Submitted:
18 May 2023
Posted:
19 May 2023
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Results
2.1. Obtaining and Identification of Isoespintanol
2.2. Antifungal Susceptibility Testing
| Candida tropicalis | ISO | ||
|---|---|---|---|
| MIC90 | MIC50 | MFC | |
| CLI 001 | 470 | 261.2 | 500 |
| CLI 002 | 326.6 | 59.38 | 350 |
| CLI 003 | 413.3 | 124.4 | 400 |
| CLI 004 | 420.8 | 121.5 | 450 |
| CLI 005 | 500 | 234.6 | 500 |
| CLI 006 | 463.9 | 179.8 | 450 |
| CLI 007 | 391.6 | 107 | 400 |
2.3. Effect of ISO on the formation of biofilms
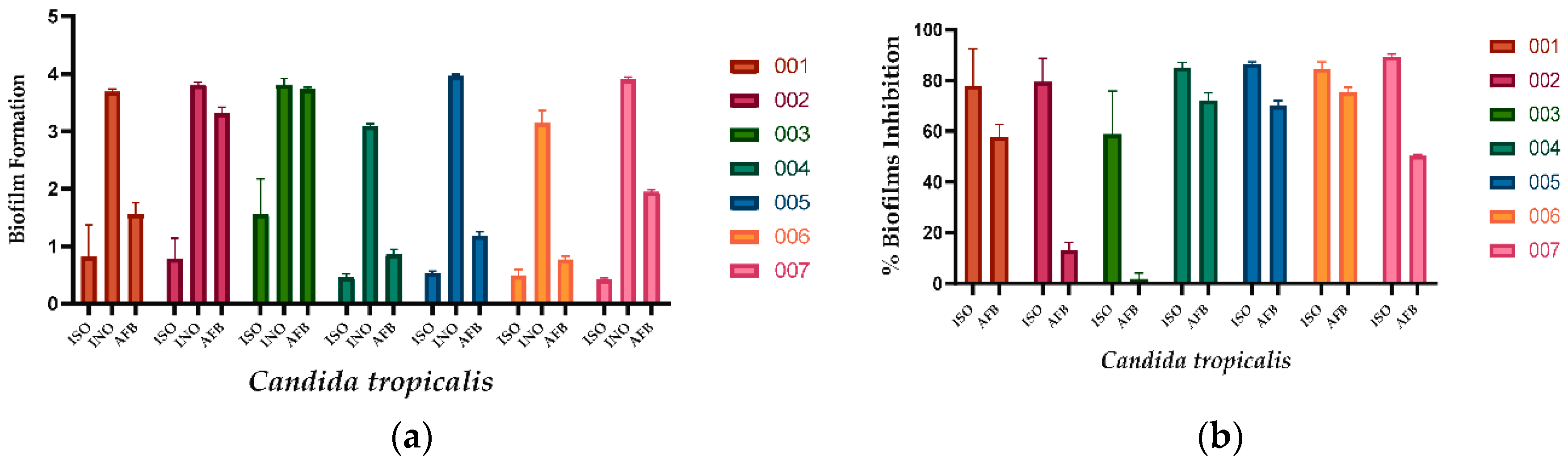
| Candida tropicalis | ISO | AFB |
|---|---|---|
| 001 | 77.80 | 57.70 |
| 002 | 79.48 | 12.86 |
| 003 | 59.18 | 1.87 |
| 004 | 85.09 | 72.23 |
| 005 | 86.46 | 70.09 |
| 006 | 84.38 | 75.45 |
| 007 | 89.35 | 50.30 |
2.4. Effect of ISO on mitochondrial membrane potential (∆Ψm)
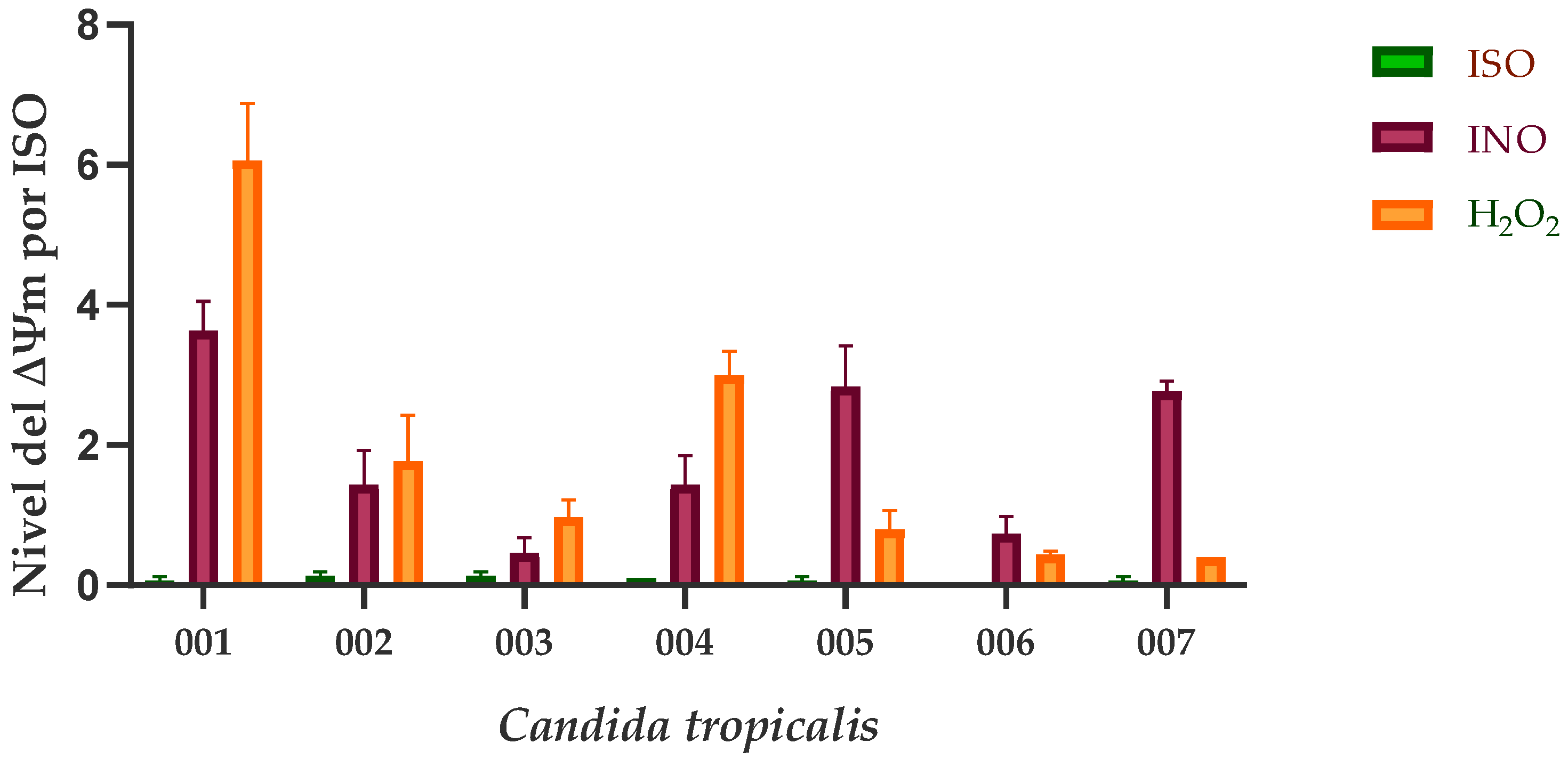
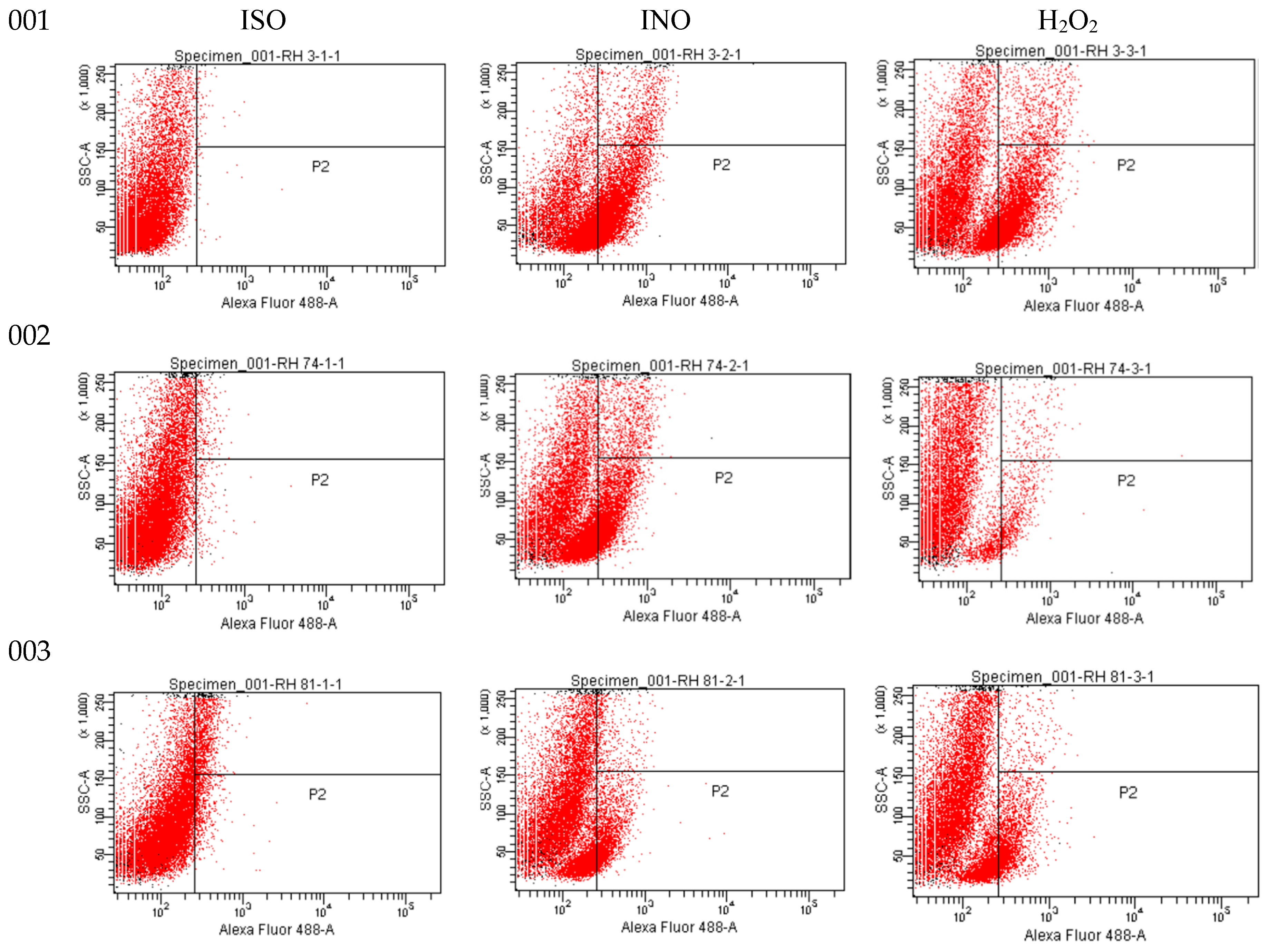
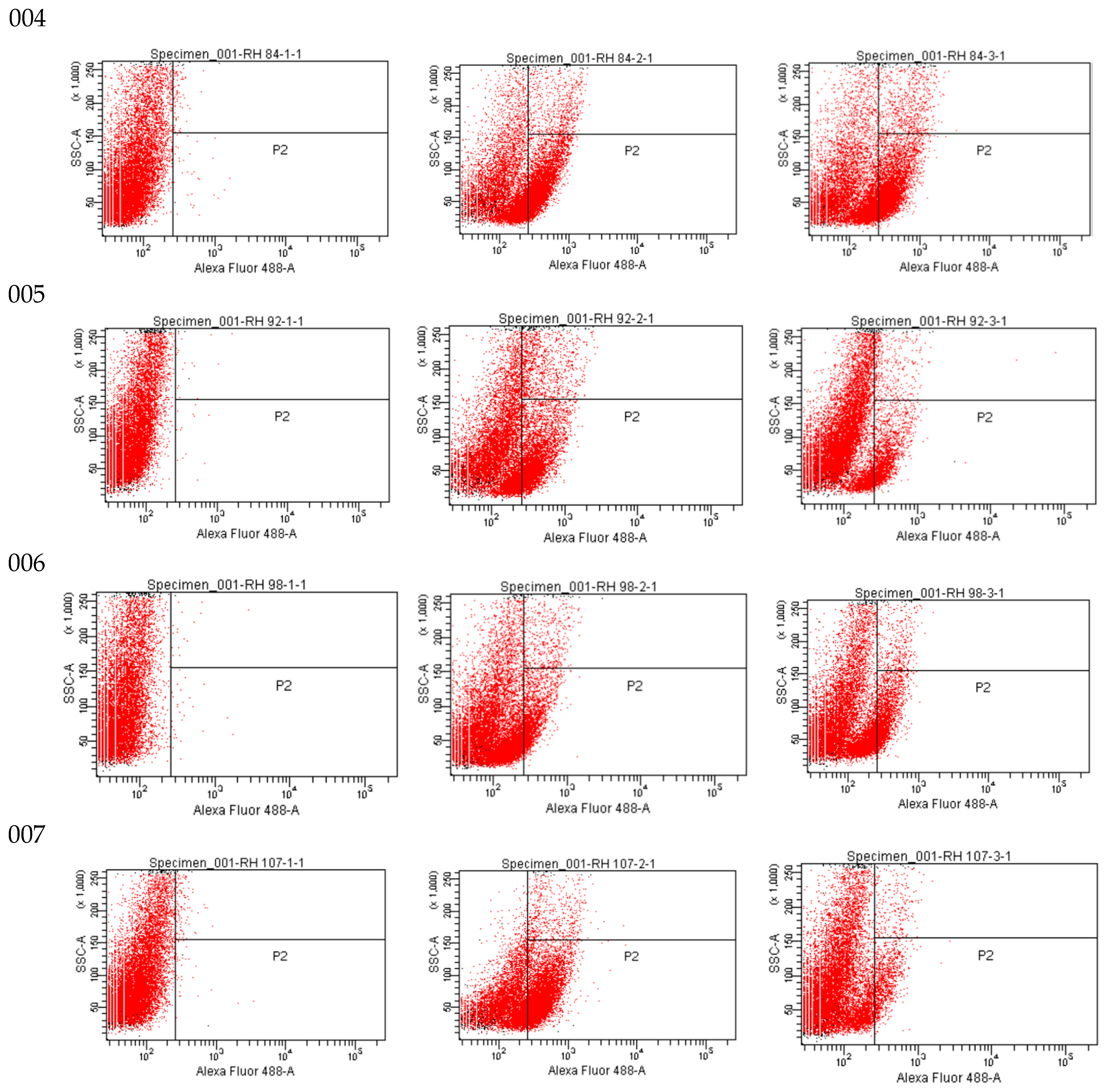
2.5. Effect of ISO on cell wall integrity
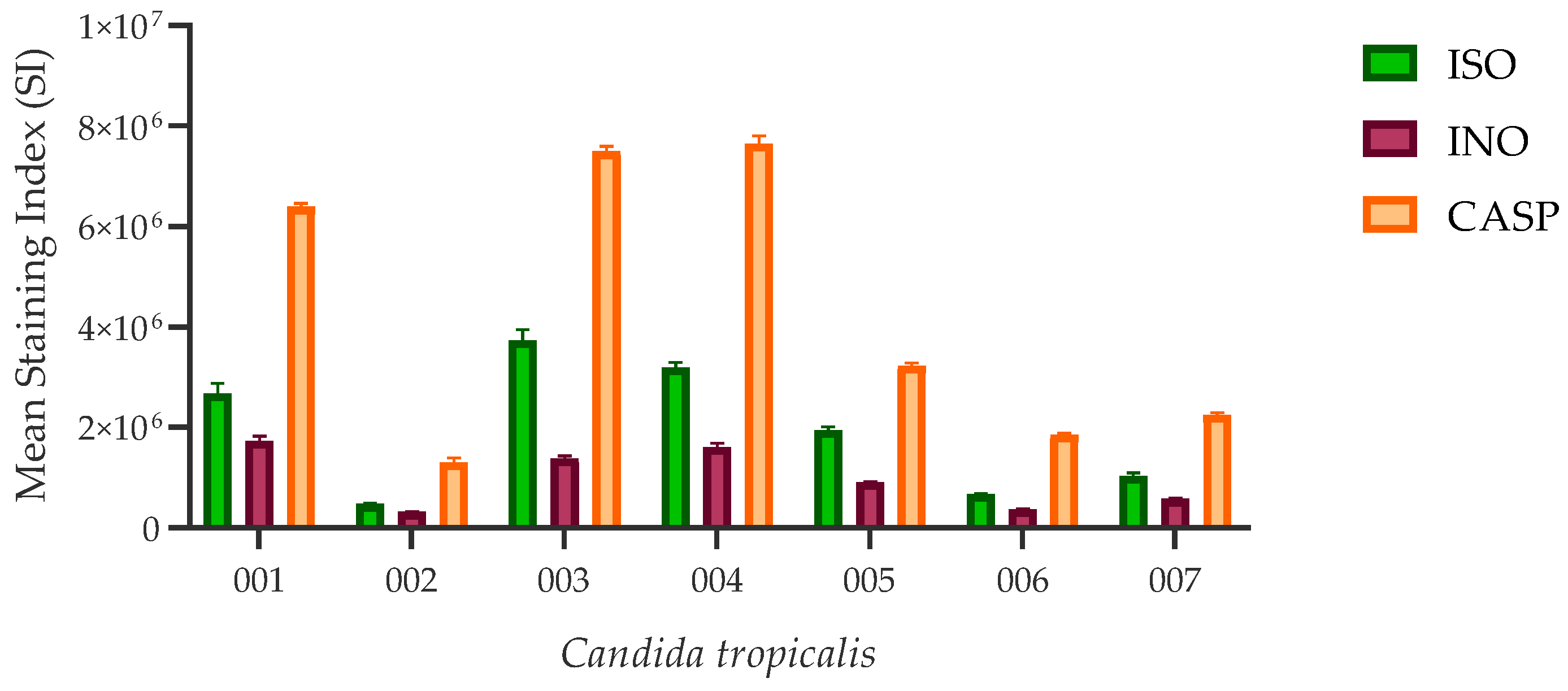
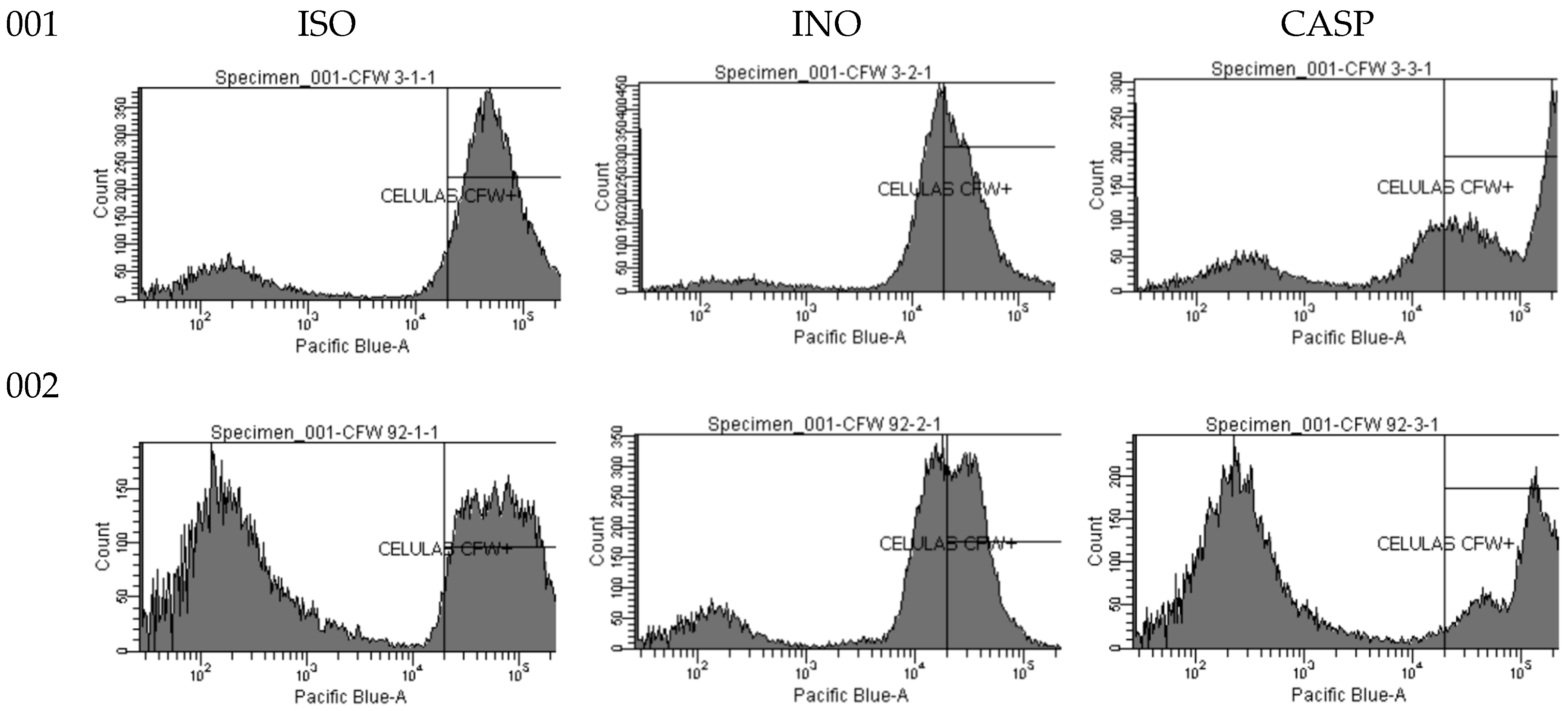
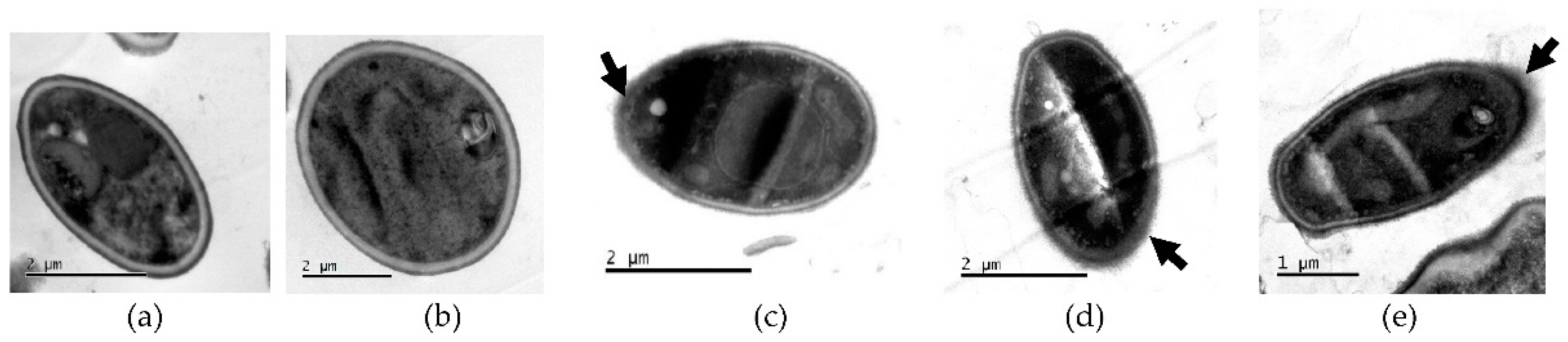
2.5.1. Transmission Electron Microscopy (TEM)
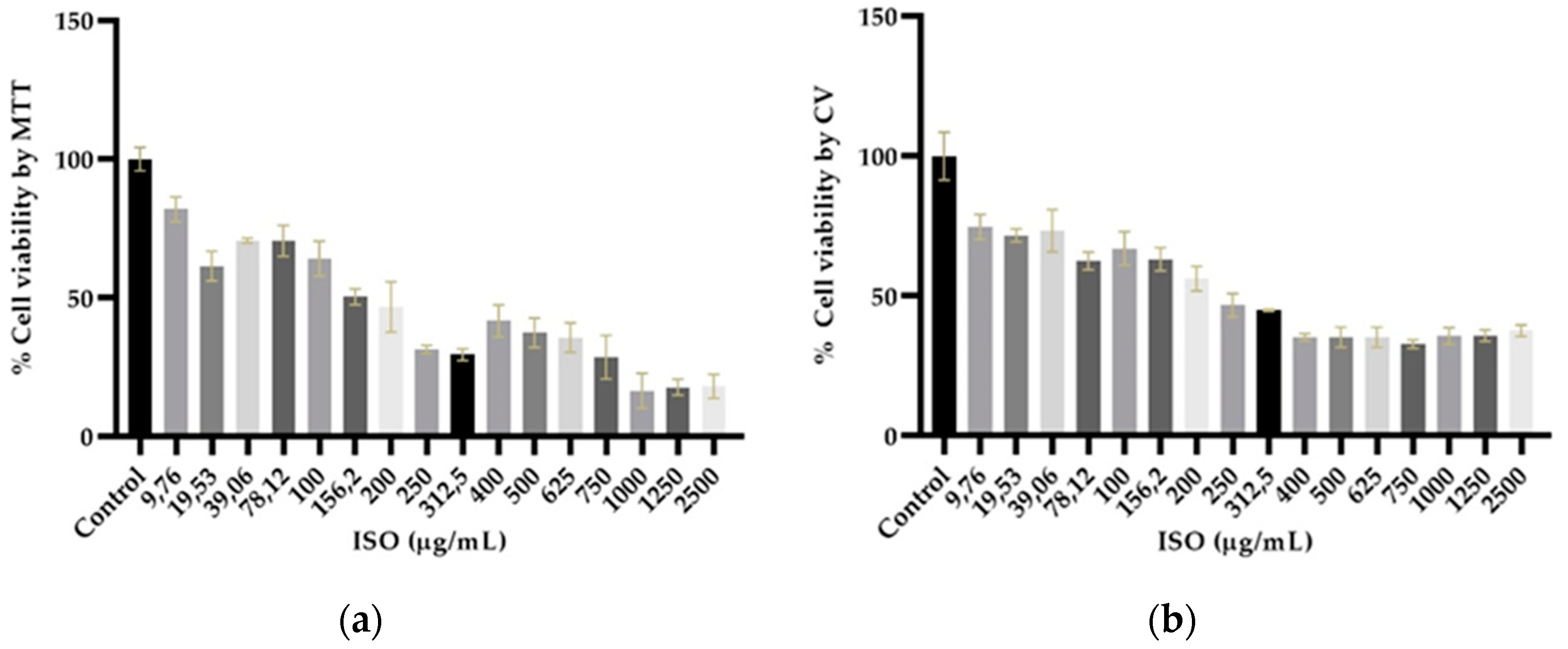
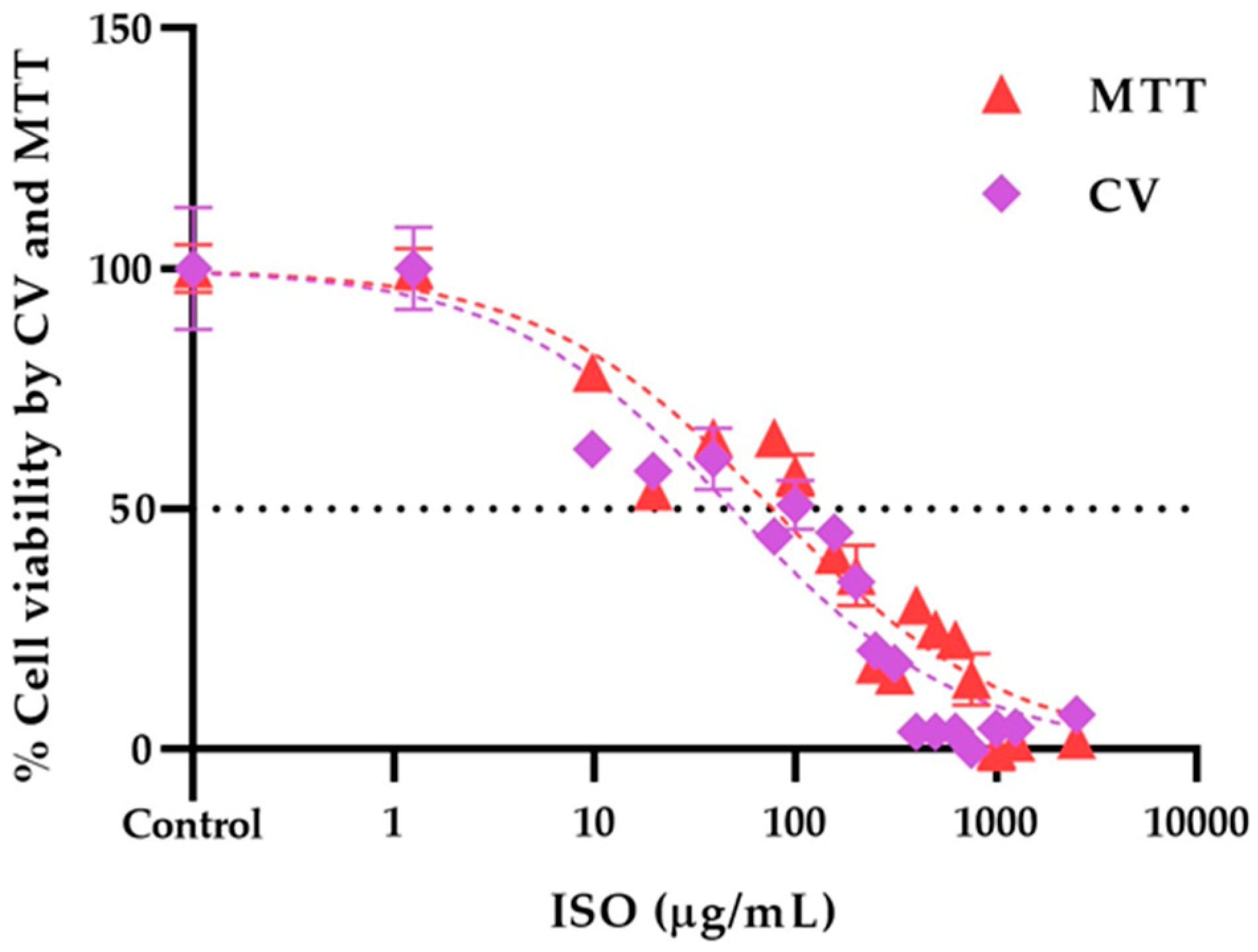
2.6. Isoespintanol cytotoxicity assays
3. Discussion
4. Materials and Methods
4.1. Reagents
4.2. Strains
4.3. Antifungal Susceptibility Testing
4.4. Effect of ISO on the formation of biofilms
4.5. Effect of ISO on mitochondrial membrane potential (∆Ψm)
4.6. Effect of ISO on cell wall integrity
4.6.1. Transmission Electron Microscopy (TEM)
4.7. Isoespintanol cytotoxicity assays
4.7.1. Cell culture
4.7.2. MTT assay
4.7.3. Crystal violet assay (CV)
4.8. Data Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- El-kholy, M. A.; Helaly, G. F.; El Ghazzawi, E. F.; El-sawaf, G.; Shawky, S. M. Virulence factors and antifungal susceptibility profile of C. tropicalis isolated from various clinical specimens in Alexandria, Egypt. J. Fungi 2021, 7 (5), 351. https://doi.org/10.3390/jof7050351. [CrossRef]
- Zuza-Alves, D. L.; Sila-Rocha, W. P.; Chaves, G. An update on Candida tropicalis based on basic and clinical approaches. Front. Microbiol. 2017, 8. https://doi.org/10.3389/fmicb.2017.01927. [CrossRef]
- Munhoz-Alves, N.; Nishiyama Mimura, L. A.; Viero, R. M.; Bagagli, E.; Schatzmann. Candida tropicalis systemic infection redirects leukocyte infiltration to the kidneys attenuating encephalomyelitis. J. Fungi 2021, 7, 757. https://doi.org/10.3390/jof7090757. [CrossRef]
- Silva, S.; Negri, M.; Henriques, M.; Oliveira, R.; Williams, D. W.; Azeredo, J. Candida glabrata, Candida parapsilosis and Candida tropicalis: Biology, epidemiology, pathogenicity and antifungal resistance. FEMS Microbiol. Rev. 2012, 36 (2), 288–305. https://doi.org/10.1111/j.1574-6976.2011.00278.x. [CrossRef]
- Cortés, J. A.; Ruiz, J. F.; Melgarejo-Moreno, L. N.; Lemos, E. V. Candidemia in Colombia. Biomedica 2020, 40 (1), 195–207. https://doi.org/10.7705/biomedica.4400. [CrossRef]
- Avato, P. Editorial to the Special Issue –“Natural products and drug discovery”. Molecules 2020, 25, 1128. https://doi.org/10.3390/molecules25051128. [CrossRef]
- Morales, I.; De La Fuente, J.; Sosa, V. Componentes de Eupatorium saltense. An Asoc Quim Argent 1991, 79 (3), 141–144.
- Hocquemiller, R.; Cortes, D.; Arango, G. J.; Myint, S. H.; Cave, A. Isolement et synthese de l’espintanol, nouveau monoterpene antiparasitaire. J. Nat. Prod. 1991, 54, 445–452.
- Rojano, B.; Pérez, E.; Figadère, B.; Martin, M. T.; Recio, M. C.; Giner, R.; Ríos, J. L.; Schinella, G.; Sáez, J. Constituents of Oxandra Cf. xylopioides with anti-inflammatory activity. J. Nat. Prod. 2007, 70 (5), 835–838. https://doi.org/10.1021/np060333v. [CrossRef]
- Rojano, B. A.; Gaviria, C. A.; Gil, M. A.; Saéz, J. A.; Schinella, G. R.; Tournier, H. Antioxidant activity of the isoespintanol in diferent media. Vitae 2008, 15 (1), 173–181.
- Gavilánez Buñay, T. C.; Colareda, G. A.; Ragone, M. I.; Bonilla, M.; Rojano, B. A.; Schinella, G. R.; Consolini, A. E. Intestinal, urinary and uterine antispasmodic effects of isoespintanol, metabolite from Oxandra xylopioides leaves. Phytomedicine 2018, 51, 20–28. https://doi.org/10.1016/j.phymed.2018.06.001. [CrossRef]
- Rinaldi, G. J.; Rojano, B.; Schinella, G.; Mosca, S. M. Participation of NO in the vasodilatory action of isoespintanol. Vitae 2019, 26, 78–83. https://doi.org/10.17533/udea.vitae.v26n2a03. [CrossRef]
- González Arbeláez, L.; Ciocci Pardo, A.; Fantinelli, J. C.; Rojano, B.; Schinella, G.; Mosca, S. M. Isoespintanol, a monoterpene isolated from Oxandra cf xylopioides, ameliorates the myocardial ischemia-reperfusion injury by AKT/PKCε/ENOS-dependent pathways. Naunyn-Schmiedeberg’s Arch Pharmacol 2020, 393 (4), 629–638. https://doi.org/10.1007/s00210-019-01761-9. [CrossRef]
- Usuga, A.; Tejera, I.; Gómez, J.; Restrepo, O.; Rojano, B.; Restrepo, G. Cryoprotective effects of ergothioneine and isoespintanol on canine semen. Animals 2021, 11, 2757. https://doi.org/10.3390/ani11102757. [CrossRef]
- Rojano, B. A.; Montoya, S.; Yépez, F.; Saez, J. Evaluación de isoespintanol aislado de Oxandra Cf. xylopioides (Annonaceae) sobre Spodoptera frugiperda J.E. Smith (Lepidoptera: Noctuidae); 2007; Vol. 60.
- Arango, N.; Vanegas, N.; Saez, J.; García, C.; Rojano, B. Actividad antifungica del isoespintanol sobre hongos del género Colletotricum. Sci. Tech. 2007, 33, 279–280. https://doi.org/10.22517/23447214.6055. [CrossRef]
- Contreras Martínez, O. I.; Angulo Ortíz, A.; Santafé Patiño, G. Antibacterial screening of isoespintanol, an aromatic monoterpene isolated from Oxandra xylopioides Diels. Molecules 2022, 27 (22). https://doi.org/10.3390/molecules27228004. [CrossRef]
- Contreras Martínez, O. I.; Ortíz, A. A.; Patiño, G. S. Antifungal potential of isoespintanol extracted from Oxandra xylopioides Diels (Annonaceae) against intrahospital isolations of Candida SPP. Heliyon 2022, 8 (10). https://doi.org/10.1016/j.heliyon.2022.e11110. [CrossRef]
- Contreras, O.; Angulo, A.; Santafé, G. Mechanism of antifungal action of monoterpene isoespintanol against clinical isolates of Candida tropicalis. Molecules 2022, 27, 5808. https://doi.org/10.3390/molecules27185808. [CrossRef]
- Kawai, A.; Yamagishi; Mikamo, H. Time-lapse tracking of Candida tropicalis biofilm formation and the antifungal efficacy of liposomal amphotericin B. Jpn. J. Infect. Dis. 2017, 70, 559–564. https://doi.org/10.7883 yoken.JJID.2016.574.
- Guembe, M.; Cruces, R.; Peláez, T.; Mu, P.; Bouza, E. Assessment of biofilm production in Candida isolates according to species and origin of infection. Enferm. Infecc. Microbiol. Clin. 2017, 35 (1), 37–40. https://doi.org/10.1016/j.eimce.2017.01.011. [CrossRef]
- Tascini, C.; Sozio, E.; Corte, L.; Sbrana, F. The role of biofilm forming on mortality in patients with candidemia : a study derived from real world data. Infect. Dis. (Auckl). 2017, 50 (3) (0), 1–6. https://doi.org/10.1080/23744235.2017.1384956. [CrossRef]
- Karpiński, T. M.; Ożarowski, M.; Seremak-Mrozikiewicz, A.; Wolski, H.; Adamczak, A. Plant preparations and compounds with activities against biofilms formed by Candida Spp. J. Fungi 2021, 7 (5), 1–13. https://doi.org/10.3390/jof7050360. [CrossRef]
- Tariq, S.; Wani, S.; Rasool, W.; Shafi, K.; Bhat, M. A.; Prabhakar, A.; Shalla, A. H.; Rather, M. A. A Comprehensive review of the antibacterial, antifungal and antiviral potential of essential oils and their chemical constituents against drug-resistant microbial pathogens. Microb. Pathog. 2019, 134 (June), 103580. https://doi.org/10.1016/j.micpath.2019.103580. [CrossRef]
- Chang, C. K.; Kao, M. C.; Lan, C. Y. Antimicrobial activity of the peptide Lfcinb15 against Candida albicans. J. Fungi 2021, 7 (7). https://doi.org/10.3390/jof7070519. [CrossRef]
- Hussain, S. Measurement of nanoparticle-induced mitochondrial membrane potential alterations. In Nanotoxicity Methods and Protocols, 1st ed.; Qunwei Zhang., Eds.; Publisher: Humana New York, NY, USA, 2018; Volume 1894, pp. 123–131. https://doi.org/10.1007/978-1-4939-8916-4. [CrossRef]
- Sakamuru, S.; Attene-Ramos, M. S.; Xia, M. Mitochondrial membrane potential assay. Methods Mol Biol. 2016, 1473 (5), 17–22. https://doi.org/10.1007/978-1-4939-6346-1. [CrossRef]
- Marika, G. J.; Saez, G. T.; O’Connor, J.-E. A fast kinetic method for assessing mitochondrial membrane potential in isolated hepatocytes with rhodamine 123 and flow cytometry. Cytometry 1994, 15 (4), 335–342. https://doi.org/10.1002/cyto.990150409. [CrossRef]
- Johnson, L. V. V.; Walsh, M. L. L.; Bockus, B. J.; Chen, L. B. Monitoring of relative mitochondrial membrane potential in living cells by fluorescence microscopy. J. Cell Biol. 1981, 88 (3), 526–535. https://doi.org/10.1083/jcb.88.3.526. [CrossRef]
- Baracca, A.; Sgarbi, G.; Solaini, G.; Lenaz, G. Rhodamine 123 as a probe of mitochondrial membrane potential: evaluation of proton flux through F0 during ATP synthesis. Biochim. Biophys. Acta - Bioenerg. 2003, 1606 (1–3), 137–146. https://doi.org/10.1016/S0005-2728(03)00110-5. [CrossRef]
- Zorova, L. D.; Popkov, V. A.; Plotnikov, E. Y.; Silachev, D. N.; Pevzner, I. B.; Jankauskas, S. S.; Babenko, V. A.; Zorov, S. D.; Balakireva, A. V.; Juhaszova, M.; Sollott, S. J.; Zorov, D. B. Mitochondrial membrane potential. Anal. Biochem. 2018, 552, 50–59. https://doi.org/10.1016/j.ab.2017.07.009. [CrossRef]
- Holanda, M. A.; da Silva, C. R.; Neto, J. B.; do AV Sa, L. G.; do Nascimento, F. B.; Barrosos, D.; da Silva, L.; Cándido, T. M.; Leitao, A. D.; Barbosa, A. D.; de Moraes, M. O.; CC, B.; Nobre, H. B. Evaluation of the antifungal activity in vitro of midazolam against fluconazole-resistant Candida spp. isolates. Future Microbiol. 2021, 16, 71–81. https://doi.org/10.2217/fmb-2020-0080. [CrossRef]
- Lu, J.; Wu, L.; Wang, X.; Zhu, J.; Du, J.; Shen, B. Detection of mitochondria membrane potential to study CLIC4 knockdown-induced HN4 cell apoptosis in vitro. J. Vis. Exp. 2018, 2018 (137), 1–8. https://doi.org/10.3791/56317. [CrossRef]
- Hwang, I.; Lee, J.; Jin, H.-G.; Woo, E.-R.; Lee, D. G. Amentoflavone stimulates mitochondrial dysfunction and induces apoptotic cell death in Candida albicans. Mycopathologia 2012, 173, 207–218. https://doi.org/10.1007/s11046-011-9503-x. [CrossRef]
- Klis, F. M. Review: Cell wall assembly in yeast. Yeast 1994, 10 (7), 851–869. https://doi.org/10.1002/yea.320100702. [CrossRef]
- Munro, C. A.; Gow, N. A. R. Chitin synthesis in human pathogenic fungi. Med. Mycol. Suppl. 2001, 39 (1), 41–53. https://doi.org/10.1080/mmy.39.1.41.53. [CrossRef]
- Ram, A. F. J.; Arentshorst, M.; Damveld, R. A.; vanKuyk, P. A.; Klis, F. M.; van den Hondel, C. A. M. J. J. The cell wall stress response in Aspergillus niger involves increased expression of the glutamine: fructose-6-phosphate amidotransferase-encoding gene (GfaA) and increased deposition of chitin in the cell wall. Microbiology 2004, 150 (10), 3315–3326. https://doi.org/10.1099/mic.0.27249-0. [CrossRef]
- Hagen, S.; Marx, F.; Ram, A. F.; Meyer, V. The antifungal protein AFP from Aspergillus giganteus inhibits chitin synthesis in sensitive fungi. Appl. Environ. Microbiol. 2007, 73 (7), 2128–2134. https://doi.org/10.1128/AEM.02497-06. [CrossRef]
- Costa de Oliveira, S.; Silva, A.; Miranda, I.; Salvador, A.; Azevedo, M.; Munro, C.; Rodríguez, A.; Pina-Vaz, C. Determination of chitin content in fungal cell wall: an alternative flow cytometric method. Cytom. part A 2013, 83A, 324–328. https://doi.org/10.1002/cyto.a.22250. [CrossRef]
- Walker, L. A.; Munro, C. A.; De Bruijn, I.; Lenardon, M. D.; McKinnon, A.; Gow, N. A. R. Stimulation of chitin synthesis rescues Candida albicans from echinocandins. PLoS Pathog. 2008, 4 (4). https://doi.org/10.1371/journal.ppat.1000040. [CrossRef]
- Fotakis, G.; Timbrell, J. A. In vitro cytotoxicity assays: comparison of LDH, neutral red, MTT and protein assay in hepatoma cell lines following exposure to cadmium chloride. Toxicol. Lett. 2006, 160 (2), 171–177. https://doi.org/10.1016/j.toxlet.2005.07.001. [CrossRef]
- Sliwka, L.; Wiktorska, K.; Suchocki, P.; Milczarek, M.; Mielczarek, S.; Lubelska, K.; Cierpial, T.; Lyzwa, P.; Kielbasinski, P.; Jaromin, A.; Flis, A.; Chilmonczyk, Z. The comparison of MTT and CVS assays for the assessment of anticancer agent interactions. PLoS One 2016, 11. https://doi.org/10.1371/journal.pone.0155772. [CrossRef]
- Feoktistova, M.; Geserick, P.; Leverkus, M. Crystal violet assay for determining viability of cultured cells. Cold Spring Harb. Protoc. 2016, 2016 (4), 343–346. https://doi.org/10.1101/pdb.prot087379. [CrossRef]
- Weyermann, J.; Lochmann, D.; Zimmer, A. A practical note on the use of cytotoxicity assays. Int. J. Pharm. 2005, 288 (2), 369–376. https://doi.org/10.1016/j.ijpharm.2004.09.018. [CrossRef]
- Marquez-Fernandez, M.; Munoz-Lasso, D.; Bautista Lopez, J.; Zapata, K.; Puertas Mejia, M.; Lopez-Alarcon, C.; Rojano, B. A. Effect of isoespintanol isolated from Oxandra Cf. xylopioides against DNA damage of human lymphocytes. Pak. J. Pharm. Sci 2018, 31, 1777–1782.
- Zapata, K.; Arias, J.; Cortés, F.; Alarcon, C.; Durango, D.; Rojano, B. Oxidative stabilization of palm olein with isoespintanol (2-isopropyl-3,6-dimethoxy-5-methylphenol) isolated from Oxandra Cf xylopioides. J. Med. Plants Res. 2017, 11, 218–225.
- Cantón, E.; Martín, E.; Espinel-Ingroff, A. Métodos estandarizados por el CLSI para el estudio de la sensibilidad a los antifúngicos (Documentos M27-A3, M38-A y M44-A). Rev. Iberoam. Micol. 2007.
- Rodriguez-tudela, J. L. Method for determination of minimal inhibitory concentration ( MIC ) by broth dilution of fermentative yeasts. Clin. Microbiol. Infect. 2003, No. August. https://doi.org/10.1046/j.1469-0691.2003.00789.x. [CrossRef]
- Hoch, H. C.; Galvani, C. D.; Szarowski, D. H.; Turner, J. N. Two new fluorescent dyes applicable for visualization of fungal cell walls. Mycologia 2005, 97 (3), 580–588. https://doi.org/10.1080/15572536.2006.11832788. [CrossRef]
- Monheit, J. G.; Brown, G.; Kott, M. M.; Schmidt, W. A.; Moore, D. G. Calcofluor white detection of fungi in cytopathology. Am. J. Clin. Pathol. 1986, 85 (2), 222–225. https://doi.org/10.1093/ajcp/85.2.222. [CrossRef]
- Araldi, R. P.; dos Santos, M. O.; Barbon, F. F.; Manjerona, B. A.; Meirelles, B. R.; de Oliva Neto, P.; da Silva, P. I.; dos Santos, L.; Camargo, I. C. C.; de Souza, E. B. Analysis of antioxidant, cytotoxic and mutagenic potential of Agave sisalana perrine extracts using vero cells, human lymphocytes and mice polychromatic erythrocytes. Biomed. Pharmacother. 2018, 98 (January), 873–885. https://doi.org/10.1016/j.biopha.2018.01.022. [CrossRef]
- Hussein, H. A.; Maulidiani, M.; Abdullah, M. A. Microalgal metabolites as anti-cancer/anti-oxidant agents reduce cytotoxicity of elevated silver nanoparticle levels against non-cancerous VERO cells. Heliyon 2020, 6 (10), e05263. https://doi.org/10.1016/j.heliyon.2020.e05263. [CrossRef]
- Motlhatlego, K.; Ali, M.; Leonard, C.; Eloff, J.; McGaw, L. Inhibitory effect of newtonia extracts and myricetin-3-O-rhamnoside (myricitrin) on bacterial biofilm formation. BMC Complement. Med. Ther. 2020, 20, 358. https://doi.org/10.1186/s12906-020-03139-4. [CrossRef]
- Negrette-Guzmán, M.; Huerta-Yepez, S.; Vega, M. I.; León-Contreras, J. C.; Hernández-Pando, R.; Medina-Campos, O. N.; Rodríguez, E.; Tapia, E.; Pedraza-Chaverri, J. Sulforaphane induces differential modulation of mitochondrial biogenesis and dynamics in normal cells and tumor cells. Food Chem. Toxicol. 2017, 100, 90–102. https://doi.org/10.1016/j.fct.2016.12.020. [CrossRef]
- Mosmann, T. Rapid colorimetric assay for cellular growth and survival: application to proliferation and cytotoxicity assays. J. Immunol. 1983, 65, 55–63.
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





