Submitted:
12 May 2023
Posted:
12 May 2023
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Results
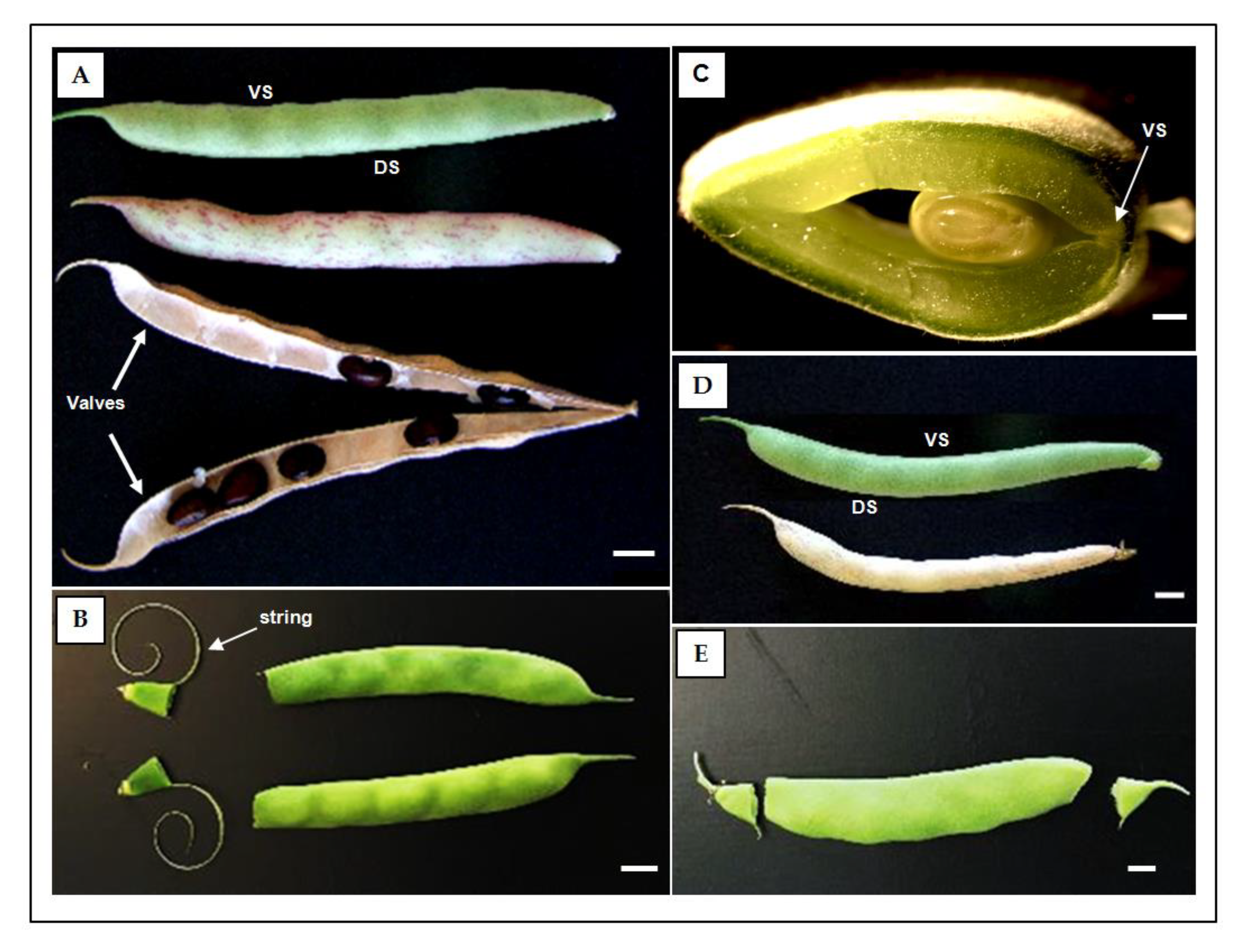
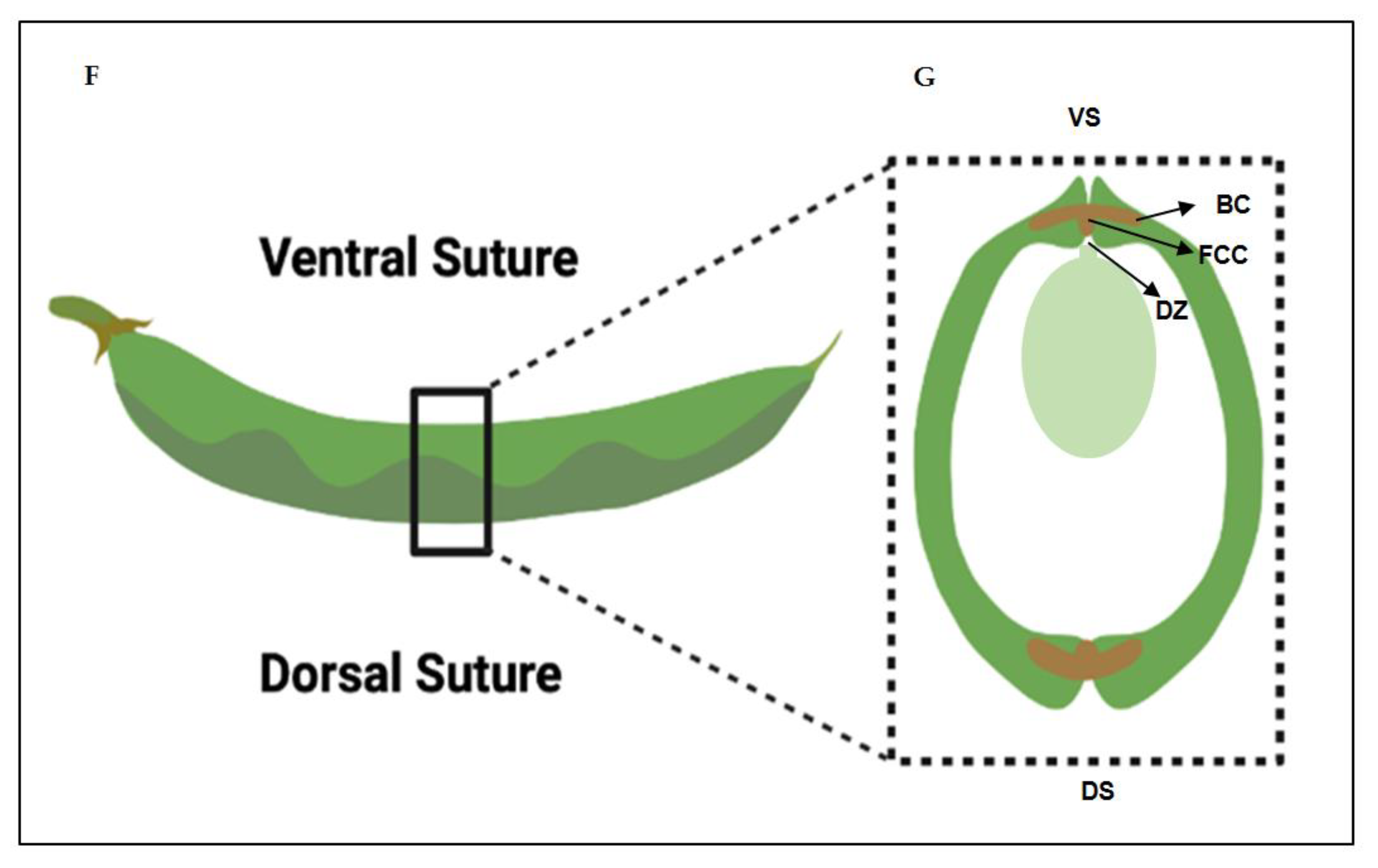
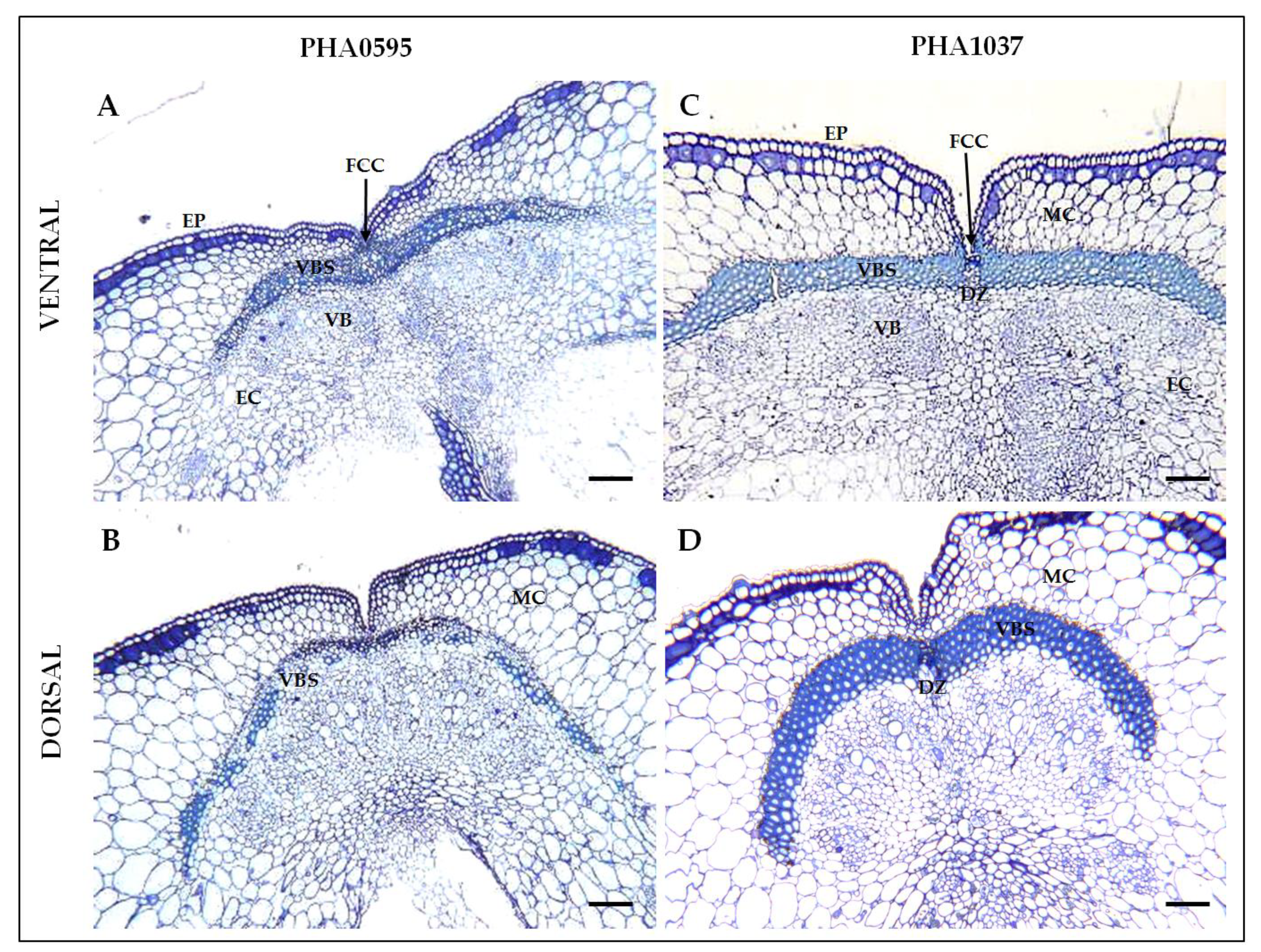
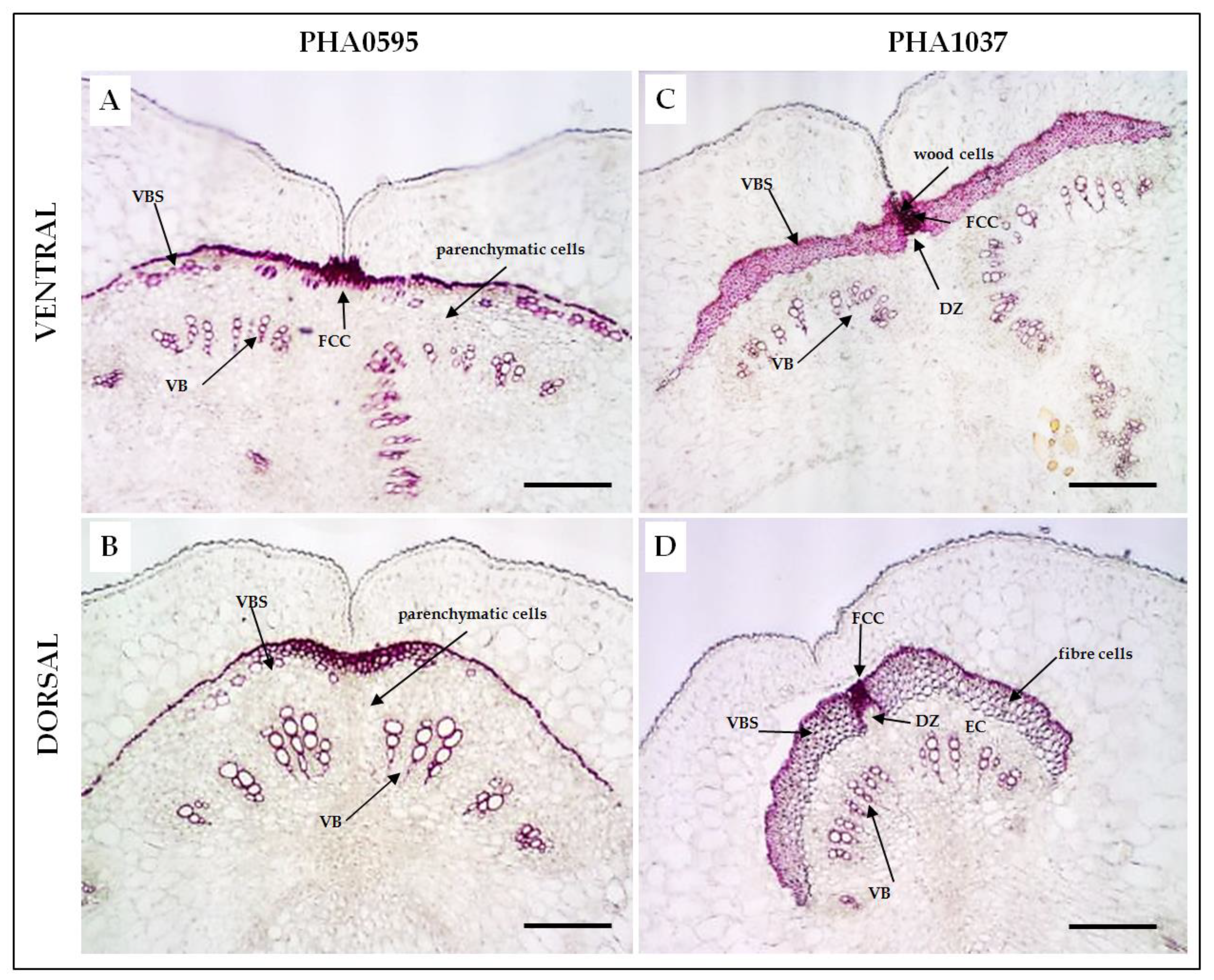
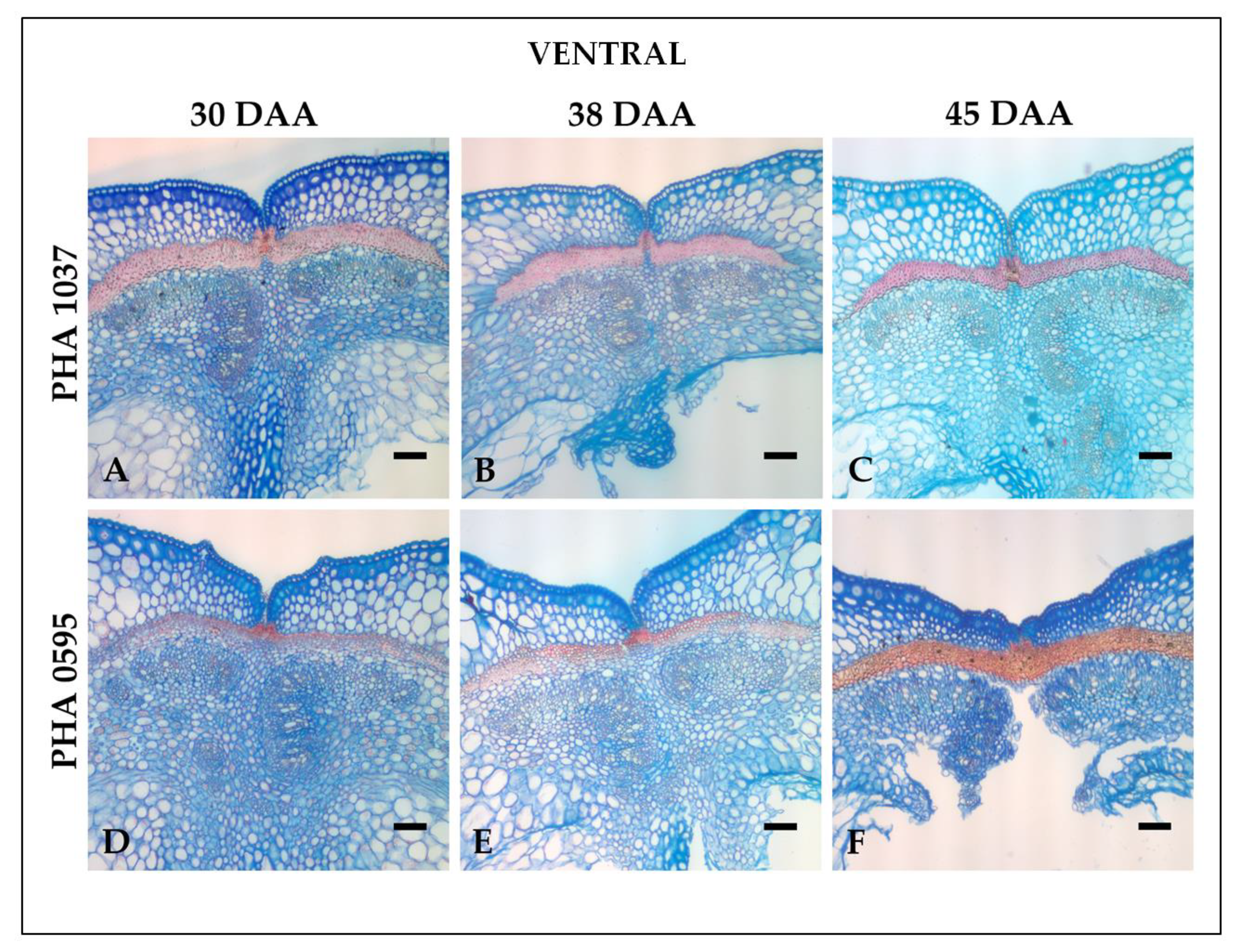
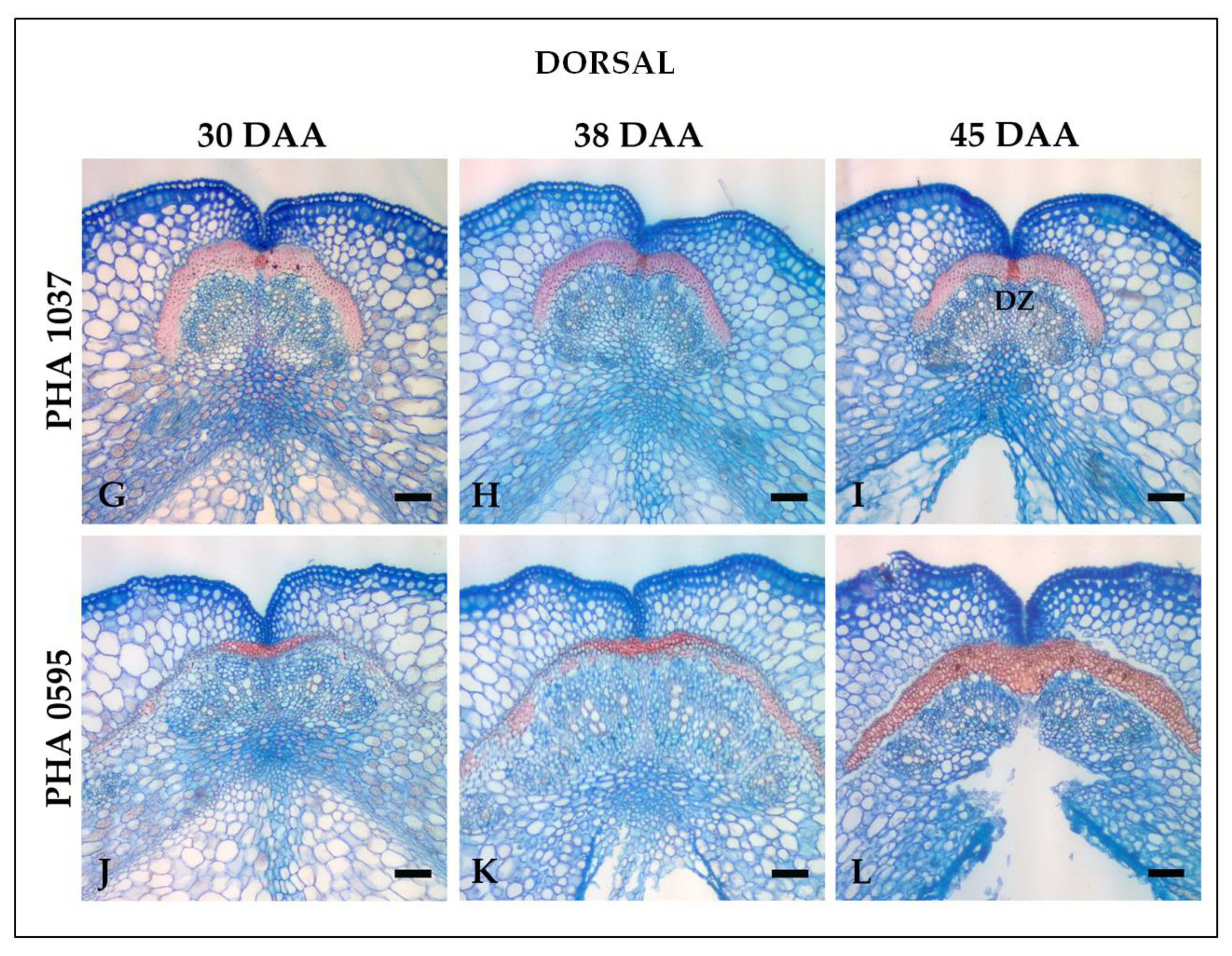
2.1. Histological characterization of the dehiscence zone in the common bean pod
2.2. Identification of the dehiscent structures underlying spatial and temporal fruit development
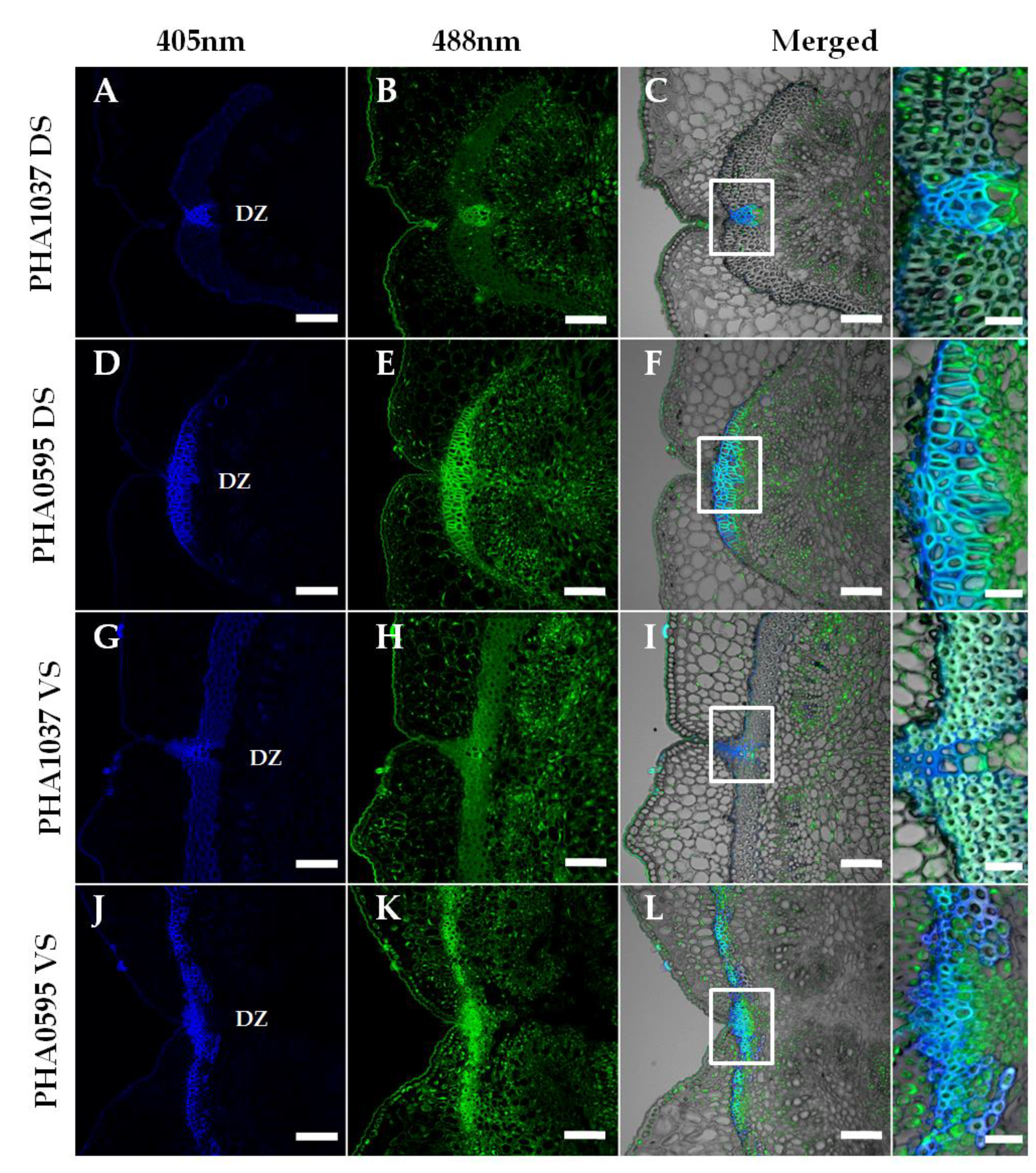
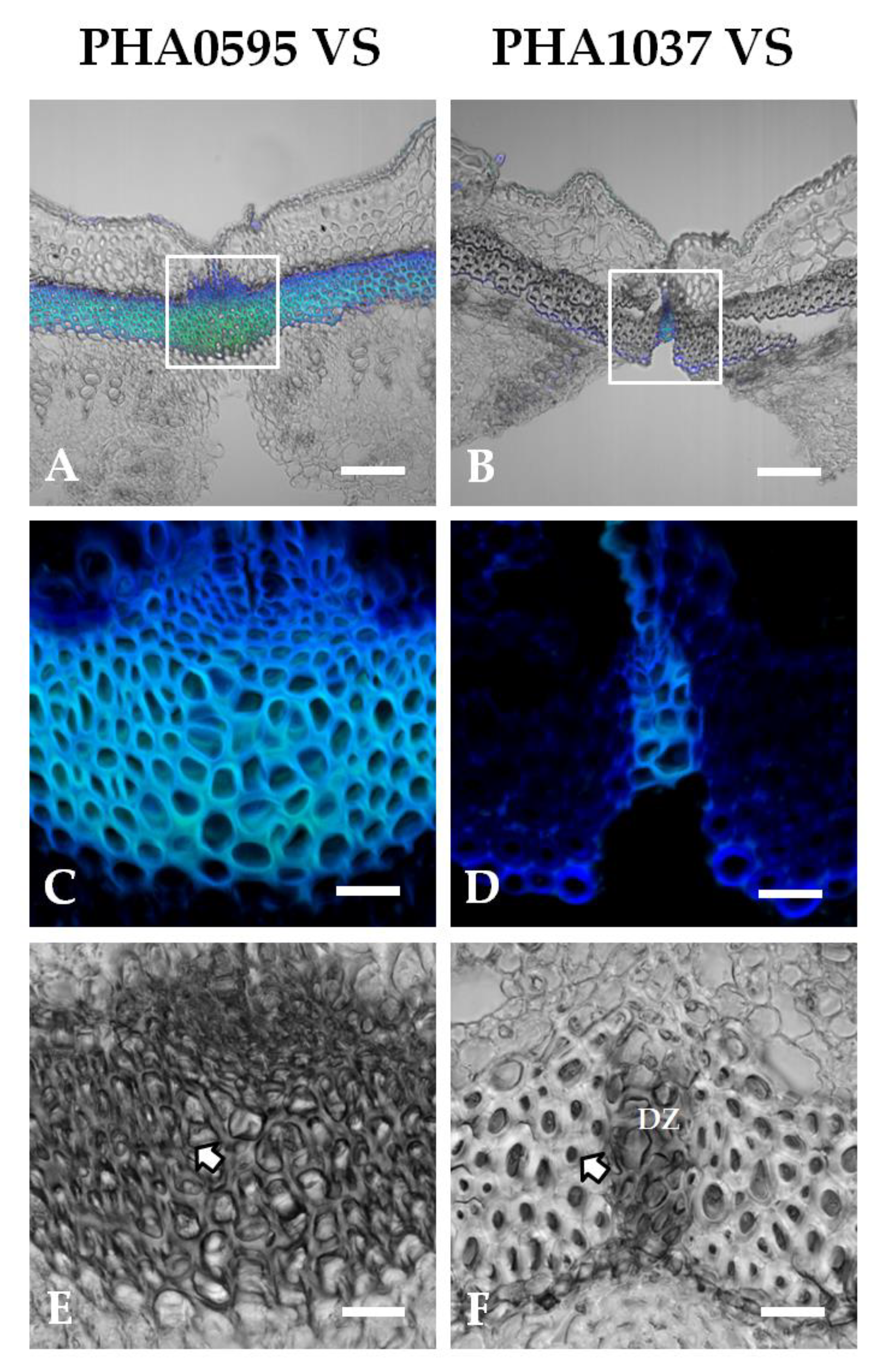
2.3. Association of fruit dehiscent structures with autofluorescence through pod maturation
3. Discussion
4. Materials and Methods
4.1. Plant growth and phenotype evaluation
4.2. Sample preparation, tissue embedding, blocking, and sectioning
4.3. Histochemical staining procedure and light microscopy
4.4. Confocal microscopy
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Doebley, J.F.; Gaut, B.S.; Smith, B.D. The molecular genetics of crop domestication. Cell. 2006, 127, 1309–21. [Google Scholar] [CrossRef] [PubMed]
- Purugganan, M.; Fuller, D.Q. The nature of selection during plant domestication. Nature. 2009, 457, 843–8. [Google Scholar] [CrossRef] [PubMed]
- Parker, T.A.; Sassoum, L.; Gepts, P. Pod shattering in grain legumes: emerging genetic and environment-related patterns. The Plant Cell. 2021, 33, 179–199. [Google Scholar] [CrossRef]
- Parker, T.A.; Berny Mier, T.; Palkovic, A.; Jernstedt, J.; Gepts, P. Pod indehiscence is a domestication and aridity resilience trait in common bean. New Phytol. 2020, 225(1), 558–570. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.; Tu, B.; Liu, C.; Liu, X. Pod anatomy, morphology and dehiscing forces in pod dehiscence of soybean (Glycine max (L.) Merrill). Flora. [CrossRef]
- Lush, W.M.; Evans, L.T. The domestication and improvement of cowpeas (Vigna unguiculata (L.) WALP.). Euphytica. [CrossRef]
- Gepts, P.; Debouck, D.G. Origin, domestication, and evolution of the common bean, Phaseolus vulgaris. 1991, In Common beans: research for crop improvement. CAB, Oxon, UK. Voysest, O.; Van Schoonhoven, A. Eds.; 1991; pp. 7–53.
- Romkaew, J.; Nagaya, Y.; Goto, M.; Suzuki, K.; Umezaki, T. Pod dehiscence in relation to chemical components of pod shell in soybean. Plant Prod. Sci. 2008, 11, 278–282. [Google Scholar] [CrossRef]
- Prakken, R. Inheritance of colours and pod characters in Phaseolus vulgaris L. Genetica. 1934, 16, 177–296. [Google Scholar] [CrossRef]
- Koinange, E.M.S. , Singh, S.P.; Gepts, P. Genetic control of the domestication syndrome in common bean. Crop Sci. 1996, 36, 1037–1045. [Google Scholar] [CrossRef]
- Kongjaimun, A.; Somta, P.; Tomooka, N.; Kaga, A.; Vaughan, D.A.; Srinives, P. QTL mapping of pod tenderness and total soluble solid in yardlong bean [Vigna unguiculata (L.) Walp. subsp. unguiculata cv. -gr. sesquipedalis]. Euphytica. [CrossRef]
- Suanum, W.; Somta, P.; Kongjaimun, A.; Yimram, T.; Kage, A.; Tomooka, N. Co-localization of QTLs for pod fiber content and pod shattering in F2 and backcross populations between yardlong bean and wild cowpea. Mol. Breed. 2016, 36, 80. [Google Scholar] [CrossRef]
- Takahashi, Y.; Kongjaimun, A.; Muto, C.; Kobayashi, Y.; Kumagai, M.; Sakai, H.; Satou, K.; Teruya, K.; Shiroma, A.; Shimoji, M.; et al. Same Locus for Non-shattering Seed Pod in Two Independently Domesticated Legumes, Vigna angularis and Vigna unguiculata. Front. Genet. 2020, 11, 748. [Google Scholar] [CrossRef]
- Ogutcen, E.; Pandey, A.; Khan, M.K.; Marques, E.; Penmetsa, R.V.; Kahraman, A.; von Wettberg, E.J. Pod shattering: a homologous series of variation underlying domestication and an avenue for crop improvement. Agronomy. 2018, 8, 137. [Google Scholar] [CrossRef]
- Di Vittori, V.; Gioia, T.; Rodriguez, M.; Bellucci, E.; Bitocchi, E.; Nanni, L.; Attene, G.; Rau, D.; Papa, R. Convergent evolution of the seed shattering trait. Genes. 2019, 10, 68. [Google Scholar] [CrossRef] [PubMed]
- Spence, J.; Vercher, Y.; Gates, P.; Harris, N. ‘Pod shatter’ in Arabidopsis thaliana, Brassica napus and B. juncea. J. Microsc. 1996, 181(2), 195–203. [Google Scholar] [CrossRef]
- Balanzà, V.; Roig-Villanova, I.; Di Marzo, M. , Masiero, S., and Colombo, L. Seed abscission and fruit dehiscence required for seed dispersal rely on similar genetic networks. Development. 2016, 2016. 143, 3372–3381. [Google Scholar] [CrossRef]
- Zhang, Y.; Shen, Y.Y.; Wu, X.M.; Wang, J.B. The basis of pod dehiscence: anatomical traits of the dehiscence zone and expression of eight pod shatter-related genes in four species of Brassicaceae. Biol. Plant. 2016, 60, 343–354. [Google Scholar] [CrossRef]
- Meakin, P.J.; Roberts, J.A. Dehiscence of fruit in oilseed rape (Brassica napus L.) II. The role of cell wall degrading enzymes and ethylene. J. Exp. Bot. 1990, 41, 1003–1011. [Google Scholar] [CrossRef]
- Østergaard, L.; Borkhardt, B.; Ulvskov, P. In ’Dehiscence’ in Plant Cell Separation and Adhesion, eds. Roberts, J.A.; Gonzalez-Carranza, Z.H.,Victoria: Blackwell Publishing. 2007, pp. 137–163.
- Morgan, C.L.; Bruce, D.M.; Child, R.; Ladbrooke, Z.L.; Arthur, A.E. Genetic variation for pod shatter resistance among lines of oilseed rape developed from synthetic B. napus. Field Crops Res. 1998, 58, 153–165. [Google Scholar] [CrossRef]
- Christiansen, L.C.; Dal Degan, F.; Ulvskov, P.; Borkhardt, B. Examination of the dehiscence zone in soybean pods and isolation of a dehiscence-related endopolygalacturonase gene. Plant Cell Environ. 2002, 25, 479–490. [Google Scholar] [CrossRef]
- Østergaard, L.; Kempin, S.A.; Bies, D.; Klee, H.J.; Yanofsky, M.F. Pod shatter-resistant Brassica fruit produced by ectopic expression of the FRUITFULL gene. Plant Biotechnol. J. 2006, 4, 45–51. [Google Scholar] [CrossRef]
- Tiwari, S.; Bhatia, V. Characters of pod anatomy associated with pod shattering in soybean. Ann. Bot. 1995, 76, 483–485. [Google Scholar] [CrossRef]
- Ferrándiz, C. Regulation of fruit dehiscence in Arabidopsis. J. Exp. Bot. 2002, 53, 2031–8. [Google Scholar] [CrossRef]
- Yang, J.B.; Wright, R.L.; McGraw, R.L. Seed pod dehiscence in birdsfoot trefoil, Lotus conimbricensis, and their interspecific somatic hybrid. Can. J. Plant Sci. 1990, 70, 279–284. [Google Scholar] [CrossRef]
- Fourquin, C.; del Cerro, C.; Victoria, F.C.; Vialette-Guiraud, A.; de Oliveira, A.C.; Ferrandiz, C. A change in SHATTERPROOF protein lies at the origin of a fruit morphological novelty and a new strategy for seed dispersal in the Medicago genus. Plant Physiol. 2013, 162, 907–17. [Google Scholar] [CrossRef] [PubMed]
- Dong, Y.; Yang, X.; Liu, J.; Wang, B.H.; Liu, B.L.; Wang, Y.Z. Pod dehiscence resistance associated with domestication is mediated by a NAC gene in soybean. Nat. Commun. 2014, 5, 3352. [Google Scholar] [CrossRef] [PubMed]
- Dong, Y.; Wang, Y.Z. Seed shattering: from models to crops. Front. Plant Sci. 2015, 6, 476. [Google Scholar] [CrossRef]
- 30. Li, .LF.; Olsen, K.M. To Have and to Hold: Selection for Seed and Fruit Retention During Crop Domestication. Curr. Top. Dev. Biol. [CrossRef]
- Ballester, P.; Ferrándiz, C. Shattering fruits: variations on a dehiscent theme. Curr. Opin. Plant Biol. 2017, 35, 68–75. [Google Scholar] [CrossRef]
- Funatsuki, H.; Suzuki, M.; Hirose, A.; Inaba, H.; Yamada, T.; Hajika, M.; Komatsu, K.; Katayama, T.; Sayama, T.; Ishimoto, M.; Fujino, K. Molecular basis of a shattering resistance boosting global dissemination of soybean. Proc. Natl. Acad. Sci. USA. 2014, 111, 17797–17802. [Google Scholar] [CrossRef]
- Murgia, M.L.; Attene, G.; Rodriguez, M.; Bitocchi, E.; Bellucci, E.; Fois, D.; Nanni, L.; Gioia, T.; Albani, D.M.; Papa, R.; Rau, D. A Comprehensive Phenotypic Investigation of the "Pod-Shattering Syndrome" in Common Bean. Front Plant Sci. 2017, 8, 251. [Google Scholar] [CrossRef]
- Morgan, C.L.; Ladbrooke, Z.L.; Bruce, D.M.; Child, R.; Arthur, A.E. Breeding oilseedrape for pod shattering resistance. J. Agric. Sci. 2000, 135, 347–359. [Google Scholar] [CrossRef]
- Davies, G.C.; Bruce, D.M. Fracture mechanics of oilseed rape pods. J. Mater. Sci. 1997, 32, 5895–5899. [Google Scholar] [CrossRef]
- Dong, D.; Dong, R.; Wang, Y.; Nie, B.; Liu, Z. Study on pod development and ventral suture structure of Vicia sativa cultivar Lanjian NO. 3. Acta. Bot. Boreali-Occidentalia Sin, 1382. [Google Scholar]
- Tsuchiya, T. Physiological and genetic analysis of pod shattering in soybean. Jarq-Japan. Agric. Res. Q, 21.
- Suzuki, M.; Fujino, K.; Funatsuki, H. A major soybean QTL, qPDH1, controls pod dehiscence without marked morphological change. Plant Prod. Sci. 2009, 12, 217–223. [Google Scholar] [CrossRef]
- Boerjan, W.; Ralph, J.; Baucher, M. Lignin biosynthesis. Annu Rev Plant Biol. 2003, 54, 519–46. [Google Scholar] [CrossRef] [PubMed]
- Donaldson, L. Autofluorescence in Plants. Molecules. 2020, 25, 2393. [Google Scholar] [CrossRef] [PubMed]
- Donaldson, L.A. Lignification and lignin topochemistry – an ultrastructural view. Phytochemistry. 2001, 57, 859–873. [Google Scholar] [CrossRef] [PubMed]
- Pesquet, E.; Ranocha, P.; Legay, S.; Digonnet, C.; Barbier, O.; Pichon, M.; Goffner, D. Novel markers of xylogenesis in zinnia are differentially regulated by auxin and cytokinin. Plant Physiol. 2005, 139, 1821–39. [Google Scholar] [CrossRef] [PubMed]
- Liljegren, S. Phloroglucinol stain for lignin. Cold Spring Harb. Protoc, 4954. [Google Scholar] [CrossRef]
- Tolivia, D.; Tolivia, J. Fasga: a new polychromatic method for simultaneous and differential staining of plant tissues. J. Microsc. 1987, 148, 113–117. [Google Scholar] [CrossRef]
- Fernández de C., F.; Gepts, P.; López, M. In Etapas de desarrollo de la planta de fríjol común (Phaseolus vulgaris L.). Centro Internacional de Agricultura Tropical (CIAT), Cali, CO. 1986, 33 p.
- Debouck, D.G.; Hidalgo, R. Morfología de la planta de frijol común. In Frijol: Investigación y producción. Programa de las Naciones Unidas (PNUD); Centro Internacional de Agricultura Tropical (CIAT), Cali, CO. 1985, pp. 7–41.
- Carlson, J.B.; Lersten, N.R. Reproductive morphology. In Soybeans: improvement, production, and uses. Shibles, R.M.; Harper, J.E.; Wilson, R.F.; Shoemaker, R.C.; Eds. 2004, 16, 59–95. [Google Scholar] [CrossRef]
- Bonawitz, N.D.; Kim, J.I.; Tobimatsu, Y.; Ciesielski, P.N.; Anderson, N.A.; Ximenes, E. , et al. Disruption of Mediator rescues the stunted growth of a lignin-deficient Arabidopsis mutant. Nature. [CrossRef]
- Vanholme, R.; Demedts, B.; Morreel, K.; Ralph, J.; Boerjan, W. Lignin biosynthesis and structure. Plant Physiol. 2010, 153, 895–905. [Google Scholar] [CrossRef]
- Graham, E.T.; Trentham, W.R. Staining paraffin extracted, alcohol rinsed plant tissue with an aqueous mixture of three dyes. Biotechnol. Histochem. 1998, 73, 178–185. [Google Scholar] [CrossRef]
- Rogers, L.A.; Campbell, M.M. The genetic control of lignin deposition during plant growth and development. New Phytol. 2004, 164, 17–30. [Google Scholar] [CrossRef]
- Drijfhout, E. Influence of temperature on string formation of beans (Phaseolus vulgaris). Euphytica. 1970, 19, 145–151. [Google Scholar] [CrossRef]
- Thurling, N.; Howieson, J. Genotypic variation in shattering resistance in spring rape. Australasian Plant Breed. Newslett. 1982, 32, 95–6. [Google Scholar]
- Liljegren, S.; Ditta, G.; Eshed, Y. SHATTERPROOF MADS-box genes control seed dispersal in Arabidopsis. Nature. [CrossRef]
- Di Vittori, V.; Bitocchi, E.; Rodriguez, M.; Alseekh, S.; Bellucci, E.; Nanni, L. Pod indehiscence in common bean is associated to the fine regulation of PvMYB26 and a non-functional abscission layer. J. Exp. Bot. 2021. 72, 1093. [Google Scholar]
- Kadkol, G.P.; Beilharz, V.C.; Halloran, G.M.; Macmilla, R.H. Anatomical Basis of Shatter-resistance in the Oilseed Brassicas. Aust. J. Bot. 1986, 34, 595–601. [Google Scholar] [CrossRef]
- Aguilar-Benitez, D.; Rubio, J.; Millán, T. Genetic analysis reveals PDH1 as a candidate gene for control of pod dehiscence in chickpea. Mol Breed. 2020, 40, 40. [Google Scholar] [CrossRef]
- Donaldson, L.A.; Radotic, K. Fluorescence lifetime imaging of lignin autofluorescence in normal and compression wood. J. Microsc. 2013, 251, 178–187. [Google Scholar] [CrossRef] [PubMed]
- Tu, B.; Liu, C.; Wang, X.; Li, Y.; Zhang, Q.; Liu, X.; Herbert, S.J. Greater Anatomical Differences of Pod Ventral Suture in Shatter-Susceptible and Shatter-Resistant Soybean Cultivars. Crop Sci. 2019, 59, 2784–2793. [Google Scholar] [CrossRef]
- Jia, C. , Dong, D.; Zhou, Q.; Searle, I.R., Liu, Z. Significant cell differences in pod ventral suture in shatter-resistant and shatter-susceptible common vetch accessions. Crop Sci, 1749; 61. [Google Scholar] [CrossRef]
- Bertioli, D.J.; Moretzsohn, M.C.; Madsen, H.; Sanda, N.; Leal-Bertioli, S.C.; Guimaraes, P.M.; Hougaard, B.K.; Fredslund, J.; Schauser, L.; Nielsen, A.M.; et al. An analysis of synteny of Arachis with Lotus and Medicago sheds new light on the structure, stability and evolution of legume genomes. BMC Genomics. 2009, 10, 45. [Google Scholar] [CrossRef]
- Parker, T.; Cetz, J.; de Sousa, L.L.; Kuzay, S.; Lo, S.; de Oliveira Floriani, T.; et al. Loss of pod strings in common bean is associated with gene duplication, retrotransposon insertion, and overexpression of PvIND. New Phytol. 2022, 235, 2454–2465. [Google Scholar] [CrossRef]
- Dong, D.; Yan, L.; Dong, R.; Liu, W.; Wang, Y.; Liu, Z. Evaluation and analysis of pod dehiscence factors in shatter-susceptible and shatter-resistant common vetch. Crop Sci. 2017, 57, 2770–2776. [Google Scholar] [CrossRef]
- Romkaew, J.; Umezaki, T. Pod dehiscence in soybean: Assessing methods and varietal difference. Plant Prod. Sci. 2006, 2006. 9, 373–382. [Google Scholar] [CrossRef]
- Zhang, J.; Singh, A.K. Genetic control and geo-climate adaptation of pod dehiscence provides novel insights into soybean domestication. G3 Genes, Genomes, Genetics, 10. [CrossRef]
- Mitra, P.P.; Loque, D. Histochemical staining of Arabidopsis thaliana secondary cell wall elements. J. Vis. Exp. 2014, 87, e51381. [Google Scholar] [CrossRef]
- Sessions, R.A.; Zambryski, P.C. Arabidopsis gynoecium structure in the wild and in ettin mutants. Development. 1995, 121, 1519–1532. [Google Scholar] [CrossRef] [PubMed]
- Zúñiga-Mayo, V.M.; Marsch-Martinez, N.; De Folter, S. JAIBA, a class-II HD-ZIP transcription factor involved in the regulation of meristematic activity, and important for correct gynoecium and fruit development in Arabidopsis. Plant J. 2012, 71, 314–326. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).




