Submitted:
12 April 2023
Posted:
12 April 2023
You are already at the latest version
Abstract
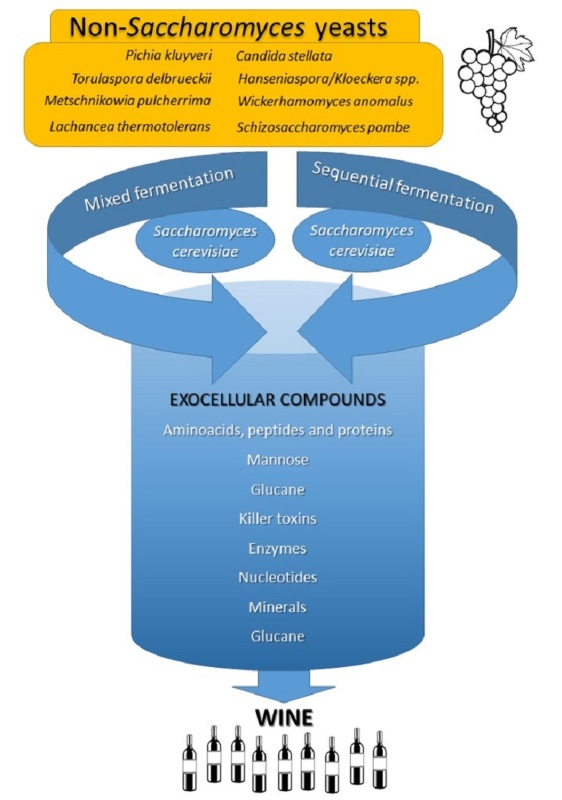
Keywords:
1. Introduction
2. Saccharomyces cerevisiae
3. Non-Saccharomyces yeasts
3.1. Schizosaccharomyces pombe
3.2. Pichia kluyveri
3.3. Torulaspora delbrueckii
3.4. Wickerhamomyces anomalus
3.5. Metschnikowia pulcherrima
3.6. Hanseniaspora/Kloeckera spp.
3.7. Lachancea thermotolerans
3.8. Candida stellata
4. Conclusions
Funding
Conflicts of Interest
References
- Pretorius, I.S. Tailoring wine yeast for the new millennium: novel approaches to the ancient art of winemaking. Yeast 2000, 16, 675–729. [Google Scholar] [CrossRef] [PubMed]
- Fleet, G.H. Yeast interactions and wine flavour. International journal of food microbiology 2003, 86, 11–22. [Google Scholar] [CrossRef] [PubMed]
- Maicas, S. Advances in wine fermentation. Fermentation 2021, 7. [Google Scholar] [CrossRef]
- Barata, A.; Malfeito-Ferreira, M.; Loureiro, V. The microbial ecology of wine grape berries. International journal of food microbiology 2012, 153, 243–259. [Google Scholar] [CrossRef]
- Maicas, S. The use of alternative technologies to develop malolactic fermentation in wine. Applied Microbiology and Biotechnology 2001, 56, 35–39. [Google Scholar] [CrossRef] [PubMed]
- Lonvaud-Funel, A. Lactic acid bacteria in the quality improvement and depreciation of wine. Antonie van Leeuwenhoek 1999, 76, 317–331. [Google Scholar] [CrossRef] [PubMed]
- Boulton, R. The application of microbiology in the wine industry. Critical reviews in biotechnology 1996, 16, 305–322. [Google Scholar]
- Di Maio, S.; Polizzotto, G. Native yeasts for wine fermentation: A review. Fermentation 2021, 7, 28. [Google Scholar] [CrossRef]
- Belda, I.; Ruiz, J.; Alastruey-Izquierdo, A.; Navascués, E.; Marquina, D.; Santos, A.; Calderón, F. Unraveling the contribution of indigenous and co-inoculated yeast to wine fermentation. Food microbiology 2020, 92, 103571. [Google Scholar] [CrossRef]
- Knight, S.; Klaere, S.; Fedrizzi, B.; Goddard, M.R.; Jiranek, V. Regional microbial signatures positively correlate with differential wine phenotypes: evidence for a microbial aspect to terroir. Scientific reports 2018, 8, 1–12. [Google Scholar] [CrossRef]
- Lencioni, L.; Romani, C.; Gobbi, M.; Capece, A.; Tirelli, A. Candida zemplinina and Saccharomyces cerevisiae mixed fermentations to enhance complexity and variability of wines from Malvasia grapes. Food microbiology 2020, 87, 103390. [Google Scholar] [CrossRef]
- Longo, R.; Capece, A.; Comi, G. Selection of indigenous Saccharomyces cerevisiae strains to make Amarone wine. Annals of Microbiology 2018, 68, 479–487. [Google Scholar] [CrossRef]
- Rojo Bezares, B.; Sanchez-Patàn, F.; Palop, M.L.; López-Alfaro, I. Diversity and dynamics of yeast communities in wine fermentation processes. Annals of Microbiology 2018, 68, 363–374. [Google Scholar] [CrossRef]
- Santamaría, P.; López-Rituerto, E.; Garijo, P. Wine fermentation microbiome: a landscape from different Spanish wine denominations. Food microbiology 2019, 82, 43–50. [Google Scholar] [CrossRef]
- Terrat, S.; Lhomme, E.; Tarnus, E.; Bargier, C.; Thomas, S.; Maman, S.; Alexandre, H. Environmental microbiology reveals complex microbial communities in native and inoculated fermentations for Banyuls wine. PloS one 2020, 15, e0231164. [Google Scholar] [CrossRef]
- Deed, R.C.; Fedrizzi, B.; Gardner, J.M.; Jiranek, V.; Borneman, A.R. Variability of wine yeast strain populations across seasons and locations in an Australian vineyard. Frontiers in Microbiology 2021, 12, 1285. [Google Scholar] [CrossRef]
- Fuss, A.; du Toit, M. The impact of grape variety, harvest year and yeast strain on the yeast population during the fermentation of South African Pinotage wines. South African Journal of Enology and Viticulture 2021, 42, 1–10. [Google Scholar] [CrossRef]
- Maicas, S. The role of yeasts in fermentation processes. Microorganisms 2020, 8, 1–8. [Google Scholar] [CrossRef]
- Tofalo, R.; Perpetuini, G.; Rossetti, A.P.; Gaggiotti, S.; Piva, A.; Olivastri, L.; Cichelli, A.; Compagnone, D.; Arfelli, G. Impact of Saccharomyces cerevisiae and non-Saccharomyces yeasts to improve traditional sparkling wines production. Food Microbiology 2022, 108. [Google Scholar] [CrossRef]
- Ciani, M.; Capece, A.; Comitini, F.; Canonico, L.; Siesto, G.; Romano, P. Yeast interactions in inoculated wine fermentation. Frontiers in Microbiology 2016, 7. [Google Scholar] [CrossRef]
- Tofalo, R.; Schirone, M.; Fasoli, G.; Perpetuini, G.; Perpetuini, F.; Corsetti, A.; Suzzi, G. The contribution of non-Saccharomyces yeasts to wine aroma. Annals of Microbiology 2019, 69, 1027–1041. [Google Scholar] [CrossRef]
- Medina-Trujillo, L.; González-Robles, I.A.; Durán-Cruz, M.J.; Escalante-Minakata, P. Diversity and dynamics of yeast communities during fermentation of “Criolla” grape musts. Frontiers in Microbiology 2018, 9, 2570. [Google Scholar] [CrossRef]
- Masneuf-Pomarède, I.; Bely, M.; Marullo, P.; Albertin, W. The genetics of non-conventional wine yeasts: current knowledge and future challenges. Frontiers in Microbiology 2019, 10, 2766. [Google Scholar] [CrossRef] [PubMed]
- Jolly, N.P.; Varela, C.; Pretorius, I.S. Not your ordinary yeast: Non-Saccharomyces yeasts in wine production uncovered. FEMS Yeast Research 2014, 14, 215–237. [Google Scholar] [CrossRef] [PubMed]
- Pérez-Martín, F.; Medina-Trujillo, L.; Escott, C.; Rodríguez, M.E.; García-Martínez, T. Use of non-Saccharomyces yeasts alone or in combination with Saccharomyces cerevisiae during alcoholic fermentation of Tempranillo wine: impact on aroma compounds. Food research international 2019, 121, 613–620. [Google Scholar] [CrossRef]
- González-Arenzana, L.; Moreno-Arribas, M.V. Wine microbiome: a dynamic world of microbial interactions. Crit Rev Food Sci Nutr 2018, 58, 2371–2381. [Google Scholar] [CrossRef]
- Jolly, N.P.; Augustyn, O.P.H. The use of non-Saccharomyces yeast species in wine production. South African Journal of Enology and Viticulture 2018, 39, 137–146. [Google Scholar] [CrossRef]
- Padilla, B.; Gil, J.V.; Manzanares, P. Challenges of the Non-Conventional Yeast Wickerhamomyces anomalus in Winemaking. Fermentation 2018, 4. [Google Scholar] [CrossRef]
- Pretorius, I.S. Tailoring wine yeast for the new millennium: novel approaches to the ancient art of winemaking. Yeast 2019, 36, 191–202. [Google Scholar] [CrossRef]
- Varela, C.; Borneman, A.R. Yeast found on grapes and in musts during winemaking. Yeast 2018, 35, 111–128. [Google Scholar] [CrossRef]
- Grangeteau, C.; Gerhards, D.; Rousseaux, S.; von Wallbrunn, C.; Alexandre, H.; Guilloux-Benatier, M. Contribution of non-Saccharomyces yeasts to wine volatile and sensory diversity: A study on Lachancea thermotolerans, Metschnikowia pulcherrima and Candida zemplinina. Food Microbiology 2020, 91, 103499. [Google Scholar]
- Liu, J.; Zhu, B.; Zhang, Z. Impact of Non-Saccharomyces Yeasts on Volatile Compound Profiles in Wine Fermentation: A Review. Frontiers in Bioengineering and Biotechnology 2021, 9, 629219. [Google Scholar]
- Varela, C. Sensory impact of non-Saccharomyces yeasts in wine. Current Opinion in Food Science 2019, 28, 1–6. [Google Scholar]
- Padilla, B.; Gil, J.V.; Manzanares, P. Past and Future of Non-Saccharomyces Yeasts: From Spoilage Microorganisms to Biotechnological Tools for Improving Wine Aroma Complexity. Frontiers in Microbiology 2016, 7. [Google Scholar] [CrossRef] [PubMed]
- Vegi, A.; Alexopoulos, A.; Chrysanthopoulos, P.K.; Koutinas, A.A.; Kookos, I.K. The Potential of Non-Saccharomyces Yeasts in Beer Production. Fermentation 2018, 4, 1. [Google Scholar]
- Fleet, G.H. Non-Saccharomyces yeasts: the underestimated and indispensable contribution of early co-fermentations in wine. Journal of Industrial Microbiology & Biotechnology 2019, 46, 1201–1219. [Google Scholar]
- Contreras, A.; Escott, C.; Cuesta, I.; Torija, M.J.; Gonzalez-Arenzana, L.; Garijo, P.; Lopez-Alfaro, I.; Lopez, R.; Suarez-Lepe, J.A. Exploring the potential of non-Saccharomyces yeasts for cider fermentation: a comparative study with Saccharomyces cerevisiae. Food Microbiology 2021, 95, 103685. [Google Scholar] [CrossRef]
- Gallone, B.; Steensels, J.; Prahl, T.; Soriaga, L.; Saels, V.; Herrera-Malaver, B.; Merlevede, A.; Roncoroni, M.; Voordeckers, K.; Miraglia, L.; et al. Domestication and divergence of Saccharomyces cerevisiae beer yeasts. Cell 2016, 166, 1397–1410. [Google Scholar] [CrossRef]
- Liu, S.Q. Non-Saccharomyces Yeasts: The Secondary Players in Wine Fermentation. In Wine Science: Principles and Applications, 3ed.; 2018; pp. 353–383. [Google Scholar]
- Schüller, D.; Lombardero, J.; Guillamón, J.M.; del Olmo, M.L. Exploring the potential of non-Saccharomyces yeasts for the reduction of alcohol content in wine. Applied Microbiology and Biotechnology 2019, 103, 9269–9277. [Google Scholar]
- Padilla, B.; Gil, J.V.; Manzanares, P. The impact of non-Saccharomyces yeast species in the production of sparkling wines. Foods 2020, 9, 1252. [Google Scholar]
- Fleet, G.H. Wine yeasts for the future. FEMS Yeast Research 2008, 8, 979–995. [Google Scholar] [CrossRef] [PubMed]
- Peinado, R.A.; Moreno, J.J.; Maestre, O.; Mauricio, J.C. Removing gluconic acid by using different treatments with a Schizosac- charomyces pombe mutant: Effect on fermentation byproducts. Food Chemistry 2007, 104, 457–465. [Google Scholar] [CrossRef]
- Palomero, F.; Morata, A.; Benito, S.; Calderón, F.; Suárez-Lepe, J. New genera of yeasts for over-lees aging of red wine. Food Chemistry 2009, 112, 432–441. [Google Scholar] [CrossRef]
- Palomero, F.; Ntanos, K.; Morata, A.; Benito, S.; Suárez-Lepe, J. Reduction of wine 4-ethylphenol concentration using lyophilized yeast as a bioadsorbent: influence on anthocyanin content and chromatic variables. Eur Food Res Technol 2011, 232, 971–977. [Google Scholar] [CrossRef]
- Benito, S.; Hofmann, T.; Laier, M.; Lochbühler, B.; Schüttler, A.; Ebert, K.; Fritsch, S.; Röcker, J.; Rauhut, D. Effect on quality and composition of Riesling wines fermented by sequential inoculation with non-Saccharomyces and Saccharomyces cerevisiae. Eur Food Res Technol 2015, 241, 707–717. [Google Scholar] [CrossRef]
- Sgouros, G.; Mallouchos, A.; Dourou, D.; Banilas, G.; Chalvantzi, I.; Kourkoutas, Y.; Nisiotou, A. Torulaspora delbrueckii May Help Manage Total and Volatile Acidity of Santorini-Assyrtiko Wine in View of Global Warming. Foods 2023, 12, 191. [Google Scholar] [CrossRef]
- Silva-Sousa, F.; Fernandes, T.; Pereira, F.; Rodrigues, D.; Rito, T.; Camarasa, C.; Franco-Duarte, R.; Sousa, M.J. Torulaspora delbrueckii Phenotypic and Metabolic Profiling towards Its Biotechnological Exploitation. J Fungi (Basel) 2022, 8, 569. [Google Scholar] [CrossRef]
- Wang, R.; Sun, J.; Lassabliere, B.; Yu, B.; Liu, S.Q. Fermentation characteristics of four non-Saccharomyces yeasts in green tea slurry. Food Microbiol 2020, 92, 103609. [Google Scholar] [CrossRef]
- Ramírez, M.; Velázquez, R.; Maqueda, M.; Zamora, E.; López-Piñeiro, A.; Hernández, L.M. Influence of the dominance of must fermentation by Torulaspora delbrueckii on the malolactic fermentation and organoleptic quality of red table wine. Int J Food Microbiol 2016, 238, 311–319. [Google Scholar] [CrossRef]
- Corbu, V.M.; Csutak, O. Molecular and Physiological Diversity of Indigenous Yeasts Isolated from Spontaneously Fermented Wine Wort from Ilfov County, Romania. Microorganisms 2022, 11, 37. [Google Scholar] [CrossRef]
- Ramírez, M.; Velázquez, R.; López-Piñeiro, A.; Martínez, A. Genome Features of a New Double-Stranded RNA Helper Virus (LBCbarr) from Wine Torulaspora delbrueckii Killer Strains. International Journal of Molecular Sciences 2021, 22, 13492. [Google Scholar] [CrossRef] [PubMed]
- Heard, G.M.; Fleet, G.H. Growth of Natural Yeast Flora during the Fermentation of Inoculated Wines. Applied and Environmental Microbiology 1985, 50, 727–728. [Google Scholar] [CrossRef] [PubMed]
- Díaz, C.; Molina, A.M.; Nähring, J.; Fischer, R. Characterization and Dynamic Behavior of Wild Yeast during Spontaneous Wine Fermentation in Steel Tanks and Amphorae. BioMed Research International 2013, 540465. [Google Scholar] [CrossRef] [PubMed]
- Sabel, A.; Martens, S.; Petri, A.; König, H.; Claus, H. Wickerhamomyces anomalus AS1: a new strain with potential to improve wine aroma. Annals of Microbiology 2014, 64, 483–491. [Google Scholar] [CrossRef]
- Nascimento, B.L.; Martelli, E.C.; da Silva, J.C.; Delabeneta, M.F.; Rosseto, L.R.; Junges, D.S.; Paris, A.P.; Persel, C.; Paula, C.R.; Simão, R.C.; et al. Inhibition of Klebsiella pneumoniae carbapenemases by mycocins produced by Wickerhamomyces anomalus. Archives of Microbiology 2022, 204, 702. [Google Scholar] [CrossRef] [PubMed]
- Madrigal, T.; Maicas, S.; Mateo Tolosa, J.J. Glucose and Ethanol Tolerant Enzymes Produced by Pichia (Wickerhamomyces) Isolates from Enological Ecosystems. American Journal of Enology and Viticulture 2013, 64, 126–133. [Google Scholar] [CrossRef]
- Morata, A.; Loira, I.; Escott, C.; del Fresno, J.M.; Bañuelos, M.A.; Suárez-Lepe, J.A. Applications of Metschnikowia pulcherrima in Wine Biotechnology. Fermentation 2019, 5. [Google Scholar] [CrossRef]
- Mažeika, K.; Šiliauskas, L.; Skridlaite˙, G.; Matelis, A.; Garjonyte˙, R.; Paškevicˇius, A.; Melvydas, V. Features of iron accumulation at high concentration in pulcherrimin-producing Metschnikowia yeast biomass. Journal of Biological Inorganic Chemistry 2021, 26, 299–311. [Google Scholar] [CrossRef]
- Barbosa, C.; Lage, P.; Esteves, M.; Chambel, L.; Mendes-Faia, A.; Mendes-Ferreira, A. Molecular and Phenotypic Characterization of Metschnikowia pulcherrima Strains from Douro Wine Region. Fermentation 2018, 4. [Google Scholar] [CrossRef]
- Qin, T.; Liao, J.; Zheng, Y.; Zhang, W.; Zhang, X. Oenological Characteristics of Four Non-Saccharomyces Yeast Strains With /beta-Glycosidase Activity. Frontiers in Microbiology 2021, 12, 626920. [Google Scholar] [CrossRef]
- Puškaš, V.S.; Miljic´, U.D.; Djuran, J.J.; Vucˇurovic´, V.M. The aptitude of commercial yeast strains for lowering the ethanol content of wine. Food Science & Nutrition 2020, 8, 1489–1498. [Google Scholar] [CrossRef]
- Postigo, V.; Sanz, P.; Garc’ia, M.; Arroyo, T. Impact of Non-Saccharomyces Wine Yeast Strains on Improving Healthy Characteristics and the Sensory Profile of Beer in Sequential Fermentation. Foods 2022, 11, 2029. [Google Scholar] [CrossRef] [PubMed]
- Mateo, J.J.; Maicas, S.; Thiessen, C. Biotechnological characterisation of exocellular proteases produced by enological Hanseniaspora isolates. International Journal of Food Science & Technology 2015, 50, 218–225. [Google Scholar] [CrossRef]
- Vicente, J.; Baran, Y.; Navascu’es, E.; Santos, A.; Calder’on, F.; Marquina, D.; Rauhut, D.; Benito, S. Biological management of acidity in wine industry: A review. International Journal of Food Microbiology 2022, 375, 109726. [Google Scholar] [CrossRef] [PubMed]
- Morata, A.; Loira, I.; Tesfaye, W.; Bañuelos, M.A.; González, C.; Suárez Lepe, J.A. Lachancea thermotolerans Applications in Wine Technology. Fermentation 2018, 4, 53. [Google Scholar] [CrossRef]
- Vilela-Moura, A.; Schuller, D.; Mendes-Faia, A.; Côrte-Real, M. Reduction of volatile acidity of wines by selected yeast strains. Applied Microbiology and Biotechnology 2008, 80, 881–890. [Google Scholar] [CrossRef] [PubMed]
- Comitini, F.; Gobbi, M.; Domizio, P.; Romani, C.; Lencioni, L.; Mannazzu, I.; Ciani, M. Selected non-Saccharomyces wine yeasts in controlled multistarter fermentations with Saccharomyces cerevisiae. Food Microbiology 2011, 28, 873–882. [Google Scholar] [CrossRef]
- Ciani; Ferraro. Combined use of immobilized Candida stellata cells and Saccharomyces cerevisiae to improve the quality of wines. Journal of Applied Microbiology 1998, 85, 247–254. [Google Scholar] [CrossRef]
- Arevalo-Villena, M.; Bartowsky, E.; Capone, D.; Sefton, M. Production of indole by wine-associated microorganisms under oenological conditions. Food Microbiology 2010, 27, 685–690. [Google Scholar] [CrossRef]
- Magyar, I.; Tóth, T. Comparative evaluation of some oenological properties in wine strains of Candida stellata, Candida zemplinina, Saccharomyces uvarum and Saccharomyces cerevisiae. Food Microbiology 2011, 28, 94–100. [Google Scholar] [CrossRef]
- Ciani, M.; Ferraro, L.; Fatichenti, F. Influence of glycerol production on the aerobic and anaerobic growth of the wine yeast Candida stellata. Enzyme and Microbial Technology 2000, 27, 698–703. [Google Scholar] [CrossRef] [PubMed]
- van Dijken, J.P.; Scheffers, W.A. Redox balances in the metabolism of sugars by yeasts. FEMS Microbiology Reviews 1986, 1, 199–224. [Google Scholar] [CrossRef]
- Petruzzi, L.; Capozzi, V.; Berbegal, C.; Corbo, M.R.; Bevilacqua, A.; Spano, G.; Sinigaglia, M. Microbial Resources and Enological Significance: Opportunities and Benefits. Frontiers in Microbiology 2017, 8. [Google Scholar] [CrossRef] [PubMed]
- Roudil, L.; Russo, P.; Berbegal, C.; Albertin, W.; Spano, G.; Capozzi, V. Non-Saccharomyces Commercial Starter Cultures: Scientific Trends, Recent Patents and Innovation in the Wine Sector. Recent Patents on Food, Nutrition & Agriculture 2020, 11, 27–39. [Google Scholar] [CrossRef]
- Zhao, H.; Li, Y.; Liu, L.; Zheng, M.; Feng, Z.; Hu, K.; Tao, Y. Effects of inoculation timing and mixed fermentation with Pichia fermentans on Oenococcus oeni viability, fermentation duration and aroma production during wine malolactic fermentation. Food Research International 2022, 159. [Google Scholar] [CrossRef] [PubMed]
- Li, N.; Wang, L.; Yin, J.; Ma, N.; Tao, Y. Adjustment of impact odorants in Hutai-8 rose wine by co-fermentation of Pichia fermentans and Saccharomyces cerevisiae. Food Research International (Ottawa, Ont.) 2022, 153, 110959. [Google Scholar] [CrossRef] [PubMed]
- Strauss, M.; Jolly, N.; Lambrechts, M.; Van Rensburg, P. Screening for the production of extracellular hydrolytic enzymes by non-Saccharomyces wine yeasts. Journal of Applied Microbiology 2001, 91, 182–190. [Google Scholar] [CrossRef] [PubMed]
- López, S.; Mateo, J.J.; Maicas, S. Screening of Hanseniaspora Strains for the Production of Enzymes with Potential Interest for Winemaking. Fermentation 2016, 2. [Google Scholar] [CrossRef]
- Pérez, G.; Fariña, L.; Barquet, M.; Boido, E.; Gaggero, C.; Dellacassa, E.; Carrau, F. A quick screening method to identify β-glucosidase activity in native wine yeast strains: application of Esculin Glycerol Agar (EGA) medium. World Journal of Microbiology and Biotechnology 2011, 27, 47–55. [Google Scholar] [CrossRef]
- Medina, K.; Boido, E.; Fariña, L.; Gioia, O.; Gomez, M.; Barquet, M.; Gaggero, C.; Dellacassa, E.; Carrau, F. Increased flavour diversity of Chardonnay wines by spontaneous fermentation and co-fermentation with Hanseniaspora vineae. Food Chemistry 2013, 141, 2513–2521. [Google Scholar] [CrossRef]
- Maturano, Y.P.; Rodr’iguez Assaf, L.A.; Toro, M.E.; Nally, M.C.; Vallejo, M.; Castellanos de Figueroa, L.I.; Combina, M.; Vazquez, F. Multi-enzyme production by pure and mixed cultures of Saccharomyces and non-Saccharomyces yeasts during wine fermentation. International Journal of Food Microbiology 2012, 155, 43–50. [Google Scholar] [CrossRef] [PubMed]
- Sivieri, K.; Favaro-Trindade, C.S.; Grosso, C.R.F.; Grimaldi, R.; Lerayer, A.L.F. Potential probiotic characterization and in vitro evaluation of functional properties of a strain of Wickerhamomyces anomalus isolated from human milk. Journal of applied microbiology 2014, 117, 726–738. [Google Scholar]
- Jara, C.; Laurie, V.F.; Mas, A.; Romero, J.; Martinez-Moreno, R.; Acuña-Fontecilla, A.; Rodriguez, J.; Borquez, R.; Luchsinger, C.; Lopez-Miranda, J. Bioactive Compounds Produced by Yeasts: Functional and Health-Protective Benefits. Applied microbiology and biotechnology 2016, 100, 10065–10078. [Google Scholar]
- Magnusson, J.; Ström, K.; Roos, S.; Sjögren, J.; Schnürer, J. Anti-inflammatory and anti-cancer activities of peptide- and exopolysac-charide-producing lactic acid bacteria. Journal of dairy science 2016, 99, 7832–7841, Vega, F.E.; Posada, F.; Peterson, S.W.; Gianfagna, T.J.; Chaves, F. Penicillium species endophytic in coffee plants and ochratoxin A production. Mycological Progress 2015, 14, 1–12.. [Google Scholar]
- Vega, F.E.; Posada, F.; Peterson, S.W.; Gianfagna, T.J.; Chaves, F. Penicillium species endophytic in coffee plants and ochratoxin A production. Mycological Progress 2015, 14, 1–12. [Google Scholar]
- Jolly, N.P.; Augustyn, O.P.H.; Pretorius, I.S. Non-Saccharomyces yeasts in wine production: challenges and opportunities. Current Opinion in Biotechnology 2019, 56, 76–84. [Google Scholar]
- Fernandes, T.; Mateus, P.; Couto, J.A. Impact of non-Saccharomyces yeasts on wine chemistry and flavour: A review. Trends in Food Science & Technology 2021, 111, 145–162. [Google Scholar]
- Benito, S.; Palomero, F.; Calderón, F.; Palmero, D.; Suárez-Lepe, J. Selection of appropriate Schizosaccharomyces strains for winemaking. Food Microbiology 2014, 42, 218–224. [Google Scholar] [CrossRef]
- Moreno-Arribas, M.V.; Carmen Polo, M. Occurrence of lactic acid bacteria and biogenic amines in biologically aged wines. Food Microbiology 2008, 25, 875–881. [Google Scholar] [CrossRef]
- Morata, A.; Benito, S.; Loira, I.; Palomero, F.; González, M.; Suárez-Lepe, J. Formation of pyranoanthocyanins by Schizosac-charomyces pombe during the fermentation of red must. International Journal of Food Microbiology 2012, 159, 47–53. [Google Scholar] [CrossRef]
- Wang, Y.; Zhao, Y.c.; Fan, L.l.; Xia, X.d.; Li, Y.h.; Zhou, J.z. Identification and characterization of Pichia membranifaciens Hmp-1 isolated from spoilage blackberry wine. Journal of Integrative Agriculture 2018, 17, 2126–2136. [Google Scholar] [CrossRef]
- Spencer, J.; Ragout de Spencer, A.; Laluce, C. Non-conventional yeasts. Applied Microbiology and Biotechnology 2002, 58, 147–156. [Google Scholar] [CrossRef] [PubMed]
- Vicente, J.; Calderón, F.; Santos, A.; Marquina, D.; Benito, S. High Potential of Pichia kluyveri and Other Pichia Species in Wine Technology. International Journal of Molecular Sciences 2021, 22. [Google Scholar] [CrossRef] [PubMed]
- Lago, J.C.; Tavares, C.; Silva, J.A.; Araújo, F.F.; Guedes de Pinho, P. Sequential inoculation of Pichia kluyveri and Saccharomyces cerevisiae as a strategy for enhancing flavour complexity and improving fermentation efficiency in Cabernet Sauvignon wines. Food Chemistry 2019, 274, 900–911. [Google Scholar]
- Sun, Y.H.; Zhang, Q.Q.; Zhi, Y.F.; Liu, J.; Gao, Q.; Zhang, T.; Wang, X.Q.; Zhou, W. Production of 2-phenylethanol by Pichia kluyveri using the extract from Chinese jujube as substrate. Food Science and Biotechnology 2019, 28, 579–585. [Google Scholar]
- Ramírez, M.; Velázquez, R. The Yeast Torulaspora delbrueckii: An Interesting But Difficult-To-Use Tool for Winemaking. Fermentation 2018, 4. [Google Scholar] [CrossRef]
- Lencioni, L.; Romani, C.; Gobbi, M.; Comitini, F.; Ciani, M.; Domizio, P. Exploring the potential of Torulaspora delbrueckii in mixed fermentations with two Saccharomyces cerevisiae strains for the production of craft beers. Food Microbiology 2018, 74, 51–61. [Google Scholar] [CrossRef]
- Gobbi, M.; Comitini, F.; Domizio, P.; Romani, C.; Lencioni, L.; Mannazzu, I.; Ciani, M. Selected non-Saccharomyces wine yeasts in controlled multistarter fermentations with Saccharomyces cerevisiae. Food Microbiology 2020, 88, 103402. [Google Scholar]
- Belda, I.; Ruiz, J.; Esteban-Fernández, A.; Navascués, E.; Marquina, D.; Santos, A.; Moreno-Arribas, M.V. Microbial contribution to wine aroma and its intended use for wine quality improvement. Molecules 2017, 22, 189. [Google Scholar] [CrossRef]
- Oliveira, J.M.; Semedo-Lemsaddek, T.; Barros, D.R. Molecular detection of Wickerhamomyces anomalus in wine spoilage. Journal of Food Science and Technology 2018, 55, 3358–3363. [Google Scholar]
- Muñoz, R.; González, R.; Benito, S.; Palomero, F.; Morata, A. The impact of Wickerhamomyces anomalus on wine quality and safety. Foods 2019, 8, 132. [Google Scholar]
- Du Toit, M.; Engelbrecht, L. Lactic Acid Bacteria and Yeasts of Wine Grapes: Sensitive and Detectable Bacteria. Fermentation 2019, 5. [Google Scholar] [CrossRef]
- International Organisation of Vine and Wine. Compendium of International Methods of Wine and Must Analysis; International Organisation of Vine and Wine, 2018. [Google Scholar]
- Guzzon, R. Non-sulfur dioxide microbial inhibitors for winemaking: a review. Beverages 2019, 5. [Google Scholar] [CrossRef]
- Cappello, M.S.; Bleve, G.; Grieco, F.; Dellaglio, F. Use of non-Saccharomyces yeasts and oenological tannins in red wine vinifications: Influence on colour, astringency and sensory properties. Food microbiology 2017, 62, 271–282. [Google Scholar] [CrossRef]
- Fernández, M.; Úbeda, J.; Briones, A. Typing of non-Saccharomyces yeasts with enzymatic activities of interest in wine-making. International Journal of Food Microbiology 2000, 59, 29–36. [Google Scholar] [CrossRef]
- Lleixà, J.; Martín, V.; Portillo, M.d.C.; Carrau, F.; Beltran, G.; Mas, A. Comparison of Fermentation and Wines Produced by Inoculation of Hanseniaspora vineae and Saccharomyces cerevisiae. Frontiers in Microbiology 2016, 7. [Google Scholar] [CrossRef]
- Porter, T.J.; Divol, B.; Setati, M.E. Lachancea yeast species: Origin, biochemical characteristics and oenological significance. Food Research International 2019, 119, 378–389. [Google Scholar] [CrossRef]
- Zott, K.; Thibon, C.; Bely, M.; Lonvaud-Funel, A.; Dubourdieu, D.; Masneuf-Pomarede, I. The grape must non-Saccharomyces microbial community: Impact on volatile thiol release. International Journal of Food Microbiology 2011, 151, 210–215. [Google Scholar] [CrossRef]
- Vaquero, C.; Escott, C.; Heras, J.M.; Carrau, F.; Morata, A. Co-inoculations of Lachancea thermotolerans with different Hanseniaspora spp.: Acidification, aroma, biocompatibility, and effects of nutrients in wine. Food Research International 2022, 161. [Google Scholar] [CrossRef]
- Estela-Escalante, W.D.; Moscosa-Santillán, M.; González-Ramírez, J.E.; Rosales-Mendoza, S. Evaluation of the Potential Production of Ethanol by Candida Zemplinina Yeast with Regard to Beer Fermentation. Journal of the American Society of Brewing Chemists 2017, 75, 130–135. [Google Scholar] [CrossRef]
- Jolly, N.; Augustyn, O.; Pretorius, I. The occurrence of non-Saccharomyces species over three vintages in four vineyards and grape musts from four production regions of the Western Cape, South Africa. South Afr J Enol Viticult. 2003, 24, 35–42. [Google Scholar] [CrossRef]
- Verduyn, C. Physiology of yeasts in relation to biomass yields. Antonie van Leeuwenhoek 1991, 60, 325–353. [Google Scholar] [CrossRef] [PubMed]
| Yeast | Activity | References |
|---|---|---|
| Schizosaccharomyces pombe | Reduce malic acid content | [42] |
| Great autolytic release of polysaccharides | [43,44] | |
| Reduce wine 4-ethylphenol concentration | [45,46] | |
| Torulaspora delbrueckii | Reduce volatile acidity | [47,48] |
| Increase the concentration of some minor lactones and esters | [49,50] | |
| Killer strains | [51,52] | |
| Wickerhamomyces anomalus | Tolerate up to 12.5% (v/v) ethanol | [53,54] |
| Produce lethal toxins | [55,56] | |
| Source of different enzymes | [34,57] | |
| Metschnikowia pulcherrima | Production of pulcherrimin | [58,59] |
| β-glucosidase activity | [60,61] | |
| Good candidate for to obtaining wine with low ethanol content | [62,63] | |
| Lachancea thermotolerans | Produce lactic acid | [64,65] |
| Produce low volatile acidity | [66,67] | |
| Express extracellular enzymatic activities | [67,68] | |
| Candida stellata | Positively affect the taste and flavor of alcoholic beverages | [69,70] |
| Strong fructophilic and osmophilic character | [71,72] | |
| Positive Crabtree yeast | [72,73] | |
| Pichia kluyveri | Supply of thiols, terpenes and fruity esters | [74,75] |
| Ferment glucose but hardly other sugar molecules. | [76,77] | |
| Produce extracellular enzymes | [42,78] | |
| Hanseniaspora | High volatile acidity production | [78,79] |
| Produce extracellular enzymes | [80,81] | |
| Proteolitic activities | [64,82] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).





