1. Introduction
Brassica rapa is a leafy vegetable which widely cultivated in China. It is a member of the cruciferous family and its consumption rank first among all vegetables in China [
1,
2]. Brassica rapa prefers cold climate, and its most suitable cultivation temperature is 20~25 °C. Heat stress often leads to drought stress, resulting in reduced yield and quality. Drought, high temperature, salinity and other abiotic stresses have reduced crop yields worldwide. Among them, the rise of atmospheric temperature caused by human activities has significantly affected the function of agricultural ecosystem. It is estimated that if human activities continue to cause global warming in the current trend, the earth temperature will likely increase by 6.4 °C [
3]. At the end of the 21st century, the melting of glaciers raised the sea level to 59 cm. It is well known that climate change increases the possibility of different natural disasters, such as floods, droughts, storms, hurricanes and changes in precipitation patterns. Because agriculture is highly dependent on climate and sensitive to agro climatic conditions, changes in temperature, humidity and rainfall will adversely affect crop productivity [
4].
Global warming caused frequent occurrences of extreme heat around the world, aggravate its damage and hindering plant growth, development and metabolism. Heat stress not only leads to abnormal growth, yellowing and withered of leaves, but also Stem thinning and more sensitive to infectious disease, which greatly reduces its quality and yield [
5]. High temperature stress will destroy leaf growth and an increase in susceptibility to infectious disease, which negatively impacts its quality and yield.
Many studies have emphasized the heat resistant gene regulation and other cellular activities at the transcriptional and metabolic levels, but identification method in agricultural production still need to be further developed. The summer weather in southern China is characterized by high temperature and frequent rainfall. Under high temperature condition, Brassica rapa often shows slow growth, yellow and rotten leaves, increased fiber content, and serious death. Cultivating heat-resistant varieties of crops and improving cultivation techniques are two effective ways to reduce high-temperature damage.
With the rapid development of molecular biology, the heat resistance mechanism in many crops has been further elaborated. However, understanding the plants’ physiological response to heat resistance is still an important prerequisite for variety identification. Understanding the physiological and biochemical reactions of plants and establishing simple and effective identification methods are still important contents of heat-resistant breeding of horticultural crops. It is worth noting that the heading character of Chinese cabbage is a good indicator of heat resistance, but the evaluation system for heat resistance of non heading Chinese cabbage has not yet been established.
In the present study, the HS and HR plants were exposed to high temperature environment (37 ◦C) for seven days. Morphological indicators such as plant height, plant width, leaf area, dry and fresh weight were determined to represent the biomass of these materials under heat stress. Next, the root activity, root tip number and root area of the plants were measured to compare the effects caused by heat stress on the root growth among these varieties. The content of Chlorophyll, soluble sugar (SS), proline, soluble protein (SP), ascorbic acid, Malondialdehyde (MDA) was measured; In addition, the gas exchange parameters, including photosynthesis, transpiration rate (E), intercellular carbon dioxide concentration (Ci) and stomatal conductance (Gs), were measured to understand contribution of the photosynthetic attributes in heat stress tolerance in brassica rapa. In order to further understand the effects of photosynthetic electron transfer and antioxidant pathway on plant heat tolerance. We select several typical heat-sensitive and heat-resistant varieties, compare the differences in antioxidant enzyme activity, including AAO, CAT, SOD and POD . Besides, the electron transfer rate and efficiency of photosynthetic system were surveyed.
The purpose of this paper is to to utilize physiological and biochemical indicators to establish an effective method for identifying heat-resistant varieties, which provided some information to explore roles of other physiological attributes in heat stress tolerance in Brassica rapa.
2. Materials and methods
2.1. Plant material and heat treatment
Twenty Brassica rapa varieties with different heat-tolerance were selected for the experiment. These accessions were collected and bred by Shanghai Academy of Agricultural Sciences. Full and healthy seeds were selected for germination and sowing . Seeds of each line were grown in a control phytotron. The ambient conditions were set as follows: 16/8 h light/dark, 150 mol/m-2/s-1 light intensity, and a 50% relative humidity level. Heat treatment were performed when the plants grow at three-leaf-stage. The seedlings were exposed to heat stress conditions at 37 °C/27 °C (light/dark) for 7 days. During heat stress, the seedlings were irrigated daily to avoid drought stress.
2.2. Measurement of morphological and physiological index
Plant height and width were determined by ruler. The dry weight (DW) and fresh weights (FW) were measured using an electronic analytical balance. The plant root activity was measured by Methylene blue. The total chlorophyll content was measured using a previously described method [
6]. The root tip number and root area were counted by WinRHIZO Software. Under the heat stress treatment, leaf samples were collected at 1, 3, 5, 7 days after treatment, frozen in liquid nitrogen, and stored in -80 °Csuper cold refrigerator . The leaf samples were collected at for physiological studies. Referring to the manufacturer’s instructions, the superoxide dismutase (SOD) activity, catalase (CAT) activity, ascorbic acid oxidase activity (AAO) activity and peroxidase (POD) activity, the content of hydrogen, proline and hydroxyl radical scavenging rate were measured by using assay kits (Comin, Suzhou, China) [
7]. The soluble sugar was measured by anthrone. The soluble protein content was measured according to the Bradford’ procedure [
8]. The determining method for the relative conductance (REC) used in this study was described by Zheng et al [
9]. The determining method of Malondialdehyde (MDA) content was described by Zhou et al. [
10].
2.3. Determination of gas exchange parameters
The photosynthetic-related parameters were calculated using the GFS-3000 and DUAL-PAM-100 measuring systems (Heinz Walz, Effeltrich, Germany). The experiment was conducted according to the manufacturer's procedures [
11]. The gas exchange parameters including photosynthetic rate (Pn), transpiration rate (E), stomatal conductance (Gs), and intercellular carbon dioxide concentration (Ci) were measured.
2.4. measurement of Electron transport rate, quantum yield and PQ Pools
Uniformly sized leaves from each plant were used for the measurement of photosynthetic system (PSII and PSI) efficiency. Before starting measurements, the plants were placed in a dark environment for 30 min. The CO2 concentration was set at approximately 400±10mmol.mol
-1. Different intensities of actinic red light were set in this experiment. The light intensity gradient was 0, 2, 13, 40, 80, 114, 157, 213, 279, 358, 592, 956, 1207 and 1537 μmol m
−2 s
−1 . The RLCs of photosynthetic parameters were measured, including the photosynthetic electron transport rate [ETR(I), ETR(II)], the effective quantum yield photochemistry [Y(I),Y(II)]; the quantum yield of PSI non-photochemical energy dissipation due to the donor-side limitation [Y(ND)], the quantum yield of regulated energy dissipation in PSII [Y(NPQ)], the quantum yield of PSI nonphotochemical energy due to the acceptor-side limitation [Y(NA)] and nonregulated heat dissipation as Y(NO) were calculated [
12]. The fourth fully expanded functional leaves from different pots were used for measurements [
13]. The complementary area between the oxidation curve of P700 after ST and MT excitation were used to calculate the functional pools sizes of intersystem electrons [
14].
2.5. Statistical analysis
One-way analysis of variance (ANOVA) was performed by IBM SPSS25.0 software (Armonk, NY, USA) . Significant differences were measured by Duncan’s multiple range test (p≤0.05). All the data contain mean values with standard error. The statistically analyzed data are shown with superscripted letters after the numbers which shows significant difference. All graphs were plotted by Graghpad prism 8 (San Diego, CA, USA). Cluster analysis was performed by Omicshare tool.
3. Results
3.1. Agronomic traits and heat damage indicators of Brassica rapa under heat stress
In this study, twenty
Brassica rapa varieties were treated with heat stress for seven days using an artificial climate chamber, and the physiological and biochemical indicators were measured to illuminate the relationship between these indicators and plant heat tolerance, On top of this, the heat-resistant (HR) and heat-sensitive (HS) Brassica rapa varieties were screened. Under high temperature treatment, the treated plants exhibited varying degrees of heat damage symptoms. Under 3 day of high temperature treatment, HR plants showed less damage, while HS plants exhibited multiple thermal injuries such as leaf shrinkage, dehydration and chlorosis, spindling, and plant growth stagnation. After 7 day of heat stress treatment, the leaves of HR plants begin to dehydrate and whiten. while HS plant showed wilt and death symptoms.The overall growth condition of the HR plant is better than that of HS plants. Heat damage index reflects the degree of heat damage to plants, which is negatively correlated with heat tolerance of plants. The heat damage index of 20 varieties of non-heading Chinese cabbage after 7 days of high temperature stress at seedling stage was counted, which were presented in
Table 1. The higher the heat damage index, the higher the degree of damage to plants under heat stress, The heat damage index was negative correlated with plant heat tolerance.According to the heat damage index, plants can be divided into HS varieties and HR varieties. The HR varieties
liehuojingang, xinxiaqing, gaohuaqing, Jiaoyang. HS varieties
xinlvxiu,
aijiaohuang and
wuyueman were representative screened from these varieties. These typical HS and HR varieties can be used as breeding materials for heat resistance breeding.
3.2. Plant growth attributes of Non-heading Chinese cabbage under heat stress
In order to survive under heat stress, plants have evolved many regulatory mechanisms to acquire heat tolerance. In order to evaluate the difference in plant biomass accumulation among HS and HR varieties under heat stress, we measured the dry and fresh weight, Plant height, plant width, leaf area (
Table 2).
The dry and fresh weight of plants reflects the biomass accumulation and growth rate of plants. As shown in
Table 1, heat stress considerably reduce the plants growth index such as the fresh and dry biomass. HR plants exhibited higher dry weight accumulation compared to the HS plants. The fresh and dry weight are proposed as good indicators for plant heat tolerance. It was observed HR plants have higher leaf area, higher plant width and height. However, the negative relation between heat damage index and these indicators are not significant. The root growth index of heat-resistant and heat-sensitive varieties under heat stress is assessed. Plant roots have multiple important functions, like absorption, fixation, transportation, synthesis, storage and reproduction. The result suggested that HR plants have a better-developed root system compared to HS plants. Stronger root activity (r=0.684) and higher root shoot ratio (r=-0.631) of HR plants helps plants absorb inorganic salts and water, and synthesize amino acids.
3.3. The chlorophyll content, soluble sugar and soluble protein and the MDA of Non-heading Chinese cabbage under heat stress
Higher plants use chlorophyll to absorb light energy for photosynthesis and provide necessary materials and energy for life activities. Therefore, maintaining stable chlorophyll content is essential for the normal progress of plant photosynthesis [
15]. Heat stress remarkably reduced photosynthetic pigments. As shown in
Table 3, HR plants under heat stress showed a higher chlorophyll level relative to HS cultivars with different range of chlorophyll a (0.46-1.04 mg/g), chlorophyll b (0.17-0.38 mg/g). Especially, the difference between different varieties is more significant in term of chlorophyll a content. Previous study found that the HR varieties have a relatively higher chlorophyll content compared to HS varieties. These may be explained that the HS varieties have damaged chlorophyll synthesis pathway which lead to a decreased chlorophyll level. It can be seen from
Table 4 that the chlorophyll content of all varieties showed a downward trend under the high temperature treatment for 7 days. Numerous stresses often trigger the occurrence of photoinhibition in plants, further degrading chlorophyll and photosynthetic system antenna proteins. Chlorophyll a (r=-0.836) and Chlorophyll b (r=-0.797) are negatively correlated with the heat damage index, and the negative correlation between Chlorophyll a and the heat damage index is higher, indicating that Chlorophyll a content can better reflect the heat damage degree of plants.
The SS and SP content of different Brassica rapa materials under heat stress were determined. From the
Table 4, the SS content in the four varieties of
Aoxia,
Xinxiaqing,
jiaoyang and
liehuojingang were significantly higher, the SS content of HS varieties
xinlvxiu,
aijiaohuang and
wuyueman was lower than other varieties. The present studies have reported the accumulation of SS in cell can greatly enhance the stress tolerance of plants [
16]. Furthermore, higher SS content play important role in the maintenance of proline content and the balance of ROS catabolism.
The higher concentration of SS in HR plants is conducive to maintaining cell homeostasis and normal metabolism. In this study, The difference of SP content between different varieties is more obvious. SS and SP are deemed to function as osmotic regulatory substances in vivo, protecting the integrity of cell membranes, and helping plants maintain osmotic homeostasis under heat stress [
17,
18,
19,
20]. The SP level of HR varieties is twice than that of HS varieties. This may be explained that the protein degradation rate is lower and the synthesis rate is higher in HR plants compared to HS plants. Therefore, higher SS and SP level helps protect cell membrane system and further enhance the heat tolerance. They coordinately maintain osmoregulation and avoid damage to protein structure within the cell. Our data showed that the heat damage index was highly negatively correlated with the content of SS (r=-0.86) and SP (r=-0.695) . In addition, a significant negative correlation was proved between ascorbic acid and the heat damage index of plants, indicating that heat-resistant varieties have higher levels of ascorbic acid (r=-0.859) and stronger antioxidant abilities. The SS and ascorbic acid level has been proposed as important indicators to identify the degree of heat damage.
Table 3.
The content of MDA, chlorophyll a, chlorophyll b, ascorbic acid of Brassica rapa cultivars with elevated heat tolerance under heat stress conditions.
Table 3.
The content of MDA, chlorophyll a, chlorophyll b, ascorbic acid of Brassica rapa cultivars with elevated heat tolerance under heat stress conditions.
| Serial number |
MDA |
Chlb |
Chla |
Ascorbic acid |
| 1 |
5.50±0.07a |
0.18±0.00bcd |
0.49±0.02bcde |
0.22±0.01bcde |
| 2 |
5.49±0.04a |
0.17±0.08bcd |
0.46±0.02bcde |
0.20±0.00bcde |
| 3 |
5.73±0.14a |
0.19±0.01bcd |
0.42±0.01bcde |
0.19±0.00bcde |
| 4 |
4.49±0.06b |
0.24±0.01bc |
0.53±0.01bcde |
0.25±0.01bcde |
| 5 |
4.62±0.05b |
0.26±0.01b |
0.62±0.01bcde |
0.31±0.00bc |
| 6 |
2.85±0.33bcd |
0.37±0.01a |
0.82±0.01bc |
0.34±0.01b |
| 7 |
2.98±0.09bcd |
0.39±0.01a |
0.87±0.01b |
0.37±0.00a |
| 8 |
3.62±0.05bc |
0.36±0.00a |
0.83±0.01bc |
0.36±0.00bc |
| 9 |
3.51±0.00bc |
0.32±0.01a |
0.72±0.01bcd |
0.34±0.00bc |
| 10 |
5.01±0.00b |
0.28±0.00bc |
0.55±0.01bc |
0.21±0.02bcde |
| 11 |
3.36±2.89bc |
0.38±0.10a |
1.04±0.01a |
0.38±0.00a |
| 12 |
3.84±0.33bc |
0.29±0.00bc |
0.76±0.02bcd |
0.24±0.00bcde |
| 13 |
4.87±0.14b |
0.31±0.01b |
0.46±0.01bcde |
0.24±0.01bcde |
| 14 |
4.64±0.05b |
0.25±0.01bc |
0.60±0.02bcde |
0.30±0.00bc |
| 15 |
5.07±0.05b |
0.34±0.01b |
0.65±0.02bcde |
0.31±0.00bc |
| 16 |
4.05±0.06bc |
0.28±0.01bc |
0.47±0.01bcde |
0.24±0.00bcde |
| 17 |
3.50±0.01bc |
0.22±0.01bc |
0.46±0.02bcde |
0.20±0.00bcde |
| 18 |
4.30±0.07bc |
0.28±0.01bc |
0.59±0.00bcde |
0.29±0.00bcde |
| 19 |
4.32±0.10bc |
0.27±0.01bc |
0.63±0.01bcde |
0.31±0.00bc |
| 20 |
5.14±0.11a |
0.25±0.01bc |
0.55±0.02bcde |
0.27±0.01bcde |
Heat stress initiaHeat sHeat stress initially leads to the injury of the plasma membrane and rearrangement of the cytoskeleton. A large amount of free radicals in plants will cause membrane lipid peroxidation and produce malondialdehyde when exposed to high temperature. The membrane lipid peroxidation. Heat stress often lead to the membrane lipid peroxidation and a increased MDA level [
21].
As shown in the
Table 3, the MDA content was significantly accumulated in HS plants (
aijiaohuang,
xinlvxiu and
wuyueman). On the contrary, HR plants (
jiaoyang,
liehuojingang,
aoxia and
xinxiaqing) showed a low MDA level. It indicated that the the stability of plasma membrane is damaged in HS plants which are more sensitive to heat stress. In this study, HS plants have a higher REC level. The correlation between MDA (r=0.628) and heat damage index is not as good as other indicators.
3.4. Gas-exchange parameters of different B.rapa cultivars under heat stress
Heat stress destroys the photosynthetic system of plants, with a reduced photosynthetic rate and stomatal opening and a increase of transpiration rate. In this work, the high temperature treatment was implement on twenty B.rapa cultivars for 24 h. The gas exchange index were measured, including photosynthetic rate (Pn), transpiration rate (Tr), intercellular CO
2 concentration (Gs). The photosynthetic rate of HR plants was significantly higher compared to HS plants. Among them, the liehuojingang has the highest Pn (18.76 μmol m
−2 s
−1 ), followed by jiaoyang (16.9279 μmol m
−2 s
−1 ) and Xinxiaqing (16.1187μmol m
−2 s
−1 ). The Pn of HS plants (
xinluxiu, wuyueman,
aijiaohuang and
shanghaiqing) was approximately 10.5 μmol m
−2 s
−1 . Many study revealed that heat stress enhance the transpiration rate of plants, leading to death due to excessive dehydration [
22]. The E of these B.rapa cultivars under heat stress were measured. According to
Table 2, the E of the
liehuojingang,
jiaoyang,
xinxiaqing and
gaohuaqing were relatively higher than other varieties. The E of the
liehuojingang (4.35gm2-h) is almost twice that of the
xinlvxiu (2.24 m2-h). The gs of HR plants was significantly higher than HS varieties. Stomata are the main channels for gas exchange of plant leaves. while HR plants have a significantly higher level of Ci than that of HS plants. These results suggest that HR plants under heat stress have a stronger capacity of photosynthetic gas exchange than HS plants. In summary, the Pn (r=-0.843) are more suitable for plant heat tolerance identification than E (r=-0.679), Ci (r=-0.682) and Gs (r=-0.673).
Table 4.
Photosynthetic rate (A), transpiration rate (E), Intercellular CO2 concentration (Ci), stomatal conductance (Gs) and pearson coefficient (r) with heat damage index of Brassica rapa seedlings under heat stress, the data represent mean values ±SE from four independent experiments.
Table 4.
Photosynthetic rate (A), transpiration rate (E), Intercellular CO2 concentration (Ci), stomatal conductance (Gs) and pearson coefficient (r) with heat damage index of Brassica rapa seedlings under heat stress, the data represent mean values ±SE from four independent experiments.
| Serial number |
Pn |
Tr |
Gs |
Ci |
| 1 |
11.57±4.02 |
2.72±0.06b |
254.85±1.51bcde |
374.67±1.75bc |
| 2 |
9.94±0.53bc |
2.57±0.07b |
241.00±3.26bcde |
308.14±1.45bcde |
| 3 |
10.44±0.40bc |
2.79±0.077b |
281.04±6.86bcde |
334.81±2.08bcde |
| 4 |
11.33±0.27bc |
3.76±0.06b |
245.27±4.01bcde |
369.811±0.87bcd |
| 5 |
13.42±0.42bc |
2.91±0.01bc |
249.82±3.71bcde |
357.97±1.58bcde |
| 6 |
16.93±0.78b |
3.68±0.08bc |
395.21±3.99a |
365.38±0.73bcde |
| 7 |
15.36±0.43b |
4.15±0.04bcd |
344.28±3.01bcde |
439.41±0.43bc |
| 8 |
16.12±0.62b |
3.96±0.11bc |
372.86±2.82b |
361.53±1.01bcde |
| 9 |
14.61±0.17b |
3.74±5.60a |
270.18±3.52bcde |
369.81±0.87bcd |
| 10 |
12.15±0.53bc |
2.62±0.10bc |
237.26±5.94bcde |
336.09±2.22bcde |
| 11 |
18.76±030ac |
4.35±0.03bc |
365.24±4.79bc |
449.77±5.15a |
| 12 |
15.60±0.26b |
3.32±0.13bc |
281.91±5.27bcde |
376.78±1.50b |
| 13 |
12.750±0.25bc |
2.91±0.03bc |
284.66±3.86bcde |
384.44±1.60b |
| 14 |
13.87±0.29bc |
3.37±0.05b |
328.47±4.84bcde |
368.29±3.561bcd |
| 15 |
12.504±0.40bc |
2.46±0.02bc |
331.84±5.98bcd |
375.511±1.65bc |
| 16 |
10.47±0.39bc |
3.05±0.03b |
246.4±3.52bcde |
338.16±1.98bcde |
| 17 |
10.57±0.31b |
3.25±0.01b |
254.99±3.94bcde |
306.54±2.08bcde |
| 18 |
14.28±0.24bc |
3.03±0.03b |
266.68±4.16bcde |
337.47±4.38bcde |
| 19 |
12.75±0.25bc |
2.76±0.02bc |
244.54±3.59bcde |
346.96±2.65bcde |
| 20 |
13.51±0.28bc |
3.29±0.17b |
323.42±3.67bcde |
365.45±2.99bcde |
Heat stress considerately harm the yield of photochemistry of PS(II) in the aspect of quantum yield. The Fv/Fm value of plants can be used as an important indicator to identify the degree of multiple abiotic stresses. After heat stress treatment, the Fv/Fm values of these 20 B.rapa varieties were measured. Higher Fv/Fm values in HR plants represented a higher PSII efficacy relative to HS plants. The Fv/Fm of jiaoyang (0.33) is twice as much as aijiaohuang (0.78). Meanwhile, Fv/Fm is highly correlated with heat damage index(r=-0.735).
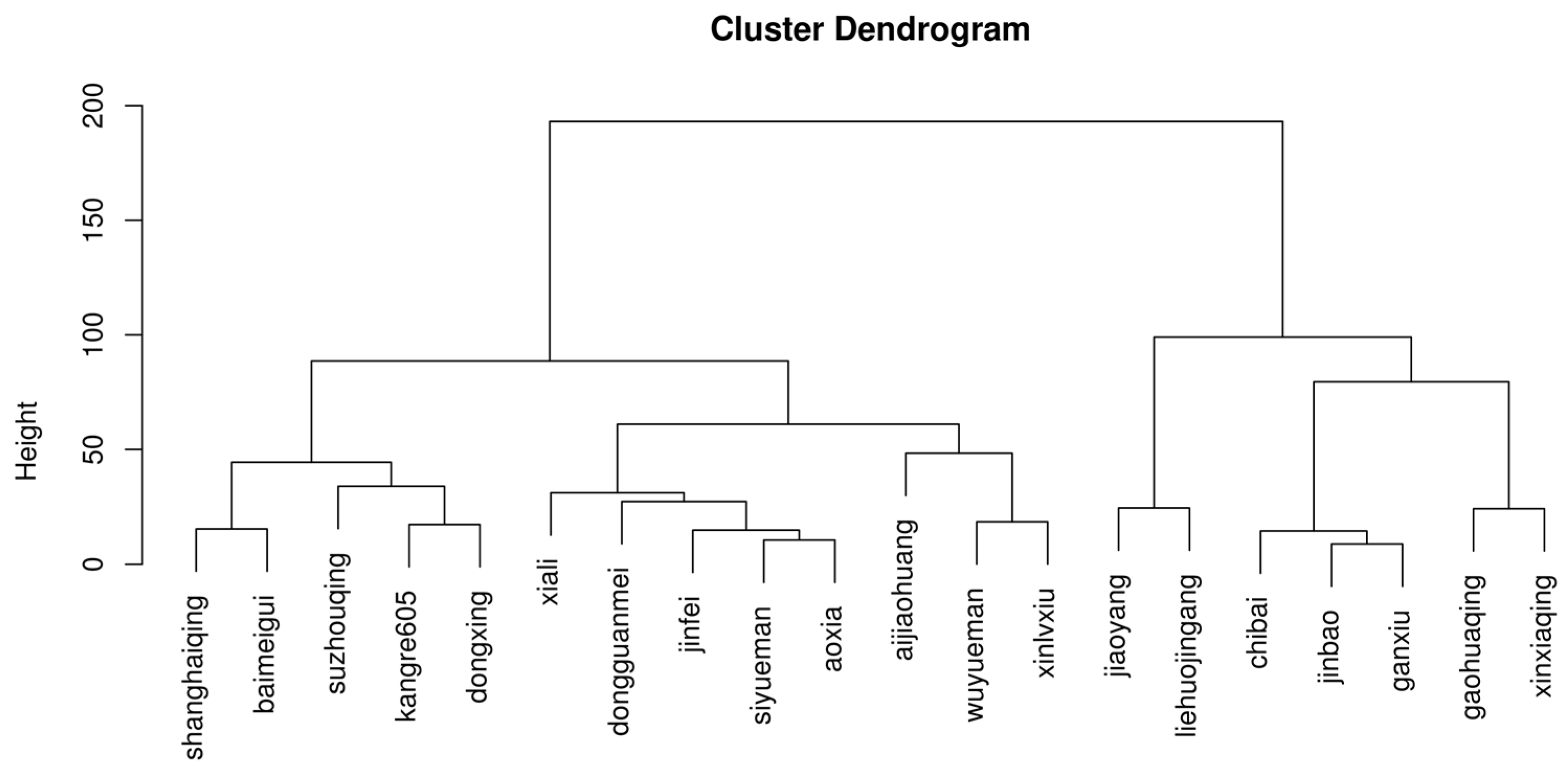
Based on these physiological indicators, cluster analysis was conducted on all non heading Chinese cabbage (
Figure 1). It was found that the heat resistance of
Xinxiaqing and
Gaohuaqing, as well as
Jiaoyang and
liehuojingang, is relatively consistent, while the sensitivity to heat is relatively consistent for May slow, dwarf yellow, and Xinlvxiu. These materials are divided into 7 heat-resistant varieties and 13 heat-sensitive varieties in total. The correlation between the heat damage index and growth physiological indicators of non heading Chinese cabbage under heat stress was also analyzed (
Table 5). intriguingly, correlation analysis shows that SS, ascorbic acid, photosynthetic rate, dry and fresh weight, and Fv/fm are useful heat tolerance indicators (
Table 5).
4. Discussion
The rapid increase in population and atmospheric pollution have led to global warming and become a major limiting factor to crop production due to global temperature rise [
23,
24,
25].
The high-throughput sequencing technology has rapidly developed in recent decades. RNA sequencing and transgenic technology have been powerful tools for explore potential molecular regulation mechanism in abiotic stress. However, using biotechnology to develop new species and applying it to crop production is still a huge challenge. Therefore, it is essential to elucidate the mechanism of heat tolerance in Brassica rapa rom the viewpoint of physiology and biochemistry in order to develop new varieties.
It is notable to understand the heat-resistant mechanism of plants at the physiological and biochemical level to study combined with molecular biology. On the other hand, it is essential to establish a simple and effective variety identification methods for variety identification.
In this experiment, we selected twenty B.rapa varieties with different heat tolerance for heat tolerance identification. Combined analyses of morphological and physiological indicators were performed to establish a simple and effective identification method. Their heat damage index was measured, and the relationship between chl content, MDA content, SS and SP content, growth attribute (including plant height, plant width, leaf area, dry and fresh weight, root-shoot ratio, root activity, etc.), gas exchange parameters and heat damage index was explored.
We found that heat damage has a greater negative impact on the growth and development of HS varieties. Secondly, the chl content of HS was lower compared to HR plants, indicating that the chl degradation activity was more frequent in HS plants. Chlorophyll content directly influence the photosynthesis of plants, The biomass of HR plants is greater than that of HS, including dry and fresh weight, plant height, plant width, leaf area, Root-shoot ratio. The result suggested the fixing carbon capacity is stronger in HR plants. On top of this, there are great differences in root development between heat-resistant varieties and heat-sensitive varieties under heat stress. Root is the vegetative organ of plant for plants to absorb water and nutrients. Damaged roots will make plants produce water deficiency, innutrition, and seriously lead to death. In general, the leaf and root development of plants affect the source-flow-sink relationship and then determine the yield.
In order to further understand the differences between heat-sensitive varieties and heat-resistant varieties in the process of photosynthetic electron transfer and oxidation resistance, the antioxidant system and photosynthetic system of HR and HS varieties were analysed.
4.1. Biochemical analysis of enzymatic activities and Hydroxyl scavenging capacity
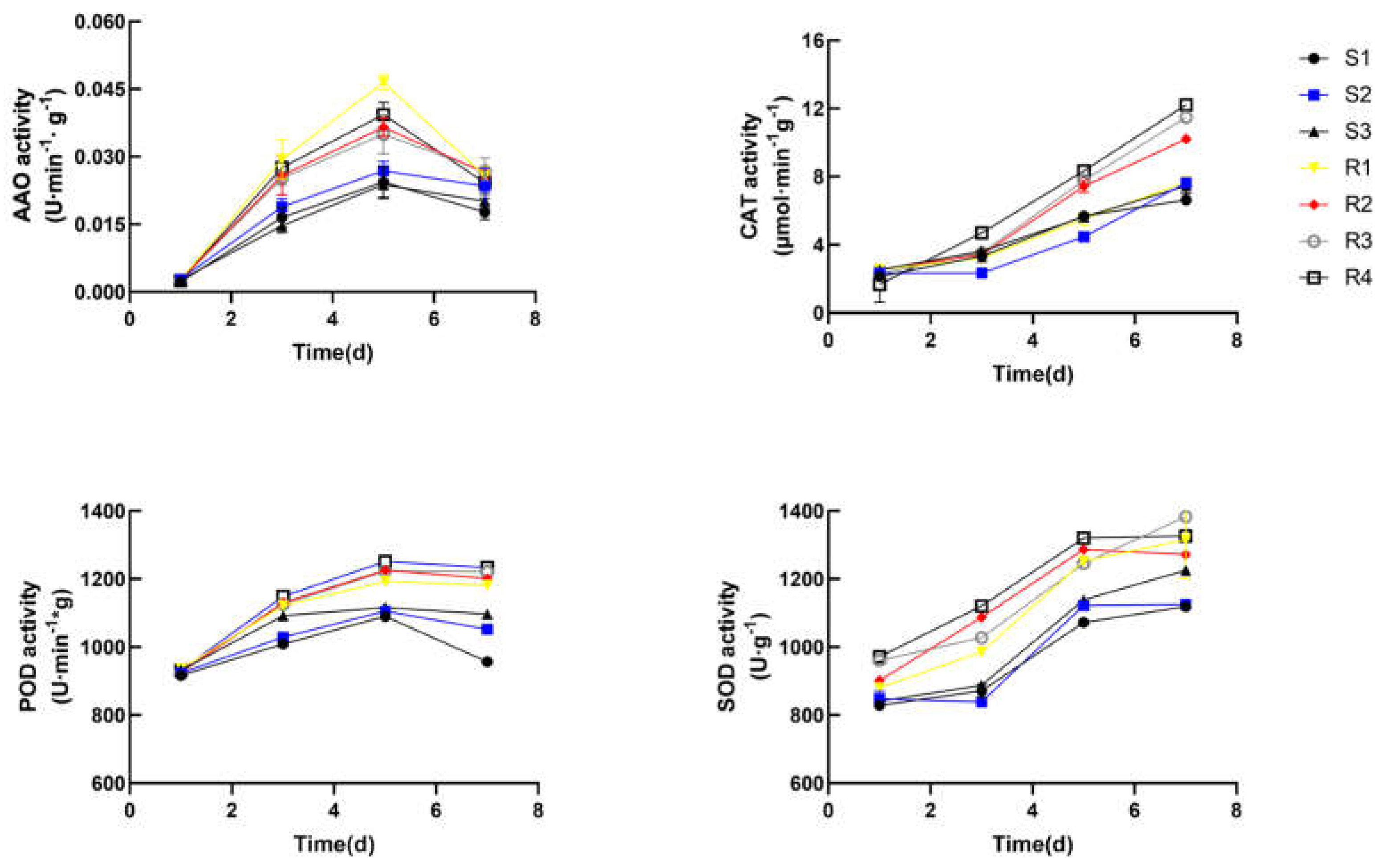
Upon exposure to high temperature environment, plants first perceive heat stress signals and trigger a series of transcriptional responses. It is widely accepted that heat stress disrupts ROS metabolic balance, resulting in its excessive accumulation and instability of the membrane system. Plants initiated antioxidant machinery which help to counteract the toxic effect of ROS [
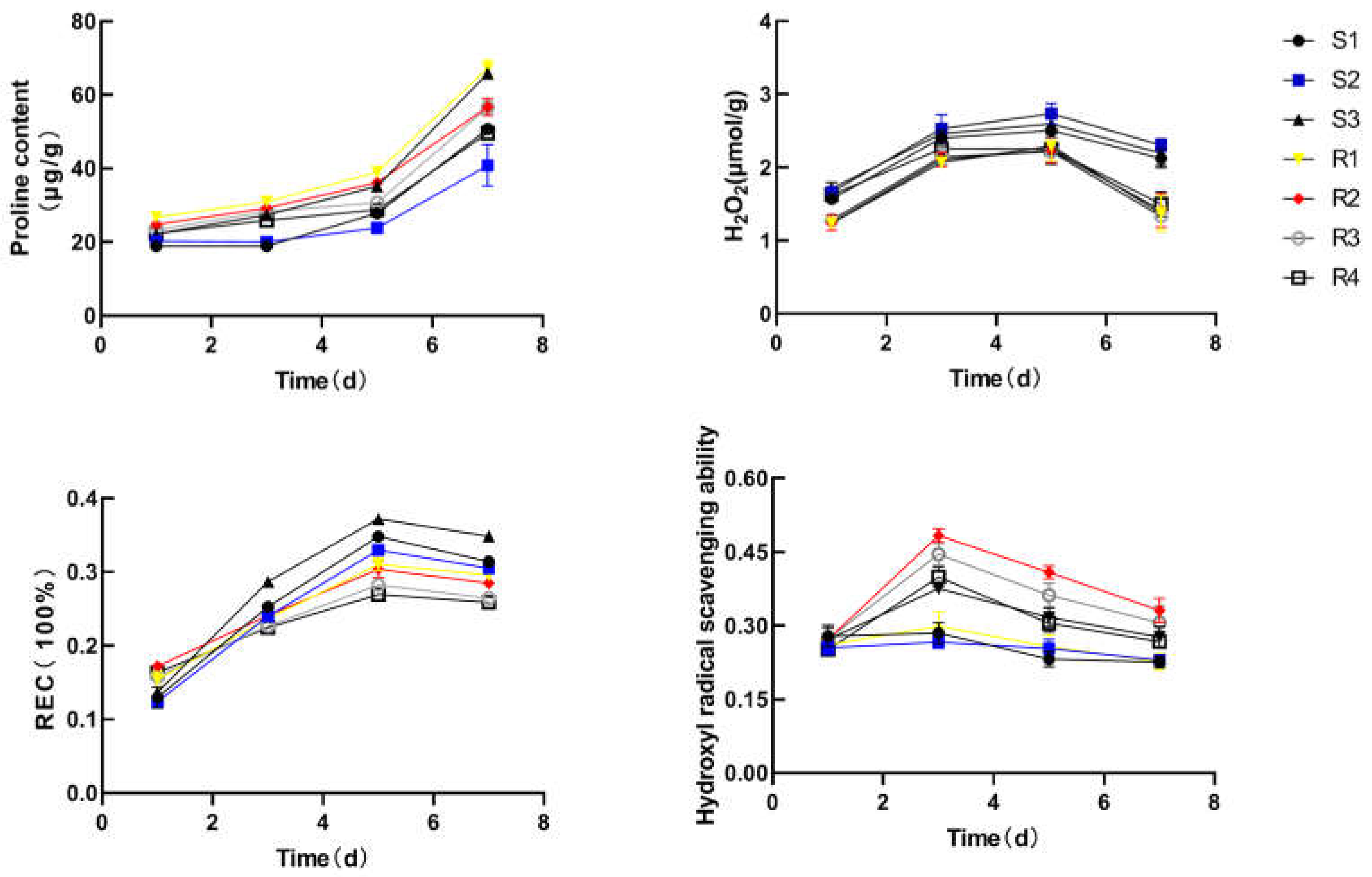
26]. Plants produce a range of antioxidants to scavenge ROS and protect cells from oxidative stress. The production of antioxidant enzyme, like superoxide catalase (CAT) ,dismutase (SOD) or peroxidase (POD), is essential for eliminating superoxide radicals. In this study, the differences of antioxidant system and photosynthetic system between heat-resistant varieties and heat-sensitive varieties under heat stress were mainly studied. The activity of peroxidase (POD), catalase (CAT), Ascorbic acid oxidase (AAO) and superoxide dismutase (SOD), the proline (Pro) content, hydrogen peroxide (H
2O
2) content, the scavenging rate of hydroxyl free radicals in HR and HS plants under heat stress were measured. As shown in
Figure 2, the trend of CAT and SOD enzyme activities is to increase gradually, while POD and AAO enzyme activities increase first and then decrease. This result shows that POD and AAO enzyme activities can only maintain for a short time in plant heat stress response. CAT and SOD enzymes may play a leading role in the enzyme activity system under continuous high temperature environment. The results showed that the protective enzyme activity of HR varieties was higher, which prevented the damage to cells by scavenging ROS. Previous findings shown that the expression of ROS scavenging genes is strongly upregulated in heat-tolerant plants, which was accordance with our data [
27].
In addition, we found that the hydroxyl radical scavenging rate of HR varieties was higher than that of HS varieties, and the H
2O
2 content of HS varieties was significantly higher than that of heat-resistant varieties, which was in line with the previous enzyme activity assay results (
Figure 3). To our knowledge, higher H
2O
2 content destroy cellular structure, produce active oxygen radicals which damage cell fate [
28].
Proline is an important amino acid which help protect the cell membrane structure and take part in antioxidant pathways [
29,
30]. Under heat stress, the accumulation of Pro gradually increased, and rose very fast after 3 d treatment. Under heat stress, Pro participate in the adjustment of osmotic pressure balance in vivo. In order to understand the differences in cell membrane permeability, the relative conductivity of the leaves of heat-resistant and heat-sensitive varieties under heat stress was also measured. The results showed that the relative conductivity of the leaves of HR and HS varieties increased continuously in the first five days, and then gradually decreased. Moreover, the relative conductivity of the leaves of heat sensitive varieties is higher than that of heat resistant varieties, indicating that HS varieties has a higher level of increase membrane permeability under heat stress. This also shows that CAT, CAT activity, hydroxyl radical scavenging rate, pro and H
2O
2 content were suitable to identify heat-tolerance in Brassica rapa varieties. ROS accumulation disrupt photosynthesis, respiration and inactivate the activity of biological macromolecules [
31]. The result suggested that HR plants possesses superior antioxidant system, indicating a stronger tolerance than HS plants.
4.2. The PSII and PSI Activity of Brassica rapa Leaves under heat stress
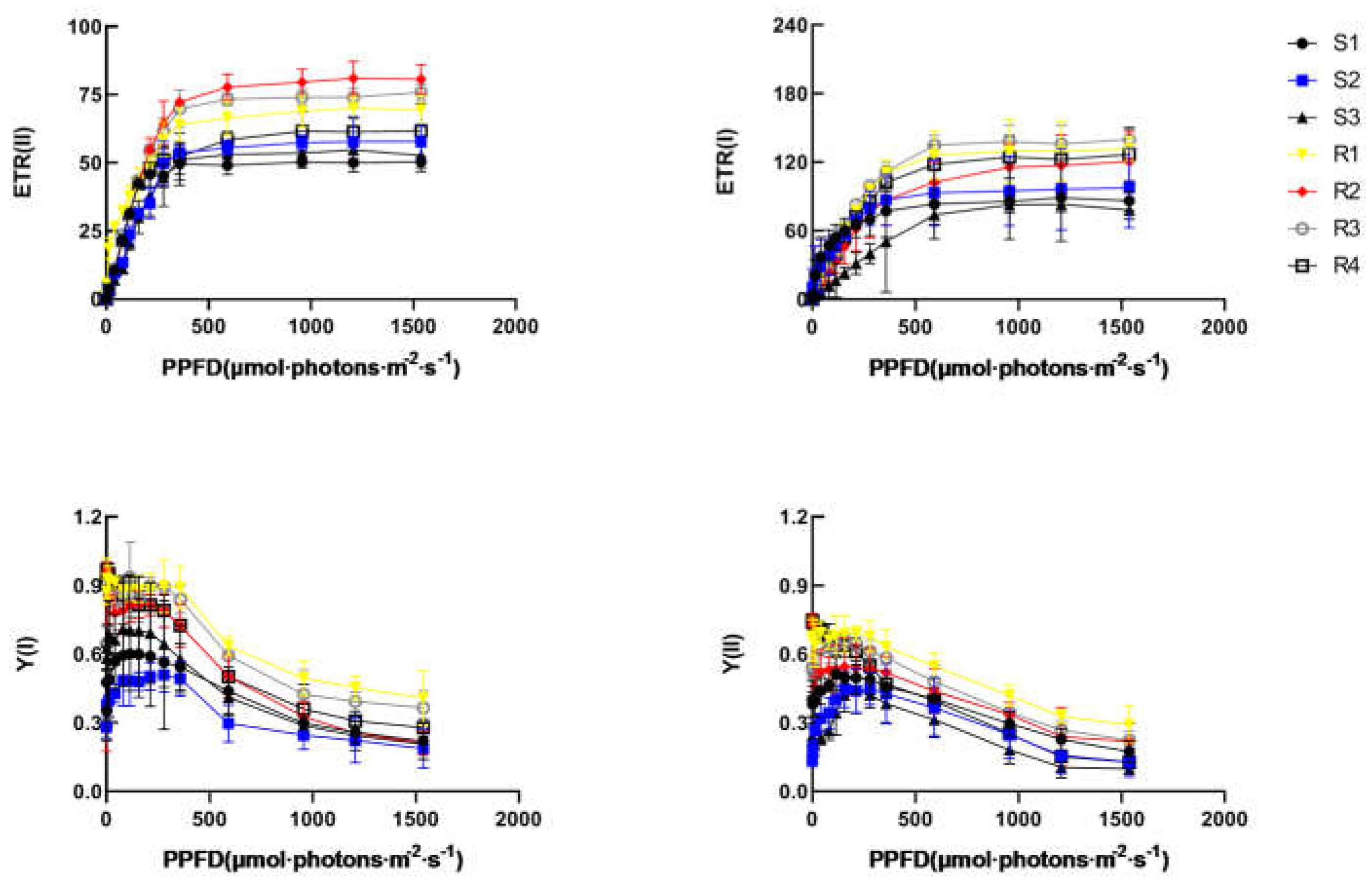
Chlorophyll fluorescence parameters has been widely used in photosynthetic research. C The primary reaction processes of photosynthesis and the processes of electron transfer can be assessed by Chlorophyll fluorescence parameters. Almost all changes in photosynthesis can be reflected by chlorophyll fluorescence. The quantum yield [Y(I),Y(II)] and electron transport rate [ETR(I), ETR(II)] of PSI and PSII in these Brassica rapa materials were measured, respectively. The processof the photosynthetic electron transport can be affected by multiple abiotic stresses, including heat, drought, cold, salinity and heavy metal. These stresses can reduce the efficiencyof photochemical reaction, further producing excess absorbed light energy and aggravating photo inhibition [
32,
33,
34,
35]. The rapid light curves (RLCs) of photosynthetic quantum yields was measured. Heat stress has destructive impact on the photosystems, especially PSII, and further causing photo inhibition. As shown in
Figure 4, ETR (II) and ETR (I) were significantly lower in HS (S1, S2, S3) plants compared to HR (R1, R2, R3, R4) plants. Our results showed that the activity of the photosynthetic electron transport was various between HS and HR varieties. HR plants can keep a higher photosynthetic efficiency and biomass accumulation compared with HS plants. The activity of PSII and PSI from B. rapa leaves in HR plants was higher than HS plants. The effective quantum yield of PSII photochemistry [Y(II)] increase initially and then decrease gradually with increasing light intensity. while Y(II) was significantly lower in HS group compared to HR group at almost all light intensities.
Figure 4.
Energy distribution in photosystems electron transport rate of Brassica rapa under heat stress.Y(II), efficient quantum yield of PSII;Y(I), quantum yield of PSI;. ETR(II), electron transport rate of PSII; ETR(I), electron transport rate of PSI.
Figure 4.
Energy distribution in photosystems electron transport rate of Brassica rapa under heat stress.Y(II), efficient quantum yield of PSII;Y(I), quantum yield of PSI;. ETR(II), electron transport rate of PSII; ETR(I), electron transport rate of PSI.
It can be seen from
Figure 4, the donor-side limitation [Y(ND)] was gradually increased with increasing light intensity. The trend of the acceptor-side limitation [Y(NA)] is opposite to Y(ND). The result indicated the HR plants have a higher level in terms of quantum yield of PSI non-photochemical energy dissipation. These results suggested that HR plants have stronger abilities to induce non-photochemical quenching of PSII and activate the self-proection of PSI. The energy utilization mechanism in plants was disturbed under heat stress, resulting in a photoinhibitory effect.
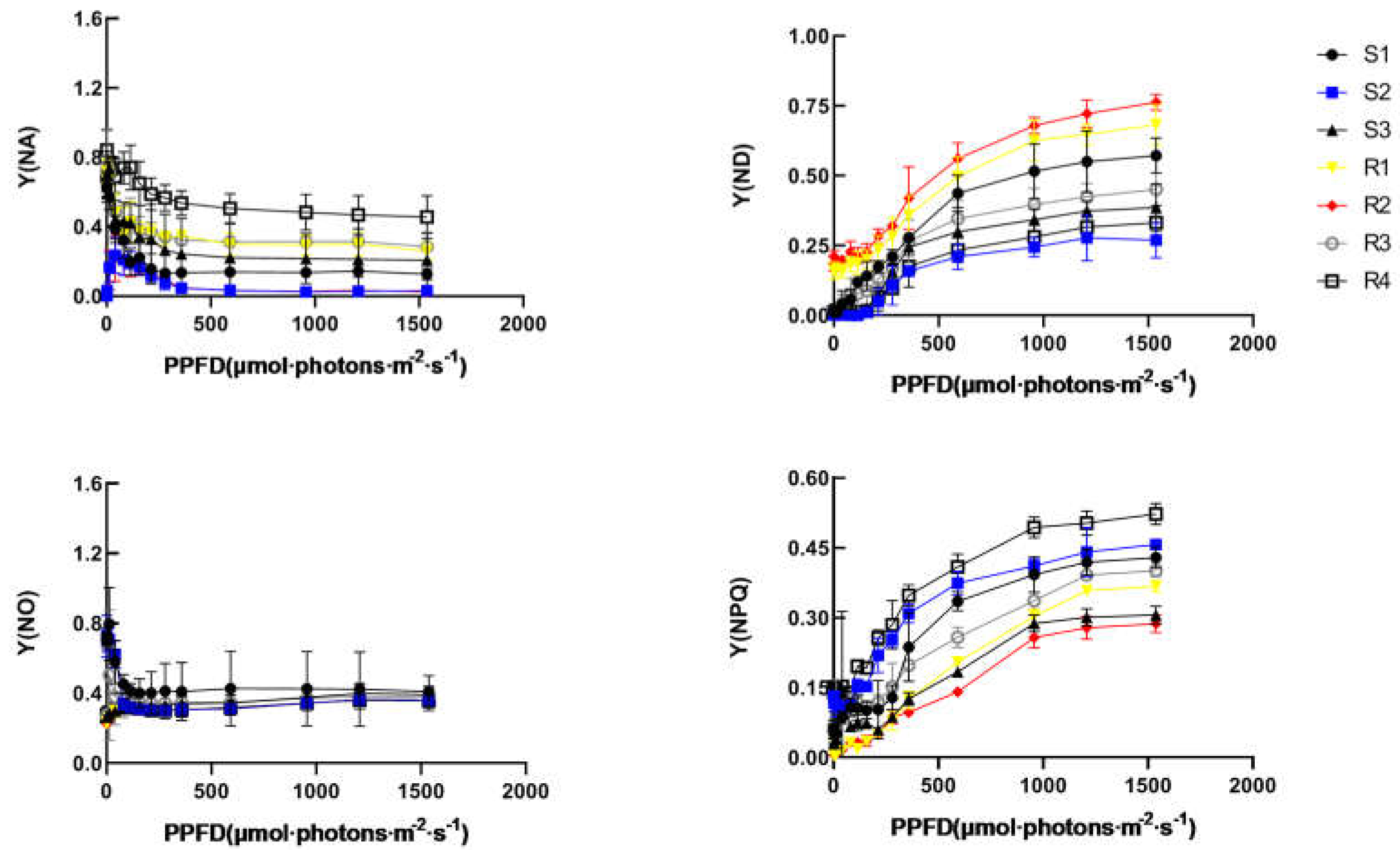
Figure 5.
Non-photochemical quenching (NPQ), Non-photoprotective heat dissipation Y(NO), the acceptor-side limitation [Y(NA)], donor side limitation of PSI Y(ND) from light curve analysis by dual-PAM of two Brassica rapa cultivars under heat stress. Data are the means of four replicates with standard errors shown by vertical bars. *indicates significant difference (P≦0.05), and **indicates a highly significant difference (P≦0.01).
Figure 5.
Non-photochemical quenching (NPQ), Non-photoprotective heat dissipation Y(NO), the acceptor-side limitation [Y(NA)], donor side limitation of PSI Y(ND) from light curve analysis by dual-PAM of two Brassica rapa cultivars under heat stress. Data are the means of four replicates with standard errors shown by vertical bars. *indicates significant difference (P≦0.05), and **indicates a highly significant difference (P≦0.01).
The quantum yield of non-regulated energy dissipation in PSII [Y(NO)] was decreased and then keep stable with increasing light intensity. R1 and R2 was significantly higher than other groups before light intensity closed to 1000 μmol m−2 s −1. With increasing light intensity, the quantum yield of regulated energy dissipation in PSII [Y(NPQ)] increased rapidly. The Y(NPQ) of jiaoyang and xinxiaqing acquired a maximum value. It suggested that HR plants have a stronger capacity to induce photoprotection of PSII. The quantum yield of PSI photo chemistry [Y(I)] decreased gradually with increasing light intensity. In addition, the functional PQ pools were significantly reduced in HS plants compared to the HR plants.
Numerous studies have focused on the mechanism of heat stress in Brassica rapa.However, few studies established a simple and effective method to identify heat resistant varieties. A large number of studies have emphasized the molecular mechanism of heat tolerance, including heat shock protein pathway, ROS pathway, hormone transduction pathway, etc. These results are of great significance and provide valuable information about the mechanism of heat tolerance in plants. Based on these funding, we investigated the physiological and biochemical response of Brassica rapa with with different heat tolerance to high-temperature stress. This paper focuses on the changes of plant osmoregulation substance catabolism, antioxidant pathway and photosynthetic pathway in adapting to heat stress. This paper provides a more accurate identification method for heat tolerance, and explains the importance of light protection and antioxidant mechanism for plant heat tolerance, so as to provide the key genes for future exploration of related pathways.
Heat stress has multiple devastating effects on the life activities of cells. The specific physiological reactions that play a decisive role in the mechanism of heat tolerance have not been clarified. In this study, the HS and HR varieties under heat stress were compared involving in multiple physiological and biochemical response. The results showed that protective enzymes and photosynthetic electron transport played important roles in the heat tolerance of Brassica rapa. Photosynthetic apparatus behaved differently in the different Brassica rapa materials under heat stress, which may play key roles in causing the differences in the heat resistance of B.rapa materials.
Our study provides valuable information on the physiobiochemical of B. rapa in response to heat stress. we propose to pay more attention to the gene regulation of antioxidant enzyme synthesis and photosynthetic electron transport pathway at the molecular level in the future research on the mechanism of heat tolerance regulation of Brassica rapa, which opens a new door to further reveal the mechanism of high temperature adaptation of horticultural crops.
Noteworthy, these findings provide us with a new light of the physiological mechanisms of heat stress on Brassica rapa and could promote the genetic modification of heat tolerance in horticultural crops.
5. Conclusion
In summary, we conclude that chlorophyll content, photosynthetic rate, the content of soluble sugar and soluble protein, the dry and fresh weight, the Fv/Fm can be used for rapid screening of heat-resistant varieties in brassica rapa. Secondly, the heat resistant variety possess a stronger antioxidant system, which produce the antioxidants to scavenge ROS in B.rapa. On the other hand, heat-resistant varieties in B.rapa acquired a stronger heat tolerance by protect electron transport system in photosynthesis and enhance antioxidant enzyme activity in ROS signaling. Our result provide theoretical basis for the future development of heat-tolerant horticulture crop germplasm resources.
Author Contributions
J. conducted the research and wrote the manuscript; Q.L N. conceived the project; J.Y. W and P. L.L performed data analysis. H. J.Y assisted in the experiments; C. L.Y performed microscopic studies; All authors read and approved the final version for publication.
Acknowledgments
This project was supported by Shanghai Agriculture Applied Technology development program, China (Grant number G.20190202).
Conflicts of Interest
The authors declare that they have no competing financial interests in this paper.
References
- Dai, Y.; Sun, X.; Wang, C.; Li, F.; Zhang, S.; Zhang, H.; Li, G.; Yuan, L.; Chen, G.; Sun, R.; et al. Gene co-expression network analysis reveals key pathways and hub genes in Chinese cabbage (Brassica rapa L.) during vernalization. BMC Genom. 2021, 22, 1–16. [Google Scholar] [CrossRef] [PubMed]
- Qi, J.; Yu, S.; Zhang, F.; Shen, X.; Zhao, X.; Yu, Y.; Zhang, D. Reference Gene Selection for Real-Time Quantitative Polymerase Chain Reaction of mRNA Transcript Levels in Chinese Cabbage (Brassica rapa L. ssp. pekinensis). Plant Mol. Biol. Rep. 2010, 28, 597–604. [Google Scholar] [CrossRef]
- Solomon, S. , Qin, D., Manning, M., Chen, Z., Marquis, M., Averyt, K. B.,... & Miller, H. L. Summary for policymakers. Climate change, 2007, 1-18.
- Qu, A.-L.; Ding, Y.-F.; Jiang, Q.; Zhu, C. Molecular mechanisms of the plant heat stress response. Biochem. Biophys. Res. Commun. 2013, 432, 203–207. [Google Scholar] [CrossRef] [PubMed]
- Yue, L.; Li, G.; Dai, Y.; Sun, X.; Li, F.; Zhang, S.; Zhang, H.; Sun, R.; Zhang, S. Gene co-expression network analysis of the heat-responsive core transcriptome identifies hub genes in Brassica rapa. Planta 2021, 253, 1–23. [Google Scholar] [CrossRef] [PubMed]
- Ni, Z.; Kim, E.-D.; Ha, M.; Lackey, E.; Liu, J.; Zhang, Y.; Sun, Q.; Chen, Z.J. Altered circadian rhythms regulate growth vigour in hybrids and allopolyploids. Nature 2008, 457, 327–331. [Google Scholar] [CrossRef] [PubMed]
- Weng, J.; Rehman, A.; Li, P.; Chang, L.; Zhang, Y.; Niu, Q. Physiological and Transcriptomic Analysis Reveals the Responses and Difference to High Temperature and Humidity Stress in Two Melon Genotypes. Int. J. Mol. Sci. 2022, 23, 734. [Google Scholar] [CrossRef] [PubMed]
- Bradford, M.M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 1976, 72, 248–254. [Google Scholar] [CrossRef] [PubMed]
- Zheng, Y.; Jia, A.; Ning, T.; Xu, J.; Li, Z.; Jiang, G. Potassium nitrate application alleviates sodium chloride stress in winter wheat cultivars differing in salt tolerance. J. Plant Physiol. 2008, 165, 1455–1465. [Google Scholar] [CrossRef]
- Zhou, W.; Leul, M. Uniconazole-induced tolerance of rape plants to heat stress in relation to changes in hormonal levels, enzyme activities and lipid peroxidation. Plant Growth Regul. 1999, 27, 99–104. [Google Scholar] [CrossRef]
- Zhang, G.; Liu, Y.; Ni, Y.; Meng, Z.; Lu, T.; Li, T. Exogenous Calcium Alleviates Low Night Temperature Stress on the Photosynthetic Apparatus of Tomato Leaves. PLOS ONE 2014, 9, e97322. [Google Scholar] [CrossRef]
- Hanelt, D. Photosynthesis assessed by chlorophyll fluorescence. In Bioassays, 2018, pp. 169-198.
- Asada, K.; Heber, U.; Schreiber, U. Pool Size of Electrons That Can Be Donated to P700+ As Determined in Intact Leaves: Donation to P700+ from Stromal Components Via the Intersystem Chain. Plant Cell Physiol. 1992, 33, 927–932. [Google Scholar] [CrossRef]
- Savitch, L.V.; Ivanov, A.G.; Gudynaite-Savitch, L.; Huner, N.P.A.; Simmonds, J. Cold Stress Effects on PSI Photochemistry in Zea mays: Differential Increase of FQR-Dependent Cyclic Electron Flow and Functional Implications. Plant Cell Physiol. 2011, 52, 1042–1054. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.-L.; Chen, J.-H.; He, N.-Y.; Guo, F.-Q. Metabolic Reprogramming in Chloroplasts under Heat Stress in Plants. Int. J. Mol. Sci. 2018, 19, 849. [Google Scholar] [CrossRef] [PubMed]
- Sami, F.; Yusuf, M.; Faizan, M.; Faraz, A.; Hayat, S. Role of sugars under abiotic stress. Plant Physiol. Biochem. 2016, 109, 54–61. [Google Scholar] [CrossRef]
- Hasanuzzaman, M.; Nahar, K.; Alam, M.M.; Roychowdhury, R.; Fujita, M. Physiological, Biochemical, and Molecular Mechanisms of Heat Stress Tolerance in Plants. Int. J. Mol. Sci. 2013, 14, 9643–9684. [Google Scholar] [CrossRef]
- Rosa, M. , Prado, C., Podazza, G., Interdonato, R., González, J. A., Hilal, M., & Prado, F. E. Soluble sugars: Metabolism, sensing and abiotic stress: A complex network in the life of plants. 2009. [Google Scholar]
- Xue, X.; Liu, A.; Hua, X. Proline accumulation and transcriptional regulation of proline biothesynthesis and degradation in Brassica napus. BMB Rep. 2009, 42, 28–34. [Google Scholar] [CrossRef]
- Yu, X.; Ali, M.M.; Li, B.; Fang, T.; Chen, F. Transcriptome data-based identification of candidate genes involved in metabolism and accumulation of soluble sugars during fruit development in ‘Huangguan’ plum. J. Food Biochem. 2021, 45, e13878. [Google Scholar] [CrossRef]
- Zou, M.; Yuan, L.; Zhu, S.; Liu, S.; Ge, J.; Wang, C. Effects of heat stress on photosynthetic characteristics and chloroplast ultrastructure of a heat-sensitive and heat-tolerant cultivar of wucai (Brassica campestris L.). Acta Physiol. Plant. 2016, 39, 1–10. [Google Scholar] [CrossRef]
- Locato, V. , De Pinto, M. C., & De Gara, L. Different involvement of the mitochondrial, plastidial and cytosolic ascorbate–glutathione redox enzymes in heat shock responses. Physiol. Plant. 2009, 135(3), 296-306.
- Yang, S.; He, M.; Zhi, Y.; Chang, S.X.; Gu, B.; Liu, X.; Xu, J. An integrated analysis on source-exposure risk of heavy metals in agricultural soils near intense electronic waste recycling activities. Environ. Int. 2019, 133, 105239. [Google Scholar] [CrossRef]
- Samanta, S.; Singh, A.; Banerjee, A.; Roychoudhury, A. Exogenous supplementation of melatonin alters representative organic acids and enzymes of respiratory cycle as well as sugar metabolism during arsenic stress in two contrasting indica rice cultivars. J. Biotechnol. 2020, 324, 220–232. [Google Scholar] [CrossRef]
- Islam, F. , Ali B., Wang J., Farooq M.A., Gill, R.A., Ali, S., Zhou, W. Combined herbicide and saline stress diferentially modulates hormonal regulation and antioxidant defense system in Oryza sativa cultivars. Plant Physiol. Biochem. 8: 2016, 107, 2016. [Google Scholar]
- Ahmad, R. , Ali S., Abid M., Rizwan M., Ali B., Tanveer A., Ahmad I., Azam M., Ghani M.A. Glycinebetaine alleviates the chromium toxicity in Brassica oleracea L. by suppressing oxidative stress and modulating the plant morphology and photosynthetic attributes. Environ. Sci. Pollut. Res. 1: 2020, 27(1), 2020. [Google Scholar]
- Almeselmani, M.; Deshmukh, P.S.; Sairam, R.K.; Kushwaha, S.R.; Singh, T.P. Protective role of antioxidant enzymes under high temperature stress. Plant Sci. 2006, 171, 382–388. [Google Scholar] [CrossRef] [PubMed]
- Chen, Z. , Song S., Wen Y., Zou Y., Liu H. Toxicity of Cu (II) to the green alga Chlorella vulgaris: a perspective of photosynthesis and oxidant stress. Environ. Sci. Pollut. Res. 1: 2016, 23(18), 2016. [Google Scholar]
- Samanta, S.; Banerjee, A.; Roychoudhury, A. Melatonin application differentially modulates the enzymes associated with antioxidative machinery and ascorbate-glutathione cycle during arsenate exposure in indica rice varieties. Plant Biol. 2020, 23, 193–201. [Google Scholar] [CrossRef] [PubMed]
- Meng, J.F. , Xu T.F., Wang Z.Z., Fang Y.L., Xi Z.M., Zhang Z.W. The ameliorative effects of exogenous melatonin on grape cuttings under water-deficient stress: antioxidant metabolites, leaf anatomy, and chloroplast morphology. J Pineal Res. 2: 2014, 57(2), 2014. [Google Scholar]
- Wahid, A.; Close, T.J. Expression of dehydrins under heat stress and their relationship with water relations of sugarcane leaves. Biol. Plant. 2007, 51, 104–109. [Google Scholar] [CrossRef]
- Meng, L. X. , Liang, X. Q., Min, L., Lu, J. X., Li, X. N., and Li, J. B. Study on preparation of xylan from high-temperature boliled pretreated sugarcane leaves. Food Ferment. Indus. 2012, 38, 105–108. [Google Scholar]
- El-Esawi, M.A.; Alayafi, A.A. Overexpression of Rice Rab7 Gene Improves Drought and Heat Tolerance and Increases Grain Yield in Rice (Oryza sativa L.). Genes 2019, 10, 56. [Google Scholar] [CrossRef]
- Kouil,R., Wientjes, E., Bultema, J.B., Croce, R., Boekema, E.J. High-light vs. low-light: Effect of light acclimation on photosystem II composition and organization in Arabidopsis thaliana[J]. Biochim. Biophys. Acta. 2013, 1827(3).
- Yamori, W. Photosynthetic response to fluctuating environments and photoprotective strategies under abiotic stress. J. Plant Res. 2016, 129, 379–395. [Google Scholar] [CrossRef]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).