Submitted:
04 May 2023
Posted:
05 May 2023
You are already at the latest version
Abstract

Keywords:
1. Introduction
2. Materials and Methods
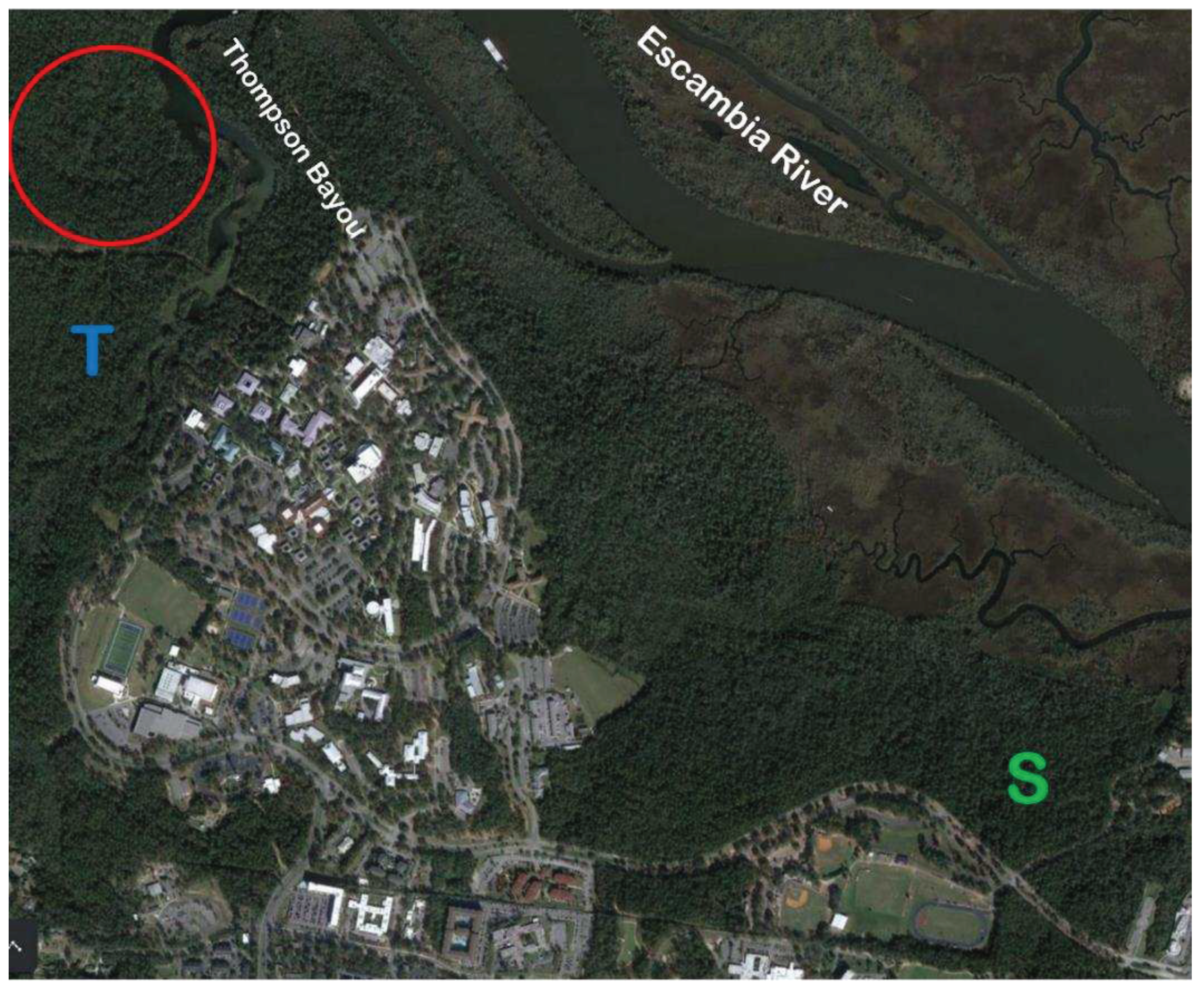
2.1. Study Site
2.2. Field Sampling
2.3. Data analysis
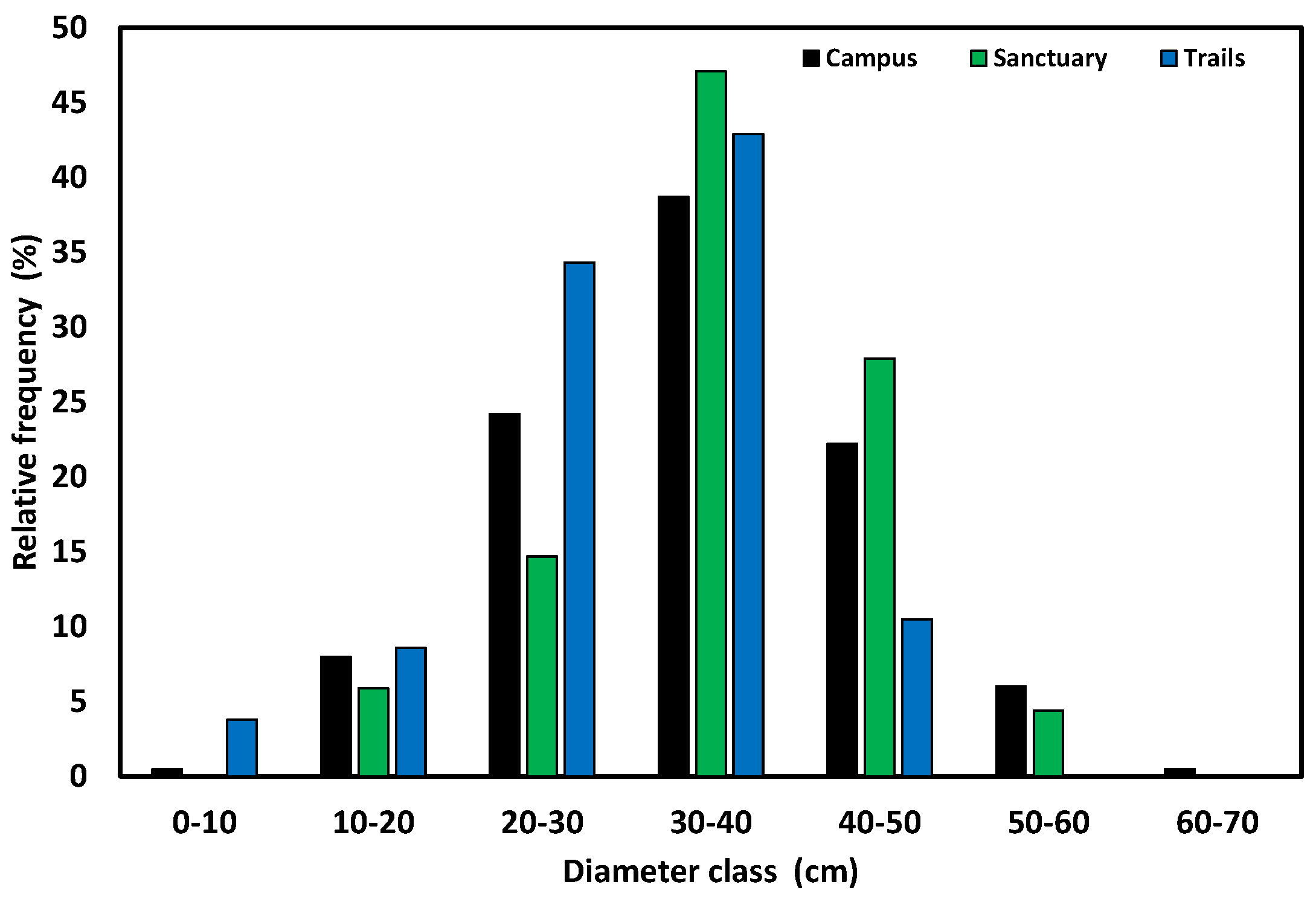
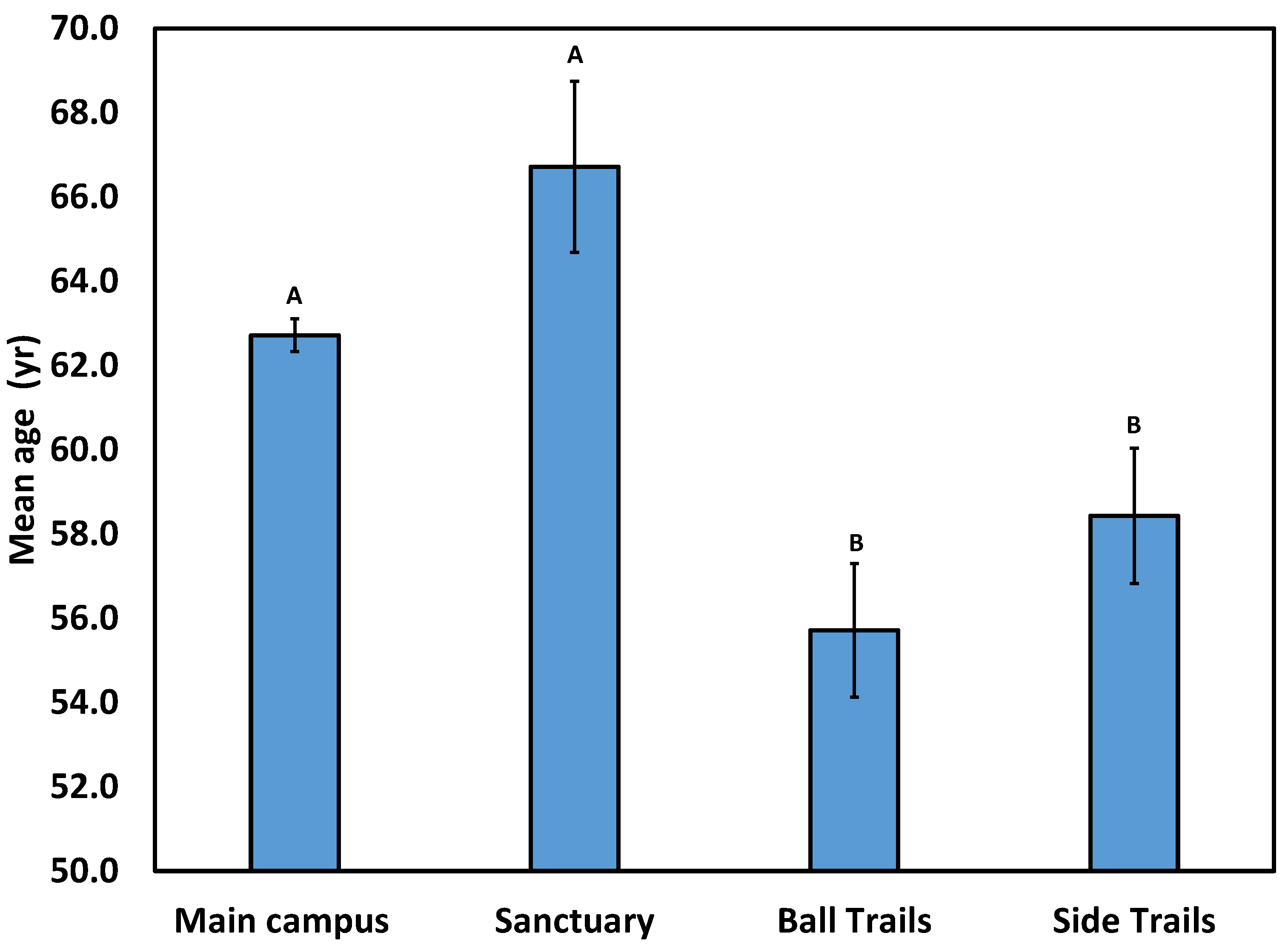
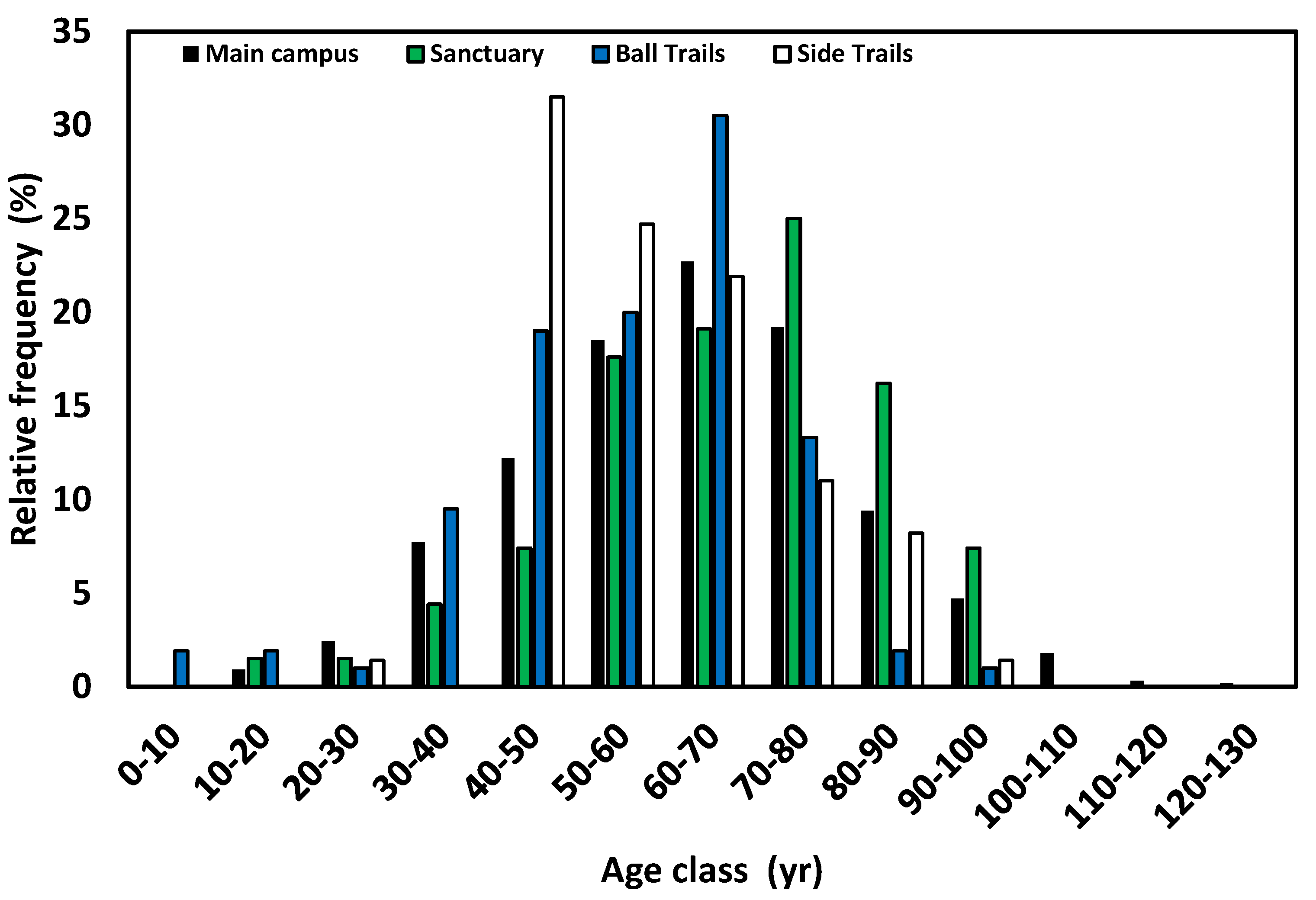
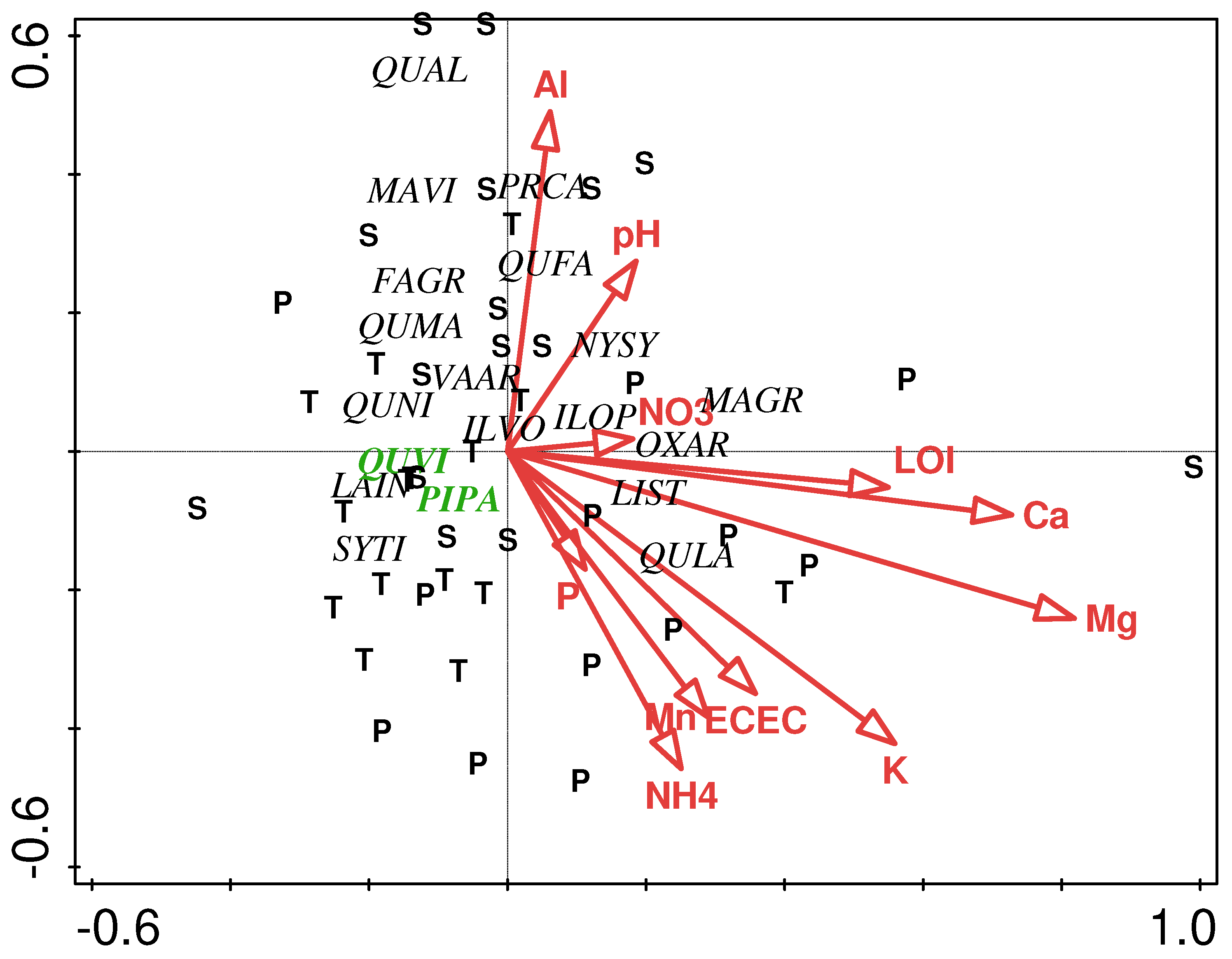
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Platt, W.J. Southeastern pine savannas. In The Savanna, Barren, and Rock Outcrop Communities of North America. Anderson, R.C.; Fralish, J.S.; Baskin, J., Eds.; Cambridge University Press, Cambridge, England, 1999; pp. 23–51.
- Zampieri, N.E. , Pau, S., and Okamoto, D.K. The impact of Hurricane Michael on longleaf pine habitats in Florida. Sci. Rep.-UK, 2020, 10, 8483. [Google Scholar] [CrossRef] [PubMed]
- Gilliam, F.S. Impacts of tropical cyclones on longleaf pine ecosystems of Florida: tropical cyclogenesis, landfall frequencies, and climate change. Front. Ecol. Evol., 2021, 9, 595791. [Google Scholar] [CrossRef]
- Zampieri, N.E.; Pau, S. The effects of fire, climate, and species composition on longleaf pine stand structure and growth rates across diverse natural communities in Florida. Forest Ecology and Management, 2022, 526, 120568. [Google Scholar] [CrossRef]
- Gilliam, F.S.; Platt, W.J. , Peet, R.K. Natural disturbances and the physiognomy of pine savannas: a phenomenological model. Appl. Veg. Sci., 2006, 9, 83–96. [Google Scholar] [CrossRef]
- Platt, W.J.; Carr, S.M.; Reilly, M.; Fahr, J. Pine savanna overstorey influences on ground-cover biodiversity. Appl. Veg. Sci., 2006, 9, 37–50. [Google Scholar] [CrossRef]
- Gilliam, F.S. Introduction: the herbaceous layer—the forest between the trees. In The Herbaceous Layer in Forests of North America. Gilliam, F.S., Ed.; Oxford University Press, Oxford, England, 2014.; pp. 1-9.
- Gilliam, F.S.; Platt, W.J. Conservation and restoration of the longleaf pine ecosystem. Appl. Veg. Sci., 2006, 9, 7–10. [Google Scholar] [CrossRef]
- Francos, M.; Stefanuto, E.B.; Úbeda, X.; Pereira, P. Long-term impact of prescribed fire on soil chemical properties in a wildland-urban interface. Northeastern Iberian Peninsula. Sci. Tot. Environ., 2019, 689, 305–311. [Google Scholar] [CrossRef]
- Barrington-Leigh, C.; Millard-Ball, A. A century of sprawl in the United States. PNAS, 2015, 112, 8244–8249. [Google Scholar] [CrossRef]
- Turner, P.V. Campus: an American planning tradition. MIT Press, Cambridge, Massachusetts, 1984.
- Roman, L.A.; Fristensky, J.P.; Eisenman, T.S.; Greenfield, E.J.; Lundgren, R.E.; Cerwinka, C.E.; Hewitt, D.A.; Welsh, C.C. Growing canopy on a College campus: understanding urban forest change through archival records and aerial photography. Environ. Manage., 2017, 60, 1042–1061. [Google Scholar] [CrossRef]
- Knight, G.R.; Oetting, J.B.; Cross, L. Atlas of Florida’s natural heritage—biodiversity, landscapes, stewardship, and opportunities. Institute of Science and Public Affairs, Florida State University, Tallahassee, FL, USA, 2011.
- Jarvis, J.E. The Next Larger Picture: an Anecdotal History of the Master Planning of the Campus of the University of West Florida and the Design and Construction of its Buildings for the Initial Thirty-Something Years; a Memoir, of Sorts. Pioneer Series/University of West Florida Foundation, Inc, Pensacola, FL, USA, 2008.
- Daubenmire, R. The Magnolia grandiflora-Quercus virginiana forest of Florida. Am. Midl. Nat., 1990, 123, 331–347. [Google Scholar] [CrossRef]
- Marse, C. Rising Fiery Constellation: a Portrait of the University of West Florida from Birth to Forty. Pioneer Series, Pensacola, FL, USA, 2007.
- Cole, K.; Bennington, C. From the ground up: natural history education in an urban campus restoration. Southeast. Nat., 2017, 16, 132–145. [Google Scholar] [CrossRef]
- Copenheaver, C.A.; Seiler, J.R.; Peterson, J.A.; Evans, A.M.; McVay, J.L.; White, J.H. Stadium woods: a dendroecological analysis of an old-growth forest fragment on a university campus. Dendrochronologia, 2014, 32, 62–70. [Google Scholar] [CrossRef]
- Gilliam, F.S.; Harmon, E.E.; Boyles, S.C. The University of West Florida campus ecosystem study: gopher tortoise and longleaf pine populations in an urban interface. Urban Ecosyst., 2020, 23, 355–362. [Google Scholar] [CrossRef]
- Gilliam, F.S.; Detzel, S.J.; Bray, K.D.; Major, E.A. The University of West Florida campus ecosystem study: the college/university campus as a unit for study of the ecology of longleaf pine. Urban Ecosyst., 2021, 24, 1073–1082. [Google Scholar] [CrossRef]
- Gilliam, F.S.; Patten, H.N.; Rabinowitz, S.K. The University of West Florida campus ecosystem study: age-diameter and growth relationships of longleaf pine using hurricane-induced windthrows. Urban Ecosyst., 2022, 25, 839–848. [Google Scholar] [CrossRef]
- Gilliam, F.S.; Hargis, E.A.; Rabinowitz, S.K.; Davis, B.C.; Sweet, L.L.; Moss, J.A. Soil microbiomes of hardwood- versus pine-dominated stands: linkage with overstory species. Ecosphere, 2023; in press. [Google Scholar]
- Gilliam, F.S.; Hardin, J.W.; Williams, J.A; Lackaye, R.L. The University of West Florida Campus Ecosystem Study: spatial and temporal variation in water quality of Thompson Bayou. Water, 2022, 14, 2916. [Google Scholar] [CrossRef]
- Addington, R.N.; Knapp, B.O.; Sorrell, G.G.; Elmore, M.L.; Wang, G.G.; Walker, J.L. Factors affecting broadleaf woody vegetation in upland pine forests managed for longleaf pine restoration. For. Ecol. Manag., 2015, 354, 130–138. [Google Scholar] [CrossRef]
- Gilliam, F.S.; Platt, W.J. Effects of long-term fire exclusion on tree species composition and stand structure in an old-growth longleaf pine forest. Plant Ecol., 1999, 140, 15–26. [Google Scholar] [CrossRef]
- Hiers, J.K.; Walters, J.R.; Mitchel, R.J.; Varner, J.M.; Conner, L.M.; Blanc, L.A.; Stowe, J. Ecological value of retaining pyrophytic oaks in longleaf pine ecosystems. J. Wildlife. Manage., 2014, 78, 383–393. [Google Scholar] [CrossRef]
- Provencher, L.; Litt, A.R.; Gordon, D.R. Predictors of species richness in Northwest Florida longleaf pine sandhills. Conserv. Biol., 2003, 17, 1660–1671. [Google Scholar] [CrossRef]
- Varner, J.M.; Gordon, D.R.; Putz, F.E.; Hiers, J.K. Restoring fire to long-unburned Pinus palustris ecosystems: novel fire effects and consequences for long-unburned ecosystems. Restor. Ecol., 2005, 13, 536–544. [Google Scholar] [CrossRef]
- Platt, W.J.; Evans, G.W.; Rathbun, S.L. The population dynamics of a long-lived conifer (Pinus palustris). Am. Nat., 1988, 131, 491–525. [Google Scholar] [CrossRef]
- Noel, J.M.; Platt, W.J.; Moser, E.B. Characteristics of old and second growth stands of longleaf pine (Pinus palustris) in the Gulf coastal region of the U.S.A. Cons. Biol., 1998, 12, 533–548. [Google Scholar]
- Hine, A.C. Geologic History of Florida: Major Events That Formed the Sunshine State. University Press of Florida, Gainesville, FL, USA, 2013.
- USDA. 2004. Soil survey of Escambia County, Florida. United States Department of Agriculture, Natural Resources Conservation Service, Washington, DC, USA.
- Zar, J.H. Biostatistical Analysis, 5th ed.; Prentice-Hall: Upper Saddle River, NJ, USA, 2009. [Google Scholar]
- Šmilauer, P.; Lepš, J. Multivariate Analysis of Ecological Data Using CANOCO 5, 2nd ed. Cambridge University Press: Cambridge, England, 2014.
- Barbour, M.G.; Burk, J.H; Pitts, W.D.; Gilliam, F.S.; Schwartz, M.W. Terrestrial plant ecology, 3rd Edition. Benjamin/Cummings Publishing Company, Inc.: Menlo Park, CA, USA, 1999.
- Gilliam, F.S.; Saunders, N.E. Making more sense of the order: A review of Canoco for Windows 4.5, PC-ORD version 4 and SYN-TAX 2000. J. Veg. Sci., 2003, 14, 297–304. [Google Scholar] [CrossRef]
- Moore, L.D.; Van Stan II, J.T.; Gay, T.E.; Rosier, C.; Wu, T. Alteration of soil chitinolytic bacterial and ammonia oxidizing archaeal community diversity by rainwater redistribution in an epiphyte-laden Quercus virginiana canopy. Soil Biol. Biochem., 2016, 100, 33–41. [Google Scholar] [CrossRef]
- Muller, R.N. Nutrient relations of the herbaceous layer in deciduous forest ecosystems. In The Herbaceous Layer in Forests of North America. Gilliam, F.S., Ed.; Oxford University Press, Oxford, England, 2014.; pp. 13-34.
- Marschner, H . Mineral Nutrition of Higher Plants, 3rd edn. Academic Press, London, England, 2012.
- Schroth, A.W.; Friedland, A.J.; Bostick, B.C. Macronutrient depletion and redistribution in soils under conifer and northern hardwood forests. Can. J. For. Res., 2007, 71, 457–468. [Google Scholar] [CrossRef]
- West, J.B.; Espeleta, J.F.; Donovan, L.A. Fine root production and turnover across a complex edaphic gradient of a Pinus palustris—Aristida stricta savanna ecosystem. For. Ecol. Manag., 2004, 189, 397–405. [Google Scholar] [CrossRef]
- Cipollini, M.; Felch, P.; Dingley, N.R.; Maddox, C. Changes in tree canopy, understory vegetation, and fuel composition after 10 years of restoration management in an old-growth mountain longleaf pine forest. Nat. Areas J., 2019, 39, 197–211. [Google Scholar] [CrossRef]
- Cipollini, M.L.; Culberson, J.; Stripplehoff, C.; Baldvins, T.; Miller, K. Herbaceous plants and grasses in a mountain longleaf pine forest undergoing restoration: a survey and comparative study. Southeast. Nat., 2012, 11, 637–668. [Google Scholar] [CrossRef]
| Status | pH | OM | NO3-N | NH4-N | Ca | K | Mg | CEC | P | Al |
| % | µg/g | µg/g | µg/g | µg/g | µg/g | meq/100 g | µg/g | µg/g | ||
| Pre (n=32) | 5.37±0.08 | 2.09±0.13 | 0.23±0.05 | 2.60±0.43 | 175.2±36.1 | 16.8±1.9 | 22.0±2.5 | 1.51±0.17 | 0.37±0.03 | 52.0±3.8 |
| **** | * | NS | * | **** | **** | **** | NS | ** | **** | |
| Post (n=42) | 4.67±0.03 | 1.66±0.10 | 0.30±0.02 | 3.77±0.25 | 26.9±4.1 | 8.2±0.5 | 6.5±0.5 | 1.47±0.10 | 0.49±0.01 | 113.3±3.5 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





