Submitted:
05 April 2023
Posted:
06 April 2023
Read the latest preprint version here
Abstract
Keywords:
Introduction
Mitochondrial deficits and oxidative stress as close partners in Alzheimer’s disease brain damage
Impaired mitochondrial function and associated oxidative damage in Parkinson’s disease
Ca2+ dysregulation and downstream effects in Alzheimer’s disease
Altered Ca2+ homeostasis and concomitant neurotoxicity in Parkinson’s disease
Modulation of calcium signaling and homeostasis by heterocyclic compounds in Alzheimer’s disease and Parkinson’s disease
1. ANAVEX2-73
2. Caffeine
3. Diltiazem
4. Latrepirdine
5. Nifedipine
6. Nimodipine
Abbreviations
Conclusions and Future Directions
Funding support
Author contributions
References
- Cobley, J.N.; Fiorello, M.L.; Bailey, D.M. 13 reasons why the brain is susceptible to oxidative stress. Redox Biol 2018, 15, 490-503. [CrossRef]
- Beal, M.F.; Chiluwal, J.; Calingasan, N.Y.; Milne, G.L.; Shchepinov, M.S.; Tapias, V. Isotope-reinforced polyunsaturated fatty acids improve Parkinson's disease-like phenotype in rats overexpressing alpha-synuclein. Acta Neuropathol Commun 2020, 8, 220. [CrossRef]
- Butterfield, D.A.; Mattson, M.P. Apolipoprotein E and oxidative stress in brain with relevance to Alzheimer's disease. Neurobiol Dis 2020, 138, 104795. [CrossRef]
- Niedzielska, E.; Smaga, I.; Gawlik, M.; Moniczewski, A.; Stankowicz, P.; Pera, J.; Filip, M. Oxidative Stress in Neurodegenerative Diseases. Mol Neurobiol 2016, 53, 4094-4125. [CrossRef]
- Tapias, V.; Hu, X.; Luk, K.C.; Sanders, L.H.; Lee, V.M.; Greenamyre, J.T. Synthetic alpha-synuclein fibrils cause mitochondrial impairment and selective dopamine neurodegeneration in part via iNOS-mediated nitric oxide production. Cell Mol Life Sci 2017, 74, 2851-2874. [CrossRef]
- Yoboue, E.D.; Sitia, R.; Simmen, T. Redox crosstalk at endoplasmic reticulum (ER) membrane contact sites (MCS) uses toxic waste to deliver messages. Cell Death Dis 2018, 9, 331. [CrossRef]
- van der Reest, J.; Lilla, S.; Zheng, L.; Zanivan, S.; Gottlieb, E. Proteome-wide analysis of cysteine oxidation reveals metabolic sensitivity to redox stress. Nat Commun 2018, 9, 1581. [CrossRef]
- Xiao, H.; Jedrychowski, M.P.; Schweppe, D.K.; Huttlin, E.L.; Yu, Q.; Heppner, D.E.; Li, J.; Long, J.; Mills, E.L.; Szpyt, J.; et al. A Quantitative Tissue-Specific Landscape of Protein Redox Regulation during Aging. Cell 2020, 180, 968-983 e924. [CrossRef]
- Doulias, P.T.; Tenopoulou, M.; Greene, J.L.; Raju, K.; Ischiropoulos, H. Nitric oxide regulates mitochondrial fatty acid metabolism through reversible protein S-nitrosylation. Sci Signal 2013, 6, rs1. [CrossRef]
- Song, I.K.; Lee, J.J.; Cho, J.H.; Jeong, J.; Shin, D.H.; Lee, K.J. Degradation of Redox-Sensitive Proteins including Peroxiredoxins and DJ-1 is Promoted by Oxidation-induced Conformational Changes and Ubiquitination. Sci Rep 2016, 6, 34432. [CrossRef]
- Lu, T.; Pan, Y.; Kao, S.Y.; Li, C.; Kohane, I.; Chan, J.; Yankner, B.A. Gene regulation and DNA damage in the ageing human brain. Nature 2004, 429, 883-891. [CrossRef]
- Rao, V.K.; Carlson, E.A.; Yan, S.S. Mitochondrial permeability transition pore is a potential drug target for neurodegeneration. Biochim Biophys Acta 2014, 1842, 1267-1272. [CrossRef]
- Abe, K.; Kimura, H. Amyloid beta toxicity consists of a Ca(2+)-independent early phase and a Ca(2+)-dependent late phase. J Neurochem 1996, 67, 2074-2078.
- Corona, C.; Pensalfini, A.; Frazzini, V.; Sensi, S.L. New therapeutic targets in Alzheimer's disease: brain deregulation of calcium and zinc. Cell Death Dis 2011, 2, e176. [CrossRef]
- Espana, J.; Valero, J.; Minano-Molina, A.J.; Masgrau, R.; Martin, E.; Guardia-Laguarta, C.; Lleo, A.; Gimenez-Llort, L.; Rodriguez-Alvarez, J.; Saura, C.A. beta-Amyloid disrupts activity-dependent gene transcription required for memory through the CREB coactivator CRTC1. J Neurosci 2010, 30, 9402-9410. [CrossRef]
- Verma, M.; Callio, J.; Otero, P.A.; Sekler, I.; Wills, Z.P.; Chu, C.T. Mitochondrial Calcium Dysregulation Contributes to Dendrite Degeneration Mediated by PD/LBD-Associated LRRK2 Mutants. J Neurosci 2017, 37, 11151-11165. [CrossRef]
- Querfurth, H.W.; LaFerla, F.M. Alzheimer's disease. N Engl J Med 2010, 362, 329-344. [CrossRef]
- Swerdlow, R.H.; Khan, S.M. The Alzheimer's disease mitochondrial cascade hypothesis: an update. Exp Neurol 2009, 218, 308-315. [CrossRef]
- Swerdlow, R.H.; Khan, S.M. A "mitochondrial cascade hypothesis" for sporadic Alzheimer's disease. Med Hypotheses 2004, 63, 8-20. [CrossRef]
- Swerdlow, R.H.; Burns, J.M.; Khan, S.M. The Alzheimer's disease mitochondrial cascade hypothesis. J Alzheimers Dis 2010, 20 Suppl 2, S265-279. [CrossRef]
- Buckner, R.L.; Snyder, A.Z.; Shannon, B.J.; LaRossa, G.; Sachs, R.; Fotenos, A.F.; Sheline, Y.I.; Klunk, W.E.; Mathis, C.A.; Morris, J.C.; et al. Molecular, structural, and functional characterization of Alzheimer's disease: evidence for a relationship between default activity, amyloid, and memory. J Neurosci 2005, 25, 7709-7717. [CrossRef]
- Greicius, M.D.; Srivastava, G.; Reiss, A.L.; Menon, V. Default-mode network activity distinguishes Alzheimer's disease from healthy aging: evidence from functional MRI. Proc Natl Acad Sci U S A 2004, 101, 4637-4642. [CrossRef]
- Putcha, D.; Eckbo, R.; Katsumi, Y.; Dickerson, B.C.; Touroutoglou, A.; Collins, J.A. Tau and the fractionated default mode network in atypical Alzheimer's disease. Brain Commun 2022, 4, fcac055. [CrossRef]
- Kim, E.J.; Cho, S.S.; Jeong, Y.; Park, K.C.; Kang, S.J.; Kang, E.; Kim, S.E.; Lee, K.H.; Na, D.L. Glucose metabolism in early onset versus late onset Alzheimer's disease: an SPM analysis of 120 patients. Brain 2005, 128, 1790-1801. [CrossRef]
- Smith, G.S.; de Leon, M.J.; George, A.E.; Kluger, A.; Volkow, N.D.; McRae, T.; Golomb, J.; Ferris, S.H.; Reisberg, B.; Ciaravino, J.; et al. Topography of cross-sectional and longitudinal glucose metabolic deficits in Alzheimer's disease. Pathophysiologic implications. Arch Neurol 1992, 49, 1142-1150. [CrossRef]
- De Santi, S.; de Leon, M.J.; Rusinek, H.; Convit, A.; Tarshish, C.Y.; Roche, A.; Tsui, W.H.; Kandil, E.; Boppana, M.; Daisley, K.; et al. Hippocampal formation glucose metabolism and volume losses in MCI and AD. Neurobiol Aging 2001, 22, 529-539. [CrossRef]
- Gibson, G.E.; Sheu, K.F.; Blass, J.P.; Baker, A.; Carlson, K.C.; Harding, B.; Perrino, P. Reduced activities of thiamine-dependent enzymes in the brains and peripheral tissues of patients with Alzheimer's disease. Arch Neurol 1988, 45, 836-840. [CrossRef]
- Ke, Z.J.; Gibson, G.E. Selective response of various brain cell types during neurodegeneration induced by mild impairment of oxidative metabolism. Neurochem Int 2004, 45, 361-369. [CrossRef]
- Bubber, P.; Haroutunian, V.; Fisch, G.; Blass, J.P.; Gibson, G.E. Mitochondrial abnormalities in Alzheimer brain: mechanistic implications. Ann Neurol 2005, 57, 695-703. [CrossRef]
- Hirai, K.; Aliev, G.; Nunomura, A.; Fujioka, H.; Russell, R.L.; Atwood, C.S.; Johnson, A.B.; Kress, Y.; Vinters, H.V.; Tabaton, M.; et al. Mitochondrial abnormalities in Alzheimer's disease. J Neurosci 2001, 21, 3017-3023. [CrossRef]
- Silva, D.F.; Selfridge, J.E.; Lu, J.; E, L.; Cardoso, S.M.; Swerdlow, R.H. Mitochondrial abnormalities in Alzheimer's disease: possible targets for therapeutic intervention. Adv Pharmacol 2012, 64, 83-126. [CrossRef]
- Gibson, G.E.; Starkov, A.; Blass, J.P.; Ratan, R.R.; Beal, M.F. Cause and consequence: mitochondrial dysfunction initiates and propagates neuronal dysfunction, neuronal death and behavioral abnormalities in age-associated neurodegenerative diseases. Biochim Biophys Acta 2010, 1802, 122-134. [CrossRef]
- Gibson, G.E.; Zhang, H.; Sheu, K.F.; Bogdanovich, N.; Lindsay, J.G.; Lannfelt, L.; Vestling, M.; Cowburn, R.F. Alpha-ketoglutarate dehydrogenase in Alzheimer brains bearing the APP670/671 mutation. Ann Neurol 1998, 44, 676-681. [CrossRef]
- Mastrogiacomo, F.; Bergeron, C.; Kish, S.J. Brain alpha-ketoglutarate dehydrogenase complex activity in Alzheimer's disease. J Neurochem 1993, 61, 2007-2014. [CrossRef]
- Tapias, V.; Jainuddin, S.; Ahuja, M.; Stack, C.; Elipenahli, C.; Vignisse, J.; Gerges, M.; Starkova, N.; Xu, H.; Starkov, A.A.; et al. Benfotiamine treatment activates the Nrf2/ARE pathway and is neuroprotective in a transgenic mouse model of tauopathy. Hum Mol Genet 2018, 27, 2874-2892. [CrossRef]
- Bosetti, F.; Brizzi, F.; Barogi, S.; Mancuso, M.; Siciliano, G.; Tendi, E.A.; Murri, L.; Rapoport, S.I.; Solaini, G. Cytochrome c oxidase and mitochondrial F1F0-ATPase (ATP synthase) activities in platelets and brain from patients with Alzheimer's disease. Neurobiol Aging 2002, 23, 371-376. [CrossRef]
- Cottrell, D.A.; Blakely, E.L.; Johnson, M.A.; Ince, P.G.; Turnbull, D.M. Mitochondrial enzyme-deficient hippocampal neurons and choroidal cells in AD. Neurology 2001, 57, 260-264. [CrossRef]
- Cottrell, D.A.; Borthwick, G.M.; Johnson, M.A.; Ince, P.G.; Turnbull, D.M. The role of cytochrome c oxidase deficient hippocampal neurones in Alzheimer's disease. Neuropathol Appl Neurobiol 2002, 28, 390-396. [CrossRef]
- Maurer, I.; Zierz, S.; Moller, H.J. A selective defect of cytochrome c oxidase is present in brain of Alzheimer disease patients. Neurobiol Aging 2000, 21, 455-462. [CrossRef]
- Mutisya, E.M.; Bowling, A.C.; Beal, M.F. Cortical cytochrome oxidase activity is reduced in Alzheimer's disease. J Neurochem 1994, 63, 2179-2184. [CrossRef]
- Parker, W.D., Jr.; Filley, C.M.; Parks, J.K. Cytochrome oxidase deficiency in Alzheimer's disease. Neurology 1990, 40, 1302-1303. [CrossRef]
- Parker, W.D., Jr.; Mahr, N.J.; Filley, C.M.; Parks, J.K.; Hughes, D.; Young, D.A.; Cullum, C.M. Reduced platelet cytochrome c oxidase activity in Alzheimer's disease. Neurology 1994, 44, 1086-1090. [CrossRef]
- Valla, J.; Schneider, L.; Niedzielko, T.; Coon, K.D.; Caselli, R.; Sabbagh, M.N.; Ahern, G.L.; Baxter, L.; Alexander, G.; Walker, D.G.; et al. Impaired platelet mitochondrial activity in Alzheimer's disease and mild cognitive impairment. Mitochondrion 2006, 6, 323-330. [CrossRef]
- Swerdlow, R.H.; Parks, J.K.; Cassarino, D.S.; Maguire, D.J.; Maguire, R.S.; Bennett, J.P., Jr.; Davis, R.E.; Parker, W.D., Jr. Cybrids in Alzheimer's disease: a cellular model of the disease? Neurology 1997, 49, 918-925. [CrossRef]
- Mosconi, L.; de Leon, M.; Murray, J.; E, L.; Lu, J.; Javier, E.; McHugh, P.; Swerdlow, R.H. Reduced mitochondria cytochrome oxidase activity in adult children of mothers with Alzheimer's disease. J Alzheimers Dis 2011, 27, 483-490. [CrossRef]
- Mishra, P.; Chan, D.C. Mitochondrial dynamics and inheritance during cell division, development and disease. Nat Rev Mol Cell Biol 2014, 15, 634-646. [CrossRef]
- Tapias, V. Editorial: Mitochondrial Dysfunction and Neurodegeneration. Front Neurosci 2019, 13, 1372. [CrossRef]
- Youle, R.J.; van der Bliek, A.M. Mitochondrial fission, fusion, and stress. Science 2012, 337, 1062-1065. [CrossRef]
- Misgeld, T.; Schwarz, T.L. Mitostasis in Neurons: Maintaining Mitochondria in an Extended Cellular Architecture. Neuron 2017, 96, 651-666. [CrossRef]
- Franco-Iborra, S.; Vila, M.; Perier, C. Mitochondrial Quality Control in Neurodegenerative Diseases: Focus on Parkinson's Disease and Huntington's Disease. Front Neurosci 2018, 12, 342. [CrossRef]
- Wang, W.; Zhao, F.; Ma, X.; Perry, G.; Zhu, X. Mitochondria dysfunction in the pathogenesis of Alzheimer's disease: recent advances. Mol Neurodegener 2020, 15, 30. [CrossRef]
- Manczak, M.; Calkins, M.J.; Reddy, P.H. Impaired mitochondrial dynamics and abnormal interaction of amyloid beta with mitochondrial protein Drp1 in neurons from patients with Alzheimer's disease: implications for neuronal damage. Hum Mol Genet 2011, 20, 2495-2509. [CrossRef]
- Calkins, M.J.; Manczak, M.; Mao, P.; Shirendeb, U.; Reddy, P.H. Impaired mitochondrial biogenesis, defective axonal transport of mitochondria, abnormal mitochondrial dynamics and synaptic degeneration in a mouse model of Alzheimer's disease. Hum Mol Genet 2011, 20, 4515-4529. [CrossRef]
- Du, H.; Guo, L.; Yan, S.; Sosunov, A.A.; McKhann, G.M.; Yan, S.S. Early deficits in synaptic mitochondria in an Alzheimer's disease mouse model. Proc Natl Acad Sci U S A 2010, 107, 18670-18675. [CrossRef]
- Devi, L.; Prabhu, B.M.; Galati, D.F.; Avadhani, N.G.; Anandatheerthavarada, H.K. Accumulation of amyloid precursor protein in the mitochondrial import channels of human Alzheimer's disease brain is associated with mitochondrial dysfunction. J Neurosci 2006, 26, 9057-9068. [CrossRef]
- Pavlov, P.F.; Hansson Petersen, C.; Glaser, E.; Ankarcrona, M. Mitochondrial accumulation of APP and Abeta: significance for Alzheimer disease pathogenesis. J Cell Mol Med 2009, 13, 4137-4145. [CrossRef]
- Hansson Petersen, C.A.; Alikhani, N.; Behbahani, H.; Wiehager, B.; Pavlov, P.F.; Alafuzoff, I.; Leinonen, V.; Ito, A.; Winblad, B.; Glaser, E.; et al. The amyloid beta-peptide is imported into mitochondria via the TOM import machinery and localized to mitochondrial cristae. Proc Natl Acad Sci U S A 2008, 105, 13145-13150. [CrossRef]
- Lustbader, J.W.; Cirilli, M.; Lin, C.; Xu, H.W.; Takuma, K.; Wang, N.; Caspersen, C.; Chen, X.; Pollak, S.; Chaney, M.; et al. ABAD directly links Abeta to mitochondrial toxicity in Alzheimer's disease. Science 2004, 304, 448-452. [CrossRef]
- Du, H.; Guo, L.; Fang, F.; Chen, D.; Sosunov, A.A.; McKhann, G.M.; Yan, Y.; Wang, C.; Zhang, H.; Molkentin, J.D.; et al. Cyclophilin D deficiency attenuates mitochondrial and neuronal perturbation and ameliorates learning and memory in Alzheimer's disease. Nat Med 2008, 14, 1097-1105. [CrossRef]
- Canevari, L.; Clark, J.B.; Bates, T.E. beta-Amyloid fragment 25-35 selectively decreases complex IV activity in isolated mitochondria. FEBS Lett 1999, 457, 131-134. [CrossRef]
- Casley, C.S.; Canevari, L.; Land, J.M.; Clark, J.B.; Sharpe, M.A. Beta-amyloid inhibits integrated mitochondrial respiration and key enzyme activities. J Neurochem 2002, 80, 91-100. [CrossRef]
- Yao, J.; Irwin, R.W.; Zhao, L.; Nilsen, J.; Hamilton, R.T.; Brinton, R.D. Mitochondrial bioenergetic deficit precedes Alzheimer's pathology in female mouse model of Alzheimer's disease. Proc Natl Acad Sci U S A 2009, 106, 14670-14675. [CrossRef]
- Fukui, H.; Diaz, F.; Garcia, S.; Moraes, C.T. Cytochrome c oxidase deficiency in neurons decreases both oxidative stress and amyloid formation in a mouse model of Alzheimer's disease. Proc Natl Acad Sci U S A 2007, 104, 14163-14168. [CrossRef]
- Hauptmann, S.; Scherping, I.; Drose, S.; Brandt, U.; Schulz, K.L.; Jendrach, M.; Leuner, K.; Eckert, A.; Muller, W.E. Mitochondrial dysfunction: an early event in Alzheimer pathology accumulates with age in AD transgenic mice. Neurobiol Aging 2009, 30, 1574-1586. [CrossRef]
- Ronnback, A.; Pavlov, P.F.; Mansory, M.; Gonze, P.; Marliere, N.; Winblad, B.; Graff, C.; Behbahani, H. Mitochondrial dysfunction in a transgenic mouse model expressing human amyloid precursor protein (APP) with the Arctic mutation. J Neurochem 2016, 136, 497-502. [CrossRef]
- Perkins, M.; Wolf, A.B.; Chavira, B.; Shonebarger, D.; Meckel, J.P.; Leung, L.; Ballina, L.; Ly, S.; Saini, A.; Jones, T.B.; et al. Altered Energy Metabolism Pathways in the Posterior Cingulate in Young Adult Apolipoprotein E varepsilon4 Carriers. J Alzheimers Dis 2016, 53, 95-106. [CrossRef]
- Valla, J.; Yaari, R.; Wolf, A.B.; Kusne, Y.; Beach, T.G.; Roher, A.E.; Corneveaux, J.J.; Huentelman, M.J.; Caselli, R.J.; Reiman, E.M. Reduced posterior cingulate mitochondrial activity in expired young adult carriers of the APOE epsilon4 allele, the major late-onset Alzheimer's susceptibility gene. J Alzheimers Dis 2010, 22, 307-313. [CrossRef]
- Manczak, M.; Anekonda, T.S.; Henson, E.; Park, B.S.; Quinn, J.; Reddy, P.H. Mitochondria are a direct site of A beta accumulation in Alzheimer's disease neurons: implications for free radical generation and oxidative damage in disease progression. Hum Mol Genet 2006, 15, 1437-1449. [CrossRef]
- Wang, X.; Su, B.; Lee, H.G.; Li, X.; Perry, G.; Smith, M.A.; Zhu, X. Impaired balance of mitochondrial fission and fusion in Alzheimer's disease. J Neurosci 2009, 29, 9090-9103. [CrossRef]
- Wang, X.; Su, B.; Siedlak, S.L.; Moreira, P.I.; Fujioka, H.; Wang, Y.; Casadesus, G.; Zhu, X. Amyloid-beta overproduction causes abnormal mitochondrial dynamics via differential modulation of mitochondrial fission/fusion proteins. Proc Natl Acad Sci U S A 2008, 105, 19318-19323. [CrossRef]
- Manczak, M.; Kandimalla, R.; Fry, D.; Sesaki, H.; Reddy, P.H. Protective effects of reduced dynamin-related protein 1 against amyloid beta-induced mitochondrial dysfunction and synaptic damage in Alzheimer's disease. Hum Mol Genet 2016, 25, 5148-5166. [CrossRef]
- Cho, D.H.; Nakamura, T.; Fang, J.; Cieplak, P.; Godzik, A.; Gu, Z.; Lipton, S.A. S-nitrosylation of Drp1 mediates beta-amyloid-related mitochondrial fission and neuronal injury. Science 2009, 324, 102-105. [CrossRef]
- Kim, D.I.; Lee, K.H.; Gabr, A.A.; Choi, G.E.; Kim, J.S.; Ko, S.H.; Han, H.J. Abeta-Induced Drp1 phosphorylation through Akt activation promotes excessive mitochondrial fission leading to neuronal apoptosis. Biochim Biophys Acta 2016, 1863, 2820-2834. [CrossRef]
- Liu, C.; Song, X.; Nisbet, R.; Gotz, J. Co-immunoprecipitation with Tau Isoform-specific Antibodies Reveals Distinct Protein Interactions and Highlights a Putative Role for 2N Tau in Disease. J Biol Chem 2016, 291, 8173-8188. [CrossRef]
- Li, X.C.; Hu, Y.; Wang, Z.H.; Luo, Y.; Zhang, Y.; Liu, X.P.; Feng, Q.; Wang, Q.; Ye, K.; Liu, G.P.; et al. Human wild-type full-length tau accumulation disrupts mitochondrial dynamics and the functions via increasing mitofusins. Sci Rep 2016, 6, 24756. [CrossRef]
- Rhein, V.; Song, X.; Wiesner, A.; Ittner, L.M.; Baysang, G.; Meier, F.; Ozmen, L.; Bluethmann, H.; Drose, S.; Brandt, U.; et al. Amyloid-beta and tau synergistically impair the oxidative phosphorylation system in triple transgenic Alzheimer's disease mice. Proc Natl Acad Sci U S A 2009, 106, 20057-20062. [CrossRef]
- Amadoro, G.; Corsetti, V.; Atlante, A.; Florenzano, F.; Capsoni, S.; Bussani, R.; Mercanti, D.; Calissano, P. Interaction between NH(2)-tau fragment and Abeta in Alzheimer's disease mitochondria contributes to the synaptic deterioration. Neurobiol Aging 2012, 33, 833 e831-825. [CrossRef]
- Kandimalla, R.; Manczak, M.; Yin, X.; Wang, R.; Reddy, P.H. Hippocampal phosphorylated tau induced cognitive decline, dendritic spine loss and mitochondrial abnormalities in a mouse model of Alzheimer's disease. Hum Mol Genet 2018, 27, 30-40. [CrossRef]
- Jara, C.; Aranguiz, A.; Cerpa, W.; Tapia-Rojas, C.; Quintanilla, R.A. Genetic ablation of tau improves mitochondrial function and cognitive abilities in the hippocampus. Redox Biol 2018, 18, 279-294. [CrossRef]
- Vossel, K.A.; Xu, J.C.; Fomenko, V.; Miyamoto, T.; Suberbielle, E.; Knox, J.A.; Ho, K.; Kim, D.H.; Yu, G.Q.; Mucke, L. Tau reduction prevents Abeta-induced axonal transport deficits by blocking activation of GSK3beta. J Cell Biol 2015, 209, 419-433. [CrossRef]
- Zhang, B.; Higuchi, M.; Yoshiyama, Y.; Ishihara, T.; Forman, M.S.; Martinez, D.; Joyce, S.; Trojanowski, J.Q.; Lee, V.M. Retarded axonal transport of R406W mutant tau in transgenic mice with a neurodegenerative tauopathy. J Neurosci 2004, 24, 4657-4667. [CrossRef]
- Kanaan, N.M.; Morfini, G.A.; LaPointe, N.E.; Pigino, G.F.; Patterson, K.R.; Song, Y.; Andreadis, A.; Fu, Y.; Brady, S.T.; Binder, L.I. Pathogenic forms of tau inhibit kinesin-dependent axonal transport through a mechanism involving activation of axonal phosphotransferases. J Neurosci 2011, 31, 9858-9868. [CrossRef]
- Selfridge, J.E.; E, L.; Lu, J.; Swerdlow, R.H. Role of mitochondrial homeostasis and dynamics in Alzheimer's disease. Neurobiol Dis 2013, 51, 3-12. [CrossRef]
- Giraldo, E.; Lloret, A.; Fuchsberger, T.; Vina, J. Abeta and tau toxicities in Alzheimer's are linked via oxidative stress-induced p38 activation: protective role of vitamin E. Redox Biol 2014, 2, 873-877. [CrossRef]
- Bobba, A.; Amadoro, G.; Valenti, D.; Corsetti, V.; Lassandro, R.; Atlante, A. Mitochondrial respiratory chain Complexes I and IV are impaired by beta-amyloid via direct interaction and through Complex I-dependent ROS production, respectively. Mitochondrion 2013, 13, 298-311. [CrossRef]
- Lovell, M.A.; Ehmann, W.D.; Butler, S.M.; Markesbery, W.R. Elevated thiobarbituric acid-reactive substances and antioxidant enzyme activity in the brain in Alzheimer's disease. Neurology 1995, 45, 1594-1601. [CrossRef]
- McIntosh, L.J.; Trush, M.A.; Troncoso, J.C. Increased susceptibility of Alzheimer's disease temporal cortex to oxygen free radical-mediated processes. Free Radic Biol Med 1997, 23, 183-190. [CrossRef]
- Markesbery, W.R.; Lovell, M.A. Four-hydroxynonenal, a product of lipid peroxidation, is increased in the brain in Alzheimer's disease. Neurobiol Aging 1998, 19, 33-36. [CrossRef]
- Montine, K.S.; Reich, E.; Neely, M.D.; Sidell, K.R.; Olson, S.J.; Markesbery, W.R.; Montine, T.J. Distribution of reducible 4-hydroxynonenal adduct immunoreactivity in Alzheimer disease is associated with APOE genotype. J Neuropathol Exp Neurol 1998, 57, 415-425. [CrossRef]
- Youssef, P.; Chami, B.; Lim, J.; Middleton, T.; Sutherland, G.T.; Witting, P.K. Evidence supporting oxidative stress in a moderately affected area of the brain in Alzheimer's disease. Sci Rep 2018, 8, 11553. [CrossRef]
- Pratico, D.; Clark, C.M.; Lee, V.M.; Trojanowski, J.Q.; Rokach, J.; FitzGerald, G.A. Increased 8,12-iso-iPF2alpha-VI in Alzheimer's disease: correlation of a noninvasive index of lipid peroxidation with disease severity. Ann Neurol 2000, 48, 809-812.
- Quinn, J.F.; Montine, K.S.; Moore, M.; Morrow, J.D.; Kaye, J.A.; Montine, T.J. Suppression of longitudinal increase in CSF F2-isoprostanes in Alzheimer's disease. J Alzheimers Dis 2004, 6, 93-97. [CrossRef]
- Selley, M.L.; Close, D.R.; Stern, S.E. The effect of increased concentrations of homocysteine on the concentration of (E)-4-hydroxy-2-nonenal in the plasma and cerebrospinal fluid of patients with Alzheimer's disease. Neurobiol Aging 2002, 23, 383-388. [CrossRef]
- Sultana, R.; Poon, H.F.; Cai, J.; Pierce, W.M.; Merchant, M.; Klein, J.B.; Markesbery, W.R.; Butterfield, D.A. Identification of nitrated proteins in Alzheimer's disease brain using a redox proteomics approach. Neurobiol Dis 2006, 22, 76-87. [CrossRef]
- Reed, T.; Perluigi, M.; Sultana, R.; Pierce, W.M.; Klein, J.B.; Turner, D.M.; Coccia, R.; Markesbery, W.R.; Butterfield, D.A. Redox proteomic identification of 4-hydroxy-2-nonenal-modified brain proteins in amnestic mild cognitive impairment: insight into the role of lipid peroxidation in the progression and pathogenesis of Alzheimer's disease. Neurobiol Dis 2008, 30, 107-120. [CrossRef]
- Beck, S.J.; Guo, L.; Phensy, A.; Tian, J.; Wang, L.; Tandon, N.; Gauba, E.; Lu, L.; Pascual, J.M.; Kroener, S.; et al. Deregulation of mitochondrial F1FO-ATP synthase via OSCP in Alzheimer's disease. Nat Commun 2016, 7, 11483. [CrossRef]
- Good, P.F.; Werner, P.; Hsu, A.; Olanow, C.W.; Perl, D.P. Evidence of neuronal oxidative damage in Alzheimer's disease. Am J Pathol 1996, 149, 21-28.
- Smith, M.A.; Richey Harris, P.L.; Sayre, L.M.; Beckman, J.S.; Perry, G. Widespread peroxynitrite-mediated damage in Alzheimer's disease. J Neurosci 1997, 17, 2653-2657. [CrossRef]
- Castegna, A.; Thongboonkerd, V.; Klein, J.B.; Lynn, B.; Markesbery, W.R.; Butterfield, D.A. Proteomic identification of nitrated proteins in Alzheimer's disease brain. J Neurochem 2003, 85, 1394-1401. [CrossRef]
- Reed, T.T.; Pierce, W.M., Jr.; Turner, D.M.; Markesbery, W.R.; Allan Butterfield, D. Proteomic identification of nitrated brain proteins in early Alzheimer's disease inferior parietal lobule. J Cell Mol Med 2009, 13, 2019-2029. [CrossRef]
- Hensley, K.; Hall, N.; Subramaniam, R.; Cole, P.; Harris, M.; Aksenov, M.; Aksenova, M.; Gabbita, S.P.; Wu, J.F.; Carney, J.M.; et al. Brain regional correspondence between Alzheimer's disease histopathology and biomarkers of protein oxidation. J Neurochem 1995, 65, 2146-2156. [CrossRef]
- Sharma, A.; Weber, D.; Raupbach, J.; Dakal, T.C.; Fliessbach, K.; Ramirez, A.; Grune, T.; Wullner, U. Advanced glycation end products and protein carbonyl levels in plasma reveal sex-specific differences in Parkinson's and Alzheimer's disease. Redox Biol 2020, 34, 101546. [CrossRef]
- Mullaart, E.; Boerrigter, M.E.; Ravid, R.; Swaab, D.F.; Vijg, J. Increased levels of DNA breaks in cerebral cortex of Alzheimer's disease patients. Neurobiol Aging 1990, 11, 169-173. [CrossRef]
- Anderson, A.J.; Su, J.H.; Cotman, C.W. DNA damage and apoptosis in Alzheimer's disease: colocalization with c-Jun immunoreactivity, relationship to brain area, and effect of postmortem delay. J Neurosci 1996, 16, 1710-1719. [CrossRef]
- Nunomura, A.; Tamaoki, T.; Motohashi, N.; Nakamura, M.; McKeel, D.W., Jr.; Tabaton, M.; Lee, H.G.; Smith, M.A.; Perry, G.; Zhu, X. The earliest stage of cognitive impairment in transition from normal aging to Alzheimer disease is marked by prominent RNA oxidation in vulnerable neurons. J Neuropathol Exp Neurol 2012, 71, 233-241. [CrossRef]
- Mecocci, P.; MacGarvey, U.; Beal, M.F. Oxidative damage to mitochondrial DNA is increased in Alzheimer's disease. Ann Neurol 1994, 36, 747-751. [CrossRef]
- Spillantini, M.G.; Schmidt, M.L.; Lee, V.M.; Trojanowski, J.Q.; Jakes, R.; Goedert, M. Alpha-synuclein in Lewy bodies. Nature 1997, 388, 839-840. [CrossRef]
- Borghammer, P. Perfusion and metabolism imaging studies in Parkinson's disease. Dan Med J 2012, 59, B4466.
- Edison, P.; Ahmed, I.; Fan, Z.; Hinz, R.; Gelosa, G.; Ray Chaudhuri, K.; Walker, Z.; Turkheimer, F.E.; Brooks, D.J. Microglia, amyloid, and glucose metabolism in Parkinson's disease with and without dementia. Neuropsychopharmacology 2013, 38, 938-949. [CrossRef]
- Firbank, M.J.; Yarnall, A.J.; Lawson, R.A.; Duncan, G.W.; Khoo, T.K.; Petrides, G.S.; O'Brien, J.T.; Barker, R.A.; Maxwell, R.J.; Brooks, D.J.; et al. Cerebral glucose metabolism and cognition in newly diagnosed Parkinson's disease: ICICLE-PD study. J Neurol Neurosurg Psychiatry 2017, 88, 310-316. [CrossRef]
- Andersen, K.B.; Hansen, A.K.; Schacht, A.C.; Horsager, J.; Gottrup, H.; Klit, H.; Danielsen, E.H.; Poston, K.L.; Pavese, N.; Brooks, D.J.; et al. Synaptic Density and Glucose Consumption in Patients with Lewy Body Diseases: An [(11) C]UCB-J and [(18) F]FDG PET Study. Mov Disord 2023. [CrossRef]
- Dunn, L.; Allen, G.F.; Mamais, A.; Ling, H.; Li, A.; Duberley, K.E.; Hargreaves, I.P.; Pope, S.; Holton, J.L.; Lees, A.; et al. Dysregulation of glucose metabolism is an early event in sporadic Parkinson's disease. Neurobiol Aging 2014, 35, 1111-1115. [CrossRef]
- Rodriguez-Araujo, G.; Nakagami, H.; Hayashi, H.; Mori, M.; Shiuchi, T.; Minokoshi, Y.; Nakaoka, Y.; Takami, Y.; Komuro, I.; Morishita, R.; et al. Alpha-synuclein elicits glucose uptake and utilization in adipocytes through the Gab1/PI3K/Akt transduction pathway. Cell Mol Life Sci 2013, 70, 1123-1133. [CrossRef]
- Zilocchi, M.; Finzi, G.; Lualdi, M.; Sessa, F.; Fasano, M.; Alberio, T. Mitochondrial alterations in Parkinson's disease human samples and cellular models. Neurochem Int 2018, 118, 61-72. [CrossRef]
- Trimmer, P.A.; Swerdlow, R.H.; Parks, J.K.; Keeney, P.; Bennett, J.P., Jr.; Miller, S.W.; Davis, R.E.; Parker, W.D., Jr. Abnormal mitochondrial morphology in sporadic Parkinson's and Alzheimer's disease cybrid cell lines. Exp Neurol 2000, 162, 37-50. [CrossRef]
- Gold, M.; Hauser, R.A.; Chen, M.F. Plasma thiamine deficiency associated with Alzheimer's disease but not Parkinson's disease. Metab Brain Dis 1998, 13, 43-53. [CrossRef]
- Jimenez-Jimenez, F.J.; Molina, J.A.; Hernanz, A.; Fernandez-Vivancos, E.; de Bustos, F.; Barcenilla, B.; Gomez-Escalonilla, C.; Zurdo, M.; Berbel, A.; Villanueva, C. Cerebrospinal fluid levels of thiamine in patients with Parkinson's disease. Neurosci Lett 1999, 271, 33-36. [CrossRef]
- Gibson, G.E.; Kingsbury, A.E.; Xu, H.; Lindsay, J.G.; Daniel, S.; Foster, O.J.; Lees, A.J.; Blass, J.P. Deficits in a tricarboxylic acid cycle enzyme in brains from patients with Parkinson's disease. Neurochem Int 2003, 43, 129-135. [CrossRef]
- Mizuno, Y.; Matuda, S.; Yoshino, H.; Mori, H.; Hattori, N.; Ikebe, S. An immunohistochemical study on alpha-ketoglutarate dehydrogenase complex in Parkinson's disease. Ann Neurol 1994, 35, 204-210. [CrossRef]
- Miki, Y.; Tanji, K.; Mori, F.; Kakita, A.; Takahashi, H.; Wakabayashi, K. Alteration of mitochondrial protein PDHA1 in Lewy body disease and PARK14. Biochem Biophys Res Commun 2017, 489, 439-444. [CrossRef]
- Mallajosyula, J.K.; Chinta, S.J.; Rajagopalan, S.; Nicholls, D.G.; Andersen, J.K. Metabolic control analysis in a cellular model of elevated MAO-B: relevance to Parkinson's disease. Neurotox Res 2009, 16, 186-193. [CrossRef]
- Schapira, A.H.; Cooper, J.M.; Dexter, D.; Clark, J.B.; Jenner, P.; Marsden, C.D. Mitochondrial complex I deficiency in Parkinson's disease. J Neurochem 1990, 54, 823-827. [CrossRef]
- Mann, V.M.; Cooper, J.M.; Daniel, S.E.; Srai, K.; Jenner, P.; Marsden, C.D.; Schapira, A.H. Complex I, iron, and ferritin in Parkinson's disease substantia nigra. Ann Neurol 1994, 36, 876-881. [CrossRef]
- Janetzky, B.; Hauck, S.; Youdim, M.B.; Riederer, P.; Jellinger, K.; Pantucek, F.; Zochling, R.; Boissl, K.W.; Reichmann, H. Unaltered aconitase activity, but decreased complex I activity in substantia nigra pars compacta of patients with Parkinson's disease. Neurosci Lett 1994, 169, 126-128. [CrossRef]
- Krige, D.; Carroll, M.T.; Cooper, J.M.; Marsden, C.D.; Schapira, A.H. Platelet mitochondrial function in Parkinson's disease. The Royal Kings and Queens Parkinson Disease Research Group. Ann Neurol 1992, 32, 782-788. [CrossRef]
- Yoshino, H.; Nakagawa-Hattori, Y.; Kondo, T.; Mizuno, Y. Mitochondrial complex I and II activities of lymphocytes and platelets in Parkinson's disease. J Neural Transm Park Dis Dement Sect 1992, 4, 27-34. [CrossRef]
- Parker, W.D., Jr.; Parks, J.K.; Swerdlow, R.H. Complex I deficiency in Parkinson's disease frontal cortex. Brain Res 2008, 1189, 215-218. [CrossRef]
- Bindoff, L.A.; Birch-Machin, M.A.; Cartlidge, N.E.; Parker, W.D., Jr.; Turnbull, D.M. Respiratory chain abnormalities in skeletal muscle from patients with Parkinson's disease. J Neurol Sci 1991, 104, 203-208. [CrossRef]
- Haas, R.H.; Nasirian, F.; Nakano, K.; Ward, D.; Pay, M.; Hill, R.; Shults, C.W. Low platelet mitochondrial complex I and complex II/III activity in early untreated Parkinson's disease. Ann Neurol 1995, 37, 714-722. [CrossRef]
- Gonzalez-Rodriguez, P.; Zampese, E.; Stout, K.A.; Guzman, J.N.; Ilijic, E.; Yang, B.; Tkatch, T.; Stavarache, M.A.; Wokosin, D.L.; Gao, L.; et al. Disruption of mitochondrial complex I induces progressive parkinsonism. Nature 2021, 599, 650-656. [CrossRef]
- Vives-Bauza, C.; Tocilescu, M.; Devries, R.L.; Alessi, D.M.; Jackson-Lewis, V.; Przedborski, S. Control of mitochondrial integrity in Parkinson's disease. Prog Brain Res 2010, 183, 99-113. [CrossRef]
- Carelli, V.; Musumeci, O.; Caporali, L.; Zanna, C.; La Morgia, C.; Del Dotto, V.; Porcelli, A.M.; Rugolo, M.; Valentino, M.L.; Iommarini, L.; et al. Syndromic parkinsonism and dementia associated with OPA1 missense mutations. Ann Neurol 2015, 78, 21-38. [CrossRef]
- Lynch, D.S.; Loh, S.H.Y.; Harley, J.; Noyce, A.J.; Martins, L.M.; Wood, N.W.; Houlden, H.; Plun-Favreau, H. Nonsyndromic Parkinson disease in a family with autosomal dominant optic atrophy due to OPA1 mutations. Neurol Genet 2017, 3, e188. [CrossRef]
- Chu, Y.; Morfini, G.A.; Langhamer, L.B.; He, Y.; Brady, S.T.; Kordower, J.H. Alterations in axonal transport motor proteins in sporadic and experimental Parkinson's disease. Brain 2012, 135, 2058-2073. [CrossRef]
- Tapias, V.; McCoy, J.L.; Greenamyre, J.T. Phenothiazine normalizes the NADH/NAD(+) ratio, maintains mitochondrial integrity and protects the nigrostriatal dopamine system in a chronic rotenone model of Parkinson's disease. Redox Biol 2019, 24, 101164. [CrossRef]
- Goiran, T.; Eldeeb, M.A.; Zorca, C.E.; Fon, E.A. Hallmarks and Molecular Tools for the Study of Mitophagy in Parkinson's Disease. Cells 2022, 11. [CrossRef]
- Ge, P.; Dawson, V.L.; Dawson, T.M. PINK1 and Parkin mitochondrial quality control: a source of regional vulnerability in Parkinson's disease. Mol Neurodegener 2020, 15, 20. [CrossRef]
- Devi, L.; Raghavendran, V.; Prabhu, B.M.; Avadhani, N.G.; Anandatheerthavarada, H.K. Mitochondrial import and accumulation of alpha-synuclein impair complex I in human dopaminergic neuronal cultures and Parkinson disease brain. J Biol Chem 2008, 283, 9089-9100. [CrossRef]
- Bender, A.; Desplats, P.; Spencer, B.; Rockenstein, E.; Adame, A.; Elstner, M.; Laub, C.; Mueller, S.; Koob, A.O.; Mante, M.; et al. TOM40 mediates mitochondrial dysfunction induced by alpha-synuclein accumulation in Parkinson's disease. PLoS One 2013, 8, e62277. [CrossRef]
- Guardia-Laguarta, C.; Area-Gomez, E.; Rub, C.; Liu, Y.; Magrane, J.; Becker, D.; Voos, W.; Schon, E.A.; Przedborski, S. alpha-Synuclein is localized to mitochondria-associated ER membranes. J Neurosci 2014, 34, 249-259. [CrossRef]
- Di Maio, R.; Barrett, P.J.; Hoffman, E.K.; Barrett, C.W.; Zharikov, A.; Borah, A.; Hu, X.; McCoy, J.; Chu, C.T.; Burton, E.A.; et al. alpha-Synuclein binds to TOM20 and inhibits mitochondrial protein import in Parkinson's disease. Sci Transl Med 2016, 8, 342ra378. [CrossRef]
- Ganjam, G.K.; Bolte, K.; Matschke, L.A.; Neitemeier, S.; Dolga, A.M.; Hollerhage, M.; Hoglinger, G.U.; Adamczyk, A.; Decher, N.; Oertel, W.H.; et al. Mitochondrial damage by alpha-synuclein causes cell death in human dopaminergic neurons. Cell Death Dis 2019, 10, 865. [CrossRef]
- Nakamura, K.; Nemani, V.M.; Azarbal, F.; Skibinski, G.; Levy, J.M.; Egami, K.; Munishkina, L.; Zhang, J.; Gardner, B.; Wakabayashi, J.; et al. Direct membrane association drives mitochondrial fission by the Parkinson disease-associated protein alpha-synuclein. J Biol Chem 2011, 286, 20710-20726. [CrossRef]
- Pozo Devoto, V.M.; Dimopoulos, N.; Alloatti, M.; Pardi, M.B.; Saez, T.M.; Otero, M.G.; Cromberg, L.E.; Marin-Burgin, A.; Scassa, M.E.; Stokin, G.B.; et al. alphaSynuclein control of mitochondrial homeostasis in human-derived neurons is disrupted by mutations associated with Parkinson's disease. Sci Rep 2017, 7, 5042. [CrossRef]
- Krzystek, T.J.; Banerjee, R.; Thurston, L.; Huang, J.; Swinter, K.; Rahman, S.N.; Falzone, T.L.; Gunawardena, S. Differential mitochondrial roles for alpha-synuclein in DRP1-dependent fission and PINK1/Parkin-mediated oxidation. Cell Death Dis 2021, 12, 796. [CrossRef]
- Kamp, F.; Exner, N.; Lutz, A.K.; Wender, N.; Hegermann, J.; Brunner, B.; Nuscher, B.; Bartels, T.; Giese, A.; Beyer, K.; et al. Inhibition of mitochondrial fusion by alpha-synuclein is rescued by PINK1, Parkin and DJ-1. EMBO J 2010, 29, 3571-3589. [CrossRef]
- Ulusoy, A.; Rusconi, R.; Perez-Revuelta, B.I.; Musgrove, R.E.; Helwig, M.; Winzen-Reichert, B.; Di Monte, D.A. Caudo-rostral brain spreading of alpha-synuclein through vagal connections. EMBO Mol Med 2013, 5, 1119-1127. [CrossRef]
- Brzozowski, C.F.; Hijaz, B.A.; Singh, V.; Gcwensa, N.Z.; Kelly, K.; Boyden, E.S.; West, A.B.; Sarkar, D.; Volpicelli-Daley, L.A. Inhibition of LRRK2 kinase activity promotes anterograde axonal transport and presynaptic targeting of alpha-synuclein. Acta Neuropathol Commun 2021, 9, 180. [CrossRef]
- Cali, T.; Ottolini, D.; Negro, A.; Brini, M. alpha-Synuclein controls mitochondrial calcium homeostasis by enhancing endoplasmic reticulum-mitochondria interactions. J Biol Chem 2012, 287, 17914-17929. [CrossRef]
- Park, J.H.; Burgess, J.D.; Faroqi, A.H.; DeMeo, N.N.; Fiesel, F.C.; Springer, W.; Delenclos, M.; McLean, P.J. Alpha-synuclein-induced mitochondrial dysfunction is mediated via a sirtuin 3-dependent pathway. Mol Neurodegener 2020, 15, 5. [CrossRef]
- Parihar, M.S.; Parihar, A.; Fujita, M.; Hashimoto, M.; Ghafourifar, P. Mitochondrial association of alpha-synuclein causes oxidative stress. Cell Mol Life Sci 2008, 65, 1272-1284. [CrossRef]
- Valero, R.A.; Senovilla, L.; Nunez, L.; Villalobos, C. The role of mitochondrial potential in control of calcium signals involved in cell proliferation. Cell Calcium 2008, 44, 259-269. [CrossRef]
- Villalobos, C.; Nunez, L.; Montero, M.; Garcia, A.G.; Alonso, M.T.; Chamero, P.; Alvarez, J.; Garcia-Sancho, J. Redistribution of Ca2+ among cytosol and organella during stimulation of bovine chromaffin cells. FASEB J 2002, 16, 343-353. [CrossRef]
- Nunez, L.; Senovilla, L.; Sanz-Blasco, S.; Chamero, P.; Alonso, M.T.; Villalobos, C.; Garcia-Sancho, J. Bioluminescence imaging of mitochondrial Ca2+ dynamics in soma and neurites of individual adult mouse sympathetic neurons. J Physiol 2007, 580, 385-395. [CrossRef]
- Núñez, L.; Villalobos, C.; García-Sancho, J. Coupling or not coupling of mitochondria to Ca2+ sources in neurones. Soma and neurites differ. Physiol News. 2008, 70, 23-24.
- Parys, J.B.; Pereira, C.F.; Villalobos, C. The Eighth ECS Workshop on "Calcium Signaling in Aging and Neurodegenerative Diseases". Int J Mol Sci 2019, 20. [CrossRef]
- Camandola, S.; Mattson, M.P. Aberrant subcellular neuronal calcium regulation in aging and Alzheimer's disease. Biochim Biophys Acta 2011, 1813, 965-973. [CrossRef]
- Popugaeva, E.; Pchitskaya, E.; Bezprozvanny, I. Dysregulation of neuronal calcium homeostasis in Alzheimer's disease - A therapeutic opportunity? Biochem Biophys Res Commun 2017, 483, 998-1004. [CrossRef]
- Arispe, N.; Diaz, J.C.; Simakova, O. Abeta ion channels. Prospects for treating Alzheimer's disease with Abeta channel blockers. Biochim Biophys Acta 2007, 1768, 1952-1965. [CrossRef]
- Sanz-Blasco, S.; Valero, R.A.; Rodriguez-Crespo, I.; Villalobos, C.; Nunez, L. Mitochondrial Ca2+ overload underlies Abeta oligomers neurotoxicity providing an unexpected mechanism of neuroprotection by NSAIDs. PLoS One 2008, 3, e2718. [CrossRef]
- Caballero, E.; Calvo-Rodriguez, M.; Gonzalo-Ruiz, A.; Villalobos, C.; Nunez, L. A new procedure for amyloid beta oligomers preparation enables the unambiguous testing of their effects on cytosolic and mitochondrial Ca(2+) entry and cell death in primary neurons. Neurosci Lett 2016, 612, 66-73. [CrossRef]
- Calvo-Rodriguez, M.; Nunez, L.; Villalobos, C. Non-steroidal anti-inflammatory drugs (NSAIDs) and neuroprotection in the elderly: a view from the mitochondria. Neural Regen Res 2015, 10, 1371-1372. [CrossRef]
- Garcia-Martinez, E.M.; Sanz-Blasco, S.; Karachitos, A.; Bandez, M.J.; Fernandez-Gomez, F.J.; Perez-Alvarez, S.; de Mera, R.M.; Jordan, M.J.; Aguirre, N.; Galindo, M.F.; et al. Mitochondria and calcium flux as targets of neuroprotection caused by minocycline in cerebellar granule cells. Biochem Pharmacol 2010, 79, 239-250. [CrossRef]
- Perez-Alvarez, S.; Solesio, M.E.; Cuenca-Lopez, M.D.; Melero-Fernandez de Mera, R.M.; Villalobos, C.; Kmita, H.; Galindo, M.F.; Jordan, J. Pharmacological Characterization of the Mechanisms Involved in Delayed Calcium Deregulation in SH-SY5Y Cells Challenged with Methadone. Int J Cell Biol 2012, 2012, 642482. [CrossRef]
- Sanz-Blasco, S.; Calvo-Rodriguez, M.; Caballero, E.; Garcia-Durillo, M.; Nunez, L.; Villalobos, C. Is it All Said for NSAIDs in Alzheimer's Disease? Role of Mitochondrial Calcium Uptake. Curr Alzheimer Res 2018, 15, 504-510. [CrossRef]
- Atwood, C.; Bacskai, B.; Kuchibhotla, K.; Bezprozvanny, I.; Chakroborty, S.; Fagan, T.; Foskett, K.; Green, K.; Goussakov, I.; Moyer, J.; et al. Alzheimer research forum live discussion: calcium in AD pathogenesis. J Alzheimers Dis 2009, 16, 909-917. [CrossRef]
- Calvo, M.; Sanz-Blasco, S.; Caballero, E.; Villalobos, C.; Nunez, L. Susceptibility to excitotoxicity in aged hippocampal cultures and neuroprotection by non-steroidal anti-inflammatory drugs: role of mitochondrial calcium. J Neurochem 2015, 132, 403-417. [CrossRef]
- Calvo-Rodriguez, M.; Garcia-Durillo, M.; Villalobos, C.; Nunez, L. Aging Enables Ca2+ Overload and Apoptosis Induced by Amyloid-beta Oligomers in Rat Hippocampal Neurons: Neuroprotection by Non-Steroidal Anti-Inflammatory Drugs and R-Flurbiprofen in Aging Neurons. J Alzheimers Dis 2016, 54, 207-221. [CrossRef]
- Pascual, M.; Calvo-Rodriguez, M.; Nunez, L.; Villalobos, C.; Urena, J.; Guerri, C. Toll-like receptors in neuroinflammation, neurodegeneration, and alcohol-induced brain damage. IUBMB Life 2021, 73, 900-915. [CrossRef]
- Calvo-Rodriguez, M.; de la Fuente, C.; Garcia-Durillo, M.; Garcia-Rodriguez, C.; Villalobos, C.; Nunez, L. Aging and amyloid beta oligomers enhance TLR4 expression, LPS-induced Ca(2+) responses, and neuron cell death in cultured rat hippocampal neurons. J Neuroinflammation 2017, 14, 24. [CrossRef]
- Calvo-Rodriguez, M.; Garcia-Rodriguez, C.; Villalobos, C.; Nunez, L. Role of Toll Like Receptor 4 in Alzheimer's Disease. Front Immunol 2020, 11, 1588. [CrossRef]
- Calvo-Rodriguez, M.; Garcia-Durillo, M.; Villalobos, C.; Nunez, L. In vitro aging promotes endoplasmic reticulum (ER)-mitochondria Ca(2+) cross talk and loss of store-operated Ca(2+) entry (SOCE) in rat hippocampal neurons. Biochim Biophys Acta 2016, 1863, 2637-2649. [CrossRef]
- Calvo-Rodriguez, M.; Hernando-Perez, E.; Nunez, L.; Villalobos, C. Amyloid beta Oligomers Increase ER-Mitochondria Ca(2+) Cross Talk in Young Hippocampal Neurons and Exacerbate Aging-Induced Intracellular Ca(2+) Remodeling. Front Cell Neurosci 2019, 13, 22. [CrossRef]
- Calvo-Rodriguez, M.; Hernando-Perez, E.; Lopez-Vazquez, S.; Nunez, J.; Villalobos, C.; Nunez, L. Remodeling of Intracellular Ca(2+) Homeostasis in Rat Hippocampal Neurons Aged In Vitro. Int J Mol Sci 2020, 21. [CrossRef]
- Texido, L.; Martin-Satue, M.; Alberdi, E.; Solsona, C.; Matute, C. Amyloid beta peptide oligomers directly activate NMDA receptors. Cell Calcium 2011, 49, 184-190. [CrossRef]
- Caballero, E.; Hernando-Perez, E.; Tapias, V.; Calvo-Rodriguez, M.; Villalobos, C.; Nunez, L. Amyloid Beta Oligomers-Induced Ca(2+) Entry Pathways: Role of Neuronal Networks, NMDA Receptors and Amyloid Channel Formation. Biomedicines 2022, 10. [CrossRef]
- Gonzalez-Andres, P.; Fernandez-Pena, L.; Diez-Poza, C.; Villalobos, C.; Nunez, L.; Barbero, A. Marine Heterocyclic Compounds That Modulate Intracellular Calcium Signals: Chemistry and Synthesis Approaches. Mar Drugs 2021, 19. [CrossRef]
- Chan, C.S.; Guzman, J.N.; Ilijic, E.; Mercer, J.N.; Rick, C.; Tkatch, T.; Meredith, G.E.; Surmeier, D.J. 'Rejuvenation' protects neurons in mouse models of Parkinson's disease. Nature 2007, 447, 1081-1086. [CrossRef]
- Chan, C.S.; Gertler, T.S.; Surmeier, D.J. A molecular basis for the increased vulnerability of substantia nigra dopamine neurons in aging and Parkinson's disease. Mov Disord 2010, 25 Suppl 1, S63-70. [CrossRef]
- Zampese, E.; Wokosin, D.L.; Gonzalez-Rodriguez, P.; Guzman, J.N.; Tkatch, T.; Kondapalli, J.; Surmeier, W.C.; D'Alessandro, K.B.; De Stefani, D.; Rizzuto, R.; et al. Ca(2+) channels couple spiking to mitochondrial metabolism in substantia nigra dopaminergic neurons. Sci Adv 2022, 8, eabp8701. [CrossRef]
- Zampese, E.; Surmeier, D.J. Calcium, Bioenergetics, and Parkinson's Disease. Cells 2020, 9. [CrossRef]
- Yun, J.; Jeong, D.; Xie, Z.; Lee, S.; Kim, J.; Surmeier, D.J.; Silverman, R.B.; Kang, S. Palladium-Catalyzed alpha-Arylation of Cyclic beta-Dicarbonyl Compounds for the Synthesis of Ca(V)1.3 Inhibitors. ACS Omega 2022, 7, 14252-14263. [CrossRef]
- Oakes, S.A.; Scorrano, L.; Opferman, J.T.; Bassik, M.C.; Nishino, M.; Pozzan, T.; Korsmeyer, S.J. Proapoptotic BAX and BAK regulate the type 1 inositol trisphosphate receptor and calcium leak from the endoplasmic reticulum. Proc Natl Acad Sci U S A 2005, 102, 105-110. [CrossRef]
- Tu, H.; Nelson, O.; Bezprozvanny, A.; Wang, Z.; Lee, S.F.; Hao, Y.H.; Serneels, L.; De Strooper, B.; Yu, G.; Bezprozvanny, I. Presenilins form ER Ca2+ leak channels, a function disrupted by familial Alzheimer's disease-linked mutations. Cell 2006, 126, 981-993. [CrossRef]
- Lacampagne, A.; Liu, X.; Reiken, S.; Bussiere, R.; Meli, A.C.; Lauritzen, I.; Teich, A.F.; Zalk, R.; Saint, N.; Arancio, O.; et al. Post-translational remodeling of ryanodine receptor induces calcium leak leading to Alzheimer's disease-like pathologies and cognitive deficits. Acta Neuropathol 2017, 134, 749-767. [CrossRef]
- Vamvakides, A. [Anticonvulsant and forced swim anti-immobility effects of tetrahydro-N, N-dimethyl-2,2-diphenyl-3-furanemethanamine (AE37): common action mechanism?]. Ann Pharm Fr 2002, 60, 88-92.
- Hayashi, T.; Maurice, T.; Su, T.P. Ca(2+) signaling via sigma(1)-receptors: novel regulatory mechanism affecting intracellular Ca(2+) concentration. J Pharmacol Exp Ther 2000, 293, 788-798.
- Hayashi, T.; Su, T.P. Sigma-1 receptor chaperones at the ER-mitochondrion interface regulate Ca(2+) signaling and cell survival. Cell 2007, 131, 596-610. [CrossRef]
- Willems, P.H.; Valsecchi, F.; Distelmaier, F.; Verkaart, S.; Visch, H.J.; Smeitink, J.A.; Koopman, W.J. Mitochondrial Ca2+ homeostasis in human NADH:ubiquinone oxidoreductase deficiency. Cell Calcium 2008, 44, 123-133. [CrossRef]
- Hampel, H.; Williams, C.; Etcheto, A.; Goodsaid, F.; Parmentier, F.; Sallantin, J.; Kaufmann, W.E.; Missling, C.U.; Afshar, M. A precision medicine framework using artificial intelligence for the identification and confirmation of genomic biomarkers of response to an Alzheimer's disease therapy: Analysis of the blarcamesine (ANAVEX2-73) Phase 2a clinical study. Alzheimers Dement (N Y) 2020, 6, e12013. [CrossRef]
- Villard, V.; Espallergues, J.; Keller, E.; Vamvakides, A.; Maurice, T. Anti-amnesic and neuroprotective potentials of the mixed muscarinic receptor/sigma 1 (sigma1) ligand ANAVEX2-73, a novel aminotetrahydrofuran derivative. J Psychopharmacol 2011, 25, 1101-1117. [CrossRef]
- Lahmy, V.; Meunier, J.; Malmstrom, S.; Naert, G.; Givalois, L.; Kim, S.H.; Villard, V.; Vamvakides, A.; Maurice, T. Blockade of Tau hyperphosphorylation and Abeta(1)(-)(4)(2) generation by the aminotetrahydrofuran derivative ANAVEX2-73, a mixed muscarinic and sigma(1) receptor agonist, in a nontransgenic mouse model of Alzheimer's disease. Neuropsychopharmacology 2013, 38, 1706-1723. [CrossRef]
- Lahmy, V.; Long, R.; Morin, D.; Villard, V.; Maurice, T. Mitochondrial protection by the mixed muscarinic/sigma1 ligand ANAVEX2-73, a tetrahydrofuran derivative, in Abeta25-35 peptide-injected mice, a nontransgenic Alzheimer's disease model. Front Cell Neurosci 2015, 8, 463. [CrossRef]
- Goguadze, N.; Zhuravliova, E.; Morin, D.; Mikeladze, D.; Maurice, T. Sigma-1 Receptor Agonists Induce Oxidative Stress in Mitochondria and Enhance Complex I Activity in Physiological Condition but Protect Against Pathological Oxidative Stress. Neurotox Res 2019, 35, 1-18. [CrossRef]
- Maurice, T. Protection by sigma-1 receptor agonists is synergic with donepezil, but not with memantine, in a mouse model of amyloid-induced memory impairments. Behav Brain Res 2016, 296, 270-278. [CrossRef]
- Christ, M.G.; Huesmann, H.; Nagel, H.; Kern, A.; Behl, C. Sigma-1 Receptor Activation Induces Autophagy and Increases Proteostasis Capacity In Vitro and In Vivo. Cells 2019, 8. [CrossRef]
- Foscolos, G.B.; Kolocouris, N.; Fytas, G.; Marakos, P.; Pouli, N.; Vamvakides, A. Synthesis and pharmacological study of some new beta-(dialkylaminomethyl)- gamma-butyrolactones and their tetrahydrofuran analogues. Farmaco 1996, 51, 19-26. [CrossRef]
- Ashihara, H.; Suzuki, T. Distribution and biosynthesis of caffeine in plants. Front Biosci 2004, 9, 1864-1876. [CrossRef]
- Ludwig, I.A.; Clifford, M.N.; Lean, M.E.; Ashihara, H.; Crozier, A. Coffee: biochemistry and potential impact on health. Food Funct 2014, 5, 1695-1717. [CrossRef]
- Hall, S.; Desbrow, B.; Anoopkumar-Dukie, S.; Davey, A.K.; Arora, D.; McDermott, C.; Schubert, M.M.; Perkins, A.V.; Kiefel, M.J.; Grant, G.D. A review of the bioactivity of coffee, caffeine and key coffee constituents on inflammatory responses linked to depression. Food Res Int 2015, 76, 626-636. [CrossRef]
- Cedazo-Minguez, A.; Popescu, B.O.; Ankarcrona, M.; Nishimura, T.; Cowburn, R.F. The presenilin 1 deltaE9 mutation gives enhanced basal phospholipase C activity and a resultant increase in intracellular calcium concentrations. J Biol Chem 2002, 277, 36646-36655. [CrossRef]
- Smith, I.F.; Boyle, J.P.; Vaughan, P.F.; Pearson, H.A.; Cowburn, R.F.; Peers, C.S. Ca(2+) stores and capacitative Ca(2+) entry in human neuroblastoma (SH-SY5Y) cells expressing a familial Alzheimer's disease presenilin-1 mutation. Brain Res 2002, 949, 105-111. [CrossRef]
- Chan, S.L.; Mayne, M.; Holden, C.P.; Geiger, J.D.; Mattson, M.P. Presenilin-1 mutations increase levels of ryanodine receptors and calcium release in PC12 cells and cortical neurons. J Biol Chem 2000, 275, 18195-18200. [CrossRef]
- Leissring, M.A.; Akbari, Y.; Fanger, C.M.; Cahalan, M.D.; Mattson, M.P.; LaFerla, F.M. Capacitative calcium entry deficits and elevated luminal calcium content in mutant presenilin-1 knockin mice. J Cell Biol 2000, 149, 793-798. [CrossRef]
- Barrow, P.A.; Empson, R.M.; Gladwell, S.J.; Anderson, C.M.; Killick, R.; Yu, X.; Jefferys, J.G.; Duff, K. Functional phenotype in transgenic mice expressing mutant human presenilin-1. Neurobiol Dis 2000, 7, 119-126. [CrossRef]
- Etcheberrigaray, R.; Hirashima, N.; Nee, L.; Prince, J.; Govoni, S.; Racchi, M.; Tanzi, R.E.; Alkon, D.L. Calcium responses in fibroblasts from asymptomatic members of Alzheimer's disease families. Neurobiol Dis 1998, 5, 37-45. [CrossRef]
- Hirashima, N.; Etcheberrigaray, R.; Bergamaschi, S.; Racchi, M.; Battaini, F.; Binetti, G.; Govoni, S.; Alkon, D.L. Calcium responses in human fibroblasts: a diagnostic molecular profile for Alzheimer's disease. Neurobiol Aging 1996, 17, 549-555. [CrossRef]
- Ito, E.; Oka, K.; Etcheberrigaray, R.; Nelson, T.J.; McPhie, D.L.; Tofel-Grehl, B.; Gibson, G.E.; Alkon, D.L. Internal Ca2+ mobilization is altered in fibroblasts from patients with Alzheimer disease. Proc Natl Acad Sci U S A 1994, 91, 534-538. [CrossRef]
- Cheung, K.H.; Shineman, D.; Muller, M.; Cardenas, C.; Mei, L.; Yang, J.; Tomita, T.; Iwatsubo, T.; Lee, V.M.; Foskett, J.K. Mechanism of Ca2+ disruption in Alzheimer's disease by presenilin regulation of InsP3 receptor channel gating. Neuron 2008, 58, 871-883. [CrossRef]
- Leissring, M.A.; LaFerla, F.M.; Callamaras, N.; Parker, I. Subcellular mechanisms of presenilin-mediated enhancement of calcium signaling. Neurobiol Dis 2001, 8, 469-478. [CrossRef]
- Leissring, M.A.; Parker, I.; LaFerla, F.M. Presenilin-2 mutations modulate amplitude and kinetics of inositol 1, 4,5-trisphosphate-mediated calcium signals. J Biol Chem 1999, 274, 32535-32538. [CrossRef]
- Stutzmann, G.E.; Caccamo, A.; LaFerla, F.M.; Parker, I. Dysregulated IP3 signaling in cortical neurons of knock-in mice expressing an Alzheimer's-linked mutation in presenilin1 results in exaggerated Ca2+ signals and altered membrane excitability. J Neurosci 2004, 24, 508-513. [CrossRef]
- Smith, I.F.; Hitt, B.; Green, K.N.; Oddo, S.; LaFerla, F.M. Enhanced caffeine-induced Ca2+ release in the 3xTg-AD mouse model of Alzheimer's disease. J Neurochem 2005, 94, 1711-1718. [CrossRef]
- Eskelinen, M.H.; Kivipelto, M. Caffeine as a protective factor in dementia and Alzheimer's disease. J Alzheimers Dis 2010, 20 Suppl 1, S167-174. [CrossRef]
- Larsson, S.C.; Woolf, B.; Gill, D. Plasma Caffeine Levels and Risk of Alzheimer's Disease and Parkinson's Disease: Mendelian Randomization Study. Nutrients 2022, 14. [CrossRef]
- Kim, J.W.; Byun, M.S.; Yi, D.; Lee, J.H.; Jeon, S.Y.; Jung, G.; Lee, H.N.; Sohn, B.K.; Lee, J.Y.; Kim, Y.K.; et al. Coffee intake and decreased amyloid pathology in human brain. Transl Psychiatry 2019, 9, 270. [CrossRef]
- Ross, G.W.; Abbott, R.D.; Petrovitch, H.; Morens, D.M.; Grandinetti, A.; Tung, K.H.; Tanner, C.M.; Masaki, K.H.; Blanchette, P.L.; Curb, J.D.; et al. Association of coffee and caffeine intake with the risk of Parkinson disease. JAMA 2000, 283, 2674-2679. [CrossRef]
- Ascherio, A.; Zhang, S.M.; Hernan, M.A.; Kawachi, I.; Colditz, G.A.; Speizer, F.E.; Willett, W.C. Prospective study of caffeine consumption and risk of Parkinson's disease in men and women. Ann Neurol 2001, 50, 56-63. [CrossRef]
- Ascherio, A.; Chen, H.; Schwarzschild, M.A.; Zhang, S.M.; Colditz, G.A.; Speizer, F.E. Caffeine, postmenopausal estrogen, and risk of Parkinson's disease. Neurology 2003, 60, 790-795. [CrossRef]
- Powers, K.M.; Kay, D.M.; Factor, S.A.; Zabetian, C.P.; Higgins, D.S.; Samii, A.; Nutt, J.G.; Griffith, A.; Leis, B.; Roberts, J.W.; et al. Combined effects of smoking, coffee, and NSAIDs on Parkinson's disease risk. Mov Disord 2008, 23, 88-95. [CrossRef]
- Saaksjarvi, K.; Knekt, P.; Rissanen, H.; Laaksonen, M.A.; Reunanen, A.; Mannisto, S. Prospective study of coffee consumption and risk of Parkinson's disease. Eur J Clin Nutr 2008, 62, 908-915. [CrossRef]
- Qi, H.; Li, S. Dose-response meta-analysis on coffee, tea and caffeine consumption with risk of Parkinson's disease. Geriatr Gerontol Int 2014, 14, 430-439. [CrossRef]
- Altman, R.D.; Lang, A.E.; Postuma, R.B. Caffeine in Parkinson's disease: a pilot open-label, dose-escalation study. Mov Disord 2011, 26, 2427-2431. [CrossRef]
- Postuma, R.B.; Lang, A.E.; Munhoz, R.P.; Charland, K.; Pelletier, A.; Moscovich, M.; Filla, L.; Zanatta, D.; Rios Romenets, S.; Altman, R.; et al. Caffeine for treatment of Parkinson disease: a randomized controlled trial. Neurology 2012, 79, 651-658. [CrossRef]
- Arendash, G.W.; Schleif, W.; Rezai-Zadeh, K.; Jackson, E.K.; Zacharia, L.C.; Cracchiolo, J.R.; Shippy, D.; Tan, J. Caffeine protects Alzheimer's mice against cognitive impairment and reduces brain beta-amyloid production. Neuroscience 2006, 142, 941-952. [CrossRef]
- Stazi, M.; Lehmann, S.; Sakib, M.S.; Pena-Centeno, T.; Buschgens, L.; Fischer, A.; Weggen, S.; Wirths, O. Long-term caffeine treatment of Alzheimer mouse models ameliorates behavioural deficits and neuron loss and promotes cellular and molecular markers of neurogenesis. Cell Mol Life Sci 2021, 79, 55. [CrossRef]
- Cao, C.; Cirrito, J.R.; Lin, X.; Wang, L.; Verges, D.K.; Dickson, A.; Mamcarz, M.; Zhang, C.; Mori, T.; Arendash, G.W.; et al. Caffeine suppresses amyloid-beta levels in plasma and brain of Alzheimer's disease transgenic mice. J Alzheimers Dis 2009, 17, 681-697. [CrossRef]
- Laurent, C.; Eddarkaoui, S.; Derisbourg, M.; Leboucher, A.; Demeyer, D.; Carrier, S.; Schneider, M.; Hamdane, M.; Muller, C.E.; Buee, L.; et al. Beneficial effects of caffeine in a transgenic model of Alzheimer's disease-like tau pathology. Neurobiol Aging 2014, 35, 2079-2090. [CrossRef]
- Han, M.E.; Kim, H.J.; Lee, Y.S.; Kim, D.H.; Choi, J.T.; Pan, C.S.; Yoon, S.; Baek, S.Y.; Kim, B.S.; Kim, J.B.; et al. Regulation of cerebrospinal fluid production by caffeine consumption. BMC Neurosci 2009, 10, 110. [CrossRef]
- Zeitlin, R.; Patel, S.; Burgess, S.; Arendash, G.W.; Echeverria, V. Caffeine induces beneficial changes in PKA signaling and JNK and ERK activities in the striatum and cortex of Alzheimer's transgenic mice. Brain Res 2011, 1417, 127-136. [CrossRef]
- Ghoneim, F.M.; Khalaf, H.A.; Elsamanoudy, A.Z.; Abo El-Khair, S.M.; Helaly, A.M.; Mahmoud el, H.M.; Elshafey, S.H. Protective effect of chronic caffeine intake on gene expression of brain derived neurotrophic factor signaling and the immunoreactivity of glial fibrillary acidic protein and Ki-67 in Alzheimer's disease. Int J Clin Exp Pathol 2015, 8, 7710-7728.
- Han, K.; Jia, N.; Li, J.; Yang, L.; Min, L.Q. Chronic caffeine treatment reverses memory impairment and the expression of brain BNDF and TrkB in the PS1/APP double transgenic mouse model of Alzheimer's disease. Mol Med Rep 2013, 8, 737-740. [CrossRef]
- Soliman, A.M.; Fathalla, A.M.; Moustafa, A.A. Dose-dependent neuroprotective effect of caffeine on a rotenone-induced rat model of parkinsonism: A histological study. Neurosci Lett 2016, 623, 63-70. [CrossRef]
- Khadrawy, Y.A.; Salem, A.M.; El-Shamy, K.A.; Ahmed, E.K.; Fadl, N.N.; Hosny, E.N. Neuroprotective and Therapeutic Effect of Caffeine on the Rat Model of Parkinson's Disease Induced by Rotenone. J Diet Suppl 2017, 14, 553-572. [CrossRef]
- Singh, S.; Singh, K.; Patel, S.; Patel, D.K.; Singh, C.; Nath, C.; Singh, M.P. Nicotine and caffeine-mediated modulation in the expression of toxicant responsive genes and vesicular monoamine transporter-2 in 1-methyl 4-phenyl-1,2,3,6-tetrahydropyridine-induced Parkinson's disease phenotype in mouse. Brain Res 2008, 1207, 193-206. [CrossRef]
- Bagga, P.; Chugani, A.N.; Patel, A.B. Neuroprotective effects of caffeine in MPTP model of Parkinson's disease: A (13)C NMR study. Neurochem Int 2016, 92, 25-34. [CrossRef]
- Lee, K.W.; Im, J.Y.; Woo, J.M.; Grosso, H.; Kim, Y.S.; Cristovao, A.C.; Sonsalla, P.K.; Schuster, D.S.; Jalbut, M.M.; Fernandez, J.R.; et al. Neuroprotective and anti-inflammatory properties of a coffee component in the MPTP model of Parkinson's disease. Neurotherapeutics 2013, 10, 143-153. [CrossRef]
- Aguiar, L.M.; Nobre, H.V., Jr.; Macedo, D.S.; Oliveira, A.A.; Freitas, R.M.; Vasconcelos, S.M.; Cunha, G.M.; Sousa, F.C.; Viana, G.S. Neuroprotective effects of caffeine in the model of 6-hydroxydopamine lesion in rats. Pharmacol Biochem Behav 2006, 84, 415-419. [CrossRef]
- Luan, Y.; Ren, X.; Zheng, W.; Zeng, Z.; Guo, Y.; Hou, Z.; Guo, W.; Chen, X.; Li, F.; Chen, J.F. Chronic Caffeine Treatment Protects Against alpha-Synucleinopathy by Reestablishing Autophagy Activity in the Mouse Striatum. Front Neurosci 2018, 12, 301. [CrossRef]
- Schwarzschild, M.A.; Chen, J.F.; Ascherio, A. Caffeinated clues and the promise of adenosine A(2A) antagonists in PD. Neurology 2002, 58, 1154-1160. [CrossRef]
- Fuxe, K.; Ferre, S.; Canals, M.; Torvinen, M.; Terasmaa, A.; Marcellino, D.; Goldberg, S.R.; Staines, W.; Jacobsen, K.X.; Lluis, C.; et al. Adenosine A2A and dopamine D2 heteromeric receptor complexes and their function. J Mol Neurosci 2005, 26, 209-220. [CrossRef]
- Deleu, D.; Jacob, P.; Chand, P.; Sarre, S.; Colwell, A. Effects of caffeine on levodopa pharmacokinetics and pharmacodynamics in Parkinson disease. Neurology 2006, 67, 897-899. [CrossRef]
- Zajac, M.A.; Zakrzewski, A.G.; Kowal, M.G.; Narayan, S. A Novel Method of Caffeine Synthesis from Uracil. Synth. Commun. 2003, 33, 3291-3297. [CrossRef]
- Scimmi, C.; Cardinali, M.; Abenante, L.; Amatista, M.; Nacca, F.G.; Lenardao, E.J.; Sancineto, L.; Santi, C. Q-Tube®-Assisted Alkylation and Arylation of Xanthines and Other N-H-Containing Heterocycles in Water. Chemistry 2021, 3, 1126-1137. [CrossRef]
- Templ, J.; Gjata, E.; Getzner, F.; Schnürch, M. Monoselective N-Methylation of Amides, Indoles, and Related Structures Using Quaternary Ammonium Salts as Solid Methylating Agents. Org. Lett. 2022, 24, 7315-7319. [CrossRef]
- Nejatbakhsh, N.; Feng, Z.P. Calcium binding protein-mediated regulation of voltage-gated calcium channels linked to human diseases. Acta Pharmacol Sin 2011, 32, 741-748. [CrossRef]
- Kuhl, D.E.; Koeppe, R.A.; Minoshima, S.; Snyder, S.E.; Ficaro, E.P.; Foster, N.L.; Frey, K.A.; Kilbourn, M.R. In vivo mapping of cerebral acetylcholinesterase activity in aging and Alzheimer's disease. Neurology 1999, 52, 691-699. [CrossRef]
- Davis, K.L.; Mohs, R.C.; Marin, D.; Purohit, D.P.; Perl, D.P.; Lantz, M.; Austin, G.; Haroutunian, V. Cholinergic markers in elderly patients with early signs of Alzheimer disease. JAMA 1999, 281, 1401-1406. [CrossRef]
- Shinotoh, H.; Fukushi, K.; Nagatsuka, S.; Tanaka, N.; Aotsuka, A.; Ota, T.; Namba, H.; Tanada, S.; Irie, T. The amygdala and Alzheimer's disease: positron emission tomographic study of the cholinergic system. Ann N Y Acad Sci 2003, 985, 411-419.
- Sberna, G.; Saez-Valero, J.; Beyreuther, K.; Masters, C.L.; Small, D.H. The amyloid beta-protein of Alzheimer's disease increases acetylcholinesterase expression by increasing intracellular calcium in embryonal carcinoma P19 cells. J Neurochem 1997, 69, 1177-1184. [CrossRef]
- Ritz, B.; Rhodes, S.L.; Qian, L.; Schernhammer, E.; Olsen, J.H.; Friis, S. L-type calcium channel blockers and Parkinson disease in Denmark. Ann Neurol 2010, 67, 600-606. [CrossRef]
- Becker, C.; Jick, S.S.; Meier, C.R. Use of antihypertensives and the risk of Parkinson disease. Neurology 2008, 70, 1438-1444. [CrossRef]
- Mullapudi, A.; Gudala, K.; Boya, C.S.; Bansal, D. Risk of Parkinson's Disease in the Users of Antihypertensive Agents: An Evidence from the Meta-Analysis of Observational Studies. J Neurodegener Dis 2016, 2016, 5780809. [CrossRef]
- Lee, Y.C.; Lin, C.H.; Wu, R.M.; Lin, J.W.; Chang, C.H.; Lai, M.S. Antihypertensive agents and risk of Parkinson's disease: a nationwide cohort study. PLoS One 2014, 9, e98961. [CrossRef]
- Kupsch, A.; Gerlach, M.; Pupeter, S.C.; Sautter, J.; Dirr, A.; Arnold, G.; Opitz, W.; Przuntek, H.; Riederer, P.; Oertel, W.H. Pretreatment with nimodipine prevents MPTP-induced neurotoxicity at the nigral, but not at the striatal level in mice. Neuroreport 1995, 6, 621-625. [CrossRef]
- Kupsch, A.; Sautter, J.; Schwarz, J.; Riederer, P.; Gerlach, M.; Oertel, W.H. 1-Methyl-4-phenyl-1,2,3,6-tetrahydropyridine-induced neurotoxicity in non-human primates is antagonized by pretreatment with nimodipine at the nigral, but not at the striatal level. Brain Res 1996, 741, 185-196. [CrossRef]
- Mena, M.A.; Garcia de Yebenes, M.J.; Tabernero, C.; Casarejos, M.J.; Pardo, B.; Garcia de Yebenes, J. Effects of calcium antagonists on the dopamine system. Clin Neuropharmacol 1995, 18, 410-426. [CrossRef]
- Paris, D.; Bachmeier, C.; Patel, N.; Quadros, A.; Volmar, C.H.; Laporte, V.; Ganey, J.; Beaulieu-Abdelahad, D.; Ait-Ghezala, G.; Crawford, F.; et al. Selective antihypertensive dihydropyridines lower Abeta accumulation by targeting both the production and the clearance of Abeta across the blood-brain barrier. Mol Med 2011, 17, 149-162. [CrossRef]
- Anekonda, T.S.; Quinn, J.F. Calcium channel blocking as a therapeutic strategy for Alzheimer's disease: the case for isradipine. Biochim Biophys Acta 2011, 1812, 1584-1590. [CrossRef]
- Yu, J.T.; Chang, R.C.; Tan, L. Calcium dysregulation in Alzheimer's disease: from mechanisms to therapeutic opportunities. Prog Neurobiol 2009, 89, 240-255. [CrossRef]
- Anekonda, T.S.; Quinn, J.F.; Harris, C.; Frahler, K.; Wadsworth, T.L.; Woltjer, R.L. L-type voltage-gated calcium channel blockade with isradipine as a therapeutic strategy for Alzheimer's disease. Neurobiol Dis 2011, 41, 62-70. [CrossRef]
- Mok, S.S.; Clippingdale, A.B.; Beyreuther, K.; Masters, C.L.; Barrow, C.J.; Small, D.H. A beta peptides and calcium influence secretion of the amyloid protein precursor from chick sympathetic neurons in culture. J Neurosci Res 2000, 61, 449-457. [CrossRef]
- Quartermain, D.; Garcia deSoria, V. The effects of calcium channel antagonists on short- and long-term retention in mice using spontaneous alternation behavior. Neurobiol Learn Mem 2001, 76, 117-124. [CrossRef]
- Levallois, P. Alzheimer's disease and aluminum. Neurology 1997, 48, 1141-1142. [CrossRef]
- Rondeau, V.; Commenges, D.; Jacqmin-Gadda, H.; Dartigues, J.F. Relation between aluminum concentrations in drinking water and Alzheimer's disease: an 8-year follow-up study. Am J Epidemiol 2000, 152, 59-66. [CrossRef]
- Gauthier, E.; Fortier, I.; Courchesne, F.; Pepin, P.; Mortimer, J.; Gauvreau, D. Aluminum forms in drinking water and risk of Alzheimer's disease. Environ Res 2000, 84, 234-246. [CrossRef]
- Crapper, D.R.; Krishnan, S.S.; Dalton, A.J. Brain aluminum distribution in Alzheimer's disease and experimental neurofibrillary degeneration. Science 1973, 180, 511-513. [CrossRef]
- Bouras, C.; Giannakopoulos, P.; Good, P.F.; Hsu, A.; Hof, P.R.; Perl, D.P. A laser microprobe mass analysis of brain aluminum and iron in dementia pugilistica: comparison with Alzheimer's disease. Eur Neurol 1997, 38, 53-58. [CrossRef]
- Kawahara, M.; Kato, M.; Kuroda, Y. Effects of aluminum on the neurotoxicity of primary cultured neurons and on the aggregation of beta-amyloid protein. Brain Res Bull 2001, 55, 211-217. [CrossRef]
- Mantyh, P.W.; Ghilardi, J.R.; Rogers, S.; DeMaster, E.; Allen, C.J.; Stimson, E.R.; Maggio, J.E. Aluminum, iron, and zinc ions promote aggregation of physiological concentrations of beta-amyloid peptide. J Neurochem 1993, 61, 1171-1174. [CrossRef]
- Pratico, D.; Uryu, K.; Sung, S.; Tang, S.; Trojanowski, J.Q.; Lee, V.M. Aluminum modulates brain amyloidosis through oxidative stress in APP transgenic mice. FASEB J 2002, 16, 1138-1140. [CrossRef]
- Ricchelli, F.; Drago, D.; Filippi, B.; Tognon, G.; Zatta, P. Aluminum-triggered structural modifications and aggregation of beta-amyloids. Cell Mol Life Sci 2005, 62, 1724-1733. [CrossRef]
- Rani, A.; Neha; Sodhi, R.K.; Kaur, A. Protective effect of a calcium channel blocker "diltiazem" on aluminum chloride-induced dementia in mice. Naunyn Schmiedebergs Arch Pharmacol 2015, 388, 1151-1161. [CrossRef]
- Ohnishi, N.; Kusuhara, M.; Yoshioka, M.; Kuroda, K.; Soga, A.; Nishikawa, F.; Koishi, T.; Nakagawa, M.; Hori, S.; Matsumoto, T.; et al. Studies on interactions between functional foods or dietary supplements and medicines. I. Effects of Ginkgo biloba leaf extract on the pharmacokinetics of diltiazem in rats. Biol Pharm Bull 2003, 26, 1315-1320. [CrossRef]
- Guzman, J.N.; Sanchez-Padilla, J.; Chan, C.S.; Surmeier, D.J. Robust pacemaking in substantia nigra dopaminergic neurons. J Neurosci 2009, 29, 11011-11019. [CrossRef]
- Kang, S.; Cooper, G.; Dunne, S.F.; Dusel, B.; Luan, C.H.; Surmeier, D.J.; Silverman, R.B. CaV1.3-selective L-type calcium channel antagonists as potential new therapeutics for Parkinson's disease. Nat Commun 2012, 3, 1146. [CrossRef]
- Liss, B.; Striessnig, J. The Potential of L-Type Calcium Channels as a Drug Target for Neuroprotective Therapy in Parkinson's Disease. Annu Rev Pharmacol Toxicol 2019, 59, 263-289. [CrossRef]
- Mosharov, E.V.; Larsen, K.E.; Kanter, E.; Phillips, K.A.; Wilson, K.; Schmitz, Y.; Krantz, D.E.; Kobayashi, K.; Edwards, R.H.; Sulzer, D. Interplay between cytosolic dopamine, calcium, and alpha-synuclein causes selective death of substantia nigra neurons. Neuron 2009, 62, 218-229. [CrossRef]
- Graham, D.F.; Stewart-Wynne, E.G. Diltiazem-induced acute parkinsonism. Aust N Z J Med 1994, 24, 70. [CrossRef]
- Jeong, S.; Cho, H.; Kim, Y.J.; Ma, H.I.; Jang, S. Drug-induced Parkinsonism: A strong predictor of idiopathic Parkinson's disease. PLoS One 2021, 16, e0247354. [CrossRef]
- Anjaneyulu, M.; Chopra, K. Diltiazem attenuates oxidative stress in diabetic rats. Ren Fail 2005, 27, 335-344.
- Koller, P.T.; Bergmann, S.R. Reduction of lipid peroxidation in reperfused isolated rabbit hearts by diltiazem. Circ Res 1989, 65, 838-846. [CrossRef]
- Surmeier, D.J. Calcium, ageing, and neuronal vulnerability in Parkinson's disease. Lancet Neurol 2007, 6, 933-938. [CrossRef]
- Marx, M.; Weber, M.; Merkel, F.; Meyer zum Buschenfelde, K.H.; Kohler, H. Additive effects of calcium antagonists on cyclosporin A-induced inhibition of T-cell proliferation. Nephrol Dial Transplant 1990, 5, 1038-1044. [CrossRef]
- Fansa, I.; Gol, M.; Nisanoglu, V.; Yavas, S.; Iscan, Z.; Tasdemir, O. Does diltiazem inhibit the inflammatory response in cardiopulmonary bypass? Med Sci Monit 2003, 9, PI30-36.
- Dubey, L.; Hesong, Z. Anti-inflammatory action of diltiazem in patients with unstable angina. Postgrad Med J 2006, 82, 594-597. [CrossRef]
- Szabo, C.; Hasko, G.; Nemeth, Z.H.; Vizi, E.S. Calcium entry blockers increase interleukin-10 production in endotoxemia. Shock 1997, 7, 304-307. [CrossRef]
- Silei, V.; Fabrizi, C.; Venturini, G.; Salmona, M.; Bugiani, O.; Tagliavini, F.; Lauro, G.M. Activation of microglial cells by PrP and beta-amyloid fragments raises intracellular calcium through L-type voltage sensitive calcium channels. Brain Res 1999, 818, 168-170. [CrossRef]
- Daschil, N.; Obermair, G.J.; Flucher, B.E.; Stefanova, N.; Hutter-Paier, B.; Windisch, M.; Humpel, C.; Marksteiner, J. CaV1.2 calcium channel expression in reactive astrocytes is associated with the formation of amyloid-beta plaques in an Alzheimer's disease mouse model. J Alzheimers Dis 2013, 37, 439-451. [CrossRef]
- Wang, X.; Saegusa, H.; Huntula, S.; Tanabe, T. Blockade of microglial Cav1.2 Ca(2+) channel exacerbates the symptoms in a Parkinson's disease model. Sci Rep 2019, 9, 9138. [CrossRef]
- Gizur, T.; Harsànyi, K. Some Applications of Isopropenyl Acetate To O-, N-and C-Acetylation. Synthetic Communications 1990, 20, 2365-2371. [CrossRef]
- Miyata, O.; Shinada, T.; Ninomiya, I.; Naito, T. Asymmetric induction at two contiguous stereogenic centers by diastereoface differentiating nucleophilic addition reaction. Tetrahedron Letters 1991, 32, 3519-3522. [CrossRef]
- Schwartz, A.; Madan, P.B.; Mohacsi, E.; O'Brien, J.P.; Todaro, L.J.; Coffen, D.L. Enantioselective synthesis of calcium channel blockers of the diltiazem group. The Journal of Organic Chemistry 1992, 57, 851-856. [CrossRef]
- Jacobsen, E.N.; Deng, L.; Furukawa, Y.; Martínez, L.E. Enantioselective catalytic epoxidation of cinnamate esters. Tetrahedron 1994, 50, 4323-4334. [CrossRef]
- Nangia, A.; Rao, P.; Madhavi, N. ChemInform Abstract: Carbohydrates as Chiral Auxiliaries in Asymmetric Darzens Reactions: Enantioselective Synthesis of the Benzothiazepine Ring System of Diltiazem. ChemInform 2010, 27. [CrossRef]
- Imashiro, R.; Kuroda, T. Asymmetric synthesis of methyl (2R,3S)-3-(4-methoxyphenyl) glycidate, a key intermediate of diltiazem, via Mukaiyama aldol reaction. Tetrahedron Letters 2001, 42, 1313-1315. [CrossRef]
- Seki, M. A Practical Synthesis of a Key Chiral Drug Intermediate via Asymmetric Organocatalysis. Synlett 2008, 2008, 164-176. [CrossRef]
- Mordant, C.; Caño de Andrade, C.; Touati, R.; Ratovelomanana-Vidal, V.; Hassine, B.B.; Genêt, J.-P. Stereoselective Synthesis of Diltiazem via Dynamic Kinetic Resolution. Synthesis 2003, 2003, 2405-2409. [CrossRef]
- Gizur, T.; Harsányi, K.; Fogassy, E. Studies of the resolution of racemates in the Synthesis of Diltiazem. Journal für Praktische Chemie/Chemiker-Zeitung 1994, 336, 628-631. [CrossRef]
- Desai, S.B.; Argade, N.P.; Ganesh, K.N. Remarkable Chemo-, Regio-, and Enantioselectivity in Lipase-Catalyzed Hydrolysis: Efficient Resolution of (±)-threo-Ethyl 3-(4-Methoxyphenyl)-2,3-diacetoxypropionate Leading to Chiral Intermediates of (+)-Diltiazem. The Journal of Organic Chemistry 1996, 61, 6730-6732. [CrossRef]
- Yamada, S.-i.; Tsujioka, I.; Shibatani, T.; Yoshioka, R. Efficient Alternative Synthetic Route to Diltiazem via (2R, 3S)-3-(4-Methoxyphenyl)glycidamide. CHEMICAL & PHARMACEUTICAL BULLETIN 1999, 47, 146-150. [CrossRef]
- Tokdar, P.; Ranadive, P.; George, S.; Upare, A.; Vishwasrao, S.; Roy, M.; Sivaramakrishnan, H. Bakers' Yeast Mediated Stereo Selective Reduction of Cis (+) Diketolactam to Cis (+) Hydroxylactam, a Key Intermediate for Diltiazem Synthesis in a Laboratory Scale Bioreactor. International Journal of Chemical Engineering and Applications 2011, 2, 386. [CrossRef]
- Yue, X.; Li, Y.; Liu, M.; Sang, D.; Huang, Z.; Chen, F. Biocatalytic dynamic reductive kinetic resolution of aryl α-chloro β-keto esters: divergent, stereocontrolled synthesis of diltiazem, clentiazem, and siratiazem. Chemical Communications 2022, 58, 9010-9013. [CrossRef]
- Bachurin, S.; Bukatina, E.; Lermontova, N.; Tkachenko, S.; Afanasiev, A.; Grigoriev, V.; Grigorieva, I.; Ivanov, Y.; Sablin, S.; Zefirov, N. Antihistamine agent Dimebon as a novel neuroprotector and a cognition enhancer. Ann N Y Acad Sci 2001, 939, 425-435. [CrossRef]
- Lermontova, N.N.; Lukoyanov, N.V.; Serkova, T.P.; Lukoyanova, E.A.; Bachurin, S.O. Dimebon improves learning in animals with experimental Alzheimer's disease. Bull Exp Biol Med 2000, 129, 544-546. [CrossRef]
- Cano-Cuenca, N.; Solis-Garcia del Pozo, J.E.; Jordan, J. Evidence for the efficacy of latrepirdine (Dimebon) treatment for improvement of cognitive function: a meta-analysis. J Alzheimers Dis 2014, 38, 155-164. [CrossRef]
- Wu, J.; Li, Q.; Bezprozvanny, I. Evaluation of Dimebon in cellular model of Huntington's disease. Mol Neurodegener 2008, 3, 15. [CrossRef]
- Grigorev, V.V.; Dranyi, O.A.; Bachurin, S.O. Comparative study of action mechanisms of dimebon and memantine on AMPA- and NMDA-subtypes glutamate receptors in rat cerebral neurons. Bull Exp Biol Med 2003, 136, 474-477. [CrossRef]
- Lermontova, N.N.; Redkozubov, A.E.; Shevtsova, E.F.; Serkova, T.P.; Kireeva, E.G.; Bachurin, S.O. Dimebon and tacrine inhibit neurotoxic action of beta-amyloid in culture and block L-type Ca(2+) channels. Bull Exp Biol Med 2001, 132, 1079-1083. [CrossRef]
- Doody, R.S.; Gavrilova, S.I.; Sano, M.; Thomas, R.G.; Aisen, P.S.; Bachurin, S.O.; Seely, L.; Hung, D.; dimebon, i. Effect of dimebon on cognition, activities of daily living, behaviour, and global function in patients with mild-to-moderate Alzheimer's disease: a randomised, double-blind, placebo-controlled study. Lancet 2008, 372, 207-215. [CrossRef]
- Bharadwaj, P.R.; Bates, K.A.; Porter, T.; Teimouri, E.; Perry, G.; Steele, J.W.; Gandy, S.; Groth, D.; Martins, R.N.; Verdile, G. Latrepirdine: molecular mechanisms underlying potential therapeutic roles in Alzheimer's and other neurodegenerative diseases. Transl Psychiatry 2013, 3, e332. [CrossRef]
- Kieburtz, K.; McDermott, M.P.; Voss, T.S.; Corey-Bloom, J.; Deuel, L.M.; Dorsey, E.R.; Factor, S.; Geschwind, M.D.; Hodgeman, K.; Kayson, E.; et al. A randomized, placebo-controlled trial of latrepirdine in Huntington disease. Arch Neurol 2010, 67, 154-160. [CrossRef]
- Peters, O.M.; Shelkovnikova, T.; Tarasova, T.; Springe, S.; Kukharsky, M.S.; Smith, G.A.; Brooks, S.; Kozin, S.A.; Kotelevtsev, Y.; Bachurin, S.O.; et al. Chronic administration of Dimebon does not ameliorate amyloid-beta pathology in 5xFAD transgenic mice. J Alzheimers Dis 2013, 36, 589-596. [CrossRef]
- Bachurin, S.O.; Shelkovnikova, T.A.; Ustyugov, A.A.; Peters, O.; Khritankova, I.; Afanasieva, M.A.; Tarasova, T.V.; Alentov, II; Buchman, V.L.; Ninkina, N.N. Dimebon slows progression of proteinopathy in gamma-synuclein transgenic mice. Neurotox Res 2012, 22, 33-42. [CrossRef]
- Webster, S.J.; Wilson, C.A.; Lee, C.H.; Mohler, E.G.; Terry, A.V., Jr.; Buccafusco, J.J. The acute effects of dimebolin, a potential Alzheimer's disease treatment, on working memory in rhesus monkeys. Br J Pharmacol 2011, 164, 970-978. [CrossRef]
- Wang, J.; Ferruzzi, M.G.; Varghese, M.; Qian, X.; Cheng, A.; Xie, M.; Zhao, W.; Ho, L.; Pasinetti, G.M. Preclinical study of dimebon on beta-amyloid-mediated neuropathology in Alzheimer's disease. Mol Neurodegener 2011, 6, 7. [CrossRef]
- Day, M.; Chandran, P.; Luo, F.; Rustay, N.R.; Markosyan, S.; LeBlond, D.; Fox, G.B. Latrepirdine increases cerebral glucose utilization in aged mice as measured by [18F]-fluorodeoxyglucose positron emission tomography. Neuroscience 2011, 189, 299-304. [CrossRef]
- Zhang, S.; Hedskog, L.; Petersen, C.A.; Winblad, B.; Ankarcrona, M. Dimebon (latrepirdine) enhances mitochondrial function and protects neuronal cells from death. J Alzheimers Dis 2010, 21, 389-402. [CrossRef]
- Eckert, S.H.; Eckmann, J.; Renner, K.; Eckert, G.P.; Leuner, K.; Muller, W.E. Dimebon ameliorates amyloid-beta induced impairments of mitochondrial form and function. J Alzheimers Dis 2012, 31, 21-32. [CrossRef]
- Bachurin, S.O.; Shevtsova, E.P.; Kireeva, E.G.; Oxenkrug, G.F.; Sablin, S.O. Mitochondria as a target for neurotoxins and neuroprotective agents. Ann N Y Acad Sci 2003, 993, 334-344; discussion 345-339. [CrossRef]
- Steele, J.W.; Kim, S.H.; Cirrito, J.R.; Verges, D.K.; Restivo, J.L.; Westaway, D.; Fraser, P.; Hyslop, P.S.; Sano, M.; Bezprozvanny, I.; et al. Acute dosing of latrepirdine (Dimebon), a possible Alzheimer therapeutic, elevates extracellular amyloid-beta levels in vitro and in vivo. Mol Neurodegener 2009, 4, 51. [CrossRef]
- Shevtzova, E.F.; Kireeva, E.G.; Bachurin, S.O. Effect of beta-amyloid peptide fragment 25-35 on nonselective permeability of mitochondria. Bull Exp Biol Med 2001, 132, 1173-1176. [CrossRef]
- Ustyugov, A.; Shevtsova, E.; Bachurin, S. Novel Sites of Neuroprotective Action of Dimebon (Latrepirdine). Mol Neurobiol 2015, 52, 970-978. [CrossRef]
- Steele, J.W.; Gandy, S. Latrepirdine (Dimebon(R)), a potential Alzheimer therapeutic, regulates autophagy and neuropathology in an Alzheimer mouse model. Autophagy 2013, 9, 617-618. [CrossRef]
- Steele, J.W.; Ju, S.; Lachenmayer, M.L.; Liken, J.; Stock, A.; Kim, S.H.; Delgado, L.M.; Alfaro, I.E.; Bernales, S.; Verdile, G.; et al. Latrepirdine stimulates autophagy and reduces accumulation of alpha-synuclein in cells and in mouse brain. Mol Psychiatry 2013, 18, 882-888. [CrossRef]
- Yamashita, M.; Nonaka, T.; Arai, T.; Kametani, F.; Buchman, V.L.; Ninkina, N.; Bachurin, S.O.; Akiyama, H.; Goedert, M.; Hasegawa, M. Methylene blue and dimebon inhibit aggregation of TDP-43 in cellular models. FEBS Lett 2009, 583, 2419-2424. [CrossRef]
- De Jesus-Cortes, H.; Xu, P.; Drawbridge, J.; Estill, S.J.; Huntington, P.; Tran, S.; Britt, J.; Tesla, R.; Morlock, L.; Naidoo, J.; et al. Neuroprotective efficacy of aminopropyl carbazoles in a mouse model of Parkinson disease. Proc Natl Acad Sci U S A 2012, 109, 17010-17015. [CrossRef]
- Shelkovnikova, T.A.; Ustyugov, A.A.; Millership, S.; Peters, O.; Anichtchik, O.; Spillantini, M.G.; Buchman, V.L.; Bachurin, S.O.; Ninkina, N.N. Dimebon does not ameliorate pathological changes caused by expression of truncated (1-120) human alpha-synuclein in dopaminergic neurons of transgenic mice. Neurodegener Dis 2011, 8, 430-437. [CrossRef]
- Golubeva, M.I.; Shashkina, L.F.; Proinova, V.A.; Fedorova, E.A.; Nechushkina, L.V. [Preclinical study of the safety of the antihistaminic preparation dimebon]. Farmakol Toksikol 1985, 48, 114-119.
- Shadurski, K.; Danusevich, I.; Kost, A.; Vinogradova, E. Patent No. 1138164, 1985. In Proceedings of the Chem. Abstr, 1985; p. 198010.
- Gao, M.; Wang, M.; Hutchins, G.D.; Zheng, Q.-H. [11C]Dimebon, radiosynthesis and lipophilicity of a new potential PET agent for imaging of Alzheimer’s disease and Huntington’s disease. Bioorganic Med. Chem. Lett. 2010, 20, 2529-2532. [CrossRef]
- Dong, H.; Latka, R.T.; Driver, T.G. Ruthenium-Catalyzed γ-Carbolinium Ion Formation from Aryl Azides; Synthesis of Dimebolin. Org. Lett. 2011, 13, 2726-2729. [CrossRef]
- Helton, T.D.; Xu, W.; Lipscombe, D. Neuronal L-type calcium channels open quickly and are inhibited slowly. J Neurosci 2005, 25, 10247-10251. [CrossRef]
- Ohkubo, N.; Mitsuda, N.; Tamatani, M.; Yamaguchi, A.; Lee, Y.D.; Ogihara, T.; Vitek, M.P.; Tohyama, M. Apolipoprotein E4 stimulates cAMP response element-binding protein transcriptional activity through the extracellular signal-regulated kinase pathway. J Biol Chem 2001, 276, 3046-3053. [CrossRef]
- Liu, S.J.; Gasperini, R.; Foa, L.; Small, D.H. Amyloid-beta decreases cell-surface AMPA receptors by increasing intracellular calcium and phosphorylation of GluR2. J Alzheimers Dis 2010, 21, 655-666. [CrossRef]
- Thornton, C.; Bright, N.J.; Sastre, M.; Muckett, P.J.; Carling, D. AMP-activated protein kinase (AMPK) is a tau kinase, activated in response to amyloid beta-peptide exposure. Biochem J 2011, 434, 503-512. [CrossRef]
- Yoon, S.O.; Park, D.J.; Ryu, J.C.; Ozer, H.G.; Tep, C.; Shin, Y.J.; Lim, T.H.; Pastorino, L.; Kunwar, A.J.; Walton, J.C.; et al. JNK3 perpetuates metabolic stress induced by Abeta peptides. Neuron 2012, 75, 824-837. [CrossRef]
- Hettiarachchi, N.; Dallas, M.; Al-Owais, M.; Griffiths, H.; Hooper, N.; Scragg, J.; Boyle, J.; Peers, C. Heme oxygenase-1 protects against Alzheimer's amyloid-beta(1-42)-induced toxicity via carbon monoxide production. Cell Death Dis 2014, 5, e1569. [CrossRef]
- Lovell, M.A.; Abner, E.; Kryscio, R.; Xu, L.; Fister, S.X.; Lynn, B.C. Calcium Channel Blockers, Progression to Dementia, and Effects on Amyloid Beta Peptide Production. Oxid Med Cell Longev 2015, 2015, 787805. [CrossRef]
- Nakajima, M.; Miura, M.; Aosaki, T.; Shirasawa, T. Deficiency of presenilin-1 increases calcium-dependent vulnerability of neurons to oxidative stress in vitro. J Neurochem 2001, 78, 807-814. [CrossRef]
- Hefter, D.; Kaiser, M.; Weyer, S.W.; Papageorgiou, I.E.; Both, M.; Kann, O.; Muller, U.C.; Draguhn, A. Amyloid Precursor Protein Protects Neuronal Network Function after Hypoxia via Control of Voltage-Gated Calcium Channels. J Neurosci 2016, 36, 8356-8371. [CrossRef]
- Lopez, J.R.; Lyckman, A.; Oddo, S.; Laferla, F.M.; Querfurth, H.W.; Shtifman, A. Increased intraneuronal resting [Ca2+] in adult Alzheimer's disease mice. J Neurochem 2008, 105, 262-271. [CrossRef]
- Wang, Y.; Mattson, M.P. L-type Ca2+ currents at CA1 synapses, but not CA3 or dentate granule neuron synapses, are increased in 3xTgAD mice in an age-dependent manner. Neurobiol Aging 2014, 35, 88-95. [CrossRef]
- Pascual-Caro, C.; Berrocal, M.; Lopez-Guerrero, A.M.; Alvarez-Barrientos, A.; Pozo-Guisado, E.; Gutierrez-Merino, C.; Mata, A.M.; Martin-Romero, F.J. STIM1 deficiency is linked to Alzheimer's disease and triggers cell death in SH-SY5Y cells by upregulation of L-type voltage-operated Ca(2+) entry. J Mol Med (Berl) 2018, 96, 1061-1079. [CrossRef]
- Skobeleva, K.; Shalygin, A.; Mikhaylova, E.; Guzhova, I.; Ryazantseva, M.; Kaznacheyeva, E. The STIM1/2-Regulated Calcium Homeostasis Is Impaired in Hippocampal Neurons of the 5xFAD Mouse Model of Alzheimer's Disease. Int J Mol Sci 2022, 23. [CrossRef]
- Furukawa, K.; Wang, Y.; Yao, P.J.; Fu, W.; Mattson, M.P.; Itoyama, Y.; Onodera, H.; D'Souza, I.; Poorkaj, P.H.; Bird, T.D.; et al. Alteration in calcium channel properties is responsible for the neurotoxic action of a familial frontotemporal dementia tau mutation. J Neurochem 2003, 87, 427-436. [CrossRef]
- Esteras, N.; Kundel, F.; Amodeo, G.F.; Pavlov, E.V.; Klenerman, D.; Abramov, A.Y. Insoluble tau aggregates induce neuronal death through modification of membrane ion conductance, activation of voltage-gated calcium channels and NADPH oxidase. FEBS J 2021, 288, 127-141. [CrossRef]
- Graham, W.C.; Robertson, R.G.; Sambrook, M.A.; Crossman, A.R. Injection of excitatory amino acid antagonists into the medial pallidal segment of a 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine (MPTP) treated primate reverses motor symptoms of parkinsonism. Life Sci 1990, 47, PL91-97. [CrossRef]
- Brotchie, J.M.; Mitchell, I.J.; Sambrook, M.A.; Crossman, A.R. Alleviation of parkinsonism by antagonism of excitatory amino acid transmission in the medial segment of the globus pallidus in rat and primate. Mov Disord 1991, 6, 133-138. [CrossRef]
- Benazzouz, A.; Gross, C.; Feger, J.; Boraud, T.; Bioulac, B. Reversal of rigidity and improvement in motor performance by subthalamic high-frequency stimulation in MPTP-treated monkeys. Eur J Neurosci 1993, 5, 382-389. [CrossRef]
- Beurrier, C.; Congar, P.; Bioulac, B.; Hammond, C. Subthalamic nucleus neurons switch from single-spike activity to burst-firing mode. J Neurosci 1999, 19, 599-609. [CrossRef]
- Hallworth, N.E.; Wilson, C.J.; Bevan, M.D. Apamin-sensitive small conductance calcium-activated potassium channels, through their selective coupling to voltage-gated calcium channels, are critical determinants of the precision, pace, and pattern of action potential generation in rat subthalamic nucleus neurons in vitro. J Neurosci 2003, 23, 7525-7542. [CrossRef]
- Garcia, L.; Audin, J.; D'Alessandro, G.; Bioulac, B.; Hammond, C. Dual effect of high-frequency stimulation on subthalamic neuron activity. J Neurosci 2003, 23, 8743-8751. [CrossRef]
- Pasternak, B.; Svanstrom, H.; Nielsen, N.M.; Fugger, L.; Melbye, M.; Hviid, A. Use of calcium channel blockers and Parkinson's disease. Am J Epidemiol 2012, 175, 627-635. [CrossRef]
- Whyte, K.A.; Greenfield, S.A. Effects of acetylcholinesterase and butyrylcholinesterase on cell survival, neurite outgrowth, and voltage-dependent calcium currents of embryonic ventral mesencephalic neurons. Exp Neurol 2003, 184, 496-509. [CrossRef]
- Eaton, M.E.; Macias, W.; Youngs, R.M.; Rajadhyaksha, A.; Dudman, J.T.; Konradi, C. L-type Ca2+ channel blockers promote Ca2+ accumulation when dopamine receptors are activated in striatal neurons. Brain Res Mol Brain Res 2004, 131, 65-72. [CrossRef]
- Belforte, J.E.; Magarinos-Azcone, C.; Armando, I.; Buno, W.; Pazo, J.H. Pharmacological involvement of the calcium channel blocker flunarizine in dopamine transmission at the striatum. Parkinsonism Relat Disord 2001, 8, 33-40. [CrossRef]
- Wang, R.; Ma, Z.; Wang, J.; Xie, J. L-type Cav1.2 calcium channel is involved in 6-hydroxydopamine-induced neurotoxicity in rats. Neurotox Res 2012, 21, 266-270. [CrossRef]
- Yabuki, Y.; Ohizumi, Y.; Yokosuka, A.; Mimaki, Y.; Fukunaga, K. Nobiletin treatment improves motor and cognitive deficits seen in MPTP-induced Parkinson model mice. Neuroscience 2014, 259, 126-141. [CrossRef]
- Nedergaard, S.; Flatman, J.A.; Engberg, I. Nifedipine- and omega-conotoxin-sensitive Ca2+ conductances in guinea-pig substantia nigra pars compacta neurones. J Physiol 1993, 466, 727-747.
- Martella, G.; Madeo, G.; Schirinzi, T.; Tassone, A.; Sciamanna, G.; Spadoni, F.; Stefani, A.; Shen, J.; Pisani, A.; Bonsi, P. Altered profile and D2-dopamine receptor modulation of high voltage-activated calcium current in striatal medium spiny neurons from animal models of Parkinson's disease. Neuroscience 2011, 177, 240-251. [CrossRef]
- Wang, X.J.; Xu, J.X. Possible involvement of Ca2+ signaling in rotenone-induced apoptosis in human neuroblastoma SH-SY5Y cells. Neurosci Lett 2005, 376, 127-132. [CrossRef]
- Sai, Y.; Chen, J.; Ye, F.; Zhao, Y.; Zou, Z.; Cao, J.; Dong, Z. Dopamine Release Suppression Dependent on an Increase of Intracellular Ca(2+) Contributed to Rotenone-induced Neurotoxicity in PC12 Cells. J Toxicol Pathol 2013, 26, 149-157. [CrossRef]
- Daschil, N.; Humpel, C. Nifedipine and nimodipine protect dopaminergic substantia nigra neurons against axotomy-induced cell death in rat vibrosections via modulating inflammatory responses. Brain Res 2014, 1581, 1-11. [CrossRef]
- Singh, H.; Singh, K. Carbon transfer reactions with heterocycles - V. A facile synthesis of nifedipine and analogues. Tetrahedron 1989, 45, 3967-3974. [CrossRef]
- Hayashi, M.; Yamauchi, K.; Kinoshita, M. N-Alkylation of Nitrogen Heterocyclic Compounds with Dialkyl Phosphites. Bulletin of the Chemical Society of Japan 1977, 50, 1510-1512. [CrossRef]
- Palacios, F.; Herrán, E.; Rubiales, G. Reaction of N-Vinylic Phosphazenes Derived from β-Amino Acids with Aldehydes. Azadiene-Mediated Synthesis of Dihydropyridines, Pyridines, and Polycyclic Nitrogen Derivatives. The Journal of Organic Chemistry 1999, 64, 6239-6246. [CrossRef]
- Khadilkar, B.; Chitnavis, A. ChemInform Abstract: Rate Enhancement in the Synthesis of Some 4-Aryl-1,4-dihydropyridines Using Methyl 3-Aminocrotonate under Microwave Irradiation. ChemInform 2010, 26. [CrossRef]
- Anniyappan, M.; Muralidharan, D.; Perumal, P.T. SYNTHESIS OF HANTZSCH 1,4-DIHYDROPYRIDINES UNDER MICROWAVE IRRADIATION. Synthetic Communications 2002, 32, 659-663. [CrossRef]
- Khadilkar, B.M.; Gaikar, V.G.; Chitnavis, A.A. Aqueous hydrotrope solution as a safer medium for microwave enhanced hantzsch dihydropyridine ester synthesis. Tetrahedron Letters 1995, 36, 8083-8086. [CrossRef]
- Gordeev, M.F.; Patel, D.V.; Gordon, E.M. Approaches to Combinatorial Synthesis of Heterocycles: A Solid-Phase Synthesis of 1,4-Dihydropyridines. The Journal of Organic Chemistry 1996, 61, 924-928. [CrossRef]
- Balogh, M.; Gerstmans, A.; Hermecz, I. SOLID ACID CATALYZED REACTION OF AMINALS WITH METHYL 3-AMINOCROTONATE. Heterocyclic Communications 1999, 5, 89-92. [CrossRef]
- Sivamurugan, V.; Kumar, R.S.; Palanichamy, M.; Murugesan, V. Synthesis of hantzsch 1,4-dihydropyridines under solvent-free condition using zn[(L)proline]2 as lewis acid catalyst. Journal of Heterocyclic Chemistry 2005, 42, 969-974. [CrossRef]
- Maheswara, M.; Siddaiah, V.; Rao, Y.K.; Tzeng, Y.-M.; Sridhar, C. A simple and efficient one-pot synthesis of 1,4-dihydropyridines using heterogeneous catalyst under solvent-free conditions. Journal of Molecular Catalysis A: Chemical 2006, 260, 179-180. [CrossRef]
- Gupta, R.; Gupta, R.; Paul, S.; Loupy, A. Covalently Anchored Sulfonic Acid on Silica Gel as an Efficient and Reusable Heterogeneous Catalyst for the One-Pot Synthesis of Hantzsch 1,4-Dihydropyridines under Solvent-Free Conditions. Synthesis-stuttgart 2007, 2007, 2835-2838. [CrossRef]
- Arumugam, P.; Perumal, P. ChemInform Abstract: Hantzsch Synthesis of Polyhydroquinolines — A Simple, Efficient and Neat Protocol. ChemInform 2008, 39. [CrossRef]
- Kumar, A.; Maurya, R.A. Efficient Synthesis of Hantzsch Esters and Polyhydroquinoline Derivatives in Aqueous Micelles. Synlett 2008, 2008, 883-885. [CrossRef]
- Subudhi, B.B.; Sahoo, S.P. Synthesis and Evaluation of Antioxidant, Anti-inflammatory and Antiulcer Activity of Conjugates of Amino Acids with Nifedipine. Chemical and Pharmaceutical Bulletin 2011, 59, 1153-1156. [CrossRef]
- Bridgwood, K.L.; Veitch, G.E.; Ley, S.V. Magnesium Nitride as a Convenient Source of Ammonia: Preparation of Dihydropyridines. Organic Letters 2008, 10, 3627-3629. [CrossRef]
- Blajovan, R.; Modra, D. THE STUDY SYNTHESIS OF NIFEDIPINE - CALCIUM ANTAGONIST. Annals of West University of Timisoara. Series of Chemistry 2013, 22, 47-56.
- Prasanthi, G.; Prasad, K.V.S.R.G.; Bharathi, K. Synthesis, anticonvulsant activity and molecular properties prediction of dialkyl 1-(di(ethoxycarbonyl)methyl)-2,6-dimethyl-4-substituted-1,4-dihydropyridine-3,5-dicarboxylates. European Journal of Medicinal Chemistry 2014, 73, 97-104. [CrossRef]
- El-Moselhy, T.F.; Sidhom, P.A.; Esmat, E.A.; El-Mahdy, N.A. Synthesis, Docking Simulation, Biological Evaluations and 3D-QSAR Study of 1,4-Dihydropyridines as Calcium Channel Blockers. Chemical and Pharmaceutical Bulletin 2017, 65, 893-903. [CrossRef]
- Sohal, H.; Goyal, A.; Sharma, R.; Khare, R. One-pot, multicomponent synthesis of symmetrical Hantzsch 1, 4-dihydropyridine derivatives using glycerol as clean and green solvent. European Journal of Chemistry 2014, 5, 171-175. [CrossRef]
- Paraskar, A.; Sudalai, A. Cu(OTf)2 Catalyzed High Yield Synthesis of Hantzsch 1,4-Dihydropyridines. Cheminform 2007, 38. [CrossRef]
- Fedorova, O.V.; Koryakova, O.V.; Valova, M.S.; Ovchinnikova, I.G.; Titova, Y.A.; Rusinov, G.L.; Charushin, V.N. Catalytic effect of nanosized metal oxides on the Hantzsch reaction. Kinetics and Catalysis 2010, 51, 566-572. [CrossRef]
- Siddaiah, V.; Basha, G.M.; Rao, G.P.; Prasad, U.V.; Rao, R.S. PEG-Mediated Catalyst-Free Synthesis of Hantzsch 1,4-Dihydropyridines and Polyhydroquinoline Derivatives. Synthetic Communications 2012, 42, 627-634. [CrossRef]
- Maurya, M.R.; Saini, N.; Avecilla, F. Study of temperature dependent three component dynamic covalent assembly via Hantzsch reaction catalyzed by dioxido- and oxidoperoxidomolybdenum(vi) complexes under solvent free conditions. RSC Advances 2016, 6, 12993-13009. [CrossRef]
- Rekunge, D.S.; Khatri, C.K.; Chaturbhuj, G.U. Sulfated polyborate: An efficient and reusable catalyst for one pot synthesis of Hantzsch 1,4-dihydropyridines derivatives using ammonium carbonate under solvent free conditions. Tetrahedron Letters 2017, 58, 1240-1244. [CrossRef]
- Mohammadi, B.; Mohammad, S.; Jamkarani, H.; A. Kamali, T.; Nasrollahzadeh, M.; Mohajeri, A. Sulfonic acid-functionalized silica: A remarkably efficient heterogeneous reusable catalyst for the one-pot synthesis of 1, 4-dihydropyridines. Turkish Journal of Chemistry 2010, 34. [CrossRef]
- Bitaraf, M.; Amoozadeh, A.; Otokesh, S. A Simple and Efficient One-pot Synthesis of 1,4-dihydropyridines Using Nano-WO3- supported Sulfonic Acid as an Heterogeneous Catalyst under Solvent-free Conditions. Journal of the Chinese Chemical Society 2016, 63, 336-344. [CrossRef]
- Palermo, V.; Sathicq, Á.G.; Constantieux, T.; Rodríguez, J.; Vázquez, P.G.; Romanelli, G.P. First Report About the Use of Micellar Keggin Heteropolyacids as Catalysts in the Green Multicomponent Synthesis of Nifedipine Derivatives. Catalysis Letters 2016, 146, 1634-1647. [CrossRef]
- Wu, X.Y. Facile and Green Synthesis of 1,4-Dihydropyridine Derivatives in n-Butyl Pyridinium Tetrafluoroborate. Synthetic Communications 2012, 42, 454-459. [CrossRef]
- Wu, C.; Bian, Q.; Ding, T.; Tang, M.; Zhang, W.; Xu, Y.; Liu, B.; Xu, H.; Li, H.-B.; Fu, H. Photoinduced Iron-Catalyzed ipso-Nitration of Aryl Halides via Single-Electron Transfer. ACS Catalysis 2021, 11, 9561-9568. [CrossRef]
- Guidi, M.; Moon, S.; Anghileri, L.; Cambié, D.; Seeberger, P.H.; Gilmore, K. Combining radial and continuous flow synthesis to optimize and scale-up the production of medicines. Reaction Chemistry & Engineering 2021, 6, 220-224. [CrossRef]
- Xiang, S.; Fan, W.; Zhang, W.; Li, Y.; Guo, S.; Huang, D. Aqueous CO2 fixation: construction of pyridine skeletons in cooperation with ammonium cations. Green Chemistry 2021, 23, 7950-7955. [CrossRef]
- Xu, W.; Lipscombe, D. Neuronal Ca(V)1.3alpha(1) L-type channels activate at relatively hyperpolarized membrane potentials and are incompletely inhibited by dihydropyridines. J Neurosci 2001, 21, 5944-5951. [CrossRef]
- Lopez-Arrieta, J.M.; Birks, J. Nimodipine for primary degenerative, mixed and vascular dementia. Cochrane Database Syst Rev 2002, CD000147. [CrossRef]
- Ban, T.A.; Morey, L.; Aguglia, E.; Azzarelli, O.; Balsano, F.; Marigliano, V.; Caglieris, N.; Sterlicchio, M.; Capurso, A.; Tomasi, N.A.; et al. Nimodipine in the treatment of old age dementias. Prog Neuropsychopharmacol Biol Psychiatry 1990, 14, 525-551. [CrossRef]
- Tollefson, G.D. Short-term effects of the calcium channel blocker nimodipine (Bay-e-9736) in the management of primary degenerative dementia. Biol Psychiatry 1990, 27, 1133-1142. [CrossRef]
- Parnetti, L.; Senin, U.; Carosi, M.; Baasch, H. Mental deterioration in old age: results of two multicenter, clinical trials with nimodipine. The Nimodipine Study Group. Clin Ther 1993, 15, 394-406.
- Schmage, N.; Bergener, M. Global rating, symptoms, behavior, and cognitive performance as indicators of efficacy in clinical studies with nimodipine in elderly patients with cognitive impairment syndromes. Int Psychogeriatr 1992, 4 Suppl 1, 89-99.
- Pantoni, L.; del Ser, T.; Soglian, A.G.; Amigoni, S.; Spadari, G.; Binelli, D.; Inzitari, D. Efficacy and safety of nimodipine in subcortical vascular dementia: a randomized placebo-controlled trial. Stroke 2005, 36, 619-624. [CrossRef]
- Meneses, A.; Terron, J.A.; Ibarra, M.; Hong, E. Effects of nimodipine on learning in normotensive and spontaneously hypertensive rats. Behav Brain Res 1997, 85, 121-125. [CrossRef]
- Paroczai, M.; Kiss, B.; Karpati, E. Effect of RGH-2716 on learning and memory deficits of young and aged rats in water-labyrinth. Brain Res Bull 1998, 45, 475-488. [CrossRef]
- Batuecas, A.; Pereira, R.; Centeno, C.; Pulido, J.A.; Hernandez, M.; Bollati, A.; Bogonez, E.; Satrustegui, J. Effects of chronic nimodipine on working memory of old rats in relation to defects in synaptosomal calcium homeostasis. Eur J Pharmacol 1998, 350, 141-150. [CrossRef]
- Gholami Pourbadie, H.; Naderi, N.; Janahmadi, M.; Mehranfard, N.; Motamedi, F. Calcium channel blockade attenuates abnormal synaptic transmission in the dentate gyrus elicited by entorhinal amyloidopathy. Synapse 2016, 70, 408-417. [CrossRef]
- Gholamipour-Badie, H.; Naderi, N.; Khodagholi, F.; Shaerzadeh, F.; Motamedi, F. L-type calcium channel blockade alleviates molecular and reversal spatial learning and memory alterations induced by entorhinal amyloid pathology in rats. Behav Brain Res 2013, 237, 190-199. [CrossRef]
- Veng, L.M.; Mesches, M.H.; Browning, M.D. Age-related working memory impairment is correlated with increases in the L-type calcium channel protein alpha1D (Cav1.3) in area CA1 of the hippocampus and both are ameliorated by chronic nimodipine treatment. Brain Res Mol Brain Res 2003, 110, 193-202. [CrossRef]
- Topcu, A.; Saral, S.; Ozturk, A.; Saral, O.; Kaya, A.K. The effect of the calcium channel blocker nimodipine on hippocampal BDNF/Ach levels in rats with experimental cognitive impairment. Neurol Res 2023, 1-10. [CrossRef]
- Sandin, M.; Jasmin, S.; Levere, T.E. Aging and cognition: facilitation of recent memory in aged nonhuman primates by nimodipine. Neurobiol Aging 1990, 11, 573-575. [CrossRef]
- Pierrot, N.; Ghisdal, P.; Caumont, A.S.; Octave, J.N. Intraneuronal amyloid-beta1-42 production triggered by sustained increase of cytosolic calcium concentration induces neuronal death. J Neurochem 2004, 88, 1140-1150. [CrossRef]
- Ishii, M.; Hiller, A.J.; Pham, L.; McGuire, M.J.; Iadecola, C.; Wang, G. Amyloid-Beta Modulates Low-Threshold Activated Voltage-Gated L-Type Calcium Channels of Arcuate Neuropeptide Y Neurons Leading to Calcium Dysregulation and Hypothalamic Dysfunction. J Neurosci 2019, 39, 8816-8825. [CrossRef]
- Ueda, K.; Shinohara, S.; Yagami, T.; Asakura, K.; Kawasaki, K. Amyloid beta protein potentiates Ca2+ influx through L-type voltage-sensitive Ca2+ channels: a possible involvement of free radicals. J Neurochem 1997, 68, 265-271. [CrossRef]
- Brorson, J.R.; Bindokas, V.P.; Iwama, T.; Marcuccilli, C.J.; Chisholm, J.C.; Miller, R.J. The Ca2+ influx induced by beta-amyloid peptide 25-35 in cultured hippocampal neurons results from network excitation. J Neurobiol 1995, 26, 325-338. [CrossRef]
- Weiss, J.H.; Pike, C.J.; Cotman, C.W. Ca2+ channel blockers attenuate beta-amyloid peptide toxicity to cortical neurons in culture. J Neurochem 1994, 62, 372-375. [CrossRef]
- Yagami, T.; Nakazato, H.; Ueda, K.; Asakura, K.; Kuroda, T.; Hata, S.; Sakaeda, T.; Sakaguchi, G.; Itoh, N.; Hashimoto, Y.; et al. Prostaglandin E2 rescues cortical neurons from amyloid beta protein-induced apoptosis. Brain Res 2003, 959, 328-335. [CrossRef]
- Yagami, T.; Ueda, K.; Asakura, K.; Kuroda, T.; Hata, S.; Sakaeda, T.; Kambayashi, Y.; Fujimoto, M. Effects of endothelin B receptor agonists on amyloid beta protein (25-35)-induced neuronal cell death. Brain Res 2002, 948, 72-81. [CrossRef]
- Ekinci, F.J.; Ortiz, D.; Shea, T.B. Okadaic acid mediates tau phosphorylation via sustained activation of the L-voltage-sensitive calcium channel. Brain Res Mol Brain Res 2003, 117, 145-151. [CrossRef]
- Facchinetti, F.; Fasolato, C.; Del Giudice, E.; Burgo, A.; Furegato, S.; Fusco, M.; Basso, E.; Seraglia, R.; D'Arrigo, A.; Leon, A. Nimodipine selectively stimulates beta-amyloid 1-42 secretion by a mechanism independent of calcium influx blockage. Neurobiol Aging 2006, 27, 218-227. [CrossRef]
- Higham, J.P.; Hidalgo, S.; Buhl, E.; Hodge, J.J.L. Restoration of Olfactory Memory in Drosophila Overexpressing Human Alzheimer's Disease Associated Tau by Manipulation of L-Type Ca(2+) Channels. Front Cell Neurosci 2019, 13, 409. [CrossRef]
- Zheng, Z.; Wu, K.; Ruan, Q.; Li, D.; Liu, W.; Wang, M.; Li, Y.; Xia, J.; Yang, D.; Guo, J. Suppression of Selective Voltage-Gated Calcium Channels Alleviates Neuronal Degeneration and Dysfunction through Glutathione S-Transferase-Mediated Oxidative Stress Resistance in a Caenorhabditis elegans Model of Alzheimer's Disease. Oxid Med Cell Longev 2022, 8287633. [CrossRef]
- Hopp, S.C.; D'Angelo, H.M.; Royer, S.E.; Kaercher, R.M.; Crockett, A.M.; Adzovic, L.; Wenk, G.L. Calcium dysregulation via L-type voltage-dependent calcium channels and ryanodine receptors underlies memory deficits and synaptic dysfunction during chronic neuroinflammation. J Neuroinflammation 2015, 12, 56. [CrossRef]
- Sanz, J.M.; Chiozzi, P.; Colaianna, M.; Zotti, M.; Ferrari, D.; Trabace, L.; Zuliani, G.; Di Virgilio, F. Nimodipine inhibits IL-1beta release stimulated by amyloid beta from microglia. Br J Pharmacol 2012, 167, 1702-1711. [CrossRef]
- Zongfang, Z.; Wenjing, L.; Zhaomin, C.; Lei, Z. Therapeutic effect of piracetam with nimodipine on vascular dementia after cerebral infarction. Pak J Pharm Sci 2020, 33, 2405-2411.
- Sadleir, K.R.; Popovic, J.; Khatri, A.; Vassar, R. Oral nimodipine treatment has no effect on amyloid pathology or neuritic dystrophy in the 5XFAD mouse model of amyloidosis. PLoS One 2022, 17, e0263332. [CrossRef]
- Gaasch, J.A.; Geldenhuys, W.J.; Lockman, P.R.; Allen, D.D.; Van der Schyf, C.J. Voltage-gated calcium channels provide an alternate route for iron uptake in neuronal cell cultures. Neurochem Res 2007, 32, 1686-1693. [CrossRef]
- Hopp, S.C.; Royer, S.E.; D'Angelo, H.M.; Kaercher, R.M.; Fisher, D.A.; Wenk, G.L. Differential neuroprotective and anti-inflammatory effects of L-type voltage dependent calcium channel and ryanodine receptor antagonists in the substantia nigra and locus coeruleus. J Neuroimmune Pharmacol 2015, 10, 35-44. [CrossRef]
- Putzier, I.; Kullmann, P.H.; Horn, J.P.; Levitan, E.S. Cav1.3 channel voltage dependence, not Ca2+ selectivity, drives pacemaker activity and amplifies bursts in nigral dopamine neurons. J Neurosci 2009, 29, 15414-15419. [CrossRef]
- Klimaviciusa, L.; Klusa, V.; Duburs, G.; Kaasik, A.; Kalda, A.; Zharkovsky, A. Distinct effects of atypical 1,4-dihydropyridines on 1-methyl-4-phenylpyridinium-induced toxicity. Cell Biochem Funct 2007, 25, 15-21. [CrossRef]
- Singh, A.; Verma, P.; Balaji, G.; Samantaray, S.; Mohanakumar, K.P. Nimodipine, an L-type calcium channel blocker attenuates mitochondrial dysfunctions to protect against 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine-induced Parkinsonism in mice. Neurochem Int 2016, 99, 221-232. [CrossRef]
- Singh, A.; Verma, P.; Raju, A.; Mohanakumar, K.P. Nimodipine attenuates the parkinsonian neurotoxin, MPTP-induced changes in the calcium binding proteins, calpain and calbindin. J Chem Neuroanat 2019, 95, 89-94. [CrossRef]
- Nordstrom, O.; Braesch-Andersen, S.; Bartfai, T. Dopamine release is enhanced while acetylcholine release is inhibited by nimodipine (Bay e 9736). Acta Physiol Scand 1986, 126, 115-119. [CrossRef]
- Soderstrom, K.E.; O'Malley, J.A.; Levine, N.D.; Sortwell, C.E.; Collier, T.J.; Steece-Collier, K. Impact of dendritic spine preservation in medium spiny neurons on dopamine graft efficacy and the expression of dyskinesias in parkinsonian rats. Eur J Neurosci 2010, 31, 478-490. [CrossRef]
- Schuster, S.; Doudnikoff, E.; Rylander, D.; Berthet, A.; Aubert, I.; Ittrich, C.; Bloch, B.; Cenci, M.A.; Surmeier, D.J.; Hengerer, B.; et al. Antagonizing L-type Ca2+ channel reduces development of abnormal involuntary movement in the rat model of L-3,4-dihydroxyphenylalanine-induced dyskinesia. Biol Psychiatry 2009, 65, 518-526. [CrossRef]
- Sautter, J.; Kupsch, A.; Earl, C.D.; Oertel, W.H. Degeneration of pre-labelled nigral neurons induced by intrastriatal 6-hydroxydopamine in the rat: behavioural and biochemical changes and pretreatment with the calcium-entry blocker nimodipine. Exp Brain Res 1997, 117, 111-119. [CrossRef]
- Chen, T.; Yang, Y.F.; Luo, P.; Liu, W.; Dai, S.H.; Zheng, X.R.; Fei, Z.; Jiang, X.F. Homer1 knockdown protects dopamine neurons through regulating calcium homeostasis in an in vitro model of Parkinson's disease. Cell Signal 2013, 25, 2863-2870. [CrossRef]
- Li, Y.; Hu, X.; Liu, Y.; Bao, Y.; An, L. Nimodipine protects dopaminergic neurons against inflammation-mediated degeneration through inhibition of microglial activation. Neuropharmacology 2009, 56, 580-589. [CrossRef]
- Gordeev, M.F.; Patel, D.V.; Gordon, E.M. Approaches to Combinatorial Synthesis of Heterocycles: A Solid-Phase Synthesis of 1,4-Dihydropyridines. J. Org. Chem. 1996, 61, 924-928. [CrossRef]
- Balaev, A.N.; Osipov, V.N.; Fedorov, V.E. Development of nimodipine production technology. Pharm. Chem. J. 2012, 46, 285-287. [CrossRef]
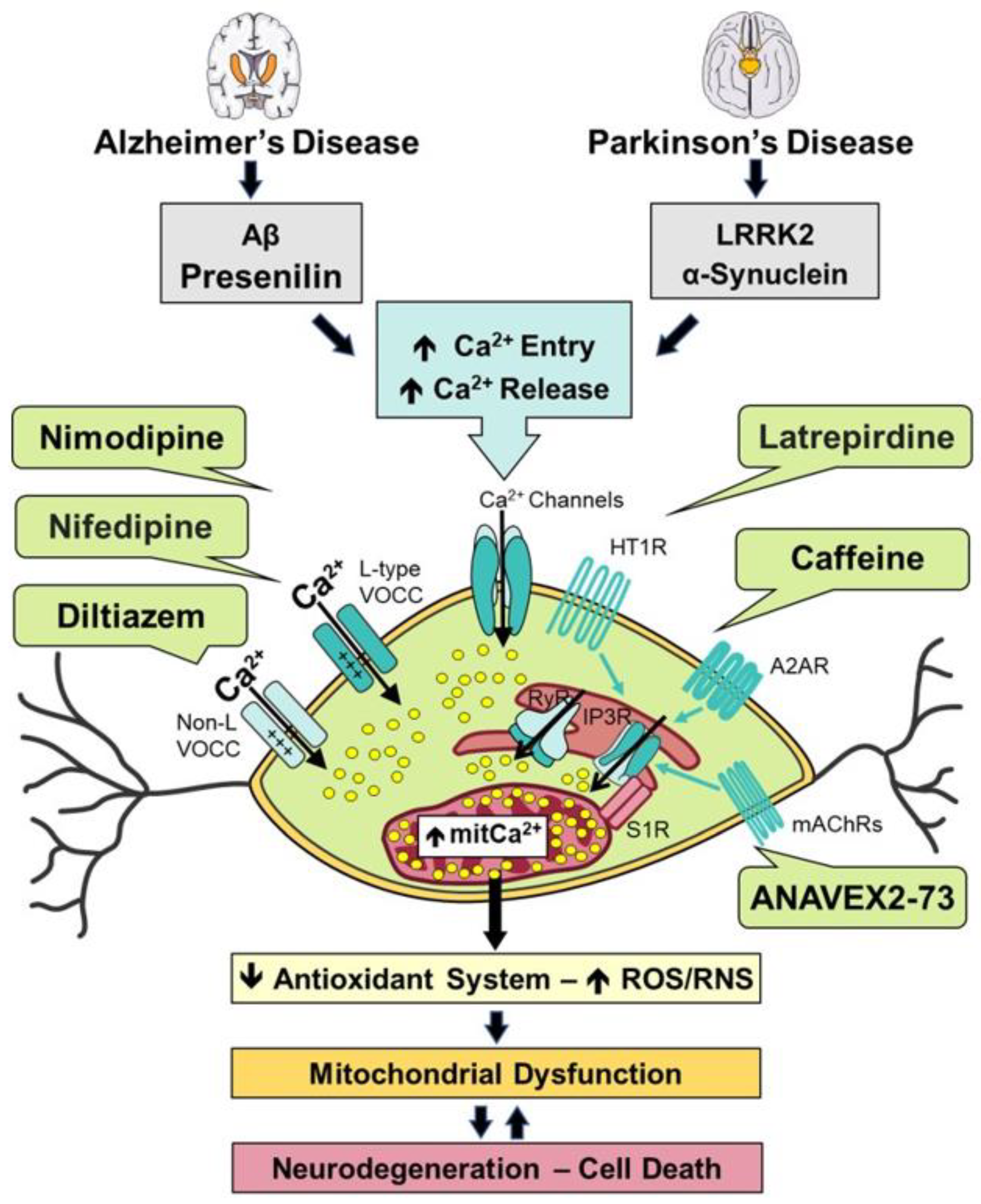
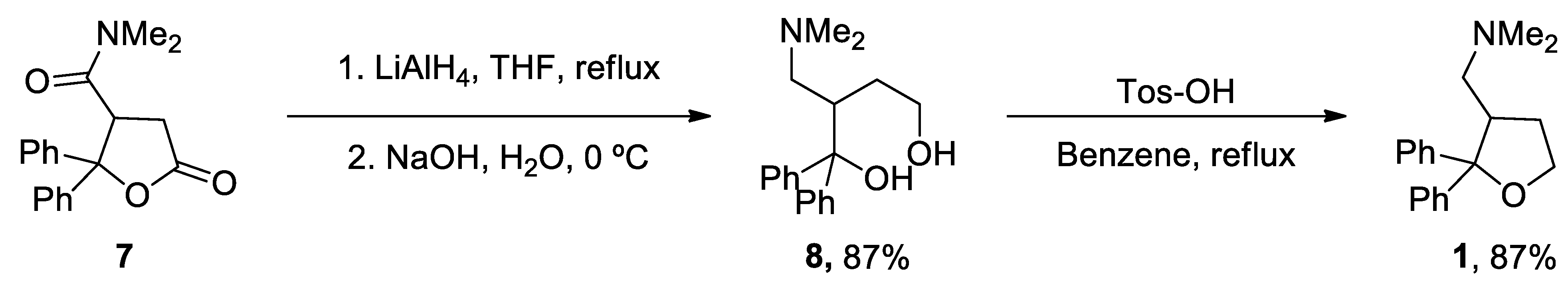
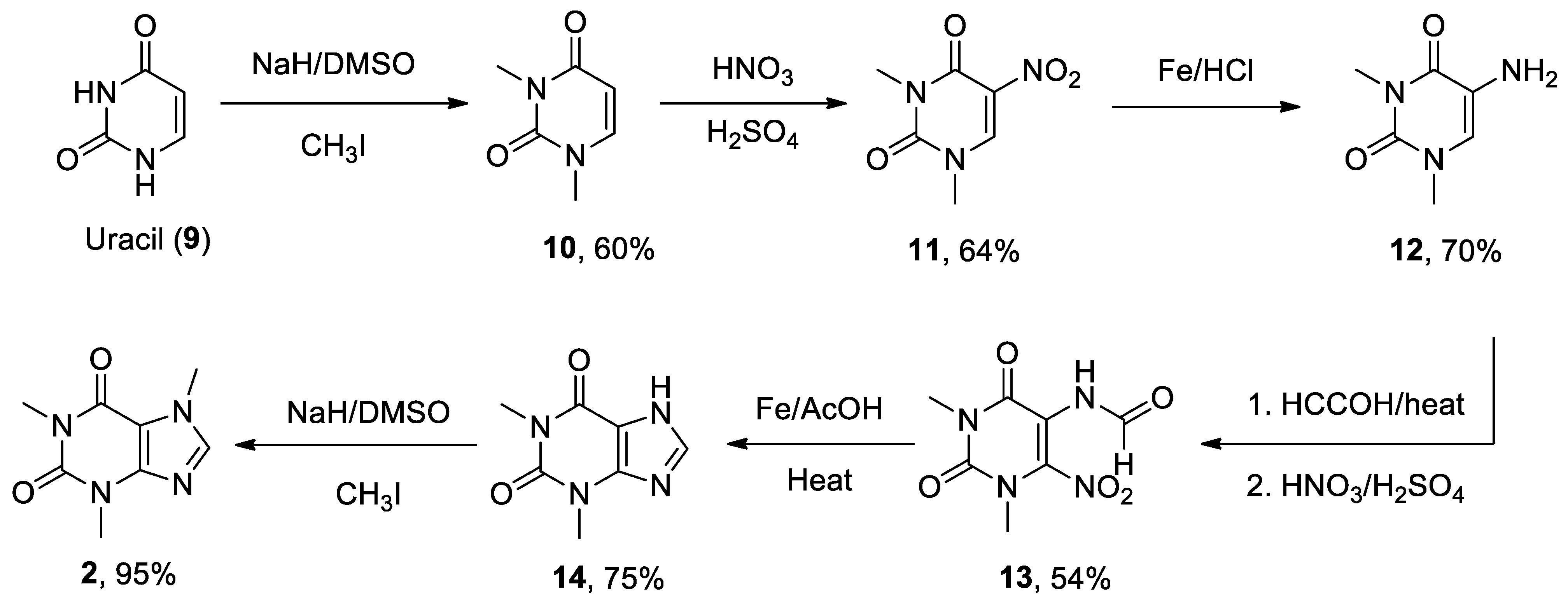
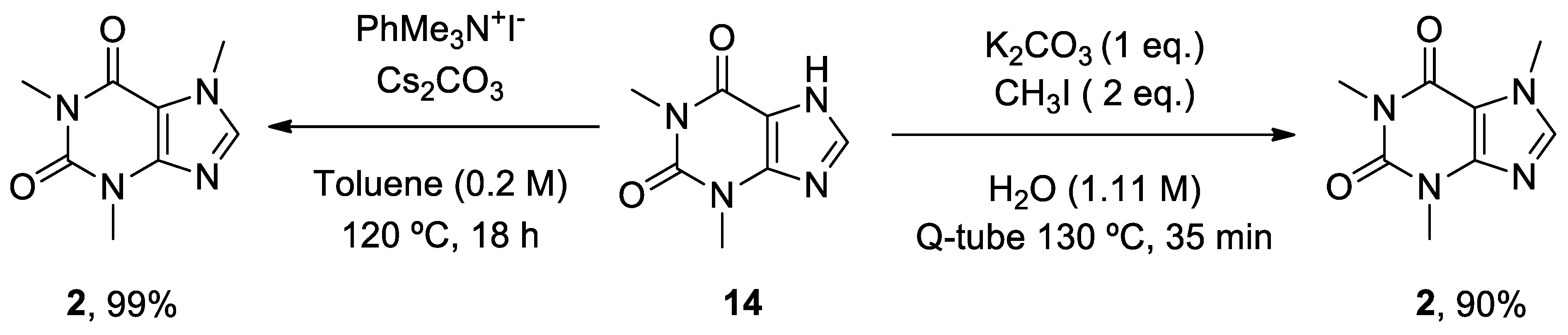
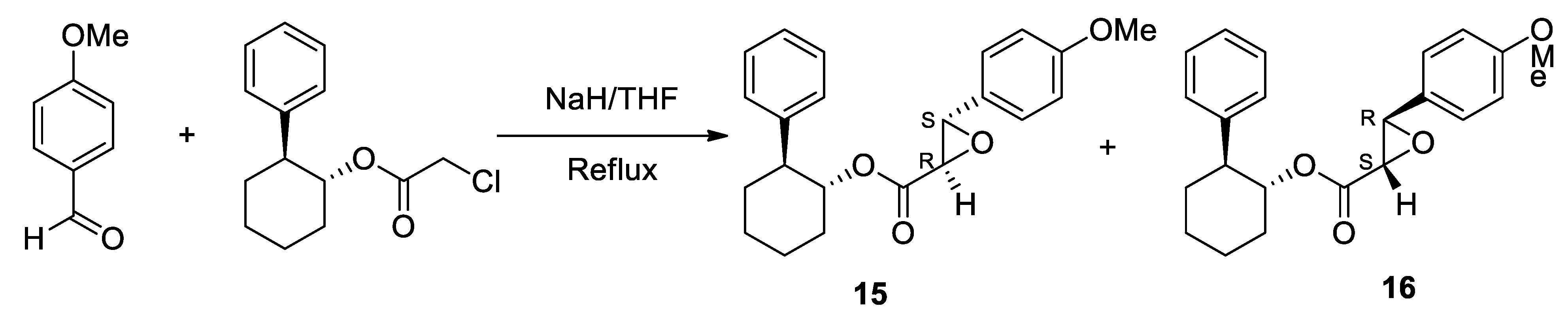
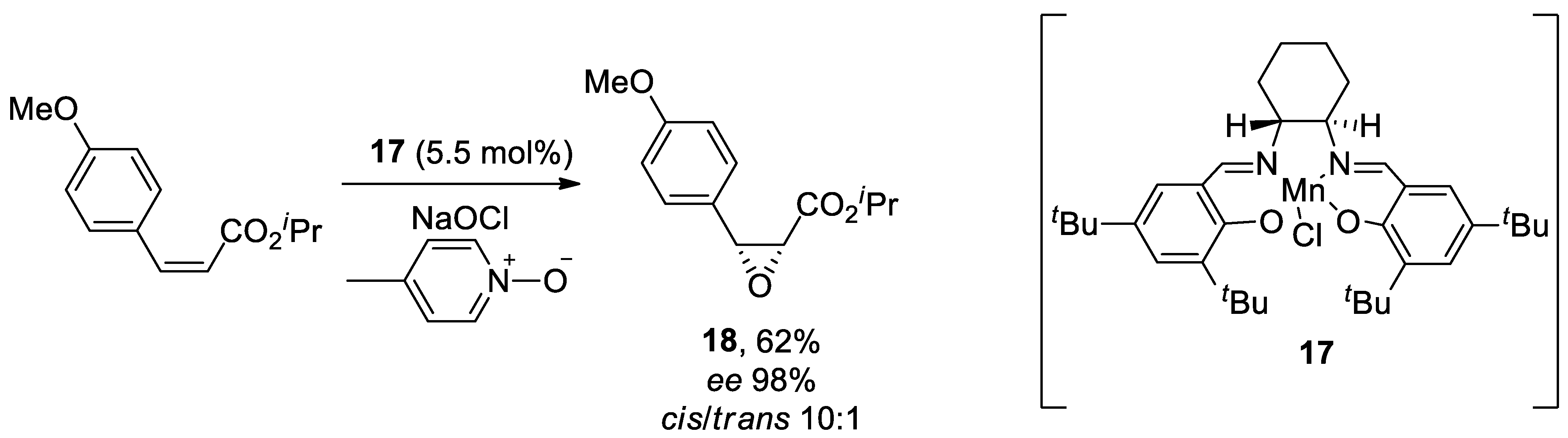
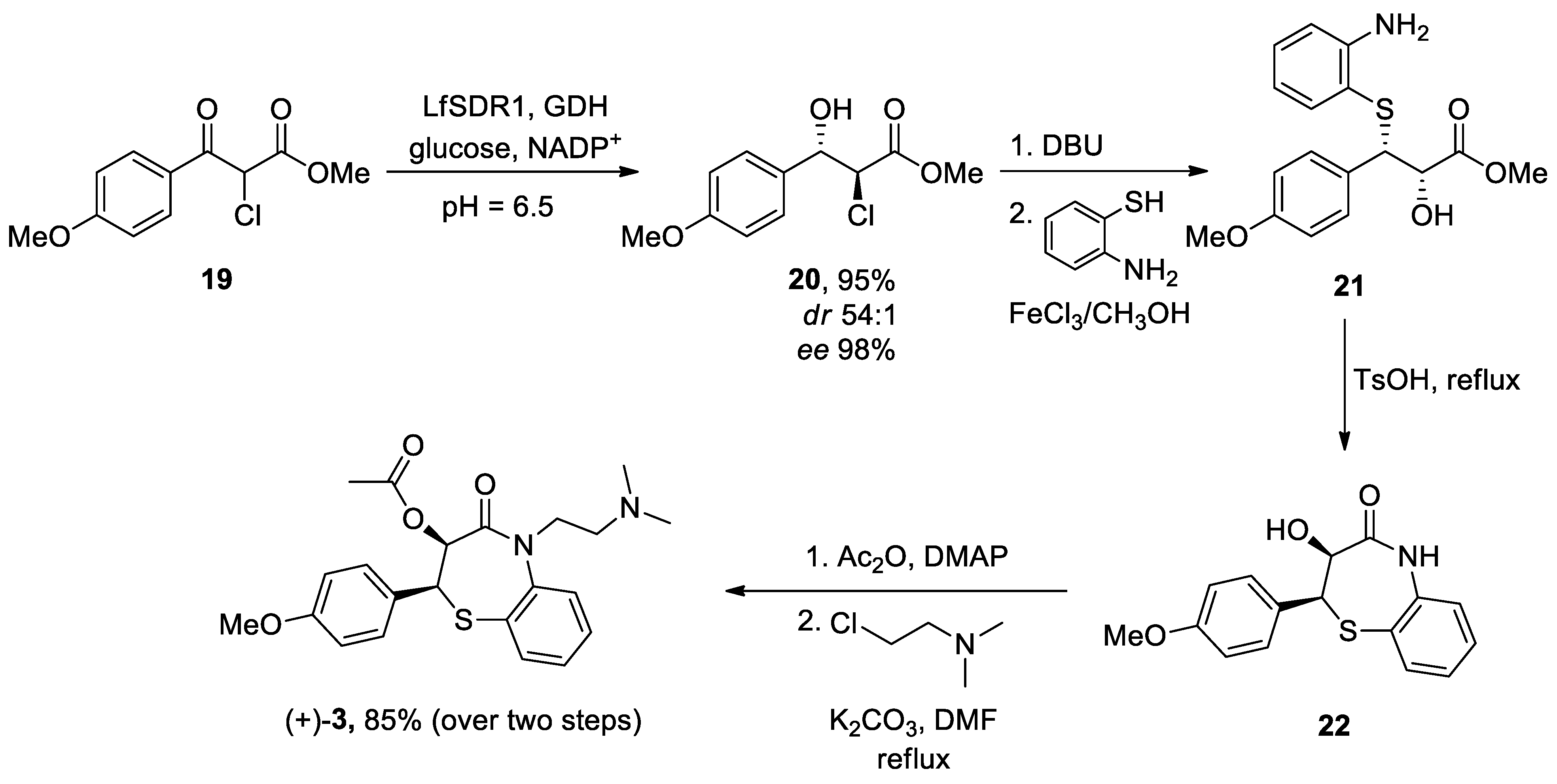
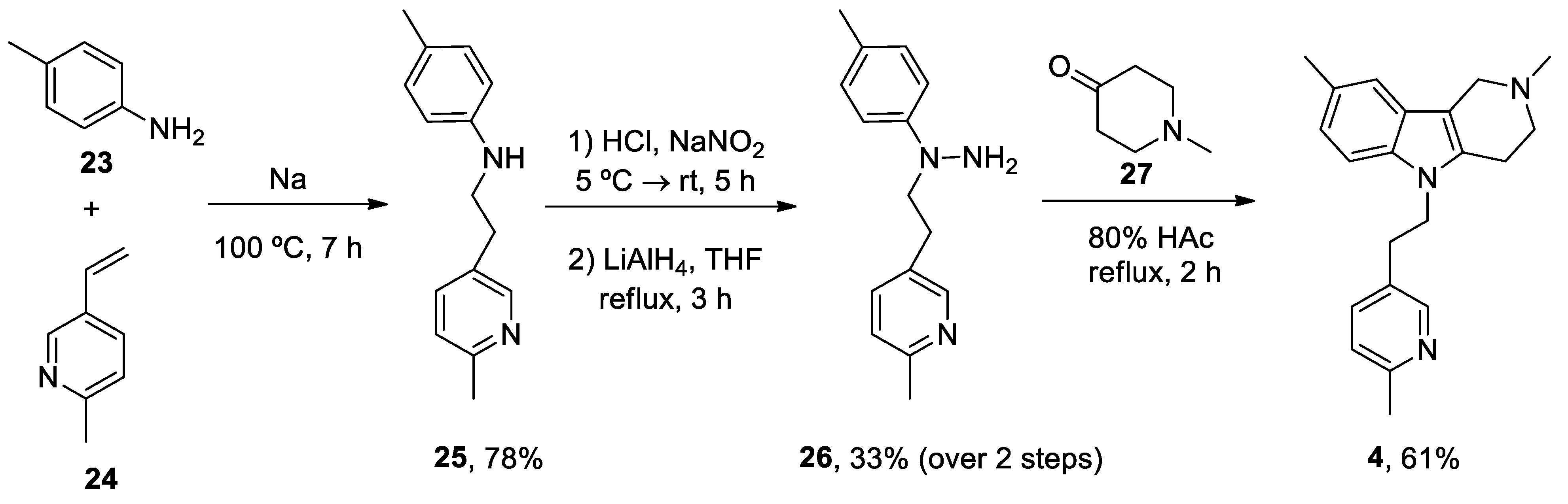
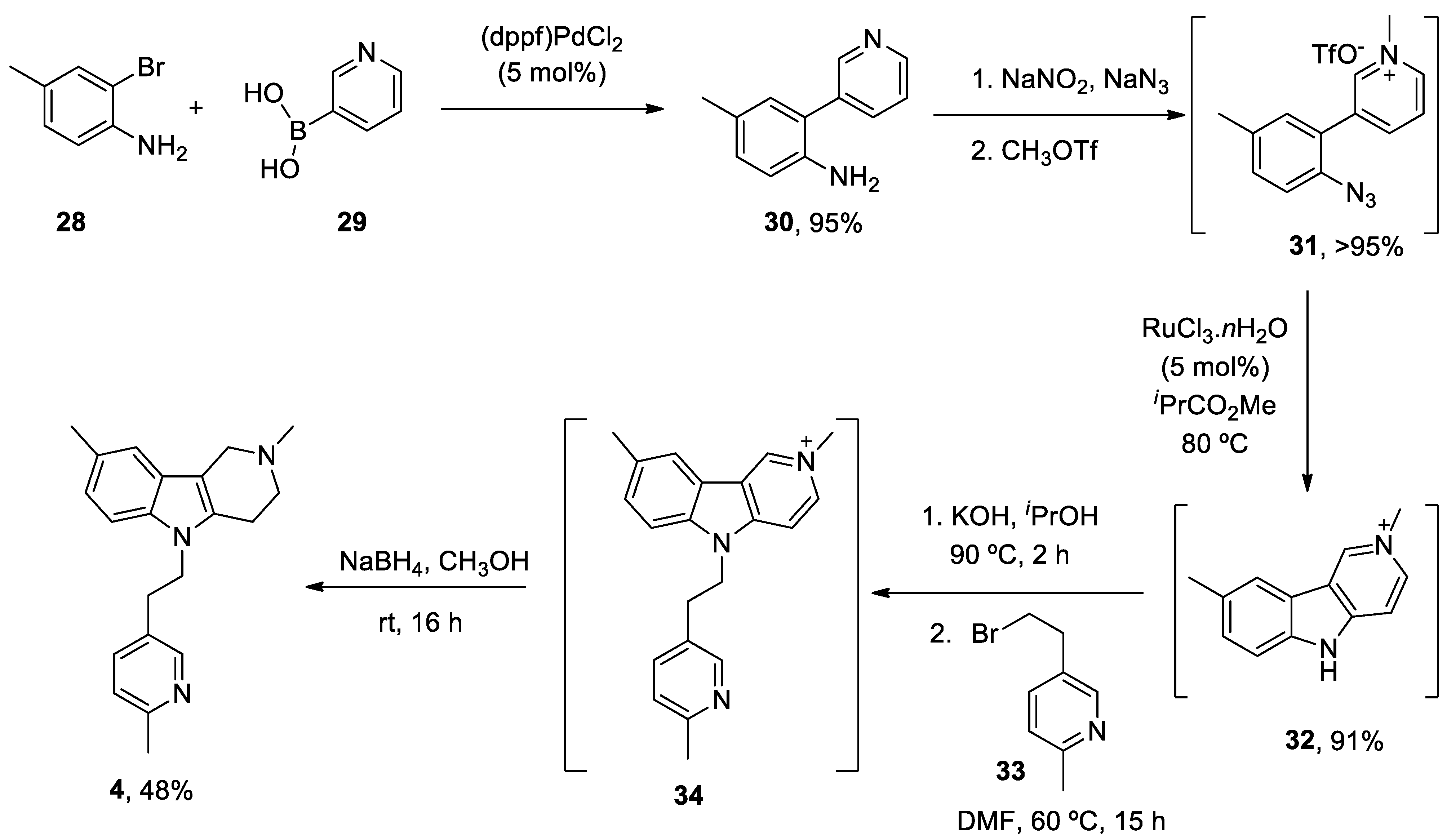
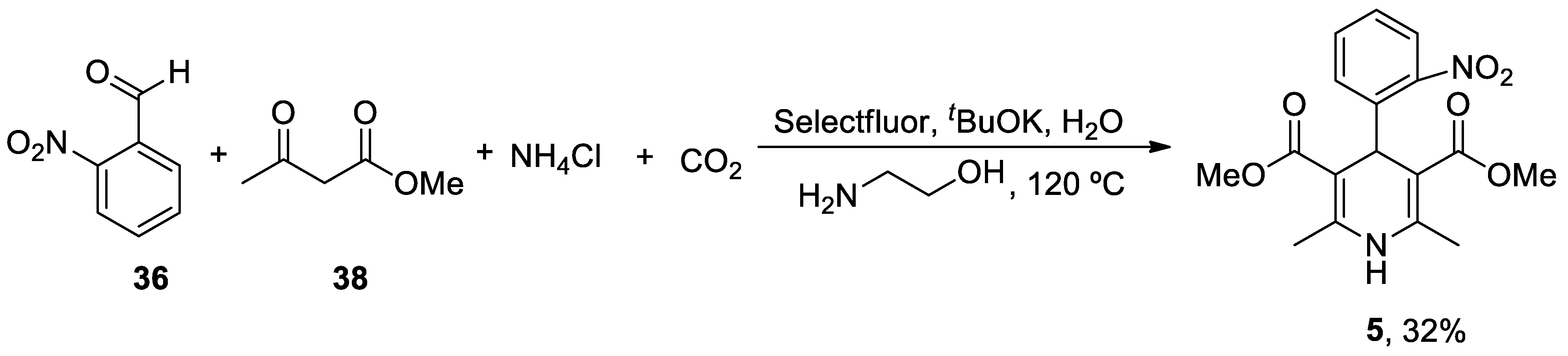
| Heterocyclic Compound | Chemical Name | Target | Proposed Mechanism | Effect on Neurodegenerative Diseases. Status of Clinical Trials |
|---|---|---|---|---|
|
1. ANAVEX2-73 (Blarcamesine) |
Tetrahydro-N,N-dimethyl-2,2-diphenyl-3-furanmethanamine hydrochloride |
mAChRs S1R |
|
Memory improvement and neuroprotective effects in AD and PD.
|
|
2. Caffeine (Mateine) |
1,3,7-Trimethyl-3,7-dihydro-1H-purine-2,6-dione |
A2AR RyR (+) |
|
Reduced risk of developing AD and PD Epidemiological studies: PD, possible motor benefits |
|
3. Diltiazen (Cardizem) |
(2S,3S)-5-(2-(Dimethylamino)ethyl)-2-(4-methoxyphenyl)-4-oxo-2,3,4,5-tetrahydrobenzo[b][1,4]thiazepin-3-yl acetate | Non-dihydro-pyridine VOCC |
|
Reduced risk of developing AD and PD Epidemiological & Randomization Studys |
|
4. Latrepirdine (Dimebon) |
3,6-Dimethyl-9-(2-methyl-pyridyl-5)-ethyl-1,2,3,4-tetrahydro-γ-carboline dihydrochloride |
H1R Other Ca2+ Channels |
|
Cognitive and psychiatric benefit in ADImprovement in cognitive decline in HD Status:
|
|
5. Nifedipine (Procardia) |
3,5-Dimethyl 2,6-dimethyl-4-(2-nitrophenyl)-1,4-dihydropyridine-3,5-dicarboxylate | L-Type VOCC |
|
Reducing risk developing AD and PD Epidemiological & Randomization Studies |
|
6. Nimodipine (Nimotop) |
3-isopropyl 5-(2-methoxyethyl) 2,6-dimethyl-4-(3-nitrophenyl)-1,4-dihydropyridine-3,5-dicarboxylate | L-Type VOCC |
|
Reducing risk developing AD and PD Epidemiological & Randomization Studies |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





