Submitted:
10 May 2023
Posted:
11 May 2023
You are already at the latest version
Abstract
Keywords:
1. Introduction
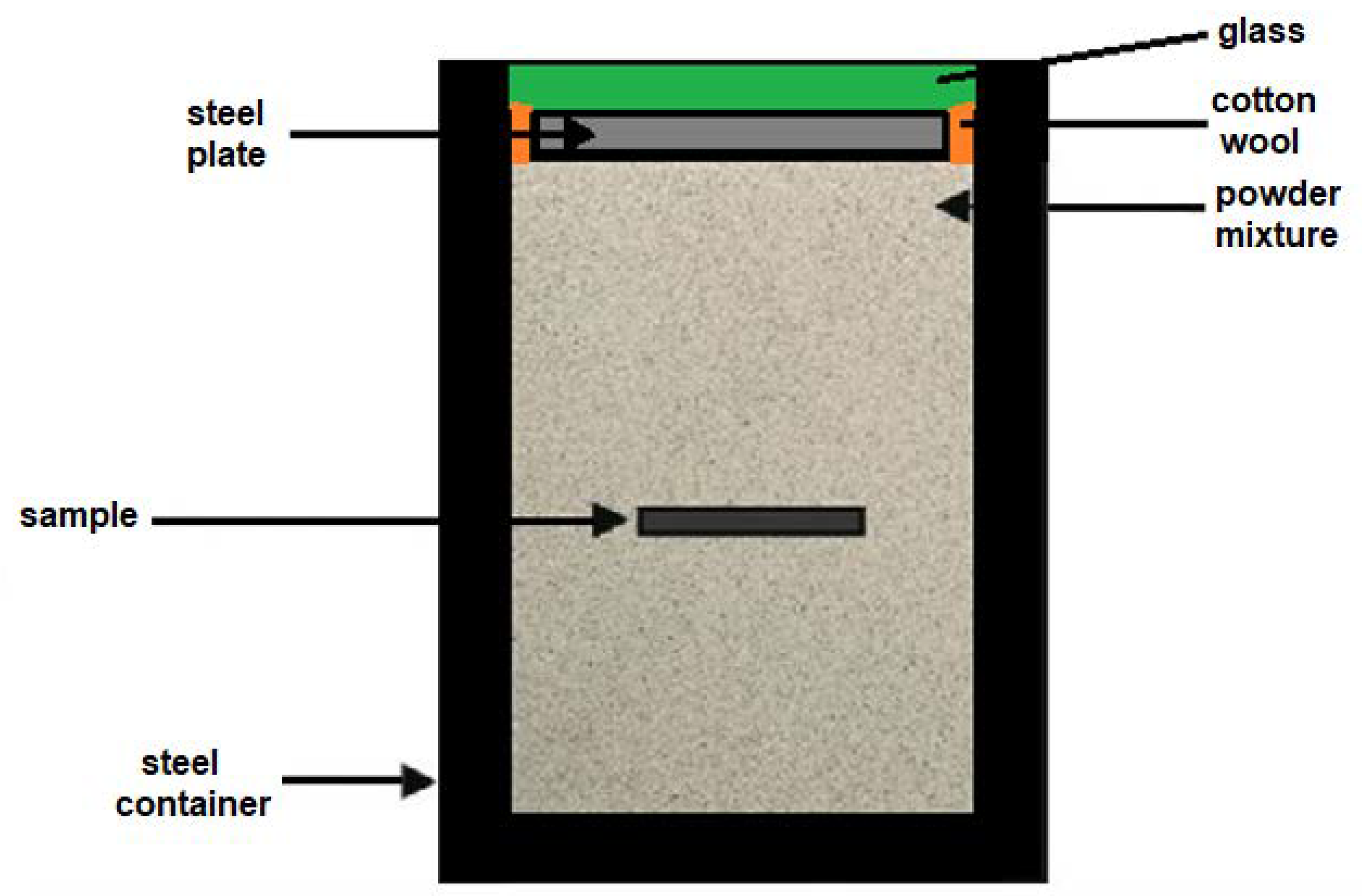
2. Materials and Methods
3. Results
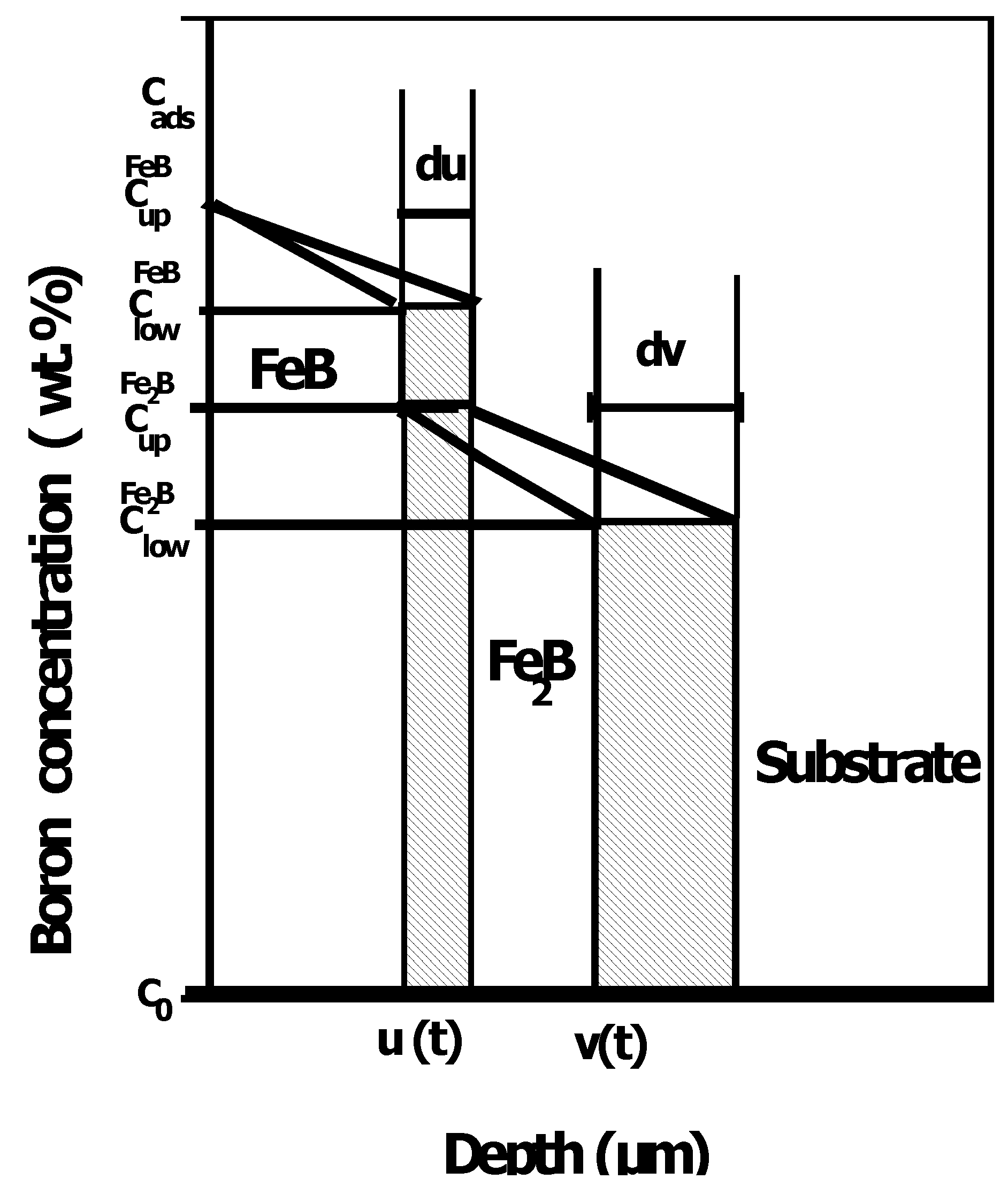
3.1. The integral diffusion model
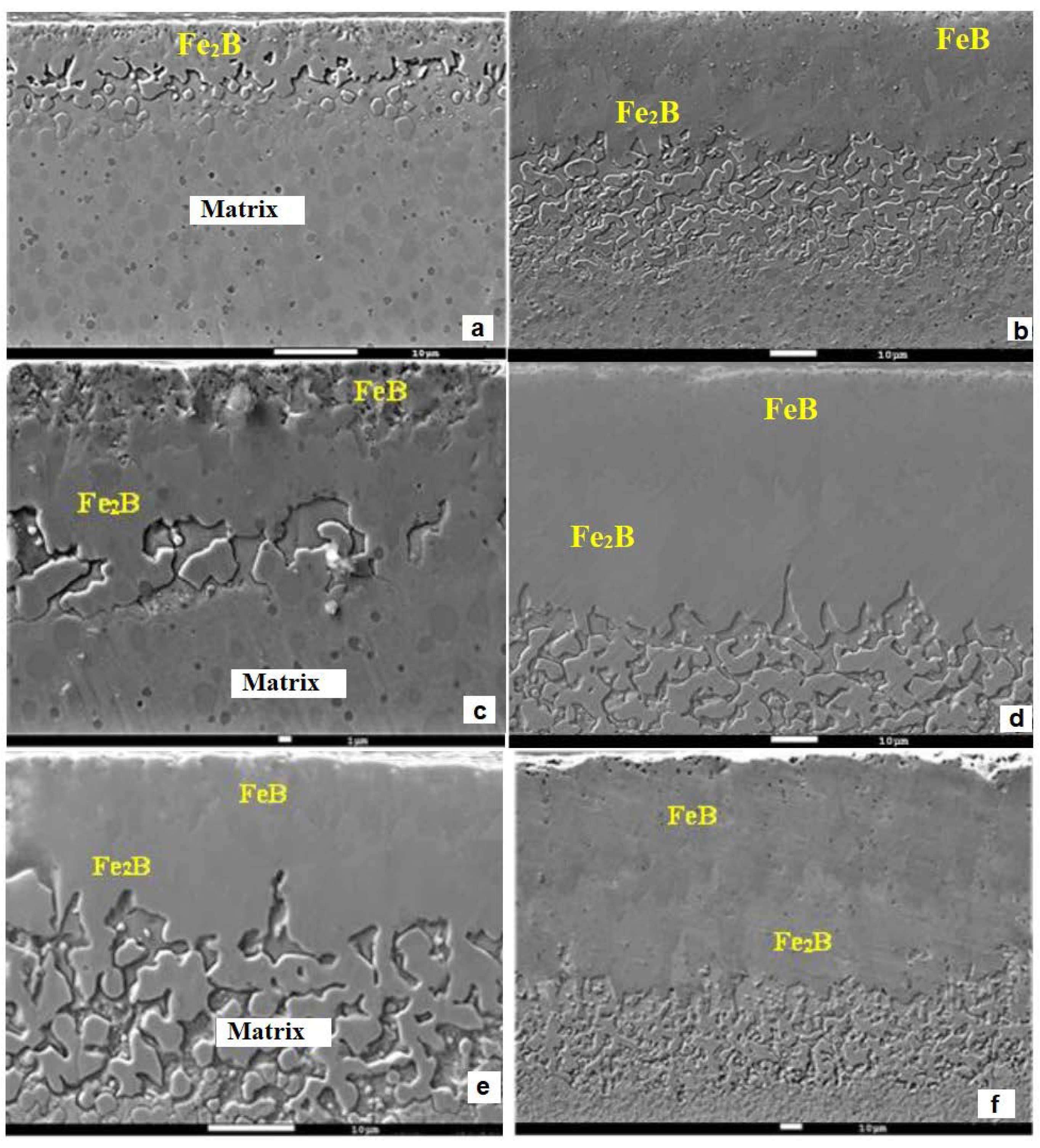
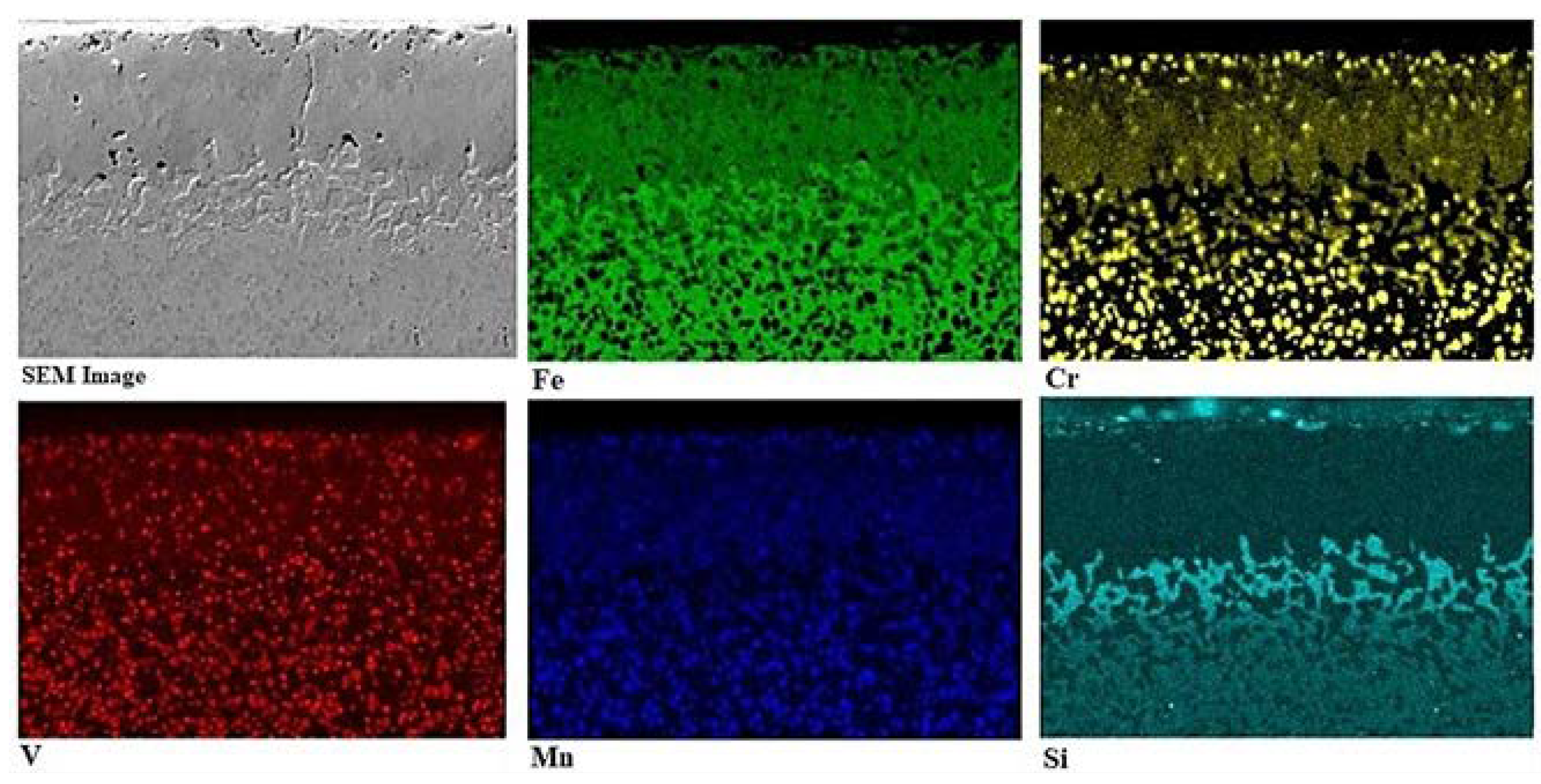
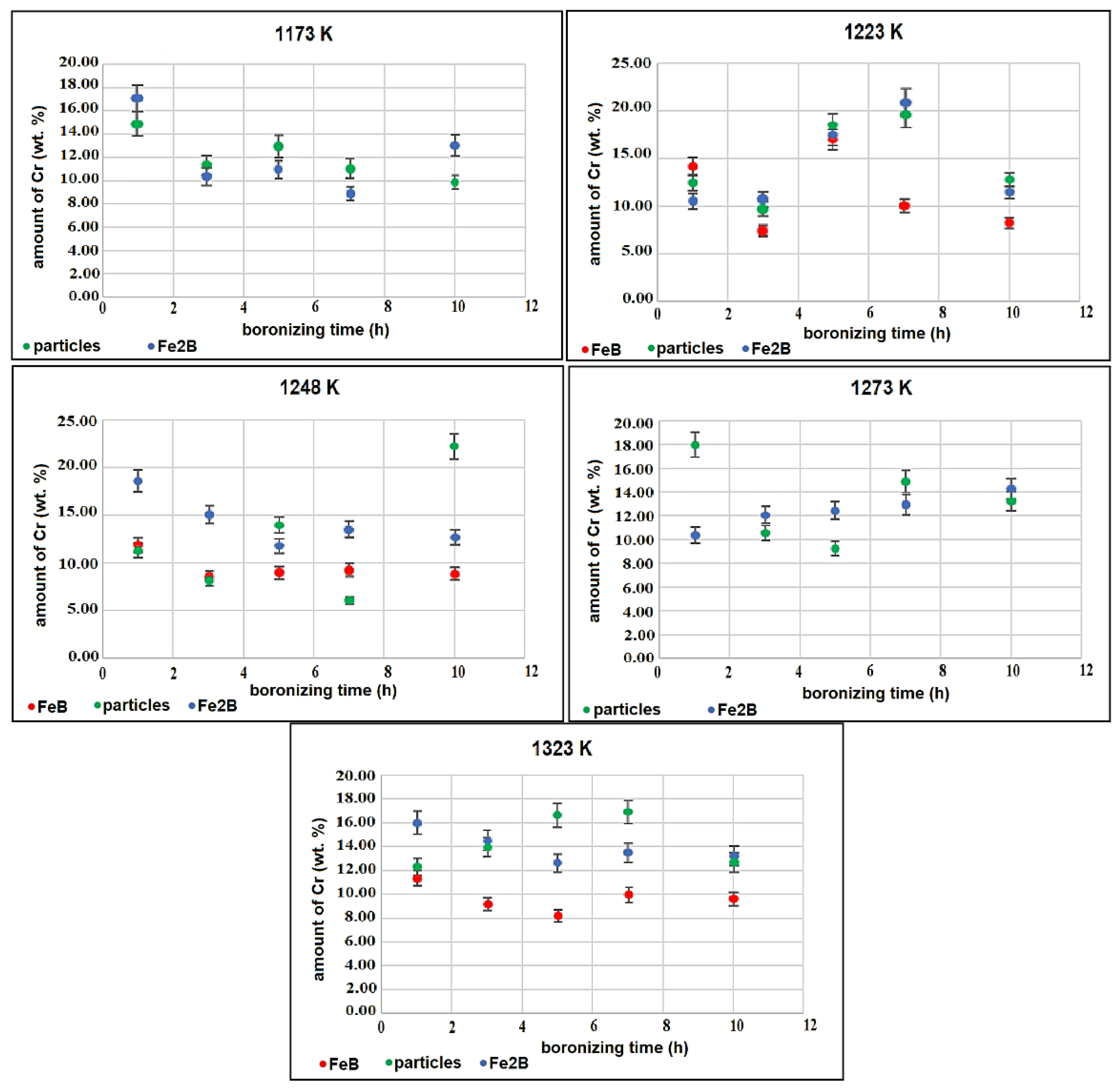
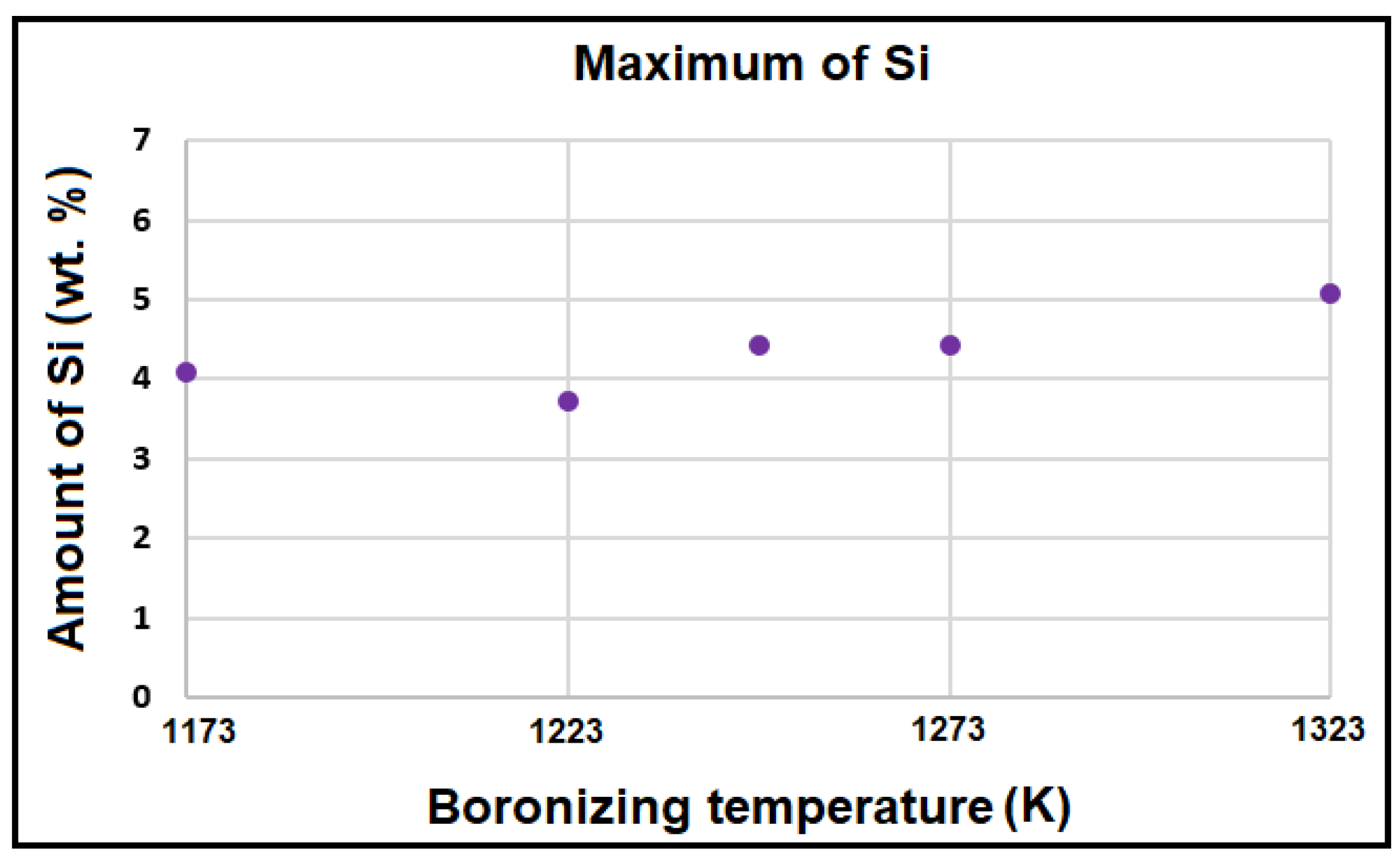
3.2. SEM examinations and EDS analysis
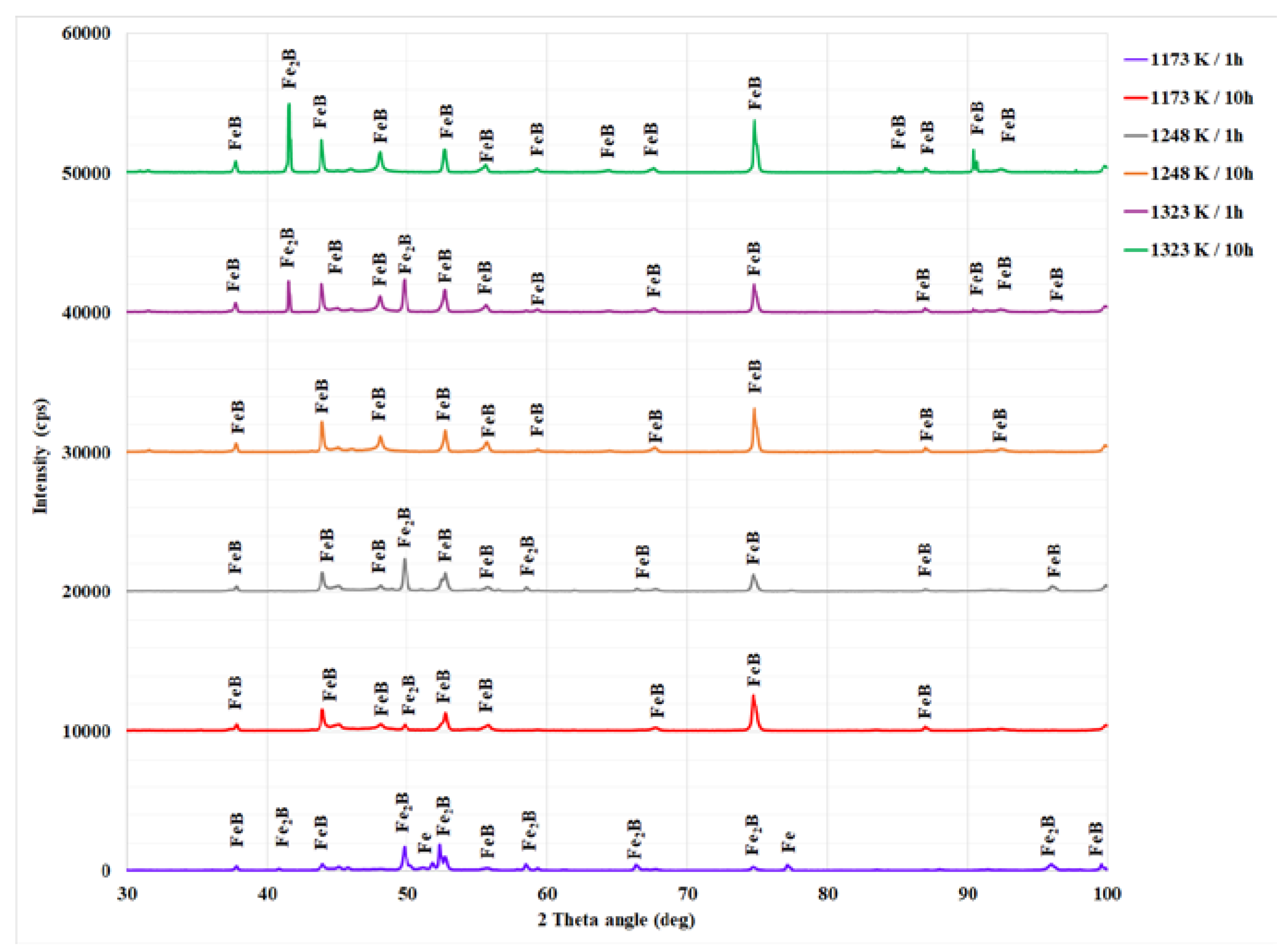
3.2. XRD Results
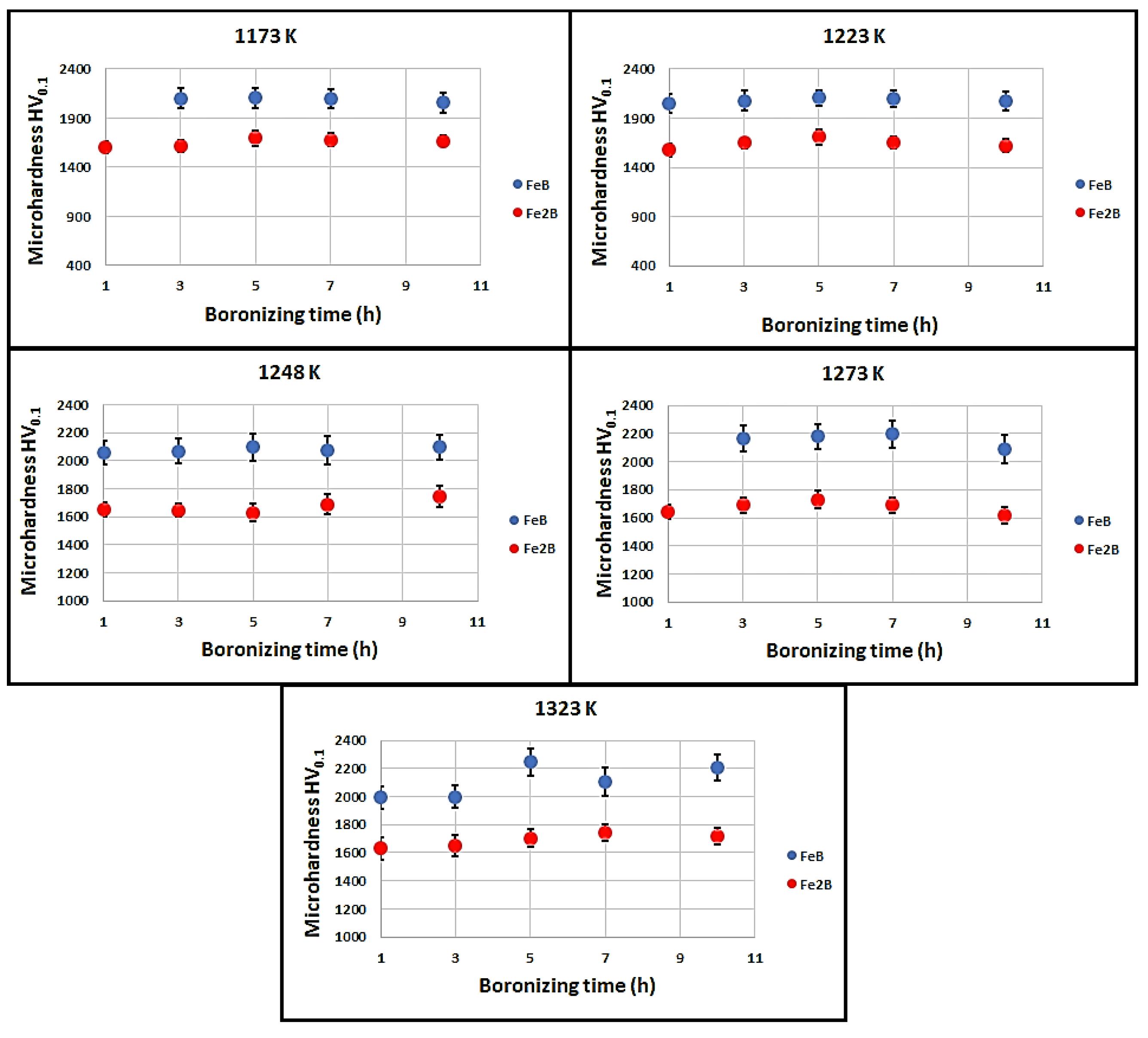
3.3. Microhardness Vickers measurements
3.4. Assessment of boron diffusion coefficients in iron borides with the integral method
4. Conclusions
References
- Keddam, M.; Kulka, M.; Makuch, N.; Pertek, A.; Maldzinski, L. A kinetic model for estimating the boron activation energies in the FeB and Fe2B layers during the gas-boriding of Armco iron: Effect of boride incubation times. Appl. Surf. Sci. 2014, 298, 155–163. [Google Scholar] [CrossRef]
- Kulka, M.; Makuch, N.; Piasecki, A. Nanomechanical characterization and fracture toughness of FeB and Fe2B iron borides produced by gas boriding of Armco iron. Surf. Coat. Technol. 2017, 325, 515–532. [Google Scholar] [CrossRef]
- Smolnikov, E.A.; Sarmanova, L.M. Study of the possibility of liquid boriding of high-speed steels. Metal Science and Heat Treatment 1982, 24, 785–788. [Google Scholar] [CrossRef]
- Nguyen, L.; Pham, N.A. study of SiC/Borax liquid boride layer on AISI H13 hot work tool steel. International Journal of Applied Engineering & Technology 2021, 23–28. [Google Scholar]
- Léon, R. Mechanical characterization of the AISI 316L alloy exposed to boriding process. Revista DYNA. 2020, 87, 34–41. [Google Scholar] [CrossRef]
- Ipek, M.; Celebi Efe, G.; Ozbek, I.; Zeytin, S.; Bindal, C. Investigation of Boronizing Kinetics of AISI 51100 Steel. J. Mater. Eng. Perform. 2012, 21, 733–738. [Google Scholar] [CrossRef]
- Gunes, I.; Ulker, S.; Taktak, S. Kinetics of plasma paste boronized AISI 8620 steel in borax paste mixtures. Protection of Metals and Physical Chemistry of Surfaces 2013, 49, 567–573. [Google Scholar] [CrossRef]
- Jiang, Y.; Bao, Y.; Wang, M. Kinetic Analysis of Additive on Plasma Electrolytic Boriding. Coatings 2017, 61, 1–9. [Google Scholar] [CrossRef]
- Jain, V.; Sundararajan, G. Influence of the pack thickness of the boronizing mixture on the boriding of steel. Surf. Coat. Technol. 2002, 149, 21–26. [Google Scholar] [CrossRef]
- Kayali, Y.; Gunes, I.; Ulu, S. Diffussion kinetics of borided AISI 52100 and AISI 440C steels. Vacuum 2012, 86, 1428–1434. [Google Scholar] [CrossRef]
- Xie, F.; Cheng, J.; Wang, S. Effects and mechanisms of an alternating current field on pack boriding. Vacuum 2018, 148, 41–47. [Google Scholar] [CrossRef]
- Okamoto, H. B-Fe (boron-iron). Journal of Phase Equilibria and Diffusion 25, 297 – 298, [online]: 2004. [viewed: 2022-11-04]. Available from: https://doi.org/10.1007/s11669-004-0128-3. [CrossRef]
- Keddam, M.; Hudáková, M.; Ptačinová, J.; Moravčík, R.; Gogola, P.; Gabalcová, Z.; Jurči, P. Characterization of boronized layers on Vanadis 6 tool steel. Surface Engineering 2020, 1–10. [Google Scholar]
- Campos-Silva, I.; Flores-Jiménez, M.; Rodríguez-Castro, G.; Hernández-Sánchez, E.; Martínez-Trinindad, J.; Tadeo-Rosas, R. Improved fracture toughness of boride coating developed with a diffusion annealing process. Surface & Coatings Technology 2013, 237, 729–439. [Google Scholar]
- Dybkov, V.I. Boriding of High Chromium Steels. Current Physical Chemistry 2016, 6, 137–144. [Google Scholar] [CrossRef]
- Dybkov, V.I. Basics of Formation of Iron Boride Coatings. Journal of Mineral Metal and Material Engineering. 2016, 2, 30–46. [Google Scholar] [CrossRef]
- Dybkov, V.I.; Lengauer, W.; Barmak, K. Formation of Boride Layers at the Fe–10 %Cr alloy–boron Interface. Journal of Alloys and Compounds. 2005, 398, 113–122. [Google Scholar] [CrossRef]
- Erdogan, M.; Gunes, I. Corrosion behavior and microstructure of borided tool steel. Revista matéria 2013, 20, 1–7. [Google Scholar] [CrossRef]
- Uslu, I.; Omert, H.; Ipek, M.; Celebi, F.G.; Ozdemir, O.; Bindal, C. A Comparison of Borides Formed on AISI 1040 and AISI P20 Steels. Mater. Des. 2007, 28, 1819–1826. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhen, Q.; Lygdenov, B.; Guriev, A.; Shun-Qi, M. Research on the technology of paste boronizing for H13 die steel. Material Science Engineering. 2019, 684, 1–5. [Google Scholar] [CrossRef]
- Orihel, P.; Drienovský, M.; Gabalcová, Z.; Jurči, P.; Keddam, M. Characterization and boron diffusion kinetics on the surface-hardened layers of Royalloy steel. Coatings 2023, 13, 113–129. [Google Scholar] [CrossRef]
- Makuch, N.; Kulka, M.; Keddam, M.; Piasecki, A. Growth kinetics, microstructure evolution and some mechanical properties of boride layers produced on X165CrV12 tool steel. Materials 2023, 16, 1–28. [Google Scholar] [CrossRef] [PubMed]
- Keddam, M.; Topuz, P.; Aydin, O. Simulation of boronizing kinetics of AISI 316 steel with the integral diffusion model. Materials Testing. 2021, 63, 906–912. [Google Scholar] [CrossRef]
- Zouzou, C.; Keddam, M. Boriding kinetics of FeB and Fe2B layers on AISI M2 steel by the integral diffusion model. Annales de Chimie-Sciences des Matériaux 2019, 43, 159–164. [Google Scholar] [CrossRef]
- Keddam, M.; Kulka, M. Simulation of the growth kinetics of FeB and Fe2B layers on AISI D2 steel by the integral method. Phys. Met. Metallogr. 2018, 119, 842–851. [Google Scholar] [CrossRef]
- Kunst, H.; Schaaber, O. Beobachtungen beim Oberflaechenborieren von Stahl. HTM Haerterei Tech. Mitt. 1967. [Google Scholar]
- Yu, L.G.; Chen, X.J.; Khor, A.K.; Sundararajan, G. FeB/Fe2B phase transformation during SPS pack-boriding: Boride layer growth kinetics. Acta Mater. 2005, 53, 2361–2368. [Google Scholar] [CrossRef]
- Okamoto, H. B-Fe (boron-iron). J Phs Eqil and Diff. 2004, 25, 297–298. [Google Scholar] [CrossRef]
- Nait Abdellah, Z.; Cherougne, R.; Keddam, M.; Bouarour, B.; Haddour, L.; Elias, A. The Phase Stability in the Fe-B Binary System: Comparison between the Interstitial and Substitutional Models. Defect and Diffusion Forum. 2012, 322, 1–9. [Google Scholar] [CrossRef]
- Goodman, T.R. Application of Integral Methods to Transient Nonlinear Heat Transfer. Adv. Heat Transfer 1964, 1, 51–122. [Google Scholar]
- Press, W.H.; Flannery, B.P.; Teukolsky, S.A. Numerical Recipes in Pascal: The Art of Scientific Computing. 1989. 1989. [Google Scholar]
- Ortiz-Domínguez, M. Modeling of the Growth Kinetics of Boride Layers in Powder-Pack Borided ASTM A36 Steel Based. Advances in Materials Science and Engineering 2019, 1–12. [Google Scholar] [CrossRef]
- Ortiz-Domínguez, M.; Elias–Espinosa, M.; Keddam, M.; Gómez–Vargas, A.; Lewis, R.; Vera–Cardénas, E. Growth kinetics and mechanical properties of Fe2B layers formed on AISI D2 steel. Indian Journal of Engineering & Materials Science 2015, 22, 231–243. [Google Scholar]
- Keddam, M.; Jurči, P. Simulating the Growth of Dual-Phase Boride Layer on AISI M2 Steel by Two Kinetic Approaches. Coatings. 2021, 11, 433. [Google Scholar] [CrossRef]
- Keddam, M.; Chegroune, R.; Kulka, M.; Taktak, S. Characterization and Diffusion Kinetics of the Plasma Paste Borided AISI 440C Steel. Transactions of the Indian Institute of Metals 2017, 70, 1377–1385. [Google Scholar] [CrossRef]
- Kartal Sireli, G.; Yuce, H.; Arslan, M. Improving the Surface Performance of Discarded AISI T1 Steel by Cathodic Reduction and Thermal Diffusion-Based Boriding. Journal of Materials Engineering and Performance 2023. [Google Scholar] [CrossRef]
- Campos-Silva, I.; Hernández-Ramirez, E.J.; Contreras-Hernández, A.; Rosales-Lopez, J.L.; Valdez-Zayas, E.; Mejía-Caballero, I.; Martínez-Trinidad, J. Pulsed-DC powder-pack boriding: Growth kinetics of boride layers on an AISI 316 L stainless steel and Inconel 718 superalloy. Surface and Coatings Technology 2021, 421. [Google Scholar] [CrossRef]
- Campos, I.; Ramírez, G.; Figueroa, U.; Martínez, J.; Morales, O. Evaluation of boron mobility on the phases FeB, Fe2B and diffusion zone in AISI 1045 and M2 steels. Applied Surface Science 2007, 253, 3469–3475. [Google Scholar] [CrossRef]
- Ramakrishnan, H.; Balasundaram, R.; Lenin, K.; Dhanapal, C.; Saravanan, S. Experimental investigation of borided kinetics on martensitic stainless steel. Materials Today: Proceedings 2022, 68, 1508–1514. [Google Scholar] [CrossRef]
- Ortiz-Domínguez, M.; Keddam, M.; Elias-Espinosa, M.; Damián-Mejía, O.; Flores-Rentería, M.A.; Arenas-Flores, A.; Hernández-Ávila, J. Investigation of boriding kinetics of AISI D2 steel. Surface Engineering 2014, 30, 490–497. [Google Scholar] [CrossRef]
- Topuz, P.; Çicek, O. Kinetic investigation of AISI 304 stainless steel boronized in indirect heated fluidized bed furnace. J. Min. Metall. 2016, 52, 63–68. [Google Scholar] [CrossRef]
- Nait Abdellah, Z.; Boumaali, B.; Keddam, M. Experimental evaluation and modelling the boronizing kinetics of AISI H13 hot work tool steel. Materials Testing 2021, 63, 1136–1141. [Google Scholar] [CrossRef]
- Kayali, Y.; Talas, Ş.; Yalcin, M.C. Diffusion Kinetics of Boronized ASP®2012 Tool Steel Produced by Powder Metallurgy. Protection of Metals and Physical Chemistry of Surfaces 2022, 58, 1036–1043. [Google Scholar] [CrossRef]
- Ortiz-Domínguez, M.; Keddam, M.; Elias-Espinosa, M.; Ramírez-Cardona, R.; Arenas-Flores, A.; Zuno-Silva, J.; Cervantes-Sodi, F.; Cardoso-Legorreta, E. Characterization and boriding kinetics of AISI T1 steel. Metallurgical Research and Technology 2019, 116. [Google Scholar] [CrossRef]
- Campos-Silva, I.; Ortiz-Domínguez, M.; Tapia-Quintero, C. Kinetics and Boron Diffusion in the FeB/Fe2B Layers Formed at the Surface of Borided High-Alloy Steel. J. of Materi Eng and Perform. 2012, 21, 1714–1723. [Google Scholar] [CrossRef]
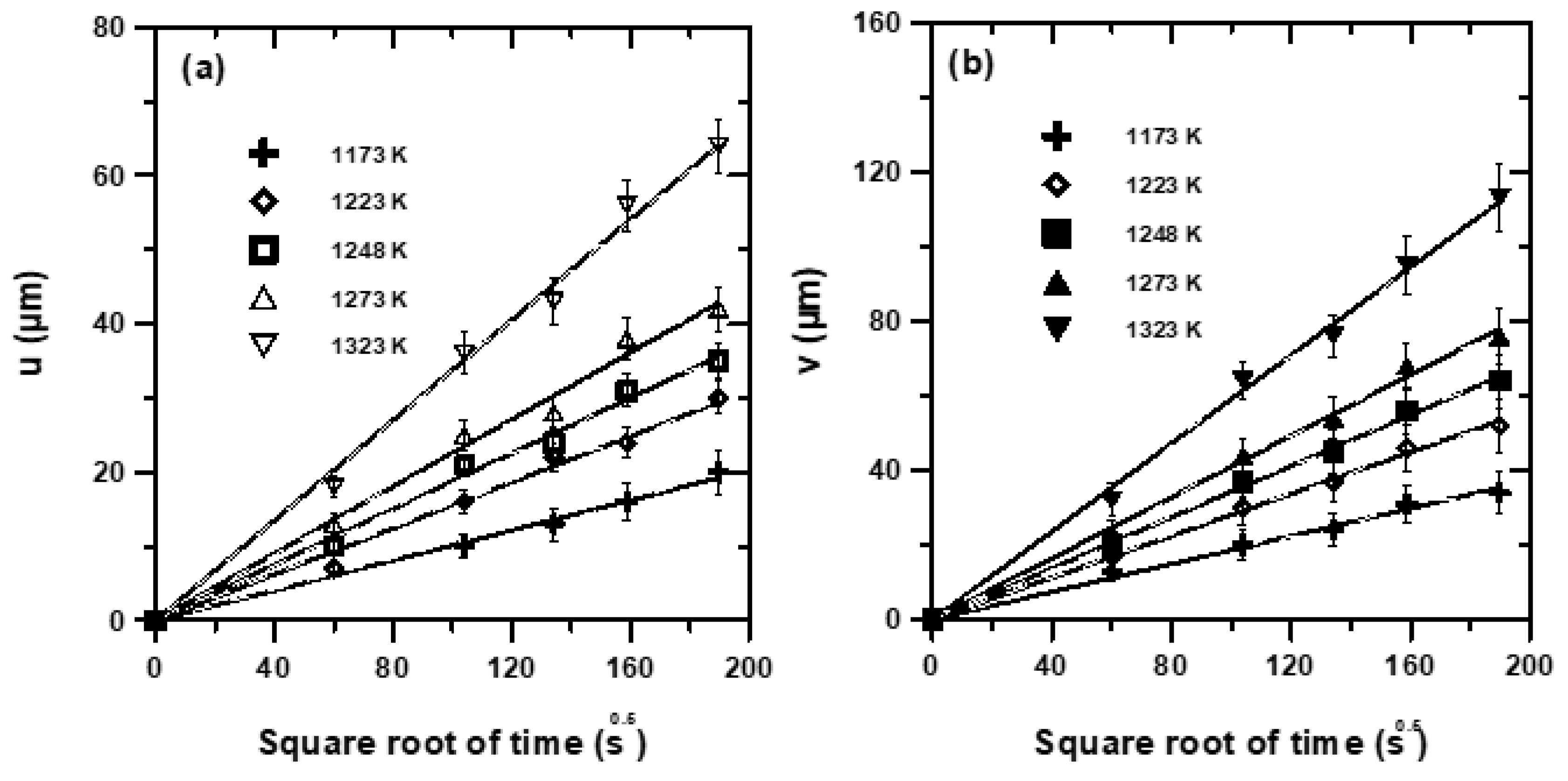
|
(µm s-0.5) at the first phase interface |
(µm s-0.5) at the second phase interface |
|
|---|---|---|
| 1173 | 0.1013 | 0.1864 |
| 1223 | 0.1559 | 0.2806 |
| 1248 | 0.1876 | 0.3431 |
| 1273 | 0.2258 | 0.4103 |
| 1323 | 0.3378 | 0.5906 |
| T (K) |
(×10-12 m2 s -1) Equation (13) |
(×10- 12 m2 s -1) Equation (14) |
parameter | parameter |
|---|---|---|---|---|
| 1173 | 0.50 | 0.32 | 0.0712 | 0.1628 |
| 1223 | 1.18 | 0.73 | 0.0716 | 0.1643 |
| 1248 | 1.73 | 1.10 | 0.0713 | 0.1632 |
| 1273 | 2.49 | 1.57 | 0.0714 | 0.1636 |
| 1323 | 5.48 | 3.14 | 0.0721 | 0.1666 |
| Steel | Boriding process | Operating parameters | Phases present | Activation energy (kJ mol-1) |
Calculation Method |
Refs. |
|---|---|---|---|---|---|---|
| AISI 440 C | PPB | 700-800°C For 3-7 h |
FeB,Fe2B,CrB, Cr2B |
134.62 | Parabolic growth law | [35] |
| AISI TI | CRTD-Bor | 850-1050°C For 0.25-1 h |
FeB and/or Fe2B | 179.05 | Parabolic growth law | [36] |
| AISI 316 L | PDCPB | 850-950°C For 0.5-2 h |
FeB,Fe2B,CrB, Cr2B |
162.7 ±7 (FeB) 171 ±5 (Fe2B) |
Bilayer model | [37] |
| AISI M2 | Paste | 950-1000°C for 2 and 6 h |
FeB,Fe2B | 257.5(FeB) 201 (Fe2B) |
Bilayer model | [38] |
| SS410 | Powder | 850-1000°C for 2-8 h |
No reported | 186.49 | Parabolic growth law | [39] |
| AISI D2 | Powder | 850-1000°C for 2-8 h |
Fe2B | 201.5 | Monolayer model | [40] |
| AISI 304 | Powder | 850-1050°C for 1-4 h |
FeB, Fe2B, Ni2B, Cr2Ni3B6 | 244 | Parabolic law | [41] |
| AISI H13 | Powder | 800-1000°C For 2-6 h |
FeB,Fe2B,CrB, Cr2B | 236.43(FeB) 233.04(Fe2B) |
MDC method | [42] |
| ASP®2012 | Powder | 850-950°C for 2-6 h |
FeB, Fe2B, CrB, Mo2B and W2B | 314.716 | Parabolic growth law | [43] |
| Royalloy | Powder | 900-1050°C for 1-10 h |
FeB, Fe2B | 242.79 (FeB) 223.0 (Fe2B) |
Integral method | [21] |
| X165CrV12 | Powder | 850-950°C for 3-9 h |
FeB,Fe2B,CrB, | 173.73 (FeB) 193.47 (Fe2B) |
Integral method | [22] |
| AISI M2 | Powder | 850-950°C for 2-6 h and 10 h |
FeB,Fe2B,CrB, Cr2B, B4V3 | 206.41 (FeB) 216.18 (Fe2B) |
Integral method | [34] |
| AISI M2 | Powder | 850-950°C for 2-6 h and 10 h |
FeB,Fe2B,CrB, Cr2B, B4V3 | 226.02 (FeB) 209.04 (Fe2B) |
Dybkov model | [34] |
| Bohler K190 | Powder | 900-1050°C for 1-10 h |
FeB,Fe2B | 204.54 (FeB) 196.67 (Fe2B) |
Integral method | This study |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





