Submitted:
07 May 2023
Posted:
09 May 2023
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Materials and Methods
2.1. Chemicals and materials
2.2. Photocatalytic Hydrogen Production
3. Results and Discussion
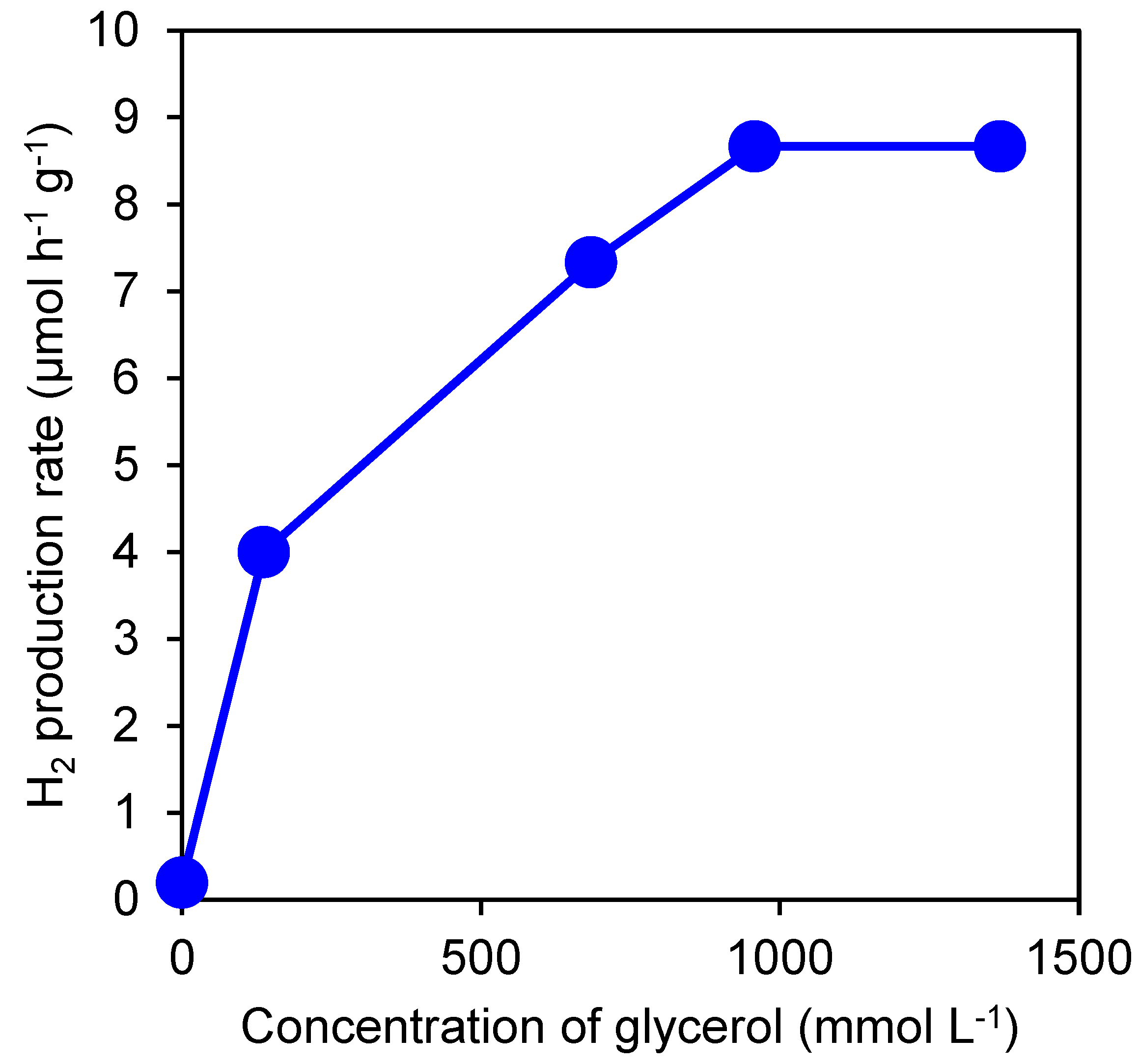
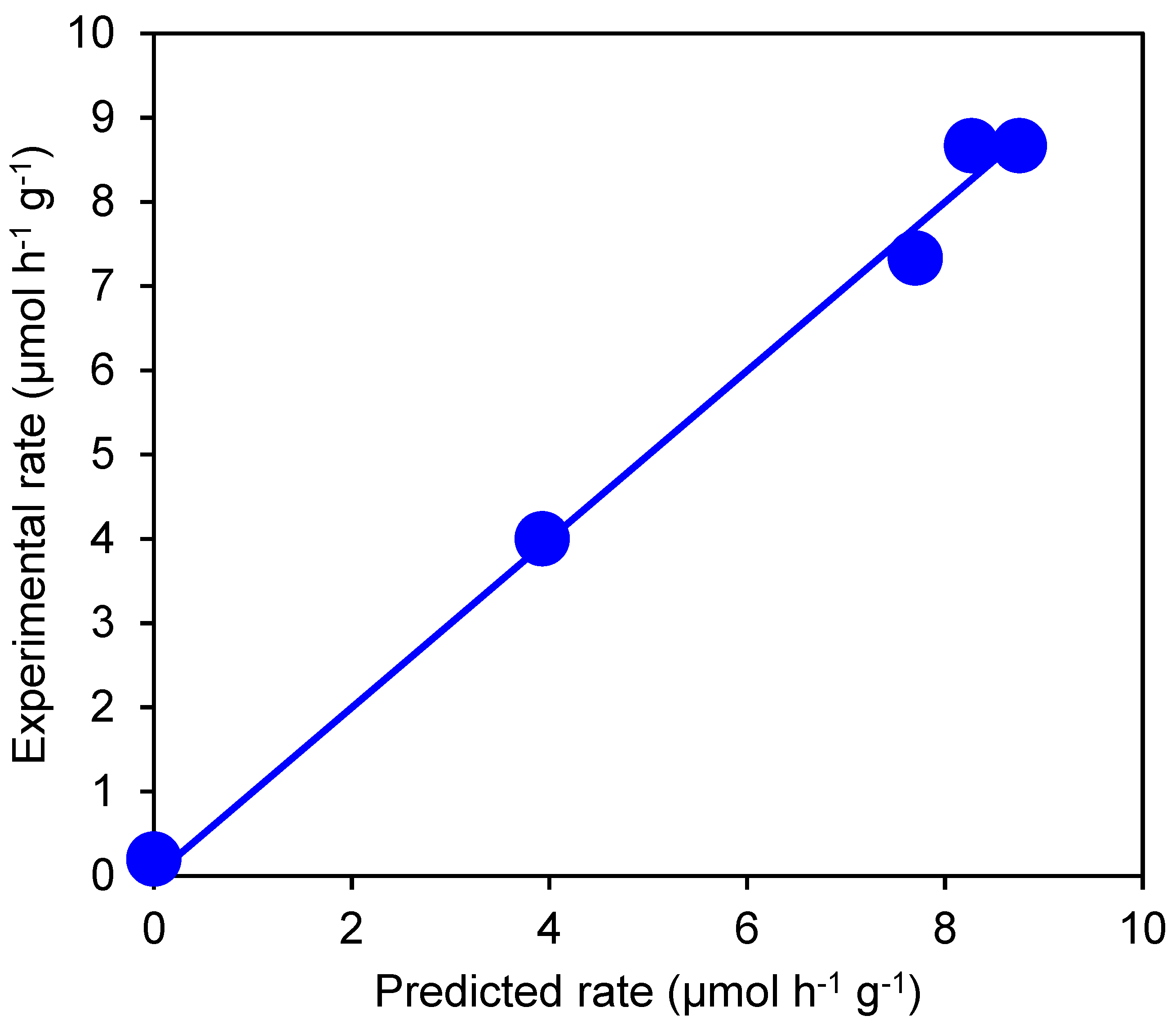
3.1. Effect of Glycerol Concentration
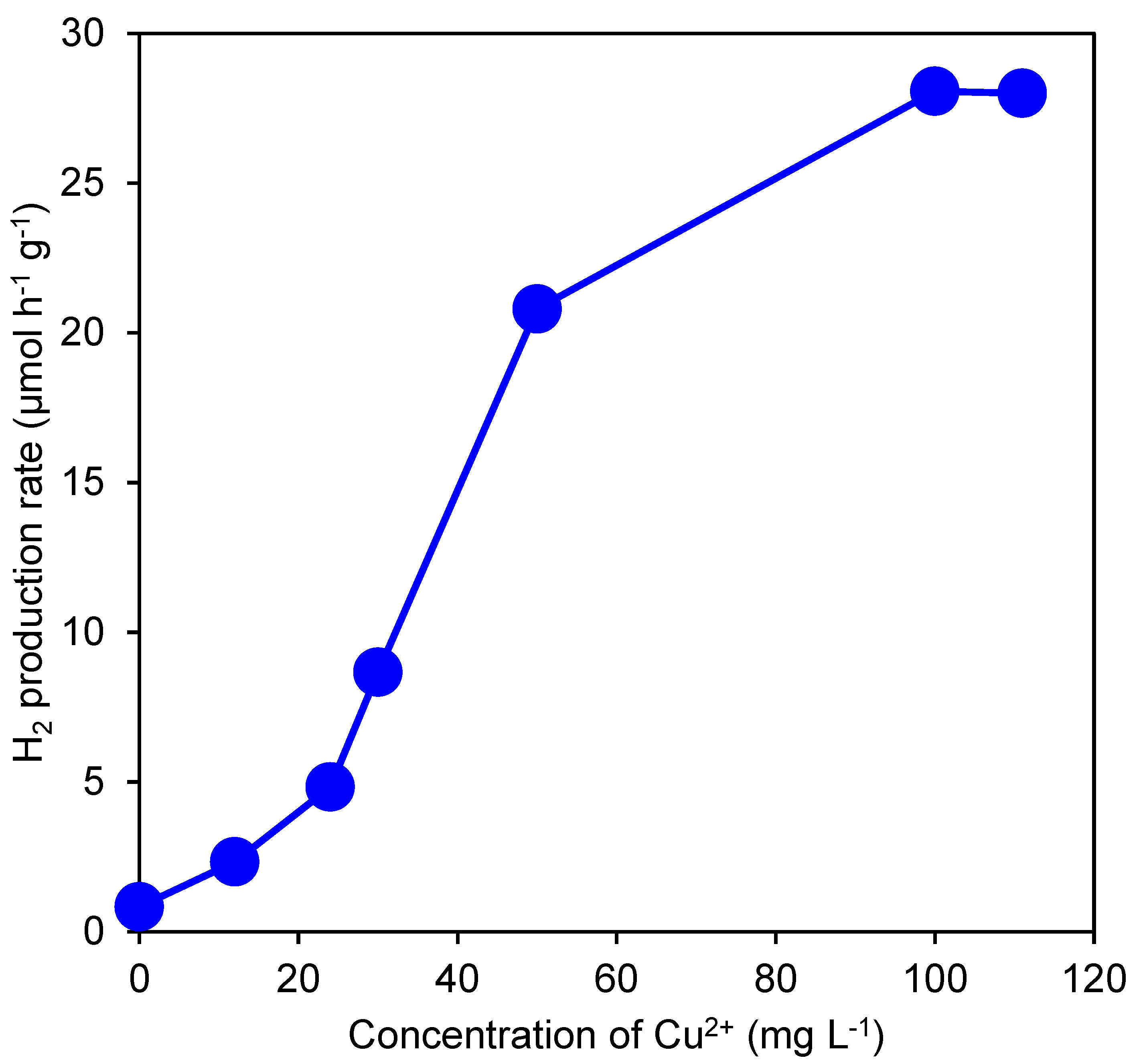
3.2. Effect of Cu Ion Concentration
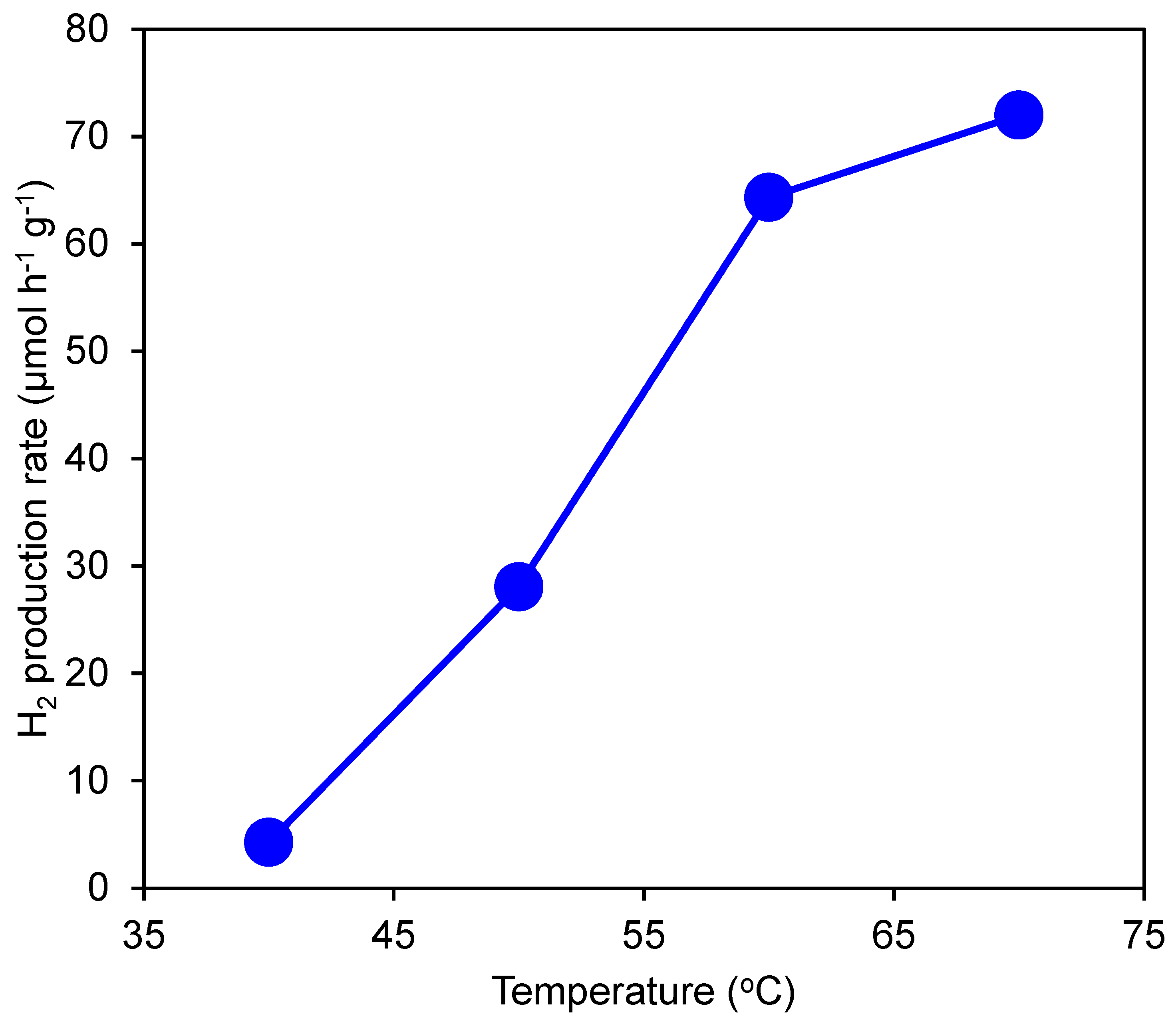
3.3. Effect of Temperature
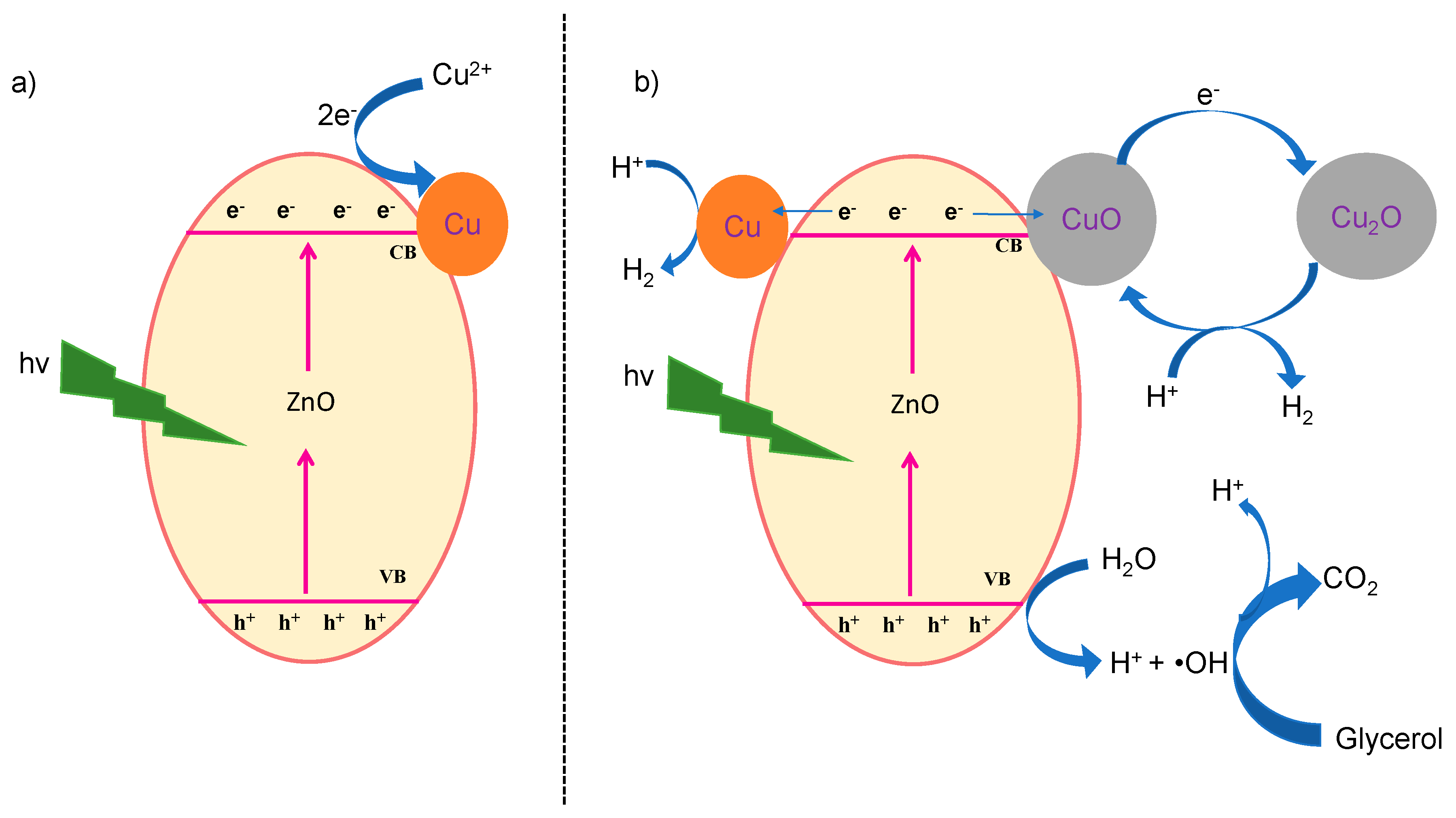
3.4. Reaction Mechanism
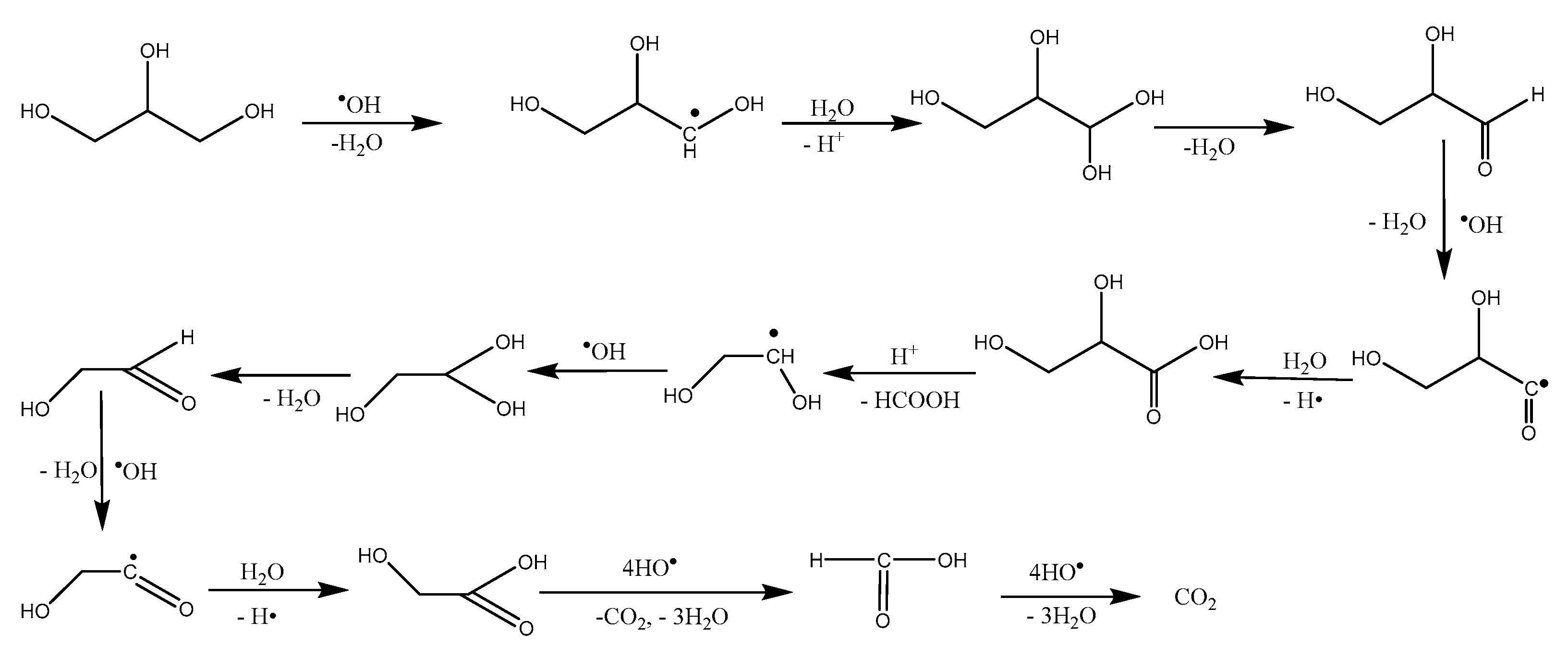
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Manzoor, M.F.; Ahmed, E.; Ahmad, M.; Ahmad, I.; Rana, A.M.; Ali, A.; Ghouri, M.I.; Manzoor, M.S.; Aziz, M.T. Enhanced Photocatalytic Activity of Hydrogen Evolution through Cu Incorporated ZnO Nano Composites. Mater. Sci. Semicond. Process 2020, 120, 105278. [CrossRef]
- Choi, S.; Do, J.Y.; Lee, J.H.; Ra, C.S.; Kim, S.K.; Kang, M. Optical Properties of Cu-Incorporated ZnO (CuxZnyO) Nanoparticles and Their Photocatalytic Hydrogen Production Performances. Mater. Chem. Phys. 2018, 205, 206–209. [CrossRef]
- Chang, C.J.; Lin, Y.G.; Weng, H.T.; Wei, Y.H. Photocatalytic Hydrogen Production from Glycerol Solution at Room Temperature by ZnO-ZnS/Graphene Photocatalysts. Appl. Surf. Sci. 2018, 451, 198–206. [CrossRef]
- Pal, D.B.; Singh, A.; Bhatnagar, A. A Review on Biomass Based Hydrogen Production Technologies. Int. J. Hydrogen Energy 2022, 47, 1461–1480. [CrossRef]
- Keskin, T.; Hallenbeck, P.C. Hydrogen Production from Sugar Industry Wastes Using Single-Stage Photofermentation. Bioresour. Technol. 2012, 112, 131–136. [CrossRef]
- Fujita, S; Kawamori, H.; Honda, D.; Yoshida, H.; Arai, M. Photocatalytic Hydrogen Production from Aqueous Glycerol Solution Using NiO/TiO2 Catalysts: Effects of Preparation and Reaction Conditions. Appl. Catal. B Environ. 2016, 181, 818–824. [CrossRef]
- Zhao, H.; Yu, X.; Li, C.F.; Yu, W.; Wang, A.; Hu, Z.Y.; Larter, S.; Li, Y.; Golam Kibria, M.; Hu, J. Carbon Quantum Dots Modified TiO2 Composites for Hydrogen Production and Selective Glucose Photoreforming. J. Energy Chem. 2022, 64, 201–208. [CrossRef]
- Zhou, C.H. (Clayton); Beltramini, J.N.; Fan, Y.X.; Lu, G.Q. (Max) Chemoselective Catalytic Conversion of Glycerol as a Biorenewable Source to Valuable Commodity Chemicals. Chem. Soc. Rev. 2008, 37, 527–549. [CrossRef]
- Hashaikeh, R.; Butler, I.S.; Kozinski, J.A. Selective Promotion of Catalytic Reactions during Biomass Gasification to Hydrogen. Energy and Fuels 2006, 20, 2743–2747. [CrossRef]
- Davda, R.R.; Shabaker, J.W.; Huber, G.W.; Cortright, R.D.; Dumesic, J.A. A Review of Catalytic Issues and Process Conditions for Renewable Hydrogen and Alkanes by Aqueous-Phase Reforming of Oxygenated Hydrocarbons over Supported Metal Catalysts. Appl. Catal. B Environ. 2005, 56, 171–186. [CrossRef]
- Dauenhauer, P.J.; Salge, J.R.; Schmidt, L.D. Renewable Hydrogen by Autothermal Steam Reforming of Volatile Carbohydrates. J. Catal. 2006, 244, 238–247. [CrossRef]
- Deluga, G.A.; Salge, J.R.; Schmidt, L.D.; Verykios, X.E. Renewable Hydrogen from Ethanol by Autothermal Reforming. Science 2004, 303, 993–997. [CrossRef]
- Byrd, A.J.; Pant, K.K.; Gupta, R.B. Hydrogen Production from Glycerol by Reforming in Supercritical Water over Ru/Al2O3 Catalyst. Fuel 2008, 87, 2956–2960. [CrossRef]
- Lyubina, T.P.; Markovskaya, D. V.; Kozlova, E.A.; Parmon, V.N. Photocatalytic Hydrogen Evolution from Aqueous Solutions of Glycerol under Visible Light Irradiation. Int. J. Hydrogen Energy 2013, 38, 14172–14179. [CrossRef]
- Liu, S.; Wang, X.; Wang, K.; Lv, R.; Xu, Y. ZnO/ZnS-PdS Core/Shell Nanorods: Synthesis, Characterization and Application for Photocatalytic Hydrogen Production from a Glycerol/Water Solution. Appl. Surf. Sci. 2013, 283, 732–739. [CrossRef]
- Wichasilp, C.; Phuruangrat, A.; Thongtem, S. Influence of PH on the Synthesis ZnO Nanorods and Photocatalytic Hydrogen Production from Glycerol Solution. J. Indian Chem. Soc. 2022, 99, 100472. [CrossRef]
- Corredor, J.; Rivero, M.J.; Rangel, C.M.; Gloaguen, F.; Ortiz, I. Comprehensive Review and Future Perspectives on the Photocatalytic Hydrogen Production. J. Chem. Technol. Biotechnol. 2019, 94, 3049–3063. [CrossRef]
- Beltram, A.; Romero-Ocaña, I.; Josè Delgado Jaen, J.; Montini, T.; Fornasiero, P. Photocatalytic Valorization of Ethanol and Glycerol over TiO2 Polymorphs for Sustainable Hydrogen Production. Appl. Catal. A Gen. 2016, 518, 167–175. [CrossRef]
- Zhao, W.; Wang, X.; Sang, H.; Wang, K. Synthesis of Bi-Doped TiO2 Nanotubes and Enhanced Photocatalytic Activity for Hydrogen Evolution from Glycerol Solution. Chinese J. Chem. 2013, 31, 415–420. [CrossRef]
- Montini, T.; Gombac, V.; Sordelli, L.; Delgado, J.J.; Chen, X.; Adami, G.; Fornasiero, P. Nanostructured Cu/TiO2 Photocatalysts for H2 Production from Ethanol and Glycerol Aqueous Solutions. ChemCatChem 2011, 3, 574–577. [CrossRef]
- Vaiano, V.; Iervolino, G. Photocatalytic Hydrogen Production from Glycerol Aqueous Solution Using Cu-Doped ZnO under Visible Light Irradiation. Appl. Sci. 2019, 9, 2741. [CrossRef]
- Suhag, M.H.; Tateishi, I.; Furukawa, M.; Katsumata, H.; Khatun, A.; Kaneco, S. Photocatalytic Hydrogen Production from Formic Acid Solution with Titanium Dioxide with the Aid of Simultaneous Rh Deposition. ChemEngineering 2022, 6, 43. [CrossRef]
- Suhag, M.H.; Tateishi, I.; Furukawa, M.; Katsumata, H.; Khatun, A.; Kaneco, S. Application of Rh/TiO2 Nanotube Array in Photocatalytic Hydrogen Production from Formic Acid Solution. J. Compos. Sci. 2022, 6, 327. [CrossRef]
- Gomathisankar, P.; Yamamoto, D.; Katsumata, H.; Suzuki, T.; Kaneco, S. Photocatalytic Hydrogen Production with Aid of Simultaneous Metal Deposition Using Titanium Dioxide from Aqueous Glucose Solution. Int. J. Hydrogen Energy 2013, 38, 5517–5524. [CrossRef]
- Gomathisankar, P.; Hachisuka, K.; Katsumata, H.; Suzuki, T.; Funasaka, K.; Kaneco, S. Enhanced Photocatalytic Hydrogen Production from Aqueous Methanol Solution Using ZnO with Simultaneous Photodeposition of Cu. Int. J. Hydrogen Energy 2013, 38, 11840–11846. [CrossRef]
- Jung, M.; Hart, J.N.; Boensch, D.; Scott, J.; Ng, Y.H.; Amal, R. Hydrogen Evolution via Glycerol Photoreforming over Cu-Pt Nanoalloys on TiO2. Appl. Catal. A Gen. 2016, 518, 221–230. [CrossRef]
- Korzhak, A. V.; Ermokhina, N.I.; Stroyuk, A.L.; Bukhtiyarov, V.K.; Raevskaya, A.E.; Litvin, V.I.; Kuchmiy, S.Y.; Ilyin, V.G.; Manorik, P.A. Photocatalytic Hydrogen Evolution over Mesoporous TiO2/Metal Nanocomposites. J. Photochem. Photobiol. A Chem. 2008, 198, 126–134. [CrossRef]
- Kim, G.; Choi, H.J.; Kim, H. Il; Kim, J.; Monllor-Satoca, D.; Kim, M.; Park, H. Temperature-Boosted Photocatalytic H2 Production and Charge Transfer Kinetics on TiO2 under UV and Visible Light. Photochem. Photobiol. Sci. 2016, 15, 1247–1253. [CrossRef]
- Toe, C.Y.; Tsounis, C.; Zhang, J.; Masood, H.; Gunawan, D.; Scott, J.; Amal, R. Advancing Photoreforming of Organics: Highlights on Photocatalyst and System Designs for Selective Oxidation Reactions. Energy Environ. Sci. 2021, 14, 1140–1175. [CrossRef]
- Avilés-García, O.; Mendoza-Zepeda, A.; Regalado-Méndez, A.; Espino-Valencia, J.; Martínez-Vargas, S.L.; Romero, R.; Natividad, R. Photo-Oxidation of Glycerol Catalyzed by Cu/TiO2. Catalysts 2022, 12, 8. [CrossRef]
- Colmenares, J.C.; Luque, R. Heterogeneous Photocatalytic Nanomaterials: Prospects and Challenges in Selective Transformations of Biomass-Derived Compounds. Chem. Soc. Rev. 2014, 43, 765–778. [CrossRef]
- Morales-Mendoza, J.E.; Herrera-Pérez, G.; Fuentes-Cobas, L.; Hermida-Montero, L.A.; Pariona, N.; Paraguay-Delgado, F. Synthesis, Structural and Optical Properties of Cu Doped ZnO and CuO–ZnO Composite Nanoparticles. Nano-Structures and Nano-Objects 2023, 34, 100967. [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).




