Submitted:
15 May 2023
Posted:
16 May 2023
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Cx26 mutations induced dysfunction and abnormal trafficking pathways
3. Possible transport pathways and controversies regarding Cxs
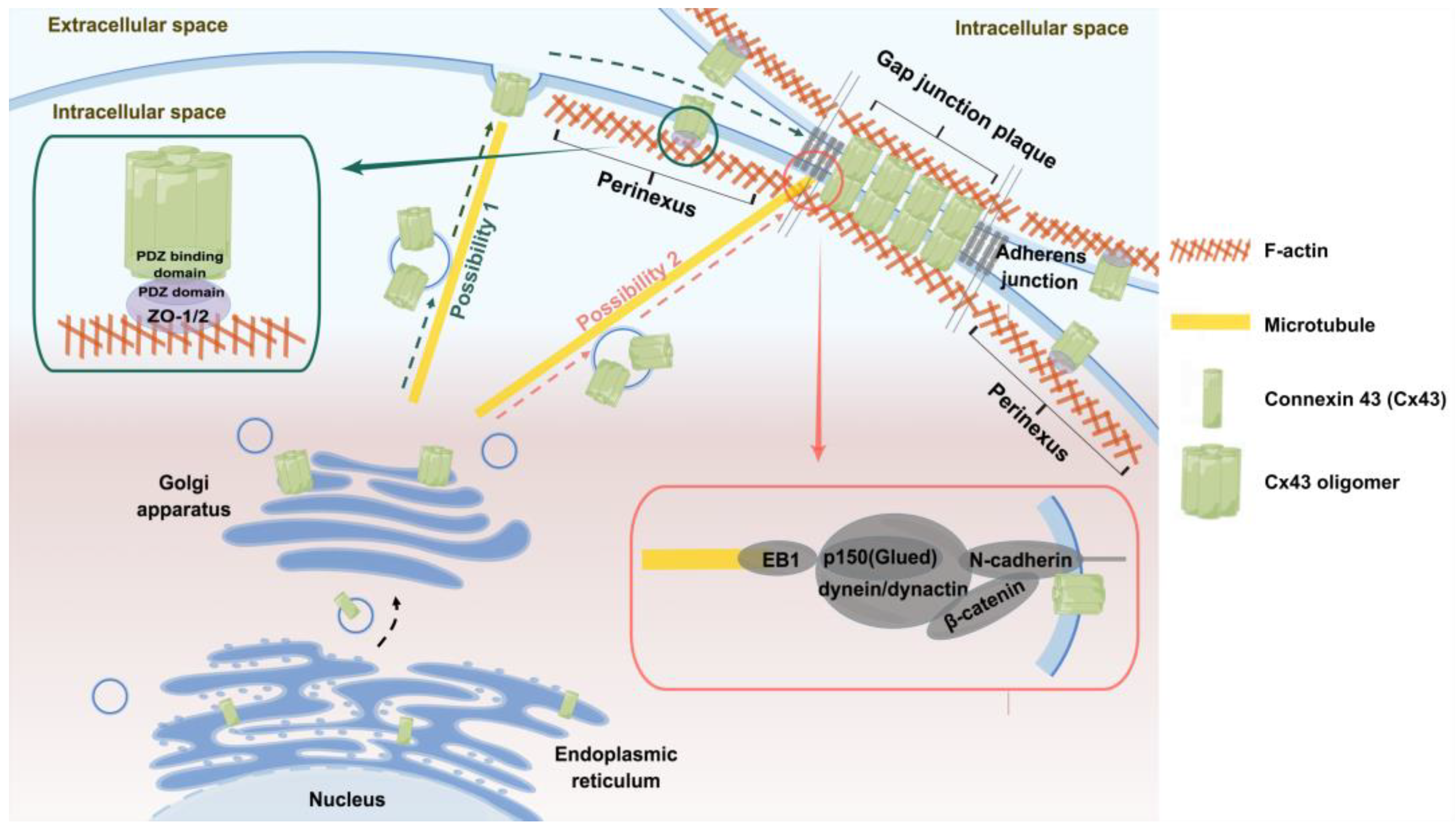
3.1. Transport pathways of Cx43
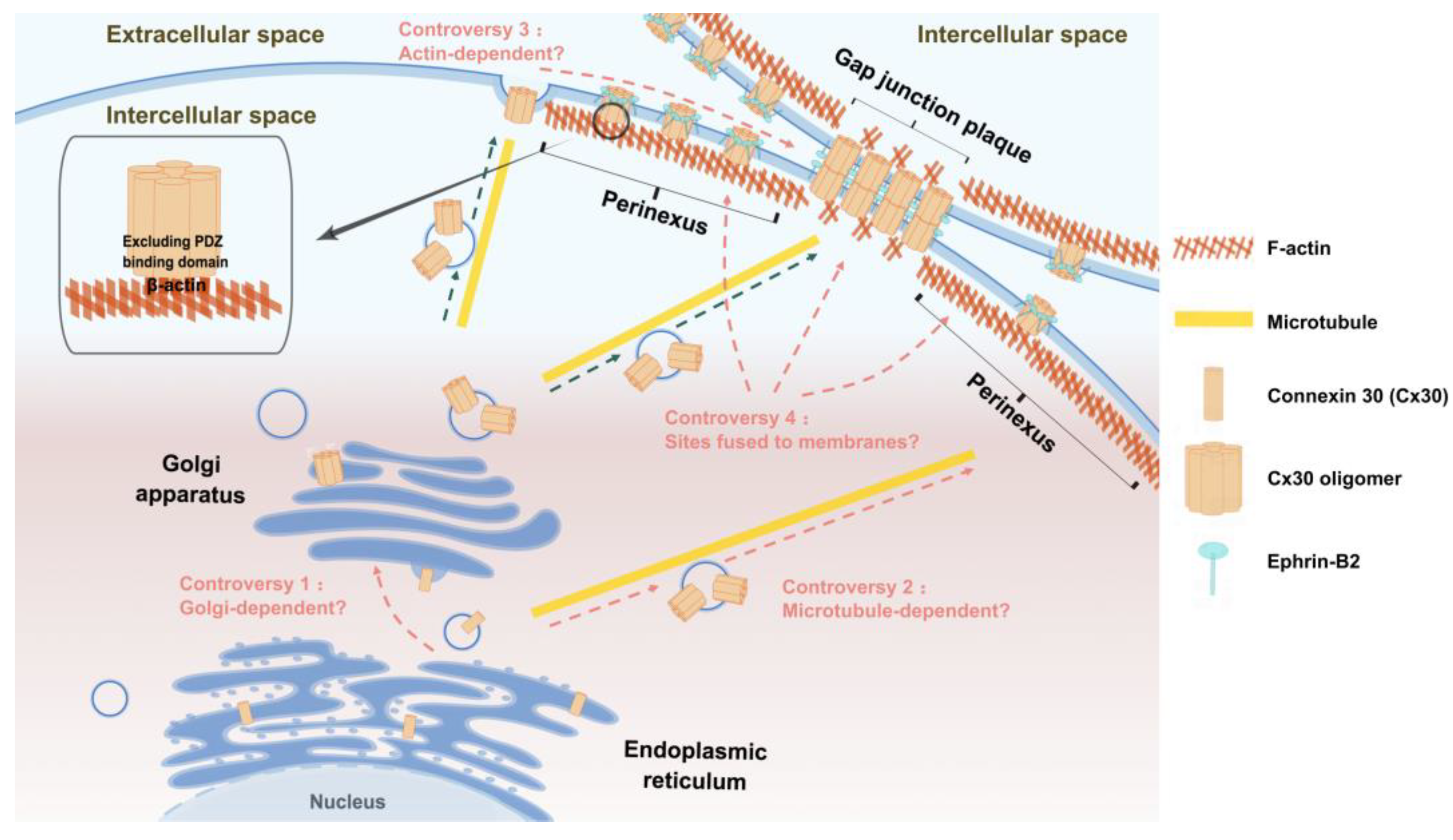
3.2. Transport pathways of Cx30
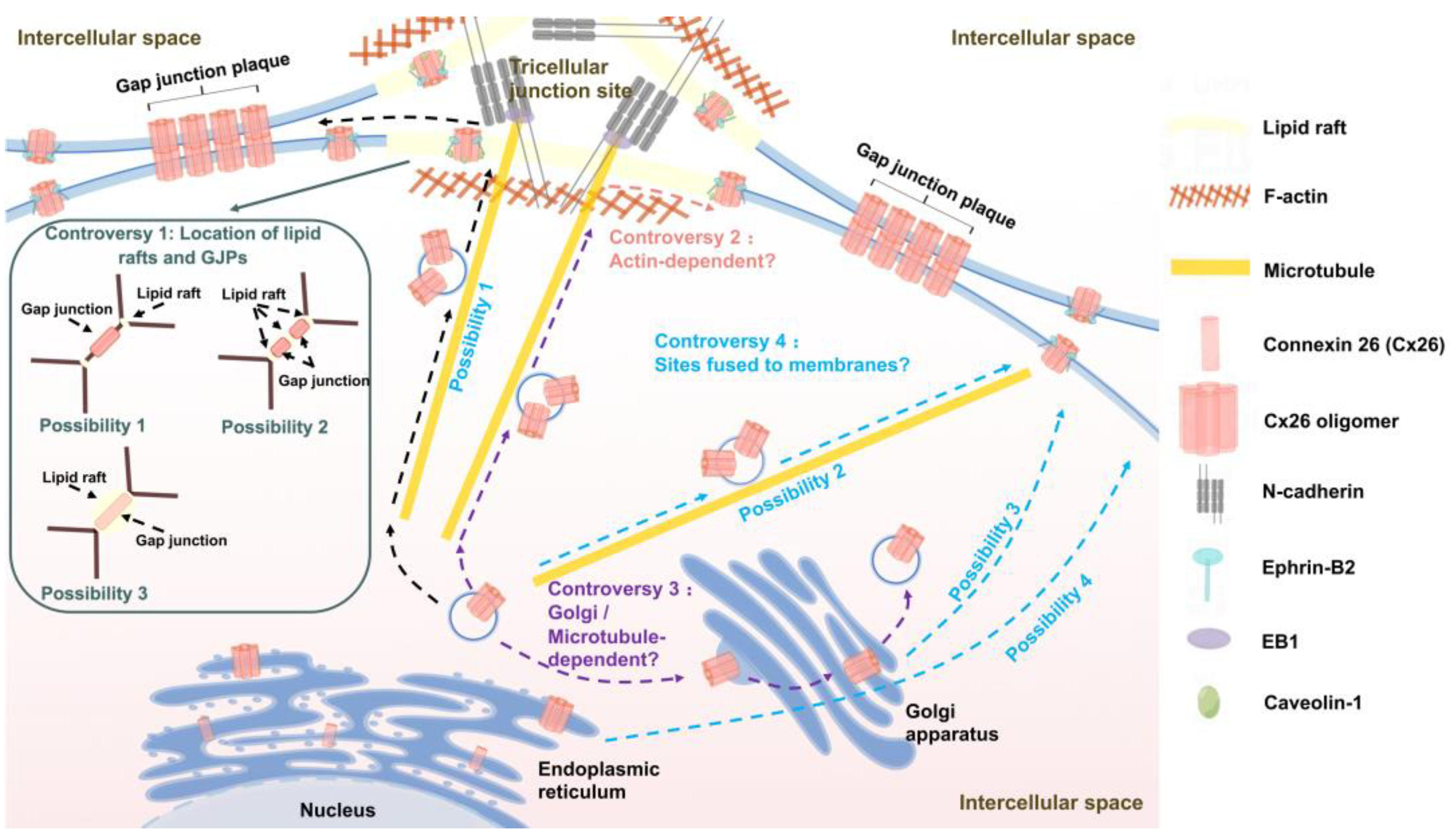
3.3. Transport pathways of Cx26
4. Molecules related to connexin transport and their roles
4.1. Cx43-targeted transport
4.2. Cx30-targeted transport
4.3. Cx26-targeted transport
| Connexins | Molecules involved in transport | Molecular function |
|---|---|---|
| Cx43 | EB1 | +TIPs, promoting microtubule anchoring to the cell membrane. |
| p150 (Glued) | Subunit of dynactin, binding to EB1 to form dynein/dunactin complexes. | |
| N-cadherin | Promoting microtubule tethering to AJs, and mediating homologous interactions between adjacent cells. | |
| β-catenin | Tethering microtubules to AJs, and acting as cytoplasmic actuators of N-cadherin/N-cadherin interactions. | |
| ZO-1/2 | Binding to the PDZ-binding motif of connexins through the PDZ domain mediates the interaction of the hemichannel with cortical actin. | |
| ephrin-B1 | Affecting intercellular GJC, which in turn affects the distribution of connexins and the formation of GJPs | |
| Cx30 | β-actin | β-actin interacts directly with Cx30 without via ZO-1/2. |
| ephrin-B2 | Preferentially interfacing with peripheral Cx30, promoting the directional movement of Cx30 across the cell membrane. And regulateing clathrin-mediated Cx30 endocytosis. | |
| Cx26 | N-cadherin | Regulating microtubule dynamics, and anchoring microtubules to the cell-cell boundary. |
| EB1 | +TIPs, promoting microtubule anchoring to the cell membrane. | |
| Caveolin-1 | Facilitating targeted transport of connexins to lipid rafts through a “piggy-back” mechanism. | |
| ephrin-B2 | Whether it is involved in the transport of connexins remains to be further investigated. |
5. Summary and perspectives
Author Contributions
Funding
Conflicts of Interest
References
- Harris, A.L. Emerging issues of connexin channels: biophysics fills the gap. Q Rev Biophys 2001, 34, 325–472. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Y.; et al. Connexin26 gap junction mediates miRNA intercellular genetic communication in the cochlea and is required for inner ear development. Sci Rep 2015, 5, 15647. [Google Scholar] [CrossRef] [PubMed]
- Elias, L.A.; Wang, D.D.; Kriegstein, A.R. Gap junction adhesion is necessary for radial migration in the neocortex. Nature 2007, 448, 901–907. [Google Scholar] [CrossRef]
- Aasen, T.; et al. Connexins: Synthesis, Post-Translational Modifications, and Trafficking in Health and Disease. Int J Mol Sci 2018, 19, 1296. [Google Scholar] [CrossRef] [PubMed]
- Kiełbowski, K.; Bakinowska, E.; Pawlik, A. The Potential Role of Connexins in the Pathogenesis of Atherosclerosis. Int J Mol Sci 2023, 24, 2600. [Google Scholar] [CrossRef]
- Martin, P.E.; et al. Multiple pathways in the trafficking and assembly of connexin 26, 32 and 43 into gap junction intercellular communication channels. J Cell Sci 2001, 114 Pt 21, 3845–3855. [Google Scholar] [CrossRef]
- Ray, A.; et al. Dileucine-like motifs in the C-terminal tail of connexin32 control its endocytosis and assembly into gap junctions. J Cell Sci 2018, 131. [Google Scholar]
- Alaei, S.R.; et al. Acetylation of C-terminal lysines modulates protein turnover and stability of Connexin-32. BMC Cell Biol 2018, 19, 22. [Google Scholar] [CrossRef]
- Liu, W.; Rask-Andersen, H. GJB2 and GJB6 gene transcripts in the human cochlea: A study using RNAscope, confocal, and super-resolution structured illumination microscopy. Front Mol Neurosci 2022, 15, 973646. [Google Scholar] [CrossRef]
- Lautermann, J.; et al. Expression of the gap-junction connexins 26 and 30 in the rat cochlea. Cell Tissue Res 1998, 294, 415–420. [Google Scholar] [CrossRef]
- Lautermann, J.; et al. Developmental expression patterns of connexin26 and -30 in the rat cochlea. Dev Genet 1999, 25, 306–311. [Google Scholar] [CrossRef]
- Forge, A.; et al. Gap junctions in the inner ear: comparison of distribution patterns in different vertebrates and assessement of connexin composition in mammals. J Comp Neurol 2003, 467, 207–231. [Google Scholar] [CrossRef] [PubMed]
- Ahmad, S.; et al. Connexins 26 and 30 are co-assembled to form gap junctions in the cochlea of mice. Biochem Biophys Res Commun 2003, 307, 362–368. [Google Scholar] [CrossRef] [PubMed]
- Korver, A.M.; et al. Congenital hearing loss. Nat Rev Dis Primers 2017, 3, 16094. [Google Scholar] [CrossRef] [PubMed]
- Kelsell, D.P.; et al. Connexin 26 mutations in hereditary non-syndromic sensorineural deafness. Nature 1997, 387, 80–83. [Google Scholar] [CrossRef]
- Avshalumova, L.; Fabrikant, J.; Koriakos, A. Overview of skin diseases linked to connexin gene mutations. Int J Dermatol 2014, 53, 192–205. [Google Scholar] [CrossRef] [PubMed]
- Lilly, E.; et al. Connexin channels in congenital skin disorders. Semin Cell Dev Biol 2016, 50, 4–12. [Google Scholar] [CrossRef]
- Xu, J.; Nicholson, B.J. The role of connexins in ear and skin physiology - functional insights from disease-associated mutations. Biochim Biophys Acta 2013, 1828, 167–178. [Google Scholar] [CrossRef]
- Yum, S.W.; Zhang, J.; Scherer, S.S. Dominant connexin26 mutants associated with human hearing loss have trans-dominant effects on connexin30. Neurobiol Dis 2010, 38, 226–236. [Google Scholar] [CrossRef]
- Kamiya, K.; et al. Assembly of the cochlear gap junction macromolecular complex requires connexin 26. J Clin Invest 2014, 124, 1598–1607. [Google Scholar] [CrossRef]
- Ahmad, S.; et al. Restoration of connexin26 protein level in the cochlea completely rescues hearing in a mouse model of human connexin30-linked deafness. Proc Natl Acad Sci U S A 2007, 104, 1337–1341. [Google Scholar] [CrossRef]
- Qu, Y.; et al. Early developmental expression of connexin26 in the cochlea contributes to its dominate functional role in the cochlear gap junctions. Biochem Biophys Res Commun 2012, 417, 245–250. [Google Scholar] [CrossRef] [PubMed]
- Ambrosi, C.; et al. Connexin43 Forms Supramolecular Complexes through Non-Overlapping Binding Sites for Drebrin, Tubulin, and ZO-1. PLoS One 2016, 11, e0157073. [Google Scholar] [CrossRef] [PubMed]
- Shaw, R.M.; Rudy, Y. Ionic mechanisms of propagation in cardiac tissue. Roles of the sodium and L-type calcium currents during reduced excitability and decreased gap junction coupling. Circ Res 1997, 81, 727–741. [Google Scholar] [CrossRef] [PubMed]
- Kaprielian, R.R.; et al. Downregulation of immunodetectable connexin43 and decreased gap junction size in the pathogenesis of chronic hibernation in the human left ventricle. Circulation 1998, 97, 651–660. [Google Scholar] [CrossRef] [PubMed]
- Abitbol, J.M.; et al. Mice harbouring an oculodentodigital dysplasia-linked Cx43 G60S mutation have severe hearing loss. J Cell Sci 2018, 131. [Google Scholar] [CrossRef]
- Hoang Dinh, E.; et al. Diverse deafness mechanisms of connexin mutations revealed by studies using in vitro approaches and mouse models. Brain Res 2009, 1277, 52–69. [Google Scholar] [CrossRef]
- Laird, D.W. Life cycle of connexins in health and disease. Biochem J 2006, 394 Pt 3, 527–543. [Google Scholar] [CrossRef]
- Mani, R.S.; et al. Functional consequences of novel connexin 26 mutations associated with hereditary hearing loss. Eur J Hum Genet 2009, 17, 502–509. [Google Scholar] [CrossRef]
- Haack, B.; et al. Deficient membrane integration of the novel p.N14D-GJB2 mutant associated with non-syndromic hearing impairment. Hum Mutat 2006, 27, 1158–1159. [Google Scholar] [CrossRef]
- Kupka, S.; et al. [Mutational analysis of the connexin26 gene in sporadic cases of moderate to profound deafness]. Hno 2000, 48, 671–674. [Google Scholar] [CrossRef] [PubMed]
- Stanghellini, I.; et al. New and rare GJB2 alleles in patients with nonsyndromic sensorineural hearing impairment: a genotype/auditory phenotype correlation. Genet Test Mol Biomarkers 2014, 18, 839–844. [Google Scholar] [CrossRef] [PubMed]
- Thomas, T.; et al. Transport and function of cx26 mutants involved in skin and deafness disorders. Cell Commun Adhes 2003, 10, 353–358. [Google Scholar] [CrossRef]
- Yotsumoto, S.; et al. Novel mutations in GJB2 encoding connexin-26 in Japanese patients with keratitis-ichthyosis-deafness syndrome. Br J Dermatol 2003, 148, 649–653. [Google Scholar] [CrossRef] [PubMed]
- Di, W.L.; et al. Connexin interaction patterns in keratinocytes revealed morphologically and by FRET analysis. J Cell Sci 2005, 118 Pt 7, 1505–1514. [Google Scholar] [CrossRef]
- Melchionda, S.; et al. Functional characterization of a novel Cx26 (T55N) mutation associated to non-syndromic hearing loss. Biochem Biophys Res Commun 2005, 337, 799–805. [Google Scholar] [CrossRef] [PubMed]
- Marziano, N.K.; et al. Mutations in the gene for connexin 26 (GJB2) that cause hearing loss have a dominant negative effect on connexin 30. Hum Mol Genet 2003, 12, 805–812. [Google Scholar] [CrossRef]
- Palmada, M.; et al. Loss of function mutations of the GJB2 gene detected in patients with DFNB1-associated hearing impairment. Neurobiol Dis 2006, 22, 112–118. [Google Scholar] [CrossRef]
- Oshima, A.; et al. Roles of Met-34, Cys-64, and Arg-75 in the assembly of human connexin 26. Implication for key amino acid residues for channel formation and function. J Biol Chem 2003, 278, 1807–1816. [Google Scholar] [CrossRef]
- Shuja, Z.; et al. Connexin26 Mutations Causing Palmoplantar Keratoderma and Deafness Interact with Connexin43, Modifying Gap Junction and Hemichannel Properties. J Invest Dermatol 2016, 136, 225–235. [Google Scholar] [CrossRef]
- Thönnissen, E.; et al. Human connexin26 (GJB2) deafness mutations affect the function of gap junction channels at different levels of protein expression. Hum Genet 2002, 111, 190–197. [Google Scholar] [CrossRef] [PubMed]
- Meşe, G.; et al. Altered gating properties of functional Cx26 mutants associated with recessive non-syndromic hearing loss. Hum Genet 2004, 115, 191–199. [Google Scholar] [CrossRef] [PubMed]
- Press, E.R.; et al. Induction of cell death and gain-of-function properties of connexin26 mutants predict severity of skin disorders and hearing loss. J Biol Chem 2017, 292, 9721–9732. [Google Scholar] [CrossRef] [PubMed]
- Zelante, L.; et al. Connexin26 mutations associated with the most common form of non-syndromic neurosensory autosomal recessive deafness (DFNB1) in Mediterraneans. Hum Mol Genet 1997, 6, 1605–1609. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Scherer, S.S.; Yum, S.W. Dominant Cx26 mutants associated with hearing loss have dominant-negative effects on wild type Cx26. Mol Cell Neurosci 2011, 47, 71–78. [Google Scholar] [CrossRef] [PubMed]
- Choung, Y.H.; Moon, S.K.; Park, H.J. Functional study of GJB2 in hereditary hearing loss. Laryngoscope 2002, 112, 1667–1671. [Google Scholar] [CrossRef] [PubMed]
- Frei, K.; et al. A novel connexin 26 mutation associated with autosomal recessive sensorineural deafness. Audiol Neurootol 2004, 9, 47–50. [Google Scholar] [CrossRef]
- Brown, C.W.; et al. A novel GJB2 (connexin 26) mutation, F142L, in a patient with unusual mucocutaneous findings and deafness. J Invest Dermatol 2003, 121, 1221–1223. [Google Scholar] [CrossRef]
- Cook, J.; de Wolf, E.; Dale, N. Cx26 keratitis ichthyosis deafness syndrome mutations trigger alternative splicing of Cx26 to prevent expression and cause toxicity in vitro. R Soc Open Sci 2019, 6, 191128. [Google Scholar] [CrossRef]
- Shi, X.; et al. Polymorphism of the 86th amino acid in CX26 protein and hereditary deafness. J Otol 2016, 11, 84–87. [Google Scholar] [CrossRef]
- Jara, O.; et al. Critical role of the first transmembrane domain of Cx26 in regulating oligomerization and function. Mol Biol Cell 2012, 23, 3299–3311. [Google Scholar] [CrossRef] [PubMed]
- Xiao, Z.; et al. Impaired membrane targeting and aberrant cellular localization of human Cx26 mutants associated with inherited recessive hearing loss. Acta Otolaryngol 2011, 131, 59–66. [Google Scholar] [CrossRef] [PubMed]
- Maslova, E.A.; Orishchenko, K.E.; Posukh, O.L. Functional Evaluation of a Rare Variant c.516G>C (p.Trp172Cys) in the GJB2 (Connexin 26) Gene Associated with Nonsyndromic Hearing Loss. Biomolecules 2021, 11, 61. [Google Scholar] [CrossRef] [PubMed]
- Su, C.C.; et al. Mutation R184Q of connexin 26 in hearing loss patients has a dominant-negative effect on connexin 26 and connexin 30. Eur J Hum Genet 2010, 18, 1061–1064. [Google Scholar] [CrossRef]
- Rodriguez-Paris, J.; et al. Comparative functional characterization of novel non-syndromic GJB2 gene variant p.Gly45Arg and lethal syndromic variant p.Gly45Glu. PeerJ 2016, 4, e2494. [Google Scholar] [CrossRef]
- Choi, S.Y.; et al. Different functional consequences of two missense mutations in the GJB2 gene associated with non-syndromic hearing loss. Hum Mutat 2009, 30, E716–E727. [Google Scholar] [CrossRef]
- Aypek, H.; Bay, V.; Meşe, G. Altered cellular localization and hemichannel activities of KID syndrome associated connexin26 I30N and D50Y mutations. BMC Cell Biol 2016, 17, 5. [Google Scholar] [CrossRef]
- Albuloushi, A.; et al. A heterozygous mutation in GJB2 (Cx26F142L) associated with deafness and recurrent skin rashes results in connexin assembly deficiencies. Exp Dermatol 2020, 29, 970–979. [Google Scholar] [CrossRef]
- Ambrosi, C.; et al. Analysis of trafficking, stability and function of human connexin 26 gap junction channels with deafness-causing mutations in the fourth transmembrane helix. PLoS One 2013, 8, e70916. [Google Scholar] [CrossRef]
- Shi, X.; et al. A Novel GJB2 compound heterozygous mutation c.257C>G (p.T86R)/c.176del16 (p.G59A fs*18) causes sensorineural hearing loss in a Chinese family. J Clin Lab Anal 2018, 32, e22444. [Google Scholar] [CrossRef]
- Lauf, U.; et al. Dynamic trafficking and delivery of connexons to the plasma membrane and accretion to gap junctions in living cells. Proc Natl Acad Sci U S A 2002, 99, 10446–10451. [Google Scholar] [CrossRef] [PubMed]
- Gaietta, G.; et al. Multicolor and electron microscopic imaging of connexin trafficking. Science 2002, 296, 503–507. [Google Scholar] [CrossRef] [PubMed]
- Laird, D.W. Connexin phosphorylation as a regulatory event linked to gap junction internalization and degradation. Biochim Biophys Acta 2005, 1711, 172–182. [Google Scholar] [CrossRef] [PubMed]
- Schmoranzer, J.; et al. Imaging constitutive exocytosis with total internal reflection fluorescence microscopy. J Cell Biol 2000, 149, 23–32. [Google Scholar] [CrossRef] [PubMed]
- Toomre, D.; et al. Fusion of constitutive membrane traffic with the cell surface observed by evanescent wave microscopy. J Cell Biol 2000, 149, 33–40. [Google Scholar] [CrossRef] [PubMed]
- Xiao, F.; et al. Detailed regulatory mechanism of the interaction between ZO-1 PDZ2 and connexin43 revealed by MD simulations. PLoS One 2011, 6, e21527. [Google Scholar] [CrossRef] [PubMed]
- Drees, F.; et al. Alpha-catenin is a molecular switch that binds E-cadherin-beta-catenin and regulates actin-filament assembly. Cell 2005, 123, 903–915. [Google Scholar] [CrossRef]
- Noren, N.K.; et al. Cadherin engagement regulates Rho family GTPases. J Biol Chem 2001, 276, 33305–33308. [Google Scholar] [CrossRef]
- Shaw, R.M.; et al. Microtubule plus-end-tracking proteins target gap junctions directly from the cell interior to adherens junctions. Cell 2007, 128, 547–560. [Google Scholar] [CrossRef]
- Jordan, K.; et al. Trafficking, assembly, and function of a connexin43-green fluorescent protein chimera in live mammalian cells. Mol Biol Cell 1999, 10, 2033–2050. [Google Scholar] [CrossRef]
- Benedetti, E.L.; et al. Structural organization of gap junctions as revealed by freeze-fracture and SDS fracture-labeling. Eur J Cell Biol 2000, 79, 575–582. [Google Scholar] [CrossRef] [PubMed]
- Hülser, D.F.; Rehkopf, B.; Traub, O. Dispersed and aggregated gap junction channels identified by immunogold labeling of freeze-fractured membranes. Exp Cell Res 1997, 233, 240–251. [Google Scholar] [CrossRef] [PubMed]
- Quist, A.P.; et al. Physiological role of gap-junctional hemichannels. Extracellular calcium-dependent isosmotic volume regulation. J Cell Biol 2000, 148, 1063–1074. [Google Scholar] [CrossRef] [PubMed]
- Kamermans, M.; et al. Hemichannel-mediated inhibition in the outer retina. Science 2001, 292, 1178–1180. [Google Scholar] [CrossRef] [PubMed]
- Contreras, J.E.; et al. Metabolic inhibition induces opening of unapposed connexin 43 gap junction hemichannels and reduces gap junctional communication in cortical astrocytes in culture. Proc Natl Acad Sci U S A 2002, 99, 495–500. [Google Scholar] [CrossRef] [PubMed]
- Qu, C.; Gardner, P.; Schrijver, I. The role of the cytoskeleton in the formation of gap junctions by Connexin 30. Exp Cell Res 2009, 315, 1683–1692. [Google Scholar] [CrossRef]
- Defourny, J.; Thelen, N.; Thiry, M. Cochlear connexin 30 homomeric and heteromeric channels exhibit distinct assembly mechanisms. Mech Dev 2019, 155, 8–14. [Google Scholar] [CrossRef]
- Kelly, J.J.; et al. Cx30 exhibits unique characteristics including a long half-life when assembled into gap junctions. J Cell Sci 2015, 128, 3947–3960. [Google Scholar] [CrossRef]
- Defourny, J.; Thelen, N.; Thiry, M. Actin-independent trafficking of cochlear connexin 26 to non-lipid raft gap junction plaques. Hear Res 2019, 374, 69–75. [Google Scholar] [CrossRef]
- Defourny, J.; Thiry, M. Tricellular adherens junctions provide a cell surface delivery platform for connexin 26/30 oligomers in the cochlea. Hear Res 2021, 400, 108137. [Google Scholar] [CrossRef]
- Defourny, J.; Thiry, M. Recent insights into gap junction biogenesis in the cochlea. Dev Dyn 2023, 252, 239–246. [Google Scholar] [CrossRef] [PubMed]
- Moll, T.; et al. Membrane lipid raft homeostasis is directly linked to neurodegeneration. Essays Biochem 2021, 65, 999–1011. [Google Scholar]
- Schubert, A.L.; et al. Connexin family members target to lipid raft domains and interact with caveolin-1. Biochemistry 2002, 41, 5754–5764. [Google Scholar] [CrossRef] [PubMed]
- Fielding, C.J.; Fielding, P.E. Cholesterol and caveolae: structural and functional relationships. Biochim Biophys Acta 2000, 1529, 210–222. [Google Scholar] [CrossRef] [PubMed]
- Thévenin, A.F.; et al. Proteins and mechanisms regulating gap-junction assembly, internalization, and degradation. Physiology (Bethesda) 2013, 28, 93–116. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.Z.; et al. F-Actin Dysplasia Involved in Organ of Corti Deformity in Gjb2 Knockdown Mouse Model. Front Mol Neurosci 2021, 14, 808553. [Google Scholar] [CrossRef]
- Thomas, T.; et al. Mechanisms of Cx43 and Cx26 transport to the plasma membrane and gap junction regeneration. J Cell Sci 2005, 118 Pt 19, 4451–4462. [Google Scholar] [CrossRef]
- Song, X.; et al. Phase separation of EB1 guides microtubule plus-end dynamics. Nat Cell Biol 2023, 25, 79–91. [Google Scholar] [CrossRef]
- Askham, J.M.; et al. Evidence that an interaction between EB1 and p150(Glued) is required for the formation and maintenance of a radial microtubule array anchored at the centrosome. Mol Biol Cell 2002, 13, 3627–3645. [Google Scholar] [CrossRef]
- Villari, G.; et al. Distinct retrograde microtubule motor sets drive early and late endosome transport. Embo j 2020, 39, e103661. [Google Scholar] [CrossRef]
- Hayashi, I.; et al. Structural basis for the activation of microtubule assembly by the EB1 and p150Glued complex. Mol Cell 2005, 19, 449–460. [Google Scholar] [CrossRef] [PubMed]
- Bellett, G.; et al. Microtubule plus-end and minus-end capture at adherens junctions is involved in the assembly of apico-basal arrays in polarised epithelial cells. Cell Motil Cytoskeleton 2009, 66, 893–908. [Google Scholar] [CrossRef] [PubMed]
- Koonce, M.P.; Samsó, M. Of rings and levers: the dynein motor comes of age. Trends Cell Biol 2004, 14, 612–619. [Google Scholar] [CrossRef] [PubMed]
- Dujardin, D.L.; Vallee, R.B. Dynein at the cortex. Curr Opin Cell Biol 2002, 14, 44–49. [Google Scholar] [CrossRef] [PubMed]
- Frenzel, E.M.; Johnson, R.G. Gap junction formation between cultured embryonic lens cells is inhibited by antibody to N-cadherin. Dev Biol 1996, 179, 1–16. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.; et al. Structure of the second PDZ domain from human zonula occludens 2. Acta Crystallogr Sect F Struct Biol Cryst Commun 2009, 65 Pt 4, 327–330. [Google Scholar] [CrossRef]
- Hervé, J.C.; et al. Influence of the scaffolding protein Zonula Occludens (ZOs) on membrane channels. Biochim Biophys Acta 2014, 1838, 595–604. [Google Scholar] [CrossRef]
- Ishii, M.; et al. EphB signaling inhibits gap junctional intercellular communication and synchronized contraction in cultured cardiomyocytes. Basic Res Cardiol 2011, 106, 1057–1068. [Google Scholar] [CrossRef]
- Pasquale, E.B. Eph receptor signalling casts a wide net on cell behaviour. Nat Rev Mol Cell Biol 2005, 6, 462–475. [Google Scholar] [CrossRef]
- Defourny, J. Eph/ephrin signalling in the development and function of the mammalian cochlea. Dev Biol 2019, 449, 35–40. [Google Scholar] [CrossRef]
- Lévy, J.; et al. EFNB2 haploinsufficiency causes a syndromic neurodevelopmental disorder. Clin Genet 2018, 93, 1141–1147. [Google Scholar] [CrossRef] [PubMed]
- Davy, A.; Bush, J.O.; Soriano, P. Inhibition of gap junction communication at ectopic Eph/ephrin boundaries underlies craniofrontonasal syndrome. PLoS Biol 2006, 4, e315. [Google Scholar] [CrossRef] [PubMed]
- Defourny, J.; et al. Efnb2 haploinsufficiency induces early gap junction plaque disassembly and endocytosis in the cochlea. Brain Res Bull 2021, 174, 153–160. [Google Scholar] [CrossRef] [PubMed]
- Takamura, N.; Yamaguchi, Y. Involvement of caveolin-1 in skin diseases. Front Immunol 2022, 13, 1035451. [Google Scholar] [CrossRef] [PubMed]
- Ohi, M.D.; Kenworthy, A.K. Emerging Insights into the Molecular Architecture of Caveolin-1. J Membr Biol 2022, 255, 375–383. [Google Scholar] [CrossRef] [PubMed]
- Defourny, J.; et al. Cochlear supporting cell transdifferentiation and integration into hair cell layers by inhibition of ephrin-B2 signalling. Nat Commun 2015, 6, 7017. [Google Scholar] [CrossRef] [PubMed]
- Mäkinen, T.; et al. PDZ interaction site in ephrinB2 is required for the remodeling of lymphatic vasculature. Genes Dev 2005, 19, 397–410. [Google Scholar] [CrossRef]
- Dravis, C.; et al. EphB2 and ephrin-B2 regulate the ionic homeostasis of vestibular endolymph. Hear Res 2007, 223, 93–104. [Google Scholar] [CrossRef]
- Chen, J.; et al. Connexin30-Deficiency Causes Mild Hearing Loss With the Reduction of Endocochlear Potential and ATP Release. Front Cell Neurosci 2021, 15, 819194. [Google Scholar] [CrossRef]
- Cohen-Salmon, M.; et al. Connexin30 deficiency causes instrastrial fluid-blood barrier disruption within the cochlear stria vascularis. Proc Natl Acad Sci U S A 2007, 104, 6229–6234. [Google Scholar] [CrossRef]
- Chang, Q.; et al. Gap junction mediated intercellular metabolite transfer in the cochlea is compromised in connexin30 null mice. PLoS One 2008, 3, e4088. [Google Scholar] [CrossRef] [PubMed]
- Sun, L.; et al. Failure Of Hearing Acquisition in Mice With Reduced Expression of Connexin 26 Correlates With the Abnormal Phasing of Apoptosis Relative to Autophagy and Defective ATP-Dependent Ca(2+) Signaling in Kölliker’s Organ. Front Cell Neurosci 2022, 16, 816079. [Google Scholar] [CrossRef] [PubMed]
- Chen, P.; et al. Pathological mechanisms of connexin26-related hearing loss: Potassium recycling, ATP-calcium signaling, or energy supply? Front Mol Neurosci 2022, 15, 976388. [Google Scholar] [CrossRef] [PubMed]
- Wang, M.; et al. Hippo/YAP signaling pathway protects against neomycin-induced hair cell damage in the mouse cochlea. Cell Mol Life Sci 2022, 79, 79. [Google Scholar] [CrossRef] [PubMed]
- Dahl, E.; et al. Molecular cloning and functional expression of mouse connexin-30,a gap junction gene highly expressed in adult brain and skin. J Biol Chem 1996, 271, 17903–17910. [Google Scholar] [CrossRef]
- Ahmad, S.; Evans, W.H. Post-translational integration and oligomerization of connexin 26 in plasma membranes and evidence of formation of membrane pores: implications for the assembly of gap junctions. Biochem J 2002, 365 Pt 3, 693–699. [Google Scholar] [CrossRef]
- Ahmad, S.; et al. Synthesis and assembly of connexins in vitro into homomeric and heteromeric functional gap junction hemichannels. Biochem J 1999, 339 Pt 2, 247–253. [Google Scholar] [CrossRef]
- Diez, J.A.; Ahmad, S.; Evans, W.H. Assembly of heteromeric connexons in guinea-pig liver en route to the Golgi apparatus, plasma membrane and gap junctions. Eur J Biochem 1999, 262, 142–148. [Google Scholar] [CrossRef]
- Zhang, J.T.; et al. Membrane integration of in vitro-translated gap junctional proteins: co- and post-translational mechanisms. Mol Biol Cell 1996, 7, 471–482. [Google Scholar] [CrossRef]
- Leithe, E.; Mesnil, M.; Aasen, T. The connexin 43 C-terminus: A tail of many tales. Biochim Biophys Acta Biomembr 2018, 1860, 48–64. [Google Scholar] [CrossRef]
- Forge, A.; et al. Connexin30-mediated intercellular communication plays an essential role in epithelial repair in the cochlea. J Cell Sci 2013, 126 Pt 7, 1703–1712. [Google Scholar] [CrossRef]
- Jagger, D.J.; Forge, A. Connexins and gap junctions in the inner ear--it’s not just about K⁺ recycling. Cell Tissue Res 2015, 360, 633–644. [Google Scholar] [CrossRef] [PubMed]
- Sun, J.; et al. Cochlear gap junctions coassembled from Cx26 and 30 show faster intercellular Ca2+ signaling than homomeric counterparts. Am J Physiol Cell Physiol 2005, 288, C613–C623. [Google Scholar] [CrossRef] [PubMed]
- Furness, D.N. Molecular basis of hair cell loss. Cell Tissue Res 2015, 361, 387–399. [Google Scholar] [CrossRef] [PubMed]
- Fourriere, L.; et al. The role of microtubules in secretory protein transport. J Cell Sci 2020, 133. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.; et al. Molecular Pathway of Microtubule Organization at the Golgi Apparatus. Dev Cell 2016, 39, 44–60. [Google Scholar] [CrossRef]
- irestone, A.J.; et al. Small-molecule inhibitors of the AAA+ ATPase motor cytoplasmic dynein. Nature 2012, 484, 125–129. [Google Scholar] [CrossRef]
- Ezratty, E.J.; Partridge, M.A.; Gundersen, G.G. Microtubule-induced focal adhesion disassembly is mediated by dynamin and focal adhesion kinase. Nat Cell Biol 2005, 7, 581–590. [Google Scholar] [CrossRef]
- Berger, A.C.; et al. Mutations in Cx30 that are linked to skin disease and non-syndromic hearing loss exhibit several distinct cellular pathologies. J Cell Sci 2014, 127 Pt 8, 1751–1764. [Google Scholar] [CrossRef]
| Classification | Cx26 mutations | References |
|---|---|---|
| I | M1V, N14D, 30delG, 35delG, I20T, I35S, DeltaE42, D50Y, D50N, T55N, G59A, G59V, C64S, D66H, H73R, I82M, L90P, Y136X, V153I, M163V, 167delT, P173R, D179N, R184P, L214P, 235delC, E147K, F142L, A88V, A40V, N14K, T86A, A40G, R32H, S199F, 572delT, 631-632delGT, Y155X, p.W172C (c.516G>C), R184Q, p.Gly45Glu, p.T86R (c.257C>G), I30N, G12R, K188R, F191L, V198M, S199F, G200R, I203K, L205P, T208P, c.176del16 | ([29], [30], [31], [32], [33], [34], [35], [36], [37], [38], [39], [40], [41], [42], [42], [43], [44], [45], [46], [47], [48], [49], [50], [51], [52], [53], [54], [55], [56], [57], [58], [59], [60]) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





