Submitted:
08 May 2023
Posted:
09 May 2023
You are already at the latest version
Abstract

Keywords:
1. Introduction
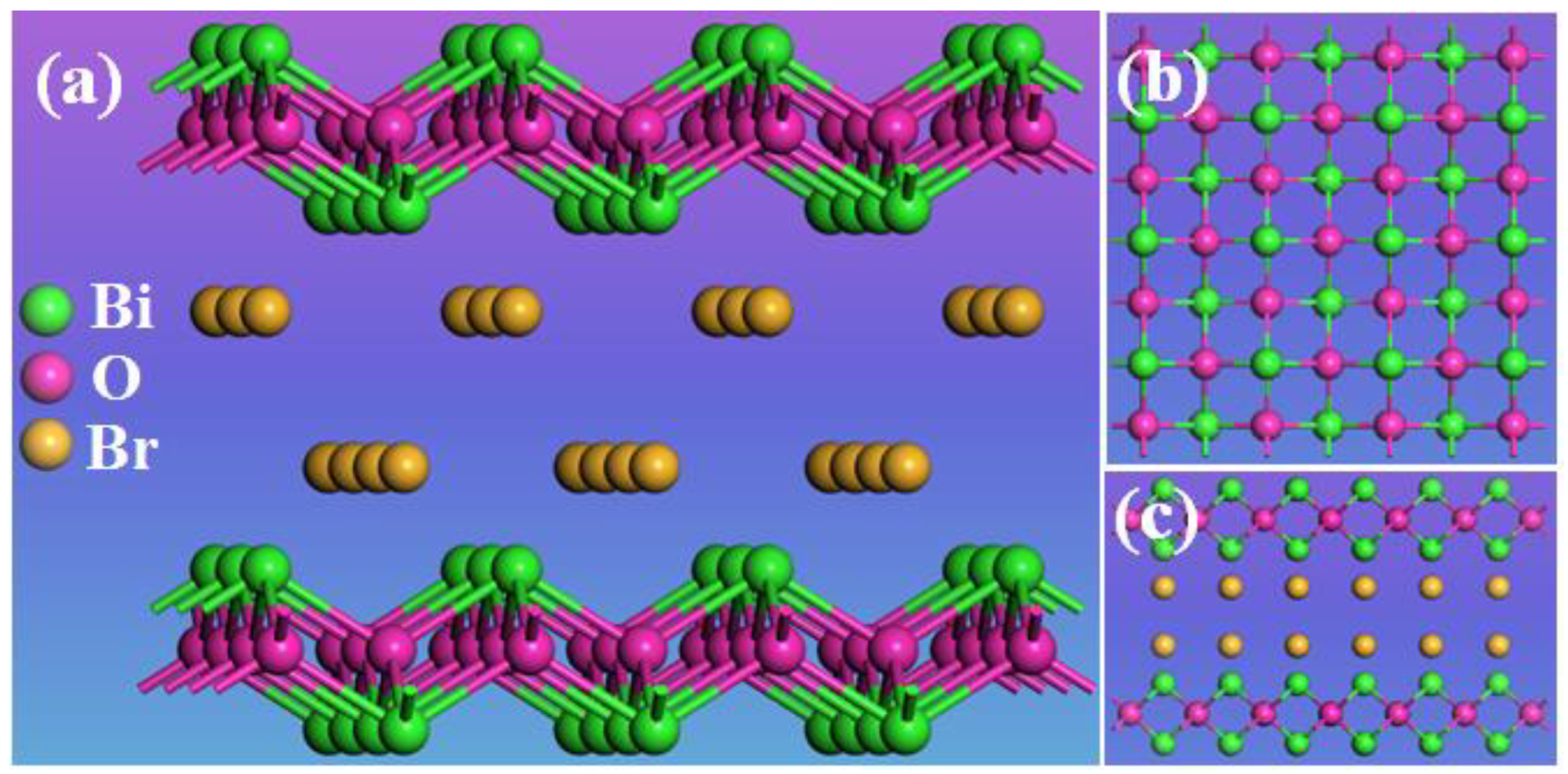
2. Results
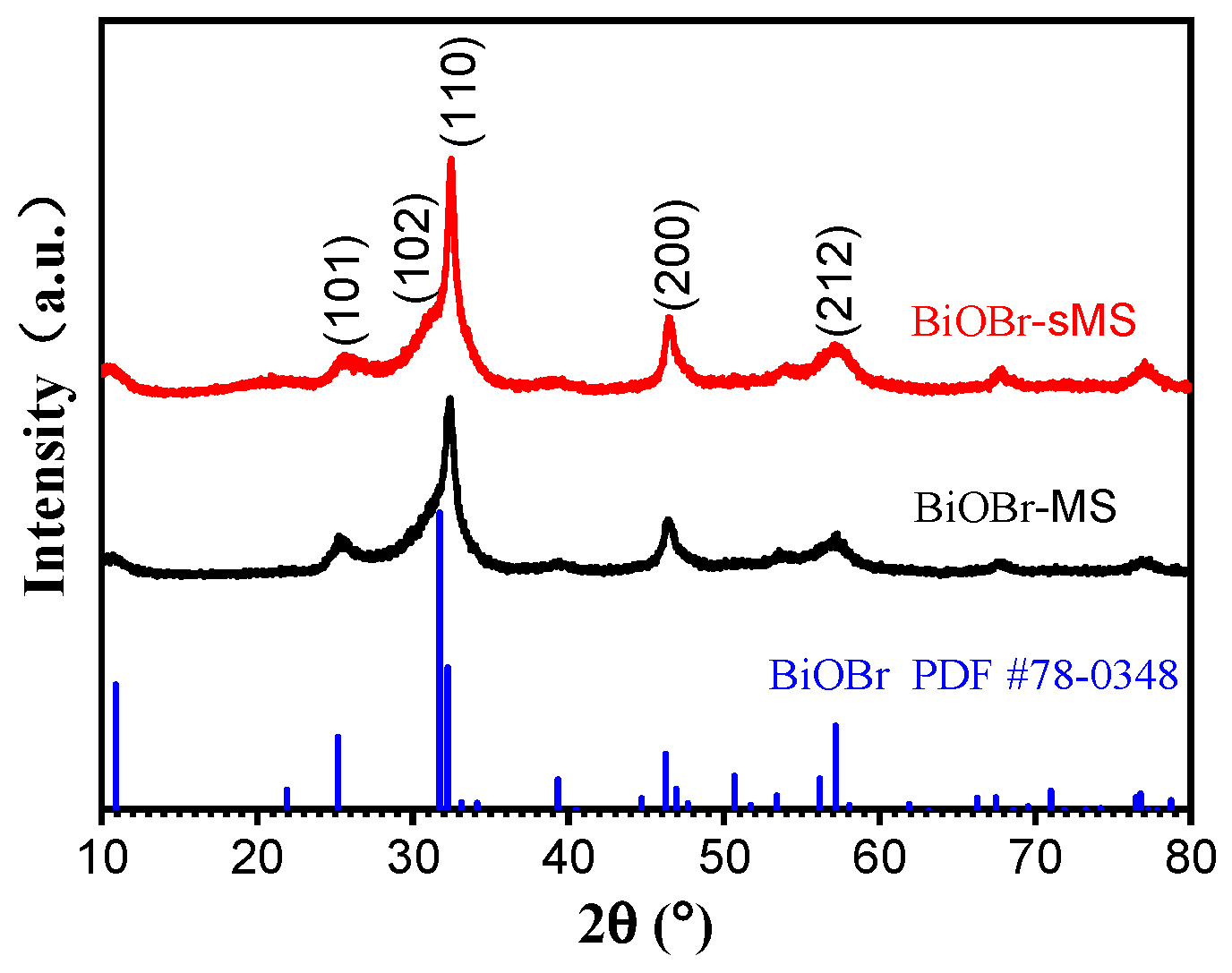
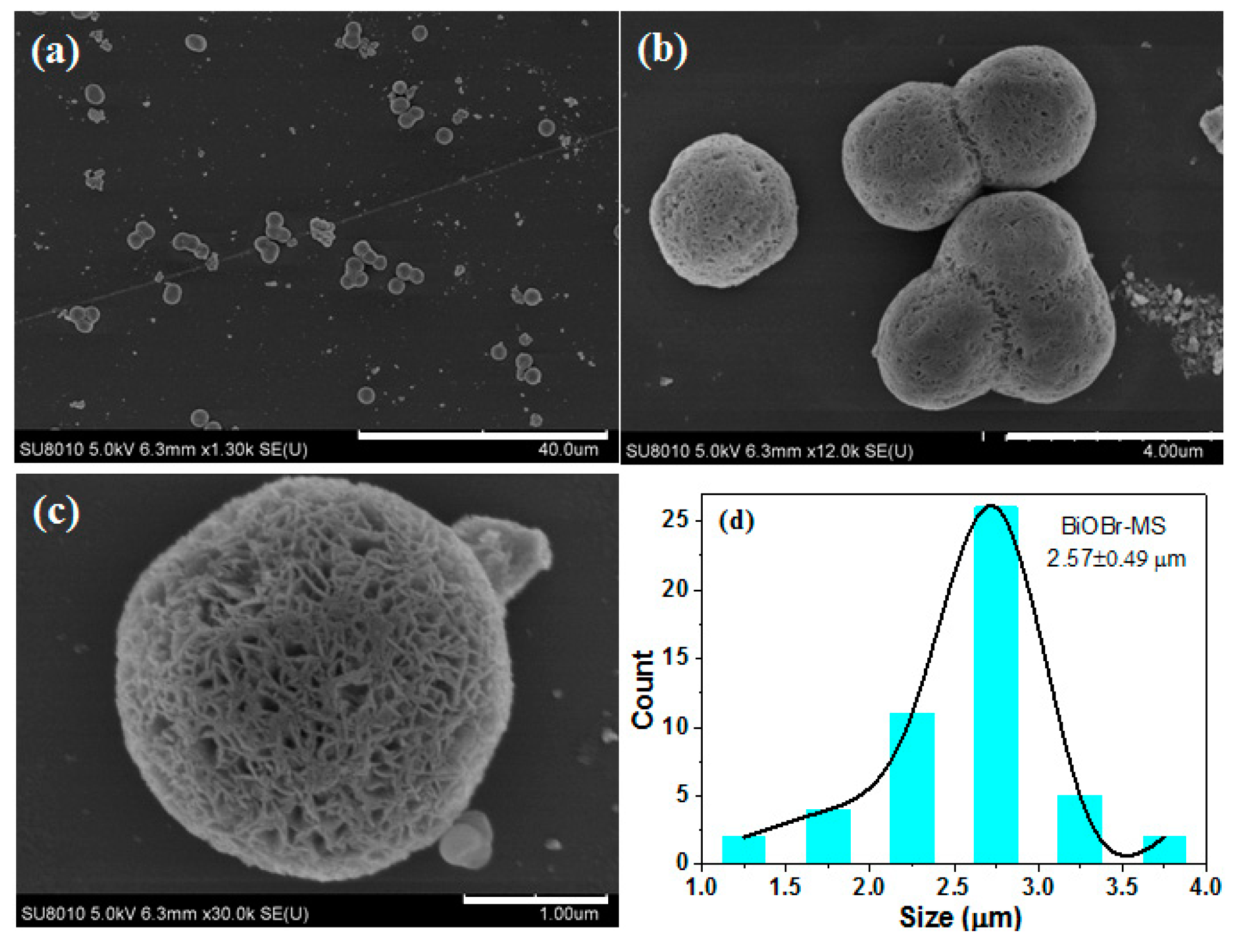
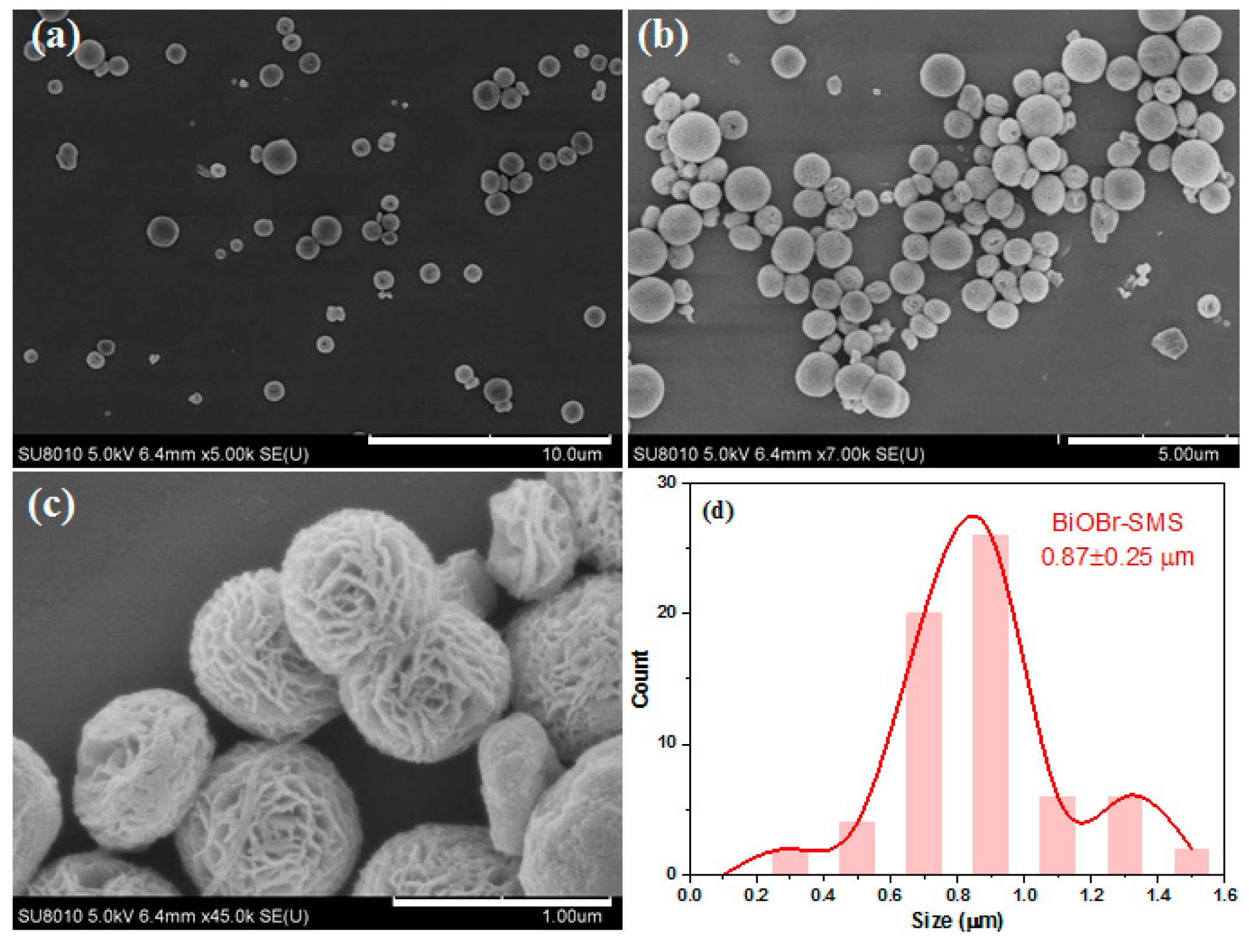
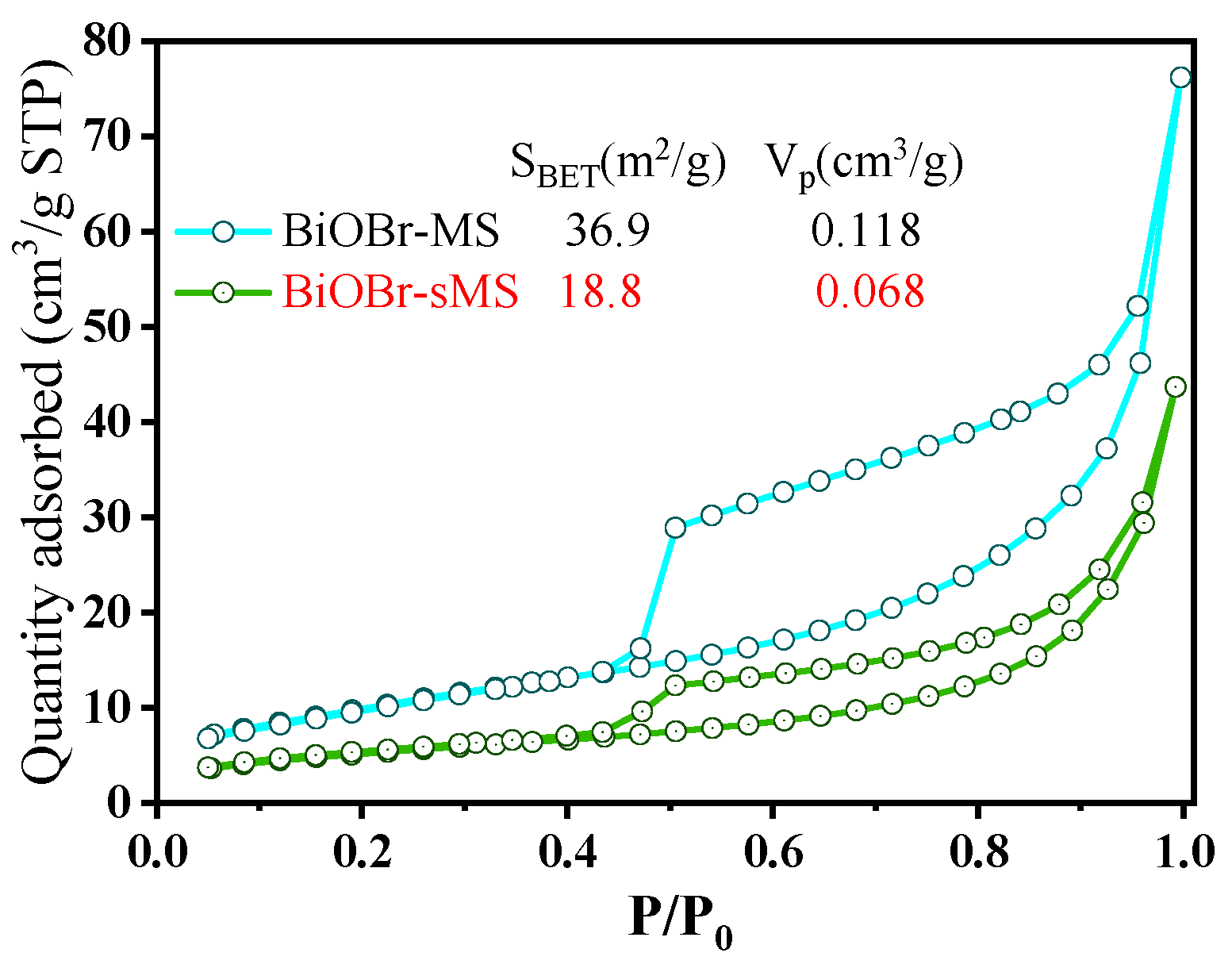
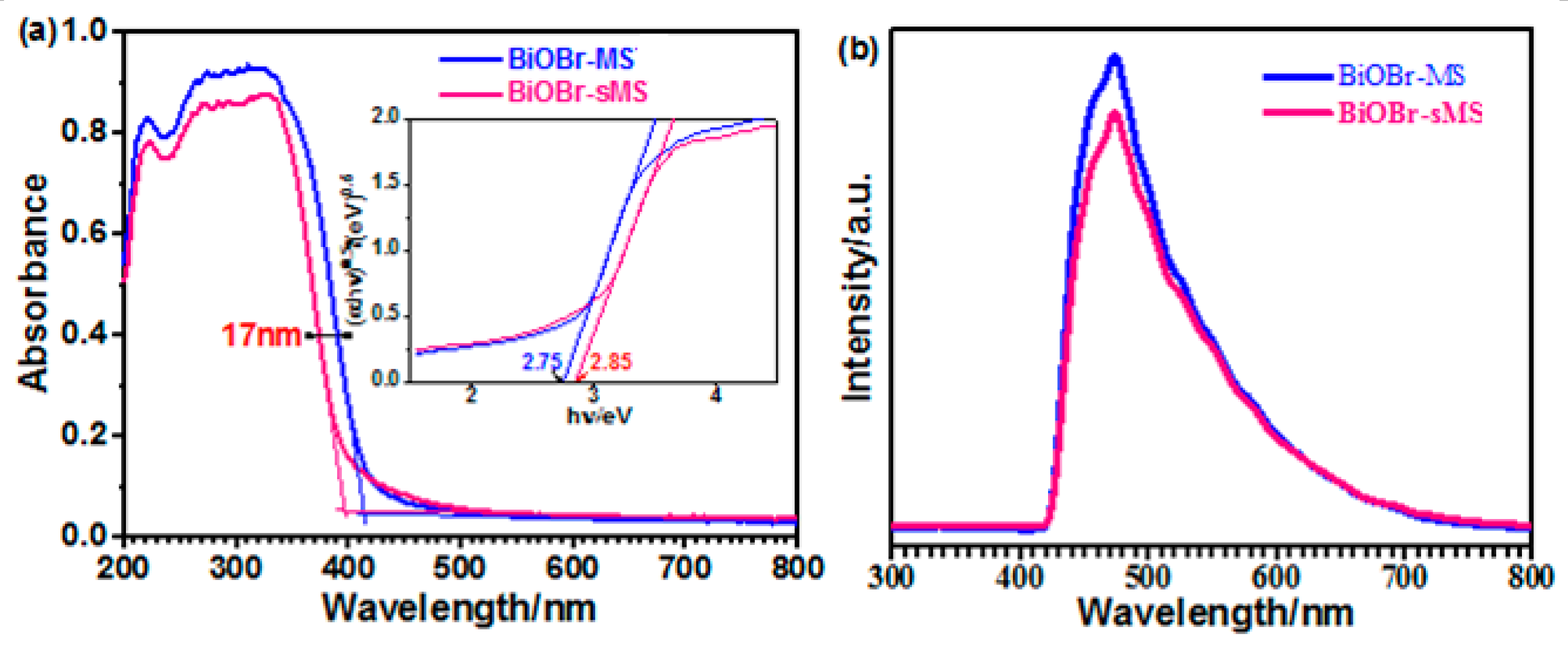
2.1. Characterization of BiOBr-(s)MS
2.2. Photocatalytic Performances of BiOBr-(s)MS
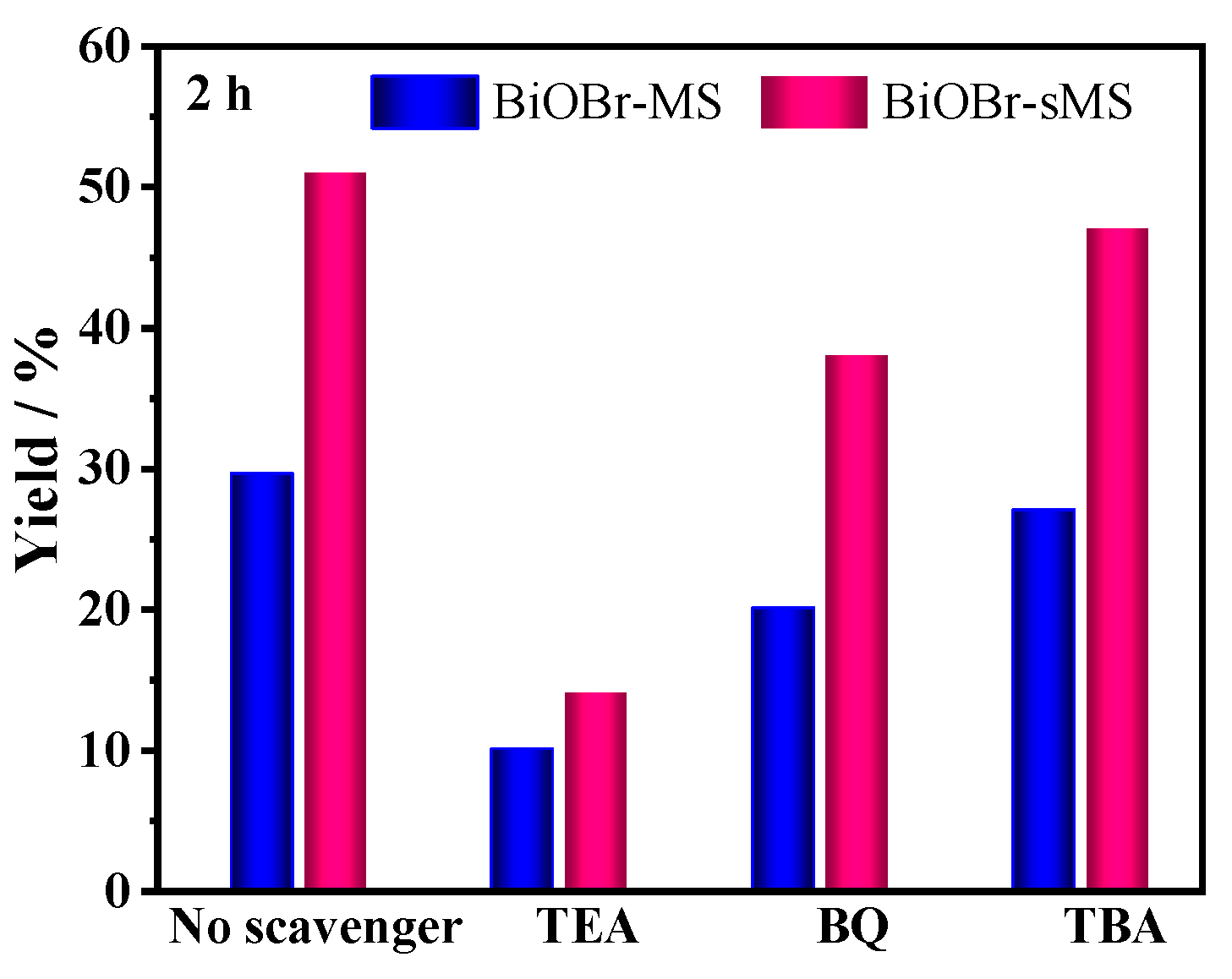
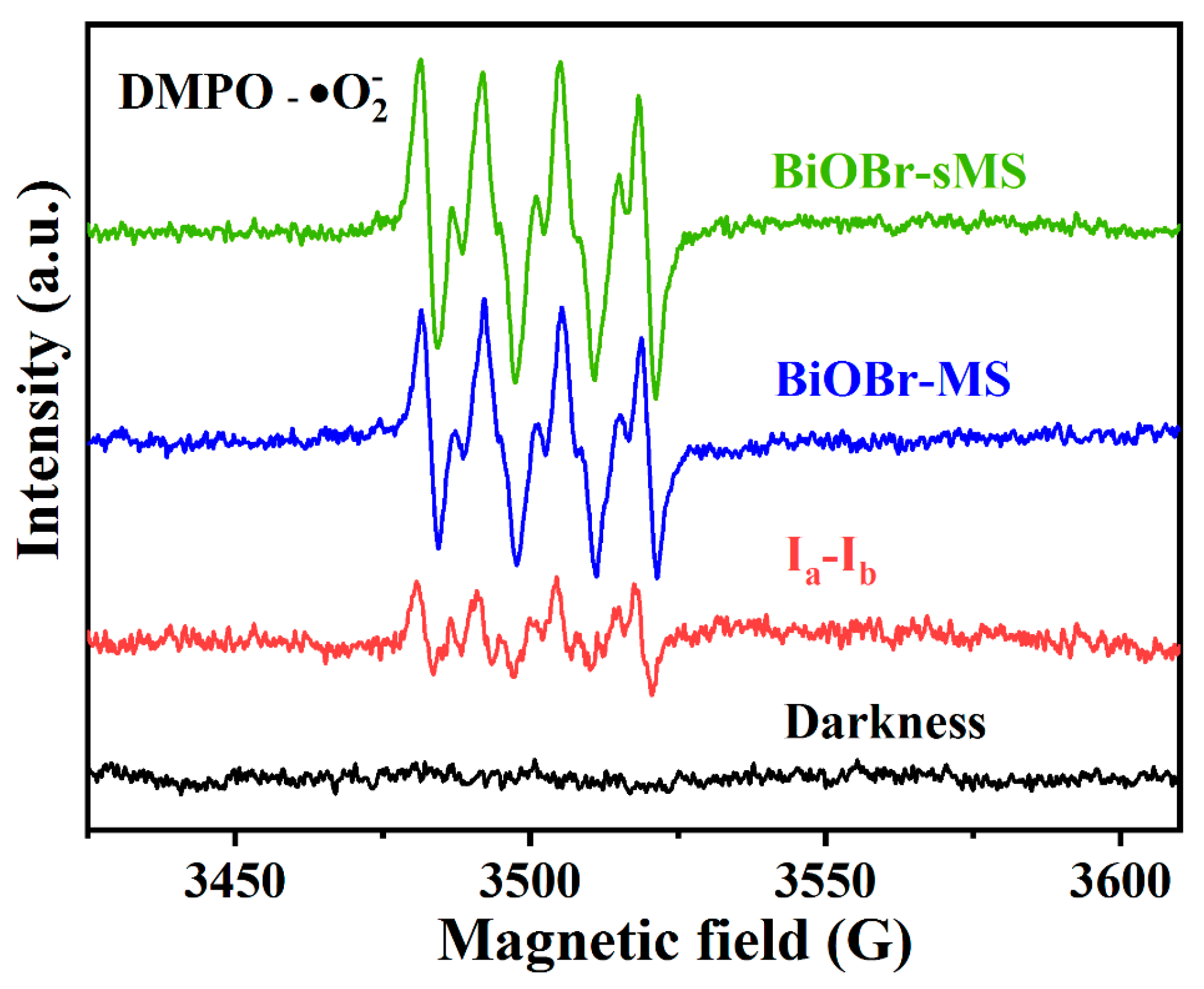
2.3. Mechanistic Studies of Enhanced Photocatalytic Activity of BiOBr-sMS
3. Materials and Methods
3.1. Chemicals Reagents
3.2. Solvothermal synthesis of BiOBr-(s)MS
3.3. Characterization
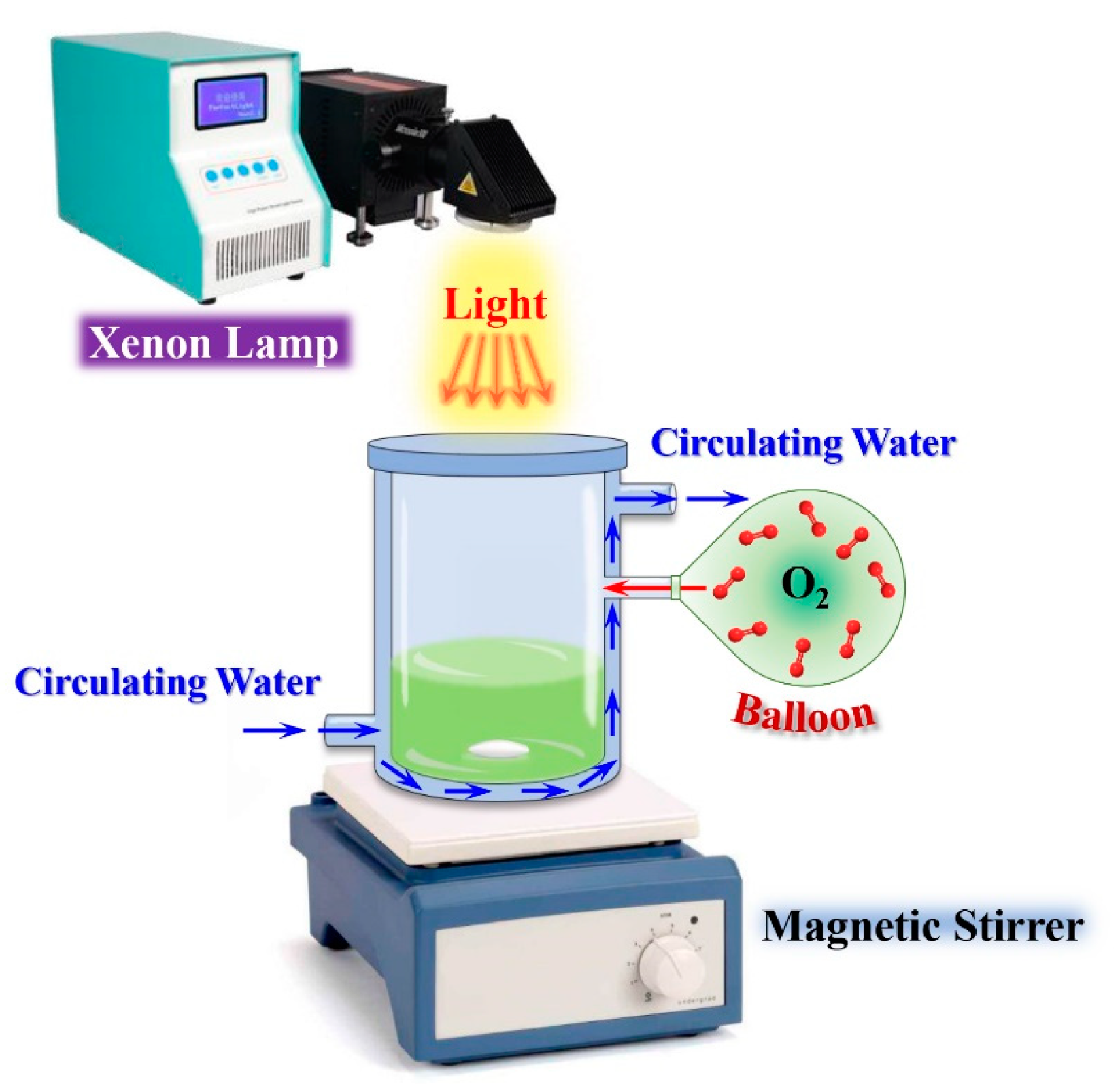
3.4. Photocatalytic Aerobic Oxidation of BzOH over BiOBr Nanostructures
3.5. Scavenging and Spin-Trapping tests
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Brühne, F.; Wright, E., Benzaldehyde, Wiley-VCH Verlag GmbH & Co. KGaA, 2000.
- Rak, M.J.; Lerro, M.; Moores, A. Hollow iron oxide nanoshells are active and selective catalysts for the partial oxidation of styrene with molecular oxygen. Chem. Commun. 2014, 50, 12482–12485. [Google Scholar] [CrossRef]
- Xiao, X.; Jiang, J.; Zhang, L. Selective oxidation of benzyl alcohol into benzaldehyde over semiconductors under visible light: The case of Bi12O17Cl2 nanobelts. Appl. Catal. B: Environ. 2013, 142-143, 487–493. [Google Scholar] [CrossRef]
- Schultz, D.M.; Yoon, T.P. Solar synthesis: Prospects in visible light photocatalysis. Science 2014, 343, 1239176. [Google Scholar] [CrossRef]
- Ma, B.; Huang, E.; Wu, G.; Dai, W.; Guan, N.; Li, L. Fabrication of WO2.72/RGO nano-composites for enhanced photocatalysis. RSC Advances 2017, 7, 2606–2614. [Google Scholar] [CrossRef]
- Li, P.; Zhao, H.; Yan, X.; Yang, X.; Li, J.; Gao, S.; Cao, R. Visible-light-driven photocatalytic hydrogen production coupled with selective oxidation of benzyl alcohol over CdS@MoS2 heterostructures. Sci China Mater 2020, 63, 2239–2250. [Google Scholar] [CrossRef]
- Karimian, D.; Yadollahi, B.; Mirkhani, V. Harvesting visible light for aerobic oxidation of alcohols by a novel and efficient hybrid polyoxometalate. Dalton Trans 2015, 44, 1709–1715. [Google Scholar] [CrossRef]
- Su, Y.; Han, Z.; Zhang, L.; Wang, W.; Duan, M.; Li, X.; Zheng, Y.; Wang, Y.; Lei, X. Surface hydrogen bonds assisted meso-porous WO3 photocatalysts for high selective oxidation of benzylalcohol to benzylaldehyde. Appl Catal B: Environ 2017, 217, 108–114. [Google Scholar] [CrossRef]
- She, H.; Zhou, H.; Li, L.; Wang, L.; Huang, J.; Wang, Q. Nickel-doped excess oxygen defect titanium dioxide for efficient selective photocatalytic oxidation of benzyl alcohol. ACS Sustain Chem Eng 2018, 6, 11939–11948. [Google Scholar] [CrossRef]
- Li, J.; Yu, Y.; Zhang, L. Bismuth oxyhalide nanomaterials: layered structures meet photocatalysis. Nanoscale 2014, 6, 8473–8488. [Google Scholar] [CrossRef]
- Chen, J.; Guan, M.; Cai, W.; Guo, J.; Xiao, C.; Zhang, G. The dominant {001} facet-dependent enhanced visible-light photoactivity of ultrathin BiOBr nanosheets. Phys Chem Chem Phys 2014, 16, 20909–20914. [Google Scholar] [CrossRef]
- Wang, F.J.; Gu, Y.Y.; Yang, Z.Y.; Xie, Y.Y.; Zhang, J.J.; Shang, X.T.; Zhao, H.B.; Zhang, Z.Z.; Wang, X.X. The effect of halogen on BiOX (X = Cl, Br, I)/Bi2WO6 heterojunction for visible-light-driven photocatalytic benzyl alcohol selective oxidation. Appl Catal A-Gen 2018, 567, 65–72. [Google Scholar] [CrossRef]
- Bisht, N.S.; Mehta, S.P.S.; Sahoo, N.G.; Dandapat, A. The room temperature synthesis of a CuO-Bi-BiOBr ternary Z-scheme photocatalyst for enhanced sunlight driven alcohol oxidation. Dalton Trans 2021, 50, 5001–5010. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Zhang, Y.; Xu, H.; Ji, X.; Gan, L.; Zhang, R. Sugar-regulated bismuth oxybromide flowers with active imprinting sites for efficient photooxidative ability. J Alloys Compd 2021, 879, 160374. [Google Scholar] [CrossRef]
- Bisht, N.S.; Pancholi, D.; Sahoo, N.G.; Melkani, A.B.; Mehta, S.P.S.; Dandapat, A. Effect of Ag–Fe–Cu tri-metal loading in bismuth oxybromide to develop a novel nanocomposite for the sunlight driven photocatalytic oxidation of alcohols. Catal Sci & Technol 2019, 9, 3923–3932. [Google Scholar]
- Wang, H.; Yong, D.; Chen, S.; Jiang, S.; Zhang, X.; Shao, W.; Zhang, Q.; Yan, W.; Pan, B.; Xie, Y. Oxygen-vacancy-mediated exciton dissociation in BiOBr for boosting charge-carrier-involved molecular oxygen activation. J Am Chem Soc 2018, 140, 1760–1766. [Google Scholar] [CrossRef]
- Dai, R.; Zhang, L.; Ning, J.; Wang, W.; Wu, Q.; Yang, J.; Zhang, F.; Wang, J.-A. New insights into tuning BiOBr photocatalysis efficiency under visible-light for degradation of broad-spectrum antibiotics: Synergistic calcination and doping. J Alloys Compd 2021, 887, 161481. [Google Scholar] [CrossRef]
- Huo, Y.; Zhang, J.; Miao, M.; Jin, Y. Solvothermal synthesis of flower-like BiOBr microspheres with highly visible-light photocatalytic performances. Appl Catal B: Environ 2012, 111-112, 334–341. [Google Scholar] [CrossRef]
- Juntrapirom, S.; Tantraviwat, D.; Anuchai, S.; Thongsook, O.; Channei, D.; Inceesungvorn, B. Boosting photocatalytic coupling of amines to imines over BiOBr: Synergistic effects derived from hollow microsphere morphology. J Environ Chem Eng 2021, 9. [Google Scholar] [CrossRef]
- Ahmad, A.; Meng, X.C.; Yun, N.; Zhang, Z.S. Preparation of hierarchical BiOBr microspheres for visible light-induced photocatalytic detoxification and disinfection. J Nanomater 2016, 2016. [Google Scholar] [CrossRef]
- Nan, Q.; Huang, S.; Zhou, Y.; Zhao, S.; He, M.; Wang, Y.; Li, S.; Huang, T.; Pan, W. Ionic liquid-assisted synthesis of porous BiOBr microspheres with enhanced visible light photocatalytic performance. Appl Organomet Chem 2018, 32, e4596. [Google Scholar] [CrossRef]
- Grabs, I.-M.; Bradtmöller, C.; Menzel, D.; Garnweitner, G. Formation mechanisms of iron oxide nanoparticles in different nonaqueous media. Cryst. Growth Des. 2012, 12, 1469–1475. [Google Scholar] [CrossRef]
- Cai, W.; Wan, J. Facile synthesis of superparamagnetic magnetite nanoparticles in liquid polyols. J. Colloid Interf. Sci. 2007, 305, 366–370. [Google Scholar] [CrossRef] [PubMed]
- Miguel-Sancho, N.; Bomati-Miguel, O.; Roca, A.G.; Martinez, G.; Arruebo, M.; Santamaria, J. Synthesis of magnetic nanocrystals by thermal decomposition in glycol media: Effect of process variables and mechanistic study. Ind. Eng. Chem. Res. 2012, 51, 8348–8357. [Google Scholar] [CrossRef]
- Zhou, S.; Wang, C.; Xia, Y. Polyol synthesis of Pd icosahedral nanocrystals: Insights into the growth mechanism and size control. Chem Mater 2022, 34, 5065–5073. [Google Scholar] [CrossRef]
- Guan, M.; Xiao, C.; Zhang, J.; Fan, S.; An, R.; Cheng, Q.; Xie, J.; Zhou, M.; Ye, B.; Xie, Y. Vacancy associates promoting solar-driven photocatalytic activity of ultrathin bismuth oxychloride nanosheets. J Am Chem Soc 2013, 135, 10411–10417. [Google Scholar] [CrossRef] [PubMed]
- Di, J.; Xia, J.; Ji, M.; Xu, L.; Yin, S.; Chen, Z.; Li, H. Bidirectional acceleration of carrier separation spatially via N-CQDs/atomically-thin BiOl nanosheets nanojunctions for manipulating active species in a photocatalytic process. J Mater Chem A 2016, 4, 5051–5061. [Google Scholar] [CrossRef]
- Sing, K.S.W. Reporting physisorption data for gas/solid systems with special reference to the determination of surface area and porosity (Recommendations 1984). Pure Appl. Chem. 1985, 57, 603–619. [Google Scholar] [CrossRef]
- Rabah, M.A.; Yasri, N.G.; Alaya, M.N. Impact of aging on the structural, textural, and acid properties of WO3-SO42--SnO2 solid acids. J. Alloy. Compd. 2019, 790, 452–465. [Google Scholar] [CrossRef]
- Ben, T.; Ren, H.; Ma, S.; Cao, D.; Lan, J.; Jing, X.; Wang, W.; Xu, J.; Deng, F.; Simmons, J.M.; Qiu, S.; Zhu, G. Targeted synthesis of a porous aromatic framework with high stability and exceptionally high surface area. Angew. Chem. Int. Ed. 2009, 48, 9457–9460. [Google Scholar] [CrossRef]
- Ali, M.A.; Asaoka, S. Ni-Mo-titania-alumina catalysts with USY zeolite for low pressure hydrodesulfurization and hydrocracking. Petroleum Science and Technology 2009, 27, 984–997. [Google Scholar] [CrossRef]
- Al-Omair, M.A.; El-Sharkawy, E.A. Removal of heavy metals via adsorption on activated carbon synthesized from solid wastes. Environ. Technol. 2007, 28, 443–451. [Google Scholar] [CrossRef] [PubMed]
- Yan, J.; Wu, G.; Guan, N.; Li, L. Nb2O5/TiO2 heterojunctions: Synthesis strategy and photocatalytic activity. Appl Catal B: Environ 2014, 152-153, 280–288. [Google Scholar] [CrossRef]
- Zhang, H.; Liu, L.; Zhou, Z. Towards better photocatalysts: first-principles studies of the alloying effects on the photocatalytic activities of bismuth oxyhalides under visible light. Phys Chem Chem Phys 2012, 14, 1286–1292. [Google Scholar] [CrossRef]
- Xiao, X.; Liu, C.; Hu, R.; Zuo, X.; Nan, J.; Li, L.; Wang, L. Oxygen-rich bismuth oxyhalides: generalized one-pot synthesis, band structures and visible-light photocatalytic properties. J Mater Chem 2012, 22, 22840–22843. [Google Scholar] [CrossRef]
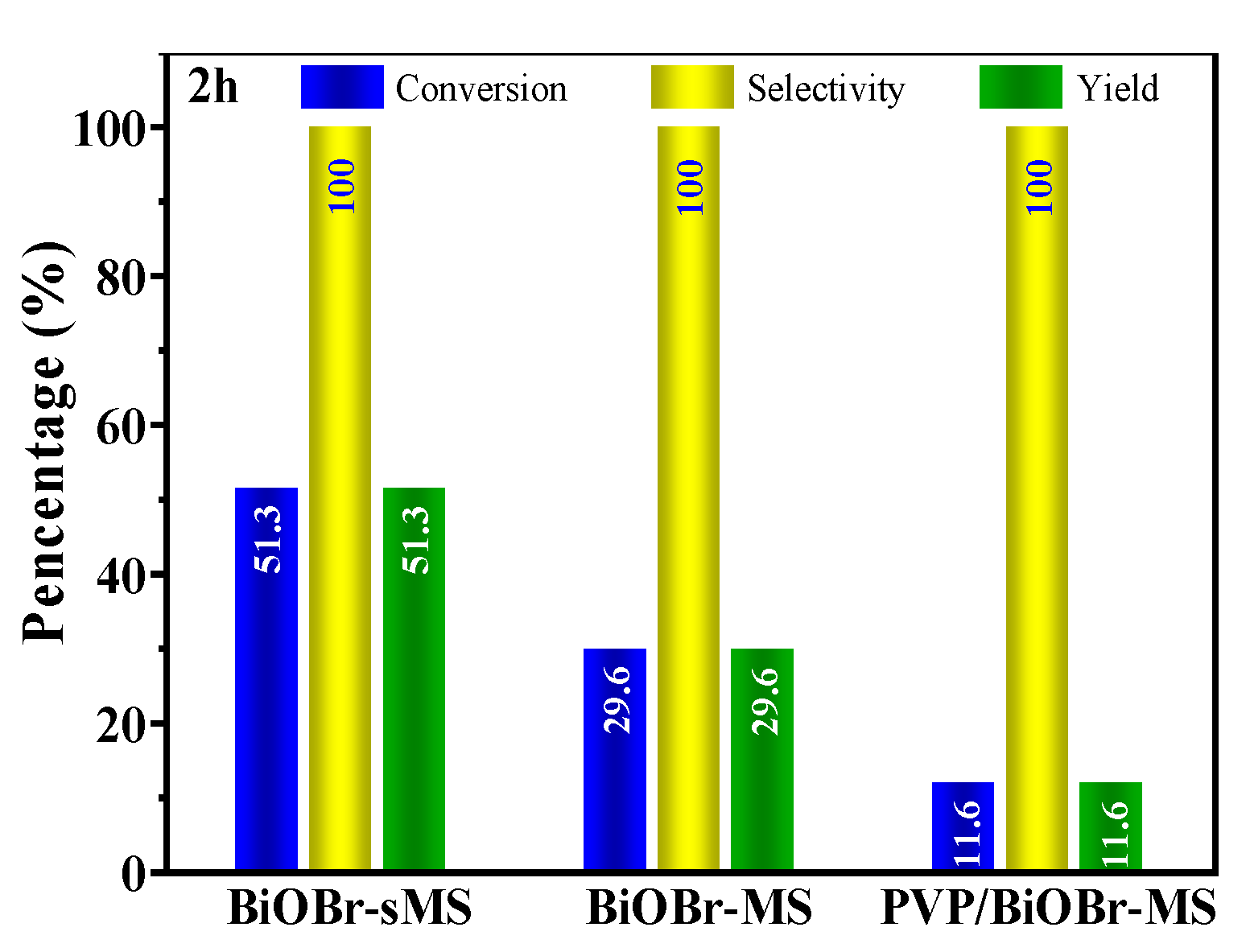
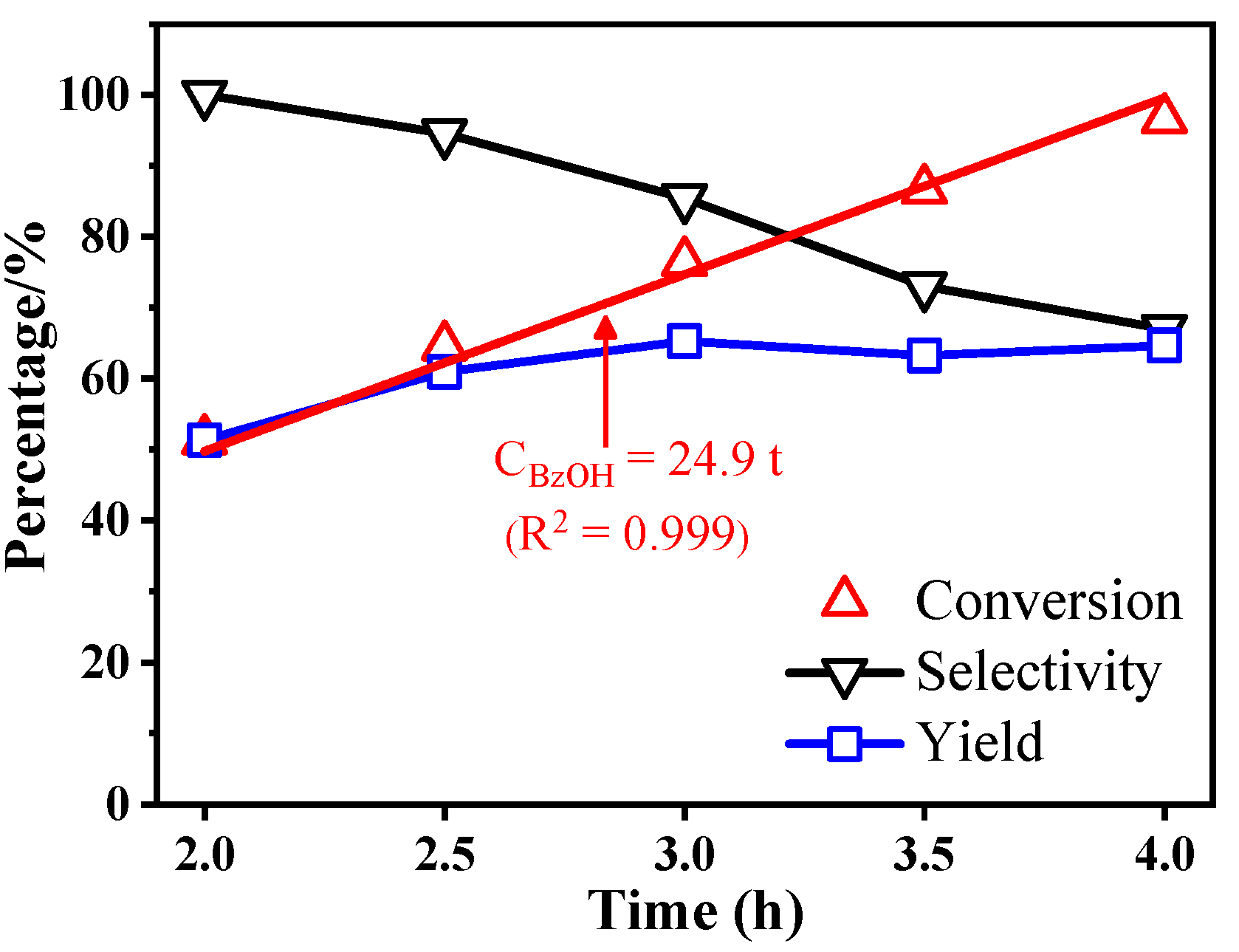
| Entry | Photocatalyst | Conditions | Convn/Sel/Yield (%) | Ref |
|---|---|---|---|---|
| 1 | BiOBr-sMS | 20mg catalyst, 0.5mmol BzOH, O2 balloon, 10mL CH3CN, 25°C, 2h | 51.3%/100%/51.3% (96.5%/67.0%/64.7% at 4h) |
This work |
| 2 | WO2.72 | 0.1g catalyst, 25mmol BzOH, O2 (20mL/min), 60mL BTF, 25oC, 8h | 9.5%/91.0%/8.6% | [5] |
| 3 | WO2.72/rGO | 0.1g catalyst, 25mmol BzOH, O2 (20mL/min), 60mL BTF, 25oC, 8h | 37.6%/92.0%/34.6% | [5] |
| 4 | Fluorinated mesoporous WO3 | 0.1g catalyst, 0.025mmol BzOH, 50mL H2O, 25oC, 4h. | ∼57%/∼99%/56% | [8] |
| 5 | Hybrid-SiW11O39 | 40mg catalyst, 1mmol BzOH, 3mL CH3CN purged with O2, 25°C, 4h | 80% yield | [7] |
| 6 | 0.8Br-BiOBr/Bi2WO6 | 20mg catalyst, 0.2mmol BzOH, 2.5mL BTF saturated with O2, 25°C, 4h | 30.9%/100%/30.9% | [12] |
| 7 | TiO2 | 0.3g catalyst, 25mmol BzOH, 27mL BTF, air bubbled at 20mL/min, 5h, (temperature unavailable) | 21.6%/91.4%/19.7% | [33] |
| 8 | Nb2O5/TiO2 | 0.3g catalyst, 25mmol BzOH, 27mL BTF, air bubbled at 20mL/min, 5h, (temperature unavailable) | 64.3%/85.1%/54.7% | [33] |
| 9 | Ni(1%)-OTiO2 | 80mg catalyst, 0.5mmol BzOH, 5mL BTF saturated with O2 (2atm) for 5 min, 1h (temperature unavailable) | 93%/99%/88% | [9] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





