Submitted:
20 April 2023
Posted:
21 April 2023
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Materials and Methods
3. Results
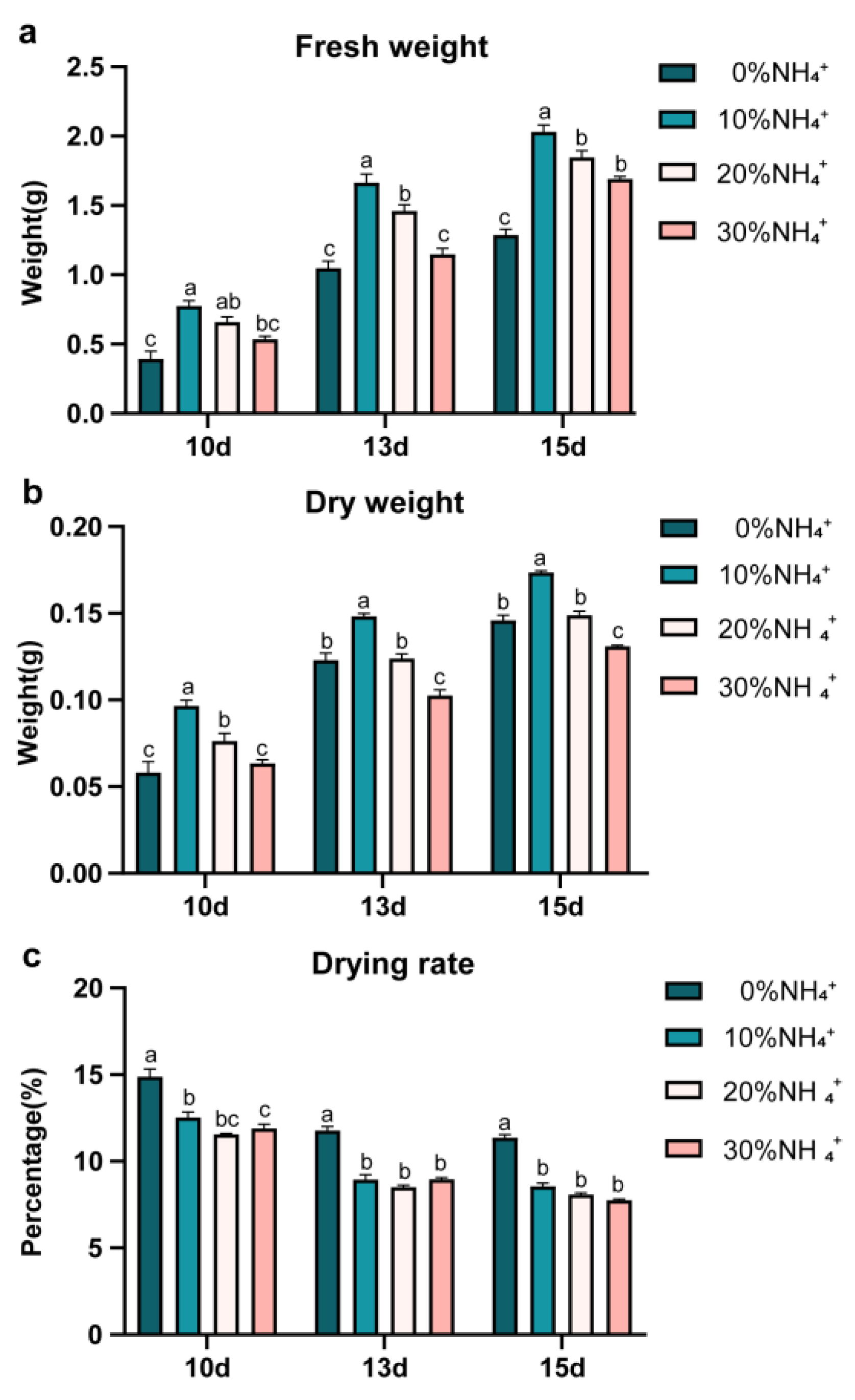
3.1. Growth of AEHR in Response to Ammonium
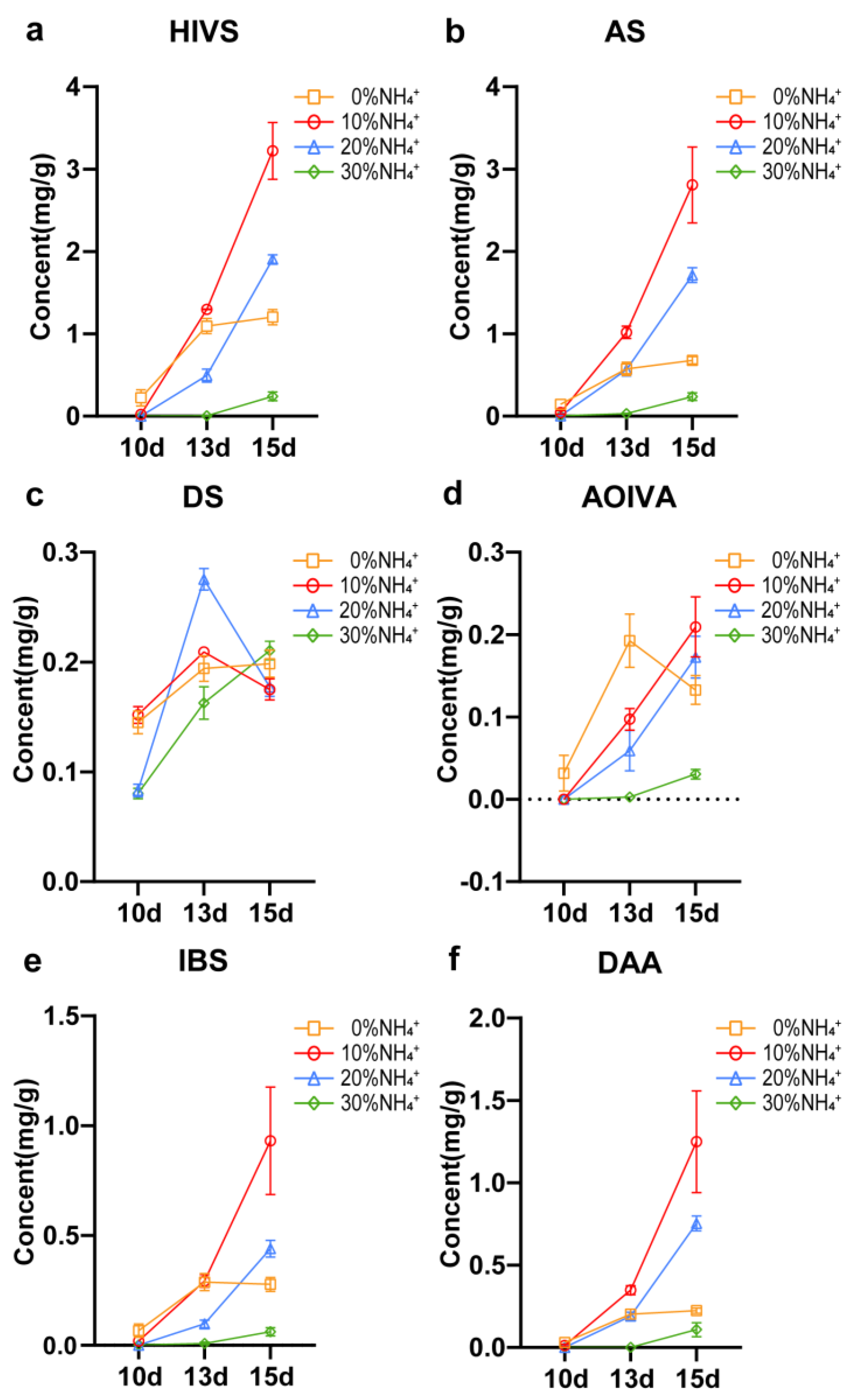
3.2. Accumulation of Shikonin in AEHR in Response to Ammonium
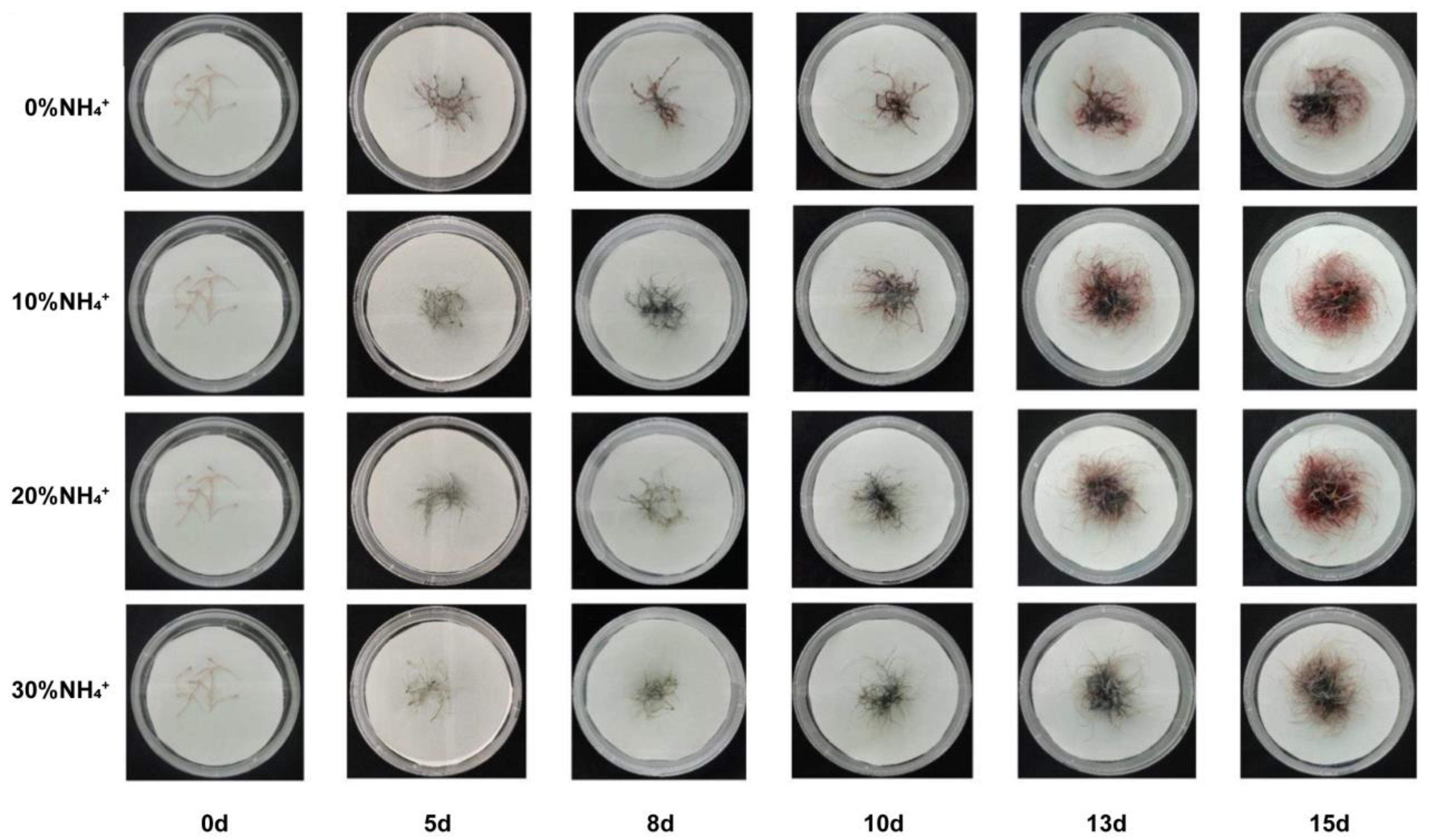
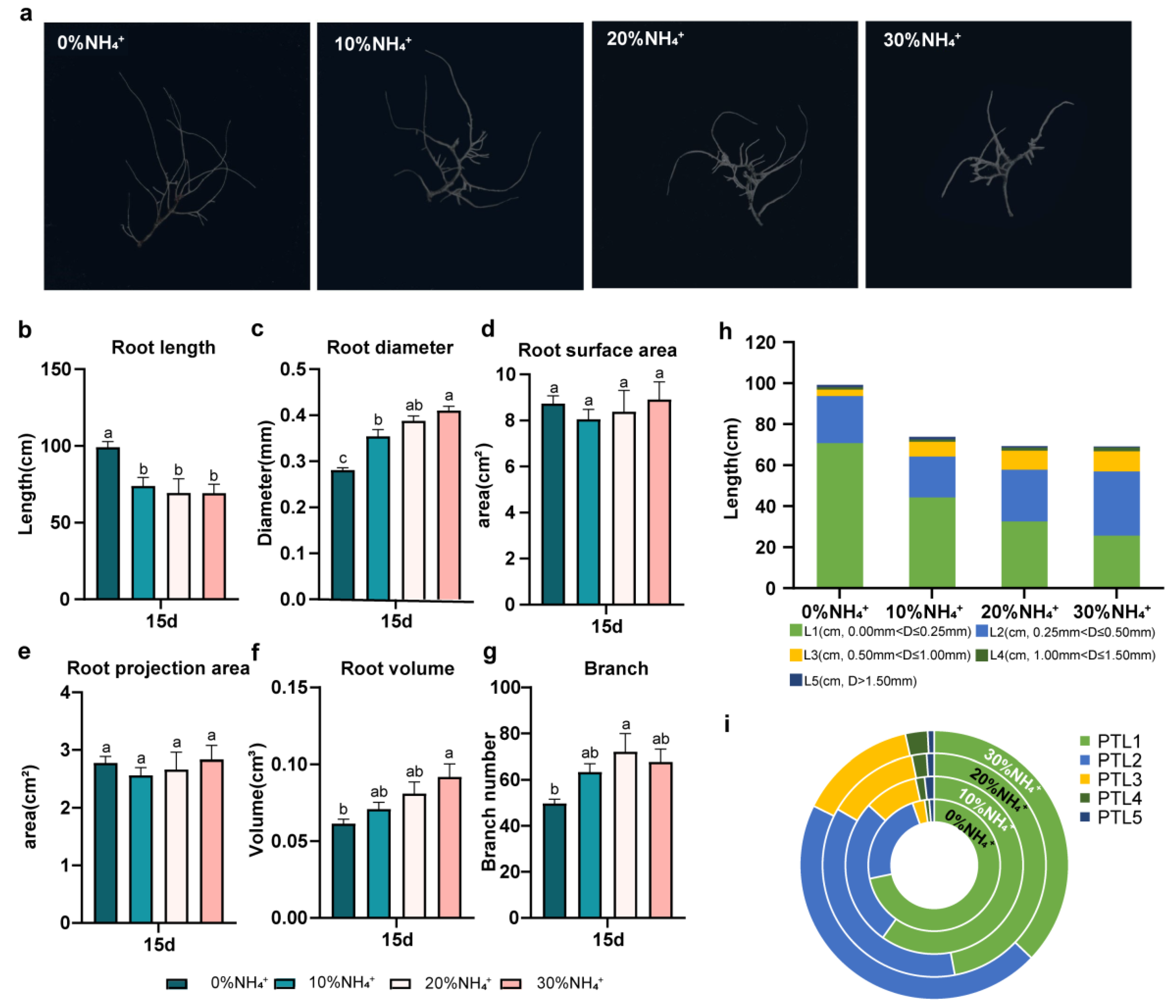
3.3. Development and Morphology of AEHR in Reponse to Ammonium
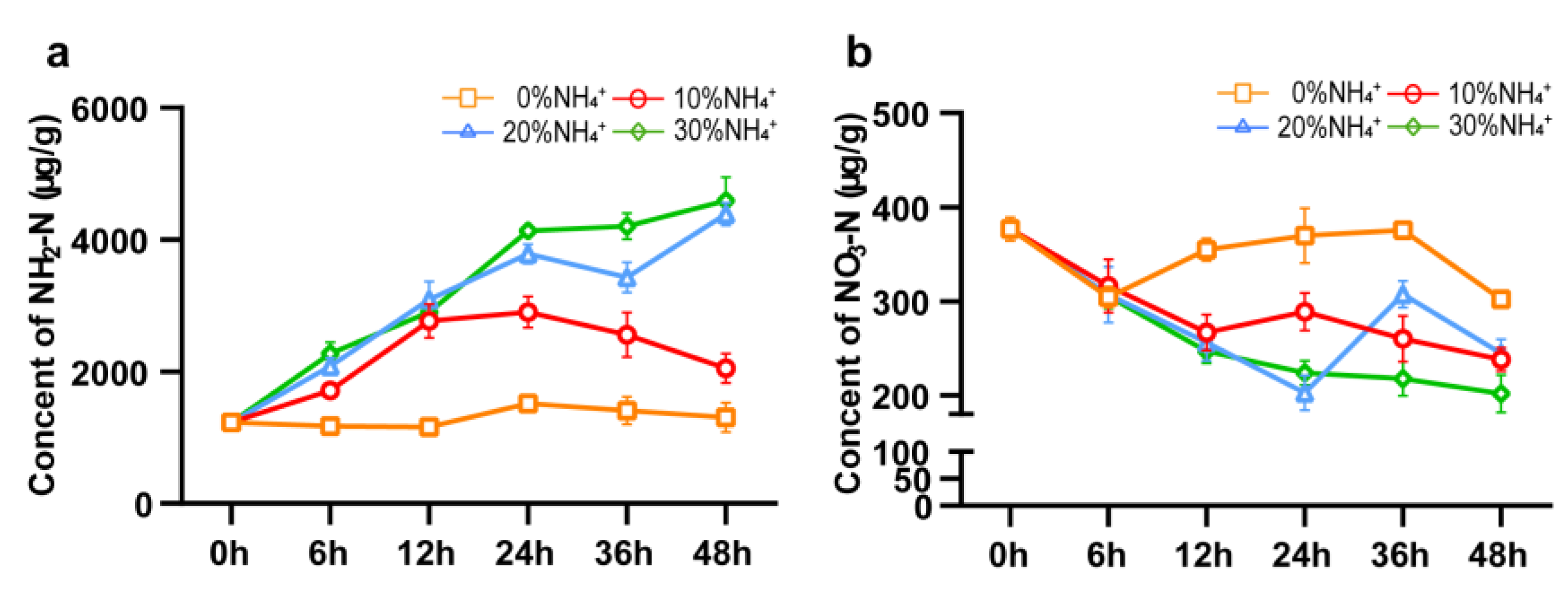
3.4. Nitrogen Assimilation in AEHR under Short-term Ammonium Treatment
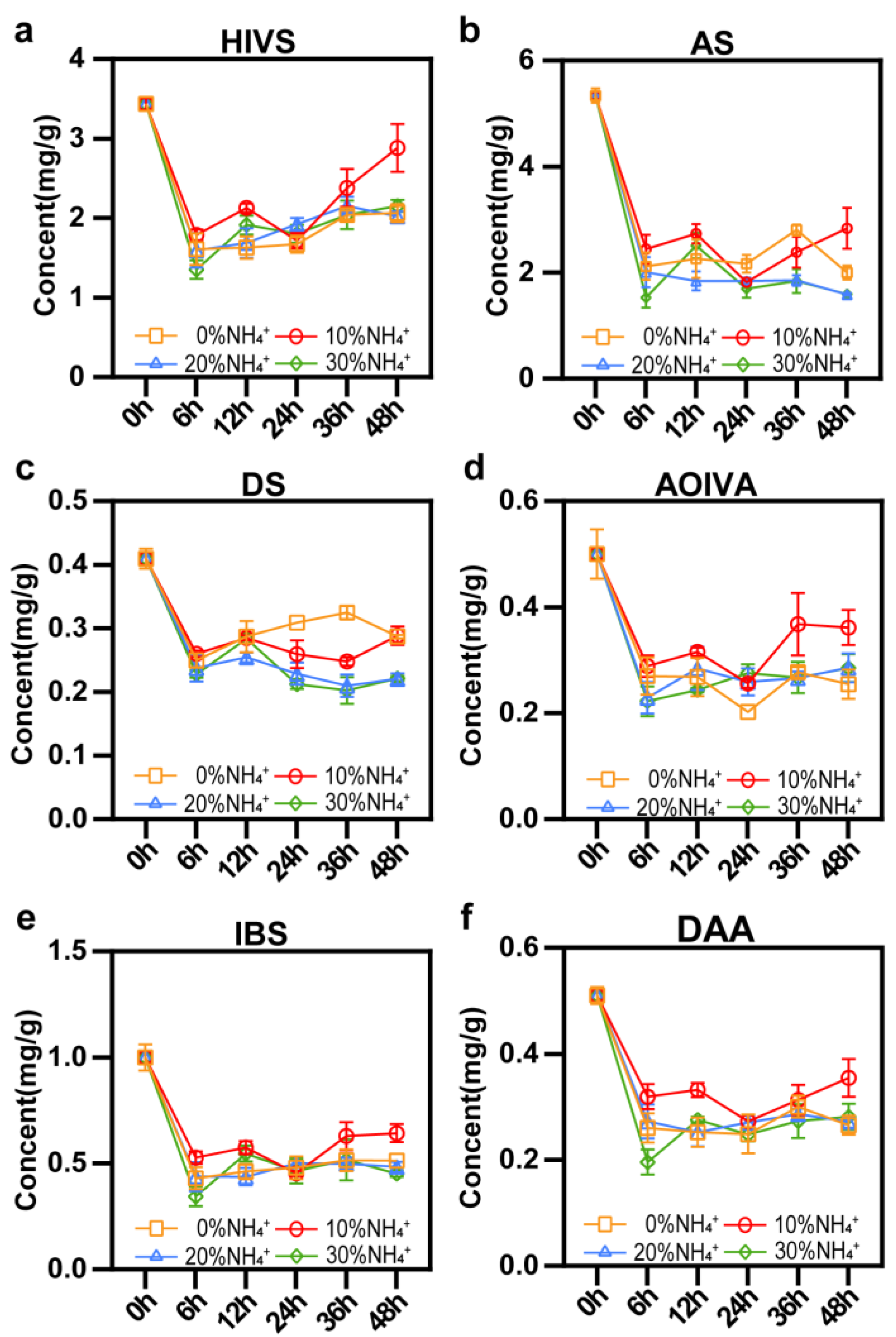
3.5. Shikonin Biosynthesis in AEHR under Short-term Ammonium Treatment
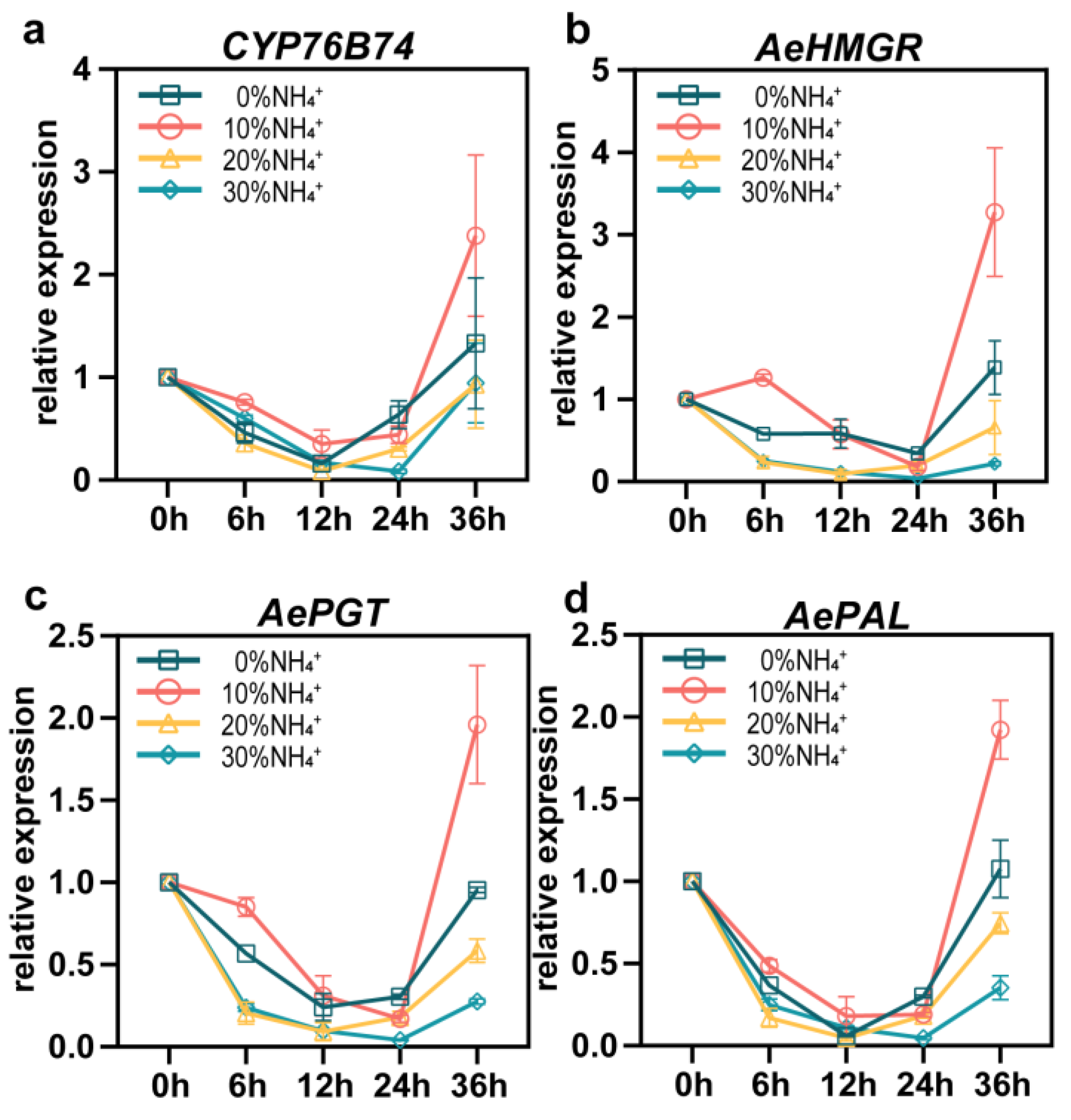
3.6. Gene Expression of Key Enzymes in Shikonin Biosynthesis Pathway
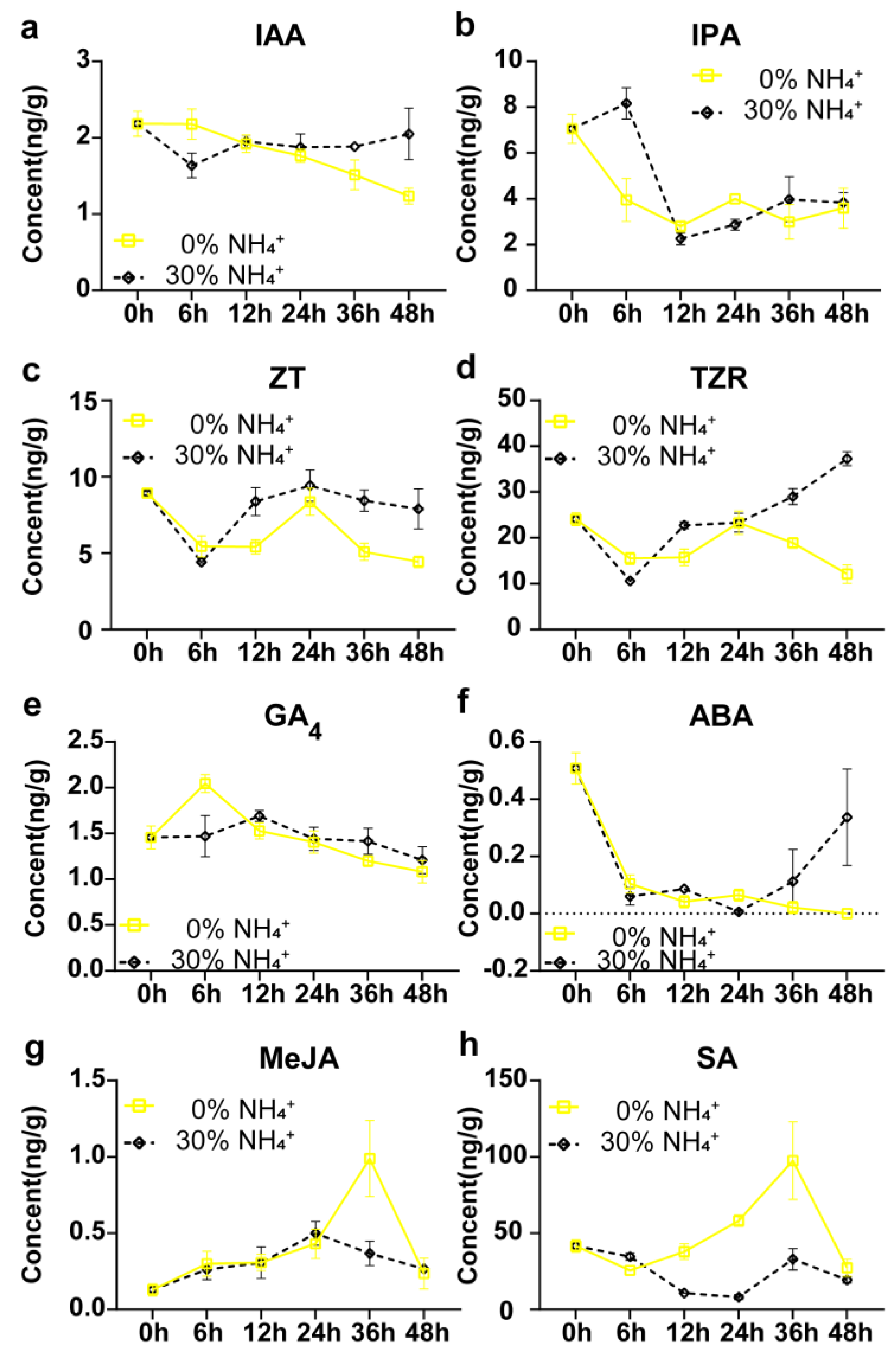
3.7. Hormone Metabolism of AEHR under NH4+ Toxicity
4. Discussion and Conclusion
4.1. Arnebiae Euchroma is an Ammonium-Sensitive Species
4.2. Compare to the Sole Nitrate Nitrogen source, A. euchroma Prefers an Appropriate Ammonium/Nitrate ratio
| Varieties | Step | Medium | NH4+(mM) | NO3-(mM) | Total content (mM) | NH4+/NO3- ratio | Reference |
| L.erythorhizon.乐suspension乐cell cultures | Two-step for growth | LS | 20.61 | 39.4 | 60.01 | 34.34% | [11] |
| MG-5 | 6.25 | 54.22 | 60.47 | 10.34% | [6] | ||
| Two-step for shikonin production | White | 0 | 3.33 | 3.33 | 0.00% | [11] | |
| M9 | 0 | 6.67 | 6.67 | 0.00% | [11] | ||
| L. erythorhizon. hairy roots | Two-step for growth | B5 | 2.02 | 24.73 | 26.75 | 7.55% | [16,31] |
| Two-step for shikonin production | M9 | 0 | 6.67 | 6.67 | 0.00% | [16,31] | |
| A. erchroma suspension cell cultures | One-step | AG-7 | 7 | 18.79 | 25.79 | 27.14% | [41,42] |
| Two-step for growth | AG-7 | 7 | 18.79 | 25.79 | 27.14% | [42] | |
| LS | 20.61 | 39.4 | 60.01 | 34.34% | [43] | ||
| MS | 20.61 | 39.4 | 60.01 | 34.34% | [44,45] | ||
| Two-step for shikonin production | M9 | 0 | 6.67 | 6.67 | 0.00% | [42,46,47,48,49,50,51] | |
| M10 | 0 | 10.97 | 10.97 | 0.00% | [43] | ||
| APM | 0 | 17.74 | 17.74 | 0.00% | [44,45] | ||
| A. erchroma hairy roots | Two-step for growth | SH | 2.61 | 24.73 | 27.34 | 9.55% | [51] |
| SH without NH4+ | 0 | 24.73 | 24.73 | 0.00% | [47,50,52] | ||
| MS without NH4+ | 0 | 18.79 | 18.79 | 0.00% | [47,52] | ||
| B5 without NH4+ | 0 | 24.73 | 24.73 | 0.00% | [47] | ||
| 1/2MS without NH4+ | 0 | 9.395 | 9.395 | 0.00% | [46,52] | ||
| LS without NH4+ | 0 | 18.79 | 18.79 | 0.00% | [47] | ||
| MG-5 without NH4+ | 0 | 47.97 | 47.97 | 0.00% | [52] | ||
| MS | 20.61 | 39.4 | 60.01 | 34.34% | [47] | ||
| B5 | 2.02 | 24.73 | 26.75 | 7.55% | [49] | ||
| Two-step for shikonin production | M9 | 0 | 6.67 | 6.67 | 0.00% | [47,50] | |
| Onosma paniculatum suspension cell cultures | Two-step for growth | B5 | 2.02 | 24.73 | 26.75 | 7.55% | [53] |
| Two-step for shikonin production | M9 | 0 | 6.67 | 6.67 | 0.00% | [53] |
4.3. Auxin and Cytokinin Might Regulate the Growth and Architecture of AEHR under NH4+ Toxicity
4.4. Shikonin Synthesis of AEHR under NH4+ Toxicity and Its Possible Hormones Regulation Mechanism
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A. Supplementary Data
References
- Shindo, S.; Hosokawa, Y.; Hosokawa, I.; Ozaki, K.; Matsuo, T. Shikonin Inhibits Inflammatory Cytokine Production in Human Periodontal Ligament Cells. Inflammation 2016, 39, 1124-1129. [CrossRef]
- Cocchi, F.; DeVico, A.L.; Yarchoan, R.; Redfield, R.; Cleghorn, F.; Blattner, W.A.; Garzino-Demo, A.; Colombini-Hatch, S.; Margolis, D.; Gallo, R.C. Higher macrophage inflammatory protein (MIP)-1alpha and MIP-1beta levels from CD8+ T cells are associated with asymptomatic HIV-1 infection. Proceedings of the National Academy of Sciences of the United States of America 2000, 97, 13812-13817. [CrossRef]
- Tong, Y.; Bai, L.; Gong, R.; Chuan, J.; Duan, X.; Zhu, Y. Shikonin Protects PC12 Cells Against β-amyloid Peptide-Induced Cell Injury Through Antioxidant and Antiapoptotic Activities. Scientific reports 2018, 8, 26. [CrossRef]
- Wang, B.; Wu, Z.; Wang, J.; Li, W.; Liu, G.; Zhang, B.; Tang, Y. Insights into the mechanism of Arnebia euchroma on leukemia via network pharmacology approach. BMC complementary medicine and therapies 2020, 20, 322. [CrossRef]
- Ahn, B.Z.; Baik, K.U.; Kweon, G.R.; Lim, K.; Hwang, B.D. Acylshikonin analogues: synthesis and inhibition of DNA topoisomerase-I. Journal of medicinal chemistry 1995, 38, 1044-1047. [CrossRef]
- Yazaki, K. Lithospermum erythrorhizon cell cultures: Present and future aspects. Plant biotechnology (Tokyo, Japan) 2017, 34, 131-142. [CrossRef]
- Papageorgiou, V.P.; Assimopoulou, A.N.; Couladouros, E.A.; Hepworth, D.; Nicolaou, K.C. The Chemistry and Biology of Alkannin, Shikonin, and Related Naphthazarin Natural Products. Angew Chem Int Ed Engl 1999, 38, 270-301. [CrossRef]
- Wang, S.; Wang, R.; Liu, T.; Lv, C.; Liang, J.; Kang, C.; Zhou, L.; Guo, J.; Cui, G.; Zhang, Y.; et al. CYP76B74 Catalyzes the 3''-Hydroxylation of Geranylhydroquinone in Shikonin Biosynthesis. Plant physiology 2019, 179, 402-414. [CrossRef]
- Tabata, M.; Mizukami, H.; Hiraoka, N.; Konoshima, M. Pigment formation in callus cultures of Lithospermum erythrorhizon. Phytochemistry 1974, 13, 927-932. [CrossRef]
- Fu, S.L.; Shang, T.M.; Xiao, P.G. Analysis of naphthoquinone pigments in several Arnebiae Radix. Acta Pharmaceutica Sinica 1984, 921-925. [CrossRef]
- Fujita, Y.; Hara, Y.; Ogino, T.; Suga, C. Production of shikonin derivatives by cell suspension cultures of Lithospermum erythrorhizon : I. Effects of nitrogen sources on the production of shikonin derivatives. Plant cell reports 1981, 1, 59-60. [CrossRef]
- Arghavani, P.; Haghbeen, K.; Mousavi, A. Enhancement of Shikalkin Production in Arnebia euchroma Callus by a Fungal Elicitor, Rhizoctonia solani. Iranian journal of biotechnology 2015, 13, 10-16. [CrossRef]
- Fang, R.; Zou, A.; Zhao, H.; Wu, F.; Zhu, Y.; Zhao, H.; Liao, Y.; Tang, R.J.; Pang, Y.; Yang, R.; et al. Transgenic studies reveal the positive role of LeEIL-1 in regulating shikonin biosynthesis in Lithospermum erythrorhizon hairy roots. BMC Plant Biol 2016, 16, 121. [CrossRef]
- Srinivasan, V.; Ryu, D.D. Improvement of shikonin productivity in Lithospermum erythrorhizon cell culture by alternating carbon and nitrogen feeding strategy. Biotechnology and bioengineering 1993, 42, 793-799. [CrossRef]
- Tani, M.; Takeda, K.; Yazaki, K.; Tabata, M. Effects of oligogalacturonides on biosynthesis of shikonin in Lithospermum cell cultures. Phytochemistry 1993, 34, 1285-1290. [CrossRef]
- Zhao, H.; Baloch, S.K.; Kong, L.R.; Zhang, W.J.; Zou, A.L.; Wang, X.M.; Qi, J.L.; Yang, Y.H. Molecular cloning, characterization, and expression analysis of LeMYB1 from Lithospermum erythrorhizon. Biologia plantarum 2014, 58, 436-444. [CrossRef]
- Sun, M.H.; Lu, X.P.; Cao, X.J.; Li, J.; Xiong, J.; Xie, X.X. Effect of different forms of nitrogen on the activity of nitrate reductase and expression of the relative genes in Citrus sinensis ×Poncirus trifoliate. Journal of Fruit Science 2017, 34, 410-417. [CrossRef]
- Wang, Q.; Wang, K.C.; Zheng, C.X.; Zhu, B.C.; Wang, Y.L.; Yang, J.W.; Wang, W. Effect of different nitrogenous forms on growth and chemical component in tuber of Pinellia pedatisecta Schott. . Journal of Plant Nutrition and Fertilizers 2014, 20, 1038-1043. [CrossRef]
- Crawford, N.M.; Glass, A.D.M. Molecular and physiological aspects of nitrate uptake in plants. Trends in plant science 1998, 3, 389-395. [CrossRef]
- Migocka, M.; Warzybok, A.; Kłobus, G. The genomic organization and transcriptional pattern of genes encoding nitrate transporters 1 (NRT1) in cucumber. Plant and Soil 2012, 364, 245-260. [CrossRef]
- Wang, S.; Guo, L.P.; Xie, T.; Yang, J.; Tang, J.F.; Li, X.; Wang, X.; Huang, L.Q. Different secondary metabolic responses to MeJA treatment in shikonin-proficient and shikonin-deficient cell lines from Arnebia euchroma (Royle) Johnst. Plant Cell, Tissue and Organ Culture (PCTOC) 2014, 119, 587-598. [CrossRef]
- Kuchipudi, S.V.; Tellabati, M.; Nelli, R.K.; White, G.A.; Perez, B.B.; Sebastian, S.; Slomka, M.J.; Brookes, S.M.; Brown, I.H.; Dunham, S.P.; et al. 18S rRNA is a reliable normalisation gene for real time PCR based on influenza virus infected cells. Virol J 2012, 9, 230. [CrossRef]
- Gazzarrini, S.; Lejay, L.; Gojon, A.; Ninnemann, O.; Frommer, W.B.; von Wirén, N. Three functional transporters for constitutive, diurnally regulated, and starvation-induced uptake of ammonium into Arabidopsis roots. Plant Cell 1999, 11, 937-948. [CrossRef]
- Xu, Q.F.; Tsai, C.L.; Tsai, C.Y. Interaction of potassium with the form and amount of nitrogen nutrition on growth and nitrogen uptake of maize. Journal of Plant Nutrition 1992, 15, 23-33. [CrossRef]
- Song, W.; Zhuang, Y.; Liu, T. CYP82AR Subfamily Proteins Catalyze C-1' Hydroxylations of Deoxyshikonin in the Biosynthesis of Shikonin and Alkannin. Organic letters 2021, 23, 2455-2459. [CrossRef]
- Yazaki, K.; Kunihisa, M.; Fujisaki, T.; Sato, F. Geranyl diphosphate:4-hydroxybenzoate geranyltransferase from Lithospermum erythrorhizon. Cloning and characterization of a ket enzyme in shikonin biosynthesis. The Journal of biological chemistry 2002, 277, 6240-6246. [CrossRef]
- Britto, D.T.; Kronzucker, H.J. NH4+ toxicity in higher plants: a critical review. Journal of Plant Physiology 2002, 159, 567-584. [CrossRef]
- Esteban, R.; Ariz, I.; Cruz, C.; Moran, J.F. Review: Mechanisms of ammonium toxicity and the quest for tolerance. Plant science : an international journal of experimental plant biology 2016, 248, 92-101. [CrossRef]
- Yoshikawa, N.; Fukui, H.; Tabata, M. Effect of gibberellin A3 on shikonin production in Lithospermum callus cultures. Phytochemistry 1986, 25, 621-622. [CrossRef]
- Kumar, P.; Saini, M.; Bhushan, S.; Warghat, A.R.; Pal, T.; Malhotra, N.; Sood, A. Effect of salicylic acid on the activity of PAL and PHB geranyltransferase and shikonin derivatives production in cell suspension cultures of Arnebia euchroma (Royle) Johnst--a medicinally important plant species. Appl Biochem Biotechnol 2014, 173, 248-258. [CrossRef]
- Zhao, H.; Chang, Q.S.; Zhang, D.X.; Fang, R.J.; Zhao, H.; Wu, F.Y.; Wang, X.M.; Lu, G.H.; Qi, J.L.; Yang, Y.H. Overexpression of LeMYB1 enhances shikonin formation by up-regulating key shikonin biosynthesis-related genes in Lithospermum erythrorhizon. Biologia plantarum 2015, 59, 429-435. [CrossRef]
- Liu, Y.; Lai, N.; Gao, K.; Chen, F.; Yuan, L.; Mi, G. Ammonium inhibits primary root growth by reducing the length of meristem and elongation zone and decreasing elemental expansion rate in the root apex in Arabidopsis thaliana. PLoS One 2013, 8, e61031. [CrossRef]
- Chen, H.; Jia, Y.; Xu, H.; Wang, Y.; Zhou, Y.; Huang, Z.; Yang, L.; Li, Y.; Chen, L.-S.; Guo, J. Ammonium nutrition inhibits plant growth and nitrogen uptake in citrus seedlings. Scientia Horticulturae 2020, 272, 109526. [CrossRef]
- Hachiya, T.; Watanabe, C.K.; Fujimoto, M.; Ishikawa, T.; Takahara, K.; Kawai-Yamada, M.; Uchimiya, H.; Uesono, Y.; Terashima, I.; Noguchi, K. Nitrate Addition Alleviates Ammonium Toxicity Without Lessening Ammonium Accumulation, Organic Acid Depletion and Inorganic Cation Depletion in Arabidopsis thaliana Shoots. Plant and Cell Physiology 2012, 53, 577-591. [CrossRef]
- Hessini, K.; Kronzucker, H.J.; Abdelly, C.; Cruz, C. Drought stress obliterates the preference for ammonium as an N source in the C(4) plant Spartina alterniflora. J Plant Physiol 2017, 213, 98-107. [CrossRef]
- Hill, T.D.; Sommer, N.R.; Kanaskie, C.R.; Santos, E.A.; Oczkowski, A.J. Nitrogen uptake and allocation estimates for Spartina alterniflora and Distichlis spicata. J Exp Mar Biol Ecol 2018, 21, 466-472. [CrossRef]
- Garnica, M.; Houdusse, F.; Zamarreno, A.M.; Garcia-Mina, J.M. The signal effect of nitrate supply enhances active forms of cytokinins and indole acetic content and reduces abscisic acid in wheat plants grown with ammonium. J Plant Physiol 2010, 167, 1264-1272. [CrossRef]
- Petersen, F.; Demann, J.; Restemeyer, D.; Ulbrich, A.; Olfs, H.W.; Westendarp, H.; Appenroth, K.J. Influence of the Nitrate-N to Ammonium-N Ratio on Relative Growth Rate and Crude Protein Content in the Duckweeds Lemna minor and Wolffiella hyalina. Plants (Basel) 2021, 10, 1741. [CrossRef]
- Mantovani, C.; Prado, R.M.; Pivetta, K.F.L. Impact of Nitrate and Ammonium ratio on Nutrition and Growth of two Epiphytic Orchids. An Acad Bras Cienc 2018, 90, 3423-3431. [CrossRef]
- Carr, N.F.; Boaretto, R.M.; Mattos, D., Jr. Coffee seedlings growth under varied NO(3)(-):NH(4)(+) ratio: Consequences for nitrogen metabolism, amino acids profile, and regulation of plasma membrane H(+)-ATPase. Plant Physiol Biochem 2020, 154, 11-20. [CrossRef]
- Hao, H.; Lei, C.; Dong, Q.; Shen, Y.; Chi, J.; Ye, H.; Wang, H. Effects of exogenous methyl jasmonate on the biosynthesis of shikonin derivatives in callus tissues of Arnebia euchroma. Appl Biochem Biotechnol 2014, 173, 2198-2210. [CrossRef]
- Kang, Q.S.; Li, H.L.; Wu, Y.L.; Lu, D.Y. Studies on cell growth and Product Synthesis in Production Stage Culture of Arnebia euchroma Cell II: Fermentation culture. Natural Product Research and Development 2003, 15, 429-432. [CrossRef]
- Ge, F.; Yuan, X.; Wang, X.; Zhao, B.; Wang, Y. Cell growth and shikonin production of Arnebia euchroma in a periodically submerged airlift bioreactor. Biotechnology letters 2006, 28, 525-529. [CrossRef]
- Malik, S.; Bhushan, S.; Sharma, M.; Singh Ahuja, P. Physico-chemical factors influencing the shikonin derivatives production in cell suspension cultures of Arnebia euchroma (Royle) Johnston, a medicinally important plant species. Cell biology international 2011, 35, 153-158. [CrossRef]
- Malik, S.; Bhushan, S.; Verma, S.C.; Sharma, N.; Sinha, A.K.; Sharma, M.; Ahuja, P.S. Production of naphthoquinone pigments in cell suspension cultures of Arnebia euchroma (Royle) Johnston: influence of pH on growth kinetics and acetylshikonin. Med Aromat Plant Sci Biotechnol 2008, 2, 43-49.
- Liang, J.W.; Li, T.; Wang, R.S.; Zhou, L.; Yang, Q.; Wang, S.; Guo, L.P. Establishment of RNA interfered hairy root system of two CYP450 genes in Arnebia euchroma and its influence. China Journal of Chinese Materia Medica 2020, 45, 3422-3431. [CrossRef]
- Lu, W.H.; Chen, Y.F.; Wang, F.; Dai, N.B.; Hao, A.H.; Li, C.F.; Jia, S.E. Effects of physical and chemical conditions on hairy root culture and content of Arnebia euchromu. Journal of Huazhong Agricultural University 2012, 31, 50-54. [CrossRef]
- Zhang, P.; Wang, F.; Zhu, C. Influence of fungal elicitor and macroporous resin on shikonin accumulation in hairy roots of Arnebia euchroma (Royle) Johnst. Chinese Journal of Biotechnology 2013, 29, 214-223. [CrossRef]
- Yang, Y.H.; Lu, J.; Zhao, Q.H.; Cao, R.Q. Effect of water extract of algae on growth and pigment formation in Lithospermuml erythrorhizon and Arnebia euchromu cell cultures. Journal of Plant Resources and Environment 1992, 1, 39-44.
- Ge, S.N.; Zhang, S.X.; Wang, F.; Wei, H.; Xie, W.L.; Xie, J.; Li, C.; Zhao, H. Effects of Exogenous Substances on the Content of Secondary Metabolites in Arnebia euchroma Johnst Hairy Roots. Food Science 2016, 37, 160-164. [CrossRef]
- Li, M.; Pan, Q.; Wang, F.; Zhang, P. Research of Effects on Arnebia euchroma (Royle)Johnst Hairy Roots Growth Factors. Xinjiang Agricultural Sciences 2012, 49, 2062-2068. [CrossRef]
- Lu, W.H.; Pan, Q.; Wang, F.; Chen, Y.F.; Dai, N.B. Influence of Culture Conditions on Arnebia euchromaHairy Roots Growth and Shikonin Content. Acta Botanica Boreali-Occidentalia Sinica 2012, 32, 1686-1691. [CrossRef]
- Yang, Y.; Zhang, H.; Cao, R. Effect of Brassinolide on Growth and Shikonin Formation in Cultured Onosma paniculatum Cells. J Plant Growth Regul 1999, 18, 89-92. [CrossRef]
- Yang, H.; von der Fecht-Bartenbach, J.; Friml, J.; Lohmann, J.U.; Neuh User, B.; Ludewig, U. Auxin-modulated root growth inhibition in Arabidopsis thaliana seedlings with ammonium as the sole nitrogen source. Funct Plant Biol 2015, 42, 239-251. [CrossRef]
- Dziewit, K.; Pěnčík, A.; Dobrzyńska, K.; Novák, O.; Szal, B.; Podgórska, A. Spatiotemporal auxin distribution in Arabidopsis tissues is regulated by anabolic and catabolic reactions under long-term ammonium stress. BMC Plant Biol 2021, 21, 602. [CrossRef]
- Péret, B.; De Rybel, B.; Casimiro, I.; Benková, E.; Swarup, R.; Laplaze, L.; Beeckman, T.; Bennett, M.J. Arabidopsis lateral root development: an emerging story. Trends in plant science 2009, 14, 399-408. [CrossRef]
- Barreto, R.F.; de Mello Prado, R.; Lúcio, J.C.B.; López-Díaz, I.; Carrera, E.; Carvalho, R.F. Ammonium Toxicity Alleviation by Silicon is Dependent on Cytokinins in Tomato cv. Micro-Tom. Journal of Plant Growth Regulation 2021, 41, 417-428. [CrossRef]
- Walch-Liu, P.; Neumann, G.; Bangerth, F.; Engels, C. Rapid effects of nitrogen form on leaf morphogenesis in tobacco. Journal of experimental botany 2000, 51, 227-237. [CrossRef]
- Fang, D.Q.; Hou, C.S.; Li, X.M.; Ye, H.C.; Li, G.F. Effects of pH and hormone on cell growth and synthesis of Shikonin derivatives in suspension culture of Arnebia euchroma. Plant Science Journal 1994, 159-164.
- Zhu, W.H.; Fan, H.X.; Hu, Q.; Zhu, H.Q. Induction culture of callus and selection of superior clones of Onosma paniculatum. Journal of Chinese Medicinal Materials 1990, 6-9. [CrossRef]
- Yan, H.Y.; Cao, R.Q. Influencing factors of Shikonin formation in callus culture of Lithospermum erythorhizon Acta Agriculturae Boreali-Sinica 2002, 116-120. [CrossRef]
- Ahmad, M.; Varela Alonso, A.; Koletti, A.E.; Rodić, N.; Reichelt, M.; Rödel, P.; Assimopoulou, A.N.; Paun, O.; Declerck, S.; Schneider, C.; et al. Dynamics of alkannin/shikonin biosynthesis in response to jasmonate and salicylic acid in Lithospermum officinale. Scientific reports 2022, 12, 17093. [CrossRef]
- Ding, J.; Shi, S.; Jiang, B.-H.; Yang, Y.-H.; Huang, J.; Shen, H.-G.; Xia, K.; Zhang, J.; Jiang, X. Effects of Methyl jasmonate with indole-3-acetic acid and 6-benzylaminopurine on the secondary metabolism of cultured Onosma paniculatum cells. In Vitro Cellular & Developmental Biology - Plant 2004, 40, 581-585. [CrossRef]
- Zhu, Y. Cloning, expression and functional analysis of LeTCP4 gene in Lithospermum erythrorhizon. Master's degree, Nanjing University,Nanjing, 2012.
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





