Submitted:
05 February 2023
Posted:
06 February 2023
You are already at the latest version
Abstract
Keywords:
Introduction
Methods
Ethical approval
Animals
Experimental design
Body weight and weight gain
Dissection
Gonadosomatic Index (GSI)
Relative organ weight
Biochemical Analysis
Catalase (CAT)
Peroxidase (POD)
Superoxide dismutase (SOD)
Thiobarbituric acid reactive substances (TBARS)
Reactive oxygen species (ROS)
Analysis of Plasma Hormonal Level
Lipid profile
Tissue histology
Statistical analysis
Ethics approval and consent to participate
Results
Body weight
Effect of daily consumption of broiler and domestic chicken meat on body weight and weight gain in the male rat
Gonadosomatic index (GSI), reproductive organ weight and testicular diameter
Accessory reproductive organ weight
Weight of Body Organs
Effect of chronic consumption of broiler and domestic chicken meat on the weight of Kidney, Heart, and Liver
Oxidative stress
Effect of chronic consumption of broiler and domestic chicken meat on antioxidant enzyme level in the testes
Hormonal Analysis
Effect of chronic consumption of broiler and domestic chicken meat on plasma level of Testosterone, Estradiol, and Progesterone
Lipid Profile
Effect of chronic consumption of broiler and domestic chicken meat on High-density lipoprotein (HDL) and Low-density lipoprotein (LDL)
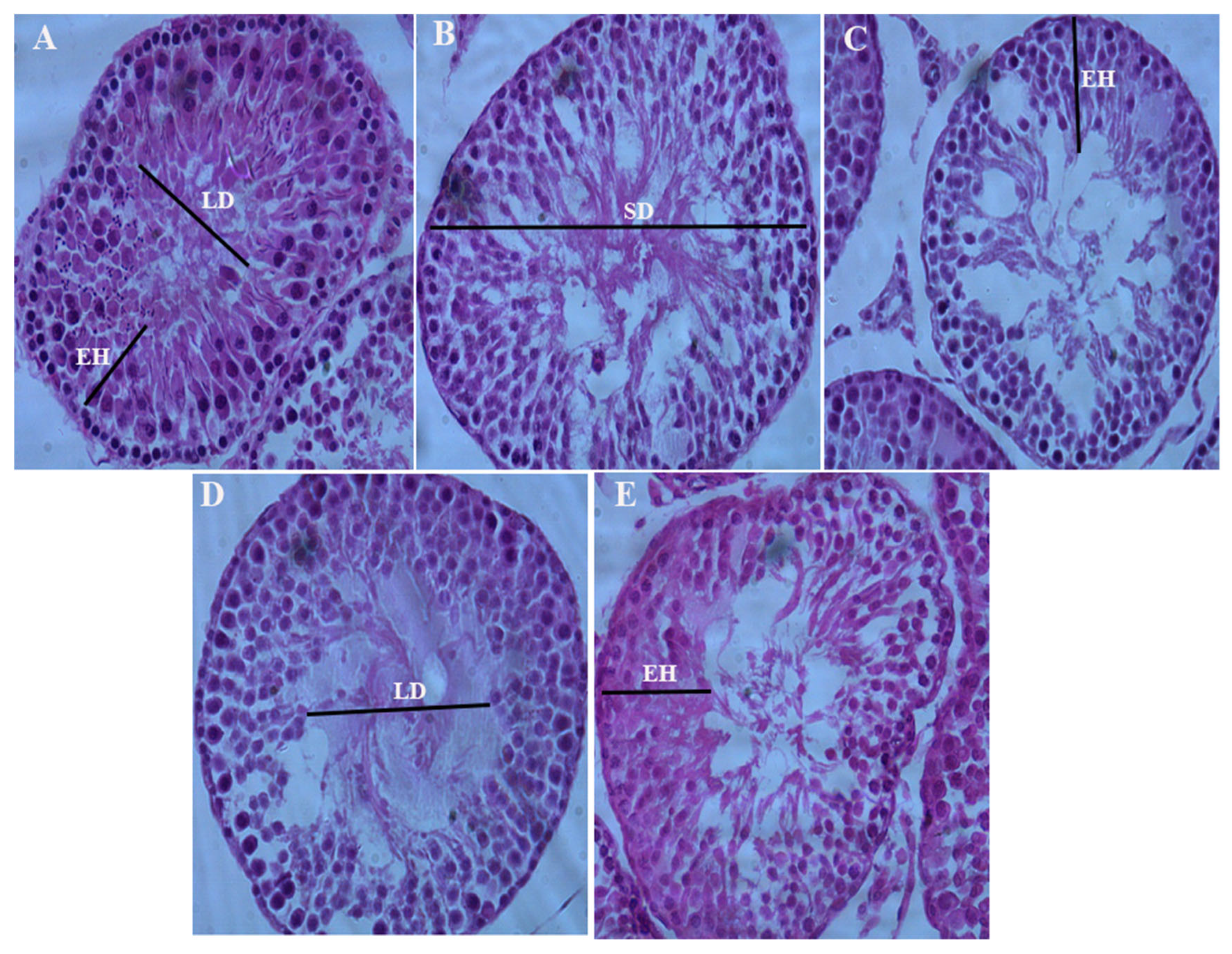
Histomorphometric Analysis
Effect of chronic consumption of broiler and domestic chicken meat on seminiferous tubule diameter, epithelial height, and lumen diameter
Discussion
Conclusions
Author Contributions
Funding
Availability of data and materials
Arrive guidelines
Conflict of interest
Consent for Publication
References
- Ahmad S, Ahmed I, Haider S, Batool Z, Ahmed F, Tabassum S, Madiha S, Perveen T, Saad BA: Effects of Consumption of Caged and Un-Caged Chicken Meat on Ovarian Health of Female Wistar Rats. Pakistan Journal of Zoology 2018, 50(2).
- Al-Nasser A, Al-Khalaifa H, Al-Saffar A, Khalil F, Albahouh M, Ragheb G, Al- Haddad A, Mashaly M: Overview of chicken taxonomy and domestication. World’s Poultry Science Journal 2007, 63(2):285-300. [CrossRef]
- Memon I, Noonari S, Asif M, Shah S, Peerzado M, Panhwar G, Sethar A, Kalwar G, Bhatti M, Jamro A: Economic analysis of poultry egg production in Quetta District Balochistan. Journal of Fisheries & Livestock Production 2015, 3(3). [CrossRef]
- Knowles TG, Kestin SC, Haslam SM, Brown SN, Green LE, Butterworth A, Pope SJ, Pfeiffer D, Nicol CJ: Leg disorders in broiler chickens: prevalence, risk factors and prevention. PloS one 2008, 3(2):e1545. [CrossRef]
- Julian R: Rapid growth problems: ascites and skeletal deformities in broilers. Poultry science 1998, 77(12):1773-1780. [CrossRef]
- Ortiz M, Valdivia F, Martínez R, Martínez de A A: Effect of clenbuterol on growth performance in broilers. Arquivo Brasileiro de Medicina Veterinária e Zootecnia 2000, 52(3):256-260. [CrossRef]
- Buttery P, Dawson J: mode of action of beta-agonists as manipulators of carcass composition. Beta-agonists and their effects on animal growth and carcass quality/edited by JP Hanrahan 1988.
- Cardoso LA, Stock MJ: Effect of clenbuterol on growth and body composition during food restriction in rats. Journal of animal science 1996, 74(9):2245-2252. [CrossRef]
- Schiavone A, Tarantola M, Perona G, Pagliasso S, Badino P, Odore R, Cuniberti B, Lussiana C: Effect of dietary clenbuterol and cimaterol on muscle composition, β-adrenergic and androgen receptor concentrations in broiler chickens. Journal of animal physiology and animal nutrition 2004, 88(3-4):94-100. [CrossRef]
- Yousefi J, Maherisis N, Telli A, Hatefinezhad K, Eshartkhah B, Saber SN: Effect of salbutamol (a beta-adrenergic agonist) on growth performance of broiler chickens. Annals of Biological Research 2011, 2:500-505.
- Lu H, Zhang H, Zhu T, Xiao Y, Xie S, Gu H, Cui M, Luo L: Metabolic effects of clenbuterol and salbutamol on pork meat studied using internal extractive electrospray ionization mass spectrometry. Scientific reports 2017, 7(1):5136. [CrossRef]
- Malucelli A, Ellendorff F, Meyer H: Tissue distribution and residues of clenbuterol, salbutamol, and terbutaline in tissues of treated broiler chickens. Journal of animal science 1994, 72(6):1555-1560. [CrossRef]
- Rondelli S, Martinez O, Garcia P: Effects of different dietary lipids on the fatty acid composition of broiler abdominal fat. Revista Brasileira de Ciência Avícola 2004, 6(3):171-175. [CrossRef]
- Ahmad S, Ahmed I, Haider S, Batool Z, Ahmed SB: Daily consumption of commercial chicken feed and meat lead to alterations in serum cholesterol and steroidal sex hormones in female rats. Pakistan journal of pharmaceutical sciences 2017, 30.
- Ahmad S, Omm-e-Hany AI, Ahmed SA, Alamgir A, Neelam A: Potential Effect of Chicken Boneless Meat on the Body Weight and Serum Cholesterol Levels of the Female Albino Wister Rats: in Direct Human Prospective Studies. American-Eurasian J. Agric Environ Sci 2016, 16(03):466-469.
- Ahmad S, Ahmed I, Haider S, Batool Z, Ahmed F, Tabassum S, Madiha S, Perveen T, Ahmed SB: Effects of Consumption of Caged and Un-Caged Chicken Meat on Ovarian Health of Female Wistar Rats. Pakistan Journal of Zoology 2018, 50(2):487-487.
- Ahmad S, Ahmed I: Response of wistar rats to broiler chicken feed and soy bean on body weight, obesity and weight of selected visceral organs. Pak J Biochem Mol Biol 2014, 47(3-4):137-140. [CrossRef]
- Emmerson D: Commercial approaches to genetic selection for growth and feed conversion in domestic poultry. Poultry Science 1997, 76(8):1121-1125. [CrossRef]
- Firman JD, Kamyab A, Leigh H: Comparison of fat sources in rations of broilers from hatch to market. Int J Poult Sci 2008, 7(12):1152-1155. [CrossRef]
- Wang Y, Lehane C, Ghebremeskel K, Crawford MA: Modern organic and broiler chickens sold for human consumption provide more energy from fat than protein. Public health nutrition 2010, 13(3):400-408. [CrossRef]
- Namekawa J, Takagi Y, Wakabayashi K, Nakamura Y, Watanabe A, Nagakubo D, Shirai M, Asai F: Effects of high-fat diet and fructose-rich diet on obesity, dyslipidemia and hyperglycemia in the WBN/Kob-Leprfa rat, a new model of type 2 diabetes mellitus. Journal of Veterinary Medical Science 2017, 79(6):988-991. [CrossRef]
- Dudek M, Kołodziejski P, Pruszyńska-Oszmałek E, Sassek M, Ziarniak K, Nowak K, Sliwowska J: Effects of high-fat diet-induced obesity and diabetes on Kiss1 and GPR54 expression in the hypothalamic–pituitary–gonadal (HPG) axis and peripheral organs (fat, pancreas and liver) in male rats. Neuropeptides 2016, 56:41-49. [CrossRef]
- Ahmad S: The effect of commercially available chicken feed and chicken meat on body weight and serum estrogen levels in female albino Wistar rats. International Journal of Livestock Production 2017, 8(2):24-27.
- Komprda T, Zelenka J, Fajmonová E, Bakaj P, Pechová P: Cholesterol content in meat of some poultry and fish species as influenced by live weight and total lipid content. Journal of agricultural and food chemistry 2003, 51(26):7692-7697. 7692. [CrossRef]
- Ullah R, Su Y, Shen Y, Li C, Xu X, Zhang J, Huang K, Rauf N, He Y, Cheng J: Postnatal feeding with high-fat diet induces obesity and precocious puberty in C57BL/6J mouse pups: a novel model of obesity and puberty. Frontiers of medicine 2017, 11(2):266-276. [CrossRef]
- Harris C, Buyken A, von Berg A, Berdel D, Lehmann I, Hoffmann B, Koletzko S, Koletzko B, Heinrich J, Standl M: Prospective associations of meat consumption during childhood with measures of body composition during adolescence: results from the GINIplus and LISAplus birth cohorts. Nutrition journal 2016, 15(1):101. [CrossRef]
- Van Hecke T, De Vrieze J, Boon N, De Vos WH, Vossen E, De Smet S: Combined Consumption of Beef-Based Cooked Mince and Sucrose Stimulates Oxidative Stress, Cardiac Hypertrophy, and Colonic Outgrowth of Desulfovibrionaceae in Rats. Molecular nutrition & food research 2019, 63(2):1800962. [CrossRef]
- Campos-Silva P, Furriel A, Costa WS, Sampaio FJ, Gregório BM: Metabolic and testicular effects of the long-term administration of different high-fat diets in adult rats. International braz j urol 2015, 41(3):569-575. [CrossRef]
- Parry SA, Hodson L: Influence of dietary macronutrients on liver fat accumulation and metabolism. Journal of Investigative Medicine 2017, 65(8):1102-1115. [CrossRef]
- “Rules and Regulations That Impact Children’s Health.”. Available online: https://www.epa.gov/children/rules-and-regulations-impact-childrens-health.
- Díaz-Rúa R, Keijer J, Palou A, van Schothorst EM, Oliver P: Long-term intake of a high-protein diet increases liver triacylglycerol deposition pathways and hepatic signs of injury in rats. The Journal of nutritional biochemistry 2017, 46:39-48. [CrossRef]
- Van Hecke T, Jakobsen LM, Vossen E, Guéraud F, De Vos F, Pierre F, Bertram HC, De Smet S: Short-term beef consumption promotes systemic oxidative stress, TMAO formation and inflammation in rats, and dietary fat content modulates these effects. Food & function 2016, 7(9):3760-3771. [CrossRef]
- Kashou AH, du Plessis SS, Agarwal A: The role of obesity in ROS generation and male infertility. In: Studies on Men’s Health and Fertility. edn.: Springer; 2012: 571-590. [CrossRef]
- Hodgson JM, Ward NC, Burke V, Beilin LJ, Puddey IB: Increased lean red meat intake does not elevate markers of oxidative stress and inflammation in humans. The Journal of nutrition 2007, 137(2):363-367. [CrossRef]
- Bronzato S, Durante A: A contemporary review of the relationship between red meat consumption and cardiovascular risk. International journal of preventive medicine 2017, 8. [CrossRef]
- Nagaoka T, Onodera H, Hayashi Y, Maekawa A: Influence of high-fat diets on the occurrence of spontaneous uterine endometrial adenocarcinomas in rats. Teratogenesis, carcinogenesis, and mutagenesis 1995, 15(4):167-177. [CrossRef]
- Heikkilä P, Kahri AI, Ehnholm C, Kovanen PT: The effect of low-and high-density lipoprotein cholesterol on steroid hormone production and ACTH-induced differentiation of rat adrenocortical cells in primary culture. Cell and tissue research 1989, 256(3):487-494. [CrossRef]
- Pelusi C, Pasquali R: The significance of low testosterone levels in obese men. Current Obesity Reports 2012, 1(4):181-190. [CrossRef]
- SCHNEIDER G, KIRSCHNER MA, BERKOWITZ R, ERTEL NH: Increased estrogen production in obese men. The Journal of Clinical Endocrinology & Metabolism 1979, 48(4):633-638. [CrossRef]
- Isidori AM, Caprio M, Strollo F, Moretti C, Frajese G, Isidori A, Fabbri A: Leptin and androgens in male obesity: evidence for leptin contribution to reduced androgen levels. The Journal of Clinical Endocrinology & Metabolism 1999, 84(10):3673-3680. [CrossRef]
- Jackie Linden. GLOBAL POULTRY TRENDS - Chicken Meat Consumption Exceeds Global Average in the Americas.the poultry Site 2014.
| Body Weight (g) | |||||
|---|---|---|---|---|---|
| Groups (n=5) |
PND 0 | PND 21 | PND 60 | PND 90 | Weight Gain |
| Control | 5.45±0.26 | 37.80±1.47 | 197.40±2.44 | 241.80±3.18 | 204.00±3.00 |
| B1 | 5.47±0.21 | 39.20±1.72 | 210.20±2.78* | 258.40±3.44** | 219.20±2.52* |
| B2 | 5.60±0.23 | 36.00±1.59 | 212.20±3.40** | 259.40±2.79** | 223.40±4.15** |
| D1 | 5.52±0.15 | 38.20±1.72 | 203.60±2.69 | 250.20±2.52 | 212.00±2.47 |
| D2 | 5.49±0.18 | 36.60±1.51 | 208.60±2.44* | 253.60±2.91* | 217.00±4.06* |
| Parameters | Groups | ||||
|---|---|---|---|---|---|
| Control | B1 | B2 | D1 | D2 | |
| Gonadosomatic index | 0.97±0.02 | 0.93±0.04 | 0.91±0.05 | 0.95±0.01 | 0.92±0.02 |
| Paired testes weight (g) | 2.35±0.03 | 2.39±0.10 | 2.35±0.12 | 2.37±0.03 | 2.33±0.04 |
| Absolute epididymal weight (g) | 1.18±0.04 | 1.07±0.03 | 1.92±0.03* | 1.13±0.08 | 1.06±0.06 |
| Relative epididymal weight (mg/g) | 4.88±0.23 | 4.14±0.12 | 5.90±0.15** | 4.50±0.31 | 3.0±0.19* |
| Longitudinal diameter of testes (mm) | 10.40±0.49 | 9.83±0.40 | 10.12±0.30 | 11.21±0.49 | 10.91±0.80 |
| Transverse diameter of testes (mm) | 6.66±0.46 | 6.30±0.46 | 7.09±0.43 | 7.40±0.40 | 6.63±0.45 |
| Seminal vesicle weight (g) | 0.93±0.03 | 0.91±0.02 | 1.5±0.03 ** | 0.64±0.02 | 0.70±0.03 * |
| Prostate weight (g) | 0.66±0.02 | 0.67±0.03 | 0.94±0.05 ** | 0.37±0.05 | 0.72±0.02* |
| Groups (n=5) |
Kidney | Heart | Liver |
|---|---|---|---|
| Control | 1.02±0.04 | 1.02±0.05 | 7.76±0.46 |
| B1 | 1.23±0.08 | 1.04±0.04 | 8.92±0.24* |
| B2 | 1.39±0.02** | 1.02±0.03 | 9.92±0.25** |
| D1 | 1.24±0.09 | 1.06±0.02 | 8.0±0.23 |
| D2 | 1.25±0.05* | 1.04±0.03 | 8.5±0.14 |
| Control | B1 | B2 | D1 | D2 | |
|---|---|---|---|---|---|
| CAT (U/mg protein) | 7.38±0.47 | 6.20±0.95 | 5.96±0.86 | 6.98±0.36 | 7.12±0.37 |
| SOD (U/mg protein) | 25.52±2.50 | 21.86±1.49 | 22.25±2.00 | 24.47±1.55 | 22.07±1.56 |
| POD (nmole) | 14.14±1.38 | 12.20±1.57 | 10.89±1.89 | 13.87±0.79 | 11.05±2.02 |
| TBARS (nM/TBAR/min/mg protein) | 16.76±1.63 | 19.16±0.95 | 17.90±0.89 | 16.79±0.77 | 17.30±0.95 |
| ROS (U/mg tissue) | 0.82±0.02 | 0.85±0.03 | 0.94±0.04* | 0.81±0.02 | 0.86±0.02 |
| HDL (mg/dL) | 60.13±2.57 | 56.44±2.63 | 53.08±2.82 | 59.09±2.50 | 58.08±2.66 |
| LDL (mg/dL) | 40.94±2.82 | 44.88±2.38 | 48.76±2.94* | 41.62±3.72 | 41.99±3.79 |
| Testosterone (nM/L) | 6.54±0.49 | 5.04±0.39 | 7.64±0.43* | 6.79±0.40 | 5.42±0.49 |
| Estradiol (pg/mL) | 7.30±0.49 | 9.60±0.74 | 10.38±0.26* | 7.44±0.40 | 7.93±1.22 |
| Progesterone (ng/mL) | 4.31±0.70 | 3.97±0.49 | 3.49±0.58 | 4.30±0.50 | 4.12±0.61 |
| LH (mU/L) | 3.39±0.8 | 3.41±0.5 | 5.95±0.7* | 3.31±0.4 | 3.85±0.9 |
| FSH (mU/L) | 34±9.5 | 36±0.2 | 38±0.4* | 34±0.1 | 36±9.0 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





