Introduction
Herbal medicines, also known as phytomedicines or botanical medicines, refer to the medicinal products of plant roots, leaves, barks, seeds, berries or flowers that can be used to treat diseases and promote health. Medicinal use of plants has a long history worldwide (Li et al., 2008). The metabolic variation in plant material due to genotypic, ecotypic, chemotypic and ontogenetic factor result in inconsistency in efficacy of herbal formulation which is one of the biggest hinderance in their wider acceptance in healthcare system. There have always been concerns about the inconsistent composition of herbal medicines and occasional cases of intoxication by adulterants and/or toxic components. Quality control of herbal medicines aims to ensure their consistency, safety and efficacy. Hence herbal products need to be standardized like pharmaceuticals to become in main-stream evidence-based drugs (Hussain et al., 2010). The standardization of herbal formulation needs a set of pharmacognostic and analytical activities. For standardization of herbal medicines, single chemical entities (marker compounds) may be used as standards in high performance liquid chromatography (HPLC) analysis which may provide additional information in the form of “chromatographic fingerprints. These marker compounds may be used to help identify herbal materials, set specifications for raw materials, standardize botanical preparations (Lazarowych and Peko, 1998; Kim et al., 2010). The markers, characteristics compounds found in the ingredients can be used as analytical markers to develop various analytical methods for herbal products standardization (Li et al., 2008). The marker-based analytical methods can also be used to understand chemical kinetics and predict shelf-life of herbal products (Khalid et al., 2011). In establishing the method for quality control, one of the key tasks is selecting appropriate monitoring markers. Since plant constituents are the major contributors of herbal functions, they can be used as the markers to monitor the function-related quality of the herbs. In general, a good marker should fulfill the criteria of specific, relatively high content with bioactivities correlated to the herbal function (Liang et al., 2009). A folklore polyherbal medicine comprising five ingredients (seeds of Tribulus terrestris, Carthamus tinctorius, Cucumis melo and Punica granatum, and seed-less dried fruit of Vitis vinifera) is being extensively used and promoted through social media in Pakistan and India for curing gout (Shaukat et al., 2020). Despite numerous pharmacological properties of ingredients and remarkable popularity of the selected polyherbal anti-gout remedy, literature contains few reports regarding antigout potential of this traditional herbal remedy (Shaukat et al., 2020; Shaukat et al., 2021). The polyherbal remedy was standardized using set of markers found in all the ingredients of the remedy (Shaukat et al., 2021). The HPLC chromatogram of the anti-gout remedy indicated a very distinct peak at 15 min (Shaukat et al., 2021). The compound exhibiting this peak if identified can be used as marker. Therefore, the compound responsible for this peak is selected for separation and characterization of a marker compound. The new developed marker compound can be used as analytical marker to standardize polyherbal anti-gout remedy
Preparation of herbal remedy
The ingredients of polyherbal anti-gout remedy includes (seeds of Tribulus terrestris, Carthamus tinctorius, Cucumis melo and Punica granatum, and seed-less dried fruit of Vitis vinifera). The traditional polyherbal remedy was prepared using method as mentioned in literature(Shaukat et al., 2020).
Chemicals and solvents
Solvents and chemicals of analytical/HPLC grade used included acetonitrile, methanol, tetrahydrofuran, ethyl acetate, acetic acid, sodium acetate (BDH Laboratory, England), TLC chromoplates (silica gel 60F254), syringe and membrane filters (0.45 µm). In-house prepared double distilled water was used, where required.
Instruments
Liquid chromatography system (Agilent Technologies, 1200 series, Germany) equipped with isocratic pump (G1310A), auto-sampler (G1329A), thermostatically controlled column oven (G1316A) and diode array detector (G1315B) were used in the current study. Other equipment used included pH meter (WTW series, Ino lab), ultrasonicator (Memmert, Germany). Other instruments include EIMS (JEOL 600H-1), NMR 600 MHz (AscendTM 600, Bruker’s Avance Neo Technology).
Isolation of analytical marker compound
The herbal medicine quantified for markers showed a distinct peak at retention time 16±0.5 min (Shaukat et al., 2021). The sample of herbal medicine was run successively and the compound peak observed at aforementioned retention time was collected as HPLC column eluent. This procedure required large number of samples run so as to collect quantity for characterization of the compound. The solvent was evaporated at temperature 40⁰C and the compound was characterized for RP-HPLC, TLC, UV-visible and FTIR profiling. Later the compound was also characterized for EIMS and NMR analysis.
Characterization of isolated compound
Purity of the compound was checked by reversed phase HPLC. Sample (20 µL) elution was done by mobile phase (isocratic) comprising aqueous acetate buffer having pH 3.6: methanol: acetonitrile: tetrahydrofuran (65:20:10:5) at 0.8 mL/min flow rate through HPLC column (Agilent TC-C18; 250 × 4.6 mm), maintained at 35ºC with photodiode array detector operating at detection beam of 330 nm and reference beam of 360 nm. The mass of the compound was determined by EIMS (JEOL 600H-1), using direct probe inlet (positive mode). The 13C NMR and 1H NMR spectra were used for structure elucidation.
Results And Discussion
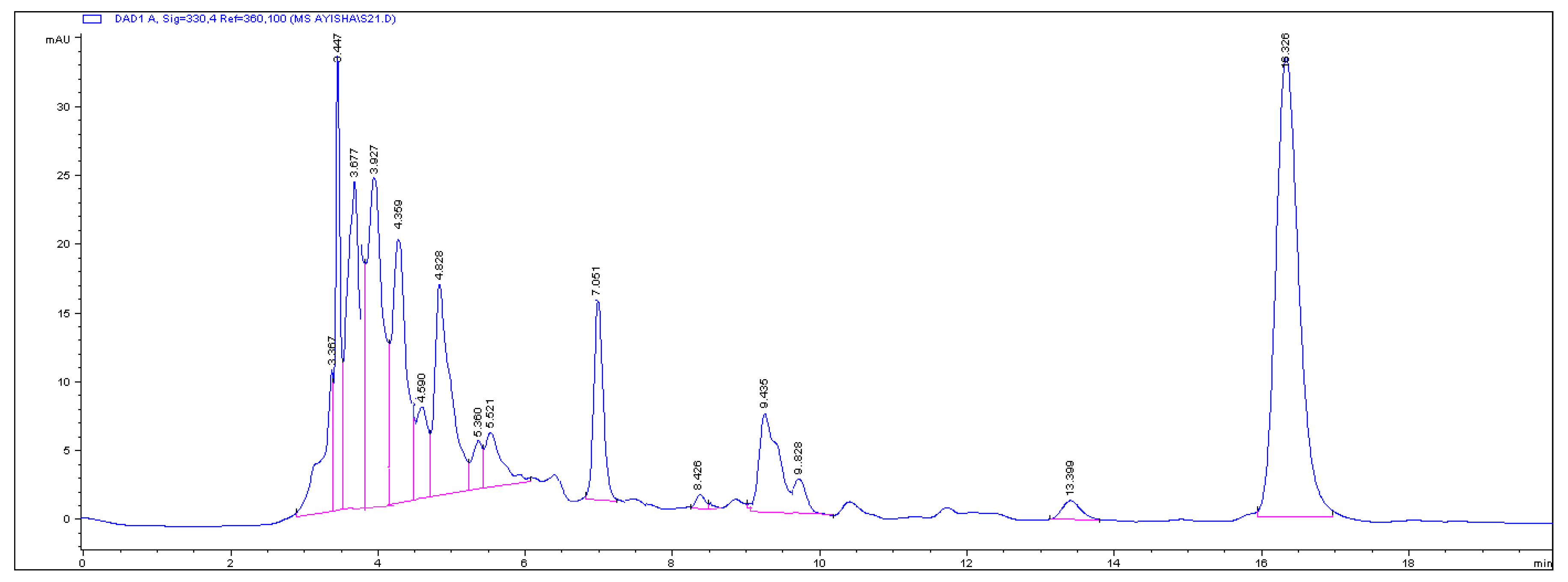
The chromatographic profile of polyherbal anti-gout remedy shown in
Figure 1 indicated a distinct peak at 15 min which corresponded to a highly non-polar compound of the sample as it retained in the column for a longer time. Therefore, it was selected for isolation and characterization of a characteristic compound that is useable to the quality control of the remedy.
The fraction corresponding to this peak was collected using HPLC and the resulting elute was fractionated by partitioning using ethyl acetate. The TLC of ethyl acetate fraction indicated the presence of a blue florescent spot observed at 366 nm which was not found in the aqueous fraction (
Figure 2).
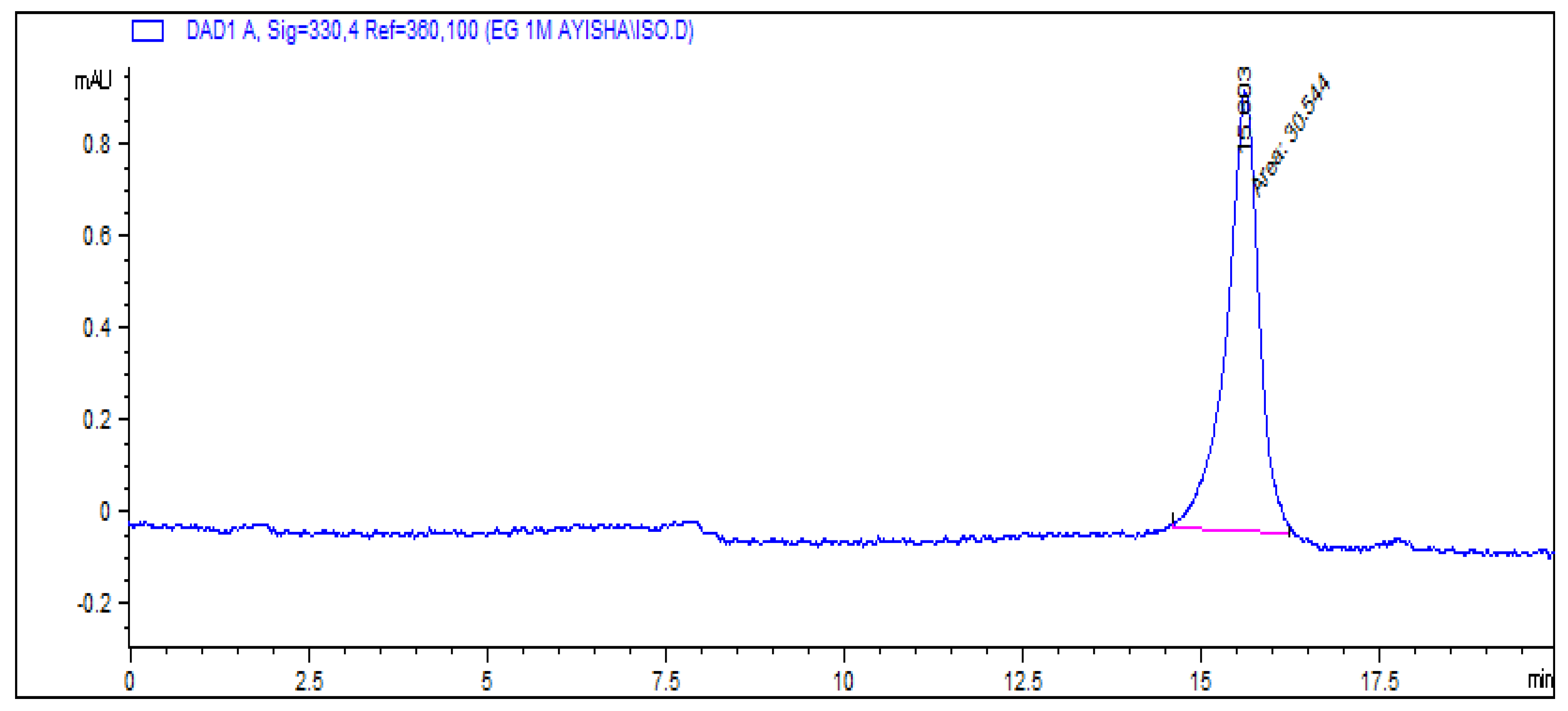
So, the ethyl acetate fraction was dried in vacuo and the residue was reconstituted with the mobile phase and analyzed using the chromatographic conditions mentioned in literature, which resulted in a single and pure peak (
Figure 3). Then, the amount of the compound was increased to identify it and this compound was termed as C-15.
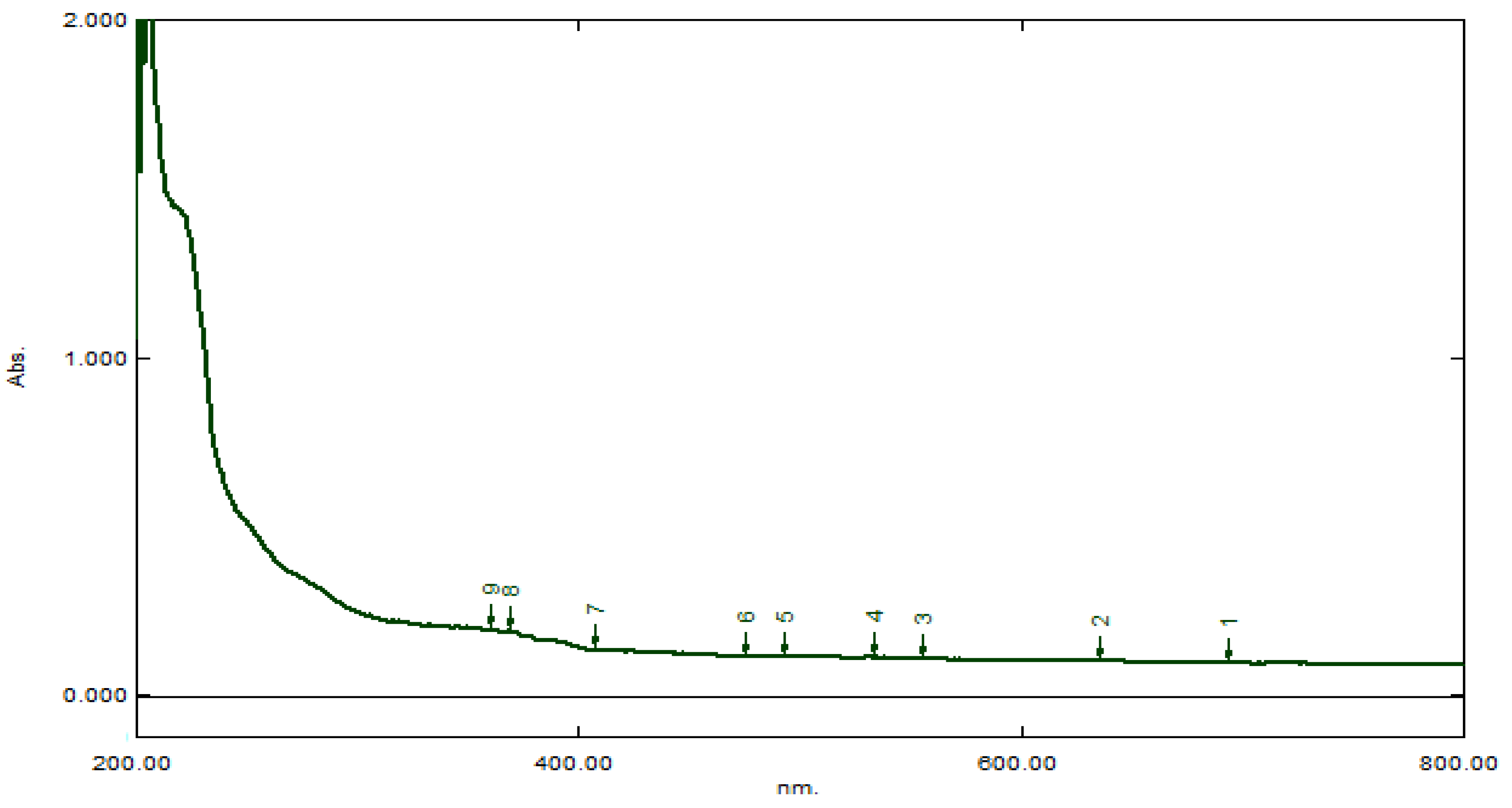
The isolated compound was gummy in nature and dark brown in color. It was soluble both in chloroform and ethyl acetate, whereas insoluble in water and sparingly soluble in methanol. This solubility profile confirmed the non-polar nature of the compound. In UV region, the compound indicated maximum absorbance at 215 and 380 nm (
Figure 4).
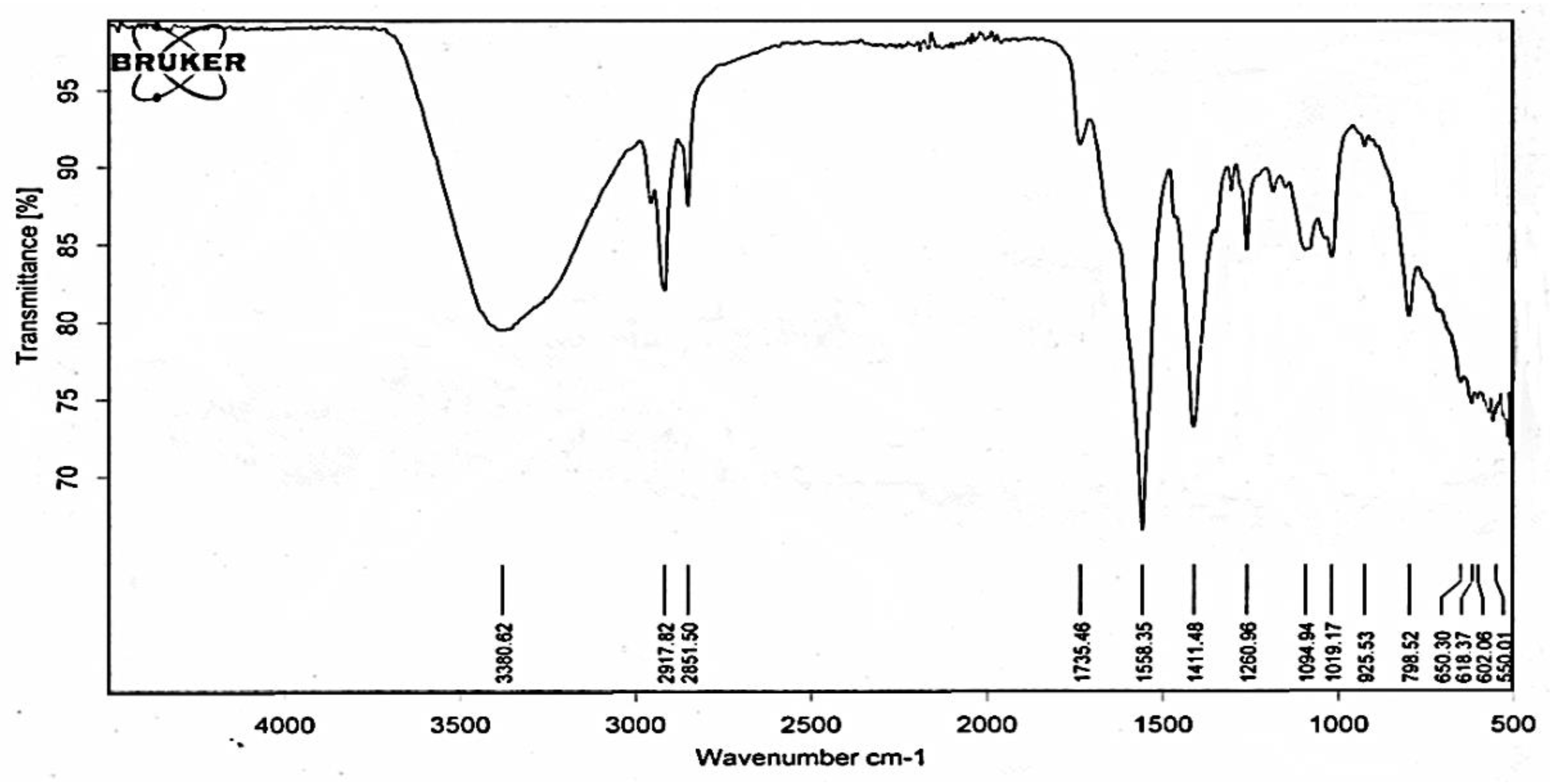
The absorbance at 215 nm indicated that the compound had pentacyclic triterpene moiety, whereas the absorbance at 380 nm indicated the presence of unsaturation and lone pair having atoms/substituents. ATR-IR spectrum (
Figure 5) indicated bands (ν cm
-1) at 330.62 (-OH stretching), 2917 (C-H stretching of CH
2), 2851 (CH stretching of CH
3), 1735, 1260 (ester carbonyl), 1558 (C=CH stretching), 1411 (deformation vibration of CH bond of the CH
2, planar scissoring). The IR spectrum indicated the compound was ester of pentacyclic triterpene.
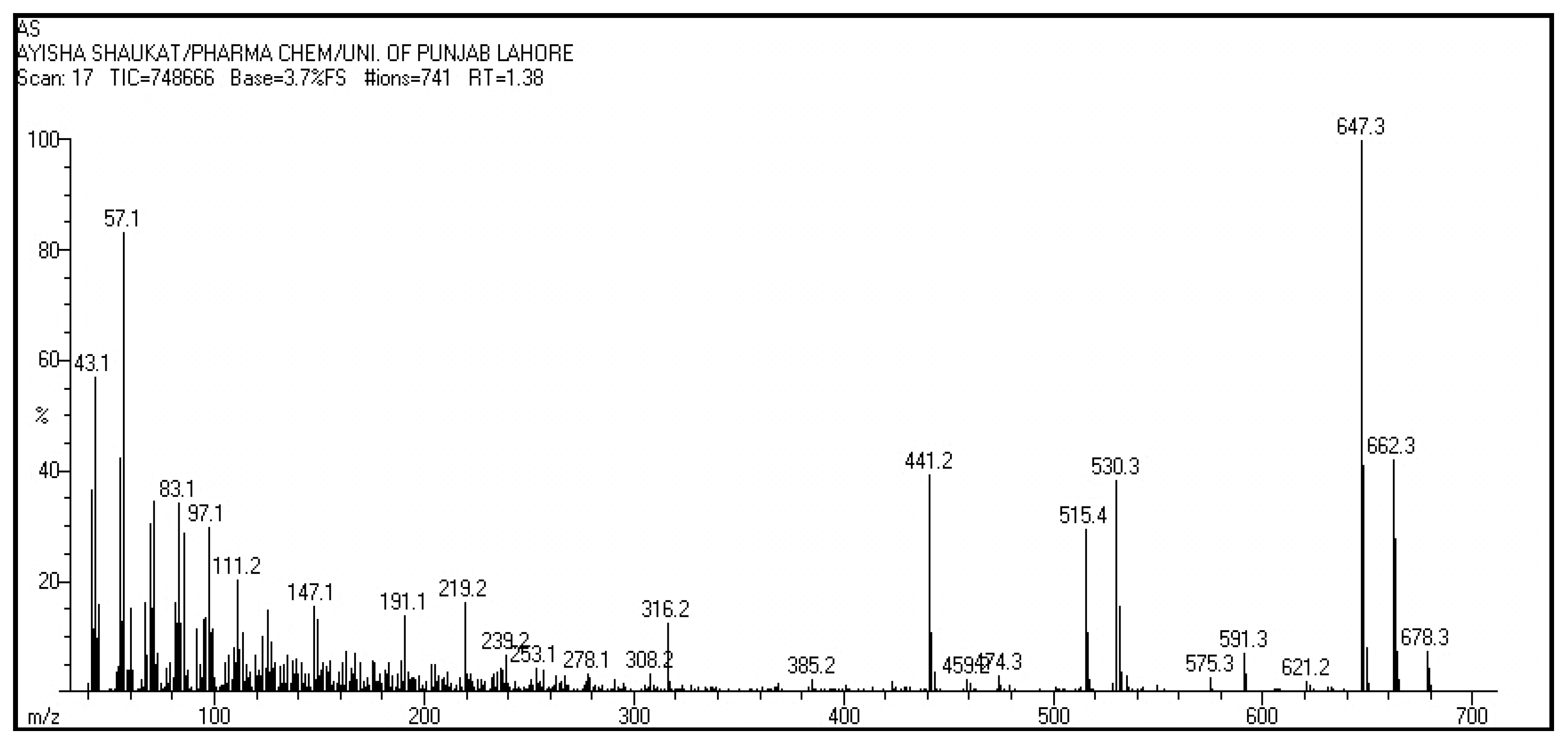
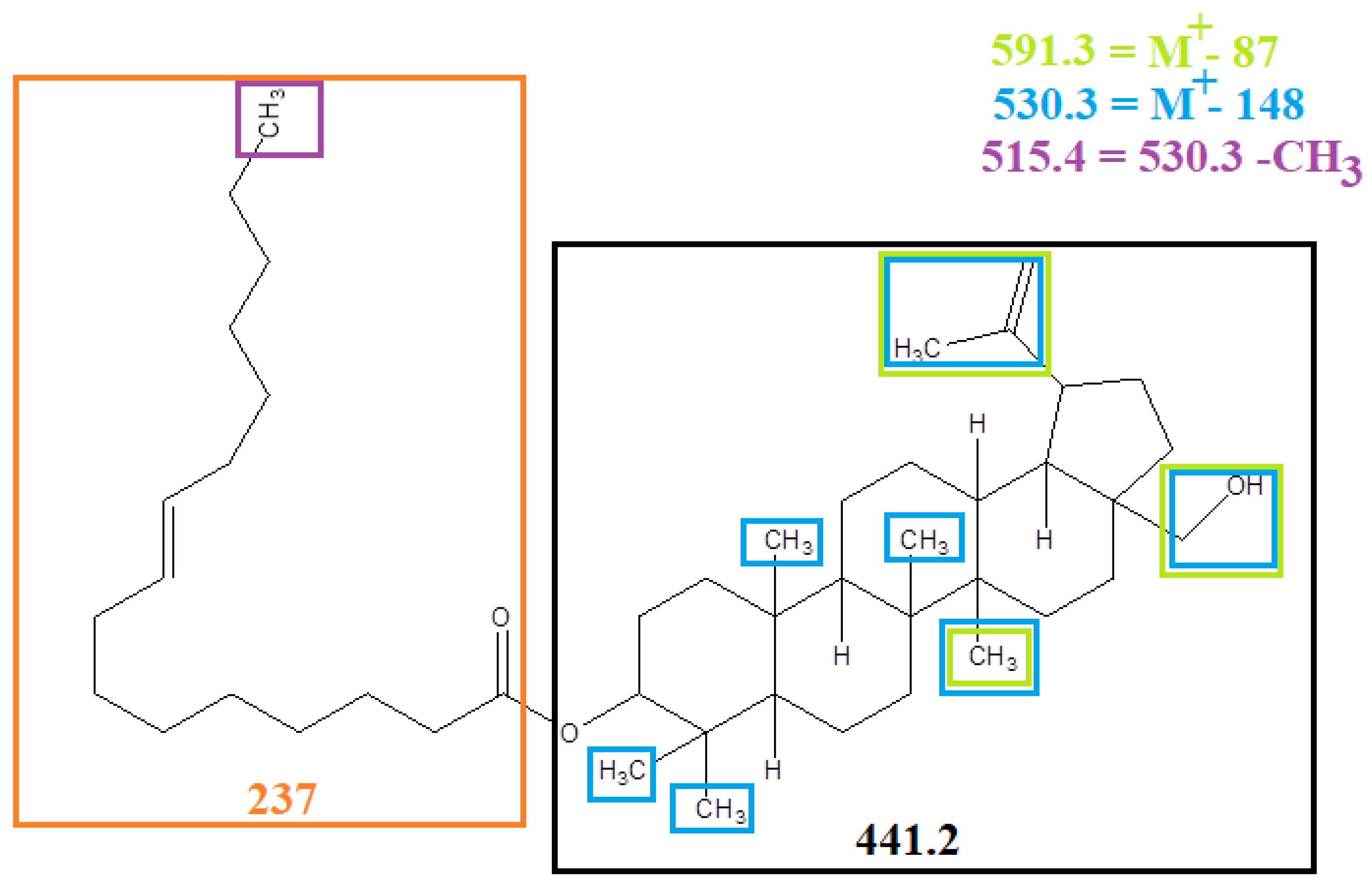
The comparison of spectra indicated that the compound may be ester of betulin and fatty acid. Liebermann Burchard reagent test also gave positive test which confirmed the presence of pentacyclic terpene. EI-MS (Positive mode) gave peak at m/z (% abundance) as 678.3 (10), 662.4 (40), 647.3 (100), 591.3 (10), 530.3 (40), 515.4 (37), 441.2 (40) as shown in
Figure 6. The fragmentation pattern is shown in
Figure 7. In EI-MS, molecular ion peak corresponded to molecular formula (C
46 H
76O
3) having m/z: 678.3 [M
+]; and peak at 441.2 corresponded to molecular formula C
30H
46O
2. These results clearly indicated that peak at 441.2 was of betulin (less one hydrogen atom due to ester linkage at OH at carbon 3). This fragment indicated the loss of a molecular part of mass 237 which confirmed that the fatty acid was of 16 carbon. The 16-carbon fatty acid was palmitic acid which suggested that the fragment was palmitoyl (C
16H
31O) molecular mass of 239. However, the fragment loss producing has molecular mass of 441.3 was of 237. It indicated that the fatty acid was mono-unsaturated that could be palmitoleic acid.
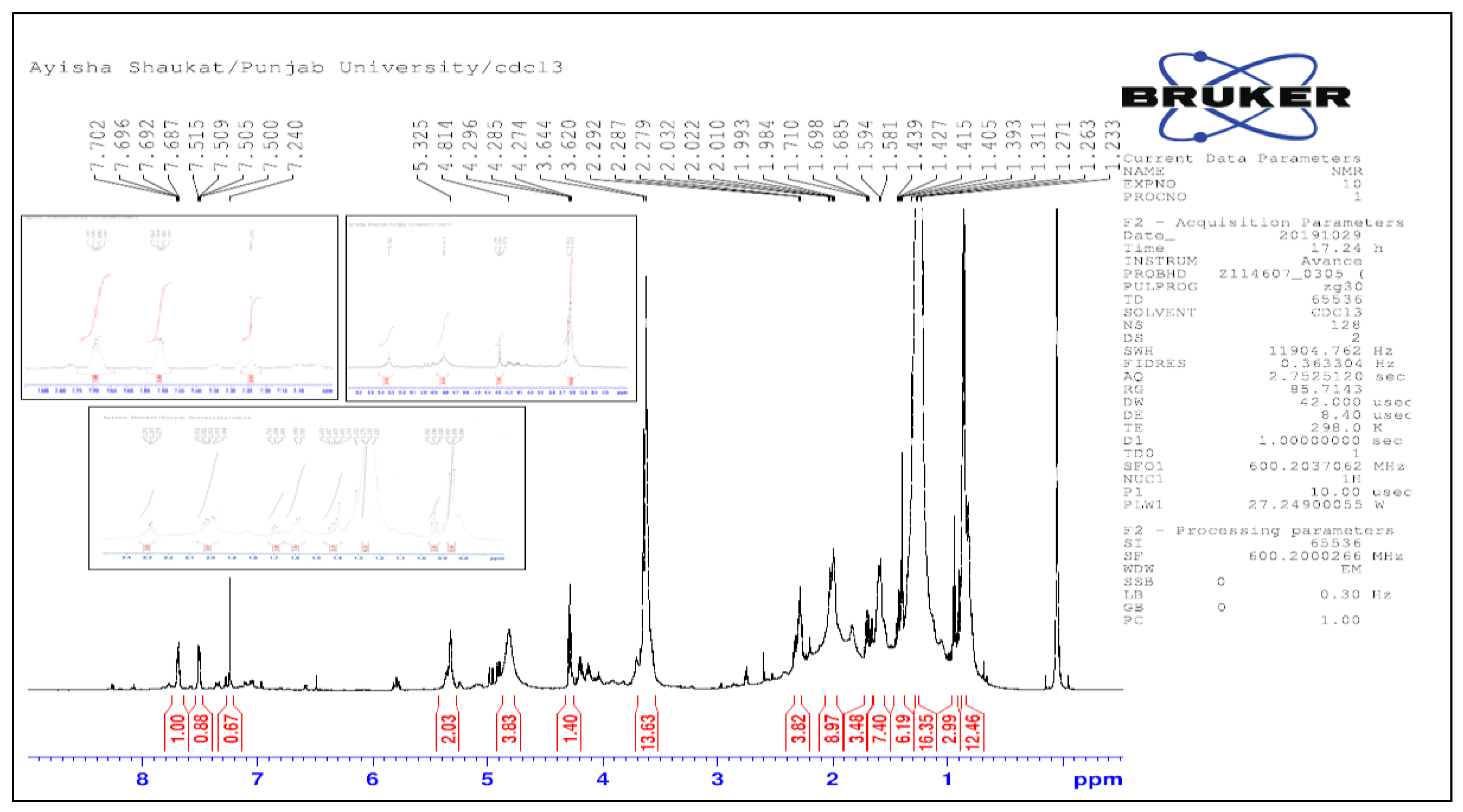
1H NMR (CDCl
3, 600 MHz) 0.87 (m 3H, 5H), 0.89 (CH
3, H-16
/), 0.9 (s, 3H), 0.99 (9s, 6H), 1.0 (s, 3H), 1.03-1.95 (m 10H, CH
2), 1.28 (br. S, H-15
/ to H-4
/, 24 H), 1.3(m 1H, CH), 1.45 (s, 3H), 1.60 (m 1H, CH), 1.65 (m, 1H, CH), 1.70 (s, 3H, C
3OH
3), 1.75 (t, H-3
/), 2.06 (s 3H, CH
3CO), 2.088 (s, 3H, CH
3CO), 2.3 (t, H-2
/); 2.47 (td, 1H, 19-H), 3.87-4.29 (d. 2H each, 28-H), 4.5 (q 1H, 3-H), 4.62 and 4.72 (s, 2H each, 29-H) (
Figure 8).
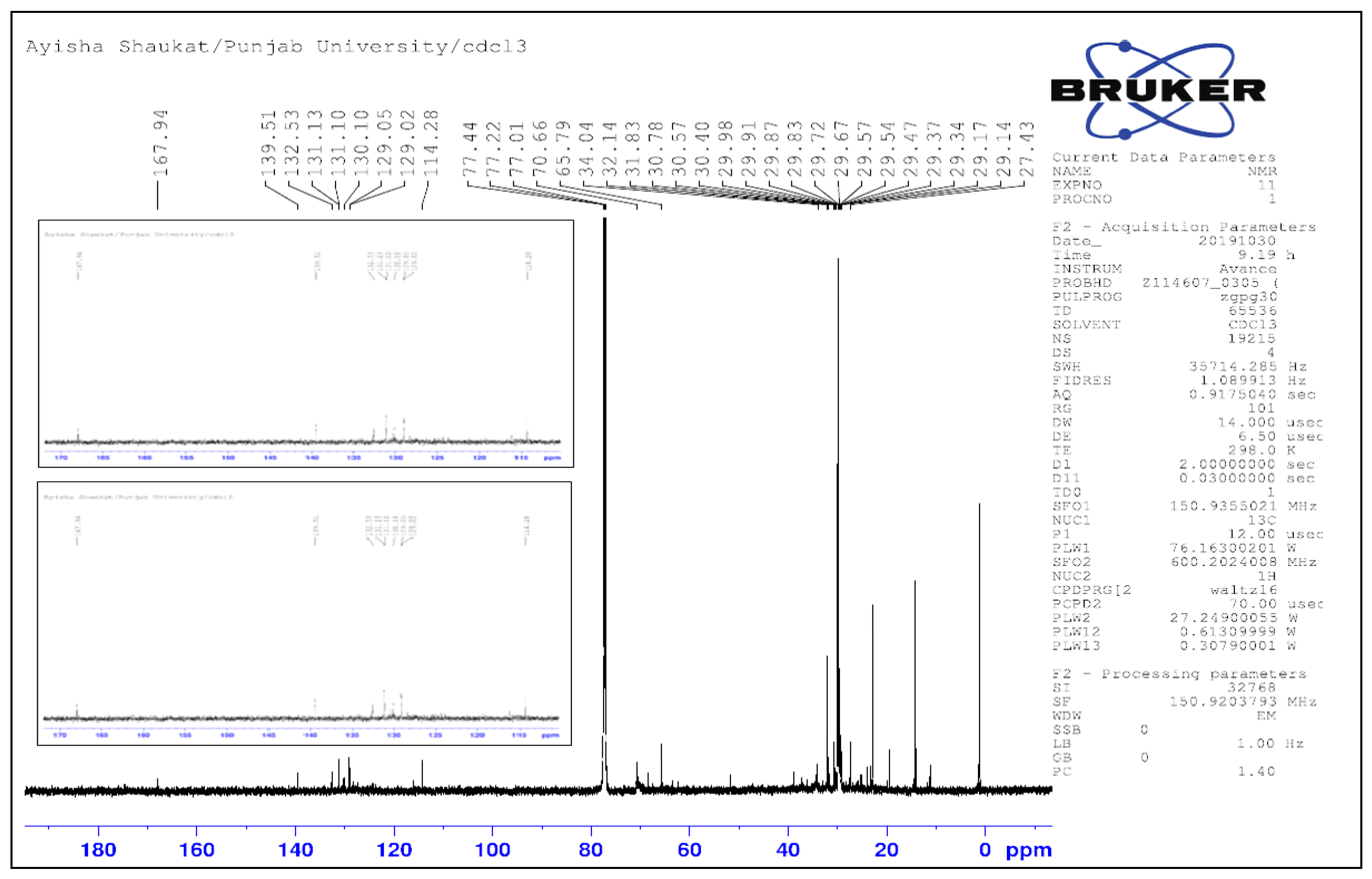
13C NMR (CDCl
3, 600 MHz) 173.5 confirmed the ester carbon (1-C
/), CH
3, 3CH
2, CH and 7C which are characteristic to pentacyclic triterpene (
Figure 9). The presence of 7 methyl groups in which singlets (0.86, 0.87, 0.9, 0.8, 0.92, 0.93) and a doublet at 0.82. Further typical reasons at 152 (C-9), 117.7 (C-11), 123.5 (C-12) and 141.67 (C-13) confirmed the pentacyclic triterpene (an ester of betulin and palmitoleic acid).
Conclusion
The isolated compound was characterized as ester of betulin and palmitoleic acid. The compound (C-15) after further NMR studies may be used as analytical marker to develop HPLC method for standardization of polyherbal medicine.
Acknowledgement
We are thankful to Punjab University College of Pharmacy University of the Punjab Lahore for provision of necessary research facilities during the study.
Conflict of Interest
The authors declare no conflict of interest.
References
- Hussain, K.; Ismail, Z.; Sadikun, A.; Ibrahim, P. Standardization and in vivo antioxidant activity of ethanol extracts of fruit and leaf of Piper sarmentosum. Planta Med. 2010, 76, 418–425. [Google Scholar] [CrossRef] [PubMed]
- Khalid, H.; Zhari, I.; Amirin, S.; Pazilah, I. Accelerated stability and chemical kinetics of ethanol extracts of fruit of Piper sarmentosum using high performance liquid chromatography. IJPR 2011, 10, 403. [Google Scholar] [PubMed]
- Kim, H.J.; Jee, E.H.; Ahn, K.S.; Choi, H.S.; Jang, Y.P. Identification of marker compounds in herbal drugs on TLC with DART-MS. Arch. Pharm. Res. 2010, 33, 1355–1359. [Google Scholar] [CrossRef] [PubMed]
- Lazarowych, N.J.; Pekos, P. Use of fingerprinting and marker compounds for identification and standardization of botanical drugs: strategies for applying pharmaceutical HPLC analysis to herbal products. Drug Inf. J. 1998, 32, 497–512. [Google Scholar] [CrossRef]
- Li, S.; Han, Q.; Qiao, C.; Song, J.; Lung Cheng, C.; Xu, H. Chemical markers for the quality control of herbal medicines: an overview. Chin. Med. 2008, 3, 1–16. [Google Scholar] [CrossRef] [PubMed]
- Liang, X.M.; Jin, Y.; Wang, Y.P.; Jin, G.W.; Fu, Q.; Xiao, Y.S. Qualitative and quantitative analysis in quality control of traditional Chinese medicines. J. Chromatogr. A 2009, 1216, 2033–2044. [Google Scholar] [CrossRef] [PubMed]
- Shaukat, A.; Hussain, K.; Bukhari, N.I.; Shehzadi, N.; Naheed, S.; Saghir, F.; Iftikhar, S.; Javed, O. In vitro anti-gout and anti-inflammatory activity of traditionally used polyherbal anti-gout remedy. Int. J. Biosci. 2020, 16, 327–335. [Google Scholar]
- Shaukat, A.; Hussain, K.; Bukhari, N.I.; Shehzadi, N.; Naheed, S.; Siddique, S.; Saghir, F.; Iftikhar, S. Evidence of anti-gout activity of a standardized traditional herbal medicine”. JAPS 2021, 31, 1836–1847. [Google Scholar]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).