Submitted:
17 May 2023
Posted:
18 May 2023
You are already at the latest version
Abstract
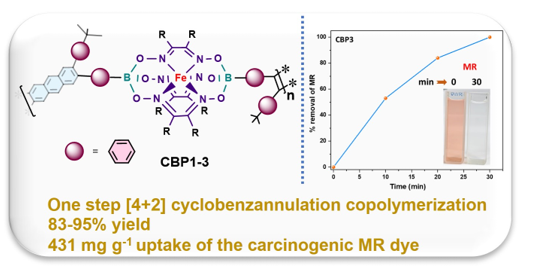
Keywords:
1. Introduction
2. Materials and Methods
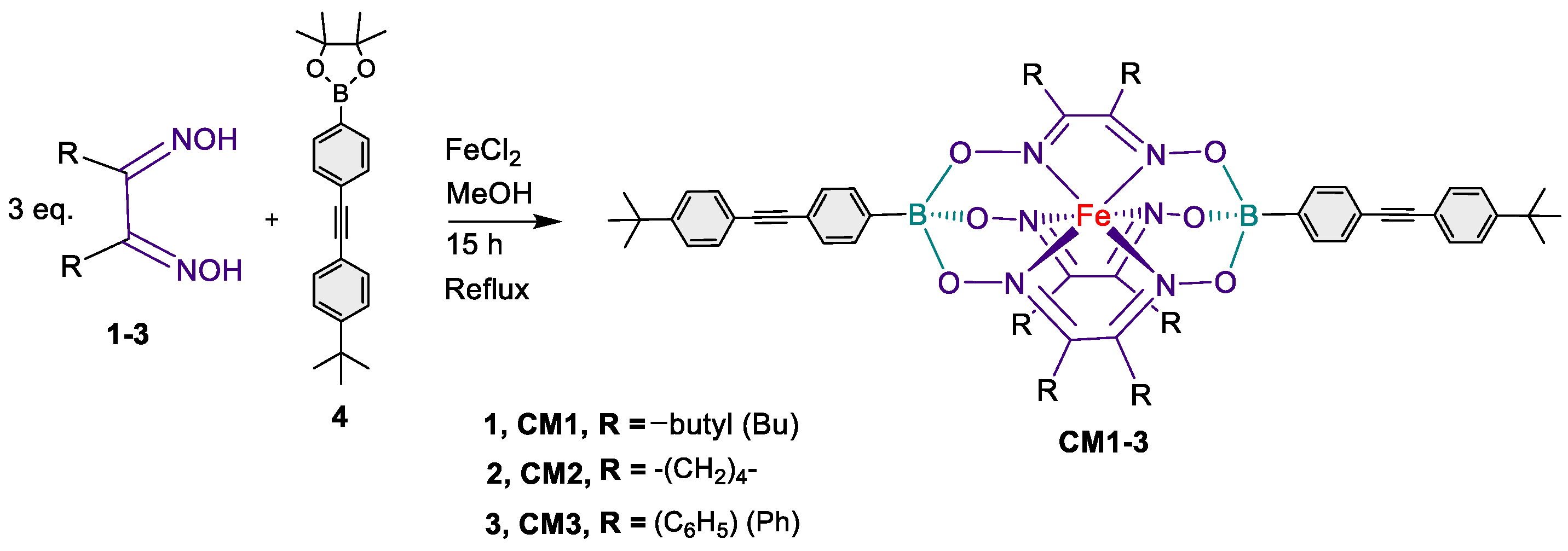
2.1. Synthesis
2.1.1. Synthesis of CM1 (Procedure A).
2.1.2. Synthesis of CM2
2.1.3. Synthesis of CM3
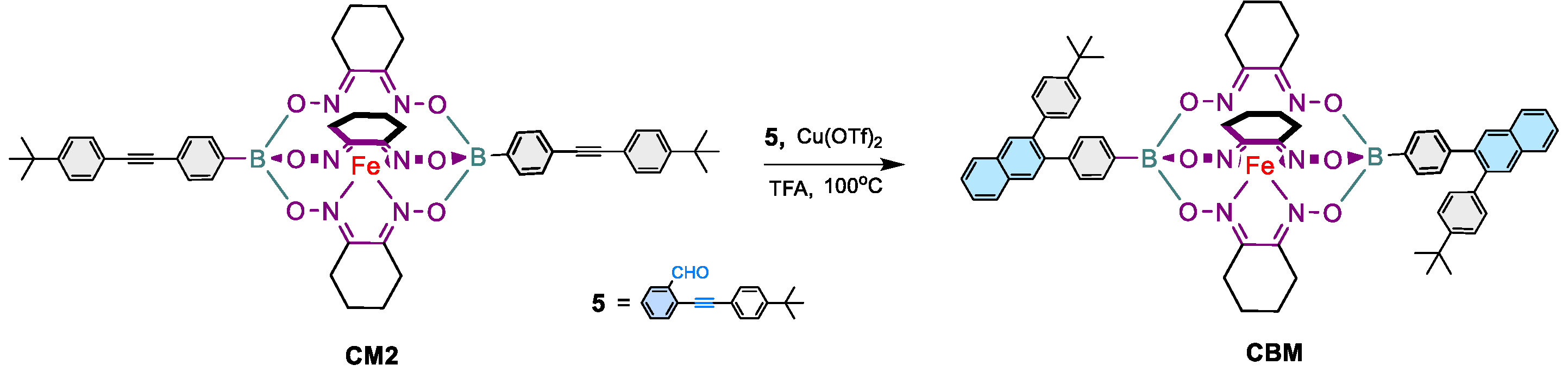
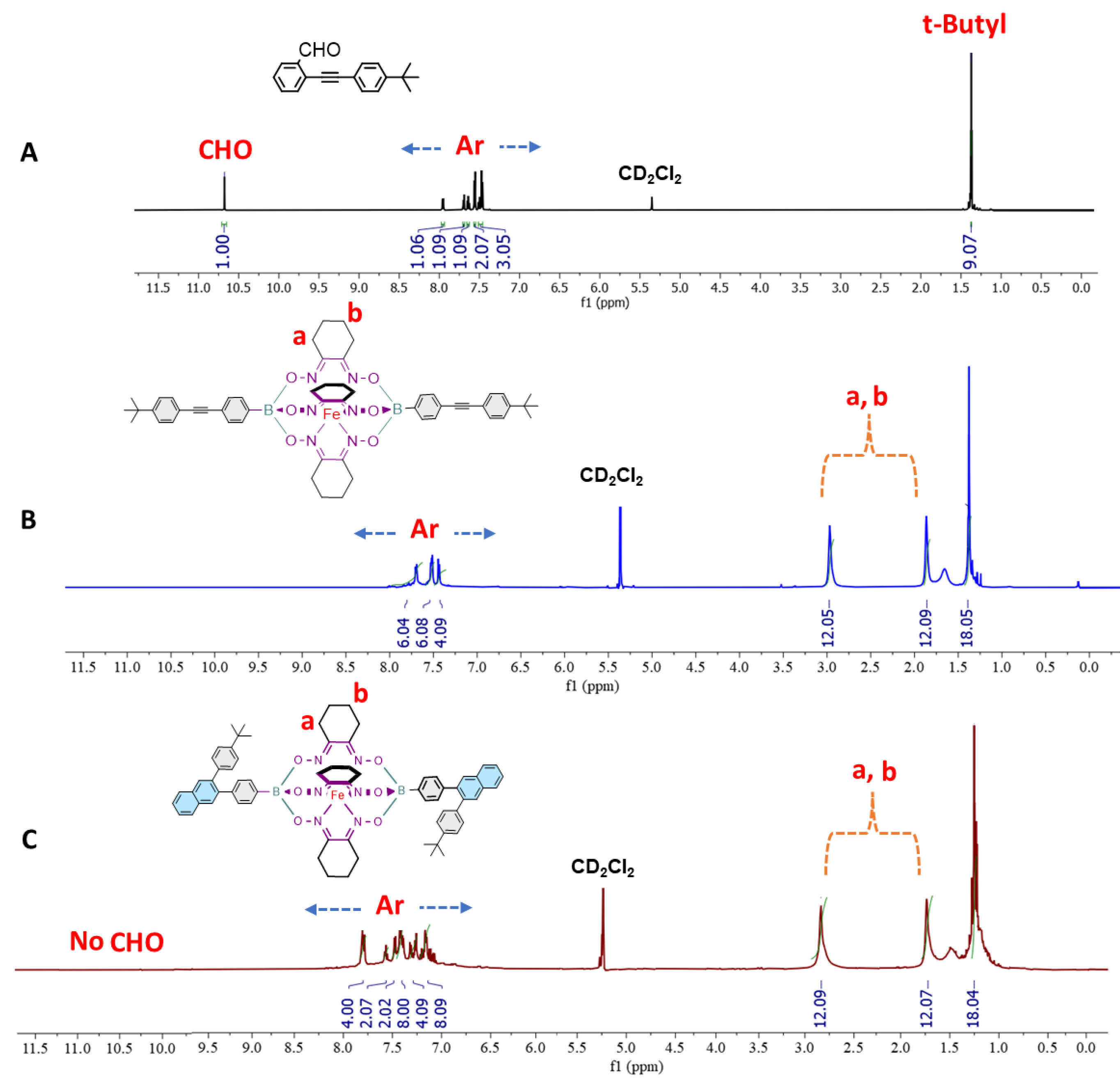
2.1.4. Synthesis of CBM
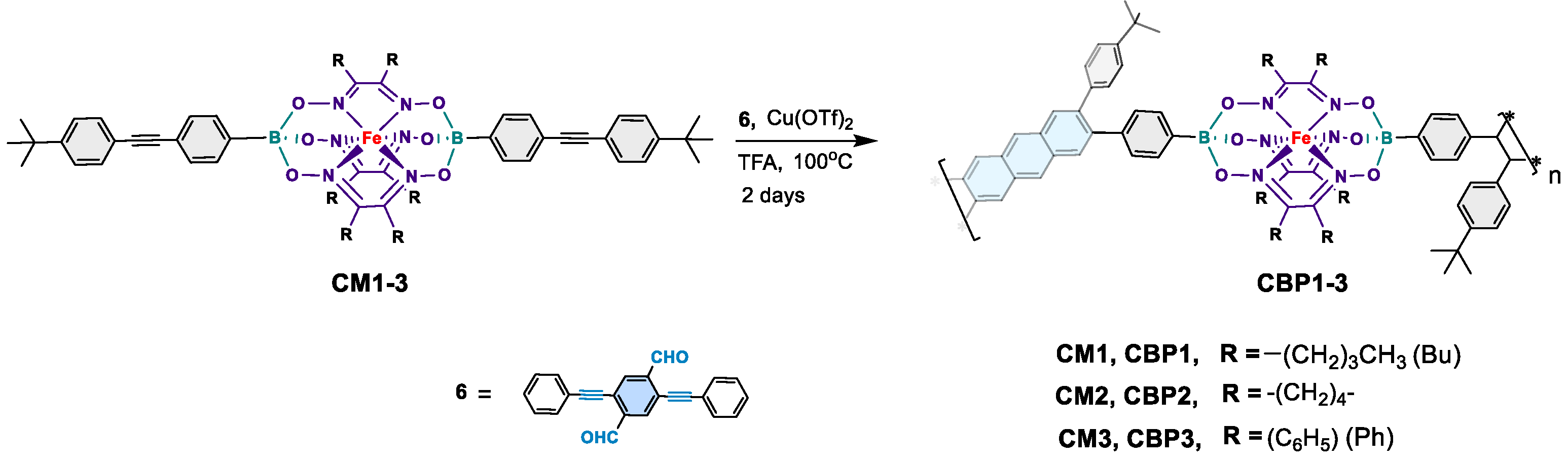
2.1.5. Synthesis of copolymer CBP1 (Procedure B)
2.1.6. Synthesis of CBP2
2.1.7. Synthesis of CBP3
3. Results and discussion
3.1. Synthesis
3.1.1. Synthesis of the prototypical monomer CBM
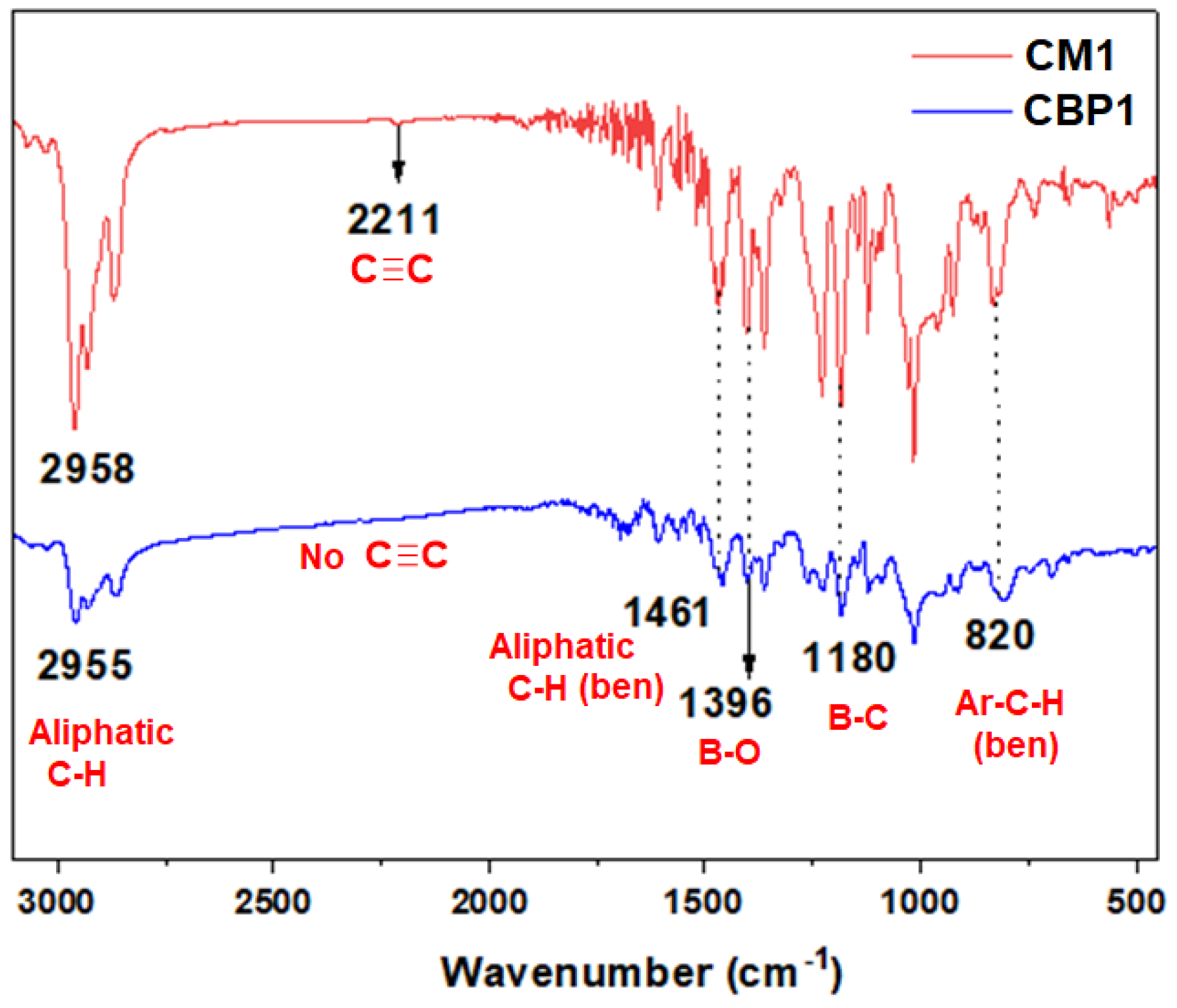
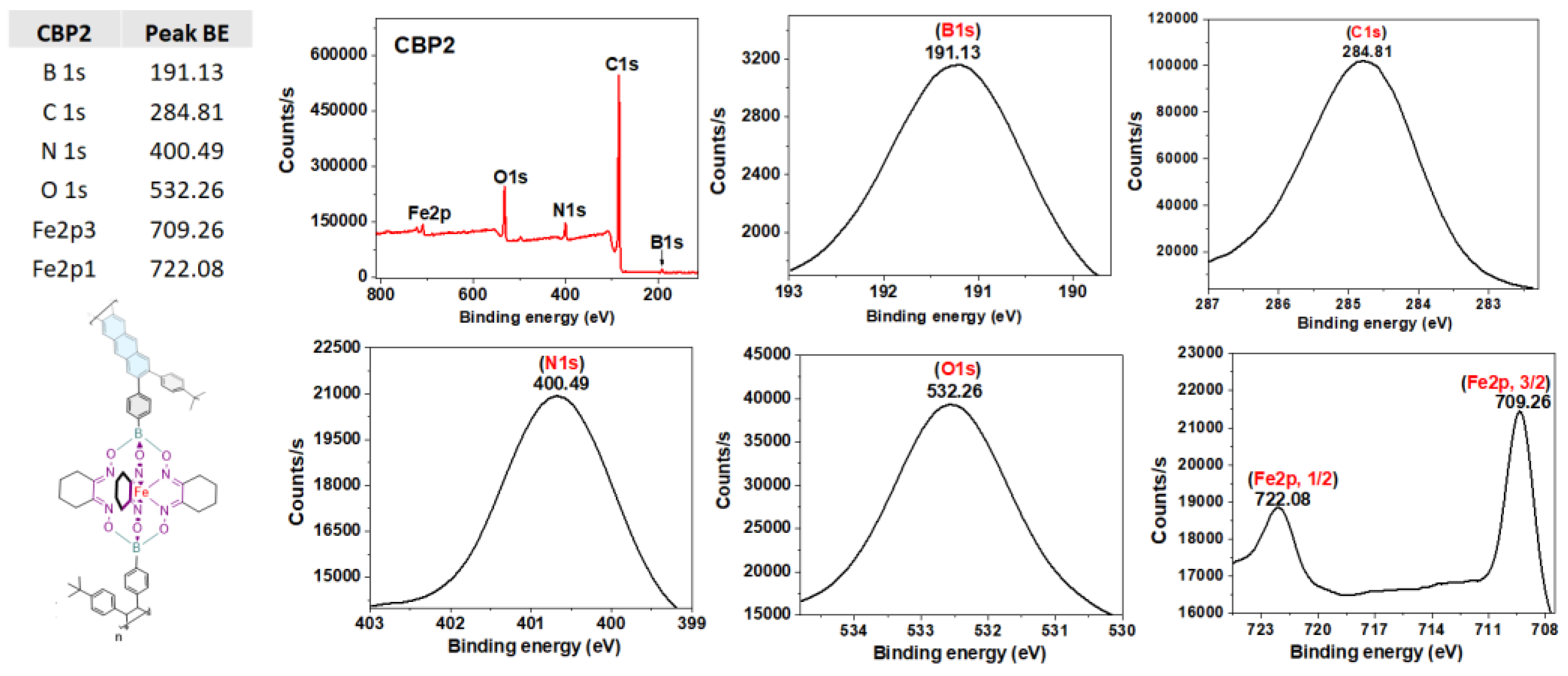
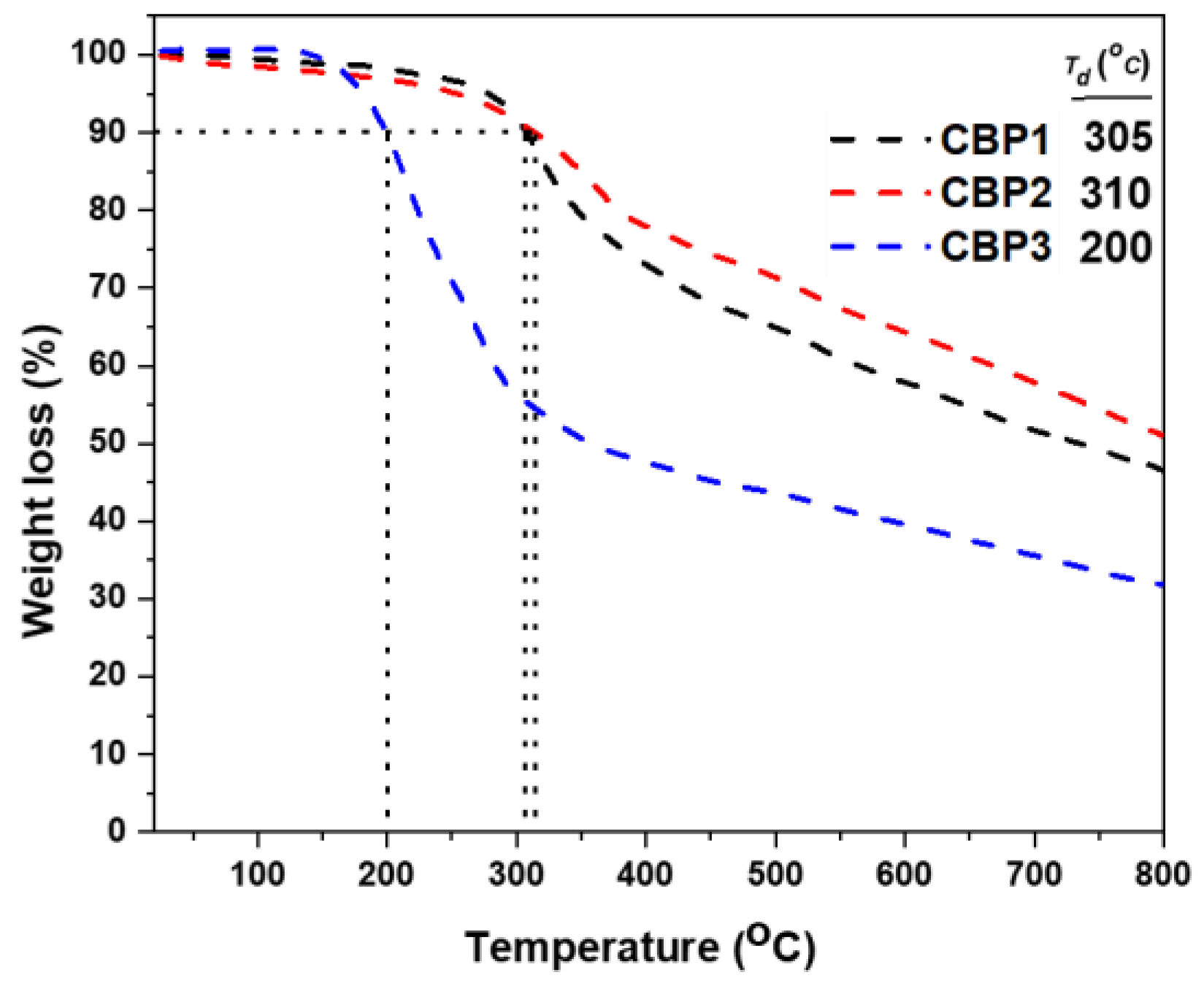
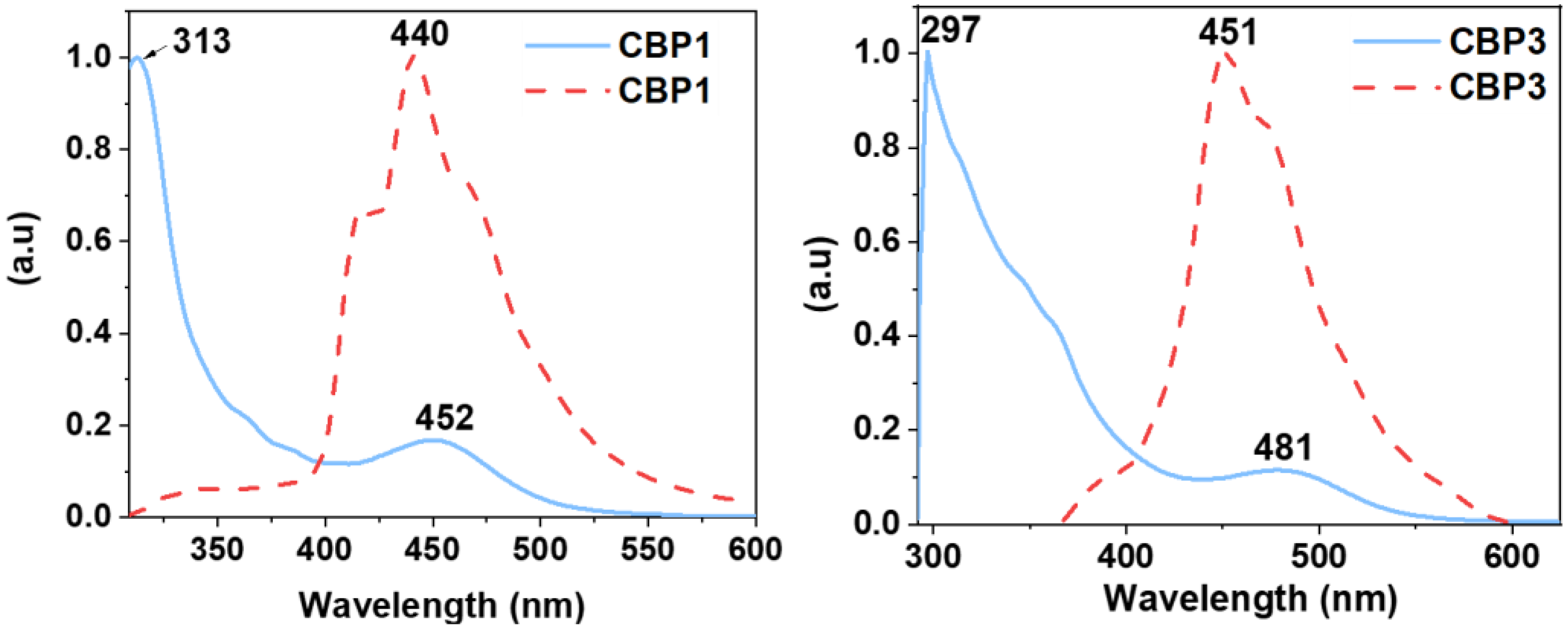
3.1.2. Synthesis of copolymers CBP1-3
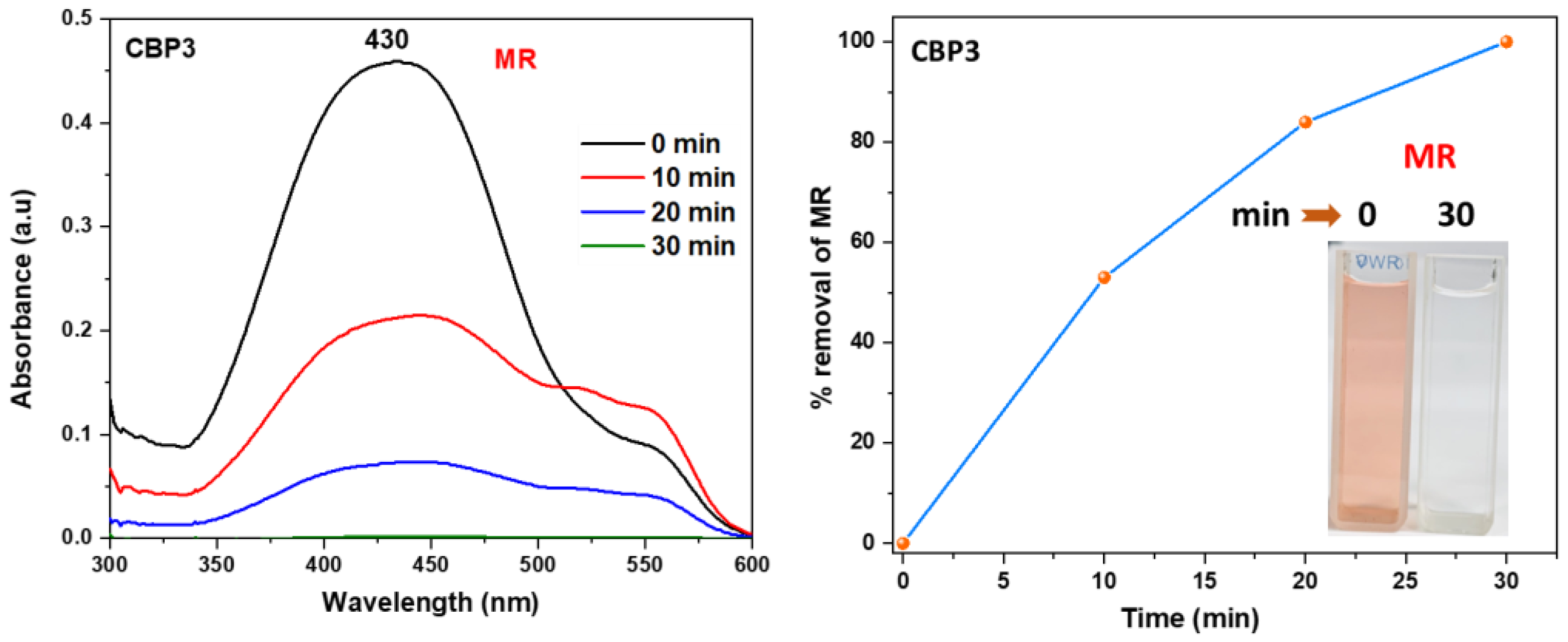
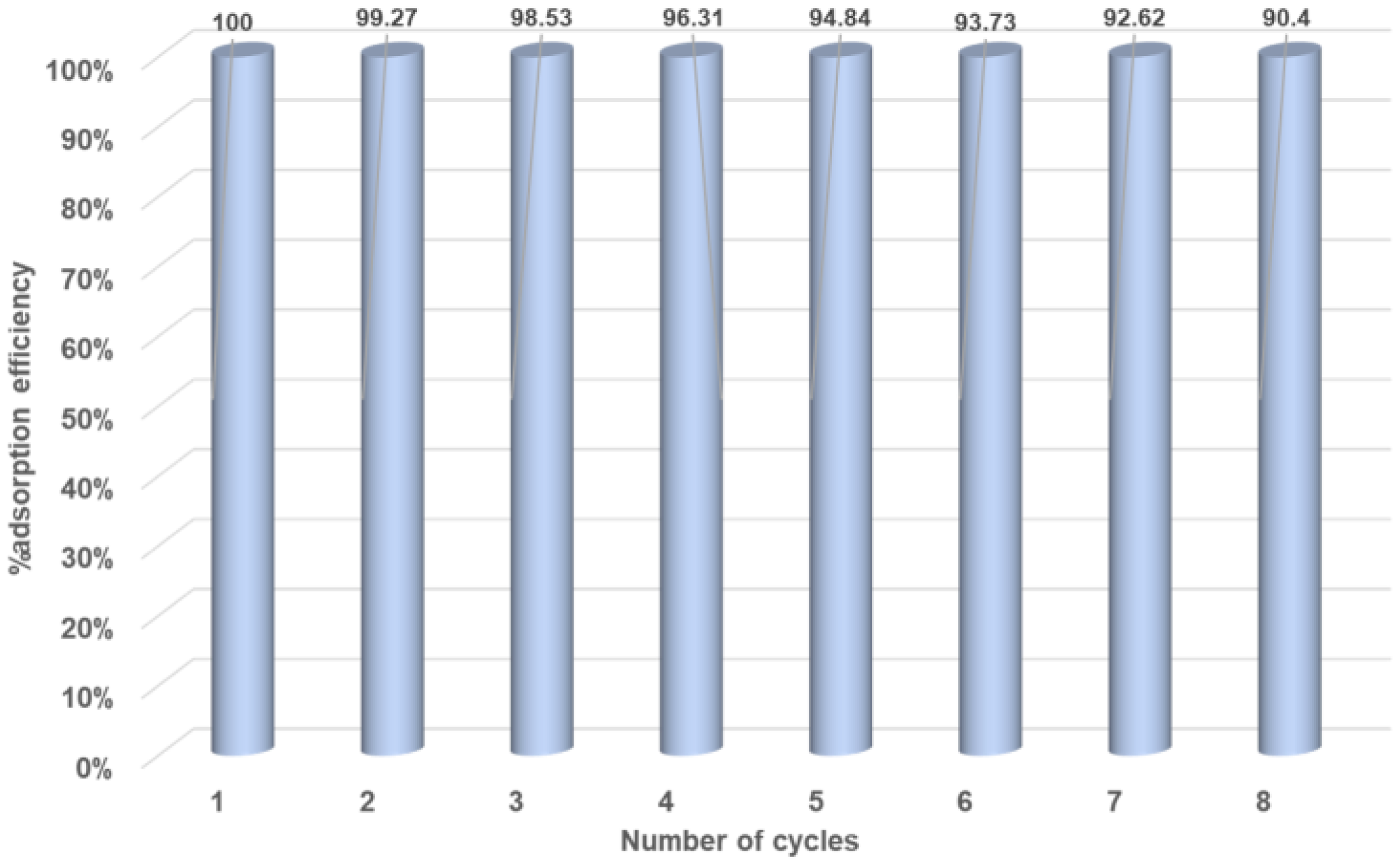
3.2. Methyl red adsorption studies
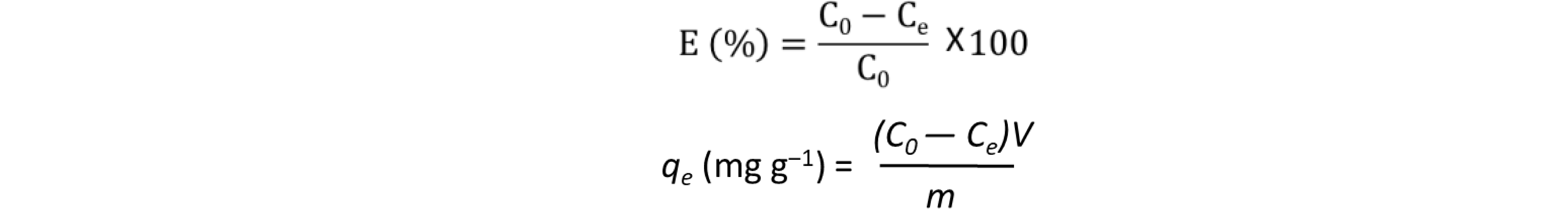
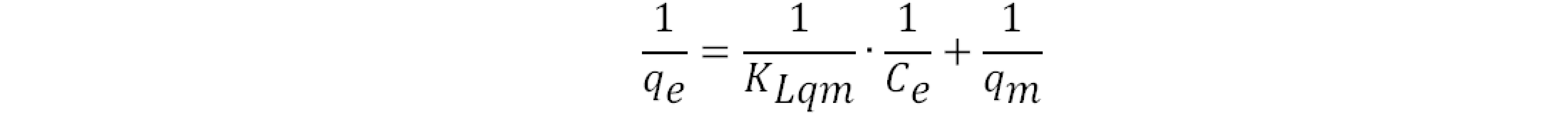
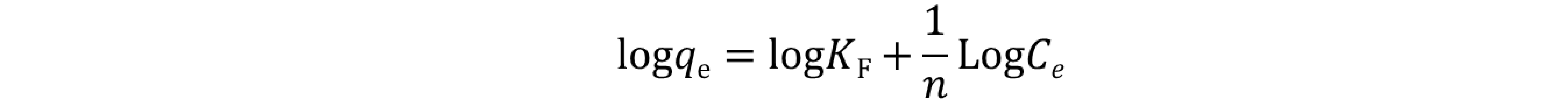 where qe (mg g‒1) denotes the equilibrium adsorption capacity, Ce (mg L‒1) represents the equilibrium dye concentration, and qm (mg g‒1) indicates the maximum adsorption capacity. KL is the Langmuir constant whereas KF and n are Freundlich constants correlated to the sorption capacity and sorption intensity, respectively (figures S30-S32 in the supporting information file). The Langmuir parameters were obtained by plotting the graph of 1/qe versus 1/Ce and those for Freundlich were derived from the plot of log qe versus log Ce (figures S30-S32 in the supporting information file). Both models were used to fit the equilibrium data obtained for MR adsorption. It is worthwhile to note that the correlation coefficient (R2) derived from the linear equation using the Langmuir model was found to be higher than that computed for the Freundlich isotherm model of MR (figure S32), thus, implying that the Langmuir isotherm is a more favorable model to illustrate the equilibrium data, and which suggests a homogenous adsorption and formation of monolayers of MR dye on the adsorbates CBP1-3. Additionally, the maximum adsorption capacity (qm) derived from the Langmuir model was found to be 199.20 mg g‒1 and 219.8 mg g‒1 for CBP2 and CBP1, respectively and it reaches 431.03 mg g‒1 for the phenyl-bearing iron(II) clathrochelate cyclobenzannulated copolymer CBP3, which to the best of our knowledge, is superior to the adsorption capacity values for most of the materials reported in the literature [5,24,48].
where qe (mg g‒1) denotes the equilibrium adsorption capacity, Ce (mg L‒1) represents the equilibrium dye concentration, and qm (mg g‒1) indicates the maximum adsorption capacity. KL is the Langmuir constant whereas KF and n are Freundlich constants correlated to the sorption capacity and sorption intensity, respectively (figures S30-S32 in the supporting information file). The Langmuir parameters were obtained by plotting the graph of 1/qe versus 1/Ce and those for Freundlich were derived from the plot of log qe versus log Ce (figures S30-S32 in the supporting information file). Both models were used to fit the equilibrium data obtained for MR adsorption. It is worthwhile to note that the correlation coefficient (R2) derived from the linear equation using the Langmuir model was found to be higher than that computed for the Freundlich isotherm model of MR (figure S32), thus, implying that the Langmuir isotherm is a more favorable model to illustrate the equilibrium data, and which suggests a homogenous adsorption and formation of monolayers of MR dye on the adsorbates CBP1-3. Additionally, the maximum adsorption capacity (qm) derived from the Langmuir model was found to be 199.20 mg g‒1 and 219.8 mg g‒1 for CBP2 and CBP1, respectively and it reaches 431.03 mg g‒1 for the phenyl-bearing iron(II) clathrochelate cyclobenzannulated copolymer CBP3, which to the best of our knowledge, is superior to the adsorption capacity values for most of the materials reported in the literature [5,24,48].4. Conclusions
Data Availability Statement
Acknowledgements
References
- Etale, A.; Onyianta, A. J.; Turner, S. R.; Eichhorn, S. J. Cellulose: A Review of Water Interactions, Applications in Composites, and Water Treatment. Chemical Reviews 2023, 123(5), 2016–2048. [Google Scholar] [CrossRef] [PubMed]
- Al-Tohamy, R.; Ali, S. S.; Li, F.; Okasha, K. M.; Mahmoud, Y. A. G.; Elsamahy, T.; Jiao, H.; Fu, Y.; Sun, J. A critical review on the treatment of dye-containing wastewater: Ecotoxicological and health concerns of textile dyes and possible remediation approaches for environmental safety. Ecotoxicology and Environmental Safety 2022, 231, 113160. [Google Scholar] [CrossRef]
- Ogugbue, C. J.; Sawidis, T. Bioremediation and Detoxification of Synthetic Wastewater Containing Triarylmethane Dyes by <Aeromonas hydrophila> Isolated from Industrial Effluent. Biotechnology Research International 2011, 2011, 967925. [Google Scholar] [CrossRef]
- Kaur, H.; Devi, N.; Siwal, S. S.; Alsanie, W. F.; Thakur, M. K.; Thakur, V. K. Metal–Organic Framework-Based Materials for Wastewater Treatment: Superior Adsorbent Materials for the Removal of Hazardous Pollutants. ACS Omega 2023, 8(10), 9004–9030. [Google Scholar] [CrossRef] [PubMed]
- Takkar, S.; Tyagi, B.; Kumar, N.; Kumari, T.; Iqbal, K.; Varma, A.; Thakur, I. S.; Mishra, A. Biodegradation of methyl red dye by a novel actinobacterium Zhihengliuella sp. ISTPL4: Kinetic studies, isotherm and biodegradation pathway. Environmental Technology & Innovation 2022, 26, 102348. [Google Scholar] [CrossRef]
- Rojas, S.; Horcajada, P. Metal–Organic Frameworks for the Removal of Emerging Organic Contaminants in Water. Chemical Reviews 2020, 120(16), 8378–8415. [Google Scholar] [CrossRef] [PubMed]
- Lellis, B.; Fávaro-Polonio, C. Z.; Pamphile, J. A.; Polonio, J. C. Effects of textile dyes on health and the environment and bioremediation potential of living organisms. Biotechnology Research and Innovation 2019, 3(2), 275–290. [Google Scholar] [CrossRef]
- Benkhaya, S.; M’Rabet, S.; El Harfi, A. Classifications, properties, recent synthesis and applications of azo dyes. Heliyon 2020, 6(1), e03271. [Google Scholar] [CrossRef]
- Overdahl, K. E.; Gooden, D.; Bobay, B.; Getzinger, G. J.; Stapleton, H. M.; Ferguson, P. L. Characterizing azobenzene disperse dyes in commercial mixtures and children’s polyester clothing. Environmental Pollution 2021, 287, 117299. [Google Scholar] [CrossRef]
- Khan, Z.; Jain, K.; Soni, A.; Madamwar, D. Microaerophilic degradation of sulphonated azo dye—Reactive Red 195 by bacterial consortium AR1 through co-metabolism. International Biodeterioration & Biodegradation 2014, 94, 167–175. [Google Scholar] [CrossRef]
- Leal Filho, W.; Perry, P.; Heim, H.; Dinis, M. A. P.; Moda, H.; Ebhuoma, E.; Paço, A. An overview of the contribution of the textiles sector to climate change. Frontiers in Environmental Science 2022, 10. [Google Scholar] [CrossRef]
- Kishor, R.; Purchase, D.; Saratale, G. D.; Saratale, R. G.; Ferreira, L. F. R.; Bilal, M.; Chandra, R.; Bharagava, R. N. Ecotoxicological and health concerns of persistent coloring pollutants of textile industry wastewater and treatment approaches for environmental safety. Journal of Environmental Chemical Engineering 2021, 9(2), 105012. [Google Scholar] [CrossRef]
- Niinimäki, K.; Peters, G.; Dahlbo, H.; Perry, P.; Rissanen, T.; Gwilt, A. The environmental price of fast fashion. Nature Reviews Earth & Environment 2020, 1 (4), 189-200. [CrossRef]
- Hossain, M. S.; Das, S. C.; Islam, J. M. M.; Al Mamun, M. A.; Khan, M. A. Reuse of textile mill ETP sludge in environmental friendly bricks—effect of gamma radiation. Radiation Physics and Chemistry 2018, 151, 77–83. [Google Scholar] [CrossRef]
- Koulini, G. V.; Laiju, A. R.; Ramesh, S. T.; Gandhimathi, R.; Nidheesh, P. V. Effective degradation of azo dye from textile wastewater by electro-peroxone process. Chemosphere 2022, 289, 133152. [Google Scholar] [CrossRef] [PubMed]
- Srinivasan, S.; Bankole, P. O.; Sadasivam, S. K. Biodecolorization and degradation of textile azo dyes using Lysinibacillus sphaericus MTCC 9523. Frontiers in Environmental Science 2022, 10. [Google Scholar] [CrossRef]
- Franca, R. D. G.; Oliveira, M. C.; Pinheiro, H. M.; Lourenço, N. D. Biodegradation Products of a Sulfonated Azo Dye in Aerobic Granular Sludge Sequencing Batch Reactors Treating Simulated Textile Wastewater. ACS Sustainable Chemistry & Engineering 2019, 7 (17), 14697-14706. [CrossRef]
- Mishra, A.; Gupta, B.; Kumar, N.; Singh, R.; Varma, A.; Thakur, I. S. Synthesis of calcite-based bio-composite biochar for enhanced biosorption and detoxification of chromium Cr (VI) by Zhihengliuella sp. ISTPL4. Bioresource Technology 2020, 307, 123262. [Google Scholar] [CrossRef] [PubMed]
- Dutta, S.; Gupta, B.; Srivastava, S. K.; Gupta, A. K. Recent advances on the removal of dyes from wastewater using various adsorbents: a critical review. Materials Advances 2021, 2(14), 4497–4531. [Google Scholar] [CrossRef]
- Wu, L.; Liu, X.; Lv, G.; Zhu, R.; Tian, L.; Liu, M.; Li, Y.; Rao, W.; Liu, T.; Liao, L. Study on the adsorption properties of methyl orange by natural one-dimensional nano-mineral materials with different structures. Scientific Reports 2021, 11(1), 10640. [Google Scholar] [CrossRef]
- Maniyam, M. N.; Ibrahim, A. L.; Cass, A. E. G. Decolourization and biodegradation of azo dye methyl red by Rhodococcus strain UCC 0016. Environ Technol 2020, 41(1), 71–85. [Google Scholar] [CrossRef]
- Baena-Baldiris, D.; Montes-Robledo, A.; Baldiris-Avila, R. Franconibacter sp., 1MS: A New Strain in Decolorization and Degradation of Azo Dyes Ponceau S Red and Methyl Orange. ACS Omega 2020, 5 (43), 28146-28157. [CrossRef]
- Ajaz, M.; Rehman, A.; Khan, Z.; Nisar, M. A.; Hussain, S. Degradation of azo dyes by Alcaligenes aquatilis 3c and its potential use in the wastewater treatment. AMB Express 2019, 9(1), 64. [Google Scholar] [CrossRef]
- Muthuraman, G.; Teng, T. T. Extraction of methyl red from industrial wastewater using xylene as an extractant. Progress in Natural Science 2009, 19(10), 1215–1220. [Google Scholar] [CrossRef]
- Shetty, S.; Baig, N.; Al-Mousawi, S.; Alameddine, B. Cover Image, Volume 139, Issue 43. Journal of Applied Polymer Science 2022, 139(43), e51150. [Google Scholar] [CrossRef]
- Losytskyy, M.; Chornenka, N.; Vakarov, S.; Meier-Menches, S. M.; Gerner, C.; Potocki, S.; Arion, V. B.; Gumienna-Kontecka, E.; Voloshin, Y.; Kovalska, V. Sensing of Proteins by ICD Response of Iron(II) Clathrochelates Functionalized by Carboxyalkylsulfide Groups Biomolecules [Online], 2020.
- Jansze, S. M.; Severin, K. Clathrochelate Metalloligands in Supramolecular Chemistry and Materials Science. Accounts of Chemical Research 2018, 51(9), 2139–2147. [Google Scholar] [CrossRef] [PubMed]
- Chen, Z.; Idrees, K. B.; Shetty, S.; Xie, H.; Wasson, M. C.; Gong, W.; Zhang, X.; Alameddine, B.; Farha, O. K. Regulation of Catenation in Metal–Organic Frameworks with Tunable Clathrochelate-Based Building Blocks. Crystal Growth & Design 2021, 21 (12), 6665-6670. [CrossRef]
- Pomadchik, A. L.; Belov, A. S.; Lebed, E. G.; Belaya, I. G.; Vologzhanina, A. V.; Voloshin, Y. Z. Dramatic Effect of A Ring Size of Alicyclic α-Dioximate Ligand Synthons on Kinetics of the Template Synthesis and of the Acidic Decomposition of the Methylboron-Capped Iron(II) Clathrochelates Molecules [Online], 2021.
- Kovalska, V.; Vakarov, S.; Losytskyy, M.; Kuperman, M.; Chornenka, N.; Toporivska, Y.; Gumienna-Kontecka, E.; Voloshin, Y.; Varzatskii, O.; Mokhir, A. Dicarboxyl-terminated iron(ii) clathrochelates as ICD-reporters for globular proteins. RSC Advances 2019, 9(42), 24218–24230. [Google Scholar] [CrossRef] [PubMed]
- Alameddine, B.; Shetty, S.; Baig, N.; Al-Mousawi, S.; Al-Sagheer, F. Synthesis and characterization of metalorganic polymers of intrinsic microporosity based on iron(II) clathrochelate. Polymer 2017, 122, 200–207. [Google Scholar] [CrossRef]
- Shetty, S.; Idrees, K. B.; Xie, H.; Alameddine, B.; Farha, O. K. Synthesis of zirconium-based metal–organic frameworks with iron(ii) clathrochelate ligands. CrystEngComm 2023, 25(10), 1550–1555. [Google Scholar] [CrossRef]
- Gong, W.; Xie, Y.; Pham, T. D.; Shetty, S.; Son, F. A.; Idrees, K. B.; Chen, Z.; Xie, H.; Liu, Y.; Snurr, R. Q.; Chen, B.; Alameddine, B.; Cui, Y.; Farha, O. K. Creating Optimal Pockets in a Clathrochelate-Based Metal–Organic Framework for Gas Adsorption and Separation: Experimental and Computational Studies. Journal of the American Chemical Society 2022, 144(8), 3737–3745. [Google Scholar] [CrossRef]
- Baig, N.; Shetty, S.; Habib, S. S.; Husain, A. A.; Al-Mousawi, S.; Alameddine, B. Synthesis of Iron(II) Clathrochelate-Based Poly(vinylene sulfide) with Tetraphenylbenzene Bridging Units and Their Selective Oxidation into Their Corresponding Poly(vinylene sulfone) Copolymers: Promising Materials for Iodine Capture. Polymers 2022, 14(18), 3727. [Google Scholar] [CrossRef]
- Shetty, S.; Baig, N.; Al-Mousawi, S.; Al-Sagheer, F.; Alameddine, B. Synthesis of secondary arylamine copolymers with Iron(II) clathrochelate units and their functionalization into tertiary Polyarylamines via Buchwald-Hartwig cross-coupling reaction. Polymer 2019, 178, 121606. [Google Scholar] [CrossRef]
- Alameddine, B.; Shetty, S.; Anju, R. S.; Al-Sagheer, F.; Al-Mousawi, S. Highly soluble metal-organic polymers based on iron(II) clathrochelates and their gelation induced by sonication. European Polymer Journal 2017, 95, 566–574. [Google Scholar] [CrossRef]
- Shetty, S.; Baig, N.; Alameddine, B. Synthesis and Iodine Adsorption Properties of Organometallic Copolymers with Propeller-Shaped Fe(II) Clathrochelates Bridged by Different Diaryl Thioether and Their Oxidized Sulfone Derivatives. Polymers 2022, 14(22), 4818. [Google Scholar] [CrossRef] [PubMed]
- Shetty, S.; Baig, N.; Moustafa, M. S.; Al-Mousawi, S.; Alameddine, B. Synthesis of Metalorganic Copolymers Containing Various Contorted Units and Iron(II) Clathrochelates with Lateral Butyl Chains: Conspicuous Adsorbents of Lithium Ions and Methylene Blue. Polymers 2022, 14(16), 3394. [Google Scholar] [CrossRef] [PubMed]
- Shetty, S.; Baig, N.; Hassan, A.; Al-Mousawi, S.; Das, N.; Alameddine, B. Fluorinated Iron(ii) clathrochelate units in metalorganic based copolymers: improved porosity, iodine uptake, and dye adsorption properties. RSC Advances 2021, 11(25), 14986–14995. [Google Scholar] [CrossRef] [PubMed]
- Baig, N.; Shetty, S.; Al-Mousawi, S.; Al-Sagheer, F.; Alameddine, B. Influence of size and nature of the aryl diborate spacer on the intrinsic microporosity of Iron(II) clathrochelate polymers. Polymer 2018, 151, 164–170. [Google Scholar] [CrossRef]
- Baig, N.; Shetty, S.; Al-Mousawi, S.; Alameddine, B. Conjugated microporous polymers using a copper-catalyzed [4 + 2] cyclobenzannulation reaction: promising materials for iodine and dye adsorption. Polymer Chemistry 2021, 12(15), 2282–2292. [Google Scholar] [CrossRef]
- Baig, N.; Shetty, S.; Tiwari, R.; Pramanik, S. K.; Alameddine, B. Aggregation-Induced Emission of Contorted Polycondensed Aromatic Hydrocarbons Made by Edge Extension Using a Palladium-Catalyzed Cyclopentannulation Reaction. ACS Omega 2022, 7(49), 45732–45739. [Google Scholar] [CrossRef] [PubMed]
- Baig, N.; Shetty, S.; Al-Mousawi, S.; Alameddine, B. Synthesis of conjugated polymers via cyclopentannulation reaction: promising materials for iodine adsorption. Polymer Chemistry 2020, 11(17), 3066–3074. [Google Scholar] [CrossRef]
- Baig, N.; Shetty, S.; Al-Mousawi, S.; Al-Sagheer, F.; Alameddine, B. Synthesis of triptycene-derived covalent organic polymer networks and their subsequent in-situ functionalization with 1,2-dicarbonyl substituents. Reactive and Functional Polymers 2019, 139, 153–161. [Google Scholar] [CrossRef]
- Baig, N.; Shetty, S.; Moustafa, M. S.; Al-Mousawi, S.; Alameddine, B. Selective removal of toxic organic dyes using Trӧger base-containing sulfone copolymers made from a metal-free thiol-yne click reaction followed by oxidation. RSC Advances 2021, 11(34), 21170–21178. [Google Scholar] [CrossRef]
- Shetty, S.; Baig, N.; Moustafa, M. S.; Al-Mousawi, S.; Alameddine, B. Sizable iodine uptake of porous copolymer networks bearing Tröger’s base units. Polymer 2021, 229, 123996. [Google Scholar] [CrossRef]
- Baig, N.; Shetty, S.; Pasha, S. S.; Pramanik, S. K.; Alameddine, B. Copolymer networks with contorted units and highly polar groups for ultra-fast selective cationic dye adsorption and iodine uptake. Polymer 2022, 239, 124467. [Google Scholar] [CrossRef]
- Gul, S.; Kanwal, M.; Qazi, R. A.; Gul, H.; Khattak, R.; Khan, M. S.; Khitab, F.; Krauklis, A. E. Efficient Removal of Methyl Red Dye by Using Bark of Hopbush Water [Online], 2022.
- Khan, E. A. ; Shahjahan; Khan, T. A. Adsorption of methyl red on activated carbon derived from custard apple (Annona squamosa) fruit shell: Equilibrium isotherm and kinetic studies. Journal of Molecular Liquids 2018, 249, 1195–1211. [Google Scholar] [CrossRef]
| Entry | Copolymer a | Time in days | CM b[M] | Yield (%) |
|---|---|---|---|---|
| 1 | CBP1 | 2 | 2.5x10‒2 | 48 |
| 2 | CBP1 | 2 | 1.25x10‒2 | 65 |
| 3 | CBP1 | 2 | 6.0x10‒3 | 83 |
| 4 | CBP2 | 2 | 6.0x10‒3 | 95 |
| 5 | CBP3 | 2 | 6.0x10‒3 | 90 |
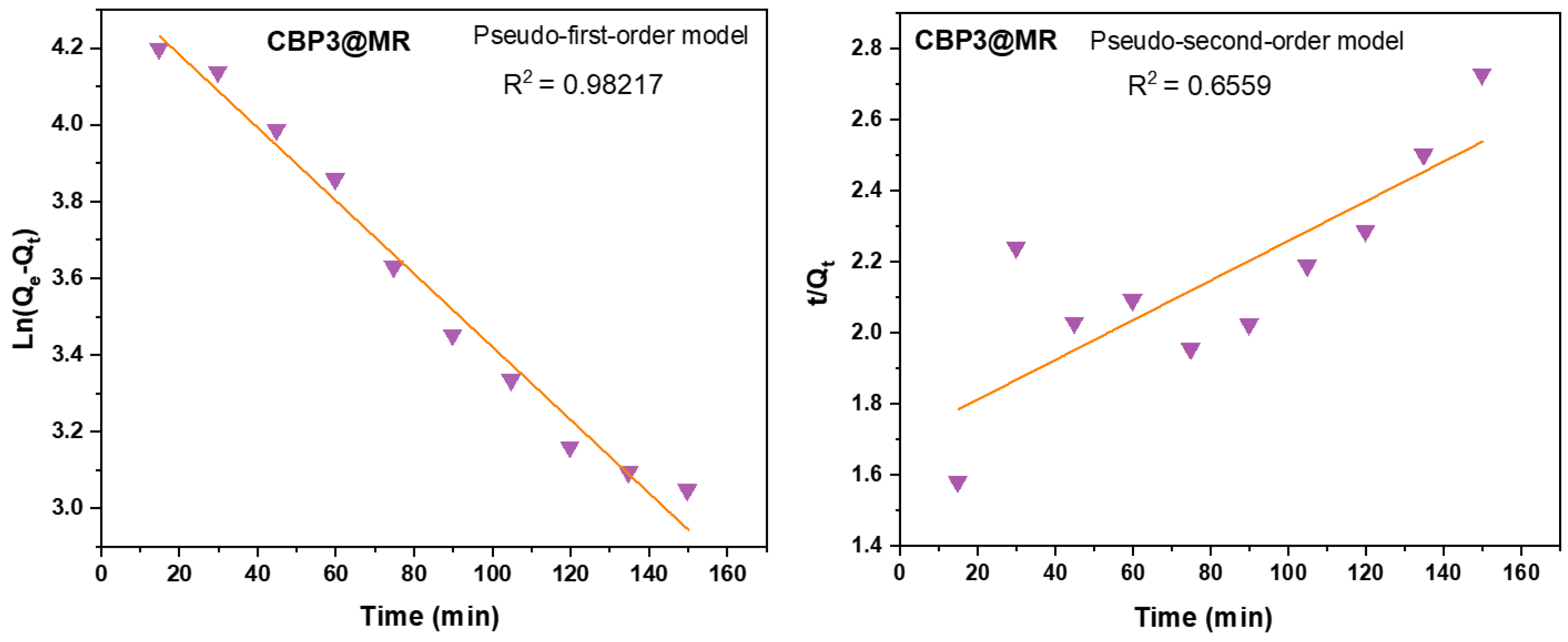
| Dye on CBP3 | Pseudo 1st order model | Pseudo 2nd order model | ||||||
| C0 (mg L‒1) |
qe, exp (mg g−1) |
qe, cal (mg g−1) |
k1 (min−1) |
R2 | qe, cal (mg g−1) |
k2 (min−1) |
R2 | |
| MR | 500 | 76 | 79.35 | -0.00013 | 0.9821 | 32116 | 1.83E-05 | 0.6559 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).





