Submitted:
21 April 2023
Posted:
23 April 2023
Read the latest preprint version here
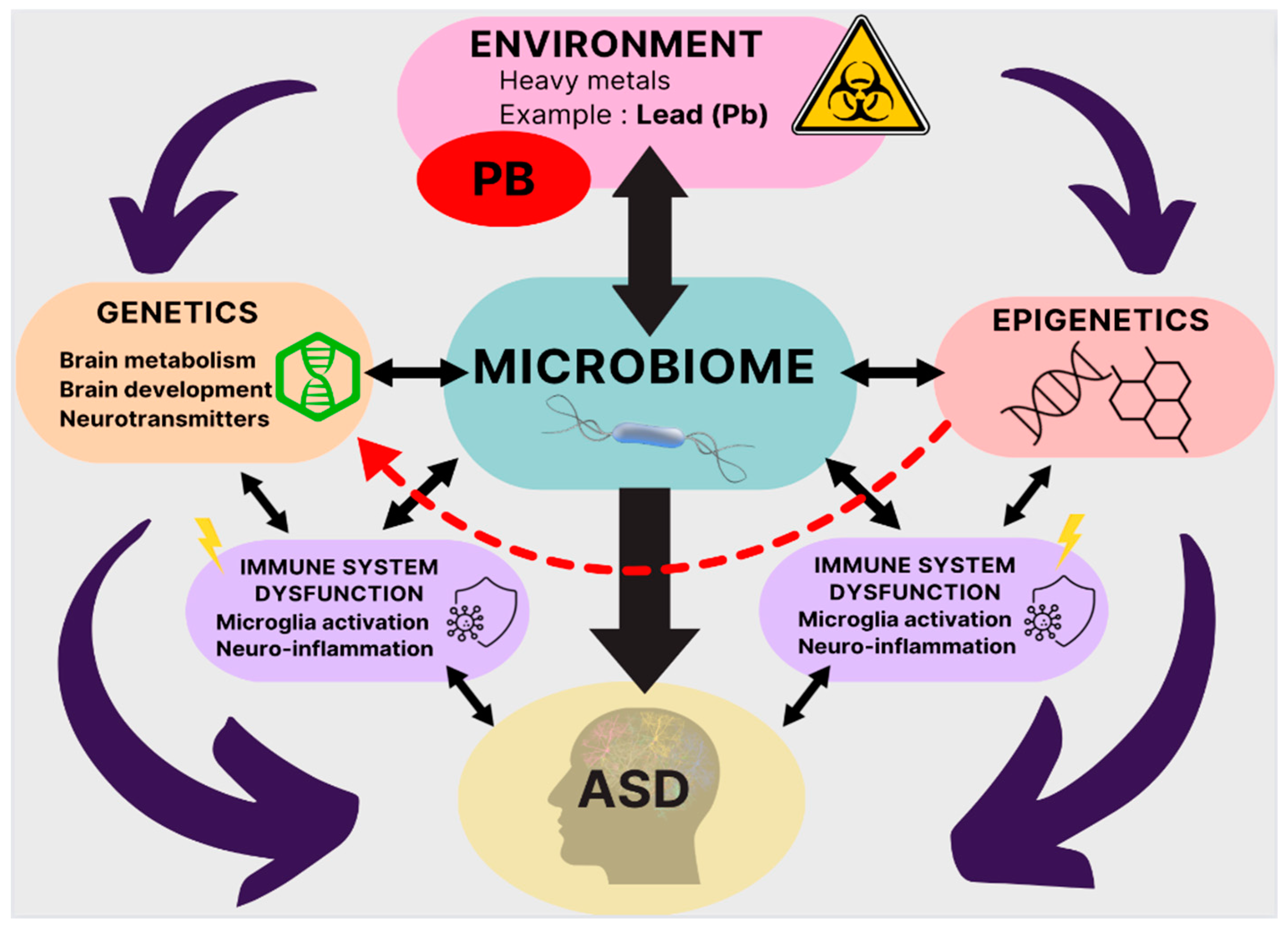
Abstract
Keywords:
1. Introduction
2. Autism Spectrum Disorder (ASD)
2.1. Neurobiological Substrates Associated with ASD
2.2. Genetics and Epigenetics in ASD
2.3. Neurotransmitters in ASD
2.4. Neuroinflammation in ASD
3. Gut Microbiota
3.1. Digestive System Innervation – Enteric Nervous System
3.2. Gut-Brain Axis (GBA)
3.3. Gut Microbiota – Genetics
3.4. Gut Microbiota – Neurotransmitters
3.5. Gut Microbiota - Short Chain Fatty Acids (SCFAs)
3.6. Gut Microbiota – Microglia
3.7. Gut Microbiota – ASD
4. SCFAs- ASD
5. LEAD (Pb)
5.1. Pb-ASD
5.2. Pb – Calcium – Neurotransmitters
5.3. Pb – Neuroinflammation
5.4. Pb – Mitochondria
5.5. Pb – Microbiota – ASD
5.6. Pb – ASD – Therapeutics
6. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Rylaarsdam, L.; Guemez-Gamboa, A. Genetic Causes and Modifiers of Autism Spectrum Disorder. Front Cell Neurosci. 2019, 13, 385. [Google Scholar] [CrossRef] [PubMed]
- Toscano, C.V.A.; Barros, L.; Lima, A.B.; Nunes, T.; Carvalho, H.M.; Gaspar, J.M. Neuroinflammation in autism spectrum disorders: Exercise as a “pharmacological” tool. Neurosci Biobehav Rev. 2021, 129, 63–74. [Google Scholar] [CrossRef] [PubMed]
- Marco, E.J.; Hinkley, L.B.; Hill, S.S.; Nagarajan, S.S. Sensory processing in autism: a review of neurophysiologic findings. Pediatr Res. 2011, 69, 48–54. [Google Scholar] [CrossRef] [PubMed]
- Murray, M.J. Attention-deficit/Hyperactivity Disorder in the context of Autism spectrum disorders. Curr Psychiatry Rep. 2010, 12, 382–388. [Google Scholar] [CrossRef] [PubMed]
- Young, S.; Hollingdale, J.; Absoud, M.; Bolton, P.; Branney, P.; Colley, W.; Craze, E.; Dave, M.; Deeley, Q.; Farrag, E.; Gudjonsson, G.; Hill, P.; Liang, H.L.; Murphy, C.; Mackintosh, P.; Murin, M.; O’Regan, F.; Ougrin, D.; Rios, P.; Stover, N.; Taylor, E.; Woodhouse, E. Guidance for identification and treatment of individuals with attention deficit/hyperactivity disorder and autism spectrum disorder based upon expert consensus. BMC Med. 2020, 18, 146. [Google Scholar] [CrossRef] [PubMed]
- Hyman, S.L.; Levy, S.E.; Myers, S.M. COUNCIL ON CHILDREN WITH DISABILITIES, SECTION ON DEVELOPMENTAL AND BEHAVIORAL PEDIATRICS. Identification, Evaluation, and Management of Children With Autism Spectrum Disorder. Pediatrics. 2020, 145, 69. [Google Scholar] [CrossRef]
- Loomes, R.; Hull, L.; Mandy, W.P.L. What Is the Male-to-Female Ratio in Autism Spectrum Disorder? A Systematic Review and Meta-Analysis. J Am Acad Child Adolesc Psychiatry, 2017, 56, 466–474. [Google Scholar] [CrossRef]
- Daniolou, S.; Pandis, N.; Znoj, H. The Efficacy of Early Interventions for Children with Autism Spectrum Disorders: A Systematic Review and Meta-Analysis. J. Clin. Med. 2022, 11, 5100. [Google Scholar] [CrossRef]
- Kemper, T.L.; Bauman, M. Neuropathology of infantile autism. J Neuropathol Exp Neurol. 1998, 57, 645–652. [Google Scholar] [CrossRef]
- Amaral DG, Schumann CM, Nordahl CW. Neuroanatomy of autism. Trends Neurosci. 2008, 31, 137–145. [CrossRef]
- Ha, S.; Sohn, I.J.; Kim, N.; Sim, H.J.; Cheon, K.A. Characteristics of Brains in Autism Spectrum Disorder: Structure, Function and Connectivity across the Lifespan. Exp Neurobiol. 2015, 24, 273–284:. [Google Scholar] [CrossRef] [PubMed]
- Weston, C.S.E. Four Social Brain Regions, Their Dysfunctions, and Sequelae, Extensively Explain Autism Spectrum Disorder Symptomatology. Brain Sci. 2019, 9, 130. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Ma, Z.H.; Xu, L.Z.; Yang, L.; Ji, Z.Z.; Tang, X.Z.; Liu, J.R.; Li, X.; Cao, Q.J.; Liu, J. Developmental brain structural atypicalities in autism: a voxel-based morphometry analysis. Child Adolesc Psychiatry Ment Health. 2022, 16, 7. [Google Scholar] [CrossRef] [PubMed]
- Hashem, S.; Nisar, S.; Bhat, A.; Yadav, S.; Azeem, M.W.; Bagga, P.; Fakhro, K.; Frenneaux, M. Genetics of structural and functional brain changes in autism spectrum disorder. Transl Psychiatry, 2020, 10, 229. [Google Scholar] [CrossRef] [PubMed]
- Qiu, S.; Qiu, Y.; Li, Y.; Cong, X. Genetics of autism spectrum disorder: an umbrella review of systematic reviews and meta-analyses. Transl Psychiatry. 2022, 12, 249. [Google Scholar] [CrossRef] [PubMed]
- Bölte, S. , Girdler S. , Marschik P. B. The contribution of environmental exposure to the etiology of autism spectrum disorder. Cell. Mol. Life Sci., 2019, 76, 1275–1297. [Google Scholar] [CrossRef]
- Wong, C.C.; Meaburn, E.L.; Ronald, A.; Price, T.S.; Jeffries, A.R.; Schalkwyk, L.C.; Plomin, R.; Mill, J. Methylomic analysis of monozygotic twins discordant for autism spectrum disorder and related behavioural traits. Mol Psychiatry. 2014, 19, 495–503. [Google Scholar] [CrossRef]
- Kubota, T.; Takae, H.; Miyake, K. Epigenetic Mechanisms and Therapeutic Perspectives for Neurodevelopmental Disorders. Pharmaceuticals 2012, 5, 369–383. [Google Scholar] [CrossRef]
- Younesian, S.; Yousefi, A.M.; Momeny, M.; Ghaffari, SH.; Bashash, D. The DNA Methylation in Neurological Diseases. Cells. 2022, 11, 3439. [Google Scholar] [CrossRef]
- Marotta, R.; Risoleo, M.C.; Messina, G.; Parisi, L.; Carotenuto, M.; Vetri, L.; Roccella, M. The Neurochemistry of Autism. Brain Sci. 2020, 10, 163. [Google Scholar] [CrossRef]
- McCarty, P.J.; Pines, A.R.; Sussman, B.L.; Wyckoff, S.N.; Jensen, A.; Bunch, R.; Boerwinkle, V.L.; Frye, R.E. Resting State Functional Magnetic Resonance Imaging Elucidates Neurotransmitter Deficiency in Autism Spectrum Disorder. J. Pers. Med. 2021, 11, 969. [Google Scholar] [CrossRef] [PubMed]
- Nisar, S.; Bhat, A.A.; Masoodi, T.; Hashem, S.; Akhtar, S.; Ali, T.A.; Amjad, S.; Chawla, S.; Bagga, P.; Frenneaux, M.P.; Reddy, R.; Fakhro, K.; Haris, M. Genetics of glutamate and its receptors in autism spectrum disorder. Mol Psychiatry 2022, 27, 2380–2392. [Google Scholar] [CrossRef] [PubMed]
- Owens, D.F.; Kriegstein, A.R. ; Is there more to GABA than synaptic inhibition? Nat. Rev. Neurosci. 2002, 3, 715–727. [Google Scholar] [CrossRef] [PubMed]
- Yizhar, O.; Fenno, L.E.; Prigge, M.; Schneider, F.; Davidson, T.J.; O’Shea, D.J.; Sohal, V.S.; Goshen, I.; Finkelstein, J.; Paz, J.T.; Stehfest, K.; Fudim, R.; Ramakrishnan, C.; Huguenard, J. R.; Hegemann, P.; Deisseroth, K. Neocortical excitation/inhibition balance in information processing and social dysfunction. Nature. 2011, 477, 171–178. [Google Scholar] [CrossRef] [PubMed]
- Siemann, J.K.; Muller, C.L.; Forsberg, C.G.; Blakely, R.D.; Veenstra-VanderWeele, J.; Wallace, M.T. An autism-associated serotonin transporter variant disrupts multisensory processing. Transl. Psychiatry. 2017, 7, e1067. [Google Scholar] [CrossRef] [PubMed]
- Abdulamir, H.A.; Abdul-Rasheed, O.F.; Abdulghani, E.A. Serotonin and serotonin transporter levels in autistic children. Saudi Med. J. 2018, 39, 487–494. [Google Scholar] [CrossRef] [PubMed]
- Yang, C.J.; Tan, H.P.; Du, Y.J. The developmental disruptions of serotonin signaling may involved in autism during early brain development. Neuroscience. 2014, 267, 1–10. [Google Scholar] [CrossRef]
- Dichter, G.S.; Damiano, C.A.; Allen, J.A. Reward circuitry dysfunction in psychiatric and neurodevelopmental disorders and genetic syndromes: Animal models and clinical findings. J. Neurodev. Disord. 2012, 4, 19. [Google Scholar] [CrossRef]
- Wang, L.; Almeida, L.E.; Spornick, N.A.; Kenyon, N.; Kamimura, S.; Khaibullina, A.; Nouraie, M.; Quezado, Z.M. Modulation of social deficits and repetitive behaviors in a mouse model of autism:The role of the nicotinic cholinergicsystem. Psychopharmacology. 2015, 232, 4303–4316. [Google Scholar] [CrossRef]
- Lee, M.; Martin-Ruiz, C.; Graham, A.; Court, J.; Jaros, E.; Perry, R.; Iversen, P.; Bauman, M.; Perry, E. Nicotinic receptor abnormalities in the cerebellar cortex in autism. Brain. 2002, 125, 1483–1495. [Google Scholar] [CrossRef]
- Takechi, K.; Suemaru, K.; Kiyoi, T.; Tanaka, A.; Araki, H. The α4β2 nicotinic acetylcholine receptor modulates autism-like behavioral and motor abnormalities in pentylenetetrazol-kindled mice. Eur. J. Pharmacol. 2016, 775, 57–66. [Google Scholar] [CrossRef] [PubMed]
- De Jaco, A.; Bernardini, L.; Rosati, J.; Maria Tata, A. Alpha-7 nicotinic receptors in nervous system disorders: From function to therapeutic perspectives. Cent. Nerv. Syst. Agents Med. Chem. (Former. Curr. Med. Chem. Cent. Nerv. Syst. Agents) 2017, 17, 100–108. [Google Scholar] [CrossRef]
- Yang, T.; Xiao, T.; Sun, Q.; Wang, K. The current agonists and positive allosteric modulators of α7 nAChR for CNS indications in clinical trials. Acta Pharm. Sin. B. 2017, 7, 611–622. [Google Scholar] [CrossRef] [PubMed]
- Deutsch, S.I; Burket, J.A. An Evolving Therapeutic Rationale for Targeting the α7Nicotinic Acetylcholine Receptor in Autism Spectrum Disorder. Curr Top Behav Neurosci. 2020, 45, 167–208. [Google Scholar] [CrossRef] [PubMed]
- Hurley, L.L.; Tizabi, Y. Neuroinflammation, neurodegeneration, and depression. Neurotox Res. 2013, 23, 131–44. [Google Scholar] [CrossRef] [PubMed]
- Bjørklund, G.; Saad, K.; Chirumbolo, S.; Kern, J.K.; Geier, D.A.; Geier, M.R.; Urbina, M.A. Immune dysfunction and neuroinflammation in autism spectrum disorder. Acta Neurobiologiae Experimentalis. 2016, 76, 257–268. [Google Scholar] [CrossRef]
- Akhondzadeh, S. Microbiota and Autism Spectrum Disorder. Avicenna J Med Biotechnol. 2019, 11, 129. [Google Scholar]
- Matta, S.M.; Hill-Yardin, E.L.; Crack, P.J. The influence of neuroinflammation in Autism Spectrum Disorder. Brain Behav Immun. 2019, 79, 75–90. [Google Scholar] [CrossRef]
- Eissa, N.; Sadeq, A.; Sasse, A.; Sadek, B. Role of Neuroinflammation in Autism Spectrum Disorder and the Emergence of Brain Histaminergic System. Lessons Also for BPSD? Front Pharmacol. 2020, 11, 886. [Google Scholar] [CrossRef]
- Wong, R.S.Y. Neuroinflammation in autism spectrum disorders: potential target for mesenchymal stem cell-based therapy. Egypt J Neurol Psychiatry Neurosurg. 2022, 58, 91. [Google Scholar] [CrossRef]
- Hughes, H.K.; Moreno, R.J.; Ashwood, P. Innate immune dysfunction and neuroinflammation in autism spectrum disorder (ASD). Brain Behav Immun. 2023, 108, 245–254. [Google Scholar] [CrossRef] [PubMed]
- Lampiasi, N.; Bonaventura, R.; Deidda, I.; Zito, F.; Russo, R. Inflammation and the Potential Implication of Macrophage-Microglia Polarization in Human ASD: An Overview. Int J Mol Sci. 2023, 24, 2703. [Google Scholar] [CrossRef] [PubMed]
- Majhi, S.; Kumar, S.; Singh, L. A Review on Autism Spectrum Disorder: Pathogenesis, Biomarkers, Pharmacological and Non-Pharmacological Interventions. CNS Neurol Disord Drug Targets. 2023, 22, 659–677. [Google Scholar] [CrossRef]
- Usui, N.; Kobayashi, H.; Shimada, S. Neuroinflammation and Oxidative Stress in the Pathogenesis of Autism Spectrum Disorder. Int J Mol Sci. 2023, 24, 5487. [Google Scholar] [CrossRef] [PubMed]
- Gevezova, M.; Sarafian, V.; Anderson, G.; Maes, M. Inflammation and Mitochondrial Dysfunction in Autism Spectrum Disorder. CNS Neurol Disord Drug Targets. 2020, 19, 320–333. [Google Scholar] [CrossRef]
- Bäckhed, F.; Ley, R.E.; Sonnenburg, J.L.; Peterson, D.A.; Gordon, J.I. Host-bacterial mutualism in the human intestine. Science (New York, N.Y.). 2005, 307, 1915–1920. [Google Scholar] [CrossRef]
- Neish, A.S. Microbes in gastrointestinal health and disease. Gastroenterology. 2009, 136, 65–80. [Google Scholar] [CrossRef]
- Zhu, B.; Wang, X.; Li, L. Human gut microbiome: the second genome of human body. Protein Cell. 2010, 8, 718–725. [Google Scholar] [CrossRef]
- Shoemaker, W.R.; Chen, D.; Garud, N.R. Comparative Population Genetics in the Human Gut Microbiome. Genome Biol Evol. 2022, 14, evab116:1–evab116:11. [Google Scholar] [CrossRef]
- Chatterjee, G.; Negi, S.; Basu, S.; Faintuch, J.; O’Donovan, A.; Shukla, P. Microbiome systems biology advancements for natural well-being. Sci Total Environ. 2022, 838, 155915. [Google Scholar] [CrossRef]
- VanEvery, H.; Franzosa, E.A.; Nguyen, L.H.; Huttenhower, C. Microbiome epidemiology and association studies in human health. Nat Rev Genet. 2023, 24, 109–124. [Google Scholar] [CrossRef] [PubMed]
- Goodrich, J.K.; Davenport, E.R.; Clark, A.G.; Ley, R.E. The Relationship Between the Human Genome and Microbiome Comes into View. Annu Rev Genet. 2017, 51, 413–433. [Google Scholar] [CrossRef] [PubMed]
- Quan, Y.; Zhang, K.X.; Zhang, H.Y. The gut microbiota links disease to human genome evolution. Trends Genet. 2023, S0168-9525(23)00032-X. [Google Scholar] [CrossRef] [PubMed]
- Prochera, A.; Rao, M. Mini-Review: Enteric glial regulation of the gastrointestinal epithelium. Neurosci Lett. 2023, 137215. [Google Scholar] [CrossRef] [PubMed]
- Seguella, L.; Palenca, I.; Franzin, S.B.; Zilli, A.; Esposito, G. Mini-review: Interaction between intestinal microbes and enteric glia in health and disease. Neurosci Lett. 2023, 806, 137221. [Google Scholar] [CrossRef]
- Vicentini, F.A.; Keenan, C.M.; Wallace, L.E.; Woods, C.; Cavin, J.; Flockton, A.R.; Macklin, W.B.; Belkind-Gerson, J.; Hirota, S.A.; Sharkey, K.A. Intestinal microbiota shapes gut physiology and regulates enteric neurons and glia. Microbiome 2021, 9, 210. [Google Scholar] [CrossRef] [PubMed]
- Ogbonnaya, E.S.; Clarke, G.; Shanahan, F.; Dinan, T.G.; Cryan, J.F.; O’Leary, O.F. Adult Hippocampal Neurogenesis Is Regulated by the Microbiome. Biol Psychiatry. 2015, 78, e7–e9. [Google Scholar] [CrossRef]
- Luczynski, P.; Whelan, S.O.; O’Sullivan, C.; Clarke, G.; Shanahan, F.; Dinan, T.G.; Cryan, J.F. Adult microbiota-deficient mice have distinct dendritic morphological changes: differential effects in the amygdala and hippocampus. Eur J Neurosci. 2016, 44, 2654–2666. [Google Scholar] [CrossRef]
- Sudo, N.; Chida, Y.; Aiba, Y.; Sonoda, J.; Oyama, N.; Yu, X.N.; Kubo, C.; Koga, Y. Postnatal microbial colonization programs the hypothalamic-pituitary-adrenal system for stress response in mice. J Physiol. 2004, 558, 263–275. [Google Scholar] [CrossRef]
- Doroszkiewicz, J.; Groblewska, M.; Mroczko, B. The Role of Gut Microbiota and Gut-Brain Interplay in Selected Diseases of the Central Nervous System. International journal of molecular sciences. 2021, 22, 10028. [Google Scholar] [CrossRef]
- Escobar, Y.H.; O’Piela, D.; Wold, L.E.; Mackos, A.R. Influence of the Microbiota-Gut-Brain Axis on Cognition in Alzheimer’s Disease. J Alzheimers Dis. 2022, 87, 17–31. [Google Scholar] [CrossRef]
- Liang, X.; Fu, Y.; Cao, W.T.; Wang, Z.; Zhang, K.; Jiang, Z.; Jia, X.; Liu, C.Y.; Lin, H.R.; Zhong, H.; Miao, Z.; Gou, W.; Shuai, M.; Huang, Y.; Chen, S.; Zhang, B.; Chen, Y.M.; Zheng, J.S. Gut microbiome, cognitive function and brain structure: a multi-omics integration analysis. Transl Neurodegener. 2022, 11, 49. [Google Scholar] [CrossRef] [PubMed]
- Queiroz, S.A.L.; Ton, A.M.M.; Pereira, T.M.C.; Campagnaro, B.P.; Martinelli, L.; Picos, A.; Campos-Toimil, M.; Vasquez, E.C. The Gut Microbiota-Brain Axis: A New Frontier on Neuropsychiatric Disorders. Front Psychiatry. 2022, 13, 872594. [Google Scholar] [CrossRef] [PubMed]
- Lopera-Maya, E.A.; Kurilshikov, A.; van der Graaf, A.; Hu, S.; Andreu-Sánchez, S.; Chen, L.; Vila, A.V.; Gacesa, R.; Sinha, T.; Collij, V.; Klaassen, M.A.Y.; Bolte, L.A.; Gois, M.F.B.; Neerincx, P.B.T.; Swertz, M.A. ; LifeLines Cohort Study; Harmsen, H.J.M.; Wijmenga, C.; Fu, J.; Weersma, R.K.; Zhernakova, A.; Sanna, S. Effect of host genetics on the gut microbiome in 7,738 participants of the Dutch Microbiome Project. Nat Genet 2022, 54, 143–151. [Google Scholar] [CrossRef] [PubMed]
- Goodrich, J.K.; Waters, J.L.; Poole, A.C.; Sutter, J.L.; Koren, O.; Blekhman, R.; Beaumont, M.; Van Treuren, W.; Knight, R.; Bell, J.T.; Spector, T.D.; Clark, A.G.; Ley, R.E. Human genetics shape the gut microbiome. Cell. 2014, 159, 789–799. [Google Scholar] [CrossRef] [PubMed]
- Goodrich, J.K.; Davenport, E.R.; Beaumont, M.; Jackson, M.A.; Knight, R.; Ober, C.; Spector, T.D.; Bell, J.T.; Clark, A.G.; Ley, R.E. Genetic Determinants of the Gut Microbiome in UK Twins. Cell Host Microbe. 2016, 19, 731–743. [Google Scholar] [CrossRef]
- Rothschild, D.; Weissbrod, O.; Barkan, E.; Kurilshikov, A.; Korem, T.; Zeevi, D.; Costea, P.I.; Godneva, A.; Kalka, I.N.; Bar, N.; Shilo, S.; Lador, D.; Vila, A.V.; Zmora, N.; Pevsner-Fischer, M.; Israeli, D.; Kosower, N.; Malka, G.; Wolf, B.C.; Avnit-Sagi, T.; Lotan-Pompan, M.; Weinberger, A.; Halpern, Z.; Carmi, S.; Fu, J.; Wijmenga, C.; Zhernakova, A.; Elinav, E.; Segal, E. Environment dominates over host genetics in shaping human gut microbiota. Nature. 2018, 555, 210–215. [Google Scholar] [CrossRef] [PubMed]
- Pivrncova, E.; Kotaskova, I.; Thon, V. Neonatal Diet and Gut Microbiome Development After C-Section During the First Three Months After Birth: A Systematic Review. Front Nutr. 2022, 9, 941549. [Google Scholar] [CrossRef]
- Kang, D.W.; Adams, J.B.; Coleman, D.M.; Pollard, E.L.; Maldonado, J.; McDonough-Means, S.; Caporaso, J.G.; Krajmalnik-Brown, R. Long-term benefit of Microbiota Transfer Therapy on autism symptoms and gut microbiota. Sci Rep. 2019, 9, 5821. [Google Scholar] [CrossRef]
- Nichols, R.G.; Davenport, E.R. The relationship between the gut microbiome and host gene expression: a review. Hum Genet. 2021, 140, 747–760. [Google Scholar] [CrossRef]
- Campisciano, G.; Biffi, S. Microbiota in vivo imaging approaches to study host-microbe interactions in preclinical and clinical setting. Heliyon. 2022, 8, e12511. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Xu, J.; Chen, Y. Regulation of Neurotransmitters by the Gut Microbiota and Effects on Cognition in Neurological Disorders. Nutrients. 2021, 13, 2099. [Google Scholar] [CrossRef] [PubMed]
- Jameson, K.G.; Olson, C.A.; Kazmi, S.A.; Hsiao, E.Y. Toward Understanding Microbiome-Neuronal Signaling. Mol Cell. 2020, 78, 577–583. [Google Scholar] [CrossRef] [PubMed]
- Strandwitz, P. Neurotransmitter modulation by the gut microbiota. Brain Res. 2018, 1693, 128–133. [Google Scholar] [CrossRef] [PubMed]
- Zhong, J-G. ; Lan, W-T.; Feng, Y-Q.; Li, Y-H.; Shen, Y-Y.; Gong, J-H.; Zou, Z.; Hou, X. Associations between dysbiosis gut microbiota and changes of neurotransmitters and short-chain fatty acids in valproic acid model rats. Front Physiol. 2023, 14, 1077821. [Google Scholar] [CrossRef]
- Wang, H.; Braun, C.; Murphy, E.F.; Enck, P. Bifidobacterium longum 1714™ Strain Modulates Brain Activity of Healthy Volunteers During Social Stress. Am J Gastroenterol. 2019, 114, 1152–1162. [Google Scholar] [CrossRef]
- Kim, C.S.; Cha, L.; Sim, M.; Jung, S.; Chun, W.Y.; Baik, H.W.; Shin, DM. Probiotic Supplementation Improves Cognitive Function and Mood with Changes in Gut Microbiota in Community-Dwelling Older Adults: A Randomized, Double-Blind, Placebo-Controlled, Multicenter Trial. J Gerontol A Biol Sci Med Sci. 2021, 76, 32–40. [Google Scholar] [CrossRef]
- Kim, I.B.; Park, S.C.; Kim, Y.K. Microbiota-Gut-Brain Axis in Major Depression: A New Therapeutic Approach. Adv Exp Med Biol. 2023, 1411, 209–224. [Google Scholar] [CrossRef]
- Evrensel, A. Microbiome-Induced Autoimmunity and Novel Therapeutic Intervention. Adv Exp Med Biol. 2023, 1411, 71–90. [Google Scholar] [CrossRef]
- Mehra, A.; Arora, G.; Sahni, G.; Kaur, M.; Singh, H.; Singh, B.; Kaur, S. Gut microbiota and Autism Spectrum Disorder: From pathogenesis to potential therapeutic perspectives. J Tradit Complement Med. 2022, 13, 135–149. [Google Scholar] [CrossRef]
- van de Wouw, M.; Schellekens, H.; Dinan, T.G.; Cryan, J.F. Microbiota-Gut-Brain Axis: Modulator of Host Metabolism and Appetite. J Nutr. 2017, 147, 727–745. [Google Scholar] [CrossRef] [PubMed]
- Unger, M.M.; Spiegel, J.; Dillmann, K.U.; Grundmann, D.; Philippeit, H.; Bürmann, J.; Faßbender, K.; Schwiertz, A.; Schäfer, K.H. Short chain fatty acids and gut microbiota differ between patients with Parkinson’s disease and age-matched controls. Parkinsonism Relat Disord. 2016, 32, 66–72. [Google Scholar] [CrossRef] [PubMed]
- Morita, C.; Tsuji, H.; Hata, T.; Gondo, M.; Takakura, S.; Kawai, K.; Yoshihara, K.; Ogata, K.; Nomoto, K.; Miyazaki, K.; Sudo, N. Gut Dysbiosis in Patients with Anorexia Nervosa. PLoS One. 2015, 10, e0145274. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.; Li, E.; Sun, Z.; Fu, D.; Duan, G.; Jiang, M.; Yu, Y.; Mei, L.; Yang, P.; Tang, Y.; Zheng, P. Altered gut microbiota and short chain fatty acids in Chinese children with autism spectrum disorder. Sci Rep. 2019, 9, 287. [Google Scholar] [CrossRef] [PubMed]
- Wu, M.; Tian, T.; Mao, Q.; Zou, T.; Zhou, C.; Xie, J.; Chen, J. Associations between disordered gut microbiota and changes of neurotransmitters and short-chain fatty acids in depressed mice. Transl Psychiatry, 2020, 10, 350. [Google Scholar] [CrossRef] [PubMed]
- Parker, A.; Fonseca, S.; Carding, S.R. Gut microbes and metabolites as modulators of blood-brain barrier integrity and brain health. Gut Microbes. 2020, 11, 135–157. [Google Scholar] [CrossRef]
- Tang, W.; Zhu, H.; Feng, Y.; Guo, R.; Wan, D. The Impact of Gut Microbiota Disorders on the Blood-Brain Barrier. Infect Drug Resist. 2020, 13, 3351–3363. [Google Scholar] [CrossRef]
- Macfabe, D.F. Short-chain fatty acid fermentation products of the gut microbiome: implications in autism spectrum disorders. Microb Ecol Health Dis. 2012, 23, 19260. [Google Scholar] [CrossRef]
- Mayer, E.A.; Padua, D.; Tillisch, K. Altered brain-gut axis in autism: comorbidity or causative mechanisms. Bioessays. 2014, 36, 933–939. [Google Scholar] [CrossRef]
- Sharon, G.; Sampson, T.R.; Geschwind, D.H.; Mazmanian, S.K. The Central Nervous System and the Gut Microbiome. Cell. 2016, 167, 915–932. [Google Scholar] [CrossRef]
- Xiong, Y.; Chen, J.; Li, Y. Microglia and astrocytes underlie neuroinflammation and synaptic susceptibility in autism spectrum disorder. Front Neurosci. 2023, 17, 1125428. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Chauhan, A.; Sheikh, A.M.; Patil, S.; Chauhan, V.; Li, X.M.; Ji, L.; Brown, T.; Malik, M. Elevated immune response in the brain of autistic patients. J Neuroimmunol. 2009, 207, 111–116. [Google Scholar] [CrossRef]
- Rossignol, D.A.; Bradstreet, J.J.; Van Dyke, K.; Schneider, C.; Freedenfeld, S.H.; O’Hara, N.; Cave, S.; Buckley, J.A.; Mumper, E.A.; Frye, R.E. Hyperbaric oxygen treatment in autism spectrum disorders. Med Gas Res. 2012, 2, 16. [Google Scholar] [CrossRef] [PubMed]
- Davoli-Ferreira, M.; Thomson, C.A.; McCoy, K.D. Microbiota and Microglia Interactions in ASD. Front Immunol. 2021, 12, 676255. [Google Scholar] [CrossRef] [PubMed]
- Schafer, D.P.; Stevens, B. Microglia Function in Central Nervous System Development and Plasticity. Cold Spring Harb Perspect Biol. 2015, 7, a020545. [Google Scholar] [CrossRef] [PubMed]
- Ibrahim, S.H.; Voigt, R.G.; Katusic, S.K.; Weaver, A.L.; Barbaresi, W.J. Incidence of gastrointestinal symptoms in children with autism: a population-based study. Pediatrics. 2009, 124, 680–686. [Google Scholar] [CrossRef] [PubMed]
- Chaidez, V.; Hansen, R.L.; Hertz-Picciotto, I. Gastrointestinal problems in children with autism, developmental delays or typical development. J Autism Dev Disord. 2014, 44, 1117–1127. [Google Scholar] [CrossRef]
- Hyman, S.L.; Levy, S.E.; Myers, S.M. COUNCIL ON CHILDREN WITH DISABILITIES, SECTION ON DEVELOPMENTAL AND BEHAVIORAL PEDIATRICS. Identification, Evaluation, and Management of Children With Autism Spectrum Disorder. Pediatrics. 2020, 145, e20193447. [Google Scholar] [CrossRef]
- Bresnahan, M.; Hornig, M.; Schultz, A.F.; Gunnes, N.; Hirtz, D.; Lie, K.K.; Magnus, P.; Reichborn-Kjennerud, T.; Roth, C.; Schjølberg, S.; Stoltenberg, C.; Surén, P.; Susser, E.; Lipkin, W.I. Association of maternal report of infant and toddler gastrointestinal symptoms with autism: evidence from a prospective birth cohort. JAMA Psychiatry. 2015, 72, 466–474. [Google Scholar] [CrossRef]
- Buie, T.; Campbell, D.B.; Fuchs, G.J., 3rd.; Furuta, G.T.; Levy, J.; Vandewater, J.; Whitaker, A.H.; Atkins, D.; Bauman, M.L.; Beaudet, A.L.; Carr, E.G.; Gershon, M.D.; Hyman, S.L.; Jirapinyo, P.; Jyonouchi, H.; Kooros, K.; Kushak, R.; Levitt, P.; Levy, S.E.; Lewis, J.D.; Murray, K.F.; Natowicz, M.R.; Sabra, A.; Wershil, B.K.; Weston, S.C.; Zeltzer, L.; Winter, H. Evaluation, diagnosis, and treatment of gastrointestinal disorders in individuals with ASDs: a consensus report. Pediatrics 2010, 125 (Suppl 1), S1–S18. [Google Scholar] [CrossRef]
- Adams, J.B.; Johansen, L.J.; Powell, L.D.; Quig, D.; Rubin, R.A. Gastrointestinal flora and gastrointestinal status in children with autism--comparisons to typical children and correlation with autism severity. BMC Gastroenterol. 2011, 11, 22. [Google Scholar] [CrossRef] [PubMed]
- Kang, D.W.; Adams, J.B.; Gregory, A.C.; Borody, T.; Chittick, L.; Fasano, A.; Khoruts, A.; Geis, E.; Maldonado, J.; McDonough-Means, S.; Pollard, E.L.; Roux, S.; Sadowsky, M.J.; Lipson, K.S.; Sullivan, M.B.; Caporaso, J.G.; Krajmalnik-Brown, R. Microbiota Transfer Therapy alters gut ecosystem and improves gastrointestinal and autism symptoms: an open-label study. Microbiome. 2017, 5, 10. [Google Scholar] [CrossRef] [PubMed]
- Iglesias-Vázquez, L.; Van Ginkel Riba, G.; Arija, V.; Canals, J. Composition of Gut Microbiota in Children with Autism Spectrum Disorder: A Systematic Review and Meta-Analysis. Nutrients. 2020, 12, 792. [Google Scholar] [CrossRef]
- Abdelli, L.S.; Samsam, A.; Naser, S.A. Propionic Acid Induces Gliosis and Neuro-inflammation through Modulation of PTEN/AKT Pathway in Autism Spectrum Disorder. Sci Rep. 2019, 9, 8824. [Google Scholar] [CrossRef] [PubMed]
- Frye, R.E.; Nankova, B.; Bhattacharyya, S.; Rose, S.; Bennuri, S.C.; MacFabe, D.F. Modulation of Immunological Pathways in Autistic and Neurotypical Lymphoblastoid Cell Lines by the Enteric Microbiome Metabolite Propionic Acid. Front Immunol. 2017, 8, 1670. [Google Scholar] [CrossRef] [PubMed]
- Choi, J.; Lee, S.; Won, J.; Jin, Y.; Hong, Y.; Hur, T.Y.; Kim, J.H.; Lee, S.R.; Hong, Y. Pathophysiological and neurobehavioral characteristics of a propionic acid-mediated autism-like rat model. PLoS One. 2018, 13, e0192925. [Google Scholar] [CrossRef]
- Finegold, S.M.; Dowd, S.E.; Gontcharova, V.; Liu, C.; Henley, K.E.; Wolcott, R.D.; Youn, E.; Summanen, P.H.; Granpeesheh, D.; Dixon, D.; Liu, M.; Molitoris, D.R.; Green, J.A. 3rd. Pyrosequencing study of fecal microflora of autistic and control children. Anaerobe. 2010, 16, 444–453. [Google Scholar] [CrossRef]
- Deng, W.; Wang, S.; Li, F.; Wang, F.; Xing, Y.P.; Li, Y.; Lv, Y.; Ke, H.; Li, Z.; Lv, P.J.; Hao, H.; Chen, Y.; Xiao, X. Gastrointestinal symptoms have a minor impact on autism spectrum disorder and associations with gut microbiota and short-chain fatty acids. Front. Microbiol. 2022, 13, 1000419. [Google Scholar] [CrossRef]
- Lobzhanidze, G.; Lordkipanidze, T.; Zhvania, M.; Japaridze, N.; MacFabe, D.F.; Pochkidze, N.; Gasimov, E.; Rzaev, F. Effect of propionic acid on the morphology of the amygdala in adolescent male rats and their behavior. Micron. 2019, 125, 102732. [Google Scholar] [CrossRef]
- Virgolini, M.B.; Aschner, M. MOLECULAR MECHANISMS OF LEAD NEUROTOXICITY. Adv Neurotoxicol. 2021, 5, 159–213. [Google Scholar] [CrossRef]
- Rădulescu, A.; Lundgren, S. A pharmacokinetic model of lead absorption and calcium competitive dynamics. Sci Rep. 2019, 9, 14225. [Google Scholar] [CrossRef] [PubMed]
- Błażewicz, A.; Grabrucker, A.M. Metal Profiles in Autism Spectrum Disorders: A Crosstalk between Toxic and Essential Metals. Int J Mol Sci. 2022, 24, 308. [Google Scholar] [CrossRef] [PubMed]
- US Centres for Disease Control and Prevention (CDC). Childhood Lead Poisoning Prevention: CDC updates blood lead reference value to 3.5µg/dL. Available online: https://www.cdc.gov/nceh/lead/news/cdc-updates-blood-lead-reference-value.html (accessed on 16 April 2023).
- Cecil, K.M.; Brubaker, C.J.; Adler, C. M.; Dietrich, K.N.; Altaye, M.; Egelhoff, J.C.; Wessel, S.; Elangovan, I.; Hornung, R.; Jarvis, K.; Lanphear, B.P. Decreased brain volume in adults with childhood lead exposure. PLoS Med 2008, 27, e112. [Google Scholar] [CrossRef] [PubMed]
- Yuan, W.; Holland, S.K.; Cecil, K.M.; Dietrich, K.N.; Wessel, S.D.; Altaye, M.; Hornung, R.W.; Ris, M.D.; Egelhoff, J.C.; Lanphear, B.P. The impact of early childhood lead exposure on brain organization: a functional magnetic resonance imaging study of language function. Pediatrics. 2006, 118, 971–977. [Google Scholar] [CrossRef] [PubMed]
- Breysse, P.N.; Cascio, W.E.; Geller, A.M.; Choiniere, C.J.; Ammon, M. Targeting Coordinated Federal Efforts to Address Persistent Hazardous Exposures to Lead. Am J Public Health. 2022, 112, S640–S646. [Google Scholar] [CrossRef] [PubMed]
- LeBlanc, T.T.; Svendsen, E.R.; Allwood, P.B. Ubiquitous Lead— A Challenge for the Future of Public Health. Am J Public Health. 2022, 112, S628–S628. [Google Scholar] [CrossRef] [PubMed]
- Fruh, V.; Rifas-Shiman, S.L.; Amarasiriwardena, C.; Cardenas, A.; Bellinger, D.C.; Wise, L.A.; White, R.F.; Wright, R.O.; Oken, E.; Claus Henn, B. Prenatal lead exposure and childhood executive function and behavioral difficulties in project viva. Neurotoxicology. 2019, 75, 105–115. [Google Scholar] [CrossRef]
- Goel, A.; Aschner, M. The Effect of Lead Exposure on Autism Development. Int. J. Mol. Sci. 2021, 22, 1637. [Google Scholar] [CrossRef]
- Roberts, A.L.; Lyall, K.; Hart, J.E.; Laden, F.; Just, A.C.; Bobb, J.F.; Koenen, K.C.; Ascherio, A.; Weisskopf, M.G. Perinatal air pollutant exposures and autism spectrum disorder in the children of Nurses’ Health Study II participants. Environ Health Perspect. 2013, 121, 978–984. [Google Scholar] [CrossRef]
- Dickerson, A.S.; Rahbar, M.H.; Bakian, A.V.; Bilder, D.A.; Harrington, R.A.; Pettygrove, S.; Kirby, R.S.; Durkin, M.S.; Han, I.; Moyé, L.A. 3rd.; Pearson, D.A.; Wingate, M.S.; Zahorodny, W.M. Autism spectrum disorder prevalence and associations with air concentrations of lead, mercury, and arsenic. Environ Monit Assess. 2016, 188, 407. [Google Scholar] [CrossRef]
- Kim, K.N.; Kwon, H.J.; Hong, Y.C. Low-level lead exposure and autistic behaviors in school-age children. Neurotoxicology. 2016, 53, 193–200. [Google Scholar] [CrossRef] [PubMed]
- Arora, M.; Reichenberg, A.; Willfors, C.; Austin, C.; Gennings, C.; Berggren, S.; Lichtenstein, P.; Anckarsäter, H.; Tammimies, K.; Bölte, S. Fetal and postnatal metal dysregulation in autism. Nat Commun. 2017, 8, 15493. [Google Scholar] [CrossRef] [PubMed]
- Saghazadeh, A.; Rezaei, N. Systematic review and meta-analysis links autism and toxic metals and highlights the impact of country development status: Higher blood and erythrocyte levels for mercury and lead, and higher hair antimony, cadmium, lead, and mercury. Prog Neuropsychopharmacol Biol Psychiatry. 2017, 79, 340–368. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Q.; Huang, D.; Xu, C.; Wang, J.; Jin, Y. Hair levels of heavy metals and essential elements in Chinese children with autism spectrum disorder. J Trace Elem Med Biol. 2021, 66, 126748. [Google Scholar] [CrossRef] [PubMed]
- Fiłon, J.; Ustymowicz-Farbiszewska, J.; Krajewska-Kułak, E. Analysis of lead, arsenic and calcium content in the hair of children with autism spectrum disorder. BMC Public Health. 2020, 20, 383. [Google Scholar] [CrossRef] [PubMed]
- Fiore, M.; Barone, R.; Copat, C.; Grasso, A.; Cristaldi, A.; Rizzo, R.; Ferrante, M. Metal and essential element levels in hair and association with autism severity. J Trace Elem Med Biol. 2020, 57, 126409. [Google Scholar] [CrossRef]
- Frye, R.E.; Cakir, J.; Rose, S.; Delhey, L.; Bennuri, S.C.; Tippett, M.; Palmer, R.F.; Austin, C.; Curtin, P.; Arora, M. Early life metal exposure dysregulates cellular bioenergetics in children with regressive autism spectrum disorder. Transl Psychiatry. 2020, 10, 223. [Google Scholar] [CrossRef]
- Williams, C.L.; Smith, S.M. Calcium dependence of spontaneous neurotransmitter release. J Neurosci Res. 2018, 96, 335–347. [Google Scholar] [CrossRef]
- Eshra, A.; Schmidt, H.; Eilers, J.; Hallermann, S. Calcium dependence of neurotransmitter release at a high fidelity synapse. Elife. 2021, 10, e70408. [Google Scholar] [CrossRef]
- Li, L.; Liu, H.; Krout, M.; Richmond, J.E.; Wang, Y.; Bai, J.; Weeratunga, S.; Collins, B.M.; Ventimiglia, D.; Yu, Y.; Xia, J.; Tang, J.; Liu, J.; Hu, Z. A novel dual Ca2+ sensor system regulates Ca2+-dependent neurotransmitter release. J Cell Biol. 2021, 220, e20200812. [Google Scholar] [CrossRef]
- Wu, C.; Sun, D. GABA receptors in brain development, function, and injury. Metab Brain Dis. 2015, 30, 367–379. [Google Scholar] [CrossRef] [PubMed]
- Shi, L.Z.; Zheng, W. Early lead exposure increases the leakage of the blood-cerebrospinal fluid barrier, in vitro. Hum Exp Toxicol. 2007, 26, 159–167. [Google Scholar] [CrossRef] [PubMed]
- Cheadle, L.; Rivera, S.A.; Phelps, J.S.; Ennis, K.A.; Stevens, B.; Burkly, L.C.; Lee, W.A.; Greenberg, M.E. Sensory Experience Engages Microglia to Shape Neural Connectivity through a Non-Phagocytic Mechanism. Neuron. 2020, 108, 451–468. [Google Scholar] [CrossRef]
- Davoli-Ferreira, M.; Thomson, C.A.; McCoy, K.D. Microbiota and Microglia Interactions in ASD. Front Immunol. 2021, 12, 676255. [Google Scholar] [CrossRef] [PubMed]
- Colonna, M.; Butovsky, O. Microglia Function in the Central Nervous System During Health and Neurodegeneration. Annu Rev Immunol. 2017, 35, 441–468. [Google Scholar] [CrossRef] [PubMed]
- O’Callaghan, J.P.; Sriram, K. Glial fibrillary acidic protein and related glial proteins as biomarkers of neurotoxicity. Expert Opin. Drug Saf. 2005, 4, 433–442. [Google Scholar] [CrossRef]
- Pathak, D.; Sriram, K. Molecular Mechanisms Underlying Neuroinflammation Elicited by Occupational Injuries and Toxicants. Int J Mol Sci. 2023, 24, 2272. [Google Scholar] [CrossRef]
- Han, Q.; Zhang, W.; Guo, J.; Zhu, Q.; Chen, H.; Xia, Y.; Zhu, G. Mitochondrion: a sensitive target for Pb exposure. J Toxicol Sci. 2021, 46, 345–358. [Google Scholar] [CrossRef]
- Sadykov, R.; Digel, I.; Artmann, A.T.; Porst, D.; Linder, P.; Kayser, P.; Artmann, G.; Savitskaya, I.; Zhubanova, A. Oral lead exposure induces dysbacteriosis in rats. J Occup Health. 2009, 51, 64–73. [Google Scholar] [CrossRef]
- Xia, J.; Lu, L.; Jin, C.; Wang, S.; Zhou, J.; Ni, Y.; Fu, Z.; Jin, Y. Effects of short term lead exposure on gut microbiota and hepatic metabolism in adult zebrafish. Comp Biochem Physiol C Toxicol Pharmacol. 2018, 209, 1–8. [Google Scholar] [CrossRef]
- Patsiou, D.; Del Rio-Cubilledo, C.; Catarino, A.I.; Summers, S.; Mohd Fahmi, A.; Boyle, D.; Fernandes, T.F.; Henry, TB. Exposure to Pb-halide perovskite nanoparticles can deliver bioavailable Pb but does not alter endogenous gut microbiota in zebrafish. Sci Total Environ. 2020, 715, 136941. [Google Scholar] [CrossRef] [PubMed]
- Kou, H.; Fu, Y.; He, Y.; Jiang, J.; Gao, X.; Zhao, H. Chronic lead exposure induces histopathological damage, microbiota dysbiosis and immune disorder in the cecum of female Japanese quails (Coturnix japonica). Ecotoxicol Environ Saf. 2019, 183, 109588. [Google Scholar] [CrossRef] [PubMed]
- Liu, W.; Feng, H.; Zheng, S.; Xu, S.; Massey, I.Y.; Zhang, C.; Wang, X.; Yang, F. Pb Toxicity on Gut Physiology and Microbiota. Front Physiol. 2021, 12, 574913:1–574913:12. [Google Scholar] [CrossRef] [PubMed]
- Zhai, Q.; Li, T.; Yu, L.; Xiao, Y.; Feng, S.; Wu, J.; Zhao, J.; Zhang, H.; Chen, W. Effects of subchronic oral toxic metal exposure on the intestinal microbiota of mice. Sci Bull (Beijing). 2017, 62, 831–840. [Google Scholar] [CrossRef] [PubMed]
- Mangalam, A.; Shahi, S.K.; Luckey, D.; Karau, M.; Marietta, E.; Luo, N.; Choung, R.S.; Ju, J.; Sompallae, R.; Gibson-Corley, K.; Patel, R.; Rodriguez, M.; David, C.; Taneja, V.; Murray, J. Human Gut-Derived Commensal Bacteria Suppress CNS Inflammatory and Demyelinating Disease. Cell Rep, 2017, 20, 1269–1277. [Google Scholar] [CrossRef] [PubMed]
- Shao, M.; Zhu, Y. Long-term metal exposure changes gut microbiota of residents surrounding a mining and smelting area. Sci. Rep. 2020, 10, 4453. [Google Scholar] [CrossRef]
- Yu, L.; Yu, Y.; Yin, R.; Duan, H.; Qu, D.; Tian, F.; Narbad, A.; Chen, W.; Zhai, Q. Dose-dependent effects of lead induced gut injuries: An in vitro and in vivo study. Chemosphere. 2021, 266, 129130. [Google Scholar] [CrossRef]
- Markowiak, P.; Śliżewska, K. Effects of Probiotics, Prebiotics, and Synbiotics on Human Health. Nutrients. 2017, 9, 1021. [Google Scholar] [CrossRef]
- Zhai, Q.; Wang, J.; Cen, S.; Zhao, J.; Zhang, H.; Tian, F.; Chen, W. Modulation of the gut microbiota by a galactooligosaccharide protects against heavy metal lead accumulation in mice. Food Funct. 2019, 10, 3768–3781. [Google Scholar] [CrossRef]
- Menees, K.B.; Otero, B.A.; Tansey, M.G. Microbiome influences on neuro-immune interactions in neurodegenerative disease. Int Rev Neurobiol. 2022, 167, 25–57. [Google Scholar] [CrossRef]
- Kumar, N.; Sahoo, N.K.; Mehan, S.; Verma, B. The importance of gut-brain axis and use of probiotics as a treatment strategy for multiple sclerosis. Mult Scler Relat Disord. 2023, 71, 104547. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).




