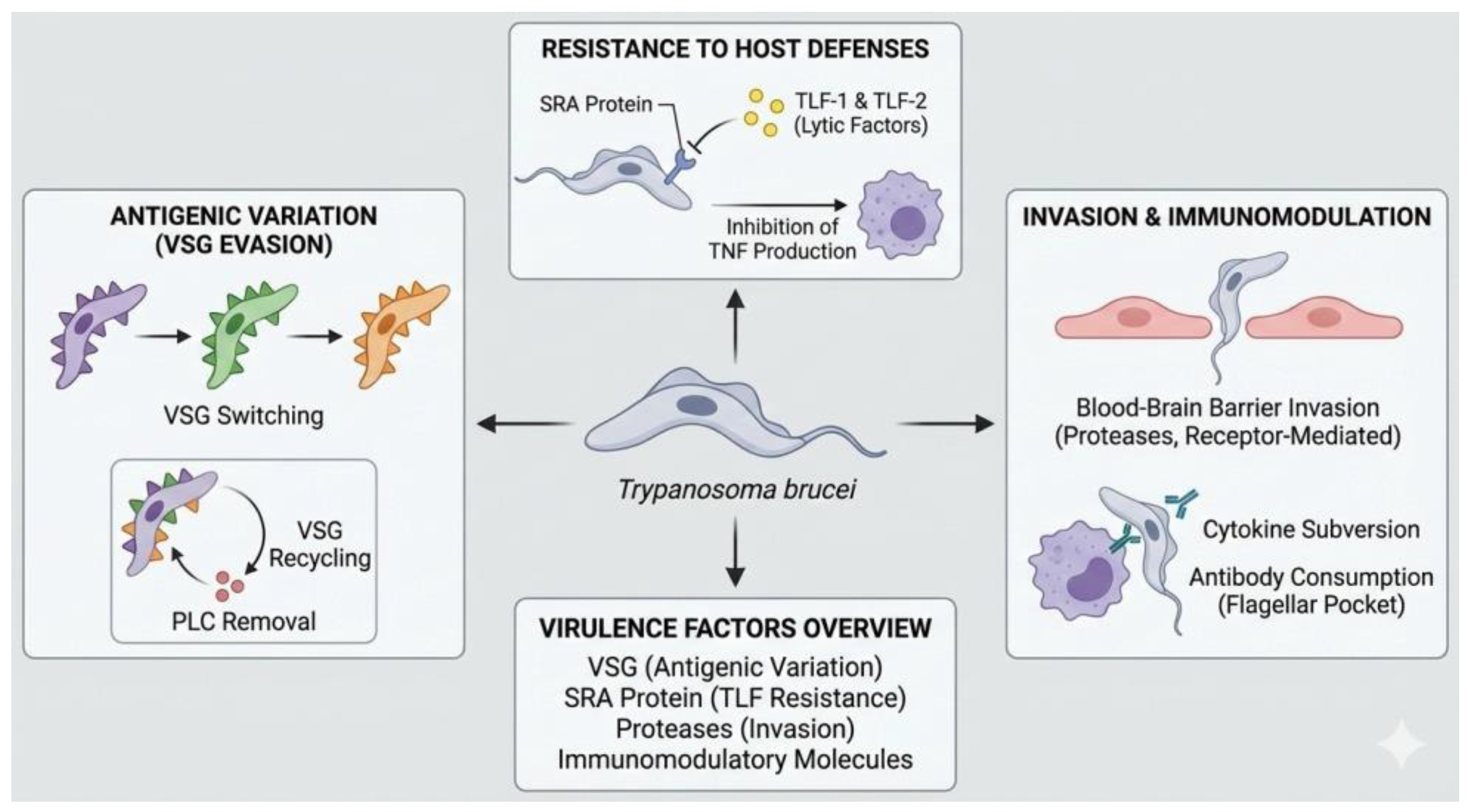
3.1. Virulence Factors
Trypanosoma brucei is an extracellular blood parasite that causes African trypanosomiasis in humans and animals. Its pathogenicity is based on a set of virulence factors that allow it to persist in the host and trigger progressive pathophysiological alterations. The central mechanism of its virulence is the antigenic variation of the variant surface glycoprotein (VSG), a protein that densely coats the parasite’s membrane and constitutes its main interface with the immune system. Although VSG is highly immunogenic
, T. brucei has the ability to periodically modify the expressed variant through genetic recombination and alternating activation of telomeric expression sites, allowing the emergence of parasitic subpopulations not recognized by previously generated antibodies. This process underpins the persistence of parasitemia and the chronicity of infection [
1].
VSG accounts for approximately 90% of the parasite’s surface proteins and performs an additional protective function by acting as a physical barrier that limits the access of complement and antibodies to invariant membrane antigens. This surface organization reduces complement-mediated lysis and opsonization, favoring the survival of the parasite in the bloodstream. Recent evidence indicates that antigenic diversification does not occur exclusively in blood, but also in extravascular tissue compartments, such as adipose tissue and other interstitial spaces, where T. brucei can persist, diversify its antigenic repertoire, and subsequently re-enter the circulation. These tissue reservoirs play a key role in both immune evasion and the development of organ damage, chronic anemia, and endothelial dysfunction, associated with sustained local inflammation and microvascular alterations [
2,
3].
Another important component of the virulence of T. brucei is the production of extracellular vesicles derived mainly from the flagellar membrane. These vesicles transport proteins and bioactive factors capable of modifying the host environment, including molecules that alter the integrity and half-life of erythrocytes, promoting their premature elimination by the reticuloendothelial system and contributing to the development of anemia. These vesicles also contain proteins involved in resistance to trypanolytic factors and in the modulation of local immune responses, which increases the parasitic load and prolongs the infection. These processes are associated with persistent systemic inflammation, mediated by proinflammatory cytokines such as TNF-alpha and IL-6, with clinical manifestations relevant to veterinary medicine and public health [
1,
4].
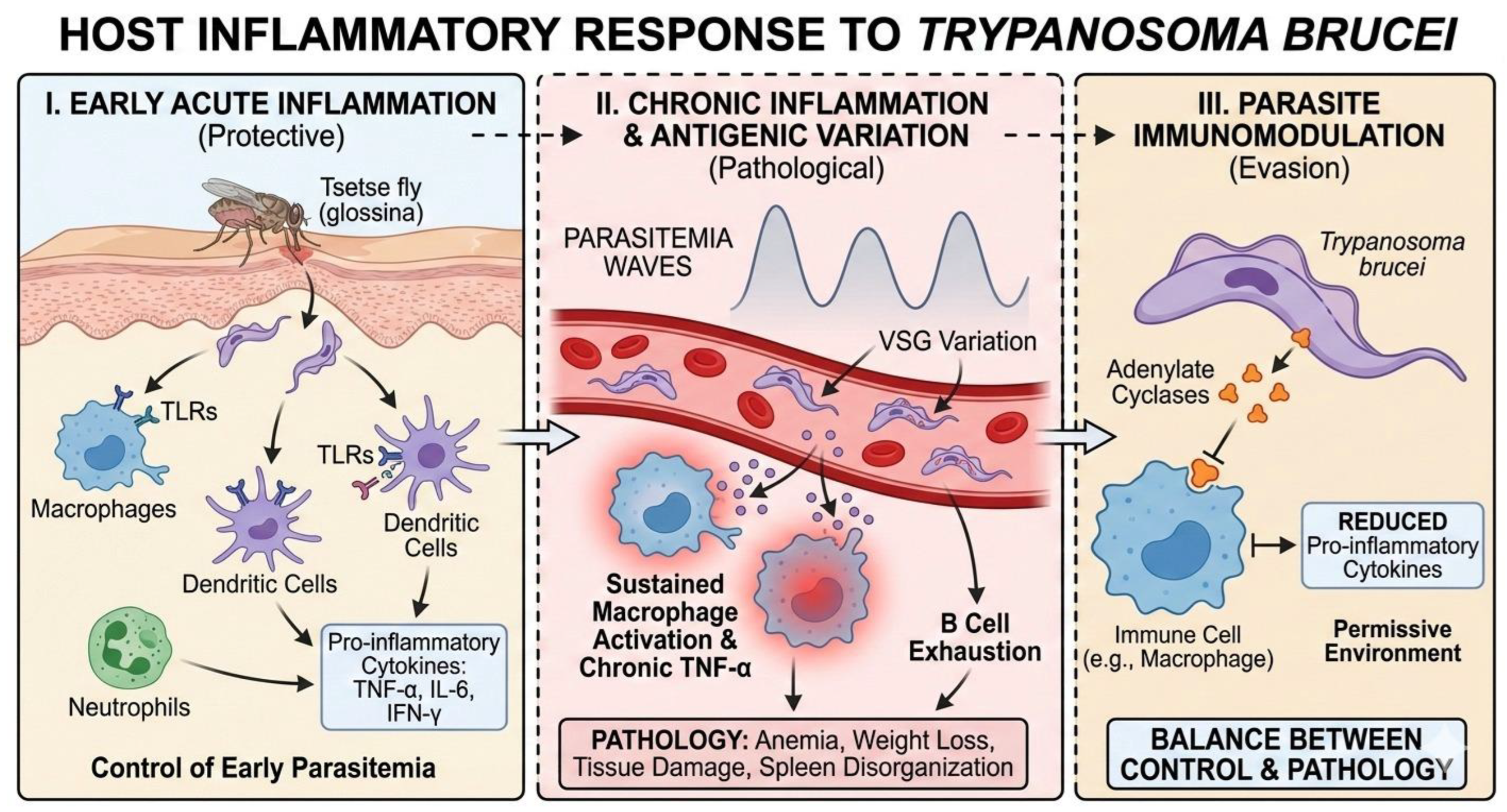
3.3. Host Inflammatory Response to Trypanosoma brucei
Infection with Trypanosoma brucei triggers a complex and highly dynamic inflammatory response in the mammalian host, which plays a dual role: initially it contributes to parasite control, but subsequently it promotes pathogenesis and disease progression. Following inoculation of the parasite by the tsetse fly, innate immunity is activated, particularly at the skin level, where macrophages, dendritic cells, and neutrophils recognize the parasite through pattern recognition receptors, such as Toll-like receptors (TLRs), inducing the early production of proinflammatory cytokines such as TNF-α, IL-6, and IFN-γ [
5].
This initial inflammatory response is crucial for limiting early parasitemia; however, T. brucei has developed effective mechanisms to resist and modulate this response. Studies have shown that sustained macrophage activation leads to chronic TNF-α production, which, while contributing to parasite control, is also associated with anemia, weight loss, and tissue damage, clinical signs characteristic of African trypanosomiasis [
6]. In this context, inflammation ceases to be protective and becomes a pathological factor.
At the systemic level, the inflammatory response is strongly influenced by the antigenic variation of the parasite, mediated by variable surface glycoproteins (VSGs). Each peak of parasitemia generates a new inflammatory surge, repeatedly stimulating the immune system and promoting a state of chronic inflammation. This constant stimulation leads to B-cell depletion and disorganization of the spleen architecture, compromising the host’s ability to mount effective immune responses against secondary infections [
7,
8].
In addition, T. brucei is capable of actively attenuating the host’s inflammatory response by releasing immunomodulatory molecules. Among these are parasitic adenylate cyclases, which interfere with the intracellular signaling of immune cells, reducing the production of proinflammatory cytokines and promoting a more permissive environment for parasite survival [
8]. Thus, the host’s inflammatory response to T. brucei represents a delicate balance between immune control and pathological damage, a direct result of an evolutionarily refined interaction between parasite and host.
Figure 2.
Host inflammatory response to Trypanosoma brucei. This panel illustrates the progression of the immune response, starting with the early activation of innate immunity mediated by TLRs and cytokines To control initial infection. It highlights the transition to a chronic phase driven by VSG-mediated antigenic variation, where sustained inflammation leads to pathology (e.g., B cell exhaustion, tissue damage). Finally, the figure depicts the active immunomodulation by the parasite via the release of adenylate cyclases, which attenuate the inflammatory response to favor parasite persistence.
Figure 2.
Host inflammatory response to Trypanosoma brucei. This panel illustrates the progression of the immune response, starting with the early activation of innate immunity mediated by TLRs and cytokines To control initial infection. It highlights the transition to a chronic phase driven by VSG-mediated antigenic variation, where sustained inflammation leads to pathology (e.g., B cell exhaustion, tissue damage). Finally, the figure depicts the active immunomodulation by the parasite via the release of adenylate cyclases, which attenuate the inflammatory response to favor parasite persistence.
3.4. Host–Parasite Interactions in Trypanosoma brucei Infection
Host–parasite interactions in Trypanosoma brucei infection are highly specialized and reflect coevolution aimed at the parasite’s persistence within the mammalian host. From the moment of transmission, T. brucei interacts closely with the host’s immune system, modulating both the innate and adaptive responses to ensure its survival and dissemination [
7].
One of the central mechanisms of this interaction is VSG-mediated antigenic variation, which allows the parasite to continuously evade the specific antibodies generated by the host. Each time the humoral response manages to control an antigenic variant, a parasitic subpopulation expresses a different VSG, restarting the cycle of infection and inflammation. This process not only ensures the persistence of the parasite, but also profoundly disrupts the host’s immunological memory [
7,
9].
Additionally, T. brucei maintains sophisticated molecular interactions with immune system cells. The release and constant turnover of VSGs not only serve to evade antibodies, but also induce polyclonal activation of B lymphocytes, contributing to progressive immunosuppression of the host [
10]. This phenomenon limits the effectiveness of specific adaptive responses and promotes susceptibility to concomitant infections, a finding of particular relevance in veterinary medicine and public health.
On the other hand, recent studies have shown that certain essential parasitic receptors remain protected from immune attack thanks to their strategic location and the dense layer of VSG that covers the surface of the parasite. This structural organization prevents antibodies from accessing vulnerable targets, reinforcing the efficiency of immune evasion and highlighting the complexity of host-parasite interactions at the molecular level [
10].
Finally, early interactions at the site of inoculation, particularly in the skin, play a decisive role in the course of infection. The local inflammatory response, modulated by components of the parasite and the vector, conditions the systemic dissemination of T. brucei and the subsequent immunological dynamics. These initial interactions represent a critical point where the biology of the parasite and the host response converge, defining the balance between control and disease progression [
5].
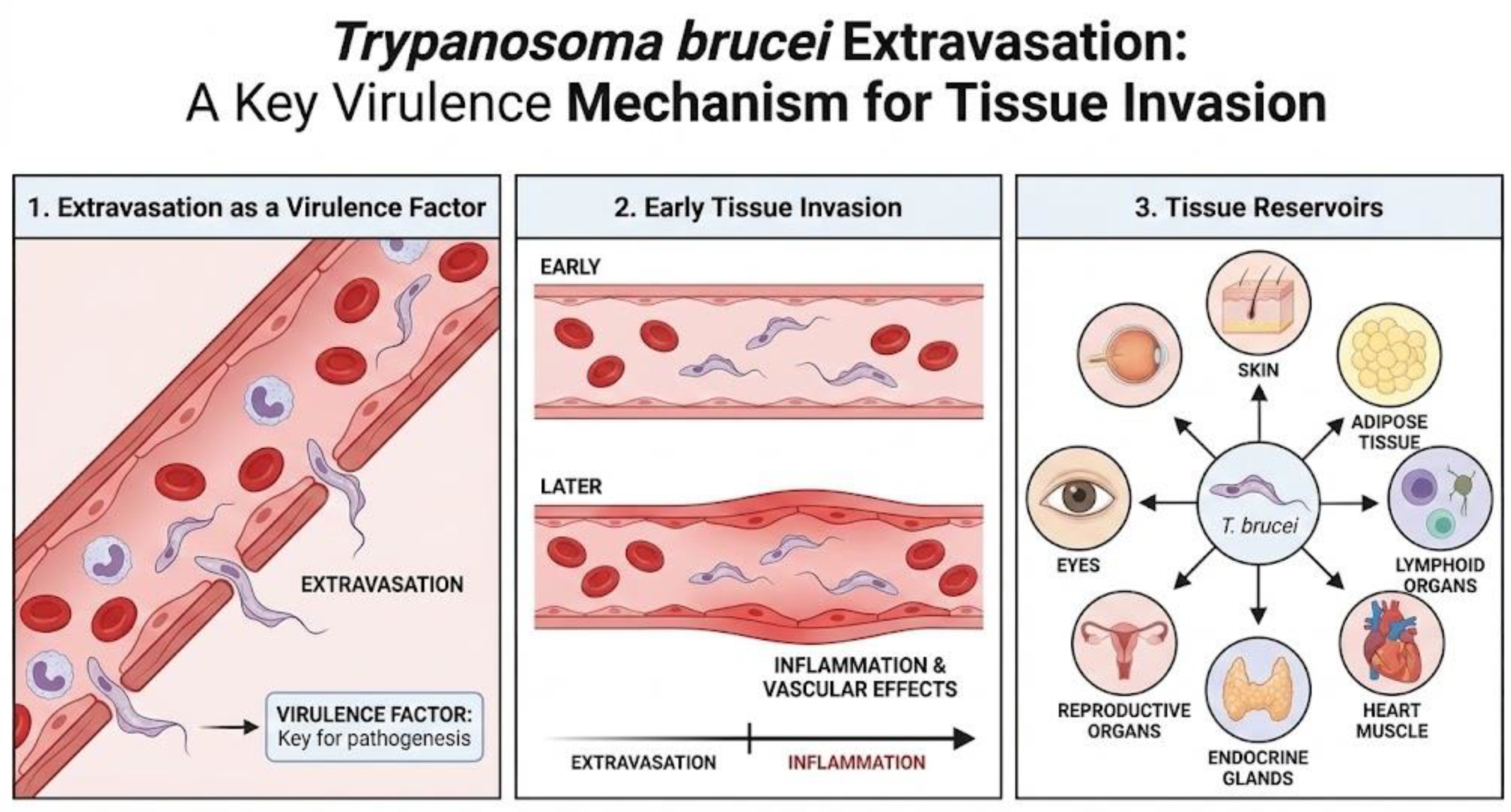
3.5. Tissue Invasion and Extravascular Residence
The symptoms produced by Trypanosoma brucei stem from its ability to extravasate and enter extravascular tissues, as has been seen in experimental models, where extravasation occurs before the vascular effects produced by inflammation, demonstrating that it is a key virulence factor [
4]. Although there is currently abundant information on the effect of the parasite on the blood and central nervous system, what is known about its effect on other tissues is not entirely clear. For this reason, studies on this subject have increased, allowing us to understand how Trypanosoma brucei reaches different tissues and uses them as reservoirs, such as the skin, adipose tissue, lymphoid organs, heart muscle, endocrine glands, reproductive organs, and even the eyes [
2].
Firstly, there is the proliferation of the parasite in the skin, a tissue considered to be an important reservoir, as there have been documented cases of individuals affected by pruritus, skin rashes, localized edema, and dermatitis. In the latter, it is suggested that the presence of trypanosomes affects the immune status of the skin, making it sensitive and vulnerable to other pathogens [
11]. On the other hand, according to one study, after simulating the vector transmission of T. brucei in human skin models, it was observed that the infectious metacyclic forms (MCF) of the parasite were activated and established a proliferative population of trypanosomes, and then slowed down their growth, metabolism, DNA synthesis, and protein translation, suggesting that they act as “resting cells,” all of which contributes to the persistence of the parasite in the skin [
12]. Similarly, another study demonstrated the presence of live, mobile parasites in the dermis and hypodermis and suggested that these trypanosomes maintain the disease foci despite screening and treatment, representing a new target for diagnosis, as skin tests should be performed to identify the presence of the parasite and prevent reinfestation [
13].
Another tissue of importance for the disease is adipose tissue. Initially considered only a lipid store, we now know that it is an immunologically active endocrine organ, as adipocytes secrete various immunomodulators. In the context of trypanosomiasis, a common sign is weight loss, which is sometimes associated with neurological disease. However, it has been suggested that the parasite consumes the host’s reserves, producing this particular sign, in addition to tissue inflammation [
14]. One study showed that adipose tissue acts as one of the main reservoirs of the parasite, reaching levels similar to those in the blood. Furthermore, the parasites present in the tissue show genetic variations compared to the blood forms [
3]. Research on trypanosomes in adipose tissue has been limited to subcutaneous fat, so there is still a lack of information on gonadal, mesenteric, and perirenal fat [
2].
Trypanosomiasis causes a variety of symptoms, including hemolytic anemia, hepatomegaly, splenomegaly, endocrine disorders, pericarditis, myocarditis, tachycardia, and congestive heart failure [
15]. In animal hosts, such as cattle, dogs, and sheep, the parasite has been observed in all layers and structures of the heart; however, there is a particular tropism for the atrial myocardium rather than the ventricular myocardium, but this cannot be generalized for all mammalian species. This could be due to the presence of collagen in the atria and the tropism that T. brucei has for it [
16]. On the other hand, damage to the heart can occur through direct parasitic action or through the host’s inflammatory response [
2].
With regard to the reproductive organs, in humans, loss of libido, impotence, menstrual and fertility problems, miscarriages, stillbirths, premature births, hair loss, gynecomastia, orchitis, and testicular atrophy have been reported [
15]. This information coincides with observations in experimental animal models, since trypanosomes were found in the tunica albuginea, pampiniform plexus, and tissue associated with the testicles in experimentally infected sheep, cattle, rabbits, and dogs. Likewise, lesions in the ovaries and uterine wall have been described in infected female dogs [
16,
17,
18]. On the other hand, a study was conducted in a murine model, where the presence of the parasite was detected in the ovaries and uterus, even when there was no active parasitemia. This suggests that the reproductive organs can act as reservoirs for trypanosomes, causing horizontal transmission of the disease and also causing relapses in patients by maintaining the infection in a latent form [
2,
3,
4,
5,
6,
7,
8,
9,
10,
11,
12,
13,
14,
15,
16,
17,
18,
19].
Symptoms often include eye conditions such as optic neuritis, diplopia, iritis, keratitis, conjunctivitis, choroidal atrophy, and iridocyclitis [15 and 20]. These signs have been studied in various animal species and have shown that the parasite enters the eye through the ciliary body and remains latent in the aqueous humor, evading pharmacological treatment, and could therefore be an anatomical reservoir. Unfortunately, no rodent models have been developed in this regard, despite the clinical importance of trypanosomes residing in the eyes [
2].
Finally, it is important to mention the presence of T. brucei in the lymphatic system. The information suggests that in this tissue, there is no true extravascular population and that the enlargement of the lymph nodes and spleen observed in affected individuals is due to the body’s immune response and not due to the direct presence of the parasite in these tissues, but it is a route by which the parasite can spread and cause relapses of the disease [
2].
3.6. Neuropathogenesis
The course of trypanosomiasis has two distinct phases: a hemolymphatic phase, in which the parasite travels through the blood and lymph, and a meningoencephalitic phase, characterized by the presence of trypanosomes in the central nervous system, causing a wide range of symptoms that vary between animals and humans. These include severe sleep disorders, neurological disorders, coma, and paralysis of the extremities as a result of myelopathy, myelitis, or peripheral motor neuron involvement. Muscle fasciculation may also be a symptom. It can also cause painful hyperesthesia in the extremities, which, when severe, is called Kerandel’s sign in advanced stages. Likewise, abnormal reflexes may occur that indicate frontal lobe involvement, such as the Pout reflex and palmo-mental reflexes [
15,
16,
17,
18,
19,
20,
21]. Despite the information on the symptoms of the disease, the mechanism by which the parasite enters the nervous system is still not known with certainty. However, possible ways have been proposed. One is that the trypanosomes enter through the fenestrated vessels of the choroid plexus, accessing the stroma before entering the blood-brain barrier and spreading with the cerebrospinal fluid through the ventricles and subarachnoid space, finally colonizing the pia mater. Another way is through the fenestrated vessels of the circumventricular organs, i.e., those with an incomplete blood-brain barrier. Finally, the parasites could also enter the neuropil directly from the blood vessels. The mechanism of entry remains a mystery, but it has been suggested that it could be a combination of the three routes [15 and 20]. On the other hand, an important factor in neuropathogenesis is the presence of immunomodulators. It has been shown that there is a proportional association between the concentration of IFN-y and TNF-⍺ and a more severe neuroinflammatory response, while higher levels of interleukin 10 and 6 are associated with milder neuroinflammation [22 and 23].
Figure 3.
Mechanism for tissue invasion and affected tissues in trypanosomiasis.
Figure 3.
Mechanism for tissue invasion and affected tissues in trypanosomiasis.