Submitted:
20 August 2024
Posted:
22 August 2024
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Materials and Methods
3. Results
3.1. Demographic characteristics
3.2. Clinical and Molecular Characteristics of ACC
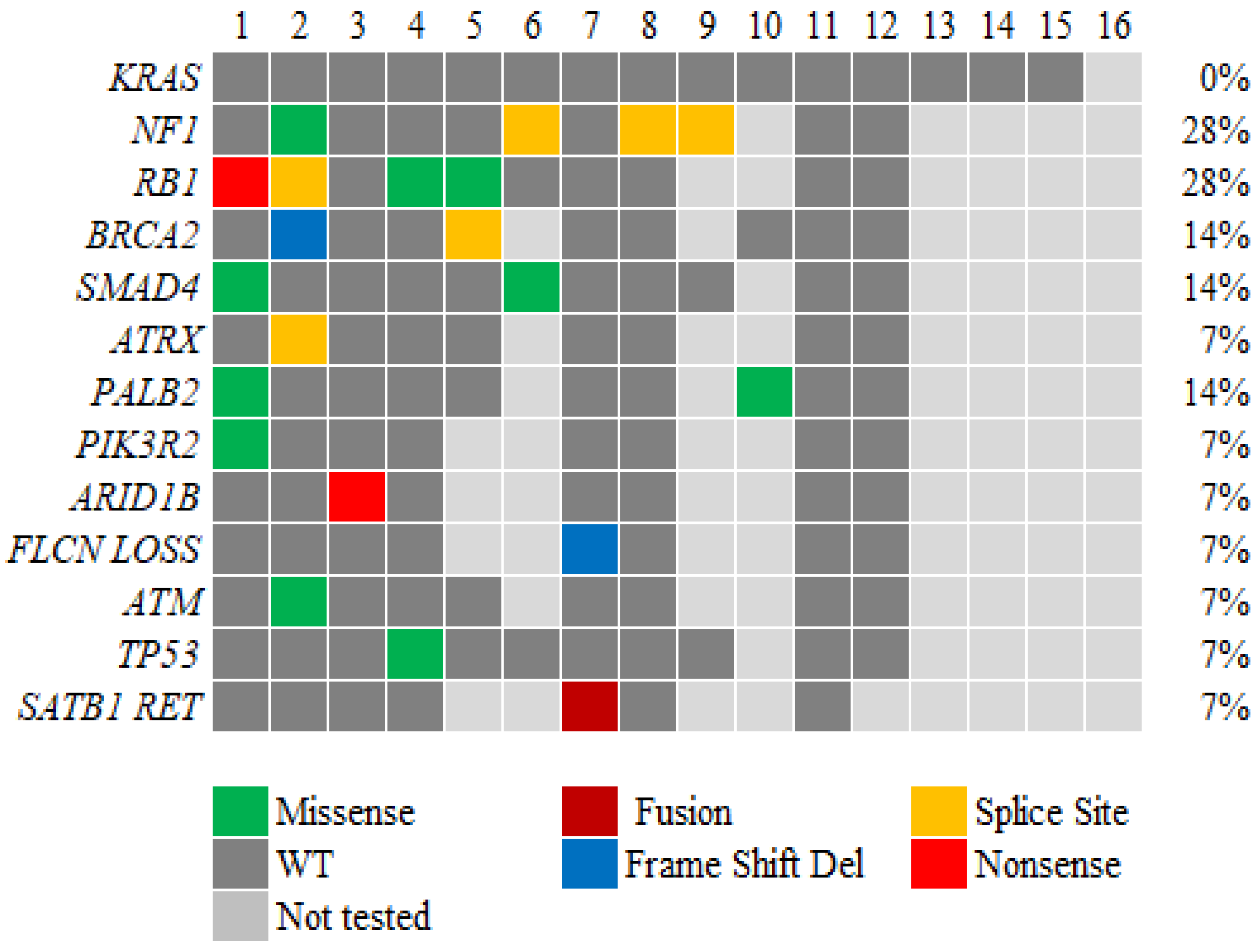
3.3. Genetic alterations in ACC
3.4. Treatment
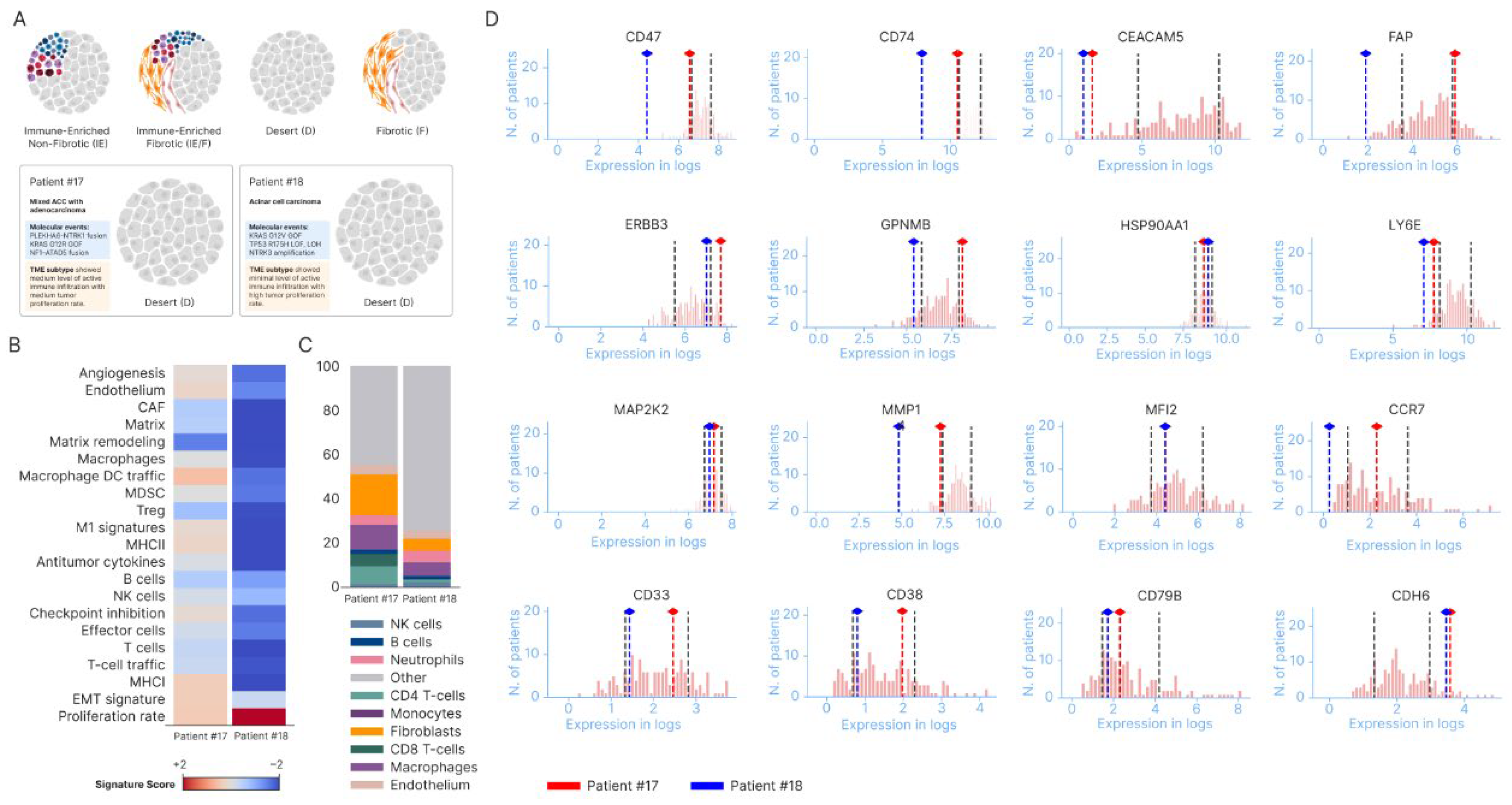
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Calimano-Ramirez, L.F.; Daoud, T.; Gopireddy, D.R.; Morani, A.C.; Waters, R.; Gumus, K.; Klekers, A.R.; Bhosale, P.R.; Virarkar, M.K. Pancreatic acinar cell carcinoma: A comprehensive review. World J. Gastroenterol. 2022, 28, 5827–5844. [Google Scholar] [CrossRef]
- Lowery, M.A.; Klimstra, D.S.; Shia, J.; Yu, K.H.; Allen, P.J.; Brennan, M.F.; O’Reilly, E.M. Acinar Cell Carcinoma of the Pancreas: New Genetic and Treatment Insights into a Rare Malignancy. Oncol. 2011, 16, 1714–1720. [Google Scholar] [CrossRef]
- Pishvaian, M.J.; Blais, E.M.; Brody, J.R.; Lyons, E.; DeArbeloa, P.; Hendifar, A.; Mikhail, S.; Chung, V.; Sahai, V.; Sohal, D.P.S.; et al. Overall survival in patients with pancreatic cancer receiving matched therapies following molecular profiling: a retrospective analysis of the Know Your Tumor registry trial. Lancet Oncol. 2020, 21, 508–518. [Google Scholar] [CrossRef] [PubMed]
- Sakakida, T.; Ishikawa, T.; Doi, T.; Morita, R.; Kataoka, S.; Miyake, H.; Yamaguchi, K.; Moriguchi, M.; Sogame, Y.; Yasuda, H.; et al. Genomic landscape and clinical features of rare subtypes of pancreatic cancer: analysis with the national database of Japan. J. Gastroenterol. 2023, 58, 575–585. [Google Scholar] [CrossRef]
- Bagaev, A.; Kotlov, N.; Nomie, K.; Svekolkin, V.; Gafurov, A.; Isaeva, O.; Osokin, N.; Kozlov, I.; Frenkel, F.; Gancharova, O.; et al. Conserved pan-cancer microenvironment subtypes predict response to immunotherapy. Cancer Cell 2021, 39, 845–865. [Google Scholar] [CrossRef] [PubMed]
- Subbiah V, Wolf J, Konda B, et al. Tumour-agnostic efficacy and safety of selpercatinib in patients with RET fusion-positive solid tumours other than lung or thyroid tumours (LIBRETTO-001): a phase 1/2, open-label, basket trial. The Lancet Oncology. 2022;23(10):1261-1273.
- Fricke, J.; Wang, J.; Gallego, N.; Mambetsariev, I.; Kim, P.; Babikian, R.; Chen, B.T.; Afkhami, M.; Subbiah, V.; Salgia, R. Selpercatinib and Pralsetinib Induced Chylous Ascites in RET-Rearranged Lung Adenocarcinoma: A Case Series. Clin. Lung Cancer 2023, 24, 666–671. [Google Scholar] [CrossRef]
- Zong, Y.; Qi, C.; Peng, Z.; Shen, L.; Zhou, J. Patients With Acinar Cell Carcinoma of the Pancreas After 2005 A Large Population Study. Pancreas 2020, 49, 781–787. [Google Scholar] [CrossRef]
- Xing-Mao, Z.; Hong-Juan, Z.; Qing, L.; Qiang, H. Pancreatic acinar cell carcinoma—case report and literature review. BMC Cancer 2018, 18, 1083. [Google Scholar] [CrossRef]
- Lowery, M.A.; Klimstra, D.S.; Shia, J.; Yu, K.H.; Allen, P.J.; Brennan, M.F.; O’Reilly, E.M. Acinar Cell Carcinoma of the Pancreas: New Genetic and Treatment Insights into a Rare Malignancy. Oncol. 2011, 16, 1714–1720. [Google Scholar] [CrossRef] [PubMed]
- Sridharan, V.; Mino-Kenudson, M.; Cleary, J.M.; Rahma, O.E.; Perez, K.; Clark, J.W.; Clancy, T.E.; Rubinson, D.A.; Goyal, L.; Bazerbachi, F.; et al. Pancreatic acinar cell carcinoma: A multi-center series on clinical characteristics and treatment outcomes. Pancreatology 2021, 21, 1119–1126. [Google Scholar] [CrossRef]
- Yamada, S.; Motegi, H.; Kurihara, Y.; Shimbo, T.; Kikuchi, I.; Wakabayashi, T.; Sato, T. A resected case of acinar cell carcinoma of the pancreas with liver metastasis following chemotherapy using modified FOLFIRINOX. Surg. Case Rep. 2023, 9, 1–10. [Google Scholar] [CrossRef]
- Skacel, M.; Ormsby, A.H.; Petras, R.E.; McMahon, J.T.; Henricks, W.H. Immunohistochemistry in the Differential Diagnosis of Acinar and Endocrine Pancreatic Neoplasms. Appl. Immunohistochem. Mol. Morphol. 2000, 8, 203–209. [Google Scholar] [CrossRef] [PubMed]
- Holen, K.D.; Klimstra, D.S.; Hummer, A.; Gonen, M.; Conlon, K.; Brennan, M.; Saltz, L.B. Clinical Characteristics and Outcomes From an Institutional Series of Acinar Cell Carcinoma of the Pancreas and Related Tumors. J. Clin. Oncol. 2002, 20, 4673–4678. [Google Scholar] [CrossRef] [PubMed]
- Said, S.; Kurtin, P.J.; Nasr, S.H.; Graham, R.P.; Dasari, S.; Vrana, J.A.; Yasir, S.; Torbenson, M.S.; Zhang, L.; Mounajjed, T.; et al. Carboxypeptidase A1 and regenerating islet-derived 1α as new markers for pancreatic acinar cell carcinoma. Hum. Pathol. 2020, 103, 120–126. [Google Scholar] [CrossRef] [PubMed]
- Ishimoto-Namiki U, Ino Y, Esaki M, Shimada K, Saruta M, Hiraoka N. Novel Insights Into Immunohistochemical Analysis For Acinar Cell Neoplasm of The Pancreas: Carboxypeptidase A2, Carboxypeptidase A1, and Glycoprotein 2. The American journal of surgical pathology. 2023;47(5):525-534.
- La Rosa S, Adsay V, Albarello L, et al. Clinicopathologic study of 62 acinar cell carcinomas of the pancreas: insights into the morphology and immunophenotype and search for prognostic markers. The American journal of surgical pathology. 2012;36(12):1782-1795.
- Strickler JH, Satake H, George TJ, et al. Sotorasib in KRAS p.G12C–Mutated Advanced Pancreatic Cancer. The New England journal of medicine. 2023;388(1):33-43.
- Yousef, A.; Yousef, M.; Chowdhury, S.; Abdilleh, K.; Knafl, M.; Edelkamp, P.; Alfaro-Munoz, K.; Chacko, R.; Peterson, J.; Smaglo, B.G.; et al. Impact of KRAS mutations and co-mutations on clinical outcomes in pancreatic ductal adenocarcinoma. npj Precis. Oncol. 2024, 8, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Hoorens, A.; Lemoine, N.R.; McLellan, E.; Morohoshi, T.; Kamisawa, T.; Heitz, P.U.; Stamm, B.; Rüschoff, J.; Wiedenmann, B.; Klöppel, G. Pancreatic acinar cell carcinoma. An analysis of cell lineage markers, p53 expression, and Ki-ras mutation.. 1993, 143, 685–98. [Google Scholar]
- Florou, V.; Elliott, A.; Bailey, M.H.; Stone, D.; Affolter, K.; Soares, H.P.; Nevala-Plagemann, C.; Scaife, C.; Walker, P.; Korn, W.M.; et al. Comparative Genomic Analysis of Pancreatic Acinar Cell Carcinoma (PACC) and Pancreatic Ductal Adenocarcinoma (PDAC) Unveils New Actionable Genomic Aberrations in PACC. Clin. Cancer Res. 2023, 29, 3408–3417. [Google Scholar] [CrossRef]
- Liu, Y.; Raimondo, M.; Wallace, M.B.; Mody, K.; Stauffer, J.A.; Zhang, L.; Ji, B.; Bi, Y. Exome Sequencing of Pancreatic Acinar Carcinoma Identified Distinctive Mutation Patterns. Pancreas 2021, 50, 1007–1013. [Google Scholar] [CrossRef]
- Furukawa, T.; Sakamoto, H.; Takeuchi, S.; Ameri, M.; Kuboki, Y.; Yamamoto, T.; Hatori, T.; Yamamoto, M.; Sugiyama, M.; Ohike, N.; et al. Whole exome sequencing reveals recurrent mutations in BRCA2 and FAT genes in acinar cell carcinomas of the pancreas. Sci. Rep. 2015, 5, srep08829. [Google Scholar] [CrossRef]
- Dae Won Kim MMP, MD; and Todd Knepper, PharmD. KRAS Wild-Type and KRAS Mutant Pancreatic Ductal Adenocarcinoma: Are These One in the Same or Separate Entities? ASCO Daily News. July 19, 2023.
- Singh, H.; Keller, R.B.; Kapner, K.S.; Dilly, J.; Raghavan, S.; Yuan, C.; Cohen, E.F.; Tolstorukov, M.; Andrews, E.; Brais, L.K.; et al. Oncogenic Drivers and Therapeutic Vulnerabilities in KRAS Wild-Type Pancreatic Cancer. Clin. Cancer Res. 2023, 29, 4627–4643. [Google Scholar] [CrossRef]
- Ramakrishnan, G.; Parajuli, P.; Singh, P.; Friend, C.; Hurwitz, E.; Prunier, C.; Razzaque, M.S.; Xu, K.; Atfi, A. NF1 loss of function as an alternative initiating event in pancreatic ductal adenocarcinoma. Cell Rep. 2022, 41, 111623–111623. [Google Scholar] [CrossRef] [PubMed]
- La Rosa, S.; Bernasconi, B.; Frattini, M.; Tibiletti, M.G.; Molinari, F.; Furlan, D.; Sahnane, N.; Vanoli, A.; Albarello, L.; Zhang, L.; et al. TP53 alterations in pancreatic acinar cell carcinoma: new insights into the molecular pathology of this rare cancer. Virchows Arch. 2015, 468, 289–296. [Google Scholar] [CrossRef] [PubMed]
- Chmielecki, J.; Hutchinson, K.E.; Frampton, G.M.; Chalmers, Z.R.; Johnson, A.; Shi, C.; Elvin, J.; Ali, S.M.; Ross, J.S.; Basturk, O.; et al. Comprehensive Genomic Profiling of Pancreatic Acinar Cell Carcinomas Identifies Recurrent RAF Fusions and Frequent Inactivation of DNA Repair Genes. Cancer Discov. 2014, 4, 1398–1405. [Google Scholar] [CrossRef] [PubMed]
- Mandelker, D.; Marra, A.; Zheng-Lin, B.; Selenica, P.; Blanco-Heredia, J.; Zhu, Y.; Gazzo, A.; Wong, D.; Yelskaya, Z.; Rai, V.; et al. Genomic Profiling Reveals Germline Predisposition and Homologous Recombination Deficiency in Pancreatic Acinar Cell Carcinoma. J. Clin. Oncol. 2023, 41, 5151–5162. [Google Scholar] [CrossRef]
- Takahashi H, Ikeda M, Shiba S, et al. Multicenter Retrospective Analysis of Chemotherapy for Advanced Pancreatic Acinar Cell Carcinoma: Potential Efficacy of Platinum- and Irinotecan-Containing Regimens. Pancreas. 2021;50(1):77-82.
- Sunami, T.; Yamada, A.; Kondo, T.; Kanai, M.; Nagai, K.; Uchida, Y.; Yokode, M.; Matsumori, T.; Uza, N.; Murakami, H.; et al. Exceptional Response of Pancreatic Acinar Cell Carcinoma and Bile Duct Cancer to Platinum-Based Chemotherapy in a Family With a Germline BRCA2 Variant. Pancreas 2022, 51, 1258–1262. [Google Scholar] [CrossRef]
- Duke, E.S.; Bradford, D.; Marcovitz, M.; Amatya, A.K.; Mishra-Kalyani, P.S.; Nguyen, E.; Price, L.S.L.; Zirkelbach, J.F.; Li, Y.; Bi, Y.; et al. FDA Approval Summary: Selpercatinib for the Treatment of Advanced RET Fusion-Positive Solid Tumors. Clin. Cancer Res. 2023, 29, 3573–3578. [Google Scholar] [CrossRef]
- Chou, A.; Brown, I.S.; Kumarasinghe, M.P.; Perren, A.; Riley, D.; Kim, Y.; Pajic, M.; Steinmann, A.; Rathi, V.; Jamieson, N.B.; et al. RET gene rearrangements occur in a subset of pancreatic acinar cell carcinomas. Mod. Pathol. 2019, 33, 657–664. [Google Scholar] [CrossRef]
- Abraham SC, Wu TT, Hruban RH, et al. Genetic and immunohistochemical analysis of pancreatic acinar cell carcinoma: frequent allelic loss on chromosome 11p and alterations in the APC/beta-catenin pathway. Am J Pathol. 2002;160(3):953-962.
- Liu W, Shia J, Gönen M, Lowery MA, O’Reilly EM, Klimstra DS. DNA mismatch repair abnormalities in acinar cell carcinoma of the pancreas: frequency and clinical significance. Pancreas. 2014;43(8):1264-1270.
- Chen, H.; Wang, B.; Zhang, Y.; Shu, Y.; Dong, H.; Zhao, Q.; Yang, C.; Li, J.; Duan, X.; Zhou, Q. A unified DNA- and RNA-based NGS strategy for the analysis of multiple types of variants at the dual nucleic acid level in solid tumors. J. Clin. Lab. Anal. 2023, 37, e24977. [Google Scholar] [CrossRef]
- Heydt, C.; Wölwer, C.B.; Camacho, O.V.; Wagener-Ryczek, S.; Pappesch, R.; Siemanowski, J.; Rehker, J.; Haller, F.; Agaimy, A.; Worm, K.; et al. Detection of gene fusions using targeted next-generation sequencing: a comparative evaluation. BMC Med Genom. 2021, 14, 1–14. [Google Scholar] [CrossRef]
- Oh, K.; Yoo, Y.J.; Torre-Healy, L.A.; Rao, M.; Fassler, D.; Wang, P.; Caponegro, M.; Gao, M.; Kim, J.; Sasson, A.; et al. Coordinated single-cell tumor microenvironment dynamics reinforce pancreatic cancer subtype. Nat. Commun. 2023, 14, 1–13. [Google Scholar] [CrossRef]
- Hong, W.C.; Lee, D.E.; Kang, H.W.; Kim, M.J.; Kim, M.; Kim, J.H.; Fang, S.; Kim, H.J.; Park, J.S. CD74 Promotes a Pro-Inflammatory Tumor Microenvironment by Inducing S100A8 and S100A9 Secretion in Pancreatic Cancer. Int. J. Mol. Sci. 2023, 24, 12993. [Google Scholar] [CrossRef] [PubMed]
- Michaels, A.D.; Newhook, T.E.; Adair, S.J.; Morioka, S.; Goudreau, B.J.; Nagdas, S.; Mullen, M.G.; Persily, J.B.; Bullock, T.N.J.; Slingluff, C.L.; et al. CD47 Blockade as an Adjuvant Immunotherapy for Resectable Pancreatic Cancer. Clin. Cancer Res. 2018, 24, 1415–1425. [Google Scholar] [CrossRef] [PubMed]
- Hilmi, M.; Delaye, M.; Muzzolini, M.; Nicolle, R.; Cros, J.; Hammel, P.; Cardot-Ruffino, V.; Neuzillet, C. The immunological landscape in pancreatic ductal adenocarcinoma and overcoming resistance to immunotherapy. Lancet Gastroenterol. Hepatol. 2023, 8, 1129–1142. [Google Scholar] [CrossRef] [PubMed]
- Bear, A.S.; Vonderheide, R.H.; O’Hara, M.H. Challenges and Opportunities for Pancreatic Cancer Immunotherapy. Cancer Cell 2020, 38, 788–802. [Google Scholar] [CrossRef] [PubMed]
- Li, K.; Tandurella, J.A.; Gai, J.; Zhu, Q.; Lim, S.J.; Thomas, D.L.; Xia, T.; Mo, G.; Mitchell, J.T.; Montagne, J.; et al. Multi-omic analyses of changes in the tumor microenvironment of pancreatic adenocarcinoma following neoadjuvant treatment with anti-PD-1 therapy. Cancer Cell 2022, 40, 1374–1391. [Google Scholar] [CrossRef]
- Gebauer, F.; Wicklein, D.; Horst, J.; Sundermann, P.; Maar, H.; Streichert, T.; Tachezy, M.; Izbicki, J.R.; Bockhorn, M.; Schumacher, U. Carcinoembryonic Antigen-Related Cell Adhesion Molecules (CEACAM) 1, 5 and 6 as Biomarkers in Pancreatic Cancer. PLOS ONE 2014, 9, e113023–e113023. [Google Scholar] [CrossRef]
- Kopetz, S.; Boni, V.; Kato, K.; Raghav, K.P.S.; Pallis, A.; Habermehl, C.; Galipelli, S.; Courlet, P.; Rivera, I.R. First-in-human trial of M9140, an anti-CEACAM5 antibody drug conjugate (ADC) with exatecan payload, in patients (pts) with metastatic colorectal cancer (mCRC). J. Clin. Oncol. 2024, 42, 3000–3000. [Google Scholar] [CrossRef]
- Zhou, W.; Han, X.; Fang, Y.; Han, S.; Cai, Y.; Kuang, T.; Lou, W.; Wang, D. Clinical Analysis of Acinar Cell Carcinoma of the Pancreas: A Single-Center Experience of 45 Consecutive Cases. Cancer Control. 2020, 27. [Google Scholar] [CrossRef]
- Goeppert, B.; Ernst, C.; Baer, C.; Roessler, S.; Renner, M.; Mehrabi, A.; Hafezi, M.; Pathil, A.; Warth, A.; Stenzinger, A.; et al. Cadherin-6 is a putative tumor suppressor and target of epigenetically dysregulated miR-429 in cholangiocarcinoma. Epigenetics 2016, 11, 780–790. [Google Scholar] [CrossRef]
- Casal JI, Bartolomé RA. Beyond N-Cadherin, Relevance of Cadherins 5, 6 and 17 in Cancer Progression and Metastasis. Int J Mol Sci. 2019;20(13).
- Luo, S.; Lin, R.; Liao, X.; Li, D.; Qin, Y. Identification and verification of the molecular mechanisms and prognostic values of the cadherin gene family in gastric cancer. Sci. Rep. 2021, 11, 1–21. [Google Scholar] [CrossRef]
- Lo, A.; Li, C.-P.; Buza, E.L.; Blomberg, R.; Govindaraju, P.; Avery, D.; Monslow, J.; Hsiao, M.; Puré, E. Fibroblast activation protein augments progression and metastasis of pancreatic ductal adenocarcinoma. J. Clin. Investig. 2017, 2. [Google Scholar] [CrossRef]
| Characteristics | Number of patients (%) |
|---|---|
| Sex | |
| Male Female |
13 (81) 3 (19) |
| Median age at diagnosis, years | 62.5 |
| Race White Hispanic Asian |
14 (88) 1 (6) 1 (6) |
| Primary tumor location Head of pancreas Body of pancreas Tail of pancreas Body and head of pancreas |
8 (50) 5 (31) 2 (13) 1 (6) |
| Tumor status at initial diagnosis Resectable/borderline resectable Metastatic |
2 (12) 14 (88) |
| Smoking status (current/former smoker) | 9 (56) |
| Alcohol status (current/former drinker) | 10 (63) |
| Characteristic | Number of patients |
|---|---|
| Histology Pure acinar cell carcinoma Mixed acinar-neuroendocrine Mixed acinar and adenocarcinoma |
14 1 1 |
| Marker positivity Trypsin Chymotrypsin Synaptophysin BCL-10 Cytokeratin 7 Alpha 1-antichymotrypsin Chromogranin Alpha 1 antitrypsin BCL-2 |
(N of positive/tested) 9/9 2 /2 5 /12 2 /2 8 /9 2 /2 3/13 1 /2 2 /2 |
| Age/Sex | First | Second | Third and beyond | OS (months) | |
|---|---|---|---|---|---|
| 1 | 59/ M | Xelox 3 cycles with stable disease, rx switch due to loss of vascular access | Gemcitabine+Xeloda->3 cycles and POD | Xeloda 1 month with POD, then FOLFIRI 4 cycles with POD, followed by Phase I | 17 |
| 2 | 62/M | Gemcitabine +Cisplatin,3 months with POD | Gemcitabine/Erlotinib 2 mo. with POD | Phase I trial | 17 |
| 3 | 53/M | Folfox 2 cycles with POD | Folfirinox2cycles POD | Gem/Abraxane,3 mo. With stable d/s. Next line Gem/Abraxane/Xeloda with continued response till 8 months, then rx break f/u POD and then Phase I | 29 |
| 4 | 70/F | Whipple surgery, recurrence in 3 yrs with liver mets rxed with partial hepatectomy | Xeloda/Gemcitabine in adj setting for 4 mo. and surveillance for 5 yrs. | Recurrence rxed with Folfox+Avastin for 3 mo. with POD followed by Gem/Abraxane, 1 mo. & DOD | 92 |
| 5 | 66/M | Folfirinox 5mo with stability, rx changed due to toxicity | Gemcitabine +Tazarotene + Xeloda for 6mo with stable d/s for 12 mo. followed by d/s POD | 33 | |
| 6 | 52/M | Folfox 2months with POD | Xeloda XRT 1month | Folfirinox 2 mo. with POD,then Gem/Abraxane 8 mo. with mixed response, then Erlotinib/Avastin 3 mo. with progression | 24 |
| 7 | 55/M | Neoadjuvant Gem/Abraxane+ Cisplatin (2 mo.) | Folfirinox 2mo with POD | Gem/Abraxane/5 FU/Cisplatin 4 mo. with mixed response, the Gem/Abraxane/5FU/Cisplatin/ Erlotinib 4 mo. mixed response and then Phase II trial | 24 |
| 8 | 67/M | Gem/Abraxane/Xeloda with response for 2 mo. followed by stable d/s for 2 mo. then POD | 7 | ||
| 9 | 72/M | Folfirinox 6 cycles with partial response | 15 | ||
| 10 | 65/F | Gem/Cis 8 cycles mixed response | Folfox 4 cycles with POD | Gem/Abraxane for 9 mo. with response | 39 |
| 11 | 49/M | Folfirinox 12 cycles with response | Xeloda maintenance with response for 17 mo. | 31 | |
| 12 | 58/F | Folfirinox 1 mo. with POD | Referred to Phase I | 26 | |
| 13 | 70/M | Folfox 10 cycles with stable response | Xelox mo. with mixed response | Folfox 2 mo. with POD then Everolimus for 6mo with POD followed by Gem/Abraxane 1 mo. with POD | 46 |
| 14 | 61/M | Folfirinox 15 mo. with response | Gem/Abraxane 2mo POD | Folfiri 2 mo. POD &Lost to f/u | 24 |
| 15 16 |
66/M 59/M |
Folfirinox 4 mo. with stable Capecitabine &Oxaliplatin for 6mo with response |
Xeloda+ XRT 1 mo. with response followed by surgery. Lost to follow up |
Adj Folfirinox 2mo f/u POD Gem/Abraxane 2 mo with POD followed by Phase I | 21 6 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).





