Submitted:
06 April 2023
Posted:
07 April 2023
Read the latest preprint version here
Abstract
Keywords:
Introduction
The Hypothesis
Evaluation of the Hypothesis
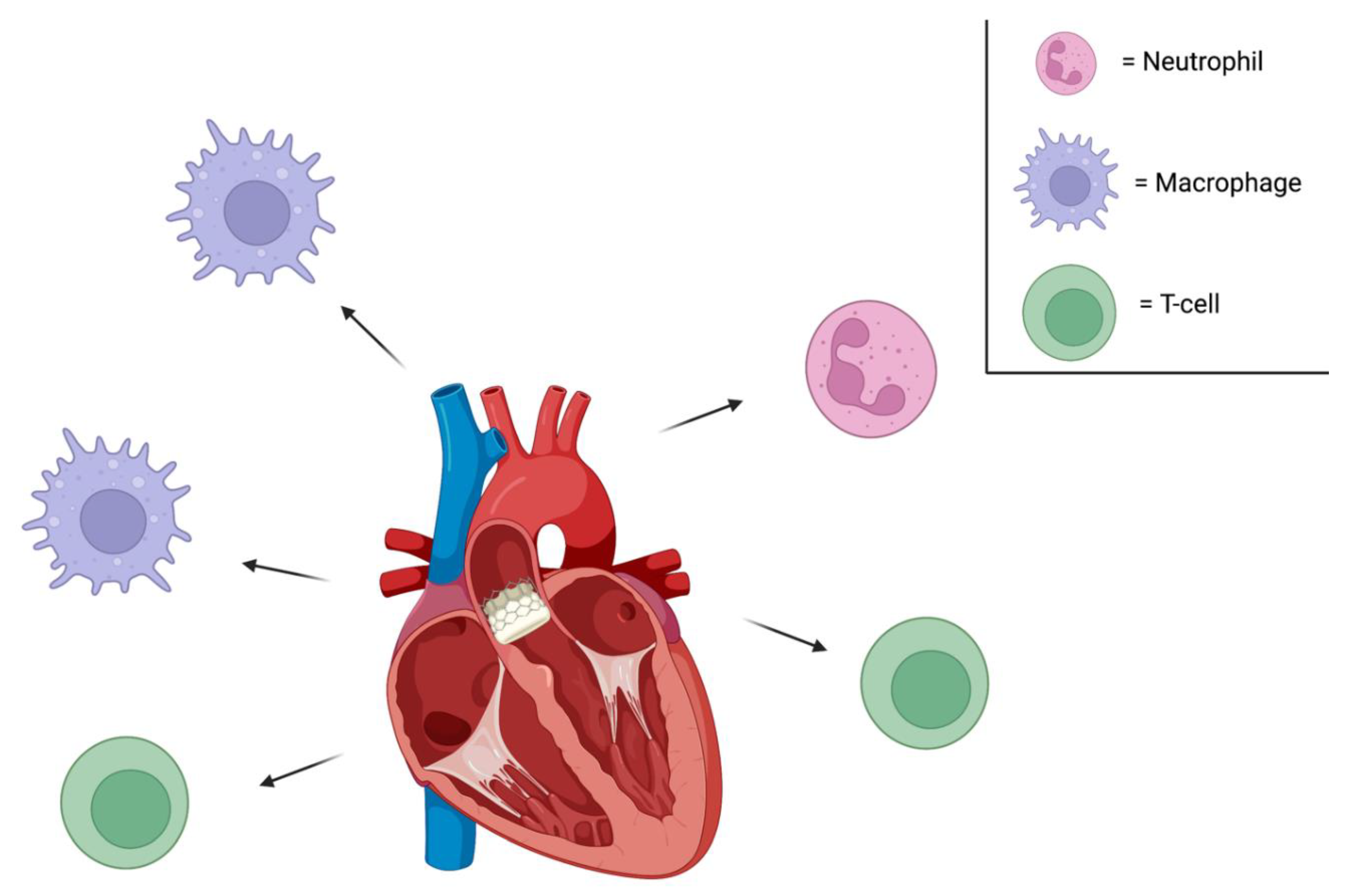
Conclusion
Funding
Acknowledgements
Competing Interests
References
- Anonymous. Facts and Benefits of Organ Donation | UF Health, University of Florida Health. n.d. Available from: https://ufhealth.org/blog/facts-and-benefits-organ-donation [Last accessed: 11/11/2022].
- Montgomery RA, Stern JM, Lonze BE, et al. Results of Two Cases of Pig-to-Human Kidney Xenotransplantation. N Engl J Med 2022;386(20):1889–1898. [CrossRef]
- Wang W, He W, Ruan Y, et al. First pig-to-human heart transplantation. Innovation (Camb) 2022;3(2):100223. [CrossRef]
- Fischer K, Kraner-Scheiber S, Petersen B, et al. Efficient production of multi-modified pigs for xenotransplantation by ‘combineering’, gene stacking and gene editing. Scientific Reports 2016;6:29081. [CrossRef]
- Fox A, Mountford J, Braakhuis A, et al. Innate and Adaptive Immune Responses to Nonvascular Xenografts: Evidence That Macrophages Are Direct Effectors of Xenograft Rejection. The Journal of Immunology 2001;166(3):2133–2140. [CrossRef]
- Zahr A, Alcaide P, Yang J, et al. Endomucin Prevents Leukocyte–Endothelial Cell Adhesion and Has a Critical Role under Resting and Inflammatory Conditions. Nat Commun 2016;7(1):10363. [CrossRef]
- Park-Windhol C, Ng YS, Yang J, et al. Endomucin Inhibits VEGF-Induced Endothelial Cell Migration, Growth, and Morphogenesis by Modulating VEGFR2 Signaling. Sci Rep 2017;7(1):17138. [CrossRef]
- Renteln M. Lipofuscin as the main driving force of current age-related disease: justification and strategies for removal. Current Aging Science (Submitted: February, 2022).
- Persaud SP, Ritchey JK, Kim S, et al. Antibody-drug conjugates plus Janus kinase inhibitors enable MHC-mismatched allogeneic hematopoietic stem cell transplantation. J Clin Invest n.d.;131(24):e145501. [CrossRef]
- Park JS, Rhau B, Hermann A, et al. Synthetic Control of Mammalian-Cell Motility by Engineering Chemotaxis to an Orthogonal Bioinert Chemical Signal. Proc Natl Acad Sci USA 2014;111(16):5896–5901. [CrossRef]
- Ma D, Hirose T, Lassiter G, et al. Kidney transplantation from triple-knockout pigs expressing multiple human proteins in cynomolgus macaques. Am J Transplant 2022;22(1):46–57. [CrossRef]
- Das S, Koyano-Nakagawa N, Gafni O, et al. Generation of human endothelium in pig embryos deficient in ETV2. Nat Biotechnol 2020;38(3):297–302. [CrossRef]
- Cohen S, Partouche S, Gurevich M, et al. Generation of vascular chimerism within donor organs. Sci Rep 2021;11(1):13437. [CrossRef]
- Scott DW. Genetic Engineering of T Cells for Immune Tolerance. Molecular Therapy - Methods & Clinical Development 2020;16:103–107. [CrossRef]
- Li M, Eckl J, Geiger C, et al. A novel and effective method to generate human porcine-specific regulatory T cells with high expression of IL-10, TGF-β1 and IL-35. Sci Rep 2017;7(1):3974. [CrossRef]
- Sachs DH. Transplantation Tolerance Through Mixed Chimerism: From Allo to Xeno. Xenotransplantation 2018;25(3):e12420. [CrossRef]
- Yamada K, Ariyoshi Y, Pomposelli T, et al. Co-transplantation of Vascularized Thymic Graft with Kidney in Pig-to-Nonhuman Primates for the Induction of Tolerance Across Xenogeneic Barriers. Methods Mol Biol 2020;2110:151–171. [CrossRef]
- Watanabe H, Ariyoshi Y, Pomposelli T, et al. Intra-bone bone marrow transplantation from hCD47 transgenic pigs to baboons prolongs chimerism to >60 days and promotes increased porcine lung transplant survival. Xenotransplantation 2020;27(1):e12552. [CrossRef]
- Pilat N, Granofszky N, Wekerle T. Combining Adoptive Treg Transfer with Bone Marrow Transplantation for Transplantation Tolerance. Curr Transplant Rep 2017;4(4):253–261. [CrossRef]
- Morsut L, Roybal KT, Xiong X, et al. Engineering Customized Cell Sensing and Response Behaviors Using Synthetic Notch Receptors. Cell 2016;164(4):780–791. [CrossRef]
- Kamano C, Vagefi PA, Kumagai N, et al. Vascularized thymic lobe transplantation in miniature swine: Thymopoiesis and tolerance induction across fully MHC-mismatched barriers. Proc Natl Acad Sci U S A 2004;101(11):3827–3832. [CrossRef]
- Bredenkamp N, Ulyanchenko S, O’Neill KE, et al. An organized and functional thymus generated from FOXN1-reprogrammed fibroblasts. Nat Cell Biol 2014;16(9):902–908. [CrossRef]
- Muthana M, Kennerley AJ, Hughes R, et al. Directing cell therapy to anatomic target sites in vivo with magnetic resonance targeting. Nature Communications 2015;6(1):8009. [CrossRef]
- Miller ST, Xavier KB, Campagna SR, et al. Salmonella typhimurium Recognizes a Chemically Distinct Form of the Bacterial Quorum-Sensing Signal AI-2. Molecular Cell 2004;15(5):677–687. [CrossRef]
- Mirouze N, Dubnau D. Chance and Necessity in Bacillus subtilis Development. Microbiology Spectrum 2013;1(1):1.1.02. [CrossRef]
- Nunes-Alves C, Nobrega C, Behar SM, et al. Tolerance has its limits: how the thymus copes with infection. Trends Immunol 2013;34(10). [CrossRef]
- Hu M, Hawthorne WJ, Yi S, et al. Cellular Immune Responses in Islet Xenograft Rejection. Frontiers in Immunology 2022;13.
- Arabi TZ, Sabbah BN, Lerman A, et al. Xenotransplantation: Current Challenges and Emerging Solutions. Cell Transplant 2023;32:09636897221148771. [CrossRef]
- Rider TH, Zook CE, Boettcher TL, et al. Broad-Spectrum Antiviral Therapeutics. PLOS ONE 2011;6(7):e22572. [CrossRef]
- Yuzefovych Y, Valdivia E, Rong S, et al. Genetic Engineering of the Kidney to Permanently Silence MHC Transcripts During ex vivo Organ Perfusion. Frontiers in Immunology 2020;11.
- Berkhout B. A Fourth Generation Lentiviral Vector: Simplifying Genomic Gymnastics. Molecular Therapy 2017;25(8):1741–1743. [CrossRef]
- Figueiredo C, Carvalho Oliveira M, Chen-Wacker C, et al. Immunoengineering of the Vascular Endothelium to Silence MHC Expression During Normothermic Ex Vivo Lung Perfusion. Hum Gene Ther 2019;30(4):485–496. [CrossRef]
- Su LT, Gopal K, Wang Z, et al. Uniform Scale-Independent Gene Transfer to Striated Muscle After Transvenular Extravasation of Vector. Circulation 2005;112(12):1780–1788. [CrossRef]
- Greelish JP, Su LT, Lankford EB, et al. Stable restoration of the sarcoglycan complex in dystrophic muscle perfused with histamine and a recombinant adeno-associated viral vector. Nat Med 1999;5(4):439–443. [CrossRef]
- Gregorevic P, Blankinship MJ, Allen JM, et al. Systemic delivery of genes to striated muscles using adeno-associated viral vectors. Nat Med 2004;10(8):828–834. [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).



