Submitted:
28 April 2023
Posted:
28 April 2023
You are already at the latest version
Abstract
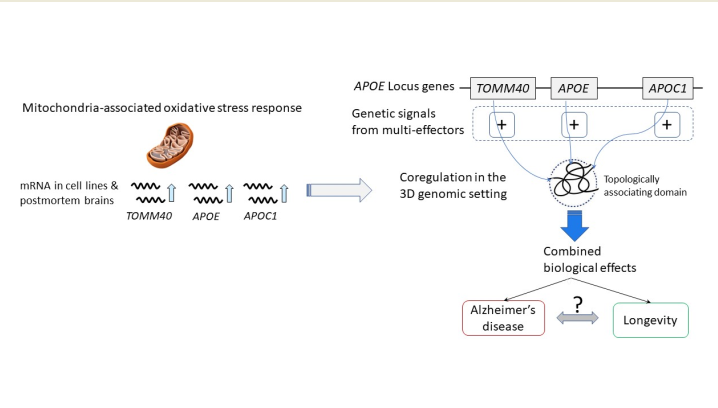
Keywords:
1. Introduction
2. Results
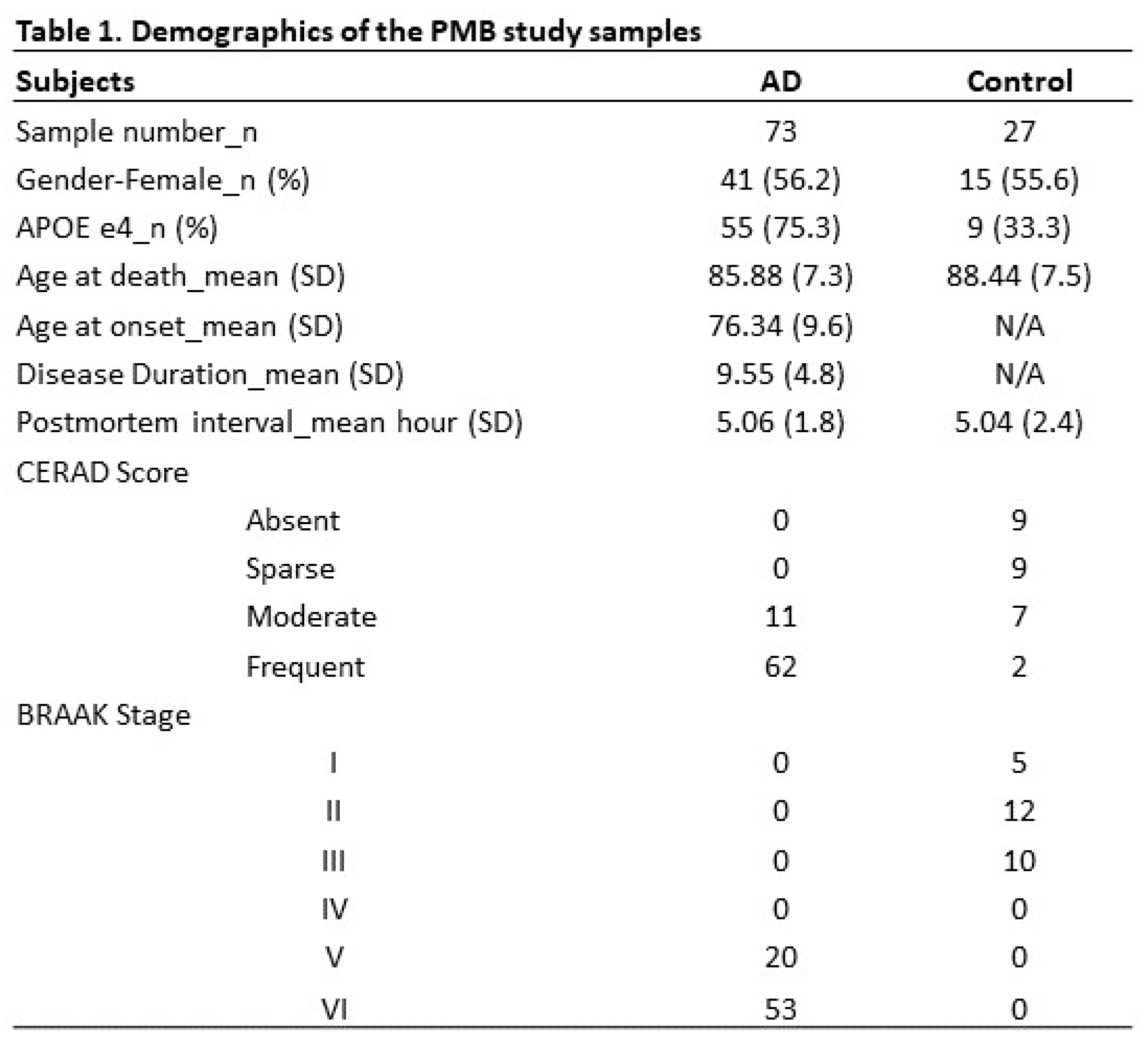
2.1. Overview of the study
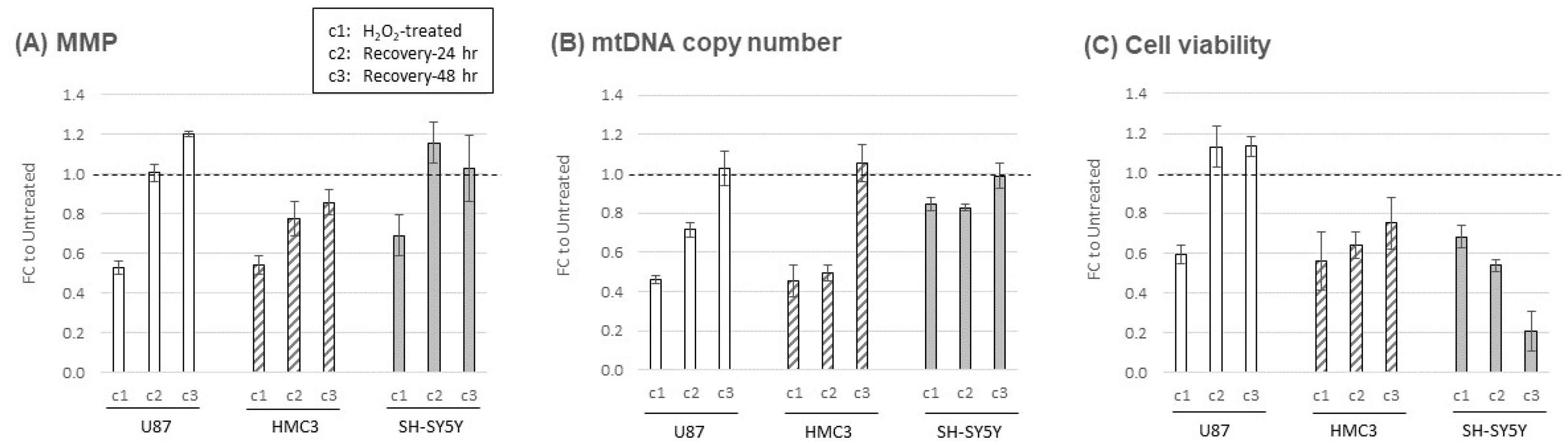
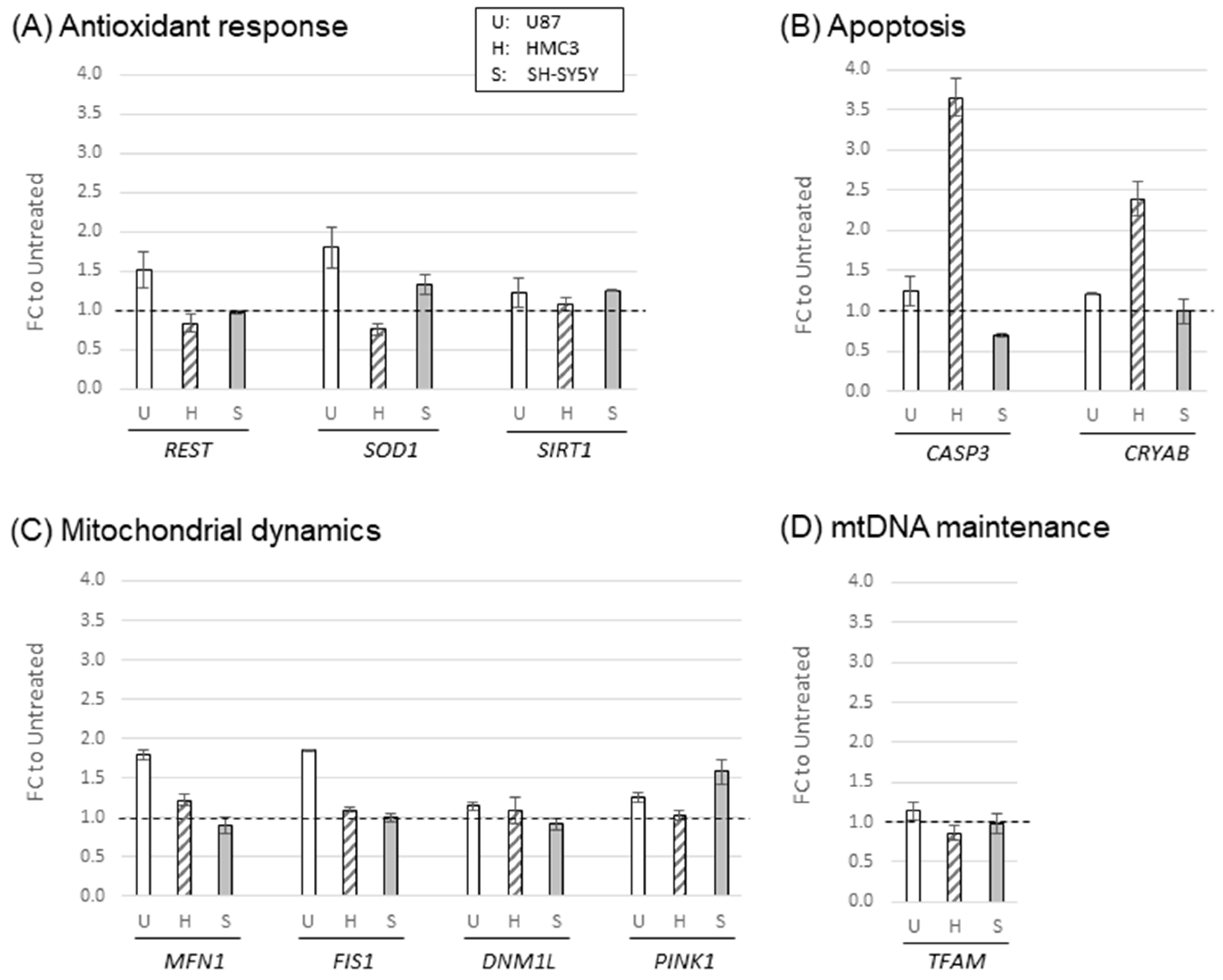
2.2. Oxidative stress-induced cellular responses in mitochondrial structure and function
2.3. Oxidative stress-induced alterations in mitochondrial structure and function-related gene expression
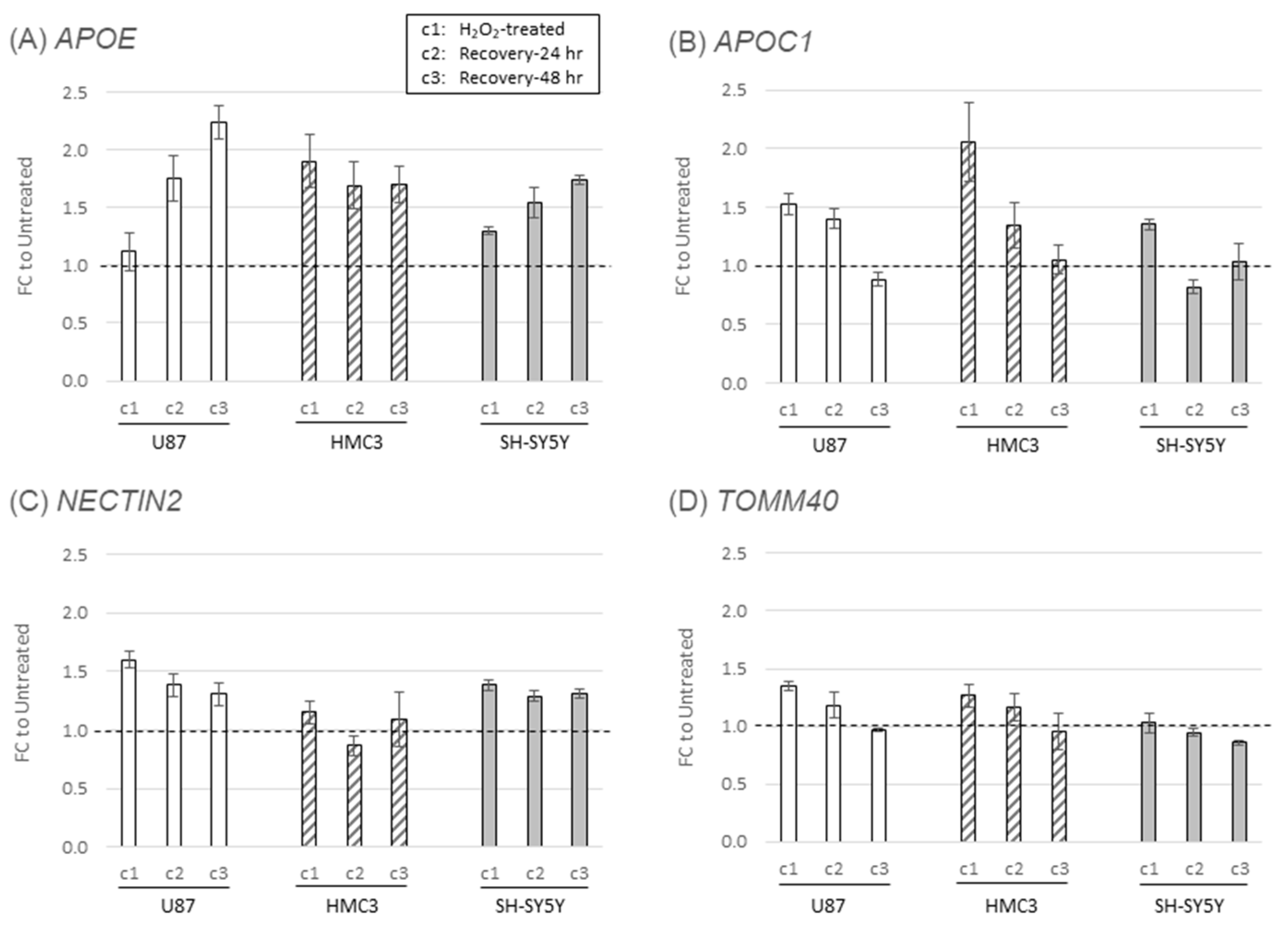
2.4. Oxidative stress-induced alterations in APOE locus gene expression
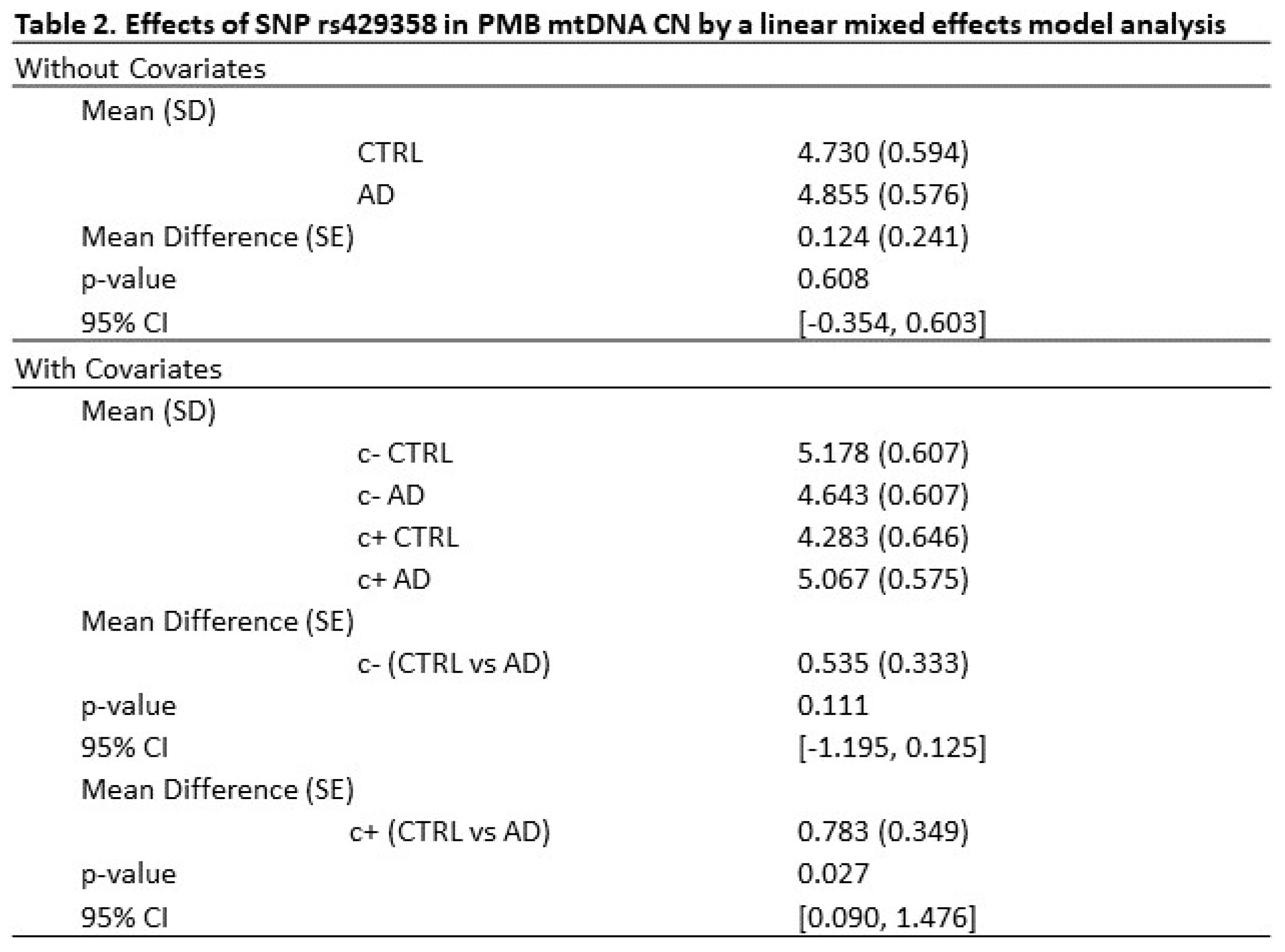
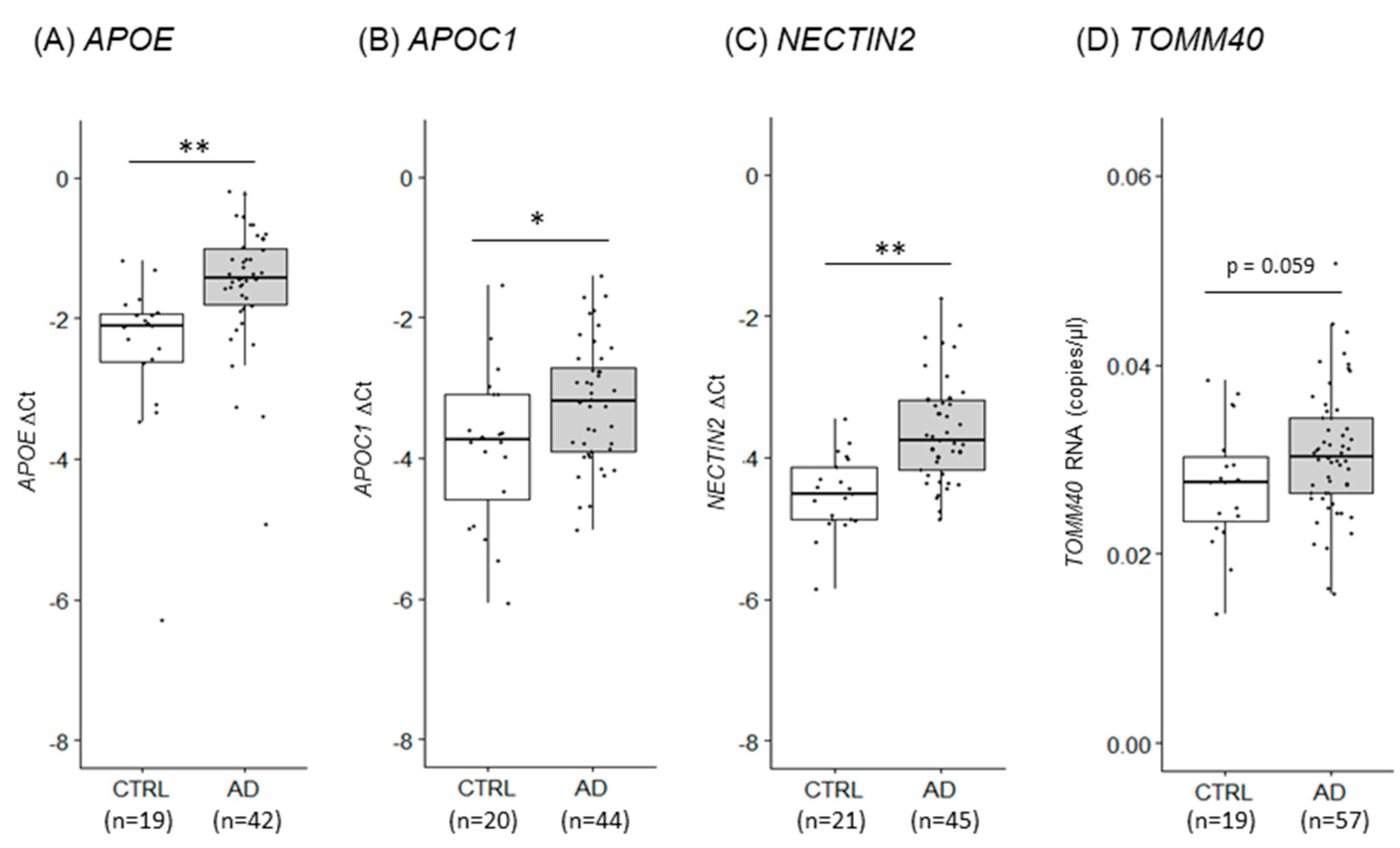
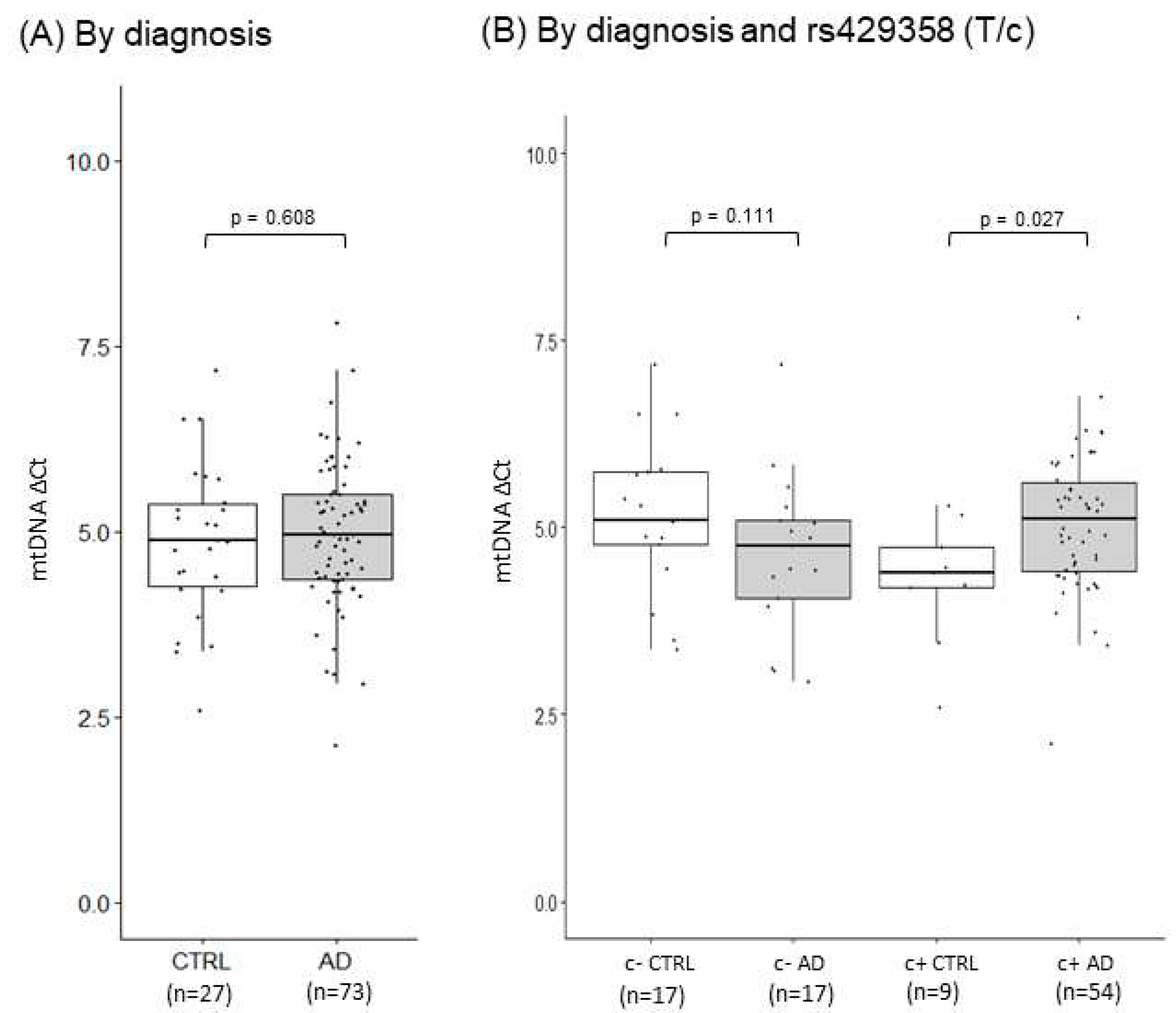
2.5. Mitochondrial function markers and gene expression in AD PMBs
2.6. 3D genome structure of the APOE locus
3. Discussion
4. Materials and Methods
4.1. Human PMB and cell lines
4.2. Hydrogen Peroxide Treatment
4.3. DNA/RNA extraction
4.4. Mitochondrial membrane potential (MMP) assay
4.5. Mitochondrial DNA copy number (mtDNA CN) assay
4.6. Cell viability assay
4.7. Gene expression by reverse transcriptase (RT) reaction and quantitative PCR (qPCR) assay
4.8. TOMM40 allelic gene expression
4.9. Genotyping of APOE, TOMM40 and APOC1
4.10. Statistical analysis and box plot
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Lambert JC, Ibrahim-Verbaas CA, Harold D, Naj AC, Sims R, Bellenguez C, et al. Meta-analysis of 74,046 individuals identifies 11 new susceptibility loci for Alzheimer’s disease. Nat Genet. 2013;45(12):1452-8. [CrossRef]
- Beecham GW, Hamilton K, Naj AC, Martin ER, Huentelman M, Myers AJ, et al. Genome-wide association meta-analysis of neuropathologic features of Alzheimer’s disease and related dementias. PLoS Genet. 2014;10(9):e1004606. [CrossRef]
- Nebel A, Kleindorp R, Caliebe A, Nothnagel M, Blanche H, Junge O, et al. A genome-wide association study confirms APOE as the major gene influencing survival in long-lived individuals. Mech Ageing Dev. 2011;132(6-7):324-30. [CrossRef]
- Lin R, Zhang Y, Yan D, Liao X, Gong G, Hu J, et al. Association of common variants in TOMM40/APOE/APOC1 region with human longevity in a Chinese population. J Hum Genet. 2016;61(4):323-8. [CrossRef]
- Yashin AI, Arbeev KG, Wu D, Arbeeva LS, Bagley O, Stallard E, et al. Genetics of Human Longevity From Incomplete Data: New Findings From the Long Life Family Study. J Gerontol A Biol Sci Med Sci. 2018;73(11):1472-81. [CrossRef]
- Deelen J, Evans DS, Arking DE, Tesi N, Nygaard M, Liu X, et al. A meta-analysis of genome-wide association studies identifies multiple longevity genes. Nat Commun. 2019;10(1):3669. [CrossRef]
- Kulminski AM, Jain-Washburn E, Philipp I, He L, Loika Y, Loiko E, et al. APOE varepsilon4 allele and TOMM40-APOC1 variants jointly contribute to survival to older ages. Aging Cell. 2022:e13730. [CrossRef]
- Bekris LM, Lutz F, Yu CE. Functional analysis of APOE locus genetic variation implicates regional enhancers in the regulation of both TOMM40 and APOE. J Hum Genet. 2012;57(1):18-25. Epub 2011/11/18. [CrossRef]
- Fuior EV, Gafencu AV. Apolipoprotein C1: Its Pleiotropic Effects in Lipid Metabolism and Beyond. Int J Mol Sci. 2019;20(23). [CrossRef]
- Heinemeyer T, Stemmet M, Bardien S, Neethling A. Underappreciated Roles of the Translocase of the Outer and Inner Mitochondrial Membrane Protein Complexes in Human Disease. DNA Cell Biol. 2019;38(1):23-40. [CrossRef]
- Lee EG, Chen S, Leong L, Tulloch J, Yu CE. TOMM40 RNA Transcription in Alzheimer’s Disease Brain and Its Implication in Mitochondrial Dysfunction. Genes (Basel). 2021;12(6). [CrossRef]
- Yu CE, Seltman H, Peskind ER, Galloway N, Zhou PX, Rosenthal E, et al. Comprehensive analysis of APOE and selected proximate markers for late-onset Alzheimer’s disease: patterns of linkage disequilibrium and disease/marker association. Genomics. 2007;89(6):655-65. [CrossRef]
- Roses A, Sundseth S, Saunders A, Gottschalk W, Burns D, Lutz M. Understanding the genetics of APOE and TOMM40 and role of mitochondrial structure and function in clinical pharmacology of Alzheimer’s disease. Alzheimers Dement. 2016;12(6):687-94. [CrossRef]
- Kujoth GC, Hiona A, Pugh TD, Someya S, Panzer K, Wohlgemuth SE, et al. Mitochondrial DNA mutations, oxidative stress, and apoptosis in mammalian aging. Science. 2005;309(5733):481-4. [CrossRef]
- Zafrilla P, Mulero J, Xandri JM, Santo E, Caravaca G, Morillas JM. Oxidative stress in Alzheimer patients in different stages of the disease. Curr Med Chem. 2006;13(9):1075-83. [CrossRef]
- Liguori I, Russo G, Curcio F, Bulli G, Aran L, Della-Morte D, et al. Oxidative stress, aging, and diseases. Clin Interv Aging. 2018;13:757-72. [CrossRef]
- Swerdlow RH. The Alzheimer’s Disease Mitochondrial Cascade Hypothesis: A Current Overview. J Alzheimers Dis. 2023. [CrossRef]
- Bereiter-Hahn J, Jendrach M. Mitochondrial dynamics. Int Rev Cell Mol Biol. 2010;284:1-65. [CrossRef]
- Juan CA, Perez de la Lastra JM, Plou FJ, Perez-Lebena E. The Chemistry of Reactive Oxygen Species (ROS) Revisited: Outlining Their Role in Biological Macromolecules (DNA, Lipids and Proteins) and Induced Pathologies. Int J Mol Sci. 2021;22(9). [CrossRef]
- Verri M, Pastoris O, Dossena M, Aquilani R, Guerriero F, Cuzzoni G, et al. Mitochondrial alterations, oxidative stress and neuroinflammation in Alzheimer's disease. Int J Immunopathol Pharmacol. 2012;25(2):345-53. [CrossRef]
- Flannery PJ, Trushina E. Mitochondrial dynamics and transport in Alzheimer’s disease. Mol Cell Neurosci. 2019;98:109-20. [CrossRef]
- Butterfield DA, Halliwell B. Oxidative stress, dysfunctional glucose metabolism and Alzheimer disease. Nat Rev Neurosci. 2019;20(3):148-60. [CrossRef]
- Hirai K, Aliev G, Nunomura A, Fujioka H, Russell RL, Atwood CS, et al. Mitochondrial abnormalities in Alzheimer’s disease. J Neurosci. 2001;21(9):3017-23. [CrossRef]
- Kwong JQ, Beal MF, Manfredi G. The role of mitochondria in inherited neurodegenerative diseases. J Neurochem. 2006;97(6):1659-75. [CrossRef]
- Wang X, Su B, Zheng L, Perry G, Smith MA, Zhu X. The role of abnormal mitochondrial dynamics in the pathogenesis of Alzheimer’s disease. J Neurochem. 2009;109 Suppl 1(Suppl 1):153-9. [CrossRef]
- Kapogiannis D, Mattson MP. Disrupted energy metabolism and neuronal circuit dysfunction in cognitive impairment and Alzheimer’s disease. Lancet Neurol. 2011;10(2):187-98. [CrossRef]
- Swerdlow RH, Burns JM, Khan SM. The Alzheimer’s disease mitochondrial cascade hypothesis: progress and perspectives. Biochim Biophys Acta. 2014;1842(8):1219-31. [CrossRef]
- Hoogenraad NJ, Ward LA, Ryan MT. Import and assembly of proteins into mitochondria of mammalian cells. Biochim Biophys Acta. 2002;1592(1):97-105. [PubMed]
- Hill K, Model K, Ryan MT, Dietmeier K, Martin F, Wagner R, et al. Tom40 forms the hydrophilic channel of the mitochondrial import pore for preproteins [see comment]. Nature. 1998;395(6701):516-21. [CrossRef] [PubMed]
- Suzuki H, Okazawa Y, Komiya T, Saeki K, Mekada E, Kitada S, et al. Characterization of rat TOM40, a central component of the preprotein translocase of the mitochondrial outer membrane. J Biol Chem. 2000;275(48):37930-6. [CrossRef] [PubMed]
- Chacinska A, Koehler CM, Milenkovic D, Lithgow T, Pfanner N. Importing mitochondrial proteins: machineries and mechanisms. Cell. 2009;138(4):628-44. [CrossRef]
- Bender A, Desplats P, Spencer B, Rockenstein E, Adame A, Elstner M, et al. TOM40 mediates mitochondrial dysfunction induced by alpha-synuclein accumulation in Parkinson’s disease. PLoS One. 2013;8(4):e62277. [CrossRef]
- Anandatheerthavarada HK, Devi L. Mitochondrial translocation of amyloid precursor protein and its cleaved products: relevance to mitochondrial dysfunction in Alzheimer’s disease. Rev Neurosci. 2007;18(5):343-54. [CrossRef] [PubMed]
- Cenini G, Rub C, Bruderek M, Voos W. Amyloid beta-peptides interfere with mitochondrial preprotein import competence by a coaggregation process. Mol Biol Cell. 2016;27(21):3257-72. [CrossRef]
- Perkins M, Wolf AB, Chavira B, Shonebarger D, Meckel JP, Leung L, et al. Altered Energy Metabolism Pathways in the Posterior Cingulate in Young Adult Apolipoprotein E varepsilon4 Carriers. J Alzheimers Dis. 2016;53(1):95-106. [CrossRef]
- Valla J, Yaari R, Wolf AB, Kusne Y, Beach TG, Roher AE, et al. Reduced posterior cingulate mitochondrial activity in expired young adult carriers of the APOE epsilon4 allele, the major late-onset Alzheimer’s susceptibility gene. J Alzheimers Dis. 2010;22(1):307-13. [CrossRef]
- Reiman EM, Caselli RJ, Yun LS, Chen K, Bandy D, Minoshima S, et al. Preclinical evidence of Alzheimer’s disease in persons homozygous for the epsilon 4 allele for apolipoprotein E. N Engl J Med. 1996;334(12):752-8. [CrossRef] [PubMed]
- Small GW, Mazziotta JC, Collins MT, Baxter LR, Phelps ME, Mandelkern MA, et al. Apolipoprotein E type 4 allele and cerebral glucose metabolism in relatives at risk for familial Alzheimer disease. JAMA. 1995;273(12):942-7. [PubMed]
- Carrieri G, Bonafe M, De Luca M, Rose G, Varcasia O, Bruni A, et al. Mitochondrial DNA haplogroups and APOE4 allele are non-independent variables in sporadic Alzheimer’s disease. Hum Genet. 2001;108(3):194-8. [PubMed]
- Maruszak A, Canter JA, Styczynska M, Zekanowski C, Barcikowska M. Mitochondrial haplogroup H and Alzheimer’s disease--is there a connection? Neurobiol Aging. 2009;30(11):1749-55. [CrossRef] [PubMed]
- Hsieh TS, Fudenberg G, Goloborodko A, Rando OJ. Micro-C XL: assaying chromosome conformation from the nucleosome to the entire genome. Nat Methods. 2016;13(12):1009-11. [CrossRef] [PubMed]
- Krietenstein N, Abraham S, Venev SV, Abdennur N, Gibcus J, Hsieh TS, et al. Ultrastructural Details of Mammalian Chromosome Architecture. Mol Cell. 2020;78(3):554-65 e7. [CrossRef]
- Nuytemans K, Lipkin Vasquez M, Wang L, Van Booven D, Griswold AJ, Rajabli F, et al. Identifying differential regulatory control of APOE varepsilon4 on African versus European haplotypes as potential therapeutic targets. Alzheimers Dement. 2022;18(10):1930-42. [CrossRef]
- Meng G, Xu H, Lu D, Li S, Zhao Z, Li H, et al. Three-dimensional chromatin architecture datasets for aging and Alzheimer’s disease. Sci Data. 2023;10(1):51. [CrossRef]
- Periasamy A, Mitchell N, Zaytseva O, Chahal AS, Zhao J, Colman PM, et al. An increase in mitochondrial TOM activates apoptosis to drive retinal neurodegeneration. Sci Rep. 2022;12(1):21634. [CrossRef]
- Shao D, Gao Z, Zhao Y, Fan M, Zhao X, Wei Q, et al. Sulforaphane Suppresses H(2)O(2)-Induced Oxidative Stress and Apoptosis via the Activation of AMPK/NFE2L2 Signaling Pathway in Goat Mammary Epithelial Cells. Int J Mol Sci. 2023;24(2). [CrossRef]
- Kovalevich J, Santerre M, Langford D. Considerations for the Use of SH-SY5Y Neuroblastoma Cells in Neurobiology. Methods Mol Biol. 2021;2311:9-23. [CrossRef] [PubMed]
- Pratiwi R, Nantasenamat C, Ruankham W, Suwanjang W, Prachayasittikul V, Prachayasittikul S, et al. Mechanisms and Neuroprotective Activities of Stigmasterol Against Oxidative Stress-Induced Neuronal Cell Death via Sirtuin Family. Front Nutr. 2021;8:648995. [CrossRef]
- Lingappa S, Shivakumar MS, Manivasagam T, Somasundaram ST, Seedevi P. Neuroprotective Effect of Epalrestat on Hydrogen Peroxide-Induced Neurodegeneration in SH-SY5Y Cellular Model. J Microbiol Biotechnol. 2021;31(6):867-74. [CrossRef]
- Santello M, Toni N, Volterra A. Astrocyte function from information processing to cognition and cognitive impairment. Nat Neurosci. 2019;22(2):154-66. [CrossRef] [PubMed]
- Kamradt MC, Chen F, Cryns VL. The small heat shock protein alpha B-crystallin negatively regulates cytochrome c- and caspase-8-dependent activation of caspase-3 by inhibiting its autoproteolytic maturation. J Biol Chem. 2001;276(19):16059-63. [CrossRef] [PubMed]
- Morrow G, Samson M, Michaud S, Tanguay RM. Overexpression of the small mitochondrial Hsp22 extends Drosophila life span and increases resistance to oxidative stress. FASEB J. 2004;18(3):598-9. [CrossRef] [PubMed]
- Yin J, Reiman EM, Beach TG, Serrano GE, Sabbagh MN, Nielsen M, et al. Effect of ApoE isoforms on mitochondria in Alzheimer disease. Neurology. 2020;94(23):e2404-e11. [CrossRef]
- Kloske CM, Barnum CJ, Batista AF, Bradshaw EM, Brickman AM, Bu G, et al. APOE and immunity: Research highlights. Alzheimers Dement. 2023. [CrossRef] [PubMed]
- Wolfe CM, Fitz NF, Nam KN, Lefterov I, Koldamova R. The Role of APOE and TREM2 in Alzheimer’s Disease-Current Understanding and Perspectives. Int J Mol Sci. 2018;20(1). [CrossRef]
- Gratuze M, Leyns CEG, Holtzman DM. New insights into the role of TREM2 in Alzheimer’s disease. Mol Neurodegener. 2018;13(1):66. [CrossRef]
- Mills EL, Kelly B, O’Neill LAJ. Mitochondria are the powerhouses of immunity. Nat Immunol. 2017;18(5):488-98. [CrossRef] [PubMed]
- Wilson JL, Mayr HK, Weichhart T. Metabolic Programming of Macrophages: Implications in the Pathogenesis of Granulomatous Disease. Front Immunol. 2019;10:2265. [CrossRef]
- Wu B, Zhong C, Lang Q, Liang Z, Zhang Y, Zhao X, et al. Poliovirus receptor (PVR)-like protein cosignaling network: new opportunities for cancer immunotherapy. J Exp Clin Cancer Res. 2021;40(1):267. [CrossRef]
- Zhu Y, Paniccia A, Schulick AC, Chen W, Koenig MR, Byers JT, et al. Identification of CD112R as a novel checkpoint for human T cells. J Exp Med. 2016;213(2):167-76. [CrossRef]
- Joshi CJ, Ke W, Drangowska-Way A, O’Rourke EJ, Lewis NE. What are housekeeping genes? PLoS Comput Biol. 2022;18(7):e1010295. [CrossRef]
- Chen YC, Chang SC, Lee YS, Ho WM, Huang YH, Wu YY, et al. TOMM40 Genetic Variants Cause Neuroinflammation in Alzheimer’s Disease. Int J Mol Sci. 2023;24(4). [CrossRef]
- Linnertz C, Anderson L, Gottschalk W, Crenshaw D, Lutz MW, Allen J, et al. The cis-regulatory effect of an Alzheimer’s disease-associated poly-T locus on expression of TOMM40 and apolipoprotein E genes. Alzheimers Dement. 2014;10(5):541-51. [CrossRef]
- Filograna R, Mennuni M, Alsina D, Larsson NG. Mitochondrial DNA copy number in human disease: the more the better? FEBS Lett. 2021;595(8):976-1002. [CrossRef]
- Liou CW, Chen SH, Lin TK, Tsai MH, Chang CC. Oxidative Stress Biomarkers and Mitochondrial DNA Copy Number Associated with APOE4 Allele and Cholinesterase Inhibitor Therapy in Patients with Alzheimer’s Disease. Antioxidants (Basel). 2021;10(12). [CrossRef]
- Mathew A, Lindsley TA, Sheridan A, Bhoiwala DL, Hushmendy SF, Yager EJ, et al. Degraded mitochondrial DNA is a newly identified subtype of the damage associated molecular pattern (DAMP) family and possible trigger of neurodegeneration. J Alzheimers Dis. 2012;30(3):617-27. [CrossRef] [PubMed]
- Wilkins HM, Weidling IW, Ji Y, Swerdlow RH. Mitochondria-Derived Damage-Associated Molecular Patterns in Neurodegeneration. Front Immunol. 2017;8:508. [CrossRef]
- Dekker J, Rippe K, Dekker M, Kleckner N. Capturing chromosome conformation. Science. 2002;295(5558):1306-11. [CrossRef] [PubMed]
- Lieberman-Aiden E, van Berkum NL, Williams L, Imakaev M, Ragoczy T, Telling A, et al. Comprehensive mapping of long-range interactions reveals folding principles of the human genome. Science. 2009;326(5950):289-93. [CrossRef]
- Dixon JR, Selvaraj S, Yue F, Kim A, Li Y, Shen Y, et al. Topological domains in mammalian genomes identified by analysis of chromatin interactions. Nature. 2012;485(7398):376-80. [CrossRef]
- Nora EP, Lajoie BR, Schulz EG, Giorgetti L, Okamoto I, Servant N, et al. Spatial partitioning of the regulatory landscape of the X-inactivation centre. Nature. 2012;485(7398):381-5. [CrossRef]
- Sexton T, Yaffe E, Kenigsberg E, Bantignies F, Leblanc B, Hoichman M, et al. Three-dimensional folding and functional organization principles of the Drosophila genome. Cell. 2012;148(3):458-72. [CrossRef] [PubMed]
- Zhan Y, Mariani L, Barozzi I, Schulz EG, Bluthgen N, Stadler M, et al. Reciprocal insulation analysis of Hi-C data shows that TADs represent a functionally but not structurally privileged scale in the hierarchical folding of chromosomes. Genome Res. 2017;27(3):479-90. [CrossRef]
- Sun F, Chronis C, Kronenberg M, Chen XF, Su T, Lay FD, et al. Promoter-Enhancer Communication Occurs Primarily within Insulated Neighborhoods. Mol Cell. 2019;73(2):250-63 e5. [CrossRef]
- Bendl J, Hauberg ME, Girdhar K, Im E, Vicari JM, Rahman S, et al. The three-dimensional landscape of cortical chromatin accessibility in Alzheimer’s disease. Nat Neurosci. 2022;25(10):1366-78. [CrossRef]
- Li K, Chi R, Liu L, Feng M, Su K, Li X, et al. 3D genome-selected microRNAs to improve Alzheimer’s disease prediction. Front Neurol. 2023;14:1059492. [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).



