscent. Seeds 12–22, 0.7–0.9 × 0.3–0.5 mm, ellipsoid to ovoid-ellipsoid, sulcate on one side, pale brown, darker at the funicular part, developing rapidly even before flower has completely withered; testa thin, not mucilaginous, with surface weakly and irregularly ornamented with a subrectangular-reticulate pattern, finely striate, almost smooth, only sparsely covered on the funicular side with small papillae 3.5–9 μm long, subhomogeneous, conical-obtuse.
Etymology: The specific epithet (nummularius, –a, –um = coin-bearing) refers to the shape of leaves and bracts, usually suborbicular and flattened, resembling coins.
Phenology: Flowering in late October–early January (occasionally in July–August), fruiting in November–February (occasionally in August–September).
Habitat and distribution:
Frankenia nummularia grows in saline, well-drained sandy, azonal soils of salt pans, intermittent saline riverbeds and ravines in inland regions, at elevations of 250–1380 m above sea level (
Figure 4b). The known distribution of the species extends through most of the SW part of the Karoo Region in western South Africa, ranging from Vanrhynsdorp and Riversdale District in the Western Cape to Calvinia and Victoria West District in the Northern Cape Province (
Figure 3), a territory mostly included in the Nama-Karoo (NK) Biomes and reaching the southern Succulent-Karoo Biomes (mostly the SKk, SKt and SKv Bioregions)
sensu Mucina and Rutherford [
14], where it occurs concretely in to the so-called “Bushmanland vloere” (code AZi 5). In those regions, the climate is semiarid to arid to arid, continental (not or scarcely ameliorated by the ocean influences), with average temperatures ranging from –5°C in winter to 43°C in summer and frosts being usual in the higher areas. The average annual precipitation varies between 100 mm and 500 mm, though rather differently distributed, the rainfall occurring mostly during late summer (December to April) with a peak in March [
14].
Notes: Wild populations of
F. nummularia include numerous individuals covering a large territory in South Africa, and no special threats are known so far to occur that might lead to any inferred decline in either the number of populations or the number of individuals. Therefore, its conservation status is suggested here as Least Concern (LC) according to IUCN [
42].
Other studied materials: South Africa. Northern Cape Province: 3021 (Vanwyksvlei): Rietspoort (-CB), (I, d1), 3000–4000 ft elev., 30 November 1826, J.F.Drège 2648 (765) (P05038732!); 3119 (Calvinia): Namaqualand, river bed W of Brandkop (-AC), 9 December 1946, F.M.Leighton 2441 (BOL!); 3119 (Calvinia): Zwart Doorn River, W of Brandkop (-AC), 9 December 1946, R.H.Compton 18893 (BOL!; NBG! excluding two annual plants); 3120 (Williston): An der Dualls Slangenfontein [Slangfontein] (-BD), (I, d1 d), 3000–4000 ft elev., 17 November 1826, J.F.Drège s.n. (P05038728!); 3120 (Williston): Sandwef [sic] on road to Brandvlei (-AC), 29 November 1986, G.Germishuizen 4011 (NBG!); 3120 (Williston): Hantam, Kookfontein farm, Kookfontein rivier at crossing R-354 (-CA), 31°43’35”S 20°14’07”E, 1082 m elev., in saline substrate of ravine, 25 August 2022, M.Martínez Azorín et al. s.n. (ABH83290!); 3121 (Fraserburg): Karoo Region, near Fraserburg (-DC), 4200 ft elev., January 1888, H.Bolus 10381 (NBG!); 3121 (Victoria West): Little Namaqualand, Bed of Brakrivier (-BD), 1600 ft elev., 11 December 1908, H.H.W.Pearson 4864 (K!); 3123 (Victoria West): Central Cape, Victoria West District, Hutchinson, Zeisiesfontein (-AC), 1260 m elev., E.M.Nortje 10 (NBG!); 3220 (Sutherland): Tankwa Karoo, between Middlepos and Ganaga Pass (-CB), 32°37’46.7”S 20°21’40.5”E, 573 m elev., 26 August 2022, M.Martínez Azorín et al. s.n. (ABH83295!); 3221 (Merweville): Fraserburg, Ratelfontein vel “Balmoral” (-BA), 4500 ft elev., January 1888, H.Bolus 10381 (BOL!, NBG!); 3221 (Merweville), Upper Region, Kopjies Kraal, river bed (-BA), 2000 ft elev., 12 December 1908, H.H.W.Pearson 4886 (K!). Capland: Boschjemanskarroo [probably near Bitterfontein], 3000–4000 ft elev., November, J.F.Drège s.n. (HBG516896!). Boschjemans-karroo oder Onderbokkeveld, [3000–4000 ft elev., November], J.F.Drège 6242 (P05038787!). Afrique austral, Herb. J. Hennecart, Drège, locum, 69 n° 1, [probably near Platberg], November 1838–1839, J.F.Drège (K!, P05038788!). Western Cape Province: 3118 (Vanrhynsdorp): Knersvlakte, Kalkgat farm (-BB), 255 m elev., 18 June 1987, C.Boucher 5175 (NBG!); 3219 (Wuppertal): SW Cape Region, foot of Katbakkies Pass, east side, at Skitterykloof picnic site (-DC), 1800 ft elev., 6 January 1976, H.C.Taylor 9049 (NBG!, K!); 3219 (Wuppertal): Karoo, Beukesfontein, sandy river bed (-CD), 1420 ft elev., 1 December 1908, H.H.W.Pearson 5005 (BOL!, K!, P05038725!); 3219 (Wuppertal), Central Karoo District: Pappekuil [Papekuil] (-BC), not far from river, in sand, 950 ft elev., 3 November 1908, H.H.W.Pearson 3985 (K!); 3219 (Wuppertal): Karoo: North of Gansfontein, river bed (-DA), 1200 ft elev., bush 1–1½ ft elev., 2 December 1908, H.H.W.Pearson 3984 (K!); 3319 (Worcester): Worcester, near Mowers station (-DA), 10 November 1964, Van Breda 1758 (NBG!); 3320 (Montagu): Laingsburg, Wittebergen near Matjiesfontein (-BA), October 1908, R.Marloth 11442 (NBG!); 3321 (Ladismith): Riversdale Div., Klein Karroo, damp places in river beds (-CC?), 1200 ft elev., October 1924, J.Muir 3546 (BOL!).
Unidentified sites: South Africa. Cap de Bonne Espérance, collection de Drège s.n. (HBG516896!, P05038791!). Cape, Drège s.n. (K!). Cap, 1838, Drège s.n. (P05144899!). Afr[ica]. austr[alis]., 1836, J.F.Drège s.n., sub F. nodiflora (K!, P05038789!).
Digital iNaturalist images:South Africa.
Eastern Cape Province. 3323 (Willowmore): Graaff-Reinet, Dr Beyers Naudé Local Municipality, Timbila Nature reserve, Grootrivier bed (-BB), 33°11’14”S 23°53’16”E, 580 m elev., 29 September 2019,
K.Jolliffe s.n. (v.v.):
https://www.inaturalist.org/observations/33692903.
3.3.3. Frankenia namaquana M.B.Crespo, M.Á.Alonso, Mart.-Azorín, J.L.Villar & Mucina, sp. nov.
Type: South Africa. Northern Cape: Namaqualand, Steinkopf, 11 December 1897, Schlechter 40 (holo.: BOL!; iso.: P05038802!).
−F. capitata auct. pl. atque F. repens auct. pl.
Diagnosis: Planta speciosa a F. repenti caulibus valde lignosis et calycibus dense puberulis (interdum etiam costis hirsutis) remote accedenti, sed ab ea distinctissima et bene distinguenda caulibus erectis non radicantibus; petalis brevioribus 5.5.–6.5 mm long. (non 9–11 mm); calycibus minoribus 3-4 mm long. (non 6-8 mm); et praesertim seminum testa papillis brevioribus, 10–43 μm long, heterogeneis, aliis globosis aliis conico-obtusis (non papillis longioribus 55–110 μm, subhomogeneis, omnibus conico-obtusis).
Description: Shrub densely branched, tap-rooted, woody at the base with grey bark, glabrous to sparsely hairy. Stems non-rooting, erect to ascending, fruticose, usually with erect, often divaricate branches 15–45 cm long; young branchlets with internodes up to 20 mm long, yellowish to reddish, ± densely pubescent (rarely glabrescent), covered all around with homogeneous indumentum of minute claviform or hooked trichomes (ca. 0.1–0.2 mm) and longer complanate trichomes (up to 0.4 mm long), denser below nodes and somewhat retrorse to patent. Leaves opposite-decussate, patent to erect-patent, deep-green, somewhat glaucescent, glabrous on the upper side and ± densely papillate beneath, mostly with scattered whitish depositions; petiole 0.3–0.5 × 0.2–0.5 mm, flattened, tapering distally; sheath extending along margins of petiole almost to blade, densely ciliate, with 6–10 pairs of cilia 0.4–1.2 mm long, unequal, filiform, whitish; leaf blade 2–4 × 1–2 mm, broadly oblong to elliptic, rounded to subacute at apex, somewhat fleshy, often discolorous, abaxially paler with minute glands usually bearing salt depositions, margins strongly revolute hiding almost completely the abaxial side, somewhat thickened; midrib thickened, tapering slightly towards apex, continuous with petiole and raised abaxially, extending about half the blade length; young leaves on short shoots fasciculate, similar but smaller than those on long shoots and sometimes narrower. Flowers pentamerous, perfect, borne commonly in dichasial groups, usually branched, with erect branchlets up to 15 mm long, but often crowded in compact inflorescences; the lowermost flowers in reduced groups, briefly pedunculate, the uppermost dichasia often denser and subcorymbose (the central flower always sessile, the lateral ones with pedicels 0.5–1 mm long). Floral bracts 2, 2–3.5 mm long, leaf-like, erect-patent to erect, connate at base, enveloping basal part of calyx for ca. 0.5 mm. Bracteoles 2, 1–2 mm long, bract-like but smaller, about half the length of calyx, adnate to the calyx base and alternating with bracts. Calyx 3–4 × 0.8–1.5 mm, fusiform-tubular to gradually fusiform after anthesis, untwisted, straight, indurate, with 5 prominent thickened ribs, densely papillate (papillae whitish, minute, claviform or globose) between ribs but sparsely hirtellous on ribs (trichomes whitish, up to 0.2 mm long), sometimes with scattered whitish depositions on the upper part; teeth 5, 0.8–1.2 mm long, triangular, acute or briefly mucronulate (mucro ca. 0.2 mm long), minutely papillate, often yellowish, not recurved after anthesis. Petals 5, 5.5–6.5 × 0.9–1.3 mm, long obovate-cuneate, whitish to purplish; claw 4–5 × 0.8–1 mm, narrowly cuneate, imperceptibly tapering to blade, whitish, hidden into the calyx tube; ligule 2–3 × 0.3–0.5 mm, narrowly oblanceolate, longitudinally adnate to claw, the free apex ca. 0.5 mm long, ovate-acute to acuminate, entire to slightly denticulate on margins; blade 2.3–4 × 1.5–2 mm, broadly obovate to suborbicular with rounded to truncate, irregularly erose-denticulate apex. Stamens 6, in 2 unequal whorls, long exserted, overtopping 1.5–2.5 mm the calyx teeth at anthesis; filaments 6–8 mm long, expanded ca. 0.5 mm wide in the lower half, but gradually tapering and filiform in the distal half, pinkish to bluish-pink; anthers 0.4–0.6 mm long, ellipsoid, versatile, yellow. Ovary ellipsoid, subtrigonous, with 3 carpels; placentae 3, parietal-basal, extending up to the lower half to two thirds of carpel wall length, ventral traces moderately to highly branched; ovules 5–6 per placenta, attached along most of placenta by erect funiculi 0.2–0.4 mm long. Style 7–8 mm long, terete, somewhat sigmoid at the base, exserted and elongating up to 11 mm after pollination, whitish; style branches 3, filiform, 0.9–1.5 mm long, whitish; stigmas slightly clavate. Capsule 2.5–3.5 × 1–2 mm, ovoid-ellipsoid, hidden into calyx tube, dark reddish-brown, early dehiscent. Seeds 1–3, 0.7–1.1 × 0.3–0.4 mm, ellipsoid to ovoid-ellipsoid, sulcate on one side, pale brown, darker at the funicular part, developing rapidly even before flower has completely withered; testa thin, not mucilaginous, with surface weakly and irregularly ornamented with a subrectangular-reticulate pattern, finely striate, sparsely covered with medium-sized papillae 10–43 μm long, heterogeneous, globose to conical-obtuse, more densely disposed on the distal part.
Etymology: The specific epithet (namaquanus, –a, –um = Namaqualander) refers to Namaqualand, a semi-desert region of NW South Africa to which the new species is native.
Phenology: Flowering in late October–early January (occasionally in July–August), fruiting in November–February (occasionally in August–September).
Habitat and distribution:
Frankenia namaquana grows in saline, well-drained sandy, azonal soils of salt pans, waadis, intermittent riverbeds and ravines in inland regions, at elevations of 300–600 m above sea level (
Figure 5). The known distribution of the species is restricted to the NW part of the Karoo Region in NW South Africa, ranging from Eksteenfontein and Steinkopf to Klipfontein in the Northern Cape Province (
Figure 2), a territory included in the northern Succulent-Karoo (SK) biomes (mostly in the SKn, SKr and SKs bioregions)
sensu Mucina and Rutherford [
14], where it specifically inhabits the so-called “Namaqualand Salt Pans” (code AZi 2). In those areas, the climate is subdesert to arid and continental (not or scarcely ameliorated by the ocean influence), with average temperatures ranging from 5°C in winter to 30°C (or even more) in summer, and frosts being absent or scarce but much varying between years. The average annual precipitation ranges around 70–200 mm, though rather differently distributed, and with occasional local rains reaching about 300 mm; the rainfall occurs mostly during winter (May to September) with a peak in June, and episodic drought periods well below 100 mm per year being frequent [
14].
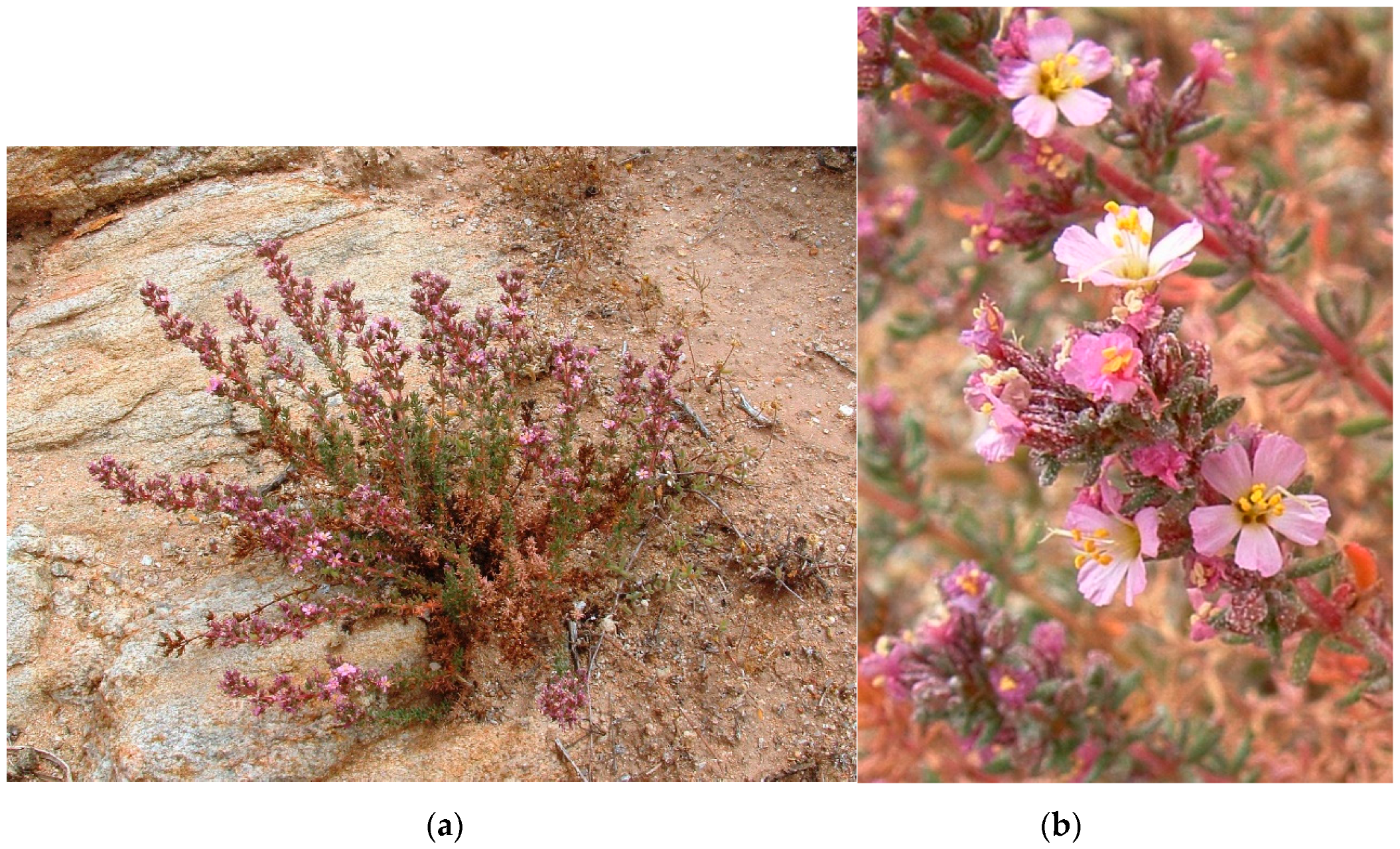
Figure 5.
Frankenia namaquana sp. nov. (a) Plant in habitat, near Springbok (Northern Cape Province); (b) Details of the inflorescences at anthesis (photos A. le Roux, 1 November 2014).
Figure 5.
Frankenia namaquana sp. nov. (a) Plant in habitat, near Springbok (Northern Cape Province); (b) Details of the inflorescences at anthesis (photos A. le Roux, 1 November 2014).
Notes: Wild populations of
F. namaquana include numerous individuals covering a large territory in NW South Africa, and no special threats are known so far to occur that might lead to any inferred decline in either the number of populations or the number of individuals. Therefore, its conservation status is suggested here as Least Concern (LC) according to IUCN [
42].
Studied material:South Africa.Northern Cape Province: 2817 (Vioolsdrift): Skoverfontein, ca 12 km NW of Eksteenfontein (-CC), P15, 28°45’50"S 17°09’15"E, 448 m elev., 19 August 2022, M.Martínez Azorín et al. s.n. (ABH831912!); 2817 (Vioolsdrift): Skoverfontein, ca 12 km NW of Eksteenfontein (-CC), P16, 28°46’25"S 17°09’41"E, 460 m elev., 19 August 2022, M.Martínez Azorín et al. s.n. (ABH83196!); 2817 (Vioolsdrift): Skoverfontein, ca 12 km NW of Eksteenfontein (-CC), P17, 28°46’33"S 17°10’18"E, 481 m elev., 19 August 2022, M.Martínez Azorín et al. s.n. (ABH83198!); 2917 (Springbok): Namaqualand, Steinkopf (-BC), 11 December 1897, Schlechter 40 (BOL!, P05038802!); Namaland Minor [near Springbok], W.C.Scully 9 (BOL!, P05038765!); 3017 (Hondeklipbaai): Klipfontein, 2–3 km N of Klipfontein, S of Kersboshoek (-BD), 30°28’43.3"S 17°49’40.9”E, 309 m elev., 30 August 2017, M.Martínez Azorín et al. s.n. (ABH76891!).
Digital iNaturalist images: South Africa.
Northern Cape Province. 2817 (Vioolsdrif): Namaqualand, Eksteenfontein, Sendelingsdrif (-CC), 28°46’29”S 17°09’45”E, 458 m, wadi bed, 12 October 2018,
S.Swanepoel s.n. (v.v.):
https://www.inaturalist.org/observations/17553648; 2917 (Springbok): Namaqualand, Komaggas area, NW Oubeep (-DC), 29°51’41.36”S 17°33’53.26”E, 581 m elev., river bed, 16 March 2017,
N.Helme s.n. (v.v.):
https://www.inaturalist.org/observations/11292132.