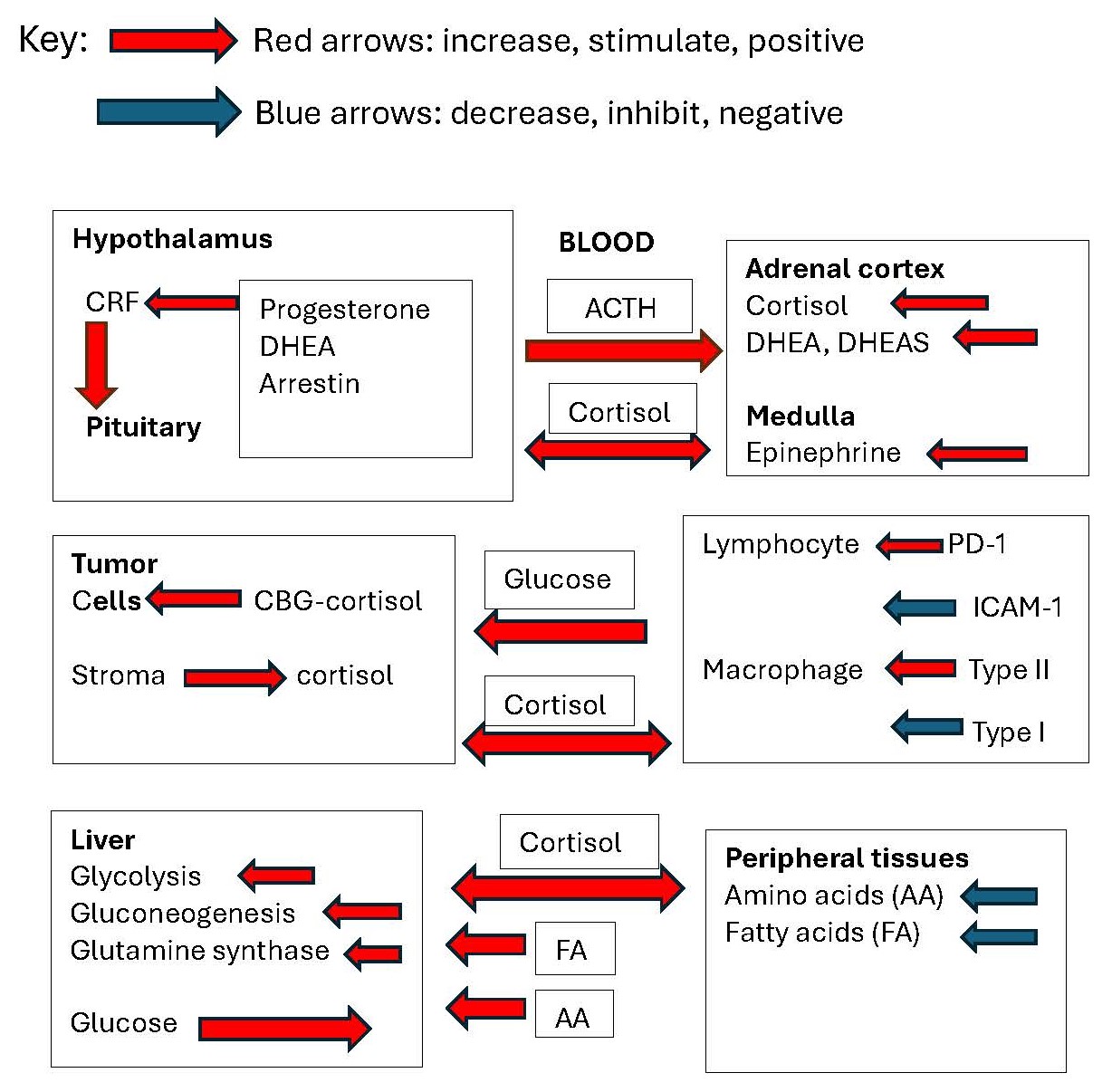
1. Formation and Secretion of Adrenocorticosteroids
Steroid secretion from the adrenal cortex is promoted by a series of events beginning with internal or external stimuli that alter body homeostasis [
1]. Such stimuli increase the transcription and release of hypothalamic corticotropin hormone (CRH), urocortin 1 (UCN1), urocortin 2 (UCN2), and urocortin 3 (UCN3) from neurons originating from the paraventricular nucleus of the hypothalamus. These tropic factors bind to CRH-R, a G-protein coupled receptor (GPCR), for the release of adrenocorticotropic hormone (ACTH). Negative feedback by the cortisol-occupied glucocorticoid receptor (GR) at this level suppresses the release of CRH and other tropic factors.
Stress increases several stimulatory factors, including epinephrine, norepinephrine, and glutamate, via inhibition by GABAergic neurons, all of which may contribute to modulation of CRH release from the hypothalamus. In addition to CRH, neurons of the paraventricular nucleus (PVN) contain arginine vasopressin, oxytocin, neurotensin, enkephalin, and cholecystokinin, each with separate or modifying activities. Stress is an important positive stimulus for CRH; however, factors that inhibit negative feedback of the hypothalamic-pituitary-adrenal (HPA) pathway are also important in increasing the activity of CRH.
Information on the pathway of cortisol formation in the adrenal cortex is abundantly available; however, some information on specific aspects is appropriate for this review. Cortisol is synthesized in the adrenal cortex from cholesterol which is stored in the form of cholesterol esters. Adrenocorticotropic hormone (ACTH) stimulates steroid synthesis by binding to the melanocortin 2 receptor (MC2R), a G protein-coupled receptor located on the surface of adrenal cortex cells. This interaction activates adenylyl cyclase, leading to the production of cyclic adenosine monophosphate (cAMP). cAMP then activates protein kinase A (PKA), which phosphorylates specific transcription factors and ultimately stimulates the conversion of free cholesterol to pregnenolone by P450scc oxidoreductase. The rate of formation of further metabolites of pregnenolone is dependent on the rate of pregnenolone formation and the activity of specific cytochrome P450 enzymes and is not individually promoted by ACTH. Hydroxylations at the C21 carbon of the steroid by CYP21A2 and at C11 by CYP11B1 occurs during the formation of both cortisol and corticosteroids. Hydroxylation of pregnenolone at carbon 17 (C17) by CYP17 is necessary for cortisol biosynthesis but not for the synthesis of the other major glucocorticoid, corticosterone. Cleavage of the side-chain of pregnenolone leads to the formation of the C-19 steroids, primarily dehydroepiandosterone (DHEA) and androstenedione. DHEA is then largely converted to DHEA sulfate before release from the adrenal cortex. The ratio of DHEA to cortisol secretion from the adrenal cortex depends on the activity of the 17,20-lyase activity of CYP17A1.
In addition to modulation within the hypothalamic-pituitary system, sympathetic innervation of the adrenal gland via the splanchnic nerve increases the sensitivity of the adrenal gland to ACTH [
2]. Under non-stress conditions, when elevated steroid secretion is not required, splanchnic neural activity appears to be inhibitory, whereas during stress conditions, when elevated steroid secretion is necessary, neural activity is excitatory. In earlier studies, splanchnicectomy was shown to have an inhibitory effect on corticosterone secretion after a variety of stressors in several species but had little effect on secretion in non-stressed animals [
3]. There are many factors that determine the rate and duration of cortisol secretion.
1.1. Extra-Adrenal Sources of Cortisol
Thorough reviews of extra-adrenal steroidogenesis in several different tissues have been published by Taves et al. and Gomez-Sanchez et al. [
4,
5]. They have described studies demonstrating de novo biosynthesis of corticosteroids in primary lymphoid organs, the intestine, skin, brain, and adipose tissue; however, the precursor for the synthesis of these steroids has not been established. Evidence for local synthesis includes the detection of steroidogenic enzymes and high local corticosteroid levels after adrenalectomy. Numerous studies have shown the presence of CYP11A and mRNAs of other enzymes necessary for biosynthesis of pregnenolone and corticosterone in lymphoid tissues and other sites, and the stimulation of corticosterone by CRH [
6] and ACTH [
7]. Interestingly, 21-hydroxylase in the human brain and other extra-adrenal tissues differs from that in adrenal CYP21 [
8,
9]. It is present as a CYP2D6 isoform and is also abundantly expressed in lymphoid tissues [
9].
The presence of local biosynthesis of corticosterone or aldosterone in adipose tissue [
10], keratinocytes [
11] or monocyte-macrophage lineage cells [
12] may be relevant to growth of solid tumors in that, as membrane-permeable steroids, adrenocortical steroids produced in the surrounding adipose tissue may reduce the ability of tumor-infiltrating lymphocytes (TILs) to act on cells expressing tumor antigens. Acharya et al. [
12] showed that genetic ablation of steroidogenesis in monocyte-macrophage lineage cells from colon cancer cells led to expression of multiple immune checkpoint receptors and promoted the induction of dysfunction-associated genes upon T cell activation. Thus, locally produced steroids appear to be a component of the promotion of tumor growth by macrophages.
1.2. Stimuli for Activation of the Hypothalamic-Pituitary-Adrenal (HPA) Axis
Any alteration in homeostasis can stimulate the HPA axis. Major depression and other psychiatric conditions are associated with hypercortisolism [
13,
14]. Conditions that chronically activate the hypothalamic–pituitary–adrenal axis (HPA) result in functional hypercortisolism and usually occur in cases of major depression, anorexia nervosa, bulimia nervosa, alcoholism, diabetes mellitus, simple obesity, polycystic ovary syndrome, obstructive sleep apnea syndrome, panic disorder, generalized anxiety disorder, shift work, and end-stage renal disease [
15]. Cessato et al. listed other high-risk conditions: arterial hypertension, osteoporosis and fractures, mood disorders, unusual infections and thrombotic events, and adrenal and pituitary incidentalomas [
16]. Whether the disease causes cortisol elevation or whether cortisol contributes to the disease is not clear for individual diseases. Abraham et al. [
17] found no effect of obesity on urinary cortisol, and dexamethasone responses were not associated with BMI or weight. However, salivary cortisol was increased as a function of increasing BMI (P < 0.0001). Surprisingly, an inflammatory condition, rheumatoid arthritis, is associated with slightly lower levels of cortisol [
18] and significantly lower levels of androgens [
19]. Erratic cortisol secretion in major depression may be a more characteristic feature of HPA axis dysregulation than hypercortisolism [
20] as also shown in patients with metastatic breast cancer [
21] and in some other conditions. It is clear that not only the concentration but the pattern of secretion may be important in the response to cortisol [
22].
Experimentally, severe physical and psychological stress during military survival training [
23] and long distance running exercise [
24] strongly stimulates cortisol elevation. The experience of free fall from the airplane (skydiving), which involves little physical exertion, also causes a large increase in serum and salivary cortisol [
25].
Increased ACTH, whether due to stress or by administration, increases the secretion of all adrenocortical steroids. Cortisol formed in the fasciculata of the adrenal cortex acts to increase DHEA secretion in the reticularis zone by inhibiting 3β-hydroxysteroid dehydrogenase [
26]. Administration of prednisone or similar steroidal drugs provides glucocorticoid activity but suppresses ACTH and adrenal steroidogenesis. Therefore, the responses to ACTH and prednisone differ in that the glucocorticoid activity of the administered synthetic drugs is not opposed by DHEA and DHEA sulfate, nor is the activity of medullary chromaffin cells increased [
27].
Many acute as well as chronic conditions bring about elevated adrenal secretions or those that are highly fluctuating. In some conditions a decrease in serum levels of cortisol may occur over time, sometimes referred to as “adrenal fatigue” (“AF”). However, there is no evidence that continuous stimulation of the HPA axis brings about exhaustion of the ability of the adrenal to secrete adrenocorticoids [
28].
1.3. Role of the 11-Hydroxysteroid Dehydrogenases (HSD11βs)
After secretion, oxidation of cortisol by 11β-hydroxysteroid dehydrogenase (HSD11β) type 2 may occur. This produces cortisone, the inactive form of the glucocorticoid. The inactivation of cortisol is important for reducing glucocorticoid activity in organs such as the kidney, where mineralocorticoid activity may be bolstered by high concentrations of circulating cortisol. HSD11β type 2 also protects adult lungs from the anti-proliferative effects of cortisol [
29] and protects the brain from the mineralocorticoid activity of cortisol [
30].
Alternatively, cortisone may be reduced to cortisol by HSD11β type 1, which can increase glucocorticoid activity in the liver, adipose tissue, muscle, and adipose tissue where it serves metabolic functions. HSD11β type 1 can be induced by TNFα [
31], inflammatory cytokines, and glucocorticoids in skin fibroblasts, synovial fibroblasts, and osteoblasts [
31]. Cytokines also induce HSD11β type 1 in macrophages [
32,
33], T- and B-cells, and dendritic cells [
34,
35], thus maintaining the active form of cortisol for its suppressive effects on the immune system.
Thus, cortisol aids in maintaining the active, reduced form of the steroid, while inflammatory cytokines are prevalent.
2. Dis-Regulation of Salivary and Serum Cortisol in Breast Cancer
A high proportion of women with metastatic breast cancer have been shown to have disrupted circadian rhythms (
Figure 1) by Sephton et al. [
36]. A total of 104 patients contributed data to the current analyses, while 21 were excluded because they were taking hydrocortisone-based medications. Subjects were also excluded if they had one of the following: 1) active cancers within the past 10 years other than breast cancer, basal cell or squamous cell carcinomas of the skin, or in situ cancer of the cervix; 2) positive supraclavicular lymph nodes as the only metastatic lesion at the time of diagnosis; or 3) a concurrent medical condition likely to influence short-term survival. excluded if they had one of the following: 1) active cancers within the past 10 years other than breast cancer, basal cell or squamous cell carcinomas of the skin, or in situ cancer of the cervix; 2) positive supraclavicular lymph nodes as the only metastatic lesion at the time of diagnosis; or 3) a concurrent medical condition likely to influence short-term survival. The effect of any treatments that the patients were receiving was not investigated.
Although there is an appearance of elevated salivary cortisol, the area under the curve of samples collected at 0800, 1200, 1700, and 2100 h was not statistically greater in patients in the upper half of the median than in those in the lower half, nor were the mean levels of salivary cortisol in the two groups significantly different. The failure to find a difference was largely due to the much greater variability (approximately 50-fold greater) in the group with flattened profiles than in the group with more normal circadian patterns.
As an indication of the greater effect of cortisol in patients with flattened profiles, the proportion of NK cells was decreased as measured by flow cytometry (CD56 positive, CD3 negative cells) compared to that in patients with more normal circadian patterns (P = 0.007), and cytolytic activity, determined by the chromium release method, was also decreased (P < 0.05).
In addition, the shape of the cortisol slope predicted subsequent survival for up to 7 years in patients with metastatic breast cancer. Earlier mortality occurred among patients with relatively “flat” rhythms, indicating a lack of normal diurnal variation (Cox proportional hazards, P = 0.0036) [
37].
A second study by the same group compared breast cancer patients with a group of normal controls [
37]. Participants included 17 metastatic breast cancer patients and 31 healthy female controls. Cancer treatment was not evaluated. The variability in cortisol concentrations is not limited to saliva. Touitou et al. [
38] found that eight out of 13 patients with breast cancer had deeply altered serum cortisol circadian patterns. The modifications were either high levels along the 24 h scale and/or erratic peaks and troughs and/or flattened profiles. Within 24 h, variations in tumor marker antigens as large as 70% were observed although no typical individual circadian patterns were found. While changes in absorption or clearance could explain the variation, the fluctuations appeared to be too rapid to be explained by those variables. Changes in feedback may explain the extreme variation; however, there are no experimental data to support this conjecture.
In non-metastatic breast cancer Wang et al. [
39] confirmed that lower cortisol levels were also associated with increased survival; 66 postmenopausal were diagnosed with estrogen receptor positive invasive, non-metastatic breast carcinoma, had the tumor surgically removed, and were scheduled for postoperative local or locoregional radiotherapy and adjuvant endocrine treatment. Serum samples were collected at six different time points [before the start of radiotherapy (as baseline), immediately after radiotherapy, and then 3, 6, 12 months, and 7-12 years after radiotherapy]. Serum steroid hormone concentrations that were measured before and immediately after radiotherapy differed between relapse and relapse-free patients [(accuracy 68.1%, p = 0.02, and 63.2%, p = 0.03, respectively, partial least squares discriminant analysis (PLS-DA)]. Baseline cortisol levels were lower in patients who relapsed than in those who did not (p < 0.05). Initially the Kaplan–Meier analysis showed that patients with high baseline concentrations of cortisol (≥median) had a significantly lower risk of breast cancer recurrence than patients with low cortisol levels (<median) (p = 0.02). During follow-up, there was a decrease in cortisol and cortisone concentrations in relapse-free patients, whereas these steroid hormones increased in patients who relapsed.
The association between flattened salivary cortisol patterns was confirmed in a more recent study of 217 patients with newly diagnosed metastatic renal cell carcinoma that had a life expectancy of greater than 4 months [
22]. Thus, the effect on salivary and plasma cortisol is not limited to metastatic breast cancer.
It is apparent that cancer patients with flattened, highly variable, diurnal cortisol patterns have decreased proportions of NK cells compared to those in patients with more normal circadian patterns. Salivary and serum cortisol levels are often elevated in the early stages of breast cancer and relapse is associated with maintenance of the elevated levels over time.
2.1. Role of Ultradian Rhythms of Cortisol Secretion
This extremely rapid pulsatile pattern may alter the expression of several genes. According to Lightman and Conway-Campbell [
40] dynamics of glucocorticoids interacting with their receptors represents a dynamic system that results in equilibrium throughout the neuroendocrine system. The dynamic state of cortisol levels is influenced by feed-forward and feedback loops, and by a random probability distribution at the level of DNA binding. The authors proposed that this continuous oscillatory activity is crucial for the optimal responsiveness of glucocorticoid-sensitive neural processes. Ultradian glucocorticoid rhythms are highly conserved across mammalian species; however, their functional significance is not fully understood. Flynn et al. [
44] demonstrated that pulsatile corticosterone replacement in adrenalectomized rats induced a pattern of glucocorticoid receptor (GR) binding at approximately 3,000 genomic sites in the liver at the pulse peak that was subsequently not found during the pulse nadir. In contrast, constant corticosterone replacement induced prolonged binding at most sites. In addition, each pattern induced markedly different transcriptional responses. In contrast to frequent pulses of secretion, constant corticosterone exposure induced significant effects on RNA polymerase II occupancy at the majority of gene targets, thus acting as a continuous regulatory signal for both the transactivation and repression of glucocorticoid target genes. The importance of circadian and ultradian patterns which alter glucocorticoid responses cannot be overemphasized and may be the primary HPA defect in women with metastatic breast cancer.
2.2. Role of Obesity in Breast Cancer Progression
Obesity may be one of the factors that may result in elevated cortisol levels in patients with metastatic breast cancer. Obesity is a risk factor for the development of hormone receptor-positive breast cancer in postmenopausal women but not in premenopausal women and has been associated with an increased risk of recurrence and reduced survival in postmenopausal but not premenopausal women [
41]. In humans, insulin resistance, associated with elevated cortisol concentrations, leads to obesity. Subclinical inflammation in visceral and subcutaneous adipose tissue of obese patients is commonly characterized by necrotic adipocytes surrounded by macrophages that form crown-like structures (CLS) [
42]. Such structures have been found in breast tissue of nearly 50% of obese patients at surgery. The severity of breast inflammation, defined as the CLS-B index, was correlated with the body mass index (P < 0.001) and adipocyte size (P = 0.01). NF-κB binding activity and elevated aromatase expression and activity were observed in the inflamed breast tissue of these women [
42]. Cortisol inhibits NF-κB at the transcriptional level [
43] and may inhibit the function of M1 macrophages (those characterized by high levels of proinflammatory cytokines) in this situation.
2.3. Associated Findings in Other Systems
Changes in the circadian rhythm of cortisol concentrations in patients with metastatic breast cancer are similar to those in patients with mild autonomous cortisol secretion (MACS), with elevated afternoon/evening serum or salivary cortisol concentrations. MACS patients often have adrenocortical adenomas and abnormal urine steroid profiles, but differ from metastatic breast cancer patients in that they have high glucocorticoid to androgen ratios and generally have higher nocturnal cortisol production [
44]. MACS patients had only 38% as much DHEA in 24-h urine as controls [
44] while metastatic breast cancer patients had significantly elevated DHEA serum levels [
45]. Androgen suppression in patients with MACS is contrary to the general stress response [
22], which may be explained by the fact that the source of glucocorticoids is often an adrenocortical adenoma. Further studies should be conducted in this area. Aberrant cortisol circadian rhythms have also been associated with other circadian abnormalities [
46], making it possible that these results reflect circadian abnormalities extending beyond the adrenocortical axis. Aberrant cortisol rhythms may be associated with hypersensitivity to stress [
47] or may disrupt other endocrine and immune rhythms [
48]. Although the disrupted patterns of serum cortisol in shift workers are more extreme than those associated with metastatic breast cancer, the associations with reduced immune tolerance [
49], impaired glucose metabolism, and increased inflammation [
50,
51] may apply to some degree.
Effects of abnormal hormonal patterns in combination with the psychological stress of cancer, may cause deterioration of disease resistance capabilities [
52].
3. Biochemical Activities of Cortisol
Cortisol is a lipid-soluble steroid with a solubility of 25 mM in ethanol and water solubility of less than 0.003 mM. It can diffuse across plasma membranes to bind to the glucocorticoid receptor without the need for a transport protein. Approximately 80 percent of cortisol in the circulation is bound to corticosteroid-binding globulin (CBG) and 15% to albumin. The remainder is free and capable of diffusing across the plasma membranes. CBG is a 50-60 kDa glycoprotein that binds cortisol at a 1:1 molar ratio with a Ka of 76 × 10
6 L/mol. It also binds progesterone with a Ka of 24 × 10
6 L/mol [
53].
Glucocorticoid receptors (GRs) are located in the cell cytoplasm and belong to the thyroid/retinoic acid receptor superfamily (NR3C1) of ligand-dependent transcription factors [
54]. GR is expressed in two isoforms: GRα and GRβ. GRα binds glucocorticoids (GCs) and are able to activate transcription; GRβ does not bind to any ligand and fails to activate transcription. Higher levels of GRβ lead to increased cortisol resistance. The type 2 receptor GRα is expressed in almost all tissues and cells and is responsible for most of the glucocorticoid effects in many different cell types. Type 1 GRα receptors bind GC, and mineralocorticoids (MC) with similar affinity are primarily involved in sodium and potassium transport activity. Binding of GCs to GRs results in the dissociation of heat shock proteins prior to translocation to the nucleus. Activation of GR target gene promoter regions occurs by the interaction of the zinc-finger domains of classic homodimeric GRα with specific GC-responsive elements (GREs) in the 5’ promoter region of a set of genes, thereby activating transcription [
58].
GR can also activate transcriptional networks via protein–protein interactions such as those with AP-1 (Jun/Fos) and NF-κB, and can result in a positive or negative transcriptional outcome depending on the target gene and cellular context [
55,
56]. The promoter regions of several genes involved in inflammatory processes, including cytokines, chemokines, and adhesion molecules, contain specific DNA sequences to which pro-inflammatory transcription factors NF-κB and/or AP-1 can bind [
57]. GR-GC binding to both AP-1 and NF-κB sites results in transrepression of the target gene. These genes play a central role in regulating inflammatory responses, particularly through the control of cytokine and chemokine gene expression [
58].
GCs maintain energy homeostasis and, during stress conditions, maintain elevated blood glucose levels to facilitate fight-or-flight responses [
59,
60,
61] by promoting gluconeogenesis and glycogen storage in the liver and by inhibiting glucose uptake by skeletal muscle and adipose tissue. GCs also stimulate pancreatic alpha cells to release glucagon, thereby promoting hyperglycemia and insulin resistance. Chronically high GC levels consequently promote the development of metabolic diseases. In addition, GCs induce reactive oxygen/nitrogen species and DNA damage. They also inhibit cyclooxygenase-2 and phospholipase A2, thus inhibiting inflammation by suppressing prostaglandin, leukotriene, and thromboxane formation [
43].
In the CNS, GCs influence a variety of functions, including learning and memory consolidation [
62] as well as mood and psychiatric disorders [
63]. Depression has been associated with elevated circulating levels of cortisol due to reduced negative feedback of cortisol and to mineralocorticoid receptor dysfunction [
14].
Cortisol plays an important role in limiting the access of leukocytes to sites of infection or cancer activity [
54]. It inhibits TNF-α and IL-1β cytokine production; it reduces the effects of ICAM-1 on the transmigration of neutrophils and monocytes across the endothelium; and it reduces the effects of VCAM-1 on the migration of lymphocytes, monocytes, and eosinophils.
Cortisol enhances the barrier properties of epithelial tissues by increasing the expression of tight junction (TJ) proteins [
64]. The endothelial cells within the capillary network are sealed by tight junctions that restrict paracellular permeability [
64]. Shang et al. [
65] concluded that both claudin-3 and claudin-4 mediate interactions with other cells in vivo, restraining growth and metastatic potential by sustaining the expression of E-cadherin and limiting β-catenin signaling. However, claudins may also have signaling activities that promote cell proliferation. How cortisol-induced expression of TJ proteins is involved in cell proliferation, transformation, and metastasis is not well understood [
66]. Nevertheless, experimental evidence suggests that TJ proteins are important players in the initiation and progression of cancer. The overall effect of cortisol may depend on the circumstances or stages of tumor development.
The full spectrum of activities has been described in detail in reviews by Sapolsky et al. [
22], Coutinho et al. [
67] and earlier by these investigators [
68]. They have described permissive, suppressive, stimulatory, and preparative functions of cortisol and proposed that stress-induced increases in glucocorticoid levels protect not against the source of stress itself but rather against the body’s normal reactions to stress, preventing those reactions from overshooting and threatening homeostasis [
68].
They proposed a hormetic effect, such that at lower concentrations, cortisol may be permissive, and at higher concentrations, it becomes suppressive. They also suggest that some enzymes, such as glutamine synthetase, are rapidly induced by glucocorticoids and that detoxification mediators are released during stress-induced activation of primary defense mechanisms. These mediators can lead to tissue damage if left unchecked [
22]. Thus, cortisol may play a beneficial role by protecting against toxic responses to stress, but persistently high doses may suppress beneficial responses to stress.
Glucocorticoids also have stimulatory and permissive functions. Patients with Addison’s disease or panhypopituitarism are notorious for growth retardation and intolerance to infection [
69], indicating an essential role of adrenocorticosteroid hormones in their stimulatory or permissive functions. Patients with primary adrenal insufficiency frequently experience fatigue, fever, infection, vomiting and diarrhea [
70]. Certainly, glucocorticoids play an important role in maintaining fluid balance, adequate glucose levels, and the release of serum immunoglobulins from the storehouse of immunoglobulins in lymphoid tissue [
71].
Cortisol Association with Illness
Many of the diseases which have been designated as ‘cortisol-potentiated’ occur most frequently in the elderly and are generally associated with age-related conditions, e.g., type II diabetes, obesity, osteoporosis, endogenous depression, cardiovascular disease [
72].
While serum cortisol concentrations are maintained with age [
73], DHEA and DHEA sulfate, which counteract many functions of cortisol, decline with age [
74]. Thus, there is a loss of immune-activating DHEA with a relative abundance of immunosuppressive effects of cortisol. In a study by Parker et al. [
75] basal serum cortisol levels were markedly increased in severe illness, whereas levels of the adrenal androgens DHEA and DHEAS tended to be lower (P < 0.01). The DHEA to cortisol and DHEAS to cortisol ratios were 4.4 and 3.4 times lower, respectively, in the ill group compared with those in the normal group (P < 0.001). Acute stimulation with adrenocorticotropic hormone (ACTH) demonstrated that the increase in cortisol was the same in normal and severely ill patients. However, the acute increase in serum DHEA concentration was significantly lower (P <0.05) in severely ill patients, indicating a decreased ACTH-stimulable DHEA reserve. Daily basal urinary unconjugated DHEA excretion was decreased, while cortisol excretion was increased, thereby decreasing the DHEA-to-cortisol ratio in the ill group by 85.4% (P < 0.001) relative to that in the normal group.
4. Suppression of Negative Feedback of Cortisol in the Hypothalamus
Circulating glucocorticoids are the primary negative feedback regulators of ACTH release. Glucocorticoids decrease the hypothalamic levels of CRH [
76] by reducing CRH mRNA [
77]. Glucocorticoids also act at the pituitary level by inhibiting the effect of CRH on POMC transcription and ACTH release [
78]. Cortisol negative feedback of ACTH secretion involves GR acting on negative GREs in the promoter region of POMC. Inhibition at this level suppresses not only the effect of CRH on ACTH secretion but suppresses other agonists such as arginine vasopressin (AVP) and the CRH-related peptides urocortin 1 (UCN1), urocortin 2 (UCN2), and urocortin 3 (UCN3).
In women with metastatic breast cancer, the afternoon/evening suppression of cortisol levels that normally occurs is reduced. As mentioned above, it is possible that GR availability to cortisol may be reduced by the binding of other steroids, or the interaction of the GR with the GRE in the promoter regions of the POMC and CRH genes may be reduced. It is also possible that the negative feedback of CRH on the CRH-R is deficient [
90].
To evaluate the feedback regulation of cortisol secretion, dexamethasone, a 1.0 mg dose was administered to metastatic breast cancer patients at 2200 h [
79]. Salivary cortisol concentrations measured during the following day were found to be significantly suppressed in patients that had shown typically decreasing concentrations of salivary cortisol with time of day (P < 0.01), but salivary cortisol in patients with flattened salivary cortisol patterns was not suppressed, indicating failure of normal hypothalamic negative feedback in patients with flatter slopes.
Ceulemans et al. [
80] also found that surgical patients with a lack of plasma cortisol suppression in the dexamethasone suppression test (DST) were associated with increased acute anxiety as determined by the State-Trait Inventory on the State scale (The Trait scale was not different) as well as elevated plasma cortisol levels. Depression assessed using the Hamilton scale was not significant. Other observations have also suggested that physiological or psychiatric stress may inhibit DST [
81,
82]. For example, in an experimental model employing rhesus monkeys, resistance to DST was associated with mean arousal ratings after nasogastric tube insertion [
83]. Although the DST is a proven method for determining whether excessive levels of cortisol are caused by a pituitary tumor, the reason for the reduction in negative feedback associated with metastatic breast cancer or major depression is not known.
Many aspects of feedback regulation complicate the investigation of this observation. The increase in cortisol levels in women with flattened profiles may be due to factors outside the hypothalamic-pituitary axis (HPA). Spiegel et al. [
79] carried out a CRH activation test (following 1.5 mg of dexamethasone to ensure that the effect was due to exogenous CRH in 93 metastatic breast cancer patients. ACTH and cortisol levels in serum measured at intervals from -16 to 120 min were highly correlated (r = 0.66, p < 0.0001, N = 74), indicating that the cortisol response of the adrenal gland could be attributed to CRH acting on ACTH, and no other stimuli were required [
79].
Reverse Effects of Stress on Cortisol Levels
Van der Pompe et al. [
84] measured the serum ACTH and cortisol levels in women with early breast cancer, metastatic breast cancer, and controls. Prior to a stress test, serum cortisol levels were significantly elevated in women with metastatic breast cancer, but ACTH levels were not different from those in controls. A stress test in which the participants recited a self-composed story resulted in a 23% decrease in serum cortisol in women with metastatic breast cancer and a 12% decline in women with early stage breast cancer after 37 min. ACTH was increased in women with early stage breast cancer and controls but remained unchanged in women with metastatic breast cancer during this time. The authors suggested that the decrease in serum cortisol may be due to increased metabolic clearance or it may be sequestered in sites outside the circulation (
Figure 2). These observations on stress in women with metastatic breast cancer should be replicated in future studies. The observations of both cortisol elevation and suppression without concomitant changes in ACTH levels indicate that CRH and ACTH are not the only important factors regulating cortisol secretion.
Other studies have confirmed that some types of stress may suppress serum cortisol levels. For example, lower plasma cortisol levels were observed in medical students preparing for a skydiving event compared to a similar control group of students, and there was a progressive decline in cortisol in blood samples taken from an indwelling catheter at 15-min intervals from the students traveling to the skydiving site [
25]. Another sample was taken immediately before entering the airplane for the skydive (
Figure 3), arrow on the graph). Four additional samples were taken at 15-min intervals beginning when the volunteers touched the ground after the skydive. Even when entering the airplane, cortisol levels were not elevated. Only the actual experience of skydiving caused a rapid and profound increase in plasma cortisol levels. Similarly, a situation has been described in which soldiers waiting for an enemy attack had lower than normal glucocorticoid production, whereas the responsible officers had high levels [
85]. In addition, radio officers on planes involved in practice landings on aircraft carriers did not have elevated urinary glucocorticoids, but the pilots who were conducting the landings did, despite the fact that radio officers were as much at risk as pilots [
86]. It may be that people who have the ability to alter their situation produce a cortisol response and those who cannot do not produce a cortisol response. Repeated exposure without the ability to adapt may also cause a failure of the cortisol response. Animal studies may be relevant in this regard. In rats with evidence of a hyperactive pituitary-adrenal system, repeated exposure to stress results in decreased corticosterone levels [
87]. Constant high levels of CRH, which may be present during chronic stress, may cause downregulation of the CRH receptor in the pituitary [
88].
Stress may result in elevated concentrations of serum free cortisol by interference with cortisol negative feedback at hypothalamic or pituitary levels. However, some forms of stress may act through mechanisms outside the HPA axis to suppress serum levels of cortisol. Although serum cortisol and ACTH are highly correlated in most physiological conditions, changes in CBG binding or metabolism may cause this relationship to fail.
5. Other Interacting Factors
5.1. Progesterone
Progesterone is known to compete for the glucocorticoid receptor but inhibition varies by cell type. Katula et al. [
88] found that the relative binding affinity of progesterone for the GR in cultured lymphoma cells was 25% that of dexamethasone. A hundred-fold excess of progesterone inhibited induction of tyrosine aminotransferase in HTC cells by only 44%, yet a ten-fold excess of progesterone inhibited binding of dexamethasone in these cells by 70% [
89]. Thus, the effect was disproportionately less than would be predicted by binding affinity of progesterone. Potentially progesterone or its metabolite allopregnanolone may inhibit the activity of cortisol in its role in feedback inhibition of ACTH secretion but at normal plasma concentrations this effect would appear to be minimal. Nevertheless, during pregnancy, when plasma progesterone concentrations are elevated, serum cortisol levels (both CBG-bound and free) are increased. This has been observed in both rats [
90] and humans [
91] suggesting that progesterone interferes with cortisol negative feedback on ACTH secretion.
In addition, progesterone may have stimulatory effects in the brain on the stress release of CRH. In the ewe chronic progesterone administration increased the response to a hypotensive stressor [
92]. Plasma progesterone concentrations were exogenously increased to a mean of 7.6 ng/ml by subcutaneous implants. This was a significant increase over the 2.3 ng/ml in untreated ewes. The treatment had no effect on plasma cortisol or glucose concentrations. However, the ACTH response to a stressor (nitroprusside induced hypotension) was approximately 80% greater in the progesterone treated ewes. Because AVP was also increased in the treated ewes, it appears that the effect was at a hypothalamic or higher level. This is consistent with effects of progesterone and its metabolite allopregnanolone as modulators of GABA(A) receptors in the brain [
93].
Progesterone acting via its metabolite allopregnanolone also increases serotonin turnover in the brain [
94], and serotonin stimulates CRH transcription [
95]. Jorgensen et al. increased neuronal 5-HT content in the rat by systemic administration of 5-hydroxytryptophan (5-HTP) in combination with the 5-HT reuptake inhibitor fluoxetine. Expression of rCRH mRNA in the paraventricular nucleus (PVN) was increased by 64%; pro-opiomelanocortin (POMC) mRNA in the anterior pituitary lobe was increased by 17% and ACTH secretion was stimulated five-fold [
95].
In addition to having a significant affinity for the cortisol receptor, a reciprocal relationship may exist between glucocorticoid and progestin binding sites; therefore, the binding of one class of steroid to its own site results in a decrease in the affinity of the other site for its respective class of steroid [
101]. Thus, the progesterone receptor complex may indirectly decrease the ability of the cortisol-GR complex to inhibit ACTH secretion.
Progesterone has been shown to be abundantly secreted in response to stress in rodents [
96] and there is evidence that stress increases progesterone, at least in the follicular phase of the menstrual cycle of women [
97]. Salivary concentrations of progesterone were highly correlated with salivary cortisol but the time-course of response to a cold pressor test was different with cortisol reaching high levels at 15 min post stress and progesterone reaching highest levels at 42 min [
97]. Also, Childs et al. [
98] using the Trier Social StressTest, [
93] found that women in the follicular phase of the menstrual cycle responded to the stressor with increased concentrations of serum progesterone. At 10 min after completion of the tasks, subjects had an average of 510 pg/mL progesterone while controls had 400 pg/ml (P < 0.05). Although not determined, the source of progesterone in this case is most likely the adrenal gland; it is, of course, an intermediate in the biosynthesis of corticosterone and cortisol. This response to low levels of progesterone may also apply to postmenopausal women.
Synthetic progestins may actually promote feedback inhibition of ACTH. Administration of a synthetic progestin (megestrol, 160 mg/day) decreased serum cortisol levels by 76%. This was partly due to a 25% decrease in the CBG [
93].
Progesterone is increased in gestation and may be increased with stress. It may act in one of several ways to either antagonize the feedback effect of cortisol on ACTH secretion or to increase ACTH secretion itself through an increase hypothalamic serotonin secretion.
5.2. Dehydroepiandrosterone (DHEA)
Although adrenal cells that produce DHEA and cortisol reside mainly in different parts of the adrenal cortex, there is no evidence that a separate DHEA-stimulating factor exists. Adrenal DHEA biosynthesis is dependent upon ACTH stimulation. Nevertheless, the secretion of cortisol and DHEA at times diverge as a function of steroid 17,20-lyase activity [
99]. This is apparent with age, when the serum concentrations of DHEA decline after the age of 30 [
100] while cortisol remains relatively unchanged [
101], and when serum DHEA increases during the first two decades of life more rapidly than serum cortisol [
102].
DHEA plays a significant role as an important modifier of the actions of cortisol [
72]. Cortisol is anti-inflammatory and immunosuppressive [
67] whereas DHEA activates the immune system [
103,
104]. The increased ratio of cortisol to DHEA with advanced age increases the exposure to the chronic effects of cortisol in the elderly and may be part of the reason that the incidence of cancer increases with age.
5.3. β-Arrestin
Elevated levels of cortisol increase the transcription of β-arrestin-1 and inhibit the transcription of β-arrestin-2 by liganded GR acting on GREs in intron-1 and intron-11, respectively [
108].
β-Arrestins act to regulate cortisol secretion by suppressing propagation of the CRH signal. Both β-arrestin isoforms have a negative effect on activated G-protein coupled receptors (GPCRs), suppressing the progression of POMC transcription [
105,
106], and the effect may be increased by as much as 30-fold following phosphorylation of the receptor by G-protein receptor kinases [
107]. In the process they shift the balance of CRH signaling from G protein-dependent coupling toward more β-arrestin-1–dependent signaling (activation of ERK).
In this way promotion of β-arrestin by cortisol may participate in the negative feedback of cortisol on ACTH secretion. On the other hand, insufficient stimulation of β-arrestin-1 by cortisol or alteration in the affinity of GPCR by reduction in G-protein-receptor kinase activity would increase CRH-1 activity at its receptor, increasing ACTH (
Figure 4). More work needs to be done on the stimuli that alter transcription of the β-arrestins.
6. Mechanisms by Which Cortisol Modifies Cancer Risk
6.1. Cortisol Effects on Metabolism
In neoplastically transformed cells, genetic mutations have occurred, resulting in metabolic reprogramming, which enables continuous cell proliferation and survival. Many processes undergo reprogramming. Among them are glucose metabolism, fatty acid metabolism, glutamine metabolism, and pathways that directly affect the tumor microenvironment [
108]. Cortisol plays an important role in the metabolic changes under conditions in which cortisol secretion is increased. It increases glucose availability through glycogenolysis, gluconeogenesis, lipolysis, decreases in insulin sensitivity, and inhibition of glucose uptake by the skeletal muscle and adipose tissue. The increased availability of glucose provides a substrate for aerobic glycolysis, a process promoted by cancer cells (the Warburg effect). Cortisol also promotes glutamine synthetase biosynthesis for the conversion of glutamate to glutamine, an essential energy source for cancer cells.
6.2. Interaction of Cortisol with IGF-I
Cortisol inhibits growth hormone-promoted transcription of IGF-I, and immunoreactive IGF-I levels in fibroblast cultures decrease in response to glucocorticoids [
109]. The converse is also true. Early work in the author’s lab demonstrated the role of growth hormone (GH) in opposing the effect of cortisol on leukocytopoiesis. Homogenates of the anterior pituitary, but not of the posterior pituitary or skeletal muscle, prevented cortisol-induced leukopenia in hypophysectomized, adrenalectomized rats. Growth hormone of rat origin (NIH-GH-S9) also opposed cortisol-induced leukopenia in hypophysectomized rats [
110]. Other studies have shown stimulation of DNA synthesis in human T-lymphocytes by growth hormones [
111] and enhancement of cytotoxic activity of human natural killer (NK) cells [
112]. This action is mediated by the local production of IGF-I. Growth stimulation is completely inhibited by preincubation with either an antibody to the IGF-I receptor or IGF-I itself [
113].
The relationship is complex because growth hormones stimulate the formation of IGF-I. Administration of GH may result in GH-receptor activation or secondarily may increase IGF-I transcription; both may stimulate kinase pathways, e.g., phosphoinositide 3-kinase (PI3K) and MAPK. While IGF-I acts to counteract the lymphocytolytic action of cortisol, cortisol has been shown to be necessary for the ontogeny of pituitary somatotrophs [
114].
According to Basu et al. [
115] information is emerging in several organ systems that support the role of GH-regulated inflammation in cancer. Overall, it is evident that autocrine- or paracrine-derived GH can potentially exacerbate local inflammation in the tumor microenvironment.
6.3. Potentiation of Stress Responses by Catecholamines
Stimulation of the adrenal gland by ACTH results in increased concentrations of cortisol, DHEA, and DHEA sulfate within the adrenal cortex and medulla. Increasing concentrations of DHEA sulfate stimulate norepinephrine biosynthesis in the adrenal medulla by increasing both mRNA and protein levels of tyrosine hydroxylase [
27]. It also promotes trafficking of catecholamine vesicles [
27]. In addition, cortisol, which is present in high concentrations in blood traversing the medulla, increases the expression of phenylethanolamine N-methyltransferase, which is essential for the conversion of norepinephrine to epinephrine, the main catecholamine released from the adrenal medulla in response to stress [
116].
In addition, glucocorticoids increase plasma levels of catecholamines by inhibiting their extra-neuronal uptake and induce super-sensitivity to catecholamines in the heart and other organs by upregulating components of the beta-adrenoceptor signal transduction system [
117,
118]. Thus, stressors that activate the HPA axis also increase catecholamine secretion, increase blood levels of catecholamines by inhibiting uptake, and increase the response to catecholamines in some tissues.
6.4. Effects of Cortisol on the Immune System
The glucocorticoid receptor GR is ubiquitously expressed in almost all human tissues and organs, including neural stem cells and lymphoblasts. GR functions as a hormone-dependent transcription factor that regulates the expression of glucocorticoid-responsive genes, which represent 3-10% of the human genome.
Glucocorticoids can cause neutrophilic leukocytosis, eosinopenia, monocytopenia, and lymphocytopenia. Cortisol brings about neutrophilic leukocytosis by inhibiting the function of neutrophil adhesion molecules (e.g., L-selectin, integrins). Blocking the adhesion molecules prevents trans-endothelial migration, keeping neutrophils in circulation and away from inflamed tissues. Although neutrophils are major sources of pro-inflammatory cytokines, reactive oxygen species, and proteases, without tissue infiltration they are unable to amplify local inflammation or contribute to tissue or tumor damage.
Granulocytes are minimally affected but monocyte-macrophage function is sensitive to glucocorticoids. GC administration causes transient lymphocytopenia in all detectable lymphocyte subpopulations, particularly recirculating thymus-derived lymphocytes. The mechanism of lymphocytopenia is probably the redistribution of circulating cells to other body compartments [
119].
Ligand-bound GRs act as potent immunosuppressive agents that interfere with binding of AP-1 and Nf-κB to their DNA binding sites, resulting in suppression of transcription of inflammatory cytokines such as the interleukins IL-1, IL-6, IL-8, and TNFα. And they promote an anti-inflammatory Th2 reaction by inhibition of IFN-γ and stimulation of IL-4, IL-10 and TGF-β secretion [
120]. Thus, cortisol elevation acts to suppress availability of neutrophils to the antigen-expressing cells, suppresses the circulation of Th1 cells, inhibits Th1 cytokine secretions, and increases the transcription of anti-inflammatory cytokines (
Figure 5).
6.4.1. Effect of GC on Lymphocytes.
Glucocorticoids enhance cytokine production of regulatory T cells (Tregs) by upregulation of FOXP3 expression [
121]. Tregs are a specialized subpopulation of T cells that play a key role in maintaining tolerance to self-antigens and in suppression of excessive immune responses after antigenic stimulation [
122]. In addition, early steps in activation of lymphocytes may be inhibited by cAMP formation as a result of glucocorticoid exposure [
123]. Indeed, treatment of resting human lymphocytes with dexamethasone increases prostaglandin E2-stimulation of cyclic AMP accumulation in a time- and concentration-dependent manner.
Treatment with the glucocorticoid agonist dexamethasone has no significant effect on adrenoceptor density, or immunodetectable G-proteins, and pertussis toxin substrates are not affected [
123,
124]. It was concluded in these studies that dexamethasone treatment promotes cAMP formation in resting human lymphocytes by altering the adenylyl cyclase enzyme activity rather than G-proteins or hormone receptors. Thus, glucocorticoid exposure results in enhanced capability of cyclic AMP-generating agonists to inhibit the early steps of lymphocyte activation and lymphocyte proliferation.
6.4.2. Effect of Cortisol on Tumor-Infiltrating Lymphocytes
Cortisol may have a significant effect on the transfer of tumor-associated lymphocytes (TALs) or tumor-infiltrating lymphocytes (TILs) to the antigen-bearing tumor cells. The activation of the intercellular adhesion molecule-1 (ICAM-1) gene by TNFα from macrophages is an essential step in early inflammatory events, leading to the recruitment of leukocytes to the site of inflammation. TNFα activates transcription of the ICAM-1 gene via the NF-κB signaling pathway in the human ICAM-1 gene promoter region [
125]. Glucocorticoids suppress ICAM-1 transcription by the ability of ligand-bound GR to interfere with NF-κB signaling at the NF-κB binding site without increasing IkBα expression. According to Liden et al. [
126] the conformational change observed at the NF-kB binding site of GR may be controlled either by direct binding of the GR to the NF-κB complex or by GR-induced recruitment or displacement of other proteins.
6.5. The Tumor Microenvironment
The microenvironmental stroma contributes to host defense. In a dermatological study by Boxman et al. [
127], fibroblasts exposed to conditioned media derived from cultures of normal human keratinocytes or squamous carcinoma cells (SCC-4) produced elevated levels of both IL-8 and IL-6 mRNAs, as well as their respective proteins. Additional studies have shown that IL-1α derived from keratinocytes induced the proliferation of fibroblasts and the secretion of IL-6 and IL-8 in a concentration-dependent manner [
127,
128,
129]. These pro-inflammatory interleukins promote cancer progression. IL-6 modulates immune cells, stromal cells and endothelial cells to support tumor growth [
130]. IL-8 recruits neutrophils and myeloid-derived suppressor cells (MDSCs) and enhances epithelial to mesenchymal transition (EMT) [
131]. Stromal cells add to the protumor environment by their ability to express the GR. These include: fibroblasts, macrophages, adipocytes, and immune cells. Gene expression profiling studies have compared tumor-associated stroma to normal stroma and have shown that cancerous cells can dramatically increase stromal GR mRNA expression [
132,
133].
GR expression is increased significantly with grade in stroma isolated from paraffin-embedded tissues (
Figure 6), whereas progesterone receptor (PR) expression is significantly decreased compared to that in the control stroma [
133] (not shown). A combination of increased GR expression and elevated concentrations of circulating cortisol act to suppress the local immune response and promote tumor cell proliferation.
To oppose the production of inflammatory cytokines in the stroma liganded GR directly interacts with NF-κB, preventing it from binding to DNA, reducing the transcription of pro-inflammatory genes [
134]. GR activation also leads to the expression of IκB, an inhibitor that prevents NF-κB from translocating to the nucleus [
135]. As a result, in the tumor microenvironment, glucocorticoids suppress the production of the pro-inflammatory cytokines IL-2, TNFα, and IFNγ and induce the secretion of IL-10.
In addition, elevation of GC dramatically induces immune checkpoint receptors, including PD-1, Tim3, and Lag3, thereby protecting the tumor from TILs. On the other hand, loss of GR potentiates the response to immune checkpoint expression [
12]. Analysis of RNA profiles showed that Nr3c1, the gene encoding the glucocorticoid receptor, was most highly expressed in the Tim-3-PD-1+ CD8+ and Tim-3+PD-1+ CD8+TIL subsets, which contain effector and terminally dysfunctional CD8+ TILs, respectively [
136]. Thus, cortisol may protect tumor cells from TIL, allowing the tumor to progress.
The high levels of GR observed in breast cancer [
133] relates to the findings reported by Matossian et al. [
137] and the observation that high GR expression is associated with poor prognosis in women with ER negative breast cancer. In a recent study patients with untreated primary triple negative breast cancer (TNBC) a significantly higher percentages of immunosuppressive tumor-infiltrating FOXP3+ and BATF3+ cells were found in tumors with high levels of GR compared to those with low levels of GR suggesting that GR expression is associated with an immunosuppressed microenvironment [
13]. Thus, locally produced GR in the microenvironment along with endogenous cortisol results in an environment in which the host defense is compromised. On the other hand, in women with ER+ tumors interaction of liganded GR with the ERE, correlates with improved relapse-free survival in patients [
138]. Currently, glucocorticoids are commonly used in conjunction with immune checkpoint inhibitors, but the pros and cons of glucocorticoids as immunomodulators in cancer immunotherapy remain to be determined at this time [
139,
140].
Effects of Cortisol on Macrophages
Macrophages are essential components of both innate and adaptive immunity and play a central role in host defense and inflammation. Activated macrophages are divided into two subsets, (M1) and (M2). The M1 phenotype is characterized by the expression of high levels of pro-inflammatory cytokines, including IL-12, IL-23, and tumor necrosis factor-α (TNFα), as well as high nitric oxide and reactive oxygen intermediate production. In contrast, cells of the M2 phenotype typically produce IL-10 and the IL-1 receptor antagonist (IL-1Ra), and have high levels of scavenger, mannose, and galactose receptors.
The phenotypes of polarized M1 and M2 macrophages can be reversed in vitro and in vivo. Cytokines and growth factors are involved in reprogramming of M1 and M2 macrophages. Interferon-c (IFN-c) induces M1 macrophages, whereas stimulation of macrophages with IL-4 or IL-13 induces M2 macrophages [
141].
Cortisol promotes polarization of macrophages toward the M2 phenotype, which is involved in the anti-inflammatory response. Glucocorticoids also have profound effects on the antigen presentation to T cells by macrophages. Corticosteroid drugs inhibit the expression of surface I-region-associated (Ia) antigens by peritoneal macrophages both in vitro and in vivo, reduce the production of IL-1, and inhibit antigen presentation for T cell proliferation by macrophages. In one study the doses of cortisol or prednisolone, which inhibit 50% Ia expression in cultured macrophages, ranged from 2 to 5 × 10
−8 M and were well within the range of endogenous hormones [
142].
In support of the immunosuppressive activity cortisol on macrophages, cortisol upregulates HSD11β type 1 in macrophages [
32,
33], T- and B-cells, and dendritic cells [
34,
35], thus maintaining the active form of cortisol for its role in promoting the M2 macrophage phenotype.
6.6. Cortisol Acting Through Corticosteroid Binding Globulin (CBG)
Corticosteroid binding globulin (CBG) is a 50-60 kDa glycoprotein that binds cortisol at a 1:1 molar ratio with a Ka of 76 × 10
6 L/mol; it also binds progesterone with a Ka of 24 × 10
6 L/mol [
53]. About 80 percent of cortisol in the circulation is bound to CBG and 15% to albumin. The remainder is free and capable of diffusing across plasma membranes. The plasma concentration of CBG the liver is increased by estrogen in oral contraceptives or by Premarin or diethylstilbestrol by as much as 2 to 3-fold [
143] and is suppressed by glucocorticoids [
144]. However, at a cellular level neither estradiol, testosterone, nor dexamethasone alters the production of CBG by HepG2 cells [
145,
146] indicating an indirect process. CBG is produced by the kidney and other organs to some extent but this is unlikely to be the source of the increase due to estrogen administration. An effect on CBG biosynthesis by IL-6 has been shown in HepG2 cells [
147]. When Hep G2 cells were exposed to different concentrations of IL-6 for variable time intervals, IL-6 caused a dose- and time-dependent decrease in the amount of [
35S]methionine-labeled CBG immuno-precipitated from the culture medium. This effect could be greatly reduced by preincubation of IL-6 with its neutralizing antibody and reversed by removing the cytokine from the culture medium. This potentially will decrease the binding capacity of CBG and increase the availability of free cortisol in situations of prolonged stress.
Another way that CBG interacts with cortisol is in binding to hepatic plasma membranes. Scatchard analysis of the soluble receptor at 37 C showed a single set of high affinity binding sites, with a Kd of 44 nM and a binding capacity of 7.3 pmol/mg protein. The association rate constant (k1) was 0.92 × 10
5 M
−1 min
−1 at 37 °C, and the dissociation rate constant (k2) was 1.0 × 10
−3 min
−1. Only unliganded CBG was able to bind to the plasma membrane receptor. Steroids that bind to CBG, e.g. corticosterone, cortisol, and progesterone noncompetitively inhibit binding of CBG to its receptor [
148].
Under normal conditions CBG would not traverse the endothelium. However, binding of cortisol to the previously liganded CBG on the breast cancer cell line MCF-7 results in the induction of adenylate cyclase activity and the accumulation of cAMP cells [
149],
Figure 7.
The data are consistent with the hypothesis that CBG is a prohormone which is activated when cortisol becomes bound to it. In hepatocytes activation of adenylyl cyclase produces cAMP which results in glycogen breakdown and inhibition of glycogen biosynthesis, a process that may provide initial substrate for cancer growth. In the breast cancer epithelium a function that may be relevant is an effect on phosphorylation of junctional proteins (e.g., claudins, occludins), reducing epithelial barrier permeability [
150]. How cortisol-induced expression of TJ proteins is involved in cell proliferation, transformation, and metastasis is not well understood [
66]. Nevertheless, experimental evidence suggests that TJ proteins are important players in the initiation and progression of cancer.
The maintenance of the CBG-cortisol complex is dependent on a correct cellular organization of the tissue, which is largely dependent on stromal-derived chemokines [
151]. In lymphocytes membrane-bound CBG inhibits cAMP-dependent protein kinase A (PKA) [
124] by blocking Ras activation [
152], and by increasing the production of anti-inflammatory IL-10 [
153]. Thus, cortisol may act through CBG binding to inhibit host defense by suppressing lymphocyte proliferation and increasing anti-proliferative cytokines, thus protecting the tumor from the immune system.
7. Cortisol vs DHEA as Risk Factors for Breast Cancer
An increase in serum DHEA concentration in breast cancer has been considered a potential risk factor. Odds ratios as high as 4 have been associated with serum DHEA levels and breast cancer incidence in epidemiological studies. However, numerous experimental studies have shown that DHEA and DHEA sulfate (DHEAS) reduce the risk of mammary cancer (reviewed in Chatterton 2022 [
154] and 2024 [
45] and DHEA may antagonize a number of cortisol responses [
72]. The mechanisms by which DHEA suppresses tumor growth includes the non-competitive inhibition of glucose-6-phosphate dehydrogenase, inhibition of cholesterol biosynthesis, immune suppression in virally induced breast cancer, enhancement of natural killer cell cytotoxicity by both DHEA and DHEAS, suppression of IL-6, and promotion of estrogen receptor beta expression [
45].
Therefore, it has been postulated that the increased secretion of DHEA in breast cancer patients is a result of ACTH stimulation of adrenal steroidogenesis, which increases both DHEA and cortisol, with cortisol being the greater of the two, and the steroid that increases the risk of metastatic breast cancer [
45]. DHEA has the benefit of increasing host defense and by opposing metabolic of cortisol.
8. Effects of Glucocorticoids in Cancer Therapy
An interesting question is how glucocorticoids interact with or modify the effects of breast cancer prevention and cancer treatment regimens that are under study in clinical trials.
8.1. Effect of Glucocorticoids on DNA Damage Response Therapy
Siramulu et al. [
155] describe clinical trials that focus on DNA repair mechanisms after radiation exposure. It is expected that target-based radio-sensitization approaches would increase the effectiveness of radiotherapy by selectively sensitizing tumor tissue to ionizing radiation [
156]. Recently, a variety of approaches have been used to develop radiosensitizers that are highly effective and have low toxicity [
157]. A number of molecular targets that are sensitive to radiation killing are being studied. These include ATM, TR, CDK1, CDK4/6, CHK1, PARP1, WEE1, and MPS1/TTK. The drugs that are being studied include inhibitors of PARP1 (Olaprib and Niraparib) and inhibitors of HER2, EGFR, VEGF, and mTOR. The effect of glucocorticoids on these systems has not been studied directly. However, the evidence is that elevations of stress hormones will facilitate the process of DNA damage in cells exposed to radiation. Flint et al. [
158] have shown that dexamethasone or epinephrine increase the rate of damage in 3T3 cells manyfold. They further showed that anti-progestin RU486 is able to reverse the effects of the glucocorticoid and that propranolol will reverse the effects of epinephrine. The mechanism may involve an effect of stress hormones on reactive oxygen species (ROS) and reactive nitrogen species (RNS). In experiments of Flaherty et al. [
159] acute exposure to cortisol and NE significantly increased levels of ROS/RNS and DNA damage and this effect was diminished in the presence of receptor antagonists. Cortisol induced DNA damage and the production of ROS was further attenuated in the presence of an iNOS inhibitor. An increase in the expression of iNOS in response to psychological stress was observed in vivo and in cortisol-treated cells. Thus, elevations in glucocorticoids will likely make the inhibitors more effective in cell killing. This is counter to the general effect of glucocorticoids on tumor proliferation.
Hormone receptor (HR) positive breast cancers respond differently to GR [
138] than HR negative cancers. [
160]. In early stage HR negative breast cancer high glucocorticoid receptor (GR) expression is associated with increased cancer cell proliferation and with shortened relapse-free survival (RFS) [
160]. It was observed that cells derived from an adjuvant chemotherapy-treated Discovery cohort with a GR signature that contained GR activation-associated genes was found to predict the probability of relapse. A separate validation study confirmed this observation in ER negative cancer (Hazard ratio = 1.9; P = 0.012) [
160]. Although many of the factors by which the liganded GR stimulates cancer progression are not known, the immunosuppressive activity of GCs and the altered metabolism resulting in increased availability of glucose and glucosamine among other factors probably contribute to tumor growth as described earlier in this review.
On the other hand, GCs have the opposite effect on cancer cell proliferation in HR+ breast cancer. Glucocorticoids are associated with slowed ER-mediated proliferation in ER+ breast cancer cell lines and longer RFS in breast cancer patients [
138]. It is not immediately clear why the presence of estrogen dependency should reverse the expected effects of glucocorticoids. However, GR liganding with either a pure agonist or a selective GR modulator (SGRM) slowed estradiol (E2)-mediated proliferation in ER+ breast cancer models. SGRMs that antagonized transcription of GR-unique genes both promoted GR chromatin association and inhibited ER chromatin localization at common DNA enhancer sites. Further, Porter et al. [
161] found that SGRMs that antagonized transcription of GR-unique genes both promoted GR chromatin association and inhibited ER chromatin localization at common DNA enhancer sites. Gene expression analysis revealed that ER and GR co-activation decreased proliferative gene activation (compared to ER activation alone), specifically reducing CCND1, CDK2, and CDK6 gene expression. Thus, the interaction of liganded GR with ER results in inhibition of estrogen-promoted cell proliferation in estrogen-dependent breast cancer cells.
8.2. Effect of Glucocorticoids on Immune Checkpoint Inhibitor Therapy
According Acharya et al. [
12] active glucocorticoid signaling is associated with failure to respond to immune checkpoint blockade in both pre-clinical tumor models and melanoma patients. Although the immune system has the capacity to fight cancer, signals present within the tumor microenvironment (TME) actively suppress anti-tumor immune responses. In particular, CD8+ T cells, key mediators of anti-tumor immunity, undergo altered effector differentiation that culminates in the development of a dysfunctional or “exhausted” state [
162,
163].Dysfunctional CD8+ T cells exhibit defective cytotoxicity, pro-inflammatory cytokine production, and induction of the immunosuppressive cytokine interleukin (IL)-10 [
164]. Among potentially responsible factors, Nr3c1, the gene encoding the glucocorticoid receptor (GR), was found to be the most highly expressed in terminally dysfunctional Tim-3+PD-1+ CD8+ TILs. A gradient of increasing GC signaling from naïve to dysfunctional CD8+ TILs was identified [
12]. They found that repeated activation in the presence of GC profoundly suppressed the production of the pro-inflammatory cytokines IL-2, TNF- α, and IFN-γ, and induced the immune suppressive cytokine IL-10, a phenotype consistent with dampened effector function. Additionally, they found that GC treatment dramatically induced immune checkpoint receptors, including PD-1, Tim-3, and Lag-3, but not Tigit in both murine and human CD8+ T cells. The observed effects of GC were not due to reduced T cell survival or altered proliferation. Thus, glucocorticoids decrease functionality of TILs and interfere with the effectiveness of immune checkpoint blockade.
8.3. Effect of Glucocorticoids on Retinoid Drug Therapy
Brown et al. [
165] reviewed targeted therapy for breast cancer prevention. Among compounds being evaluated were inhibitors of vitamin D and retinoid receptors. For example, Hidalgo et al. [
166] found that dexamethasone increased vitamin D receptor (VDR) mediated transcription in the human CYP24A1 promoter. Both VDR protein levels and ligand binding were increased in squamous cell carcinoma in a time-dependent manner. The effect was steroid specific and not produced by dihydrotestosterone.
Bagnoud et al. [
167] studied the relationship between GR and VDR signaling. They found that vitamin D (VitD) reduces experimental autoimmune encephalomyelitis disease severity in wild-type (WT) but not in T cell-specific glucocorticoid (GC) receptor-deficient mice (GR(lck). Mice lacking the GR have diminished VitD-enhanced T cell apoptosis and T regulatory cell differentiation in vitro in CD3+ T cells. Mechanistically, VitD does not appear to signal directly via the GR, as it does not bind to the GR, does not induce its nuclear translocation, and does not modulate the expression of two GR-induced genes. However, they observed that VitD enhances VDR protein expression in CD3+ T cells from WT but not GR(lck) mice in vitro, that the GR and the VDR spatially co-localize after VitD treatment, and that VitD does not modulate the expression of two VDR-induced genes in the absence of the GR. The data suggest that a functional GR, specifically in T cells, is required for the VDR to signal appropriately to mediate the therapeutic effects of VitD.
These data indicate that the GR-GC complex is essential for VDR signaling. An increase in GR-GC may assure the activity of VDR. Because RXR requires VDR as an obligate partner to bind DNA for regulation of transcription, increases in VDR may enhance the ability of drugs like IRX4204 and LG100268 to bring about programmed cell death of cancer cells. Thus, in the pharmacologic setting, glucocorticoids act to facilitate the treatment effect. However, the negative effects of GCs on the immune system may obviate the benefit.
8.4. Effect of Glucocorticoids in PARP1-Related Drug Therapy
Promising agents are currently being tested for prevention of triple negative breast cancer. Among them are poly(ADP-ribose) polymerase inhibitors [
165]. PARP1 has a role in breast cancer, mediated by its interaction with the progesterone receptor (PR) [
15]. Initially PARP1 was discovered as part of a protein complex that could interact in vitro with ligand-activated PR and assist in DNA binding [
168]. PARP1 activation also leads to a global increase in poly(ADP-ribose (PAR) levels, modifying histones, transcription factors (TF), nuclear enzymes and nuclear structural proteins. Parylation of histones regulates chromatin structure [
169] and recruits DNA repair proteins such as p53, and has also been reported to parylate and modify the function of numerous transcription factors, including AP1, NF-κB, CTCF, and YY1 [
170].
At least six new PARP1 inhibitors have been evaluated including fluzoparib and pamiparib [
171] But because of resistance to PARP1 inhibitors, dual-target inhibitors that greatly improve the antitumor effect of PARP1 inhibitors, reduce toxicity, and evade resistance by synergistically targeting multiple targets have been developed. Combinations of PARP1 inhibitors and BRD4, PI3K, HDAC, PD-1/PD-L1 inhibitors among others have been evaluated.
There is much evidence that GCs consistently counteract the effect of cytotoxic agents, reducing cell death [
172]. The mechanism by which GCs confer protection has not been fully elucidated. However, PARP1 and GR act in opposition to each other regulating transcription factor activity such as NF-κB and AP1. While PARP1 promotes NF-κB and AP1 [
170], GC treatment induces the downregulation of the survival factor NF-κB and increases FAS and FASL leading to caspase 8 activation and apoptosis.
Inhibitors of PARP should oppose recruitment of enzymes of DNA damage repair and oppose activation of PR. GCs should facilitate the effect of PARP inhibitors by increasing the rate of DNA damage and suppressing transcription factors activation [
4]. Inhibitors of PARP1 therefore will be promoted by the effect of glucocorticoids. Both will inhibit NF-κB and AP1 and both will oppose DNA repair. Nevertheless, the dose and duration of GCs probably should be limited because of their effects on the immune and other organ systems.
Summary
Cortisol is regulated by input form the nervous system, and cortisol negative feedback is suppressed by feedback from a number of sources including DHEA and β-arrestin, and progesterone may stimulate ACTH secretion via stimulation of hypothalamic serotonin.
Cortisol is produced in a number of organ sites in addition to the adrenal cortex in the body including the immune system, and peripheral activity is further regulated by 11β-oxidoreductases.
Cortisol is often dis-regulated in metastatic breast cancer with increased variability and increased peripheral concentrations, and this is common to a number of other cancers and chronic disease states.
Cortisol modifies metabolism in a manner that supports cancer by increasing glucose and glutamine availability, but is opposed by actions of IGF-I and DHEA.
Cortisol bound to its intracellular receptor blocks transcription of Nf-κB and AP-1, reducing inflammatory cytokines and the antitumor immune activity. It also binds to membrane-bound CBG, activating adenylyl cyclase in T cells, suppressing proliferation.
Cortisol is immunosuppressive, with specific effects on lymphocytes and macrophages. It also modifies the availability of immune cells to the tumor in part by suppressing ICAM-1 and by increasing the expression of immune checkpoint receptors such as PD-1 and Tim3 and promoting Treg cell proliferation.
Cortisol is opposed by effects of DHEA and DHEA sulfate on metabolism and the immune system.
Glucocorticoid agonists may provide benefit in retinoid therapy, PARP1 therapy, and therapy in ER+ cancers but not with therapy involving DNA damage in ER negative cancer or immune checkpoint blockade therapy.
References
- Kageyama, K.; Iwasaki, Y.; Daimon, M. Hypothalamic Regulation of Corticotropin-Releasing Factor under Stress and Stress Resilience. Int. J. Mol. Sci. 2021, 22, 12242. [Google Scholar] [CrossRef]
- Zhu, Y.; Wu, X.; Zhou, R.; Sie, O.; Niu, Z.; Wang, F.; Fang, Y. Hypothalamic-Pituitary-End-Organ Axes: Hormone Function in Female Patients with Major Depressive Disorder. Neurosci. Bull. 2021, 37, 1176–1187. [Google Scholar] [CrossRef]
- Engeland, W.C. Functional innervation of the adrenal cortex by the splanchnic nerve. Horm. Metab. Res. 1998, 30, 311–314. [Google Scholar] [CrossRef]
- Taves, M.D.; Gomez-Sanchez, C.E.; Soma, K.K. Extra-adrenal glucocorticoids and mineralocorticoids: Evidence for local synthesis, regulation, and function. Am. J. Physiol. Endocrinol. Metab. 2011, 301, E11–E24. [Google Scholar] [CrossRef]
- Gomez-Sanchez, C.E.; Gomez-Sanchez, E.P. Extra-adrenal Glucocorticoid and Mineralocorticoid Biosynthesis. Endocrinology 2022, 163, bqac016. [Google Scholar] [CrossRef] [PubMed]
- Slominski, A.; Zbytek, B.; Pisarchik, A.; Slominski, R.M.; Zmijewski, M.A.; Wortsman, J. CRH functions as a growth factor/cytokine in the skin. J. Cell. Physiol. 2006, 206, 780–791. [Google Scholar] [CrossRef] [PubMed]
- Ottaviani, E.; Franchini, A.; Franceschi, C. Evolution of neuroendocrine thymus: Studies on POMC-derived peptides, cytokines and apoptosis in lower and higher vertebrates. J. Neuroimmunol. 1997, 72, 67–74. [Google Scholar] [CrossRef]
- Kishimoto, W.; Hiroi, T.; Shiraishi, M.; Osada, M.; Imaoka, S.; Kominami, S.; Igarashi, T.; Funae, Y. Cytochrome P450 2D catalyze steroid 21-hydroxylation in the brain. Endocrinology 2004, 145, 699–705. [Google Scholar] [CrossRef]
- Taylor, C.; Crosby, I.; Yip, V.; Maguire, P.; Pirmohamed, M.; Turner, R.M. A Review of the Important Role of CYP2D6 in Pharmacogenomics. Genes 2020, 11, 1295. [Google Scholar] [CrossRef] [PubMed]
- Briones, A.M.; Nguyen Dinh Cat, A.; Callera, G.E.; Yogi, A.; Burger, D.; He, Y.; Corrêa, J.W.; Gagnon, A.M.; Gomez-Sanchez, C.E.; Gomez-Sanchez, E.P.; et al. Adipocytes produce aldosterone through calcineurin-dependent signaling pathways: Implications in diabetes mellitus-associated obesity and vascular dysfunction. Hypertension 2012, 59, 1069–1078. [Google Scholar] [CrossRef]
- Phan, T.S.; Schink, L.; Mann, J.; Merk, V.M.; Zwicky, P.; Mundt, S.; Simon, D.; Kulms, D.; Abraham, S.; Legler, D.F.; et al. Keratinocytes control skin immune homeostasis through de novo-synthesized glucocorticoids. Sci. Adv. 2021, 7, eabe0337. [Google Scholar] [PubMed]
- Acharya, N.; Madi, A.; Zhang, H.; Klapholz, M.; Escobar, G.; Dulberg, S.; Christian, E.; Ferreira, M.; Dixon, K.O.; Fell, G.; et al. Endogenous Glucocorticoid Signaling Regulates CD8(+) T Cell Differentiation and Development of Dysfunction in the Tumor Microenvironment. Immunity 2020, 53, 658–671.e6. [Google Scholar]
- Parker, K.J.; Schatzberg, A.F.; Lyons, D.M. Neuroendocrine aspects of hypercortisolism in major depression. Horm. Behav. 2003, 43, 60–66. [Google Scholar] [CrossRef]
- Steckler, T.; Holsboer, F.; Reul, J.M. Glucocorticoids and depression. Baillieres Best Pract. Res. Clin. Endocrinol. Metab. 1999, 13, 597–614. [Google Scholar] [CrossRef]
- Tirabassi, G.; Boscaro, M.; Arnaldi, G. Harmful effects of functional hypercortisolism: A working hypothesis. Endocrine 2014, 46, 370–386. [Google Scholar] [PubMed]
- Ceccato, F.; Terzolo, M.; Scaroni, C. Who and how to screen for endogenous hypercortisolism in a high-risk population: A special issue of the journal of endocrinological investigations. J. Endocrinol. Investig. 2024, 48, 1–2. [Google Scholar] [CrossRef]
- Abraham, S.B.; Rubino, D.; Sinaii, N.; Ramsey, S.; Nieman, L.K. Cortisol, obesity, and the metabolic syndrome: A cross-sectional study of obese subjects and review of the literature. Obesity 2013, 21, E105–E117. [Google Scholar] [CrossRef] [PubMed]
- Zoli, A.; Lizzio, M.M.; Ferlisi, E.M.; Massafra, V.; Mirone, L.; Barini, A.; Scuderi, F.; Bartolozzi, F.; Magaró, M. ACTH, cortisol and prolactin in active rheumatoid arthritis. Clin. Rheumatol. 2002, 21, 289–293. [Google Scholar] [CrossRef]
- Masi, A.T.; Elmore, K.B.; Rehman, A.A.; Chatterton, R.T.; Goertzen, N.J.; Aldag, J.C. Lower Serum Androstenedione Levels in Pre-Rheumatoid Arthritis versus Normal Control Women: Correlations with Lower Serum Cortisol Levels. Autoimmune Dis. 2013, 2013, 593493. [Google Scholar] [CrossRef]
- Peeters, F.; Nicolson, N.A.; Berkhof, J. Levels and variability of daily life cortisol secretion in major depression. Psychiatry Res. 2004, 126, 1–13. [Google Scholar] [CrossRef]
- Cohen, L.; Cole, S.W.; Sood, A.K.; Prinsloo, S.; Kirschbaum, C.; Arevalo, J.M.; Jennings, N.B.; Scott, S.; Vence, L.; Wei, Q.; et al. Depressive symptoms and cortisol rhythmicity predict survival in patients with renal cell carcinoma: Role of inflammatory signaling. PLoS ONE 2012, 7, e42324. [Google Scholar] [CrossRef]
- Sapolsky, R.M.; Romero, L.M.; Munck, A.U. How do glucocorticoids influence stress responses? Integrating permissive, suppressive, stimulatory, and preparative actions. Endocr. Rev. 2000, 21, 55–89. [Google Scholar]
- Morgan, C.A., 3rd; Southwick, S.; Hazlett, G.; Rasmusson, A.; Hoyt, G.; Zimolo, Z.; Charney, D. Relationships among plasma dehydroepiandrosterone sulfate and cortisol levels, symptoms of dissociation, and objective performance in humans exposed to acute stress. Arch. Gen. Psychiatry 2004, 61, 819–825. [Google Scholar] [CrossRef]
- Balsalobre-Fernandez, C.; Tejero-Gonzalez, C.M.; del Campo-Vecino, J. Relationships between training load, salivary cortisol responses and performance during season training in middle and long distance runners. PLoS ONE 2014, 9, e106066. [Google Scholar] [CrossRef]
- Chatterton, R.T., Jr.; Vogelsong, K.M.; Lu, Y.C.; Hudgens, G.A. Hormonal responses to psychological stress in men preparing for skydiving. J. Clin. Endocrinol. Metab. 1997, 82, 2503–2509. [Google Scholar] [CrossRef] [PubMed]
- Topor, L.S.; Asai, M.; Dunn, J.; Majzoub, J.A. Cortisol stimulates secretion of dehydroepiandrosterone in human adrenocortical cells through inhibition of 3betaHSD2. J. Clin. Endocrinol. Metab. 2011, 96, E31–E39. [Google Scholar] [CrossRef] [PubMed]
- Charalampopoulos, I.; Dermitzaki, E.; Vardouli, L.; Tsatsanis, C.; Stournaras, C.; Margioris, A.N.; Gravanis, A. Dehydroepiandrosterone sulfate and allopregnanolone directly stimulate catecholamine production via induction of tyrosine hydroxylase and secretion by affecting actin polymerization. Endocrinology 2005, 146, 3309–3318. [Google Scholar] [CrossRef]
- Cadegiani, F.A.; Kater, C.E. Adrenal fatigue does not exist: A systematic review. BMC Endocr. Disord. 2016, 16, 48. [Google Scholar]
- Garbrecht, M.R.; Klein, J.M.; McCarthy, T.A.; Schmidt, T.J.; Krozowski, Z.S.; Snyder, J.M. 11-Beta hydroxysteroid dehydrogenase type 2 in human adult and fetal lung and its regulation by sex steroids. Pediatr. Res. 2007, 62, 26–31. [Google Scholar] [CrossRef] [PubMed]
- Edwards, C.R.; Benediktsson, R.; Lindsay, R.S.; Seckl, J.R. 11 beta-Hydroxysteroid dehydrogenases: Key enzymes in determining tissue-specific glucocorticoid effects. Steroids 1996, 61, 263–269. [Google Scholar] [CrossRef]
- Kaur, K.; Hardy, R.; Ahasan, M.M.; Eijken, M.; van Leeuwen, J.P.; Filer, A.; Thomas, A.M.; Raza, K.; Buckley, C.D.; Stewart, P.M.; et al. Synergistic induction of local glucocorticoid generation by inflammatory cytokines and glucocorticoids: Implications for inflammation associated bone loss. Ann. Rheum. Dis. 2010, 69, 1185–1190. [Google Scholar] [CrossRef]
- Thieringer, R.; Le Grand, C.B.; Carbin, L.; Cai, T.Q.; Wong, B.; Wright, S.D.; Hermanowski-Vosatka, A. 11 Beta-hydroxysteroid dehydrogenase type 1 is induced in human monocytes upon differentiation to macrophages. J. Immunol. 2001, 167, 30–35. [Google Scholar]
- Gilmour, J.S.; Coutinho, A.E.; Cailhier, J.F.; Man, T.Y.; Clay, M.; Thomas, G.; Harris, H.J.; Mullins, J.J.; Seckl, J.R.; Savill, J.S.; et al. Local amplification of glucocorticoids by 11 beta-hydroxysteroid dehydrogenase type 1 promotes macrophage phagocytosis of apoptotic leukocytes. J. Immunol. 2006, 176, 7605–7611. [Google Scholar] [CrossRef] [PubMed]
- Zhang, T.Y.; Ding, X.; Daynes, R.A. The expression of 11 beta-hydroxysteroid dehydrogenase type I by lymphocytes provides a novel means for intracrine regulation of glucocorticoid activities. J. Immunol. 2005, 174, 879–889. [Google Scholar] [CrossRef]
- Freeman, L.; Hewison, M.; Hughes, S.V.; Evans, K.N.; Hardie, D.; Means, T.K.; Chakraverty, R. Expression of 11beta-hydroxysteroid dehydrogenase type 1 permits regulation of glucocorticoid bioavailability by human dendritic cells. Blood 2005, 106, 2042–2049. [Google Scholar] [CrossRef]
- Sephton, S.E.; Sapolsky, R.M.; Kraemer, H.C.; Spiegel, D. Diurnal cortisol rhythm as a predictor of breast cancer survival. J. Natl. Cancer Inst. 2000, 92, 994–1000. [Google Scholar] [CrossRef] [PubMed]
- Abercrombie, H.C.; Giese-Davis, J.; Sephton, S.; Epel, E.S.; Turner-Cobb, J.M.; Spiegel, D. Flattened cortisol rhythms in metastatic breast cancer patients. Psychoneuroendocrinology 2004, 29, 1082–1092. [Google Scholar] [CrossRef]
- Touitou, Y.; Bogdan, A.; Levi, F.; Benavides, M.; Auzeby, A. Disruption of the circadian patterns of serum cortisol in breast and ovarian cancer patients: Relationships with tumour marker antigens. Br. J. Cancer 1996, 74, 1248–1252. [Google Scholar] [CrossRef] [PubMed]
- Wang, F.; Giskeodegard, G.F.; Skarra, S.; Engstrom, M.J.; Hagen, L.; Geisler, J.; Mikkola, T.S.; Tikkanen, M.J.; Debik, J.; Reidunsdatter, R.J.; et al. Association of serum cortisol and cortisone levels and risk of recurrence after endocrine treatment in breast cancer. Clin. Exp. Med. 2023, 23, 3883–3893. [Google Scholar] [CrossRef]
- Lightman, S.L.; Conway-Campbell, B.L. The crucial role of pulsatile activity of the HPA axis for continuous dynamic equilibration. Nat. Rev. Neurosci. 2010, 11, 710–718. [Google Scholar] [CrossRef]
- Zhao, H.; Wang, J.; Fang, D.; Lee, O.; Chatterton, R.T.; Stearns, V.; Khan, S.A.; Bulun, S.E. Adiposity Results in Metabolic and Inflammation Differences in Premenopausal and Postmenopausal Women Consistent with the Difference in Breast Cancer Risk. Horm. Cancer 2018, 9, 229–239. [Google Scholar] [CrossRef]
- Morris, P.G.; Hudis, C.A.; Giri, D.; Morrow, M.; Falcone, D.J.; Zhou, X.K.; Du, B.; Brogi, E.; Crawford, C.B.; Kopelovich, L.; et al. Inflammation and increased aromatase expression occur in the breast tissue of obese women with breast cancer. Cancer Prev. Res. 2011, 4, 1021–1029. [Google Scholar] [CrossRef]
- Scheinman, R.I.; Gualberto, A.; Jewell, C.M.; Cidlowski, J.A.; Baldwin, A.S., Jr. Characterization of mechanisms involved in transrepression of NF-kappa B by activated glucocorticoid receptors. Mol. Cell Biol. 1995, 15, 943–953. [Google Scholar] [CrossRef]
- Saini, J.; Singh, S.; Ebbehoj, A.; Zhang, C.D.; Nathani, R.; Fell, V.; Atkinson, E.; Achenbach, S.; Rivard, A.; Singh, R.; et al. Steroid Profiling and Circadian Cortisol Secretion in Patients With Mild Autonomous Cortisol Secretion: A Cross-sectional Study. J. Clin. Endocrinol. Metab. 2025, 110, 542–553. [Google Scholar] [CrossRef] [PubMed]
- Chatterton, R.T., Jr. Dehydroepiandrosterone (DHEA) in relation to breast cancer. Vitam. Horm. 2024, 129, 61–97. [Google Scholar] [PubMed]
- Mormont, M.C.; Levi, F. Circadian-system alterations during cancer processes: A review. Int. J. Cancer 1997, 70, 241–247. [Google Scholar] [CrossRef]
- Rosmond, R.; Dallman, M.F.; Bjorntorp, P. Stress-related cortisol secretion in men: Relationships with abdominal obesity and endocrine, metabolic and hemodynamic abnormalities. J. Clin. Endocrinol. Metab. 1998, 83, 1853–1859. [Google Scholar] [CrossRef]
- Kronfol, Z.; Nair, M.; Zhang, Q.; Hill, E.E.; Brown, M.B. Circadian immune measures in healthy volunteers: Relationship to hypothalamic-pituitary-adrenal axis hormones and sympathetic neurotransmitters. Psychosom. Med. 1997, 59, 42–50. [Google Scholar] [CrossRef]
- Arendt, J. Shift work: Coping with the biological clock. Occup. Med. 2010, 60, 10–20. [Google Scholar] [CrossRef]
- Chandola, T.; Brunner, E.; Marmot, M. Chronic stress at work and the metabolic syndrome: Prospective study. BMJ 2006, 332, 521–525. [Google Scholar] [CrossRef]
- Buxton, O.M.; Marcelli, E. Short and long sleep are positively associated with obesity, diabetes, hypertension, and cardiovascular disease among adults in the United States. Soc. Sci. Med. 2010, 71, 1027–1036. [Google Scholar] [CrossRef]
- McEwen, B.S. Protective and damaging effects of stress mediators. N. Engl. J. Med. 1998, 338, 171–179. [Google Scholar] [CrossRef]
- Lee, J.H.; Meyer, E.J.; Nenke, M.A.; Falhammar, H.; Torpy, D.J. Corticosteroid-binding globulin (CBG): Spatiotemporal distribution of cortisol in sepsis. Trends Endocrinol. Metab. TEM 2023, 34, 181–190. [Google Scholar] [CrossRef]
- Salvador, E.; Shityakov, S.; Forster, C. Glucocorticoids and endothelial cell barrier function. Cell Tissue Res. 2014, 355, 597–605. [Google Scholar] [CrossRef]
- De Bosscher, K.; Desmet, S.J.; Clarisse, D.; Estebanez-Perpina, E.; Brunsveld, L. Nuclear receptor crosstalk—Defining the mechanisms for therapeutic innovation. Nat. Rev. Endocrinol. 2020, 16, 363–377. [Google Scholar] [CrossRef]
- Subramaniam, N.; Cairns, W.; Okret, S. Glucocorticoids repress transcription from a negative glucocorticoid response element recognized by two homeodomain-containing proteins, Pbx and Oct-1. J. Biol. Chem. 1998, 273, 23567–23574. [Google Scholar] [CrossRef]
- Verstrepen, L.; Bekaert, T.; Chau, T.L.; Tavernier, J.; Chariot, A.; Beyaert, R. TLR-4, IL-1R and TNF-R signaling to NF-kappaB: Variations on a common theme. Cell Mol. Life Sci. 2008, 65, 2964–2978. [Google Scholar] [CrossRef] [PubMed]
- Yang-Yen, H.F.; Chambard, J.C.; Sun, Y.L.; Smeal, T.; Schmidt, T.J.; Drouin, J.; Karin, M. Transcriptional interference between c-Jun and the glucocorticoid receptor: Mutual inhibition of DNA binding due to direct protein-protein interaction. Cell 1990, 62, 1205–1215. [Google Scholar] [CrossRef] [PubMed]
- Kuo, T.; McQueen, A.; Chen, T.C.; Wang, J.C. Regulation of Glucose Homeostasis by Glucocorticoids. Adv. Exp. Med. Biol. 2015, 872, 99–126. [Google Scholar] [PubMed]
- Vegiopoulos, A.; Herzig, S. Glucocorticoids, metabolism and metabolic diseases. Mol. Cell. Endocrinol. 2007, 275, 43–61. [Google Scholar] [CrossRef]
- Joels, M. Impact of glucocorticoids on brain function: Relevance for mood disorders. Psychoneuroendocrinology 2011, 36, 406–414. [Google Scholar] [CrossRef]
- Liston, C.; Cichon, J.M.; Jeanneteau, F.; Jia, Z.; Chao, M.V.; Gan, W.B. Circadian glucocorticoid oscillations promote learning-dependent synapse formation and maintenance. Nat. Neurosci. 2013, 16, 698–705. [Google Scholar] [CrossRef]
- Olejniczak, I.; Oster, H.; Ray, D.W. Glucocorticoid circadian rhythms in immune function. Semin. Immunopathol. 2022, 44, 153–163. [Google Scholar] [PubMed]
- Forster, C. Tight junctions and the modulation of barrier function in disease. Histochem. Cell Biol. 2008, 130, 55–70. [Google Scholar] [CrossRef]
- Shang, X.; Lin, X.; Alvarez, E.; Manorek, G.; Howell, S.B. Tight junction proteins claudin-3 and claudin-4 control tumor growth and metastases. Neoplasia 2012, 14, 974–985. [Google Scholar] [CrossRef] [PubMed]
- Runkle, E.A.; Mu, D. Tight junction proteins: From barrier to tumorigenesis. Cancer Lett. 2013, 337, 41–48. [Google Scholar] [CrossRef] [PubMed]
- Coutinho, A.E.; Chapman, K.E. The anti-inflammatory and immunosuppressive effects of glucocorticoids, recent developments and mechanistic insights. Mol. Cell Endocrinol. 2011, 335, 2–13. [Google Scholar] [CrossRef]
- Munck, A.; Naray-Fejes-Toth, A. Glucocorticoids and stress: Permissive and suppressive actions. Ann. N. Y Acad. Sci. 1994, 746, 115–130; discussion 31–33. [Google Scholar]
- De La Balze, F.A.; Reifenstein, E.C., Jr.; Albright, F. Differential blood counts in certain adrenal cortical disorders (Cushing’s syndrome, Addison’s disease and panhypopituitarism). J. Clin. Endocrinol. Metab. 1946, 6, 312–319. [Google Scholar] [CrossRef]
- Gjerstad, J.K.; Lightman, S.L.; Spiga, F. Role of glucocorticoid negative feedback in the regulation of HPA axis pulsatility. Stress 2018, 21, 403–416. [Google Scholar] [CrossRef]
- Dougherty, T.F.; White, A. Pituitary-adrenal cortical control of lymphocyte structure and function as revealed by experimental X-radiation. Endocrinology 1946, 39, 370–385. [Google Scholar] [CrossRef] [PubMed]
- Hechter, O.; Grossman, A.; Chatterton, R.T., Jr. Relationship of dehydroepiandrosterone and cortisol in disease. Med. Hypotheses 1997, 49, 85–91. [Google Scholar] [CrossRef] [PubMed]
- Roelfsema, F.; van Heemst, D.; Iranmanesh, A.; Takahashi, P.; Yang, R.; Veldhuis, J.D. Impact of age, sex and body mass index on cortisol secretion in 143 healthy adults. Endocr. Connect. 2017, 6, 500–509. [Google Scholar] [CrossRef]
- Orentreich, N.; Brind, J.L.; Rizer, R.L.; Vogelman, J.H. Age changes and sex differences in serum dehydroepiandrosterone sulfate concentrations throughout adulthood. J. Clin. Endocrinol. Metab. 1984, 59, 551–555. [Google Scholar] [CrossRef]
- Parker, L.N.; Levin, E.R.; Lifrak, E.T. Evidence for adrenocortical adaptation to severe illness. J. Clin. Endocrinol. Metab. 1985, 60, 947–952. [Google Scholar] [CrossRef]
- Wynn, P.C.; Harwood, J.P.; Catt, K.J.; Aguilera, G. Corticotropin-releasing factor (CRF) induces desensitization of the rat pituitary CRF receptor-adenylate cyclase complex. Endocrinology 1988, 122, 351–358. [Google Scholar] [CrossRef]
- Beyer, H.S.; Matta, S.G.; Sharp, B.M. Regulation of the messenger ribonucleic acid for corticotropin-releasing factor in the paraventricular nucleus and other brain sites of the rat. Endocrinology 1988, 123, 2117–2123. [Google Scholar] [CrossRef]
- Oki, Y.; Peatman, T.W.; Qu, Z.C.; Orth, D.N. Effects of intracellular Ca2+ depletion and glucocorticoid on stimulated adrenocorticotropin release by rat anterior pituitary cells in a microperifusion system. Endocrinology 1991, 128, 1589–1596. [Google Scholar] [CrossRef]
- Spiegel, D.; Giese-Davis, J.; Taylor, C.B.; Kraemer, H. Stress sensitivity in metastatic breast cancer: Analysis of hypothalamic-pituitary-adrenal axis function. Psychoneuroendocrinology 2006, 31, 1231–1244. [Google Scholar] [CrossRef]
- Ceulemans, D.L.; Westenberg, H.G.; van Praag, H.M. The effect of stress on the dexamethasone suppression test. Psychiatry Res. 1985, 14, 189–195. [Google Scholar] [CrossRef] [PubMed]
- Carroll, B.J.; Greden, J.F.; Feinberg, M.; Lohr, N.; James, N.M.; Steiner, M.; Haskett, R.F.; Albala, A.A.; DeVigne, J.-P.; Tarika, J. Neuroendocrine evaluation of depression in borderline patients. Psychiatr. Clin. North. Am. 1981, 4, 89–99. [Google Scholar]
- Meltzer, H.Y.; Fang, V.S.; Tricou, B.J.; Robertson, A.; Piyaka, S.K. Effect of dexamethasone on plasma prolactin and cortisol levels in psychiatric patients. Am. J. Psychiatry 1982, 139, 763–768. [Google Scholar] [CrossRef]
- Kalin, N.H.; Cohen, R.M.; Kraemer, G.W.; Risch, S.C.; Shelton, S.; Cohen, M.; McKinney, W.T.; Murphy, D.L. The dexamethasone suppression test as a measure of hypothalamic-pituitary feedback sensitivity and its relationship to behavioral arousal. Neuroendocrinology 1981, 32, 92–95. [Google Scholar] [CrossRef]
- van der Pompe, G.; Antoni, M.H.; Heijnen, C.J. Elevated basal cortisol levels and attenuated ACTH and cortisol responses to a behavioral challenge in women with metastatic breast cancer. Psychoneuroendocrinology 1996, 21, 361–374. [Google Scholar] [CrossRef]
- Miller, R.G.; Rubin, R.T.; Clark, B.R.; Crawford, W.R.; Arthur, R.J. The stress of aircraft carrier landings. Psychosom. Med. 1970, 32, 581–588. [Google Scholar] [CrossRef]
- Bourne, P.G.; Rose, R.M.; Mason, J.W. 17-OHCS levels in combat. Arch. Gen. Psychiatry 1968, 19, 135–140. [Google Scholar] [CrossRef] [PubMed]
- De Souza, E.B.; Van Loon, G.R. Stress-induced inhibition of the corticosterone response to a subsequent stress in rats: A nonadrenocorticotropin-mediated mechanism. Endocrinology 1982, 110, 23–33. [Google Scholar] [CrossRef] [PubMed]
- Kontula, K.; Paavonen, T.; Luukkainen, T.; Andersson, L.C. Binding of progestins to the glucocorticoid receptor. Correlation to their glucocorticoid-like effects on in vitro functions of human mononuclear leukocytes. Biochem. Pharmacol. 1983, 32, 1511–1518. [Google Scholar] [CrossRef] [PubMed]
- Rousseau, G.G.; Baxter, J.D.; Tomkins, G.M. Glucocorticoid receptors: Relations between steroid binding and biological effects. J. Mol. Biol. 1972, 67, 99–115. [Google Scholar] [CrossRef]
- Brunton, P.J. Resetting the dynamic range of hypothalamic-pituitary-adrenal axis stress responses through pregnancy. J. Neuroendocrinol. 2010, 22, 1198–1213. [Google Scholar] [CrossRef]
- Nierop, A.; Wirtz, P.H.; Bratsikas, A.; Zimmermann, R.; Ehlert, U. Stress-buffering effects of psychosocial resources on physiological and psychological stress response in pregnant women. Biol. Psychol. 2008, 78, 261–268. [Google Scholar] [CrossRef]
- Keller-Wood, M. ACTH responses to hypotension and feedback inhibition of ACTH increased by chronic progesterone treatment. Am. J. Physiol. 1998, 274, R81–R87. [Google Scholar] [CrossRef] [PubMed]
- Guennoun, R. Progesterone in the Brain: Hormone, Neurosteroid and Neuroprotectant. Int. J. Mol. Sci. 2020, 21, 5271. [Google Scholar] [CrossRef] [PubMed]
- Ladisich, W. Influence of progesterone on serotonin metabolism: A possible causal factor for mood changes. Psychoneuroendocrinology 1977, 2, 257–266. [Google Scholar] [CrossRef]
- Jorgensen, H.; Knigge, U.; Kjaer, A.; Moller, M.; Warberg, J. Serotonergic stimulation of corticotropin-releasing hormone and pro-opiomelanocortin gene expression. J. Neuroendocrinol. 2002, 14, 788–795. [Google Scholar] [CrossRef]
- Brown, G.P.; Courtney, G.A.; Marotta, S.F. A comparative study of adrenal progesterone secretion during the estrous cycles of hamsters and rats. Steroids 1976, 28, 283–294. [Google Scholar] [CrossRef]
- Herrera, A.Y.; Nielsen, S.E.; Mather, M. Stress-induced increases in progesterone and cortisol in naturally cycling women. Neurobiol. Stress 2016, 3, 96–104. [Google Scholar] [CrossRef]
- Childs, E.; Dlugos, A.; De Wit, H. Cardiovascular, hormonal, and emotional responses to the TSST in relation to sex and menstrual cycle phase. Psychophysiology 2010, 47, 550–559. [Google Scholar] [CrossRef] [PubMed]
- Baulieu, E.E. Androgens and aging men. Mol. Cell. Endocrinol. 2002, 198, 41–49. [Google Scholar] [CrossRef]
- Baulieu, E.E.; Thomas, G.; Legrain, S.; Lahlou, N.; Roger, M.; Debuire, B.; Faucounau, V.; Girard, L.; Hervy, M.-P.; Latour, F.; et al. Dehydroepiandrosterone (DHEA), DHEA sulfate, and aging: Contribution of the DHEAge Study to a sociobiomedical issue. Proc. Natl. Acad. Sci. USA 2000, 97, 4279–4284. [Google Scholar] [CrossRef]
- Yiallouris, A.; Tsioutis, C.; Agapidaki, E.; Zafeiri, M.; Agouridis, A.P.; Ntourakis, D.; Johnson, E.O. Adrenal Aging and Its Implications on Stress Responsiveness in Humans. Front. Endocrinol. 2019, 10, 54. [Google Scholar] [CrossRef]
- Greaves, R.F.; Wudy, S.A.; Badoer, E.; Zacharin, M.; Hirst, J.J.; Quinn, T.; Walker, D.W. A tale of two steroids: The importance of the androgens DHEA and DHEAS for early neurodevelopment. J. Steroid Biochem. Mol. Biol. 2019, 188, 77–85. [Google Scholar] [CrossRef]
- Solerte, S.B.; Fioravanti, M.; Vignati, G.; Giustina, A.; Cravello, L.; Ferrari, E. Dehydroepiandrosterone sulfate enhances natural killer cell cytotoxicity in humans via locally generated immunoreactive insulin-like growth factor I. J. Clin. Endocrinol. Metab. 1999, 84, 3260–3267. [Google Scholar] [CrossRef]
- Mirlekar, B.; Pylayeva-Gupta, Y. IL-12 Family Cytokines in Cancer and Immunotherapy. Cancers 2021, 13, 167. [Google Scholar] [CrossRef]
- Oakley, R.H.; Olivares-Reyes, J.A.; Hudson, C.C.; Flores-Vega, F.; Dautzenberg, F.M.; Hauger, R.L. Carboxyl-terminal and intracellular loop sites for CRF1 receptor phosphorylation and beta-arrestin-2 recruitment: A mechanism regulating stress and anxiety responses. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2007, 293, R209–R222. [Google Scholar] [CrossRef]
- Kohout, T.A.; Lefkowitz, R.J. Regulation of G protein-coupled receptor kinases and arrestins during receptor desensitization. Mol. Pharmacol. 2003, 63, 9–18. [Google Scholar] [CrossRef]
- Reiter, E.; Lefkowitz, R.J. GRKs and beta-arrestins: Roles in receptor silencing, trafficking and signaling. Trends Endocrinol. Metab. TEM 2006, 17, 159–165. [Google Scholar] [CrossRef] [PubMed]
- Andryszkiewicz, W.; Gasiorowska, J.; Kubler, M.; Kublinska, K.; Palkiewicz, A.; Wiatkowski, A.; Szwedowicz, U.; Choromańska, A. Glucose Metabolism and Tumor Microenvironment: Mechanistic Insights and Therapeutic Implications. Int. J. Mol. Sci. 2025, 26, 1879. [Google Scholar] [CrossRef] [PubMed]
- McCarthy, T.L.; Centrella, M.; Canalis, E. Cortisol inhibits the synthesis of insulin-like growth factor-I in skeletal cells. Endocrinology 1990, 126, 1569–1575. [Google Scholar] [CrossRef] [PubMed]
- Chatterton, R.T., Jr.; Murray, C.L.; Hellman, L. Endocrine effects on leukocytopoiesis in the rat. I. Evidence for growth hormone secretion as the leukocytopoietic stimulus following acute cortisol-induced lymphopenia. Endocrinology 1973, 92, 775–787. [Google Scholar] [CrossRef]
- Gelato, M.C. Growth hormone-insulinlike growth factor I and immune function. Trends Endocrinol. Metab. TEM 1993, 4, 106–110. [Google Scholar] [CrossRef]
- Bozzola, M.; Valtorta, A.; Moretta, A.; Cisternino, M.; Biscaldi, I.; Schimpff, R.M. In vitro and in vivo effect of growth hormone on cytotoxic activity. J. Pediatr. 1990, 117, 596–599. [Google Scholar] [CrossRef]
- Geffner, M. Effects of growth hormone and insulin-like growth factor I on T- and B-lymphocytes and immune function. Acta Paediatr. Suppl. 1997, 423, 76–79. [Google Scholar] [CrossRef]
- Klug, S.T.; Ellestad, L.E.; Porter, T.E. Pituitary-Targeted Knockout of Glucocorticoid Receptors Disrupts Growth Hormone Expression During Embryonic Development. Endocrinology 2025, 166, bqaf119. [Google Scholar] [CrossRef]
- Basu, R.; Boguszewski, C.L.; Kopchick, J.J. Growth Hormone Action as a Target in Cancer: Significance, Mechanisms, and Possible Therapies. Endocr. Rev. 2025, 46, 224–280. [Google Scholar] [CrossRef]
- Betito, K.; Diorio, J.; Meaney, M.J.; Boksa, P. Adrenal phenylethanolamine N -methyltransferase induction in relation to glucocorticoid receptor dynamics: Evidence that acute exposure to high cortisol levels is sufficient to induce the enzyme. J. Neurochem. 1992, 58, 1853–1862. [Google Scholar] [CrossRef] [PubMed]
- Adameova, A.; Abdellatif, Y.; Dhalla, N.S. Role of the excessive amounts of circulating catecholamines and glucocorticoids in stress-induced heart disease. Can. J. Physiol. Pharmacol. 2009, 87, 493–514. [Google Scholar] [CrossRef]
- Hadcock, J.R.; Malbon, C.C. Regulation of beta-adrenergic receptors by “permissive” hormones: Glucocorticoids increase steady-state levels of receptor mRNA. Proc. Natl. Acad. Sci. USA 1988, 85, 8415–8419. [Google Scholar] [CrossRef]
- Fauci, A.S.; Dale, D.C.; Balow, J.E. Glucocorticosteroid therapy: Mechanisms of action and clinical considerations. Ann. Intern. Med. 1976, 84, 304–315. [Google Scholar] [CrossRef] [PubMed]
- Rhen, T.; Cidlowski, J.A. Antiinflammatory action of glucocorticoids--new mechanisms for old drugs. N. Engl. J. Med. 2005, 353, 1711–1723. [Google Scholar] [CrossRef] [PubMed]
- Ugor, E.; Prenek, L.; Pap, R.; Berta, G.; Ernszt, D.; Najbauer, J.; Németh, P.; Boldizsár, F.; Berki, T. Glucocorticoid hormone treatment enhances the cytokine production of regulatory T cells by upregulation of Foxp3 expression. Immunobiology 2018, 223, 422–431. [Google Scholar] [CrossRef] [PubMed]
- Sakaguchi, S.; Yamaguchi, T.; Nomura, T.; Ono, M. Regulatory T cells and immune tolerance. Cell 2008, 133, 775–787. [Google Scholar] [CrossRef]
- Michel, M.C.; Knapp, J.; Ratjen, H. Sensitization by dexamethasone of lymphocyte cyclic AMP formation: Evidence for increased function of the adenylyl cyclase catalyst. Br. J. Pharmacol. 1994, 113, 240–246. [Google Scholar] [CrossRef]
- Stork, P.J.; Schmitt, J.M. Crosstalk between cAMP and MAP kinase signaling in the regulation of cell proliferation. Trends Cell Biol. 2002, 12, 258–266. [Google Scholar] [CrossRef] [PubMed]
- van de Stolpe, A.; Caldenhoven, E.; Stade, B.G.; Koenderman, L.; Raaijmakers, J.A.; Johnson, J.P.; van der Saag, P. 12-O-tetradecanoylphorbol-13-acetate- and tumor necrosis factor alpha-mediated induction of intercellular adhesion molecule-1 is inhibited by dexamethasone. Functional analysis of the human intercellular adhesion molecular-1 promoter. J. Biol. Chem. 1994, 269, 6185–6192. [Google Scholar] [CrossRef]
- Liden, J.; Rafter, I.; Truss, M.; Gustafsson, J.A.; Okret, S. Glucocorticoid effects on NF-kappaB binding in the transcription of the ICAM-1 gene. Biochem. Biophys. Res. Commun. 2000, 273, 1008–1014. [Google Scholar] [CrossRef]
- Boxman, I.L.; Ruwhof, C.; Boerman, O.C.; Lowik, C.W.; Ponec, M. Role of fibroblasts in the regulation of proinflammatory interleukin IL-1, IL-6 and IL-8 levels induced by keratinocyte-derived IL-1. Arch. Dermatol. Res. 1996, 288, 391–398. [Google Scholar] [CrossRef]
- Postlethwaite, A.E.; Lachman, L.B.; Kang, A.H. Induction of fibroblast proliferation by interleukin-1 derived from human monocytic leukemia cells. Arthritis Rheum. 1984, 27, 995–1001. [Google Scholar] [CrossRef]
- Schmidt, J.A.; Mizel, S.B.; Cohen, D.; Green, I. Interleukin 1, a potential regulator of fibroblast proliferation. J. Immunol. 1982, 128, 2177–2182. [Google Scholar] [CrossRef] [PubMed]
- Taher, M.Y.; Davies, D.M.; Maher, J. The role of the interleukin (IL)-6/IL-6 receptor axis in cancer. Biochem. Soc. Trans. 2018, 46, 1449–1462. [Google Scholar] [CrossRef]
- Waugh, D.J.; Wilson, C. The interleukin-8 pathway in cancer. Clin. Cancer Res. Off. J. Am. Assoc. Cancer Res. 2008, 14, 6735–6741. [Google Scholar] [CrossRef]
- Finak, G.; Bertos, N.; Pepin, F.; Sadekova, S.; Souleimanova, M.; Zhao, H.; Chen, H.; Omeroglu, G.; Meterissian, S.; Omeroglu, A.; et al. Stromal gene expression predicts clinical outcome in breast cancer. Nat. Med. 2008, 14, 518–527. [Google Scholar] [CrossRef]
- Smith, R.A.; Lea, R.A.; Weinstein, S.R.; Griffiths, L.R. Progesterone, glucocorticoid, but not estrogen receptor mRNA is altered in breast cancer stroma. Cancer Lett. 2007, 255, 77–84. [Google Scholar] [CrossRef] [PubMed]
- Ray, A.; Prefontaine, K.E. Physical association and functional antagonism between the p65 subunit of transcription factor NF-kappa B and the glucocorticoid receptor. Proc. Natl. Acad. Sci. USA 1994, 91, 752–756. [Google Scholar] [CrossRef] [PubMed]
- Ayroldi, E.; Macchiarulo, A.; Riccardi, C. Targeting glucocorticoid side effects: Selective glucocorticoid receptor modulator or glucocorticoid-induced leucine zipper? A perspective. FASEB J. 2014, 28, 5055–5070. [Google Scholar] [CrossRef]
- Singer, M.; Wang, C.; Cong, L.; Marjanovic, N.D.; Kowalczyk, M.S.; Zhang, H.; Nyman, J.; Sakuishi, K.; Kurtulus, S.; Gennert, D.; et al. A Distinct Gene Module for Dysfunction Uncoupled from Activation in Tumor-Infiltrating T Cells. Cell 2016, 166, 1500–1511.e9. [Google Scholar] [CrossRef]
- Matossian, M.D.; Shiang, C.; Dolcen, D.N.; Dreyer, M.; Hatogai, K.; Hall, K.; Saha, P.; Biernacka, A.; Sweis, R.F.; Karrison, T.; et al. High tumor glucocorticoid receptor expression in early-stage, triple-negative breast cancer is associated with increased T-regulatory cell infiltration. Breast Cancer Res. Treat. 2025, 209, 563–572. [Google Scholar] [CrossRef]
- Tonsing-Carter, E.; Hernandez, K.M.; Kim, C.R.; Harkless, R.V.; Oh, A.; Bowie, K.R.; West-Szymanski, D.C.; Betancourt-Ponce, M.A.; Green, B.D.; Lastra, R.R.; et al. Glucocorticoid receptor modulation decreases ER-positive breast cancer cell proliferation and suppresses wild-type and mutant ER chromatin association. Breast Cancer Res. 2019, 21, 82. [Google Scholar] [CrossRef] [PubMed]
- Kelly, W.J.; Gilbert, M.R. Glucocorticoids and immune checkpoint inhibitors in glioblastoma. J. Neurooncol. 2021, 151, 13–20. [Google Scholar] [CrossRef] [PubMed]
- Rico, S.; Rosenberg, D.; Elkayam, O.; Gertel, S. Implications of glucocorticoid-induced co-inhibitory molecule expression on combined glucocorticoid and immune checkpoint inhibitor therapy. Immunopharmacol Immunotoxicol. 2025, 47, 599–610. [Google Scholar] [CrossRef]
- Oishi, S.; Takano, R.; Tamura, S.; Tani, S.; Iwaizumi, M.; Hamaya, Y.; Takagaki, K.; Nagata, T.; Seto, S.; Horii, T.; et al. M2 polarization of murine peritoneal macrophages induces regulatory cytokine production and suppresses T-cell proliferation. Immunology 2016, 149, 320–328. [Google Scholar] [CrossRef] [PubMed]
- Snyder, D.S.; Unanue, E.R. Corticosteroids inhibit murine macrophage Ia expression and interleukin 1 production. J. Immunol. 1982, 129, 1803–1805. [Google Scholar] [CrossRef] [PubMed]
- Musa, B.U.; Seal, U.S.; Doe, R.P.; Lamusga, R.F.; Lewis, M.D.; Glaser, R. Elevation of certain plasma proteins in man following estrogen administration: A dose-response relationship. J. Clin. Endocrinol. Metab. 1965, 25, 1163–1166. [Google Scholar] [CrossRef]
- Levin, N.; Akana, S.F.; Jacobson, L.; Kuhn, R.W.; Siiteri, P.K.; Dallman, M.F. Plasma adrenocorticotropin is more sensitive than transcortin production or thymus weight to inhibition by corticosterone in rats. Endocrinology 1987, 121, 1104–1110. [Google Scholar] [CrossRef]
- Rosner, W.; Aden, D.P.; Khan, M.S. Hormonal influences on the secretion of steroid-binding proteins by a human hepatoma-derived cell line. J. Clin. Endocrinol. Metab. 1984, 59, 806–808. [Google Scholar] [CrossRef]
- Mihrshahi, R.; Lewis, J.G.; Ali, S.O. Hormonal effects on the secretion and glycoform profile of corticosteroid-binding globulin. J. Steroid Biochem. Mol. Biol. 2006, 101, 275–285. [Google Scholar] [CrossRef]
- Bartalena, L.; Hammond, G.L.; Farsetti, A.; Flink, I.L.; Robbins, J. Interleukin-6 inhibits corticosteroid-binding globulin synthesis by human hepatoblastoma-derived (Hep G2) cells. Endocrinology 1993, 133, 291–296. [Google Scholar] [CrossRef]
- Maitra, U.S.; Khan, M.S.; Rosner, W. Corticosteroid-binding globulin receptor of the rat hepatic membrane: Solubilization, partial characterization, and the effect of steroids on binding. Endocrinology 1993, 133, 1817–1822. [Google Scholar] [CrossRef]
- Nakhla, A.M.; Khan, M.S.; Rosner, W. Induction of adenylate cyclase in a mammary carcinoma cell line by human corticosteroid-binding globulin. Biochem. Biophys. Res. Commun. 1988, 153, 1012–1018. [Google Scholar] [CrossRef]
- Adamson, R.H.; Liu, B.; Fry, G.N.; Rubin, L.L.; Curry, F.E. Microvascular permeability and number of tight junctions are modulated by cAMP. Am. J. Physiol. 1998, 274, H1885–H1894. [Google Scholar] [CrossRef] [PubMed]
- Ruddle, N.H.; Akirav, E.M. Secondary lymphoid organs: Responding to genetic and environmental cues in ontogeny and the immune response. J. Immunol. 2009, 183, 2205–2212. [Google Scholar] [CrossRef]
- Grader-Beck, T.; van Puijenbroek, A.A.; Nadler, L.M.; Boussiotis, V.A. cAMP inhibits both Ras and Rap1 activation in primary human T lymphocytes, but only Ras inhibition correlates with blockade of cell cycle progression. Blood 2003, 101, 998–1006. [Google Scholar] [CrossRef]
- Eigler, A.; Siegmund, B.; Emmerich, U.; Baumann, K.H.; Hartmann, G.; Endres, S. Anti-inflammatory activities of cAMP-elevating agents: Enhancement of IL-10 synthesis and concurrent suppression of TNF production. J. Leukoc. Biol. 1998, 63, 101–107. [Google Scholar] [CrossRef]
- Chatterton, R.T. Functions of dehydroepiandrosterone in relation to breast cancer. Steroids 2022, 179, 108970. [Google Scholar] [CrossRef] [PubMed]
- Sriramulu, S.; Thoidingjam, S.; Brown, S.L.; Siddiqui, F.; Movsas, B.; Nyati, S. Molecular targets that sensitize cancer to radiation killing: From the bench to the bedside. Biomed Pharmacother. 2023, 158, 114126. [Google Scholar] [CrossRef]
- Berry, M.R.; Fan, T.M. Target-Based Radiosensitization Strategies: Concepts and Companion Animal Model Outlook. Front. Oncol. 2021, 11, 768692. [Google Scholar] [CrossRef]
- Kvols, L.K. Radiation sensitizers: A selective review of molecules targeting DNA and non-DNA targets. J. Nucl. Med. 2005, 46, 187S–190S. [Google Scholar] [PubMed]
- Flint, M.S.; Baum, A.; Chambers, W.H.; Jenkins, F.J. Induction of DNA damage, alteration of DNA repair and transcriptional activation by stress hormones. Psychoneuroendocrinology 2007, 32, 470–479. [Google Scholar] [CrossRef] [PubMed]
- Flaherty, R.L.; Owen, M.; Fagan-Murphy, A.; Intabli, H.; Healy, D.; Patel, A.; Allen, M.C.; Patel, B.A.; Flint, M.S. Glucocorticoids induce production of reactive oxygen species/reactive nitrogen species and DNA damage through an iNOS mediated pathway in breast cancer. Breast Cancer Res. 2017, 19, 35. [Google Scholar] [CrossRef]
- West, D.C.; Kocherginsky, M.; Tonsing-Carter, E.Y.; Dolcen, D.N.; Hosfield, D.J.; Lastra, R.R.; Sinnwell, J.P.; Thompson, K.J.; Bowie, K.R.; Harkless, R.V.; et al. Discovery of a Glucocorticoid Receptor (GR) Activity Signature Using Selective GR Antagonism in ER-Negative Breast Cancer. Clin. Cancer Res. Off. J. Am. Assoc. Cancer Res. 2018, 24, 3433–3446. [Google Scholar] [CrossRef]
- Porter, B.A.; Frerich, C.; Laine, M.; Clark, A.B.; Durdana, I.; Lee, J.; Taya, M.; Sahoo, S.; Greene, G.L.; Bennett, L.; et al. Glucocorticoid Receptor Activation in Lobular Breast Cancer Is Associated with Reduced Cell Proliferation and Promotion of Metastases. Cancers 2023, 15, 4679. [Google Scholar] [CrossRef]
- Danilo, M.; Chennupati, V.; Silva, J.G.; Siegert, S.; Held, W. Suppression of Tcf1 by Inflammatory Cytokines Facilitates Effector CD8 T Cell Differentiation. Cell Rep. 2018, 22, 2107–2117. [Google Scholar] [CrossRef] [PubMed]
- Wherry, E.J.; Kurachi, M. Molecular and cellular insights into T cell exhaustion. Nat. Rev. Immunol. 2015, 15, 486–499. [Google Scholar] [CrossRef] [PubMed]
- Jin, H.T.; Anderson, A.C.; Tan, W.G.; West, E.E.; Ha, S.J.; Araki, K.; Freeman, G.J.; Kuchroo, V.K.; Ahmed, R. Cooperation of Tim-3 and PD-1 in CD8 T-cell exhaustion during chronic viral infection. Proc. Natl. Acad. Sci. USA 2010, 107, 14733–14738. [Google Scholar] [CrossRef] [PubMed]
- den Hollander, P.; Savage, M.I.; Brown, P.H. Targeted therapy for breast cancer prevention. Front. Oncol. 2013, 3, 250. [Google Scholar] [CrossRef]
- Hidalgo, A.A.; Trump, D.L.; Johnson, C.S. Glucocorticoid regulation of the vitamin D receptor. J. Steroid Biochem. Mol. Biol. 2010, 121, 372–375. [Google Scholar] [CrossRef]
- Bagnoud, M.; Remlinger, J.; Massy, M.; Lodygin, D.; Salmen, A.; Chan, A.; Lühder, F.; Hoepner, R. In Vivo and In Vitro Evidence for an Interplay between the Glucocorticoid Receptor and the Vitamin D Receptor Signaling. Cells 2023, 12, 2291. [Google Scholar] [CrossRef]
- Sartorius, C.A.; Takimoto, G.S.; Richer, J.K.; Tung, L.; Horwitz, K.B. Association of the Ku autoantigen/DNA-dependent protein kinase holoenzyme and poly(ADP-ribose) polymerase with the DNA binding domain of progesterone receptors. J. Mol. Endocrinol. 2000, 24, 165–182. [Google Scholar] [CrossRef]
- Kraus, W.L.; Lis, J.T. PARP goes transcription. Cell 2003, 113, 677–683. [Google Scholar] [CrossRef]
- Kraus, W.L. Transcriptional control by PARP-1: Chromatin modulation, enhancer-binding, coregulation, and insulation. Curr. Opin. Cell Biol. 2008, 20, 294–302. [Google Scholar] [CrossRef]
- Zhang, J.; Zhang, J.; Li, H.; Chen, L.; Yao, D. Dual-target inhibitors of PARP1 in cancer therapy: A drug discovery perspective. Drug Discov. Today 2023, 28, 103607. [Google Scholar] [CrossRef] [PubMed]
- Mitre-Aguilar, I.B.; Barrios-Garcia, T.; Ruiz-Lopez, V.M.; Cabrera-Quintero, A.J.; Mejia-Dominguez, N.R.; Ventura-Gallegos, J.L.; Moreno-Mitre, D.; Aranda-Gutierrez, A.; Mejia-Rangel, J.; Escalona-Guzman, A.R.; et al. Glucocorticoid-dependent expression of IAP participates in the protection against TNF-mediated cytotoxicity in MCF7 cells. BMC Cancer 2019, 19, 356. [Google Scholar] [CrossRef]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2026 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).