1. Introduction
Intense industrial production and agricultural activities have led to widespread cadmium contamination of agricultural soils [
1,
2]. Cadmium ions, emanating from industrial emissions, are dispersed through airborne particles that ultimately settle in the soil [
3]. Furthermore, in regions where water is scarce, cadmium-laden industrial wastewater is sometimes utilized for irrigation [
4], frequently contaminating farmland in proximity to industrial areas [
5]. Additionally, impurities containing cadmium are often present in pesticides and fertilizers. Their extensive application can result in the accumulation of cadmium in the soil, thereby polluting farmlands [
5]. Such contamination poses significant health risks to humans; indeed, the United States Environmental Protection Agency (USEPA) has classified cadmium as a "Group B1 probable human carcinogen" [
6]. This classification underscores the urgency of addressing heavy metal contamination in agricultural soils, which has emerged as a critical environmental challenge.
Cadmium contamination affects both acidic and alkaline soils. In acidic environments, cadmium predominantly occurs in its free ionic form [
7]. Here, passivating agents such as sepiolite and diatomite can immobilize cadmium effectively through adsorption and precipitation processes [
8,
9]. These passivators also contribute to an increase in soil pH, thereby precipitating cadmium, which ultimately reduces the cadmium levels in the soil [
10]. However, in alkaline soils already contaminated with cadmium, the metal often forms unstable precipitates like cadmium carbonate (CdCO
3) [
11,
12], which present significant obstacles to passivation. In contrast to acidic conditions, although passivators in alkaline soils can adsorb free cadmium ions or assist in co-precipitation, they struggle to stabilize sub-stable precipitates that can readily re-mobilize with fluctuations in external pH levels. This reactivity poses a formidable challenge in treating contaminated alkaline soils to transform sub-stable cadmium compounds into stable forms. In addition, most passivators are inherently alkaline and can be introduced to acidic soils without adverse effects [
13], but their use in alkaline soils often requires larger quantities to achieve effective passivation. Such excessive use raises the risk of soil compaction [
14], which can impede soil reuse. Therefore, the selection of appropriate passivators tailored for alkaline soils is a critical step towards successful remediation.
Passivators employed to treat cadmium contamination in soils are commonly used the clay minerals, organic materials, phosphorus-containing substances and so on [
15,
16]. Clay minerals, known for adsorption and ion exchange capabilities, effectively reduce cadmium content in soil [
17]. Organic materials, either form insoluble metal-organic complexes with cadmium ions or enhance adsorption capacities through ion exchange [
18]. For phosphorus-containing substances, they are adept at precipitating cadmium as phosphates in addition to adsorbing cadmium ions directly [
19,
20,
21]. Among them, hydroxyapatite, notable for its high specific surface area and formidable adsorption capacity for heavy metals, has emerged as a material of significant interest in recent research [
22,
23,
24]. In addition to these passivators, organic fertilizers, which are abundant in organic matter and nutrients, contribute to the improvement of soil physical properties [
25,
26,
27]. They enhance aggregate stability and contribute to a decrease in soil bulk density [
28]. Furthermore, organic fertilizers play a regulatory role in soil nutrient content and pH levels to counterbalance excessive soil alkalinity [
29].
Accordingly, the hydroxylapatite and organic fertilizer combination that we developed as used as the passivator to remediate the alkaline cadmium contaminated soil. The aim of this study was to (1) investigate the adsorption behaviors and mechanisms of hydroxylapatite and organic fertilizer combination (HO), single hydroxyapatite (HA), single organic fertilizer (OF), sepiolite (SP), and diatomite (DE) in the aqueous phase, (2) to assess the influence of these passivators on both the pH levels and aggregate properties of cadmium-contaminated soil, (3) to elucidate the passivation effects and underlying mechanisms of these passivators on cadmium contaminated soil.
2. Materials and Methods
2.1. Soil and Passivators
Soil was collected from heavy metal-polluted farmland in in Anxin County, Hebei Province, China. Cadmium contamination sites arise from intense industrial and agricultural activities. After air-drying, the soil was ground through a 2-mm sieve nylon screen. Hydroxylapatite, organic fertilizer, sepiolite, and diatomite derived from Lingshou, Hebei province of China. The basic physicochemical properties of soil and passivators were list in the
Table 1.
2.2. Kinetic and Isotherm Experiments of Aqueous-Phase Passivators Adsorption
100 mg passivators and 40 mL of 80 mg/L cadmium chloride were added into 50 mL plastic centrifuge tubes. Subsequently, the sample were sealed and shaken at 160 rpm under 25 ± 3℃. The adsorption solution was sampled at different contact time intervals (10–1440 min). After being centrifuged at 4000 rpm for 15 min and filtered through a 0.45 μm microporous membrane. The supernatant was determined for the Cd2+ concentration using inductively coupled plasma mass spectrometry (ICP-MS). In addition, adsorption thermodynamic experiment was performed. 100 mg passivators were mixed with 40 mL of cadmium chloride solution with concentration ranging between 5 and 80 mg/L in a 50 mL centrifuge tube. Similarly, samples were sealed and shaken for 24 h at 25 ± 3℃ and 160 rpm. After being centrifuged at 4000 rpm for 15 min and filtered through a 0.45 μm filter, Cd2+ concentration in supernatant was determined.
The adsorption kinetic results were fitted with pseudo-first-order (Eq. 1), pseudo-second-order equation (Eq. 2). The kinetic model equations are as follow:
Where Qe (mg/g) and Qt (mg/g) represent the Cd uptake capacity at time (min) and after reaching equilibrium, respectively; k1 and k2 are the rate constants of two models.
The isotherm experiment results were fitted using the Freundlich model (Eq. 3) and Langmuir model (Eq. 4):
2.3. Passivation Experiment
HO, HA, OF, SP and DE were used as passivators of Cd2+ in contaminated soil. The amount of added passivators was 0.5%, 1%, 3%, 5% of the soil mass. Added these passivators to a basin containing 20 g of air-dried soil samples and mixed them well was used for each test. Deionized water was added adjust the moisture content of soil up to 25%. The soil treatment without passivator was set as the blank control. The treated soil sample was placed in a constant temperature 25 ± 3℃. Samples was taken out on 7 day and frozen drought in a freeze drier for 48 h. The frozen drought samples were then ground to pass a 0.149 mm and 2 mm nylon sieve respectively for the further experiment and analysis.
2.4. Leachability of Cd
The Cd leachability of soil samples was determined by the toxicity characteristic leaching procedure (TCLP), DTPA-CaCl2-TEA (DTPA), and solubility bioavailability research consortium (SBRC). The Cd2+ concentration was determined by ICP-MS. The results of the three leaching procedures correspondingly demonstrated the leaching toxicity, availability, and bioaccessibility of the four Cd2+ exist in the soil.
The preparation of chemical extracts involved three distinct methods. For the TCLP, the extract was prepared by diluting with glacial acetic acid and deionized water, followed by a pH adjustment to 2.88 ± 0.05 using 1 M HNO3 and 1 M NaOH. In this process, 2 g of soil sample was combined with 40 mL of the TCLP extract in a plastic centrifuge tube, shaken at 180 r/min for 18 hours, and subsequently filtered through a 0.45 µm microporous membrane for collection. The DTPA extract consisted of 0.1 M triethanolamine (TEA), 0.01 M CaCl2, and 0.005 M diethylenetriaminepentaacetic acid (DTPA), with its pH adjusted to 7.3 ± 0.2 using a 1:1 (V:V) HCl solution. For the DTPA extraction, 1 g of soil sample and 25 mL of extract were added to a plastic centrifuge tube, oscillated at 180 r/min for 2 hours at 25 ± 1℃, and filtered through a 0.45 µm microporous membrane. Lastly, the SBRC extract, which emulates gastric juice, was prepared with a 0.4 M glycine solution and pH adjusted to 1.5 ± 0.05 using 1:1 (V:V) HCl. The SBRC extraction involved adding 1.0 g of soil sample to 100 mL of the extract in a plastic centrifuge tube, shaking at 40 r/min in a 37 ± 1℃ water bath for 1 hour, and filtering through a 0.45 µm microporous membrane.
3. Results
3.1. Adsorption Capacity of Passivators
The influence varying initial Cd
2+ concentrations on the adsorption efficiency of the five passivators was shown in the
Figure 1a. The increased initial Cd
2+ concentration generally led to enhanced adsorption capacities across all passivators, with HO and HA exhibiting the better performance. Conversely, the adsorption capacities for the remaining three passivators began to stabilize at an initial concentration around 20 mg/L, indicating a plateau in adsorption capacity.
To further comprehend the adsorption equilibrium under different conditions, both Langmuir and Freundlich adsorption isotherms were evaluated (
Figure 1b–1c), with the deriving parameters presented in
Table 1. The analysis inferred that the Langmuir model, which suggests monolayer adsorption on the adsorbent's surface, better aligns with the experimental data compared to the Freundlich model.
Figure 1d illustrates the impact of contact time on the adsorption of Cd
2+ ions by five distinct passivators, revealing a two-phase process characterized by a rapid initial stage followed by a slower one. During the adsorption process, HO and OF achieved equilibrium at approximately 720 min, with the equilibrium adsorption capacities recorded at 26.46 mg/g and 13.92 mg/g, respectively. In contrast, DE and SP did not reach equilibrium within the experimental timeframe. HA, demonstrated a remarkable adsorption capacity, approaching equilibrium at 1080 minutes with a capacity of 30 mg/g. Pseudo-first-order and pseudo-second-order kinetic models was used to study the adsorption kinetics of passivators, with the corresponding parameters detailed in
Table 2 and the fit curves depicted in
Figure 1e–1f. The analysis indicated that the pseudo-second-order model provided a superior fit for the adsorption kinetics of Cd
2+ on the passivators, calculating the equilibrium adsorption capacities for HO, OF, and HA at 28.14 mg/g, 14.12 mg/g, and 30.01 mg/g, respectively. This suggests that the pseudo-second-order kinetic model more accurately clarified the experimental observations, revealing that the adsorption mechanism is predominantly governed by chemisorption.
3.2. Effect of Passivators Addition on Soil pH and Aggregate
Soil pH is a significant factor affecting the distribution and mobility of heavy metal ions. The untreated soil exhibits alkalinity with a pH of 9.22 (
Figure 2a). After the addition of five different passivators, except for the HO and OF, there was a varying degree of increase in pH with increased passivator dosage. Notably, HA, SP and DE considerably raised the pH from 9.22 to 9.48, 9.43 and 9.55 at dosages of 5%, respectively. This increase is partly because the inherent pH of agents are relatively higher than that of the soil, which naturally rises with increasing dosages. Furthermore, HA, SP and DE consumes H
+ in the soil, leading to a rise in pH, which in turn charges soil colloids with more negative charges [
30], promoting the agents can adsorb more Cd
2+ or transform them into hydroxide precipitates [
31]. For the HO and OF, the contained organic fertilizer being derived from plant materials like straw, it generates organic acids during decomposition, thereby reducing the soil pH as the dosage increases [
18]; at 3% and 5% dosages, the soil pH decreased by 0.26 and 0.45 compare with CK, respectively. Both HO and OF decreased the soil pH significantly and pH decreased with the amount of HO and OF. After this decrease, the soil pH is more suitable for the growth of most plants.
Aggregates are the fundamental units of soil structure, comprising minerals and organic matter and effectively modulate conditions of water, nutrients, air, and temperature within the soil [
32]. The quantity of aggregates of varying sizes determines soil structural properties, with aggregates distribution significantly impacting soil stability, nutrient storage and erosion, soil carbon cycling, and water retention capacity [
33]. Aggregates serve as one of the crucial indicators for assessing soil fertility and quality.
Figure 2b reveals that the distribution pattern of aggregate mass percentage is consistent across six treatment groups. Aggregates sizes with less than 5–75 μm and 75–200 μm constituted the largest mass percentage, whereas those sized 250–500 μm and 500–3500 μm were the least. Compared to HO, HA, and OF, the influence of SP and DE on aggregate distribution was minor. However, the proportion within the <5 μm aggregates gradually decreased with an increase in passivator dosage. With addition of HO, HA, OF, SP, and DE, showing a reduction of 23.49%, 28.51%, 37.65%, 11.46%, and 13.13% compared to the CK at a passivator dosage of 5%, respectively. Moreover, aggregates sized 5–75 μm also mostly decreased to varying degrees after passivator addition with a 5% dosage, causing reductions of 19.29%, 10.60%, 12.83%, 3.82%, and 7.87% compared to the CK. Accordingly, passivator addition led to increases in aggregates sized 75–250 μm, 250–500 μm, and 500–3500 μm. Similar conclusions can be drawn from the fractal dimensions (
Figure 2c), which decreased with increasing passivator dosage, indicating that passivators promote inter-aggregate connectivity. The fractal dimensions for HO and OF treatments were generally lower than the other three groups, particularly when dosages were at 5%, with decreases from 2.69 to 2.64 and 2.61, respectively. The combination of soil with HO and OF transform organic fertilizer to organic matter not only can adsorb Cd
2+ but also the organic matter acts as a crucial binder for aggregates, binding particles together, the addition of these passivators leads to an increase in aggregate size [
34], which is conducive to the crops grow.
3.3. Leaching of Cd
Passivator types and dosages have varying effects on the bioavailable and leachable states of Cd
2+ in cadmium-contaminated soil. The DTPA extraction method is often used to analyze the potential availability of heavy metals in soil to plants. As illustrated in the
Figure 3a, the DTPA-Cd concentration in the CK group was 1.305 mg/kg. After the addition of five passivators, the concentration of DTPA-Cd decreased to varying extents. HA exhibited the highest soil passivation efficiency at all dosages, with reductions of 14.22%, 25.67%, 35.63%, and 42.14%, respectively. Similarly, the cadmium immobilization capacity of HO approach HA, the reduction was 6.13%, 12.26%, 25.29%, and 37.16%. OF also reduced part of DTPA-Cd concentrations, with a 3% and 5% dosage resulting in reductions of 18.39% and 19.16%. At a 5% dosage, SP and DE decreased bioavailable cadmium by 29.12% and 23.37%, respectively. The increase in the amount of HO and HA also significantly reduced the DTPA-Cd content in the soil.
As the latest statutory heavy metal pollution evaluation method in the U.S.A., the TCLP is mainly used to detect the dissolution and migration of heavy metal elements in solid media.
Figure 3b shows the results of TCLP-Cd concentration. The TCLP-Cd concentration of untreated soil was 0.45 mg/kg. After the addition of five passivators, the TCLP-Cd concentration of the soil decreased. Consistent with DTPA results, HA treated soil showing the lowest concentration at all dosages; with the addition of 3% and 5%, the leaching concentration decreased by 71.43% and 83.33% compared to the CK, respectively. HO, OF, SP and DE produced similar reductions in TCLP-Cd concentration at a 5% dosage, with decreases of 69.05%, 40.48%, 21.43% and 40.48%, respectively. With an increase in the amount of passivating agent, the soil DTPA-Cd contents decreased significantly.
The SBRC in vitro simulation method, which models the environment of gastric and intestinal fluids to leach Cd
2+ from soil, was used to assess the bioavailability of cadmium in soil. As shown in
Figure 3c, the SBRC-Cd content at CK group was 0.026 mg/kg. Among the passivators, under the HA treatment, the reduction was the largest; with the addition of 3% and 5%, the reduction was 50% and 57.69%. The reduction resulting from the HO treatment was also greater, i.e., 34.62% and 42.31% under 3% and 5% addition, respectively. The reductions under OF, SP and DE remediation were slightly lower than that under the HA and HO treatment, i.e., by 30.77%, 34.62%, and 30.77%, respectively. An increase in the amount of the passivation agent is conducive to a decrease in the soil SBRC-Cd concentration.
4. Discussion
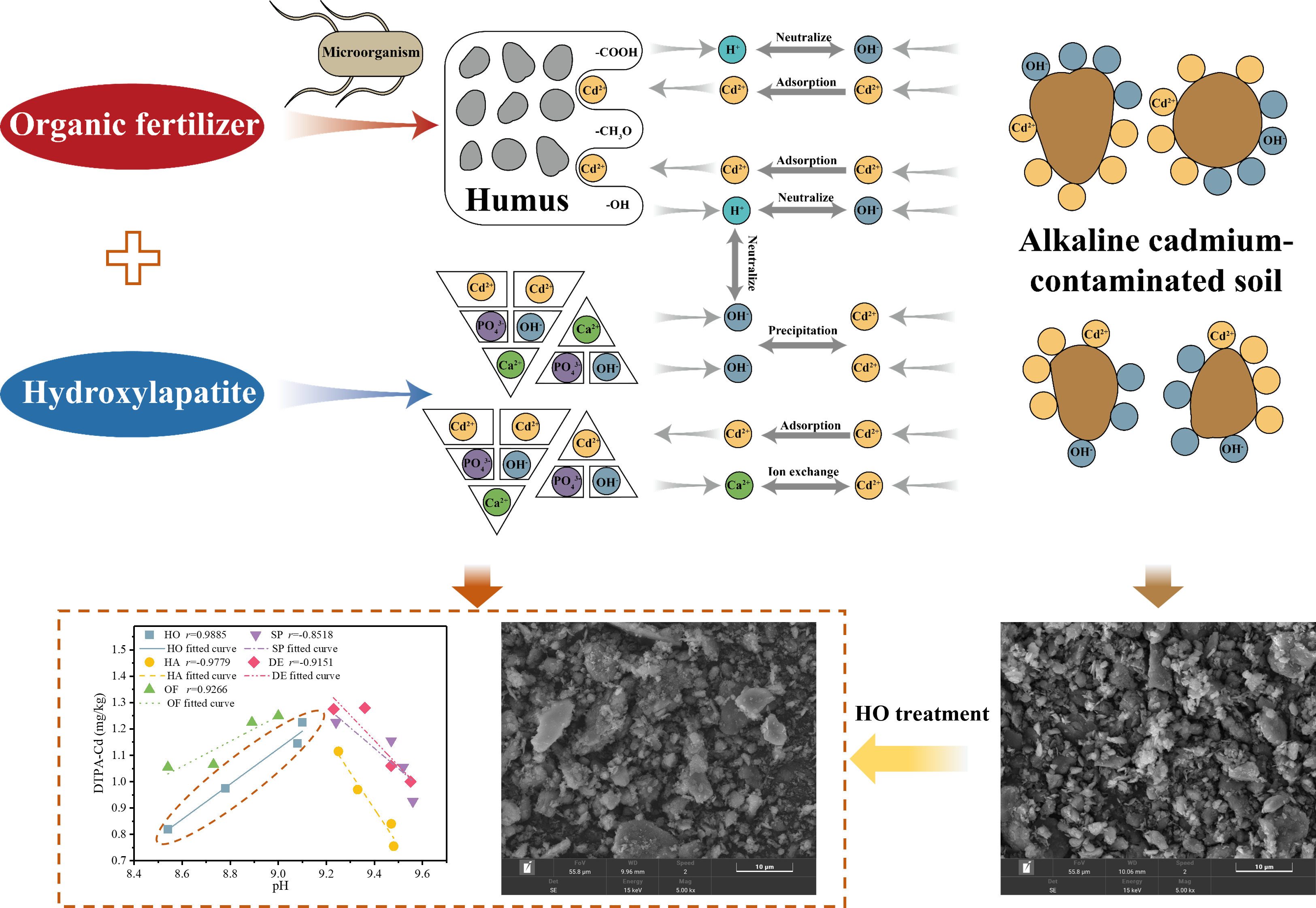
In acidic conditions, heavy metals like Cd2+ are more soluble, meaning they are more likely to exist in an ionic form within the soil solution, making them more accessible to plants and more easily removed or stabilized by chemical or physical means. Conversely, in alkaline soils, Cd2+ may form insoluble but unstable complexes, reducing its concentration in the soil solution and making removal more challenging. Therefore, dealing with weakly alkaline soils may require increased passivator usage if only alkaline passivators are applied, but using these passivators excessively on alkaline soils can cause soil compaction and fertility decline. This study suggests the combination of hydroxylapatite and organic fertilizers to treat cadmium contaminated soil.
The immobilization mechanisms of Cd
2+ by hydroxyapatite in soil primarily encompass ion exchange interactions, surface complexation of Cd
2+ on phosphate particles, partial dissolution of phosphates, and the precipitation of cadmium-containing phosphates. In comparison with control groups, contaminated soils treated with hydroxyapatite exhibit a marked increase in pH. This alteration suggests the essential role of pH modification in the immobilization processes of Cd
2+ (
Figure 4). Nonetheless, in the context of alkaline soils, the elevation in pH attributed to the incorporation of passivators may precipitate soil compaction, thereby influencing future agricultural activities. Consequently, it becomes imperative to introduce an ancillary agent for moderating the pH elevation after passivator application. Organic fertilizers emerge as an apt choice, producing humus via microbial soil activities (
Figure 5). The dissociation of –COOH and –OH within humus releases H+, effectively counterbalancing the pH augmentation induced by hydroxyapatite. Furthermore, the humic surface, endowed with oxygen-containing functional groups, exhibits properties conducive to ion exchange, complexation, and biological activity. This suggests that humus could immobilize Cd
2+ in soils through surface ion exchange and complexation mechanisms. While hydroxyapatite alone can adeptly immobilize Cd
2+ in soils, synergistic outcomes are observed when it is used with organic fertilizers. Additionally, the inclusion of organic fertilizer can partially regulate soil pH, mitigating potential soil compaction. Notably, hydroxyapatite incurs a higher cost, approximately
$1500 per ton, in stark contrast to organic fertilizer, which is derived from straw and animal manure and costs about
$80 per ton. Thus, integrating organic fertilizer not only substantially diminishes the remediation expenses of cadmium-contaminated agricultural soils but also optimizes the utilization of organic waste. Therefore, the concurrent application of hydroxyapatite and organic fertilizer holds considerable promise for remediating alkaline, cadmium-contaminated agricultural soils.
5. Conclusion
In this study, the hydroxylapatite/organic fertilizer system were developed and compare with other passivators to remediate cadmium contaminates farmland soil. The adsorption kinetic and isotherm of passivator, the effect of passivators on soil pH and aggregate, and the leachability of Cd after passivation were investigated. The following conclusions can be drawn according to experimental results and discussion:
(1) In the aqueous phase, HO successfully adsorbed Cd2+ on the surface and has superior potential Cd2+ adsorption capacity than OF, DE, and SP, with its adsorption capacity closely approach to that of HA.
(2) HO effectively lower the pH from 9.22 to 8.59 at the addition of 5% and change the aggregate distribution. The increase in the amount of passivator also significantly increased soil aggregate size.
(3) The addition of HO significantly improved the extractable contents of Cd in the soil. With the addition of 5% HO, the Cd extracted by TCLP decreased by 30.95%, 42.86%, 59.52% and 69.05%, respectively.
Therefore, based on the experiment results, HO is a highly efficient and low-cost organic-inorganic composite passivator for cadmium contaminated soils.
Author Contributions
Conceptualization, Beibei Ren and Wei Wei; methodology, Wei Wei and Mingli Wei; investigation, Beibei Ren; resources, Siguang Zhao; data curation, Beibei Ren, Wei Wei, Mingli Wei, and Siguang Zhao; writing—original draft preparation, Siguang Zhao; writing—review and editing, Beibei Ren, Wei Wei, and Mingli Wei; supervision, Siguang Zhao; funding acquisition, Siguang Zhao. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by National Natural Science Foundation of China General Program, grant number 52478388 and Open Fund Project of Jiangsu Collaborative Innovation Center for Building Energy Saving and Construction Technology, grant number SJXTBS2113.
Data Availability Statement
Dataset available on request from the authors
Conflicts of Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Abbreviations
The following abbreviations are used in this manuscript:
| MDPI |
Multidisciplinary Digital Publishing Institute |
| DOAJ |
Directory of open access journals |
| TLA |
Three letter acronym |
| LD |
Linear dichroism |
References
- Ballabio, C.; Jones, A.; Panagos, P. Cadmium in topsoils of the European Union–An analysis based on LUCAS topsoil database. Science of the Total Environment 2024, 912, 168710. [Google Scholar] [CrossRef] [PubMed]
- Song, L. P.; Zhou, J. H.; Xu, X. Y.; Na, M.; Xu, S. Q.; Huang, Y. J.; Zhang, J.; Li, X. P.; Zheng, X. Q. Inoculation of cadmium-tolerant bacteria to regulate microbial activity and key bacterial population in cadmium-contaminated soils during bioremediation. Ecotox Environ Safe 2024, 271, 115957. [Google Scholar] [CrossRef] [PubMed]
- Huang, X. H.; Li, X. Z.; Zheng, L. P.; Zhang, Y.; Sun, L.; Feng, Y. H.; Du, J. Y.; Lu, X. S.; Wang, G. Q. Comprehensive assessment of health and ecological risk of cadmium in agricultural soils across China: A tiered framework. J Hazard Mater 2024, 465, 133111. [Google Scholar] [CrossRef] [PubMed]
- Bashir, S.; Ali, U.; Shaaban, M.; Gulshan, A. B.; Iqbal, J.; Khan, S.; Husain, A.; Ahmed, N.; Mehmood, S.; Kamran, M.; Hu, H. Q. Role of sepiolite for cadmium (Cd) polluted soil restoration and spinach growth in wastewater irrigated agricultural soil. J Environ Manage 2020, 258, 110020. [Google Scholar] [CrossRef]
- Wei, X. D.; Yang, D.; Yin, X. H.; Yang, H. Q.; Fang, Y. Y.; Chen, N.; Zhang, H.; Hu, Z. Y. Comparative study of efficiencies of purification of cadmium contaminated irrigation water by different purification systems. Science of the Total Environment 2024, 907, 167941. [Google Scholar] [CrossRef]
- Huang, F. Y.; Li, Z. M.; Yang, X.; Liu, H. J.; Chen, L.; Chang, N.; He, H. R.; Zeng, Y.; Qiu, T. Y.; Fang, L. C. Silicon reduces toxicity and accumulation of arsenic and cadmium in cereal crops: A meta-analysis, mechanism, and perspective study. Science of the Total Environment 2024, 918, 170663. [Google Scholar] [CrossRef]
- Zhu, Z. Q.; Liu, H. K.; Yang, Y. H.; Zhou, X. B.; Tang, S.; Zhang, L. H.; Zhu, Y. N.; Fan, Y. M. Remediation characteristics and effects of electrokinetic-citric acid system on karst soil contaminated by arsenic and cadmium. Environ Technol Inno 2024, 33, 103483. [Google Scholar] [CrossRef]
- Qu, J.; Li, Y.; Sun, H.; Liu, R.; Han, Y.; Bi, F.; Fan, H.; Zhang, G.; Zhang, Y.; Wang, Y. Ball-milled sepiolite/phosphate rock for simultaneous remediation of cadmium-contaminated farmland and alleviation of phosphorus deficiency symptoms in pepper. Chemical Engineering Journal 2024, 150925. [Google Scholar] [CrossRef]
- Huang, C. Y.; Huang, H. L.; Qin, P. F. In-situ immobilization of copper and cadmium in contaminated soil using acetic acid-eggshell modified diatomite. J Environ Chem Eng 2020, 8((4)), 103931. [Google Scholar] [CrossRef]
- Piri, M.; Sepehr, E.; Samadi, A.; Farhadi, K. H.; Alizadeh, M. Contaminated soil amendment by diatomite: chemical fractions of zinc, lead, copper and cadmium. Int J Environ Sci Te 2021, 18((5)), 1191–1200. [Google Scholar] [CrossRef]
- Wen, J. W.; Wu, C.; Bi, X. Y.; Zhang, S. L.; Ouyang, H.; Ye, J. X.; Ohnuki, T.; Yu, Q. Q. Soil pH change induced by smelting activities affects secondary carbonate production and long-term Cd activity in subsoils. Appl Geochem 2023, 152, 105663. [Google Scholar] [CrossRef]
- Zhang, Y. N.; Zhang, Y. J.; Wu, A. G. Remediation effects and mechanisms of typical minerals combined with inorganic amendment on cadmium-contaminated soil: a field study in wheat. Environ Sci Pollut R 2023, 30((13)), 38605–38615. [Google Scholar] [CrossRef]
- Zhou, Y.; Cui, Y.; Yang, J.; Chen, L.; Qi, J.; Zhang, L.; Zhang, J.; Huang, Q.; Zhou, T.; Zhao, Y. Roles of red mud in remediation of contaminated soil in mining areas: Mechanisms, advances and perspectives. J Environ Manage 2024, 356, 120608. [Google Scholar] [CrossRef] [PubMed]
- Feng, Y.; Yang, J. J.; Aminu, D.; Han, H.; Yan, Y. B.; Wang, Y. H.; Liu, J.; Wang, X. D. Effects of hydroxyapatite on safe wheat production and soil microbial functional genes in an alkaline soil contaminated with heavy metals. Environ Res 2023, 220, 115183. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y. N.; Zhang, Y. J.; Akakuru, O. U.; Xu, X. W.; Wu, A. G. Research progress and mechanism of nanomaterials-mediated in-situ remediation of cadmium-contaminated soil: A critical review. J Environ Sci 2021, 104, 351–364. [Google Scholar] [CrossRef] [PubMed]
- Hamid, Y.; Tang, L.; Hussain, B.; Usman, M.; Lin, Q.; Rashid, M. S.; He, Z. L.; Yang, X. E. Organic soil additives for the remediation of cadmium contaminated soils and their impact on the soil-plant system: A review. Science of the Total Environment 2020, 707, 136121. [Google Scholar] [CrossRef]
- Wang, Z. L.; Zhang, T. T.; Zhao, Y. L.; Miao, Y. H.; Zhang, L. J.; Sarocchi, D.; Song, S. X.; Zhang, Q. W. Immobilization of Cd in contaminated soil by mechanically activated calcite: Sustained release activity-depended performance and mechanisms. Chemical Engineering Journal 2024, 482, 149024. [Google Scholar] [CrossRef]
- Liu, Z. T.; Ning, X.; Long, S.; Wang, S. L.; Li, S. G.; Dong, Y. W.; Nan, Z. R. Arsenic and cadmium simultaneous immobilization in arid calcareous soil amended with iron-oxidizing bacteria and organic fertilizer. Science of the Total Environment 2024, 920, 170959. [Google Scholar] [CrossRef]
- Zhang, J. X.; Dai, L. M.; Li, J. H.; Zeng, Q. Y.; Zhou, M.; Hou, H. B. Phosphate tailings-based slow-release heavy metal passivation materials: Mechanism, environmental risk, and microbial community evolution. J Clean Prod 2024, 434, 139931. [Google Scholar] [CrossRef]
- Wang, H. Y.; Liu, H.; Li, J. H.; Chen, S. N.; Zaman, Q. U.; Sultan, K.; Rehman, M.; Saud, S.; El-Kahtany, K.; Fahad, S.; Deng, G.; Chen, A. E. Combined passivators regulate physiological, antioxidant potential and metals accumulation in potato grown in metals contaminated soil. Science of the Total Environment 2024, 912, 168956. [Google Scholar] [CrossRef]
- Jiang, W.; Li, D. A.; Yang, J. L.; Ye, Y. Y.; Luo, J. W.; Zhou, X. J.; Yang, L.; Liu, Z. Z. A combined passivator of zeolite and calcium magnesium phosphate fertilizer: Passivation behavior and mechanism for Cd (II) in composting. Environ Res 2023, 231, 116306. [Google Scholar] [CrossRef] [PubMed]
- Jia, L. J.; Zhou, Y. L.; Su, Y. X.; Zhao, P.; Zhang, J. L.; Fan, W.; Yang, S. C.; Long, G. Q. Coupling raw material cultivation with nano-hydroxyapatite application to utilize and remediate severely Cd-containing soil. Process Saf Environ 2024, 184, 96–104. [Google Scholar] [CrossRef]
- Yan, Y. B.; Du, M.; Jing, L. Q.; Zhang, X. X.; Li, Q.; Yang, J. J. Green synthesized hydroxyapatite for efficient immobilization of cadmium in weakly alkaline environment. Environ Res 2023, 223, 115445. [Google Scholar] [CrossRef] [PubMed]
- Han, L. W.; Zhao, Z. J.; Li, J.; Ma, X. B.; Zheng, X.; Yue, H. Y.; Sun, G. H.; Lin, Z. Y.; Guan, S. Q. Application of humic acid and hydroxyapatite in Cd-contaminated alkaline maize cropland: A field trial. Science of the Total Environment 2023, 859, 160315. [Google Scholar] [CrossRef]
- Miao, T. L.; Jin, Z. H.; Kong, L. H.; Jin, Y.; Liu, X. S.; Qu, J. J. Effect of composite organic amendment on Cd(II) ions stabilization and microbial activity under various ammonium sulfate levels. Environ Res 2024, 247, 118194. [Google Scholar] [CrossRef]
- Iqbal, A.; Ligeng, J.; Mo, Z. W.; Adnan, M.; Lal, R.; Zaman, M.; Usman, S.; Hua, T.; Imran, M.; Pan, S. G.; Qi, J. Y.; Duan, M. Y.; Gu, Q. C.; Tang, X. R. Substation of vermicompost mitigates Cd toxicity, improves rice yields and restores bacterial community in a Cd-contaminated soil in Southern China. J Hazard Mater 2024, 465, 133118. [Google Scholar] [CrossRef]
- Yang, W. T.; Jing, H. N.; Yang, L. Y.; Li, X. X.; Chen, M. Z.; Xu, M. Q.; Chen, Y. L.; Wu, P.; Liu, H. Y.; Wang, P. Effects of rapeseed residue organic fertilizer on cadmium toxicity in soil and its uptake and transfer by rice plants (Oryza sativa L.). Land Degrad Dev 2023, 34((10)), 2791–2802. [Google Scholar] [CrossRef]
- Liu, T.; Wang, Q.; Li, Y.; Chen, Y.; Jia, B.; Zhang, J.; Guo, W.; Li, F. Y. Bio-organic fertilizer facilitated phytoremediation of heavy metal (loid) s-contaminated saline soil by mediating the plant-soil-rhizomicrobiota interactions. Science of The Total Environment 2024, 922, 171278. [Google Scholar] [CrossRef]
- Li, J.; Zhang, P. Y.; Ye, J. P.; Zhang, G. M.; Cai, Y. J. Simultaneous in-situ remediation and fertilization of Cd-contaminated weak-alkaline farmland for wheat production. J Environ Manage 2019, 250, 109528. [Google Scholar] [CrossRef]
- Li, X. Y.; Zhang, M. J.; Li, S. Y.; Wei, W. Humic acid-mediated transport of a typical soil passivation remediation product (chloropyromorphite) in saturated porous media. J Environ Sci 2024, 141, 51–62. [Google Scholar] [CrossRef]
- Wei, B. L.; Peng, Y. C.; Jeyakumar, P.; Lin, L. X.; Zhang, D. L.; Yang, M. Y.; Zhu, J. N.; Lin, C. S. K.; Wang, H. L.; Wang, Z. T.; Li, C. Soil pH restricts the ability of biochar to passivate cadmium: A meta-analysis. Environ Res 2023, 219, 115110. [Google Scholar] [CrossRef]
- Wang, Q. Y.; Wang, Q. R.; Wang, T. Y.; Zhang, S. Q.; Yu, H. W. Impacts of polypropylene microplastics on the distribution of cadmium, enzyme activities, and bacterial community in black soil at the aggregate level. Science of the Total Environment 2024, 917, 170541. [Google Scholar] [CrossRef]
- Huang, X.; Tang, S. T.; Zeng, M.; Wu, Y.; Liang, J. L.; Yuan, Y.; Yu, F. M.; Li, Y. Soil aggregate size mediates the impact of different fertilization patterns on the diazotrophic community of mine soils. Appl Soil Ecol 2024, 193, 105173. [Google Scholar] [CrossRef]
- Zhang, Y.; Li, Y.; Xu, Y.; Huang, Q.; Sun, G.; Qin, X.; Wang, L. Effects of mercapto-palygorskite application on cadmium accumulation of soil aggregates at different depths in Cd-contaminated alkaline farmland. Environ Res 2023, 216, 114448. [Google Scholar] [CrossRef]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2026 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).