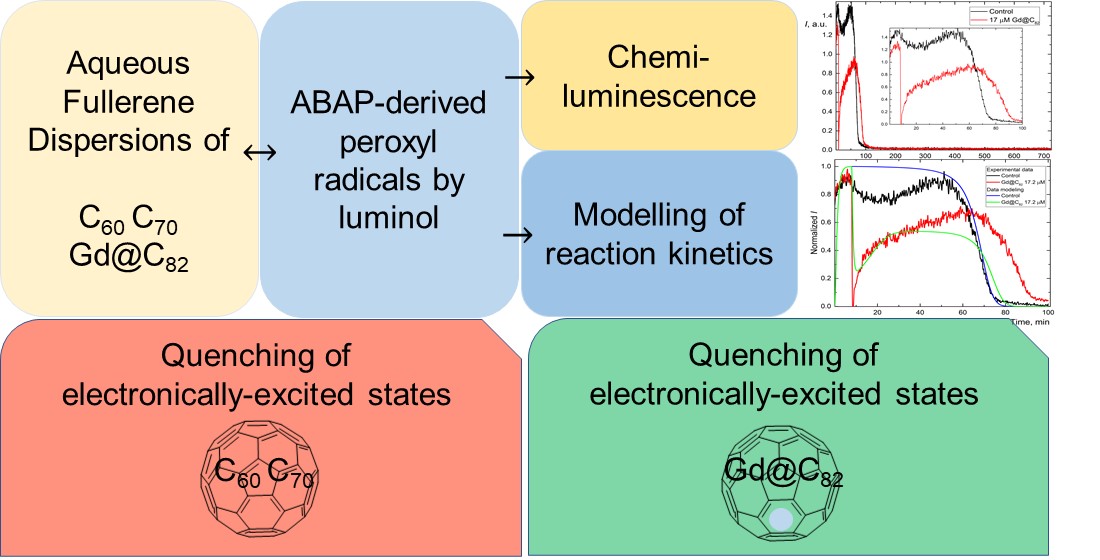
The antioxidant properties of unmodified aqueous fullerene dispersions (AFD) of C60, C70, Gd@C82 (in μM concentration range) were studied in the model of generation of organic radicals by 2,2’-azobis(2-amidinopropane) dihydrochloride (ABAP) and luminol. Purification protocols for AFDs are proposed. Based on chemiluminescence (CL) measurements, the concentration-dependent of the CL signal for any AFDs is revealed. Suppression of CL signals is up to 5 times better than Mexidol® antioxidant. The concentration of half-suppression increased in row C60<C70<Gd@C82. We further demonstrated mathematical modeling of the long-term kinetics data for C60, C70, Gd@C82 allowed fitting of CL curves. Kinetic schemes are proposed, and the constants of each reaction are estimated (~n×μM–1min–1). C60, C70, and Gd@C82 exhibit a quenching mechanism that is not an antioxidant effect. For C60 and C70, similar behavior is shown that is not quantitatively different in kinetic modeling. On the contrary, Gd@C82 has a double action: (1) quenching and (2) actual antioxidant action. Estimating quenching constants for all of the AFD types showed the same magnitude. Moreover, the antioxidant activity in Gd@C82 is 300 times greater than in the case of C60 and C70.