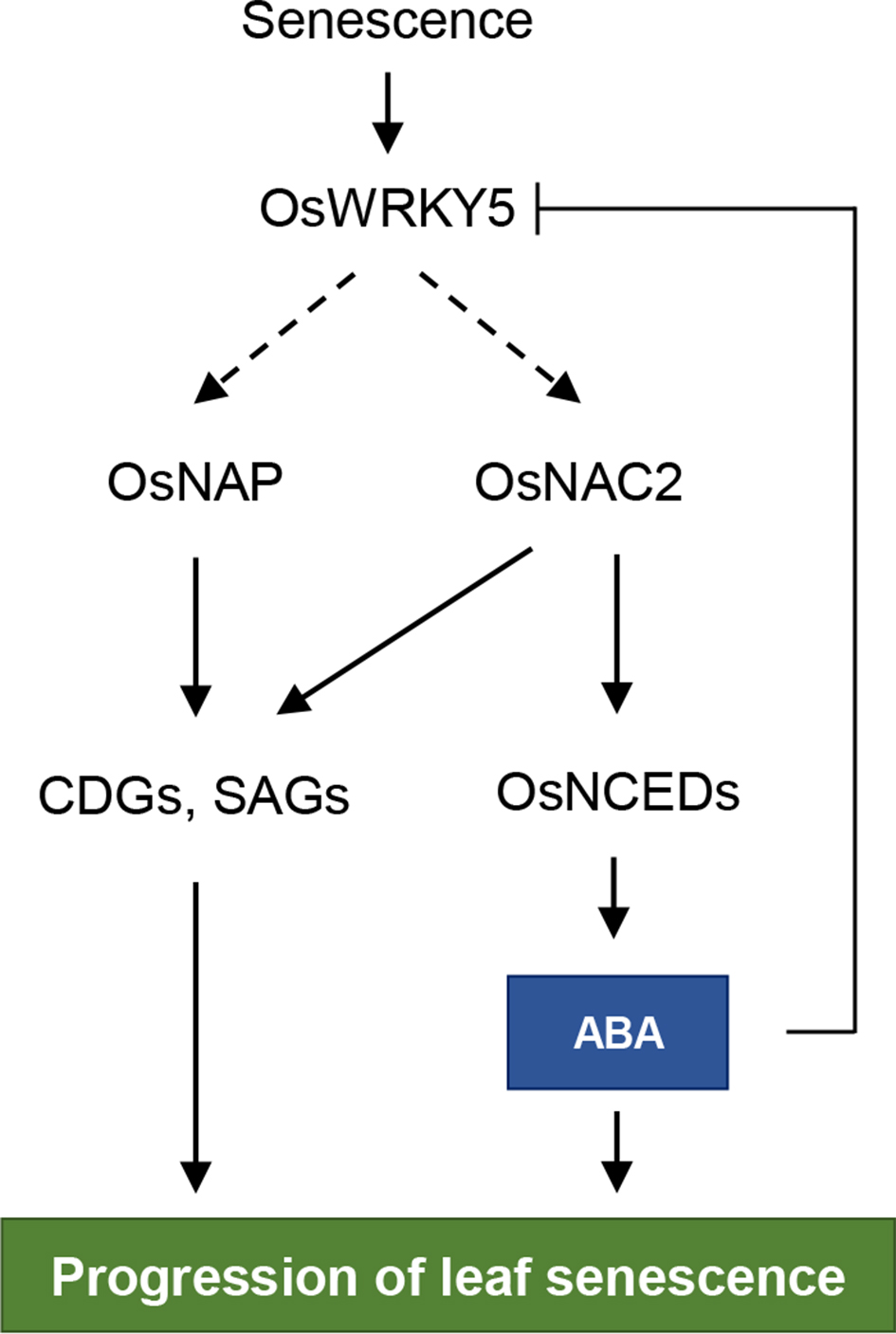
The onset of leaf senescence is triggered by external cues and internal factors such as phytohormones and signaling pathways involving transcription factors (TFs). Abscisic acid (ABA) strongly induces senescence and endogenous ABA levels are finely tuned by many senescence-associated TFs. Here, we report on the regulatory function of the senescence-induced TF OsWRKY5 TF in rice (Oryza sativa). OsWRKY5 expression was rapidly upregulated in senescing leaves, especially in yellowing sectors initiated by aging or dark treatment. A T-DNA insertion activation-tagged OsWRKY5-overexpressing mutant (termed oswrky5-D) promoted leaf senescence under natural and dark-induced senescence (DIS) conditions. By contrast, a T-DNA insertion oswrky5-knockdown mutant (termed oswrky5) retained leaf greenness during DIS. Reverse-transcription quantitative PCR (RT-qPCR) showed that OsWRKY5 upregulates the expression of genes controlling chlorophyll degradation and leaf senescence. Furthermore, RT-qPCR and yeast one-hybrid analysis demonstrated that OsWRKY5 indirectly upregulates the expression of senescence-associated NAC genes including OsNAP and OsNAC2. Precocious leaf yellowing in the oswrky5-D mutant might be caused by elevated endogenous ABA concentrations resulting from upregulated expression of ABA biosynthesis genes OsNCED3, OsNCED4, and OsNCED5, indicating that OsWRKY is a positive regulator of ABA biosynthesis during leaf senescence. Furthermore, OsWRKY5 expression was significantly suppressed by ABA treatment, indicating negative feedback regulation of OsWRKY5 expression by ABA. OsWRKY5 is a positive regulator of leaf senescence that upregulates senescence-induced NAC genes leading to expression of ABA biosynthesis and chlorophyll degradation genes.