Submitted:
19 January 2026
Posted:
19 January 2026
You are already at the latest version
Abstract
Keywords:
1. Introduction
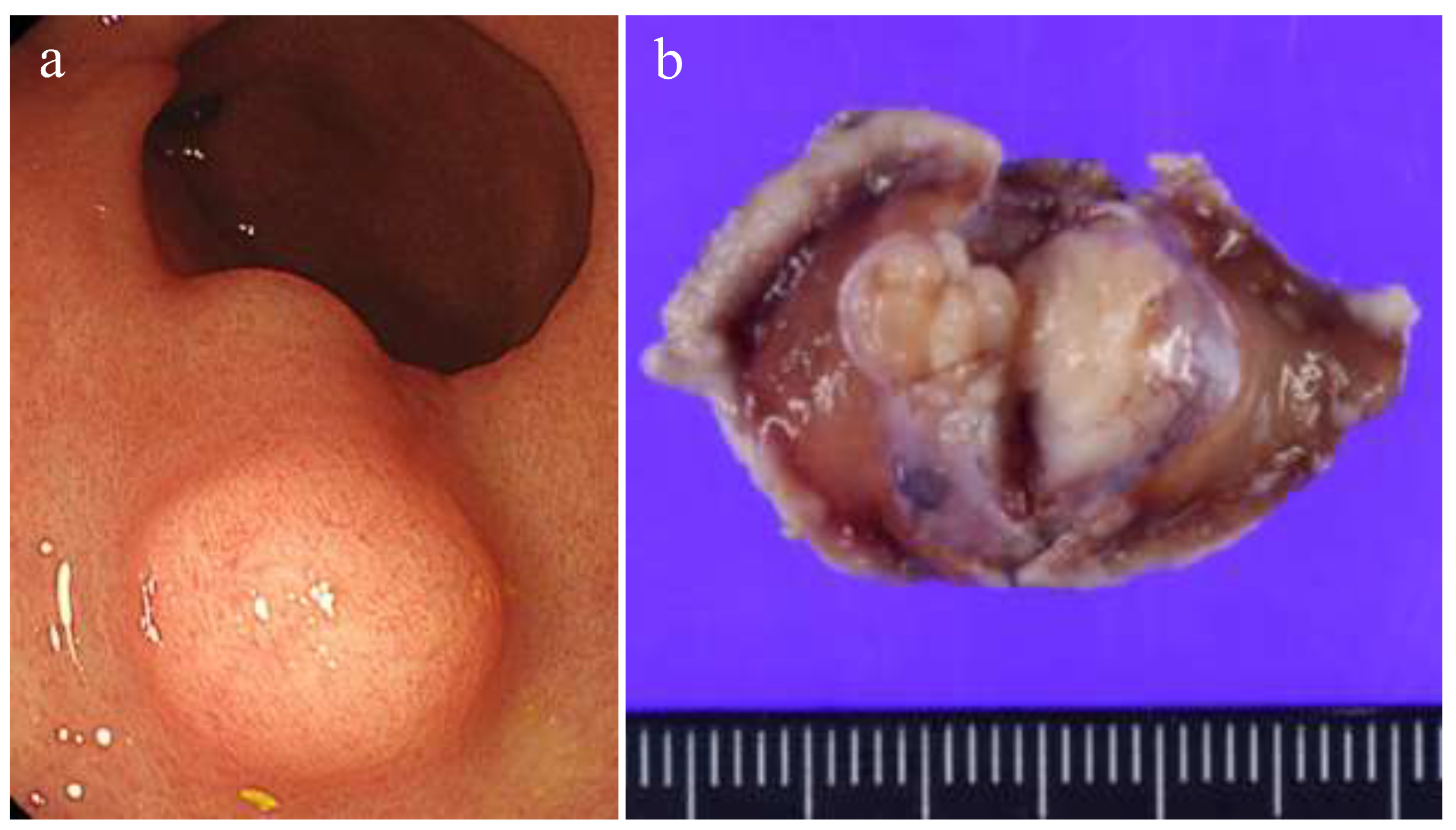
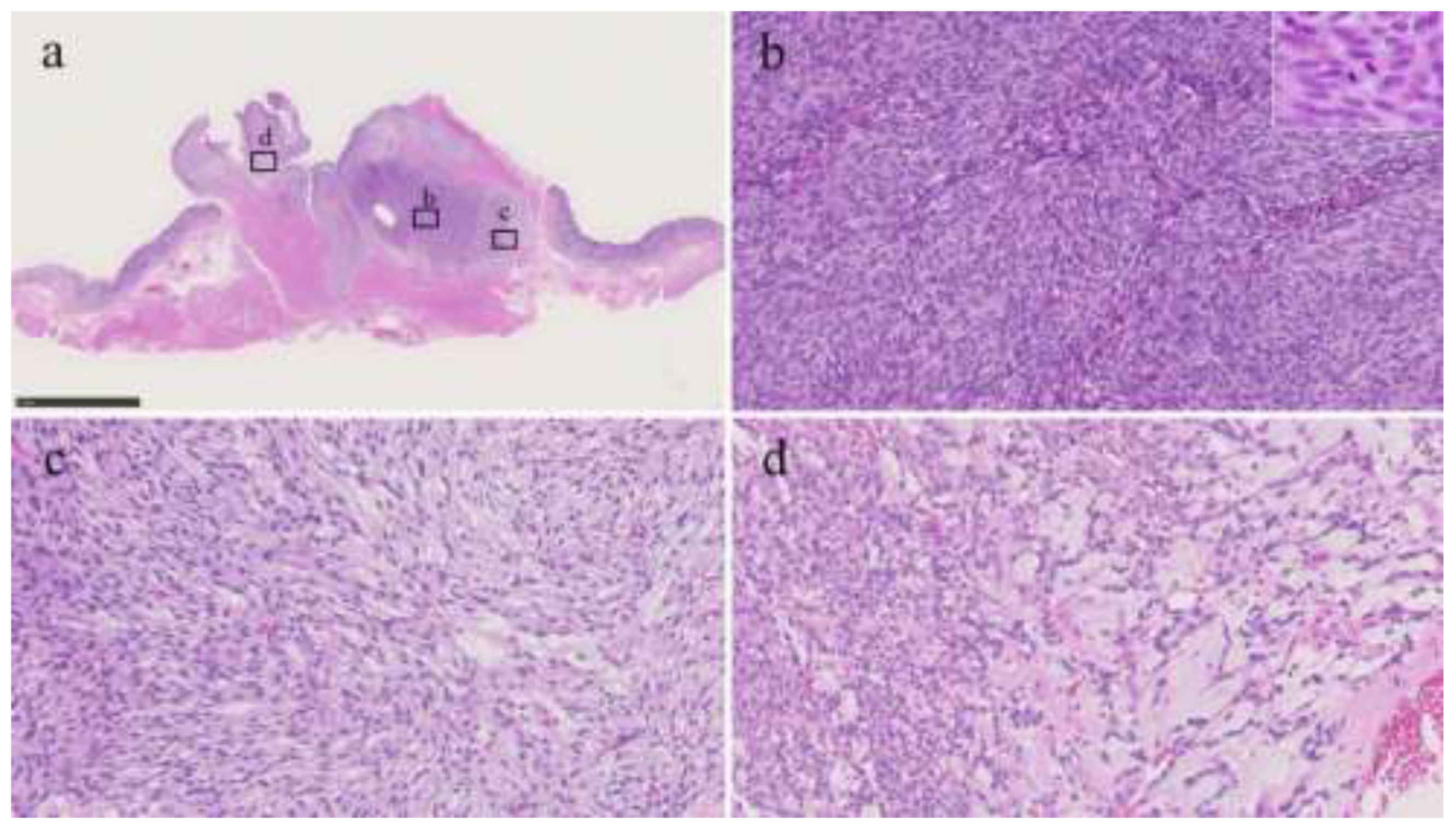
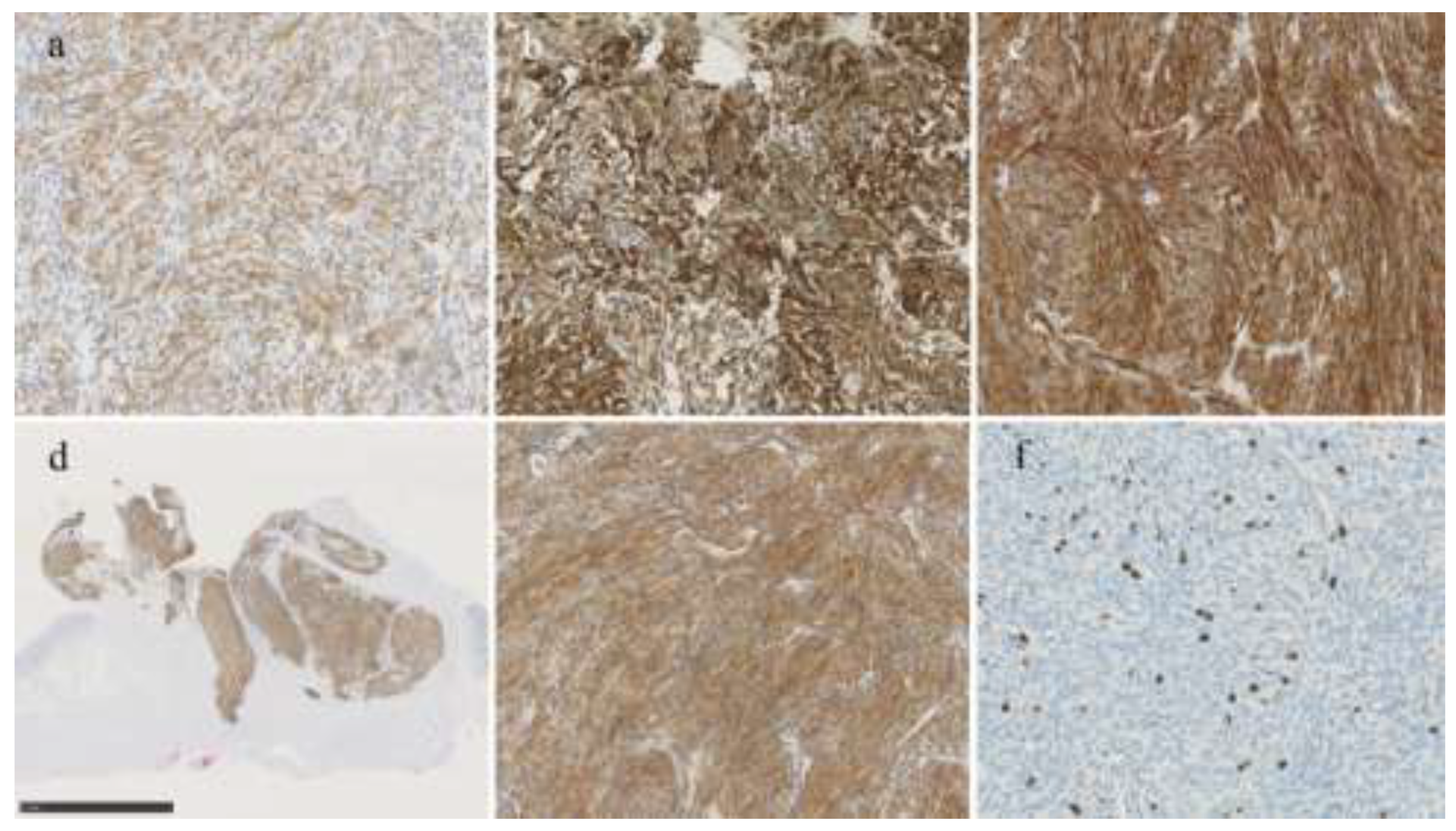
2. Case Presentation
3. Discussion
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| PFM | Plexiform fibromyxoma |
| IHC | Immunohistochemistry |
| DOG1 | Discovered on gastrointestinal stromal tumor 1 |
| SMA | Smooth muscle actin |
| SDHB | Succinate dehydrogenase subunit B |
| MALAT1 | Metastasis associated lung adenocarcinoma transcript 1 |
| GLI1 | Glioma-associated oncogene homologue 1 |
| GIST | Gastrointestinal stromal tumor |
| HE | Hematoxylin-eosin |
| SDH | Succinate dehydrogenase |
| HPF | High-power fields |
| IMT | Inflammatory myofibroblastic tumor |
| PEComa | Perivascular epithelioid cell tumor |
| CD | Cluster of differentiation |
| ICOS | Inducible T-cell co-stimulator |
| NGS | Next-generation sequencing |
| WT-1 | Wilms tumor 1 |
References
- Takahashi, Y.; Shimizu, S.; Ishida, T.; Aita, K.; Toida, S.; Fukusato, T.; Mori, S. Plexiform Angiomyxoid Myofibroblastic Tumor of the Stomach. Am. J. Surg. Pathol. 2007, 31, 724–728. [Google Scholar] [CrossRef] [PubMed]
- Miettinen, M; Makhlouf, HR; Sobin, LH; Lasota, J. Plexiform fibromyxoma: a distinctive benign gastric antral neoplasm not to be confused with a myxoid GIST. Am J Surg Pathol 2009, 33, 1624–32. [Google Scholar] [CrossRef]
- Miettinen, M; Fletcher, CD; Kindblom, LG; Tsui, WM. Mesenchymal tumors of the stomach. In WHO Classification of Tumours of the Digestive System, 4th edn; Bosman, FT, Carneiro, F, Hruban, R, Teise, ND, Eds.; IARC: Lyon, 2010; pp. 74–9. [Google Scholar]
- Arslan, M.E.; Li, H.; Fu, Z.; A Jennings, T.; Lee, H. Plexiform fibromyxoma: Review of rare mesenchymal gastric neoplasm and its differential diagnosis. World J. Gastrointest. Oncol. 2021, 13, 409–423. [Google Scholar] [CrossRef]
- Szczepanski, J.; Westerhoff, M.; Schechter, S. Plexiform Fibromyxoma: A Review and Discussion of the Differential Diagnosis of Gastrointestinal Mesenchymal Tumors. Arch. Pathol. Lab. Med. 2025, 149, e298–e304. [Google Scholar] [CrossRef]
- Spans, L.; Fletcher, C.D.; Antonescu, C.R.; Rouquette, A.; Coindre, J.; Sciot, R.; Debiec-Rychter, M. Recurrent MALAT1–GLI1 oncogenic fusion and GLI1 up-regulation define a subset of plexiform fibromyxoma. J. Pathol. 2016, 239, 335–343. [Google Scholar] [CrossRef]
- Antonescu, CR; Agaram, NP; Sung, YS; Zhang, L; Swanson, D; Dickson, BC. A distinct malignant epithelioid neoplasm with GLI1 gene rearrangements, frequent S100 protein expression, and metastatic potential: expanding the spectrum of pathologic entities with ACTB/MALAT1/PTCH1-GLI1 fusions. Am J Surg Pathol 2018, 42, 553–60. [Google Scholar] [CrossRef] [PubMed]
- Prall, O.W.J.; McEvoy, C.R.E.; Byrne, D.J.; Iravani, A.; Browning, J.; Choong, D.Y.-H.; Yellapu, B.; O’hAire, S.; Smith, K.; Luen, S.J.; et al. A Malignant Neoplasm From the Jejunum With a MALAT1-GLI1 Fusion and 26-Year Survival History. Int. J. Surg. Pathol. 2020, 28, 553–562. [Google Scholar] [CrossRef] [PubMed]
- Miettinen, M; Wang, ZF; Lasota, J. DOG1 antibody in the differential diagnosis of gastrointestinal stromal tumors: a study of 1840 cases. Am J Surg Pathol 2009, 33, 1401–8. [Google Scholar] [CrossRef] [PubMed]
- Banerjee, S; Corless, CL; Miettinen, MM; Noh, S; Ustoy, R; Davis, JL; et al. Loss of the PTCH1 subset of plexiform fibromyxoma. J Transl Med 2019, 17, 246. [Google Scholar] [CrossRef] [PubMed]
- Hu, G.; Chen, H.; Liu, Q.; Wei, J.; Feng, Y.; Fu, W.; Zhang, M.; Wu, H.; Gu, B.; Ren, J. Plexiform fibromyxoma of the stomach: a clinicopathological study of 10 cases. 2017, 10, 10926–10933. [Google Scholar] [PubMed]
- Ordóñez, N.G.; G., N. Podoplanin: A Novel Diagnostic Immunohistochemical Marker. Adv. Anat. Pathol. 2006, 13, 83–88. [Google Scholar] [CrossRef] [PubMed]
- Ebi, M.; Nagao, K.; Sugiyama, T.; Yamamoto, K.; Saito, T.; Kurahashi, S.; Yamaguchi, Y.; Adachi, K.; Tamura, Y.; Izawa, S.; et al. Gastric Plexiform Fibromyxoma Resected Using Nonexposed Endoscopic Wall-Inversion Surgery: A Case Report. Case Rep. Gastroenterol. 2022, 16, 159–164. [Google Scholar] [CrossRef] [PubMed]
- Kane, JR; Lewis, N; Lin, R; Villa, C; Larson, A; Wayne, JD; et al. Plexiform fibromyxoma with cotyledon-like serosal growth: a case report of a rare gastric tumor and review of the literature. Oncol Lett 2016, 11, 2189–94. [Google Scholar] [CrossRef] [PubMed]
- Tang, J.; Liu, F. Plexiform Fibromyxoma: A Rare Mesenchymal Tumor Found in the Esophagus. Am. J. Gastroenterol. 2019, 115, 648–648. [Google Scholar] [CrossRef] [PubMed]
| Tumors | Typical morphology | Useful immunohistochemistry | Molecular alteration |
| Plexiform fibromyxoma | Multinodular and plexiform low-power architecture; uniform spindle cells in a myxoid, fibromyxoid, or collagenous stroma | None (usually positive for SMA, occasionally positive for CD10, desmin, caldesmon, D2-40) | MALAT1::GLI1, PTCH1 inactivation |
| GIST | Intramural, submucosal, or subserosal mass; spindle cell, epithelial, or mixed morphology | Positive for KIT, DOG-1 | KIT, PDGFRA |
| SDH-deficient GIST | Characteristic epithelioid morphology and typically multinodular with plexiform mural involvement | Positive for KIT, DOG-1, loss of SDHB | SDHB, No KIT or PDGFRA mutation |
| Leiomyoma | Fascicles of spindle cells with eosinophilic cytoplasm | Positive for desmin, SMA | |
| Schwannoma | Well-circumscribed mass; areas of cellular and hypocellular spindle cells | Positive for S100 | NF1, NF2 |
| Perineurioma | Uniform spindle cells, most are small colonic polyps | Positive for EMA, Negative for S100 | NF2, BRAF (associated serrated polyp) |
| Glomus tumor | Round glomoid cells; sometimes plexiform growth | Positive for SMA, collagen IV | NOTCH, BRAF |
| PEComa | Epithelioid and/or spindle cells with granular eosinophilic-to-clear cytoplasm | Positive for smooth muscle (desmin, SMA) and melanocytic (HMB45, melan-A, PNL2, MITF, tyrosinase) markers | TSC1/2, TFE3 (minority) |
| Inflammatory fibroid polyp | Hypocellular, with short spindled-to-stellate cells, infiltration of eosinophils and lymphocytes. | Positive for CD34, PDGFRA | PDGFRA |
| Inflammatory myofibroblastic tumor | Loose fascicles of spindle cells without pleomorphism, infiltration of lymphocytes and plasma cells | Positive for SMA, ALK | ALK, ROS1 |
| Solitary fibrous tumor | Fascicles of uniform ovoid-to-spindle cells, staghorn vessels | Positive for CD34, STAT6 | NAB2::STAT6 |
| Synovial sarcoma | Spindle cell (monophasic) or spindle cell with epithelioid-to-glandular (biphasic) | None (focal positivity for keratin and EMA) | SS18::SSX1/2/4 |
| Gastroblastoma | Biphasic with spindle cells and epithelial cells | Epithelial cells are positive for CK, and spindle and epithelial cells are focally positive for CD10 and CD56 | MALAT1::GLI1 |
| Malignant epithelioid tumor with GLI1 rearrangement | Epithelioid, ovoid, round to spindle | Occasionally positive for S100, a subset case focally positive for CK | MALAT1::GLI1 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2026 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license.





