Submitted:
02 May 2023
Posted:
03 May 2023
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Materials and Methods
2.1. Study area and experimental design
2.2. Experimental design
2.3. Environmental variables
2.4. Ant survey
2.5. Survey of other arthropods
2.6. Statistical analysis
3. Results
3.1. Species richness and evenness
3.2. Species composition
3.2.1. Ordination
3.3. Beta diversity
3.4. Indicator species
4. Discussion
4.1. Ant community responses to disturbance
4.2. Indicator species
4.3. Prey, predators, and outbreaks
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Aakala, T.; Remy, C.C.; Arseneault, D.; Morin, H.; Girardin, M.P.; Gennaretti, F.; Navarro, L.; Kuosmanen, N.; Ali, A.A.; Boucher, É.; et al. Millennial-Scale Disturbance History of the Boreal Zone. In Boreal Forests in the Face of Climate Change: Sustainable Management; Girona, M.M., Morin, H., Gauthier, S., Bergeron, Y., Eds.; Advances in Global Change Research; Springer International Publishing: Cham, 2023; pp. 53–87. ISBN 978-3-031-15988-6. [Google Scholar]
- Montoro Girona, M.; Navarro, L.; Morin, H. A Secret Hidden in the Sediments: Lepidoptera Scales. Front. Ecol. Evol. 2018, 6, 2. [Google Scholar] [CrossRef]
- Navarro, L.; Morin, H.; Bergeron, Y.; Girona, M.M. Changes in Spatiotemporal Patterns of 20th Century Spruce Budworm Outbreaks in Eastern Canadian Boreal Forests. Frontiers in Plant Science 2018, 9, 1905. [Google Scholar] [CrossRef] [PubMed]
- Lavoie, J.; Montoro Girona, M.; Grosbois, G.; Morin, H. Does the Type of Silvicultural Practice Influence Spruce Budworm Defoliation of Seedlings? Ecosphere 2021, 12, e03506. [Google Scholar] [CrossRef]
- De Grandpré, L.; Marchand, M.; Kneeshaw, D.D.; Paré, D.; Boucher, D.; Bourassa, S.; Gervais, D.; Simard, M.; Griffin, J.M.; Pureswaran, D.S. Defoliation-Induced Changes in Foliage Quality May Trigger Broad-Scale Insect Outbreaks. Commun Biol 2022, 5, 463. [Google Scholar] [CrossRef] [PubMed]
- Swank, W.T.; Waide, J.B.; Crossley, D.A.; Todd, R.L. Insect Defoliation Enhances Nitrate Export from Forest Ecosystems. Oecologia 1981, 51, 297–299. [Google Scholar] [CrossRef] [PubMed]
- Hunter, M.D. Insect Population Dynamics Meets Ecosystem Ecology: Effects of Herbivory on Soil Nutrient Dynamics. Agric Forest Ent 2001, 3, 77–84. [Google Scholar] [CrossRef]
- Metcalfe, D.B.; Asner, G.P.; Martin, R.E.; Silva Espejo, J.E.; Huasco, W.H.; Farfán Amézquita, F.F.; Carranza-Jimenez, L.; Galiano Cabrera, D.F.; Baca, L.D.; Sinca, F.; et al. Herbivory Makes Major Contributions to Ecosystem Carbon and Nutrient Cycling in Tropical Forests. Ecol Lett 2014, 17, 324–332. [Google Scholar] [CrossRef]
- Metcalfe, D.B.; Crutsinger, G.M.; Kumordzi, B.B.; Wardle, D.A. Nutrient Fluxes from Insect Herbivory Increase during Ecosystem Retrogression in Boreal Forest. Ecology 2016, 97, 124–132. [Google Scholar] [CrossRef]
- Lovett, G.M.; Christenson, L.M.; Groffman, P.M.; Jones, C.G.; Hart, J.E.; Mitchell, M.J. Insect Defoliation and Nitrogen Cycling in Forests. BioScience 2002, 52, 335. [Google Scholar] [CrossRef]
- Gravel, D.; Albouy, C.; Thuiller, W. The Meaning of Functional Trait Composition of Food Webs for Ecosystem Functioning. Philosophical Transactions of the Royal Society B: Biological Sciences 2016, 371, 20150268. [Google Scholar] [CrossRef]
- Barnes, A.D.; Jochum, M.; Lefcheck, J.S.; Eisenhauer, N.; Scherber, C.; O’Connor, M.I.; de Ruiter, P.; Brose, U. Energy Flux: The Link between Multitrophic Biodiversity and Ecosystem Functioning. Trends in ecology & evolution 2018, 33, 186–197. [Google Scholar] [CrossRef]
- Kristensen, J.A.; Metcalfe, D.B.; Rousk, J. The Biogeochemical Consequences of Litter Transformation by Insect Herbivory in the Subarctic: A Microcosm Simulation Experiment. Biogeochemistry 2018, 138, 323–336. [Google Scholar] [CrossRef]
- Lovett, G.M.; Ruesink, A.E. Carbon and Nitrogen Mineralization from Decomposing Gypsy Moth Frass. Oecologia 1995, 104, 133–138. [Google Scholar] [CrossRef] [PubMed]
- Debaly, Z.M.; Marchand, P.; Girona, M.M. Autoregressive Models for Time Series of Random Sums of Positive Variables: Application to Tree Growth as a Function of Climate and Insect Outbreak. Ecological Modelling 2022, 471, 110053. [Google Scholar] [CrossRef]
- Calderón-Sanou, I.; Münkemüller, T.; Zinger, L.; Schimann, H.; Yoccoz, N.G.; Gielly, L.; Foulquier, A.; Hedde, M.; Ohlmann, M.; Roy, M.; et al. Cascading Effects of Moth Outbreaks on Subarctic Soil Food Webs. Sci Rep 2021, 11, 15054. [Google Scholar] [CrossRef] [PubMed]
- Bardgett, R.D.; Caruso, T. Soil Microbial Community Responses to Climate Extremes: Resistance, Resilience and Transitions to Alternative States. Philosophical Transactions of the Royal Society B 2020, 375, 20190112. [Google Scholar] [CrossRef] [PubMed]
- De Ruiter, P.C.; Neutel, A.-M.; Moore, J.C. Energetics, Patterns of Interaction Strengths, and Stability in Real Ecosystems. Science 1995, 269, 1257–1260. [Google Scholar] [CrossRef] [PubMed]
- Estes, J.A.; Terborgh, J.; Brashares, J.S.; Power, M.E.; Berger, J.; Bond, W.J.; Carpenter, S.R.; Essington, T.E.; Holt, R.D.; Jackson, J.B. Trophic Downgrading of Planet Earth. science 2011, 333, 301–306. [Google Scholar] [CrossRef]
- Saravesi, K.; Aikio, S.; Wäli, P.R.; Ruotsalainen, A.L.; Kaukonen, M.; Huusko, K.; Suokas, M.; Brown, S.P.; Jumpponen, A.; Tuomi, J. Moth Outbreaks Alter Root-Associated Fungal Communities in Subarctic Mountain Birch Forests. Microbial Ecology 2015, 69, 788–797. [Google Scholar] [CrossRef]
- Vindstad, O.P.L.; Schultze, S.; Jepsen, J.U.; Biuw, M.; Kapari, L.; Sverdrup-Thygeson, A.; Ims, R.A. Numerical Responses of Saproxylic Beetles to Rapid Increases in Dead Wood Availability following Geometrid Moth Outbreaks in Sub-Arctic Mountain Birch Forest. PLoS ONE 2014, 9, e99624. [Google Scholar] [CrossRef]
- Sandén, H.; Mayer, M.; Stark, S.; Sandén, T.; Nilsson, L.O.; Jepsen, J.U.; Wäli, P.R.; Rewald, B. Moth Outbreaks Reduce Decomposition in Subarctic Forest Soils. Ecosystems 2020, 23, 151–163. [Google Scholar] [CrossRef]
- Jurgensen, M.F.; Finér, L.; Domisch, T.; Kilpeläinen, J.; Punttila, P.; Ohashi, M.; Niemelä, P.; Sundström, L.; Neuvonen, S.; Risch, A.C. Organic Mound-Building Ants: Their Impact on Soil Properties in Temperate and Boreal Forests. Journal of Applied Entomology 2008, 132, 266–275. [Google Scholar] [CrossRef]
- Risch, A.C.; Jurgensen, M.F. Ants in the Soil System-a Hydrological, Chemical and Biological Approach. Journal of Applied Entomology 2008, 132, 265. [Google Scholar] [CrossRef]
- Domisch, T.; Finér, L.; Neuvonen, S.; Niemelä, P.; Risch, A.C.; Kilpeläinen, J.; Ohashi, M.; Jurgensen, M.F. Foraging Activity and Dietary Spectrum of Wood Ants ( Formica Rufa Group) and Their Role in Nutrient Fluxes in Boreal Forests. Ecological Entomology 2009, 34, 369–377. [Google Scholar] [CrossRef]
- Del Toro, I.; Ribbons, R.R.; Pelini, S.L. The Little Things That Run the World Revisited: A Review of Ant-Mediated Ecosystem Services and Disservices (Hymenoptera: Formicidae). Myrmecological News 2012, 17, 133–146. [Google Scholar]
- Folgarait, P.J. Ant Biodiversity and Its Relationship to Ecosystem Functioning: A Review. Biodiversity & Conservation 1998, 7, 1221–1244. [Google Scholar] [CrossRef]
- Hölldobler, B.; Wilson, E.O. The Ants; Harvard University Press: Cambridge, MA, USA, 1990. [Google Scholar]
- Wardle, D.A.; Hyodo, F.; Bardgett, R.D.; Yeates, G.W.; Nilsson, M.-C. Long-Term Aboveground and Belowground Consequences of Red Wood Ant Exclusion in Boreal Forest. Ecology 2011, 92, 645–656. [Google Scholar] [CrossRef] [PubMed]
- Laine, K.J.; Niemelä, P. The Influence of Ants on the Survival of Mountain Birches during an Oporinia Autumnata (Lep., Geometridae) Outbreak. Oecologia 1980, 47, 39–42. [Google Scholar] [CrossRef]
- Punttila, P.; Niemelä, P.; Karhu, K. The Impact of Wood Ants (Hymenoptera: Formicidae) on the Structure of Invertebrate Community on Mountain Birch (Betula Pubescens Ssp. Czerepanovii). In Proceedings of the Annales Zoologici Fennici; JSTOR, 2004; pp. 429–446. [Google Scholar]
- Finér, L.; Jurgensen, M.F.; Domisch, T.; Kilpeläinen, J.; Neuvonen, S.; Punttila, P.; Risch, A.C.; Ohashi, M.; Niemelä, P. The Role of Wood Ants (Formica Rufa Group) in Carbon and Nutrient Dynamics of a Boreal Norway Spruce Forest Ecosystem. Ecosystems 2013, 16, 196–208. [Google Scholar] [CrossRef]
- Andersen, A.N. Responses of Ant Communities to Disturbance: Five Principles for Understanding the Disturbance Dynamics of a Globally Dominant Faunal Group. J Anim Ecol 2019, 88, 350–362. [Google Scholar] [CrossRef]
- Kaukonen, M.; Ruotsalainen, A.L.; Wäli, P.R.; Männistö, M.K.; Setälä, H.; Saravesi, K.; Huusko, K.; Markkola, A. Moth Herbivory Enhances Resource Turnover in Subarctic Mountain Birch Forests? Ecology 2013, 94, 267–272. [Google Scholar] [CrossRef] [PubMed]
- Mikola, J.; Yeates, G.W.; Barker, G.M.; Wardle, D.A.; Bonner, K.I. Effects of Defoliation Intensity on Soil Food-Web Properties in an Experimental Grassland Community. Oikos 2001, 92, 333–343. [Google Scholar] [CrossRef]
- Pitman, R.M.; Vanguelova, E.I.; Benham, S.E. The Effects of Phytophagous Insects on Water and Soil Nutrient Concentrations and Fluxes through Forest Stands of the Level II Monitoring Network in the UK. Science of the total environment 2010, 409, 169–181. [Google Scholar] [CrossRef] [PubMed]
- Chapman, S.K.; Hart, S.C.; Cobb, N.S.; Whitham, T.G.; Koch, G.W. Insect Herbivory Increases Litter Quality and Decomposition: An Extension of the Acceleration Hypothesis. Ecology 2003, 84, 2867–2876. [Google Scholar] [CrossRef]
- Moya-Laraño, J.; Wise, D.H. Direct and Indirect Effects of Ants on a Forest-Floor Food Web. Ecology 2007, 88, 1454–1465. [Google Scholar] [CrossRef] [PubMed]
- Çakır, M. The Negative Effect of Wood Ants (Formica Rufa) on Microarthropod Density and Soil Biological Quality in a Semi-Arid Pine Forest. Pedobiologia 2019, 77, 150593. [Google Scholar] [CrossRef]
- Karhu, K.J.; Neuvonen, S. Wood Ants and a Geometrid Defoliator of Birch: Predation Outweighs Beneficial Effects through the Host Plant. Oecologia 1998, 113, 509–516. [Google Scholar] [CrossRef] [PubMed]
- Way, M.J.; Khoo, K.C. Role of Ants in Pest Management. Annu. Rev. Èntomol. 1992, 37, 479–503. [Google Scholar] [CrossRef]
- Tilman, D. Cherries, Ants and Tent Caterpillars: Timing of Nectar Production in Relation to Susceptibility of Caterpillars to Ant Predation. Ecology 1978, 59, 686–692. [Google Scholar] [CrossRef]
- Gösswald, K Die Waldameise. Band 2. Die Waldameise in Okosystem Wald, Ihr Nutzen Und Ihre Hege. Wiesbaden: Aula-Verlag. 1990, 510 S.
- Carvalho, K.S.; Vasconcelos, H.L. Forest Fragmentation in Central Amazonia and Its Effects on Litter-Dwelling Ants. Biological Conservation 1999, 91, 151–157. [Google Scholar] [CrossRef]
- Maeto, K.; Sato, S. Impacts of Forestry on Ant Species Richness and Composition in Warm-Temperate Forests of Japan. Forest Ecology and Management 2004, 187, 213–223. [Google Scholar] [CrossRef]
- Palladini, J.D.; Jones, M.G.; Sanders, N.J.; Jules, E.S. The Recovery of Ant Communities in Regenerating Temperate Conifer Forests. Forest Ecology and Management 2007, 242, 619–624. [Google Scholar] [CrossRef]
- Ewers, R.M.; Boyle, M.J.; Gleave, R.A.; Plowman, N.S.; Benedick, S.; Bernard, H.; Bishop, T.R.; Bakhtiar, E.Y.; Chey, V.K.; Chung, A.Y. Logging Cuts the Functional Importance of Invertebrates in Tropical Rainforest. Nature communications 2015, 6, 1–7. [Google Scholar] [CrossRef]
- Punttila, P.; Haila, Y.; Niemelä, J.; Pajunen, T. Ant Communities in Fragments of Old-Growth Taiga and Managed Surroundings. In Proceedings of the Annales Zoologici Fennici; JSTOR; 1994; pp. 131–144. [Google Scholar]
- Véle, A.; Holuša, J.; Horák, J. Ant Abundance Increases with Clearing Size. Journal of forest research 2016, 21, 110–114. [Google Scholar] [CrossRef]
- Grevé, M.E.; Hager, J.; Weisser, W.W.; Schall, P.; Gossner, M.M.; Feldhaar, H. Effect of Forest Management on Temperate Ant Communities. Ecosphere 2018, 9, e02303. [Google Scholar] [CrossRef]
- Yamamoto, S.-I. Forest Gap Dynamics and Tree Regeneration. Journal of forest research 2000, 5, 223–229. [Google Scholar] [CrossRef]
- Punttila, P.; Haila, Y.; Pajunen, T.; Tukia, H. Colonisation of Clearcut Forests by Ants in the Southern Finnish Taiga: A Quantitative Survey. Oikos 1991, 61, 250–262. [Google Scholar] [CrossRef]
- Schall, P.; Gossner, M.M.; Heinrichs, S.; Fischer, M.; Boch, S.; Prati, D.; Jung, K.; Baumgartner, V.; Blaser, S.; Böhm, S. The Impact of Even-Aged and Uneven-Aged Forest Management on Regional Biodiversity of Multiple Taxa in European Beech Forests. Journal of applied Ecology 2018, 55, 267–278. [Google Scholar] [CrossRef]
- Fitzgerald, T. The Tent Caterpillars; Cornell University Press: Ithaca, NY, USA, 1995. [Google Scholar]
- Moulinier, J. Impacts de La Défoliation Par La Livrée Des Forêts Sur La Mortalité Du Peuplier Faux-Tremble et La Dynamique Forestière Post-Épidémie En Forêt Boréale. 2013.
- Lach, L.; Parr, C.; Abbott, K. Ant Ecology; Oxford university press, 2010.
- Montllor, C.B.; Bernays, E.A. Invertebrate Predators and Caterpillar Foraging. 1993.
- Piñol, J.; Espadaler, X.; Cañellas, N.; MARTÍNEZ-VILALTA, J.; Barrientos, J.A.; Sol, D. Ant versus Bird Exclusion Effects on the Arthropod Assemblage of an Organic Citrus Grove. Ecological Entomology 2010, 35, 367–376. [Google Scholar] [CrossRef]
- Clark, R.E.; Farkas, T.E.; Lichter-Marck, I.; Johnson, E.R.; Singer, M.S. Multiple Interaction Types Determine the Impact of Ant Predation of Caterpillars in a Forest Community. Ecology 2016, 97, 3379–3388. [Google Scholar] [CrossRef]
- Green, G.; Sullivan, C. Ants Attacking Larvae of the Forest Tent Caterpillar, Malacosoma Disstria Hbn. (Lepidoptera: Lasiocampidae). The Canadian Entomologist 1950, 82, 94–195. [Google Scholar] [CrossRef]
- Despland, E.; Lessard, J.-P. Social Predation by Ants as a Mortality Source for an Arboreal Gregarious Forest Pest. Basic and Applied Ecology 2022, 59, 82–91. [Google Scholar] [CrossRef]
- Rosumek, F.B.; Silveira, F.A.O.; de, S. Neves, F.; de U. Barbosa, N.P.; Diniz, L.; Oki, Y.; Pezzini, F.; Fernandes, G.W.; Cornelissen, T. Ants on Plants: A Meta-Analysis of the Role of Ants as Plant Biotic Defenses. Oecologia 2009, 160, 537–549. [Google Scholar] [CrossRef]
- Romero, G.Q.; Antiqueira, P.A.; Koricheva, J. A Meta-Analysis of Predation Risk Effects on Pollinator Behaviour. PLoS ONE 2011, 6, e20689. [Google Scholar] [CrossRef]
- Moreira, X.; Mooney, K.A.; Zas, R.; Sampedro, L. Bottom-up Effects of Host-Plant Species Diversity and Top-down Effects of Ants Interactively Increase Plant Performance. Proceedings of the Royal Society B: Biological Sciences 2012, 279, 4464–4472. [Google Scholar] [CrossRef] [PubMed]
- Cerdá, X.; Dejean, A. 3. Predation by Ants on Arthropods and Other Animals. In Predation in the hymenoptera: An evolutionary perspective; Transworld Research Network: 2011.
- MFFP Aires infestées par la livrée des forêts au Québec en 2016; Gouvernement du Québec, Direction de la protection des forêts: Québec, 2016; p. 10.
- MFFP Aires infestées par la livrée des forêts au Québec en 2017; Gouvernement du Québec, Direction de la protection des forêts: Québec, 2017; p. 14.
- Bergeron, Y. Species and Stand Dynamics in the Mixed Woods of Quebec’s Southern Boreal Forest. Ecology 2000, 81, 1500–1516. [Google Scholar] [CrossRef]
- Canada, A. and A.-F. The Canadian System of Soil Classification (Second Edition). Available online: https://sis.agr.gc.ca/cansis/publications/manuals/1987-cssc-ed2/index.html (accessed on 3 November 2022).
- Vincent, J.-S.; Hardy, L. L’évolution et l’extension Des Lacs Glaciaires Barlow et Ojibway En Territoire Québécois. Géographie physique et Quaternaire 1977, 31, 357–372. [Google Scholar] [CrossRef]
- MFFP Aires infestées par la livrée des forêts au Québec en 2015; Gouvernement du Québec, Direction de la protection des forêts: Québec, 2015; p. 8.
- MFFP Aires infestées par la livrée des forêts au Québec en 2018; Gouvernement du Québec, Direction de la protection des forêts: Québec, 2019; p. 14.
- Environment Canada National Climate Data and Information Archive; Environment Canada: Ottawa, On tario, Canada, 2017.
- Lajoie, P.G. Étude Pédologique Des Comtés de Hull, Labelle et Papineau. Québec)(Ottawa: Ministère I’agriculture) 1967.
- Caron, A.; Jarry, J.J.; Despland, E. Early Instar Mortality of a Forest Pest Caterpillar: Which Mortality Sources Increase during an Outbreak Crash? Entomologia Exp Applicata 2022, 170, 268–276. [Google Scholar] [CrossRef]
- Bestelmeyer, B.T.; Agosti, D.; Alonso, L.E.; Brandão, C.R.F.; Brown, W.L.; Delabie, J.H.; Silvestre, R. Field Techniques for the Study of Ground-Dwelling Ant: An Overview, Description, and Evaluation. In Ants: Standard methods for measuring and monitoring biodiversity; 2000.
- Francœur, A. Les fourmis de la forêt boréale du Québec (Formicidae, Hymenoptera). Le Naturaliste Canadien 2001, 125, 8. [Google Scholar]
- Ellison, A.M.; Gotelli, N.J.; Farnsworth, E.J.; Alpert, G.D. A Field Guide to the Ants of New England; Yale University Press, 2012; ISBN 978-0-300-16930-0. [Google Scholar]
- Hopkin, S.P. A Key to the Collembola (Springtails) of Britain and Ireland.; FSC publications, 2007.
- Fjellberg, A. The Collembola of Fennoscandia and Denmark. Part 1: Poduromorpha Fauna. Entomologica Scandinavica. Brill Academic, Leiden 1998.
- Fjellberg, A. The Collembola of Fennoscandia and Denmark, Part II: Entomobryomorpha and Symphypleona; Brill, 2007.
- Christiansen, K.; Bellinger, P. The Collembola of North America North of the Rio Grande. A Taxonomic Analysis. Part 1. Introduction. General. Families Poduridae and Hypogastruridae. Part 2. Families Onychiuridae and Isotomidae. Part 3. Family Entomobrydae. Part 4. Families Neelidae and Sminthuridae. Glossary. Bibliography. Index. The Collembola of North America north of the Rio Grande. A taxonomic analysis. Part 1. Introduction. General. Families Poduridae and Hypogastruridae. Part 2. Families Onychiuridae and Isotomidae. Part 3. Family Entomobrydae. Part 4. Families Neelidae and Sminthuridae. Glossary. Bibliography. Index. 1980.
- Sorensen, T.A. A Method of Establishing Groups of Equal Amplitude in Plant Sociology Based on Similarity of Species Content and Its Application to Analyses of the Vegetation on Danish Commons. Biol. Skar. 1948, 5, 1–34. [Google Scholar]
- Jaccard, P. Étude Comparative de La Distribution Florale Dans Une Portion Des Alpes et Des Jura. Bull Soc Vaudoise Sci Nat 1901, 37, 547–579. [Google Scholar]
- Oksanen, J.; Blanchet, F.G.; Kindt, R.; Legendre, P.; O’hara, R.B.; Simpson, G.L.; Solymos, P.; Stevens, M.H.H.; Wagner, H. Vegan: Community Ecology Package. R Package Version 1.17-4. http://cran. r-project. org>. Acesso em 2010, 23, 2010. [Google Scholar]
- Dufrêne, M.; Legendre, P. Species Assemblages and Indicator Species: The Need for a Flexible Asymmetrical Approach. Ecological monographs 1997, 67, 345–366. [Google Scholar] [CrossRef]
- De Caceres, M.; Jansen, F. Package ‘Indicspecies. ’ indicators 2016, 8. [Google Scholar]
- R Development Core, T. A Language and Environment for Statistical Computing; The R Foundation for Statistical Computing, Department of Agronomy, Faculty …, 2017.
- Broza, M.; Izhaki, I. Post-Fire Arthropod Assemblages in Mediterranean Forest Soils in Israel. International Journal of Wildland Fire 1997, 7, 317–325. [Google Scholar] [CrossRef]
- Gardner, S.M.; Cabido, M.R.; Valladares, G.R.; Diaz, S. The Influence of Habitat Structure on Arthropod Diversity in Argentine Semi-Arid Chaco Forest. Journal of Vegetation Science 1995, 6, 349–356. [Google Scholar] [CrossRef]
- Bardgett, R.D.; Van Der Putten, W.H. Belowground Biodiversity and Ecosystem Functioning. Nature 2014, 515, 505–511. [Google Scholar] [CrossRef]
- Bardgett, R.D.; Bowman, W.D.; Kaufmann, R.; Schmidt, S.K. A Temporal Approach to Linking Aboveground and Belowground Ecology. Trends in ecology & evolution 2005, 20, 634–641. [Google Scholar] [CrossRef]
- Hedlund, K.; Griffiths, B.; Christensen, S.; Scheu, S.; Setälä, H.; Tscharntke, T.; Verhoef, H. Trophic Interactions in Changing Landscapes: Responses of Soil Food Webs. Basic and Applied Ecology 2004, 5, 495–503. [Google Scholar] [CrossRef]
- Anderson, K.E.; Russell, J.A.; Moreau, C.S.; Kautz, S.; Sullam, K.E.; Hu, Y.I.; Basinger, U.; Mott, B.M.; Buck, N.; Wheeler, D.E. Highly Similar Microbial Communities Are Shared among Related and Trophically Similar Ant Species. Molecular Ecology 2012, 21, 2282–2296. [Google Scholar] [CrossRef]
- Souza, R.F.; Anjos, D.V.; Carvalho, R.; Del-Claro, K. Availability of Food and Nesting-Sites as Regulatory Mechanisms for the Recovery of Ant Diversity after Fire Disturbance. Sociobiology 2015, 62, 1–9. [Google Scholar] [CrossRef]
- Frizzo, T.L.; Campos, R.I.; Vasconcelos, H.L. Contrasting Effects of Fire on Arboreal and Ground-Dwelling Ant Communities of a Neotropical Savanna. Biotropica 2012, 44, 254–261. [Google Scholar] [CrossRef]
- Silveira, J.M.; Louzada, J.; Barlow, J.; Andrade, R.; Mestre, L.; Solar, R.; Lacau, S.; Cochrane, M.A. A Multi-Taxa Assessment of Biodiversity Change after Single and Recurrent Wildfires in a Brazilian Amazon Forest. Biotropica 2016, 48, 170–180. [Google Scholar] [CrossRef]
- Vasconcelos, H.L.; Maravalhas, J.B.; Cornelissen, T. Effects of Fire Disturbance on Ant Abundance and Diversity: A Global Meta-Analysis. Biodiversity and Conservation 2017, 26, 177–188. [Google Scholar] [CrossRef]
- Calizza, E.; Rossi, L.; Careddu, G.; Sporta Caputi, S.; Costantini, M.L. Species Richness and Vulnerability to Disturbance Propagation in Real Food Webs. Sci Rep 2019, 9, 19331. [Google Scholar] [CrossRef]
- Cooke, B.J.; Sturtevant, B.R.; Robert, L.-E. The Forest Tent Caterpillar in Minnesota: Detectability, Impact, and Cycling Dynamics. Forests 2022, 13, 601. [Google Scholar] [CrossRef]
- Andersen, A.N. ; Penman; Debas; Houadria Ant Community Responses to Experimental Fire and Logging in a Eucalypt Forest of South-Eastern Australia. Forest Ecology and Management 2009, 258, 188–197. [Google Scholar] [CrossRef]
- York, A. Long-Term Effects of Frequent Low-Intensity Burning on Ant Communities in Coastal Blackbutt Forests of Southeastern Australia. Austral Ecology 2000, 25, 83–98. [Google Scholar] [CrossRef]
- Parr, C.L.; Robertson, H.G.; Biggs, H.C.; Chown, S.L. Response of African Savanna Ants to Long-Term Fire Regimes. Journal of Applied Ecology 2004, 630–642. [Google Scholar] [CrossRef]
- Maravalhas, J.; Vasconcelos, H.L. Revisiting the Pyrodiversity–Biodiversity Hypothesis: Long-Term Fire Regimes and the Structure of Ant Communities in a N Eotropical Savanna Hotspot. Journal of Applied Ecology 2014, 51, 1661–1668. [Google Scholar] [CrossRef]
- Johns, R.C.; Bowden, J.J.; Carleton, D.R.; Cooke, B.J.; Edwards, S.; Emilson, E.J.S.; James, P.M.A.; Kneeshaw, D.; MacLean, D.A.; Martel, V.; et al. A Conceptual Framework for the Spruce Budworm Early Intervention Strategy: Can Outbreaks Be Stopped? Forests 2019, 10, 910. [Google Scholar] [CrossRef]
- Lessard, J.-P.; Dunn, R.R.; Sanders, N.J. Temperature-Mediated Coexistence in Temperate Forest Ant Communities. Insect. Soc. 2009, 56, 149–156. [Google Scholar] [CrossRef]
- Despland, E.; Lessard, J.-P. Social Predation by Ants as a Mortality Source for an Arboreal Gregarious Forest Pest. Basic and Applied Ecology 2022, 59, 82–91. [Google Scholar] [CrossRef]
- Hoffmann, B.D.; Andersen, A.N. Responses of Ants to Disturbance in Australia, with Particular Reference to Functional Groups. Austral Ecology 2003, 28, 444–464. [Google Scholar] [CrossRef]
- Fotso Kuate, A.; Hanna, R.; Tindo, M.; Nanga, S.; Nagel, P. Ant Diversity in Dominant Vegetation Types of Southern Cameroon. Biotropica 2015, 47, 94–100. [Google Scholar] [CrossRef]
- Leal, I.R.; Filgueiras, B.K.C.; Gomes, J.P.; Iannuzzi, L.; Andersen, A.N. Effects of Habitat Fragmentation on Ant Richness and Functional Composition in Brazilian Atlantic Forest. Biodivers Conserv 2012, 21, 1687–1701. [Google Scholar] [CrossRef]
- Solar, R.R.d.C.; Barlow, J.; Andersen, A.N.; Schoereder, J.H.; Berenguer, E.; Ferreira, J.N.; Gardner, T.A. Biodiversity Consequences of Land-Use Change and Forest Disturbance in the Amazon: A Multi-Scale Assessment Using Ant Communities. Biological Conservation 2016, 197, 98–107. [Google Scholar] [CrossRef]
- Fairweather, A.D.; Lewis, J.H.; Hunt, L.; McAlpine, D.F.; Smith, M.A. Ants (Hymenoptera: Formicidae) of Rockwood Park, New Brunswick: An Assessment of Species Richness and Habitat. Northeastern Naturalist 2020, 27, 576. [Google Scholar] [CrossRef]
- Milford, E.R. Ant Communities in Flooded and Unflooded Riparian Forest of the Middle Rio Grande. The Southwestern Naturalist 1999, 278–286. [Google Scholar]
- Ellison, A.M.; Farnsworth, E.J.; Gotelli, N.J. Ant Diversity in Pitcher-Plant Bogs of Massachusetts. Northeastern Naturalist 2002, 9, 267–284. [Google Scholar] [CrossRef]
- Francoeur, A. Deux Nouvelles Fourmis Néarctiques: Leptothorax Retractus et L. Sphagnicolus (Formicidae, Hymenoptera). the Canadian entomologist 1986, 118, 1151–1164. [Google Scholar] [CrossRef]
- Francoeur, A. Revision Taxonomique Des Especes Nearctiques Du Groupe/Usca, Genre Formica (Formicidae, Hymenoptera). Mem. Soc. Ent. Queb. 3. 316 Pp.. 1979. Formicoidea. Canada and its Insect Fauna. Mem. ent. Soc. Can 1973, 108, 502–503. [Google Scholar]
- Francoeur, A. Extension de l’aire Connue de La Fourmi Myrmica Quebecensis (Formicidae, Hymenoptera). 2011.
- Oberg, E.; Del Toro, I.; Pelini, S. Characterization of the Thermal Tolerances of Forest Ants of New England. Insectes sociaux 2012, 59, 167–174. [Google Scholar] [CrossRef]
- Wheeler, G.C.; Wheeler, J. others The Ants of North Dakota; University of North Dakota Grand Forks, 1963.
- MacKay, W.P.; Mackay, E. The Ants of New Mexico (Hymenoptera: Formicidae); Edwin Mellen Press Lewiston, NY, 2002.
- Sirois, L. Impact of Fire on Picea Mariana and Pinus Banksiana Seedlings in Subarctic Lichen Woodlands. Journal of Vegetation Science 1993, 4, 795–802. [Google Scholar] [CrossRef]
- Rosengren, R. The Interaction between Red Wood Ants, Cinara Aphids, and Pines. A Ghost of Mutualism Past? In Ant-plant interactions; 1991; pp. 80–91.
- Parmentier, T.; Dekoninck, W.; Wenseleers, T. A Highly Diverse Microcosm in a Hostile World: A Review on the Associates of Red Wood Ants (Formica Rufa Group). Insect. Soc. 2014, 61, 229–237. [Google Scholar] [CrossRef]
- Kilpeläinen, J.; Finér, L.; Niemelä, P.; Domisch, T.; Neuvonen, S.; Ohashi, M.; Risch, A.C.; Sundström, L. Carbon, Nitrogen and Phosphorus Dynamics of Ant Mounds (Formica Rufa Group) in Managed Boreal Forests of Different Successional Stages. Applied Soil Ecology 2007, 36, 156–163. [Google Scholar] [CrossRef]
- Serttaş, A.; Bakar, Ö.; Alkan, U.M.; Yılmaz, A.; Yolcu, H.I.; Ipekdal, K. Nest Survival and Transplantation Success of Formica Rufa (Hymenoptera: Formicidae) Ants in Southern Turkey: A Predictive Approach. Forests 2020, 11, 533. [Google Scholar] [CrossRef]
- Stockan, J.A.; Rao, S.; Pakeman, R. Nesting Preferences of the Threatened Wood Ant Formica Exsecta (Hymenoptera: Formicidae); Implications for Conservation in Scotland. J Insect Conserv 2010, 14, 269–276. [Google Scholar] [CrossRef]
- Frouz, J. The Effect of Nest Moisture on Daily Temperature Regime in the Nests of Formica Polyctena Wood Ants. Insectes sociaux 2000, 47, 229–235. [Google Scholar] [CrossRef]
- Frouz, J.; Rybníček, M.; Cudlín, P.; Chmelíková, E. Influence of the Wood Ant, Formica Polyctena, on Soil Nutrient and the Spruce Tree Growth. Journal of Applied Entomology 2008, 132, 281–284. [Google Scholar] [CrossRef]
- Domisch, T.; Ohashi, M.; Finér, L.; Risch, A.C.; Sundström, L.; Kilpeläinen, J.; Niemelä, P. Decomposition of Organic Matter and Nutrient Mineralisation in Wood Ant (Formica Rufa Group) Mounds in Boreal Coniferous Forests of Different Age. Biol Fertil Soils 2008, 44, 539–545. [Google Scholar] [CrossRef]
- Rosengren, R.; Fortelius, W.; Lindström, K.; Luther, A. Phenology and Causation of Nest Heating and Thermoregulation in Red Wood Ants of the Formica Rufa Group Studied in Coniferous Forest Habitats in Southern Finland. In Proceedings of the Annales Zoologici Fennici; JSTOR; 1987; pp. 147–155. [Google Scholar]
- Weseloh, R.M. Simulation of Predation by Ants Based on Direct Observations of Attacks on Gypsy Moth Larvae. 1989. [CrossRef]
- Mokadam, C. Native and Non-Native Ant Impacts on Native Fungi. 2021.
- Sabelis, M.W. Predatory Arthropods. Natural enemies: The population biology of predators, parasites and diseases 1992, 225–264.
- Settle, W.H.; Ariawan, H.; Astuti, E.T.; Cahyana, W.; Hakim, A.L.; Hindayana, D.; Lestari, A.S. Managing Tropical Rice Pests through Conservation of Generalist Natural Enemies and Alternative Prey. Ecology 1996, 77, 1975–1988. [Google Scholar] [CrossRef]
- Symondson, W.; Sunderland, K.; Greenstone, M. Can Generalist Predators Be Effective Biocontrol Agents. Annu. Rev. Èntomol. 2002, 47, 561–594. [Google Scholar] [CrossRef]
- Yang, L.H. Interactions between a Detrital Resource Pulse and a Detritivore Community. Oecologia 2006, 147, 522–532. [Google Scholar] [CrossRef]
- Karban, R. Increased Reproductive Success at High Densities and Predator Satiation for Periodical Cicadas. Ecology 1982, 63, 321–328. [Google Scholar] [CrossRef]
- Sweeney, B.W.; Vannote, R.L. Population Synchrony in Mayflies: A Predator Satiation Hypothesis. Evolution 1982, 810–821. [Google Scholar] [CrossRef]
- Williams, K.S.; Smith, K.G.; Stephen, F.M. Emergence of 13-Yr Periodical Cicadas (Cicadidae: Magicicada): Phenology, Mortality, and Predators Satiation. Ecology 1993, 74, 1143–1152. [Google Scholar] [CrossRef]
- Yang, L.H.; Edwards, K.F.; Byrnes, J.E.; Bastow, J.L.; Wright, A.N.; Spence, K.O. A Meta-Analysis of Resource Pulse–Consumer Interactions. Ecological Monographs 2010, 80, 125–151. [Google Scholar] [CrossRef]
- Eveleigh, E.S.; McCann, K.S.; McCarthy, P.C.; Pollock, S.J.; Lucarotti, C.J.; Morin, B.; McDougall, G.A.; Strongman, D.B.; Huber, J.T.; Umbanhowar, J.; et al. Fluctuations in Density of an Outbreak Species Drive Diversity Cascades in Food Webs. Proceedings of the National Academy of Sciences 2007, 104, 16976–16981. [Google Scholar] [CrossRef]
- Dansereau-Macias, É.; Despland, E.; Handa, I.T. Decreased Soil Microbial Biomass and Changed Microbial Community Composition Following a Defoliation Event by the Forest Tent Caterpillar. Forests 2023, 14, 792. [Google Scholar] [CrossRef]
- Kristensen, J.Å.; Rousk, J.; Metcalfe, D.B. Below-ground Responses to Insect Herbivory in Ecosystems with Woody Plant Canopies: A Meta-analysis. J Ecol 2020, 108, 917–930. [Google Scholar] [CrossRef]
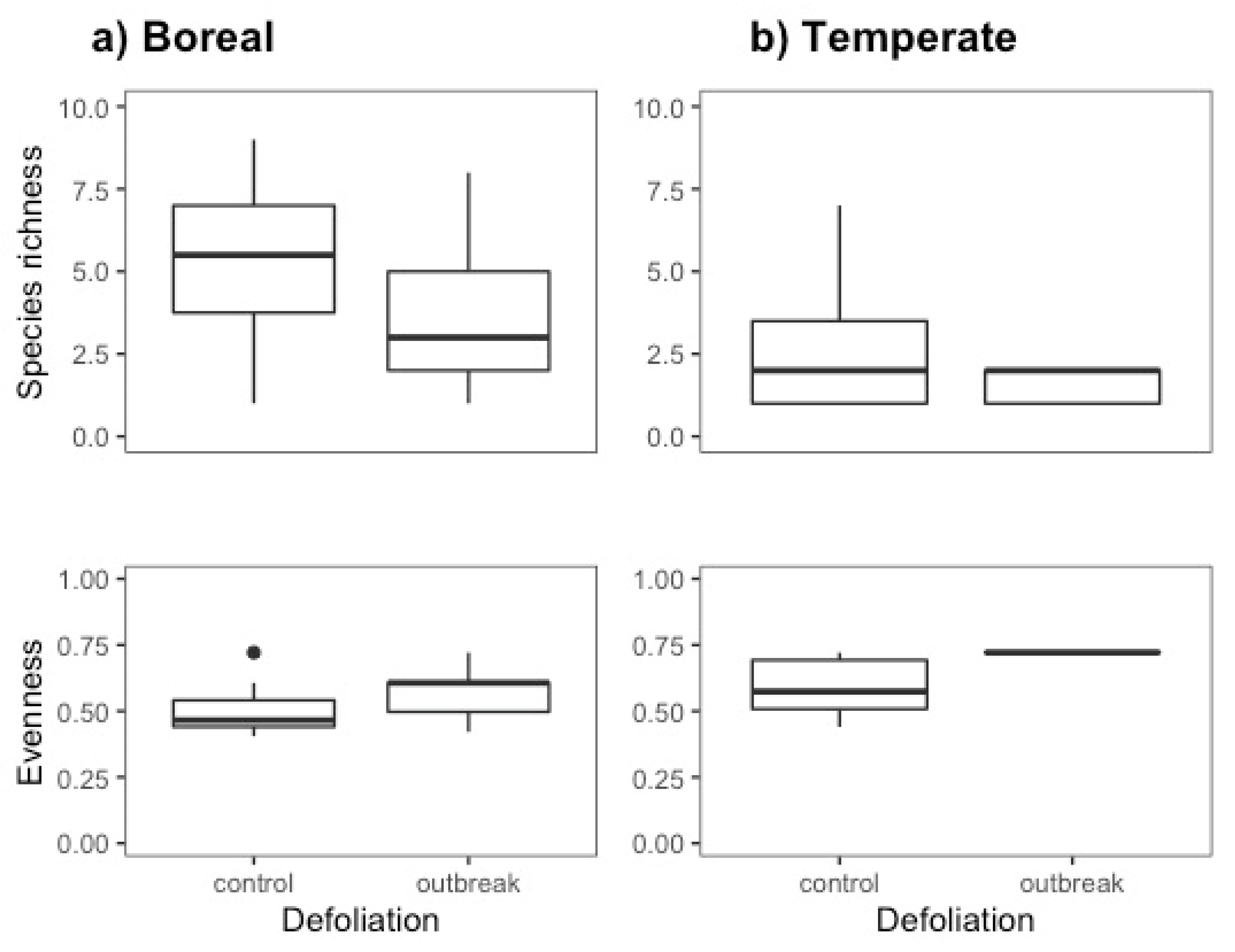
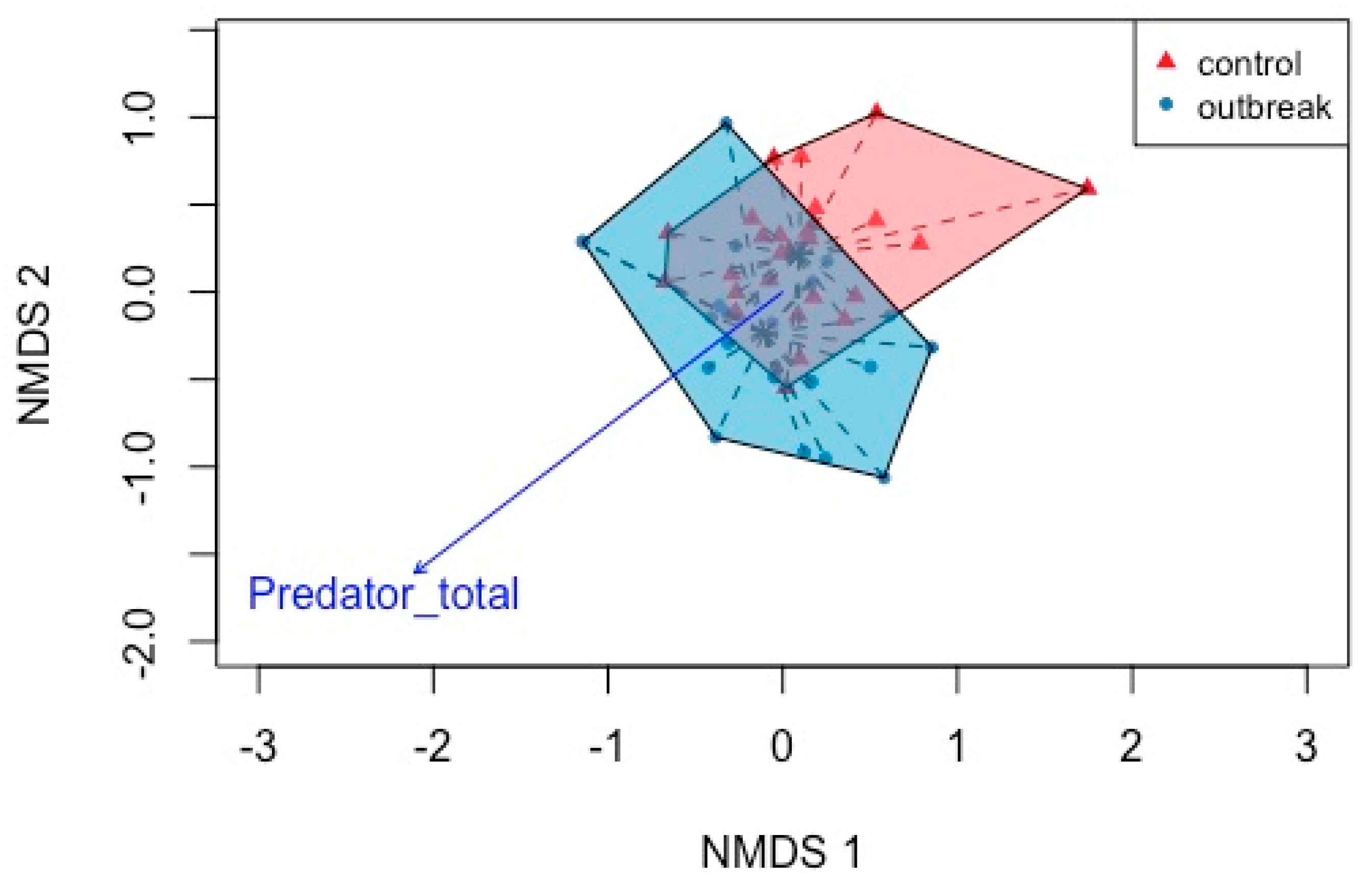
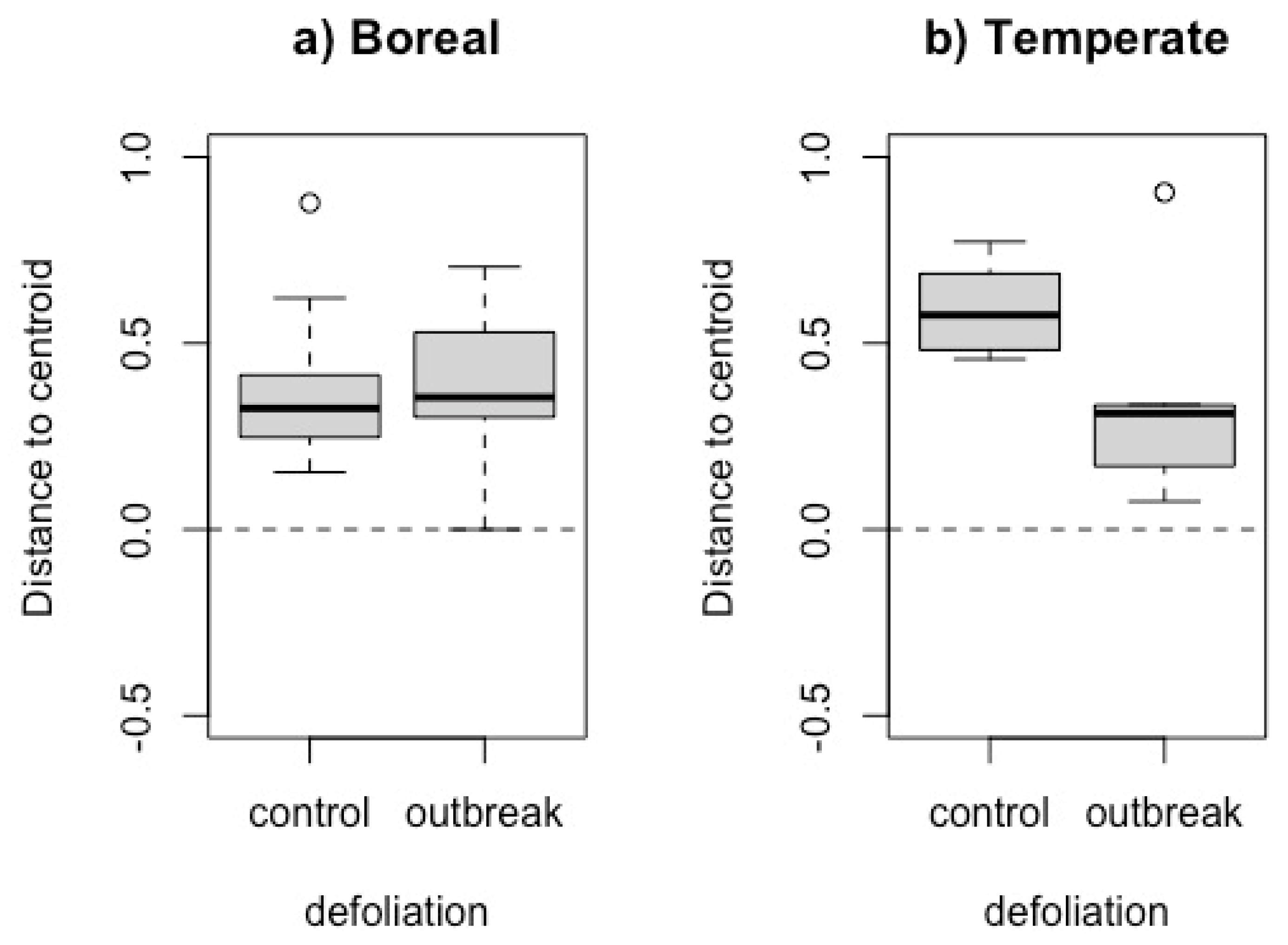
| Boreal forest | Temperate forest | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| df | F | R2 | p-value | df | F | R2 | p-value | ||
| Defoliation history | 1 | 5.3914 | 0.09096 | 0.002 | Defoliation history | 1 | 1.4536 | 0.10198 | 0.249 |
| Canopy openness | 1 | 0.9813 | 0.01656 | 0.434 | Canopy openness | 1 | 0.3802 | 0.02667 | 0.830 |
| Predators | 1 | 4.0690 | 0.06865 | 0.004 | Predators | 1 | 0.7809 | 0.05478 | 0.567 |
| Collembola (abundance) | 1 | 0.5300 | 0.00894 | 0.764 | Collembola (abundance) | 1 | 1.6389 | 0.11498 | 0.181 |
| Collembola (species richness) | 1 | 1.2997 | 0.02193 | 0.283 | |||||
| Residuals | 47 | 0.79296 | 14 | 0.70158 | |||||
| Groups | Species | A | B | Indicator value index | p-value |
|---|---|---|---|---|---|
| Control | Camponotus herculeanus | 0.7812 | 0.5714 | 0.668 | 0.002 |
| Myrmica sp1 | 0.9146 | 0.4286 | 0.626 | 0.003 | |
| Myrmica detritinodis | 0.8893 | 0.3214 | 0.535 | 0.011 | |
| Formica sp1 | 1.0000 | 0.2857 | 0.535 | 0.004 | |
| Outbreak | Formica integra | 1.0 | 0.2 | 0.447 s | 0.024 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





