Submitted:
19 May 2023
Posted:
22 May 2023
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Materials and Methods
2.1. Sample preparation
| Sample# | Ca | Co | Er |
| #01 | 0.05 | 0 | 0.95 |
| #02 | 0.08 | 0 | 0.92 |
| #03 | 0.1 | 0 | 0.9 |
| #04 | 0.95 | 0 | 0.05 |
| #05 | 0.98 | 0 | 0.02 |
| #06 | 0.05 | 0.2 | 0.75 |
| #07 | 0.2 | 0.2 | 0.6 |
| #08 | 0.55 | 0.24 | 0.21 |
| #09 | 0.65 | 0.25 | 0.1 |
| #10 | 0 | 0.3333 | 0.6667 |
| #11 | 0.3 | 0.3333 | 0.3667 |
| #12 | 0.31 | 0.3333 | 0.3567 |
| #13 | 0.32 | 0.3333 | 0.3467 |
| #14 | 0.3333 | 0.3333 | 0.3334 |
| #15 | 0.35 | 0.3333 | 0.3167 |
| #16 | 0.39 | 0.3333 | 0.2767 |
| #17 | 0.4 | 0.3333 | 0.2667 |
| #18 | 0.42 | 0.3333 | 0.2467 |
| #19 | 0.43 | 0.3333 | 0.2367 |
| #20 | 0.45 | 0.3333 | 0.2167 |
| #21 | 0.5 | 0.3333 | 0.1667 |
| #22 | 0.55 | 0.3333 | 0.1167 |
| #23 | 0.4 | 0.4 | 0.2 |
| #24 | 0.5 | 0.4 | 0.1 |
| #25 | 0.55 | 0.4 | 0.05 |
| #26 | 0.6 | 0.4 | 0 |
| #27 | 0.2 | 0.425 | 0.375 |
| #28 | 0 | 0.5 | 0.5 |
| #29 | 0.1 | 0.5 | 0.4 |
| #30 | 0.15 | 0.5 | 0.35 |
| #31 | 0.16 | 0.5 | 0.34 |
| #32 | 0.17 | 0.5 | 0.33 |
| #33 | 0.18 | 0.5 | 0.32 |
| #34 | 0.19 | 0.5 | 0.31 |
| #35 | 0.2 | 0.5 | 0.3 |
| #36 | 0.21 | 0.5 | 0.29 |
| #37 | 0.22 | 0.5 | 0.28 |
| #38 | 0.25 | 0.5 | 0.25 |
| #39 | 0.28 | 0.5 | 0.22 |
| #40 | 0.3 | 0.5 | 0.2 |
| #41 | 0.25 | 0.5714 | 0.1786 |
| #42 | 0.26 | 0.5714 | 0.1686 |
| #43 | 0.27 | 0.5714 | 0.1586 |
| #44 | 0.28 | 0.5714 | 0.1486 |
| #45 | 0.29 | 0.5714 | 0.1386 |
| #46 | 0.295 | 0.5714 | 0.1336 |
| #47 | 0.3 | 0.5714 | 0.1286 |
| #48 | 0.3572 | 0.5714 | 0.0714 |
| #49 | 0.4143 | 0.5714 | 0.0143 |
| #50 | 0.4286 | 0.5714 | 0 |
| #51 | 0.02 | 0.6 | 0.38 |
| #52 | 0.2 | 0.65 | 0.15 |
2.2. X-ray diffraction
2.3. Rietveld refinements
3. Results
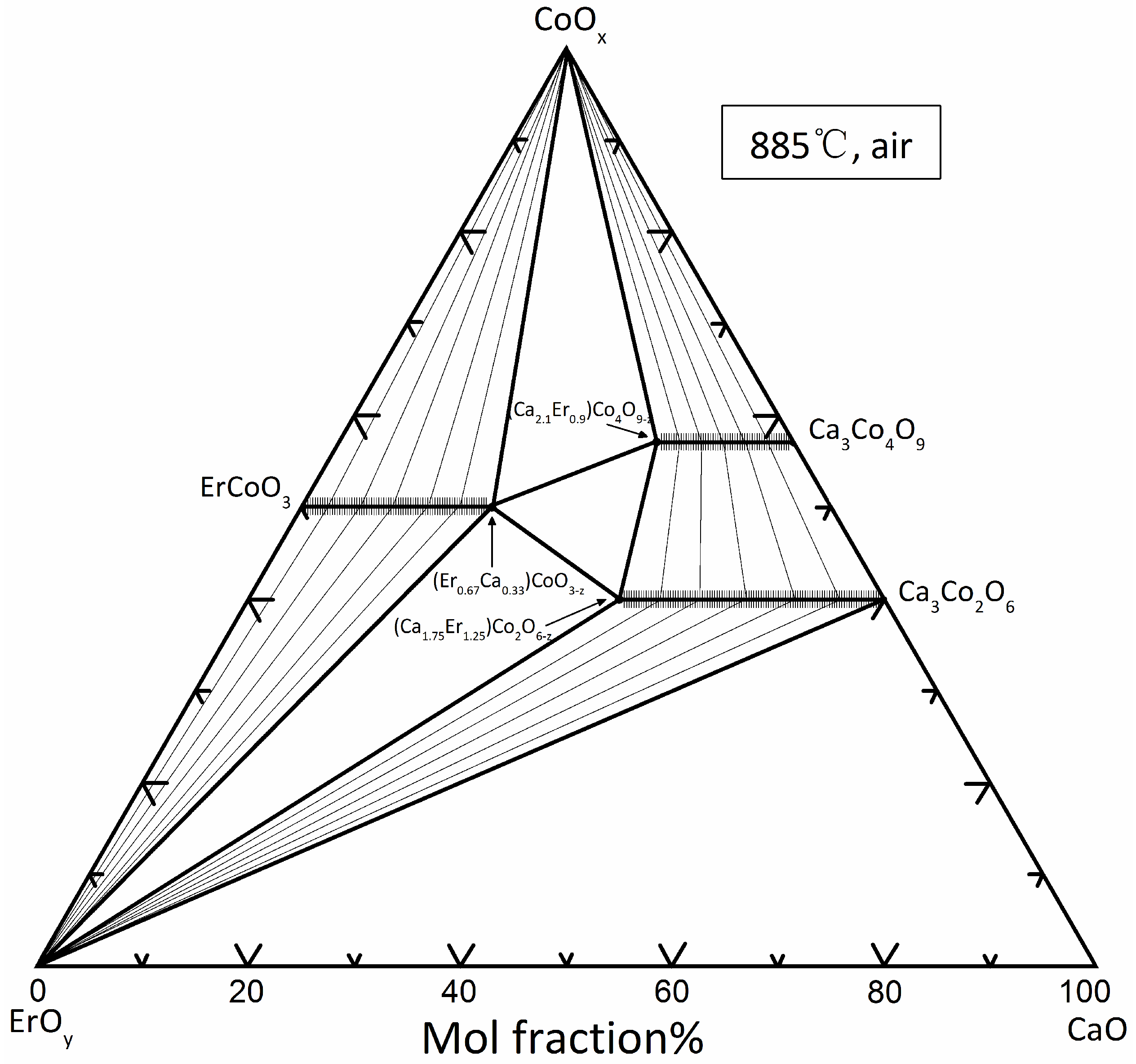
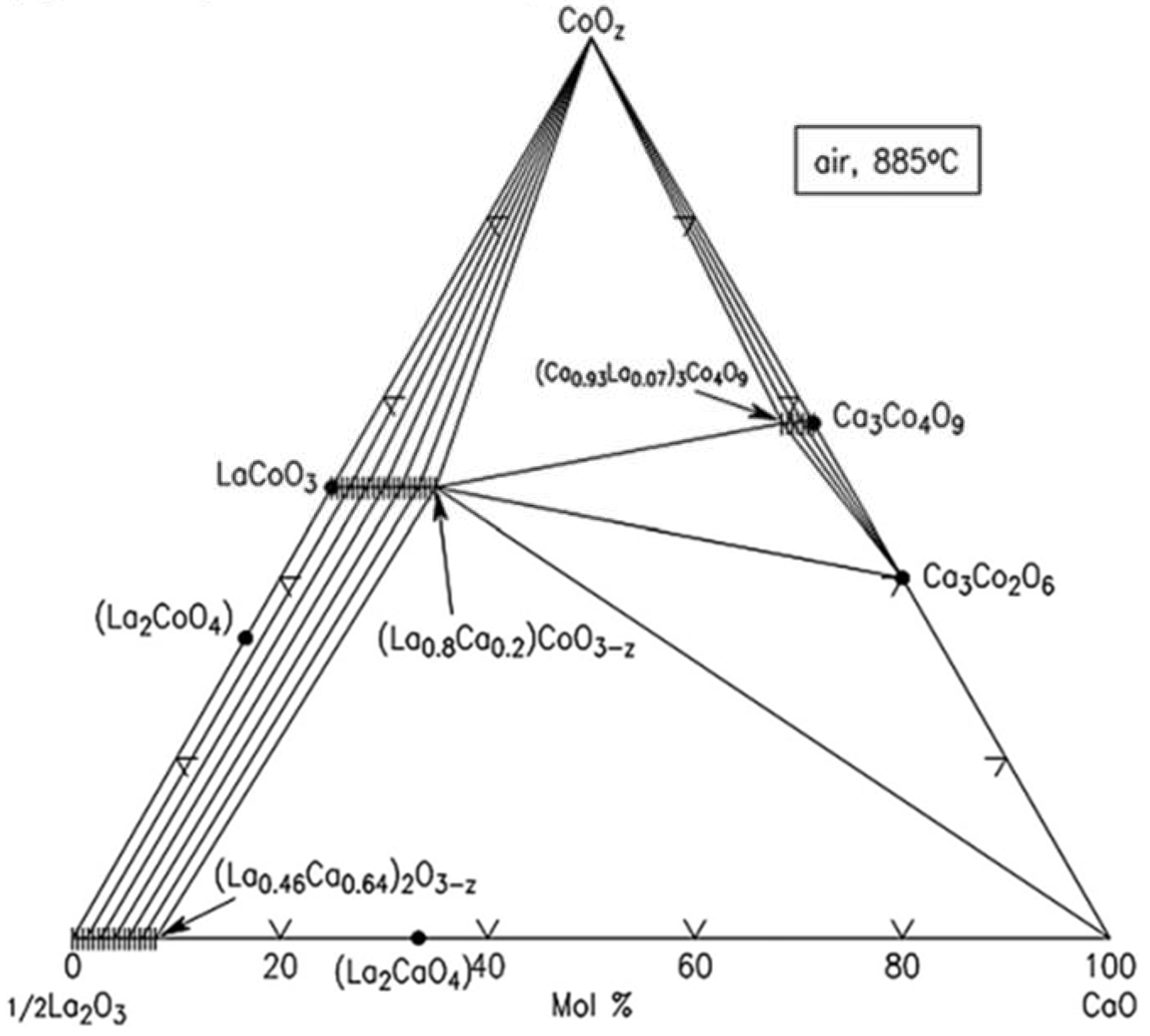
3.1. Binary oxide systems
3.1.1. CaO-CoOx
3.1.2. CaO-ErOy
3.1.3. CoOx-ErOy
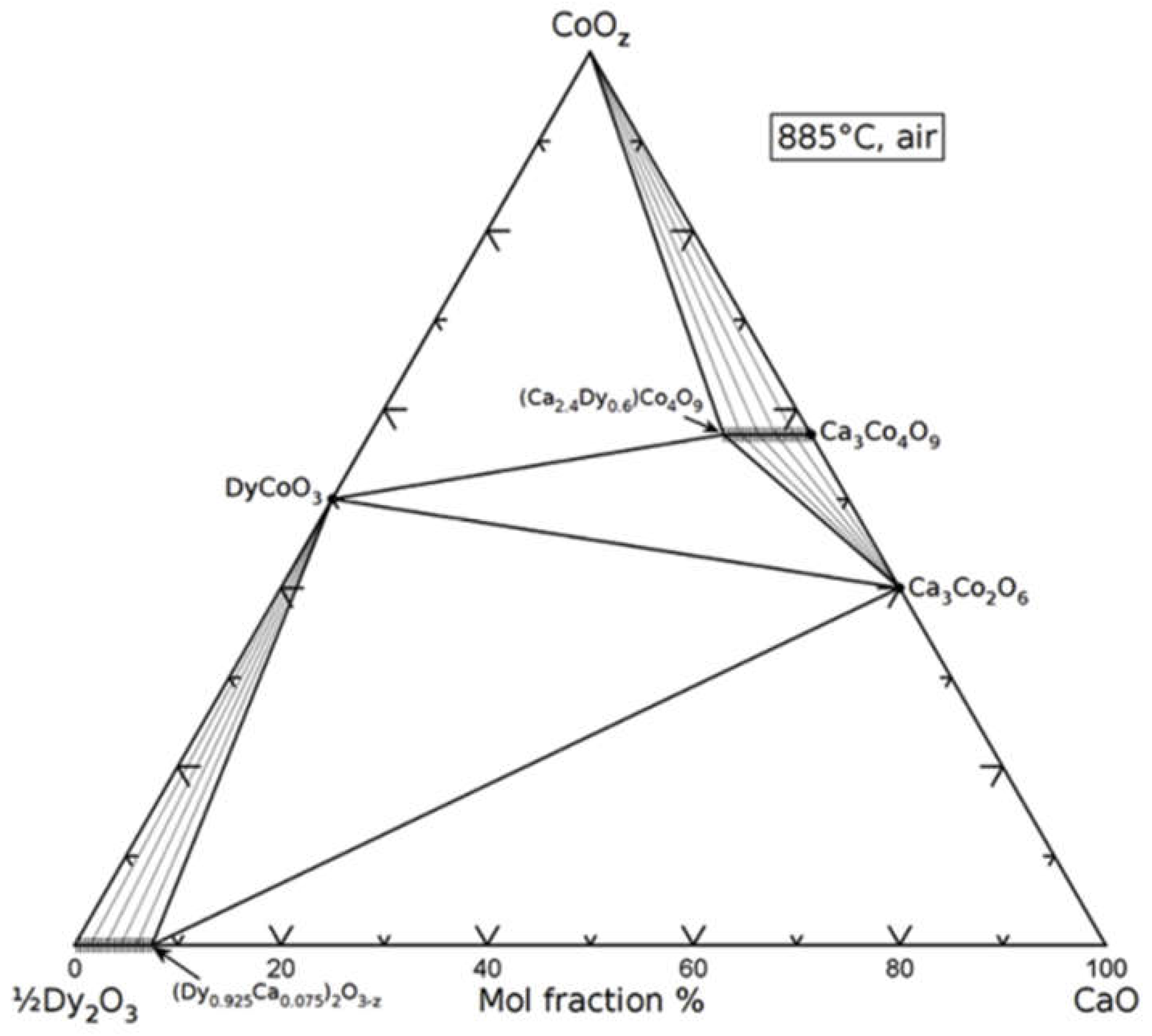
3.2. Ternary oxide systems
3.2.1. (Ca3-xErx)Co4O9-z
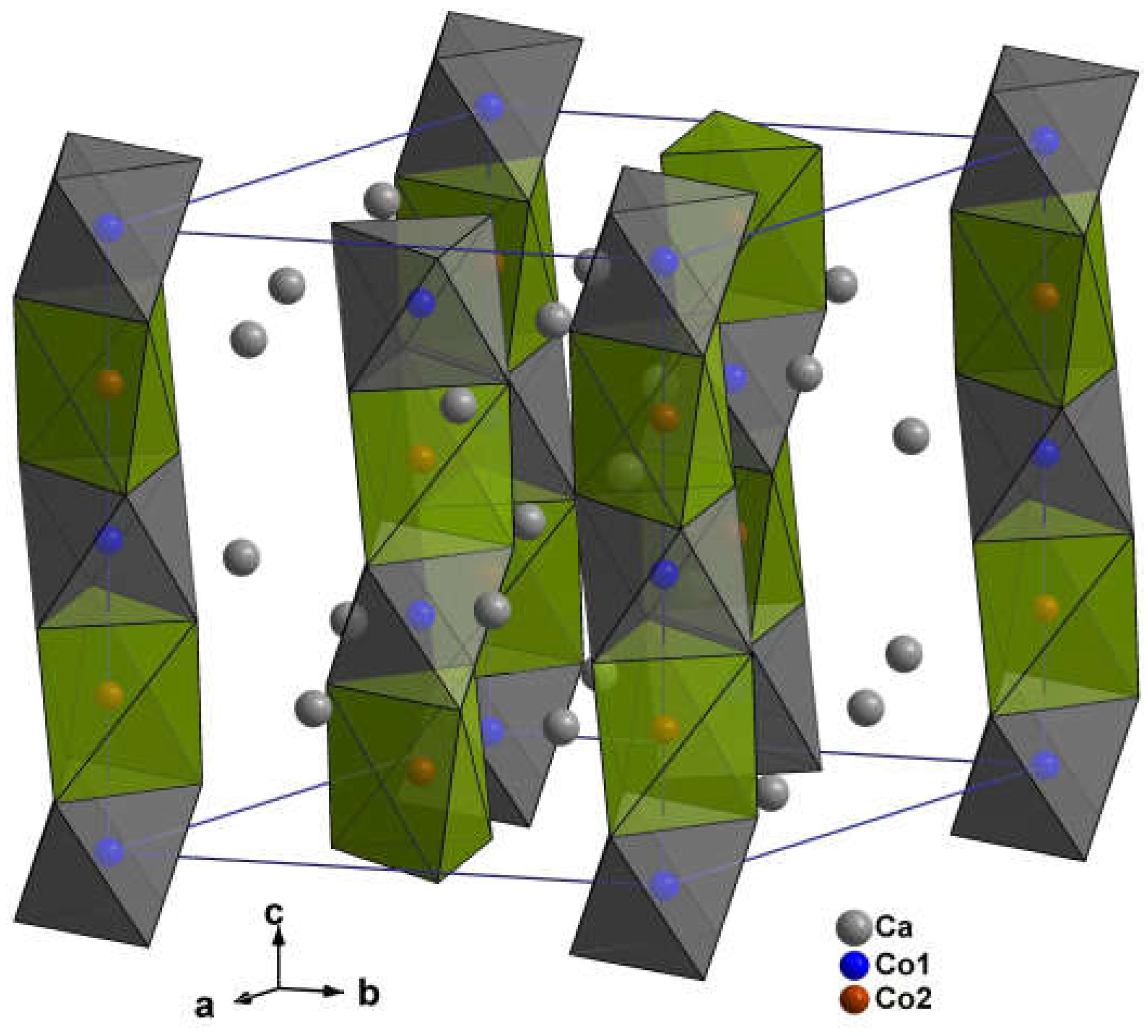
3.2.2. (Ca3-xErx)Co2O6-z
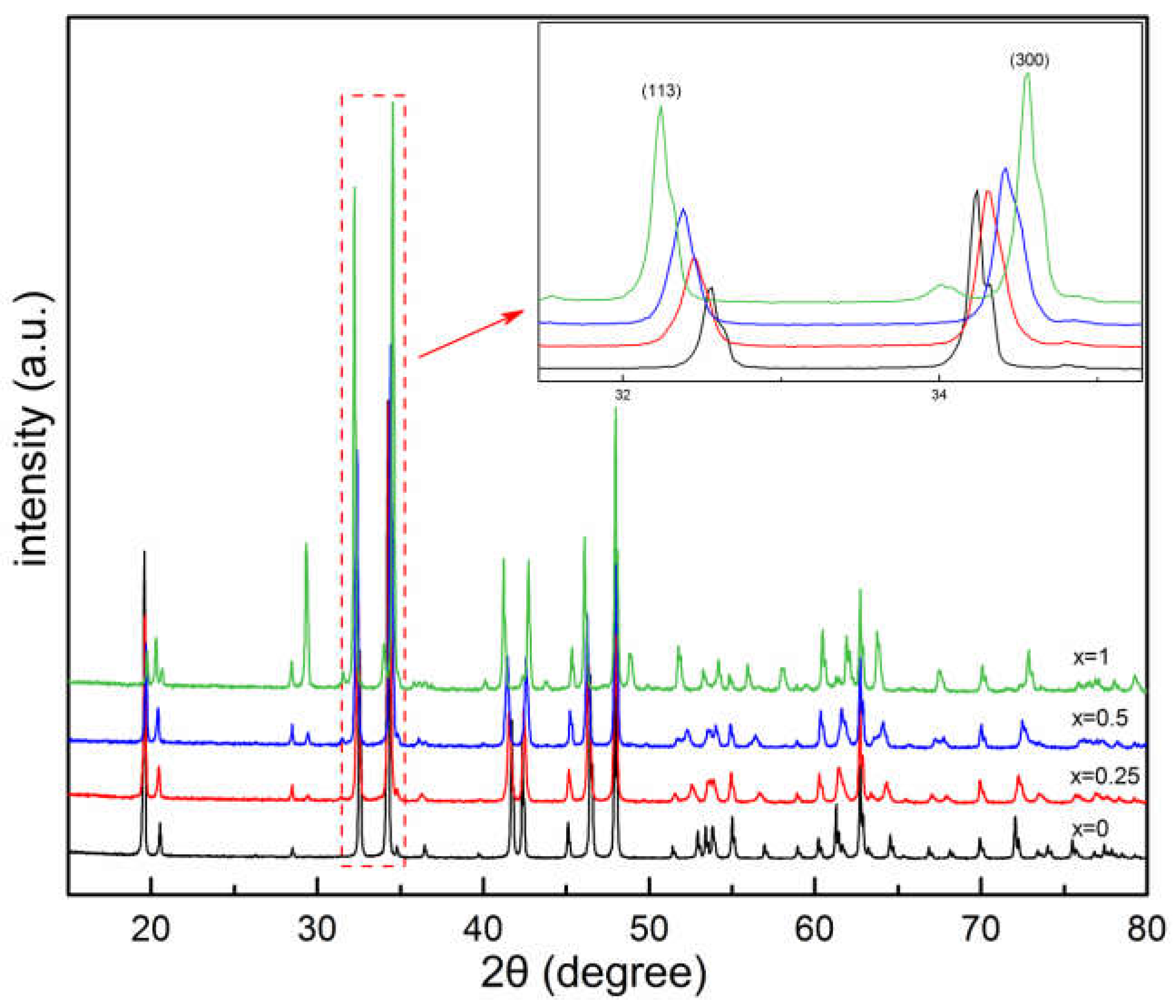
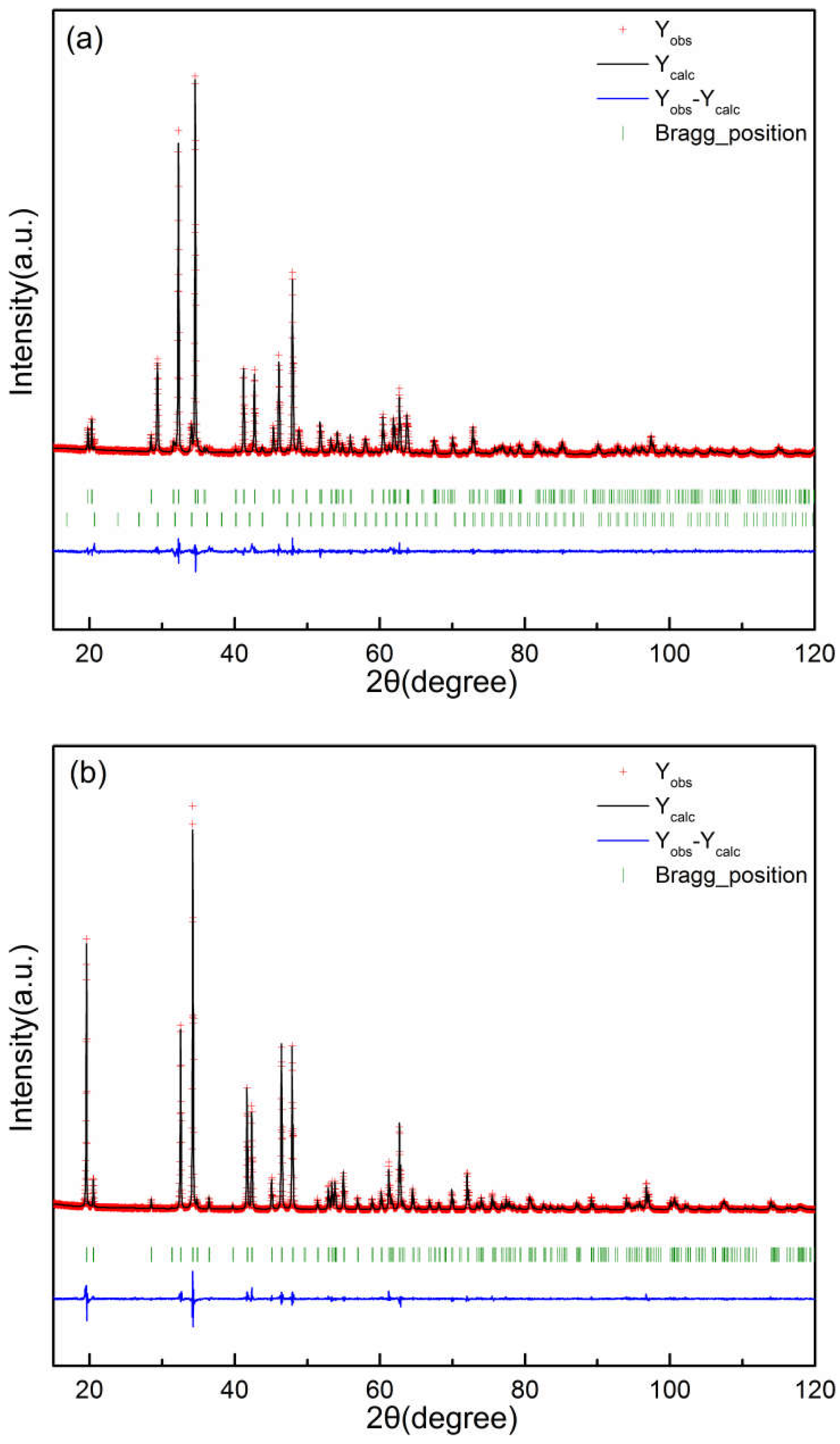
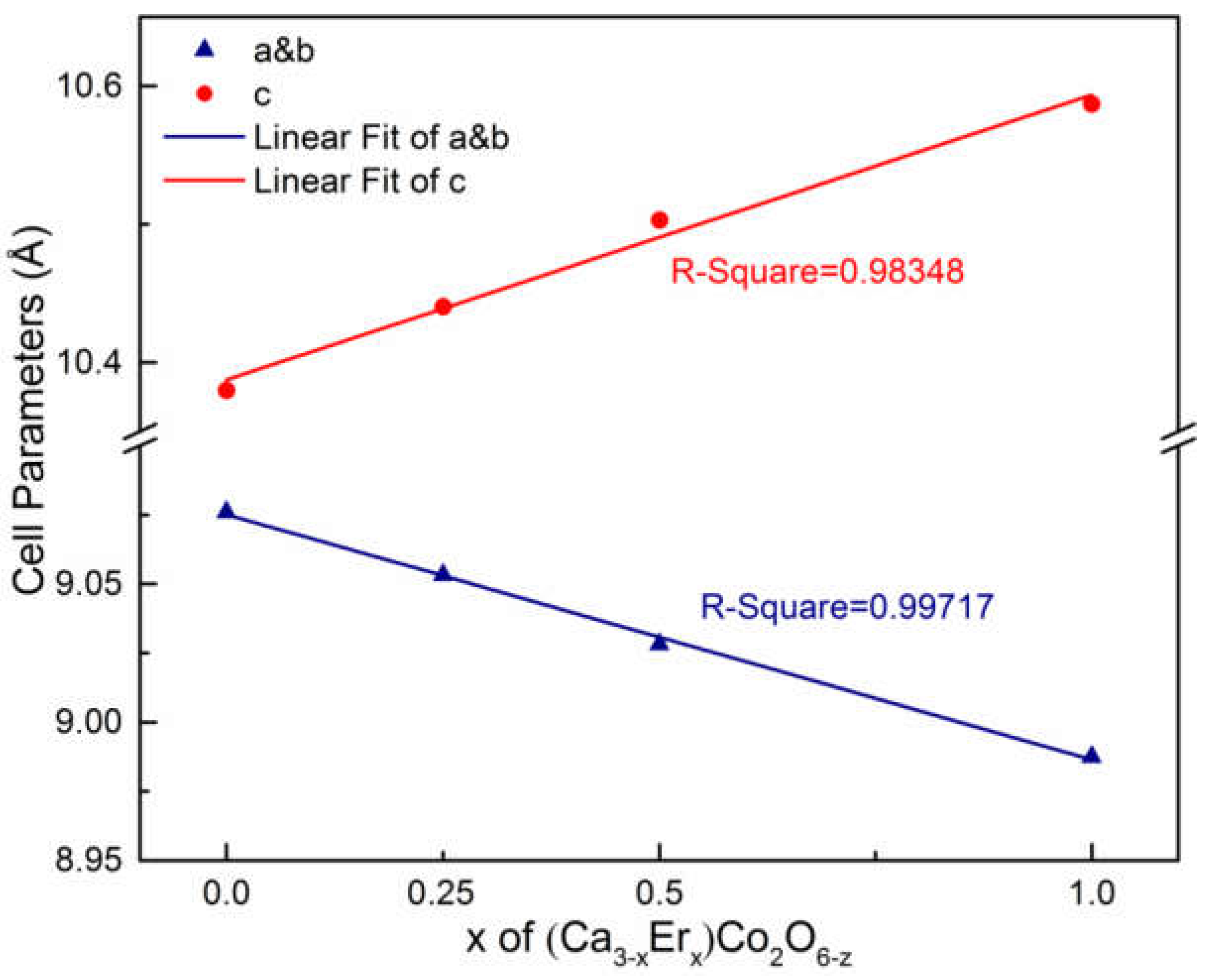
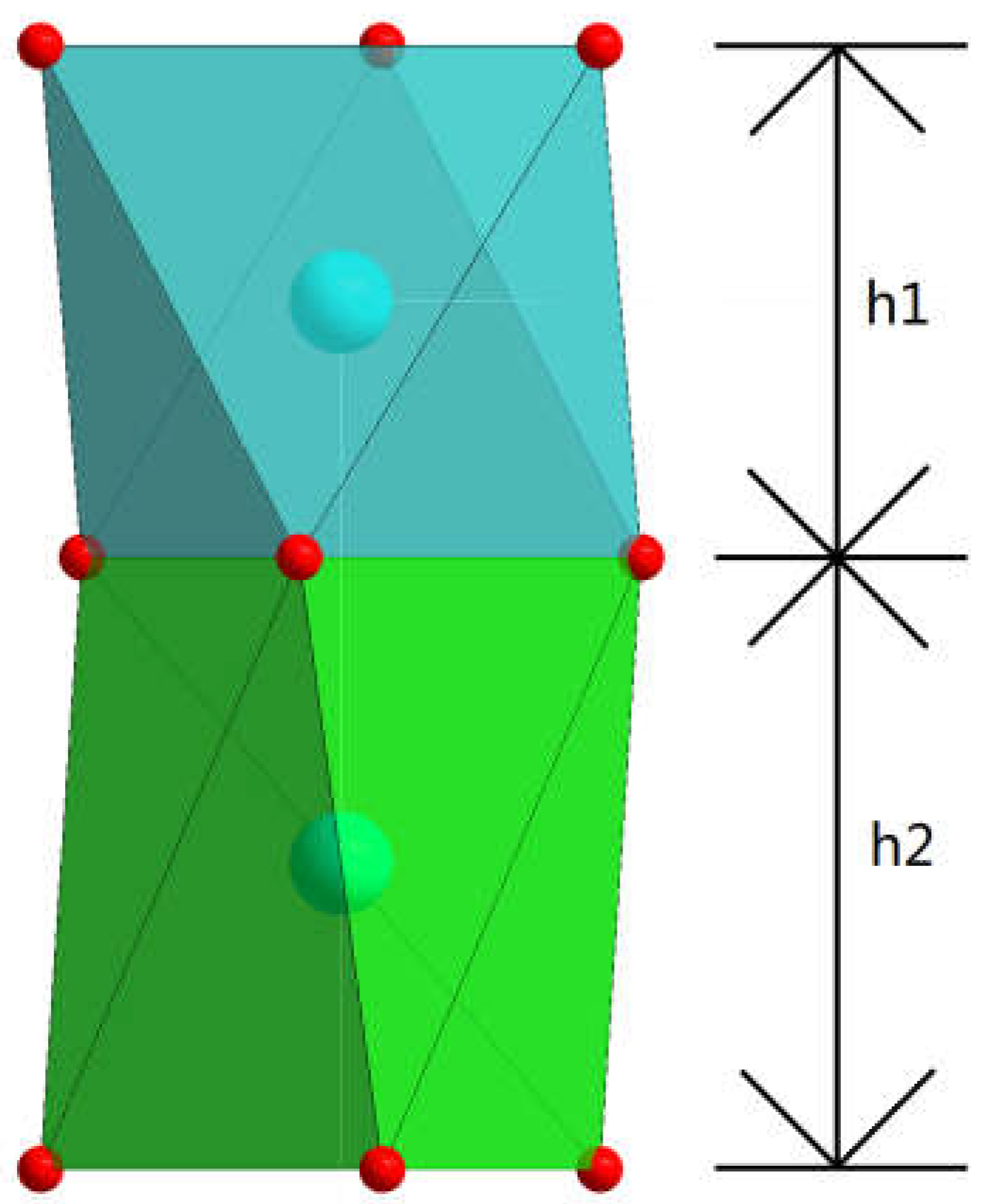
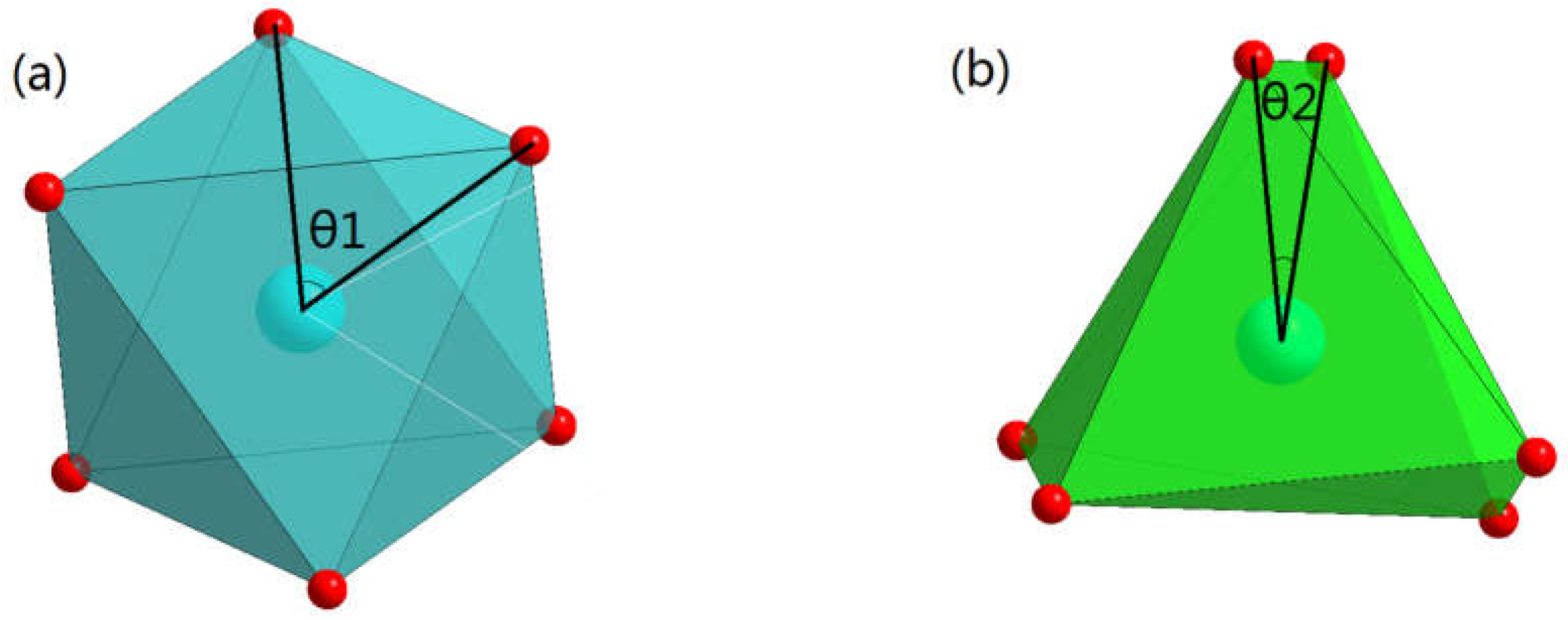
3.2.3. (Er1-xCax)CoO3-z
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Xiao Z.W.; Song Z.N.; Yan Y.F. From Lead Halide Perovskites to Lead-Free Metal Halide Perovskites and Perovskite Derivatives. Adv Mater. 2019, 31(47), pp. 1803792. [CrossRef]
- Shen L.N.; Chen R.; Zhang D.; et al. High-Performance Perovskite Photovoltaics by Heterovalent Substituted Mixed Perovskites. Adv. Funct. Mater. 2022, 32(47), pp. 2207911. [CrossRef]
- Wang J.F.; Luo S.Q.; Lin Y.; et al. Templated growth of oriented layered hybrid perovskites on 3D-like perovskites. Nat Commun. 2020, 11, pp. 582. [CrossRef]
- Mohapatra A.; Singh N.; Singh A.; et al. Solution-Processed Perovskite/Perovskite Heterostructure Via a Grafting-Assisted Transfer Technique. ACS Appl Energy Mater. 2021, 4(2), pp. 1962-1971. [CrossRef]
- Clark C.P.; Mann J.E.; Bangsund J.S.; et al. Formation of Stable Metal Halide Perovskite/Perovskite Heterojunctions. ACS Energy Lett. 2020, 5(11), pp. 3443-3451. [CrossRef]
- Riffat S.B.; Ma X. Thermoelectrics: a review of present and potential applications. Appl Therm Eng. 2003, 23(8), pp. 913-935. [CrossRef]
- Ma Z.; Wei J.T.; Song P.S.; et al. Review of experimental approaches for improving zT of thermoelectric materials. Mat Sci Semicon Proc. 2021, 121, pp. 105303. [CrossRef]
- Zhang S.H.; Niu X.B.; Xie Y.Q.; et al. High intrinsic ZT in InP3 monolayer at room temperature. J Phys-Condens Mat. 2019, 31, 365501. [CrossRef]
- Fjellva G.H.; Gulbrandsen E.; Aasland S.; et al. Crystal Structure and Possible Charge Ordering in One-Dimensional Ca3Co2O6. J Solid State Chem. 1996, 124(1), pp. 190-194. [CrossRef]
- Hardy V.; Flahaut D.; Fresard R.; et al. Anisotropic susceptibility of the geometrically frustrated spin-chain compound Ca3Co2O6. J Phys-Condens Mat. 2007, 19(14), pp. 1898-1908. [CrossRef]
- Hardy V.; Lees M.R.; Petrenko O.A.; et al. Temperature and time dependence of the field-driven magnetization steps in Ca3Co2O6 single crystals. Phys Rev B. 2004, 70(6), pp. 064424. [CrossRef]
- Masset A.C.; Michel C.; Maignan A.; et al. Misfit-layered cobaltite with an anisotropic giant magnetoresistance: Ca3Co4O9. Phys Rev B. 2000, 62(1), 166-175. [CrossRef]
- Kenfaui D.; Chateigner D.; Gomina M.; et al. Texture, mechanical and thermoelectric properties of Ca3Co4O9 ceramics. J Appl Ceram Tec. 2009, 490(1), pp. 472-479. [CrossRef]
- Saini S.; Yaddanapudi H.S.; Tian K.; et al. Terbium Ion Doping in Ca3Co4O9: A Step towards High-Performance Thermoelectric Materials. Sci Rep. 2017, 7, pp. 44621. [CrossRef]
- Ren G.K.; Lan J.L.; Zhao L.D.; et al. Layered oxygen-containing thermoelectric materials: Mechanisms, strategies, and beyond. Mater. Today 2019, 29, pp. 68-85. [CrossRef]
- Tanabe K.; Okazaki R.; et al. Optical conductivity of layered calcium cobaltate Ca3Co4O9. J Phys-Condens Mat. 2016, 085601. [CrossRef]
- Li S.W.; Funahashi R.; Matsubara I.; et al. Synthesis and thermoelectric properties of the new oxide ceramics Ca3-xSrxCo4O9+δ (x=0.0-1.0). Ceram Int. 2001, 27(3), pp. 321-324. [CrossRef]
- Bhattacharya S.; Aswal D.K.; Singh A.; et al. Anisotropic electrical transport studies of Ca3Co4O9 single crystals grown by the flux method. J Cryst Growth. 2005, 277(1-4), pp. 246-251. [CrossRef]
- Wolf M.; Rehder L.; Steinbach F.; et al. Combination of Laser and Thermal Sintering of Thermoelectric Ca3Co4O9 Films. Chem Ing Tech. 2021, 94(1), pp. 177-185. [CrossRef]
- Li Y.N.; Wu P.; Zhang S.P.; Pei Y.L.; et al. Enhanced thermoelectric properties of Ca3Co4O9 by adding nano MoSi2. Ceram Int. 2022, 48(22), pp. 33967-33975. [CrossRef]
- Terasaki I.; Sasago Y.; et al. Large thermoelectric power in NaCo2O4 single crystals. Phys Rev B. 1997, 56(20), R12685–R12687. [CrossRef]
- Wang S.F.; Venimadhav A.; Guo S.M.; et al. Structural and thermoelectric properties of Bi2Sr2Co2Oy thin films on LaAlO3 (100) and fused silica substrates. Appl Phys Lett. 2009, 94(2), pp. R12685. [CrossRef]
- Wong-Ng W.; Yan Y.; Kaduk J.A.; Tang X.F. X-ray powder diffraction reference patterns for Bi1-xPb(x)OCuSe. Power Diffr. 2006, 0885. [CrossRef]
- Wong-Ng W.; Liu G.Y.; Martin J.; et al. Phase compatibility and thermoelectric properties of compounds in the Sr–Ca–Co–O system. J Appl Phys. 2010, 107(3), pp. 188-488. [CrossRef]
- Wong-Ng W.; Luo T.; Xie W.; et al. Phase diagram, crystal chemistry and thermoelectric properties of compounds in the Ca-Co-Zn-O system. J Solid State Chem. 2011, 184(8), pp. 2159-2166. [CrossRef]
- Li S.W.; Funahashi R.; Matsubara I.; et al. Synthesis and Thermoelectric Properties of the New Oxide Materials Ca3-xBixCo4O9+δ (0.0 < x < 0.75). Chem Mater. 2000, 31(47), pp. 2424-2427. [CrossRef]
- Thorogood G.J.; Orain P.Y.; Ouvry M.; Piriou B.; Tedesco T.; Wallwork K.S.; Herrmann J.; James M. Structure, crystal chemistry and magnetism of rare earth calcium-doped cobaltates: Ln2-xCaxCoO4+δ (Ln= Pr, Nd, Sm, Eu and Gd). Solid State Sci. 2011, 13(12), pp. 2113-2123. [CrossRef]
- Wong-Ng W.; Laws W.J.; Yan Y.G. Phase diagram and crystal chemistry of the La-Ca-Co-O system. Solid State Sci. 2013, 17, pp. 107–110. [CrossRef]
- Wong-Ng W.; Laws W.J.; Lapidus S.H.; et al. Phase equilibria and crystal chemistry of the CaO-Nd2O3-CoOz system at 885℃ in air. Solid State Sci. 2014, 215, pp. 128-134. [CrossRef]
- Wong-Ng W.; Laws W.J.; Lapidus S.H.; et al. Phase equilibria and crystal chemistry of the CaO-Sm2O3-CoOz system at 885℃ in air. Solid State Sci. 2015, 48, pp. 31-38. [CrossRef]
- Wong-Ng W.; Laws W.J.; Kaduk J.A. Crystal chemistry and phase equilibria of the CaO-Eu2O3-CoOz system at 885℃ in air. Solid State Sci. 2016, 2558(16), pp. 30391. [CrossRef]
- Wong-Ng W.; Laws W.J.; Lapidus S.H.; et al. Phase equilibria and crystal chemistry of the CaO-Gd2O3-CoOz system at 885℃ in air. Solid State Sci. 2017, 72, pp. 128-134. [CrossRef]
- Wong-Ng W.; Laws W.J.; Kaduk J.A. Crystal chemistry and phase equilibria of the CaO-Dy2O3-CoOz system at 885℃ in air. Solid State Sci. 2018, 88, pp. 57-62. [CrossRef]
- Wong-Ng W.; Laws W.J.; Huang Q.; et al. Crystal chemistry and phase equilibria of the CaO-Ho2O3-CoOz system at 885℃ in air. Solid State Sci. 2020, 107, pp. 106348. [CrossRef]
- Gates-Rector S.D.; Blanton T.N. The Powder Diffraction File: A quality Materials Characterization Database. Powder Diffr. 2019, 34, pp. 352–360.
- Rietveld H.M. A method for including the line profiles of neutron powder diffraction peaks in the determination of crystal structures. Acta Crystallogr. 1966, 229, pp. 151.
- Rodriguez-Carvajal J.L. Recent advances in magnetic structure determination by neutron powder diffraction. Physica B. 1993, 19255. [CrossRef]
- Jain A.; Singh S.; Yusuf S.M. Structural and magnetic properties of spin chain compounds Ca3Co2-xFexO6, Phys.Rev.B 2006, 74 pp. 174419. [CrossRef]
- Shannon R.D. Revised Effective Ionic Radii and Systematic Studies of Interatomie Distances in Halides and Chaleogenides. Acta Cryst. 1976, A32, pp. 751-767. [CrossRef]
| x | a&b (Å) | c (Å) | Rwp | χ2 |
| 0 | 9.07628(8) | 10.38005(12) | 11.0 | 2.22 |
| 0.25 | 9.05344(21) | 10.44059(28) | 10.8 | 3.53 |
| 0.5 | 9.02907(22) | 10.50383(28) | 9.57 | 3.43 |
| 1 | 8.98770(12) | 10.58704(17) | 6.79 | 2.47 |
| x | y | z | Biso [38] | ||
| Ca3Co2O6 | |||||
| Ca | 0.36986(2) | 0 | 0.25 | 0.39 | |
| Co1 | 0 | 0 | 0 | 0.37 | |
| Co2 | 0 | 0 | 0.25 | 0.48 | |
| O | 0.17804(6) | 0.02561(8) | 0.11402(5) | 0.53 | |
| (Ca2.75Er0.25)Co2O6-z | |||||
| Ca/Er | 0.36834(3) | 0 | 0.25 | 0.39 | |
| Co1 | 0 | 0 | 0 | 0.37 | |
| Co2 | 0 | 0 | 0.25 | 0.48 | |
| O | 0.17787(11) | 0.02463(13) | 0.11268(7) | 0.53 | |
| (Ca2.5Er0.5)Co2O6-z | |||||
| Ca/Er | 0.36773(3) | 0 | 0.25 | 0.39 | |
| Co1 | 0 | 0 | 0 | 0.37 | |
| Co2 | 0 | 0 | 0.25 | 0.48 | |
| O | 0.17959(12) | 0.02464(14) | 0.11238(8) | 0.53 | |
| (Ca2Er1)Co2O6-z | |||||
| Ca/Er | 0.36688(2) | 0 | 0.25 | 0.39 | |
| Co1 | 0 | 0 | 0 | 0.37 | |
| Co2 | 0 | 0 | 0.25 | 0.48 | |
| O | 0.18265(12) | 0.02475(13) | 0.11010(7) | 0.53 | |
| x | h1(Å) | h2(Å) | h1+h2(Å) | OccCa | OccEr | θ1(degree) | θ2(degree) |
| 0 | 2.367 | 2.823 | 5.19 | 1 | 0 | 60.000(44) | 15.290(39) |
| 0.25 | 2.3529 | 2.8674 | 5.2203 | 0.916 | 0.076 | 60.000(53) | 14.683(52) |
| 0.5 | 2.3608 | 2.8911 | 5.2519 | 0.834 | 0.16 | 60.000(51) | 14.540(52) |
| 1 | 2.3312 | 2.9623 | 5.2935 | 0.666 | 0.298 | 60.000(45) | 14.349(38) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





