Submitted:
16 January 2026
Posted:
16 January 2026
You are already at the latest version
Abstract
Keywords:
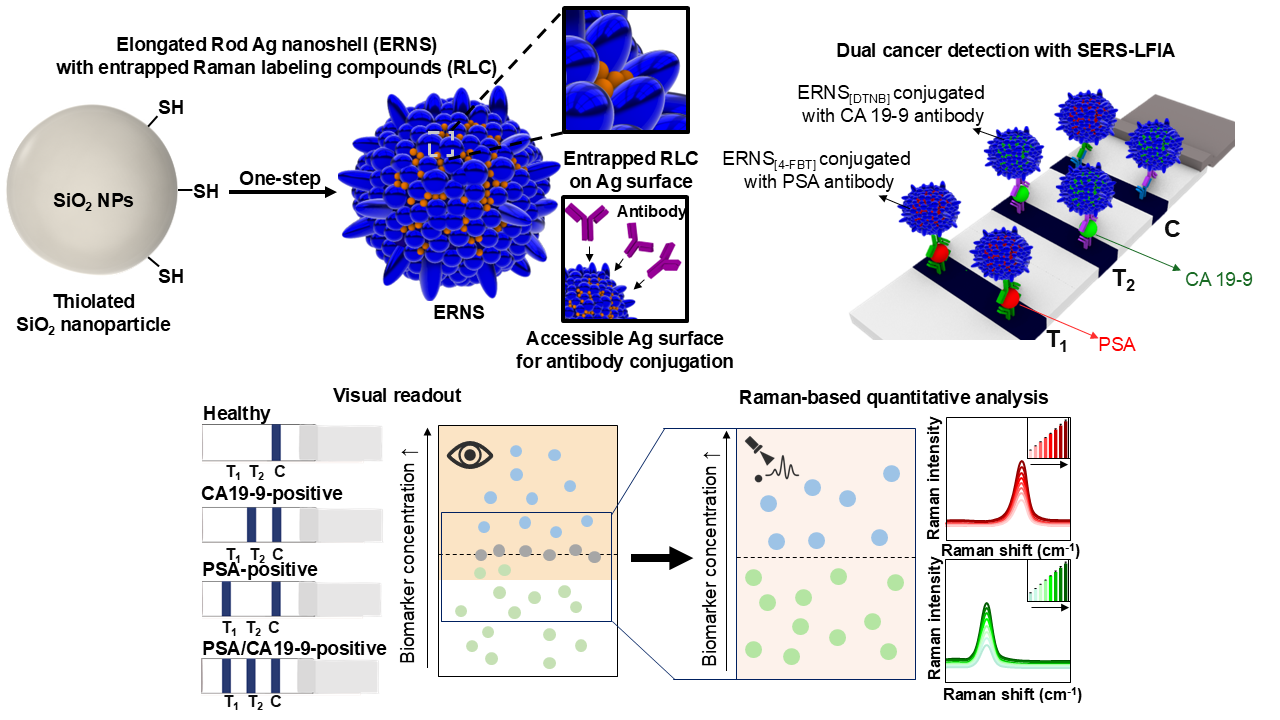
1. Introduction

2. Materials and Methods
2.1. Materials
2.2. Instruments
2.3. Synthesis and Surface Thiolation of Silica Nanoparticles
2.4. Synthesis of a One-Step ERNS (O-ERNS)
2.5. Synthesis of a Multi-Step ERNS (M-ERNS)
2.6. Characterization of Silica NPs Encapsulated with Silver Nanoshells
2.7. Antibody Conjugation onto Synthesized ERNS
2.8. Preparation of Test Strips for the ERNS-Based LFIA
2.9. Validation of Individual PSA and CA19-9 Detection via SERS-Based LFIA
2.10. Application of O-ERNS–Based LFIA for Multiplex Biomarker Detection
2.11. Calculation of Analytical Limit of Detection
3. Results and Discussion
3.1. Structural Evolution and Optical Properties of ERNS During Synthesis.
3.2. Structural and Optical Comparison of ERNS with Different RLC Incorporation Strategies and Versatility Toward Multiple RLCs.
3.3. Effect of RLC Location on Antibody Binding Behavior and SERS-LFIA Performance of ERNS.
3.4. Dual Cancer Detection SERS-LFIA Platform for Simultaneous Detection of PSA and CA19-9 Using ERNS.
4. Conclusion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Kim, Y.J.; Rho, W.-Y.; Park, S.-M.; Jun, B.-H. Optical nanomaterial-based detection of biomarkers in liquid biopsy. J. Hematol. Oncol. 2024, 17, 10. [CrossRef]
- Crosby, D.; Bhatia, S.; Brindle, K.M.; Coussens, L.M.; Dive, C.; Emberton, M.; Esener, S.; Fitzgerald, R.C.; Gambhir, S.S.; Kuhn, P.; et al. Early detection of cancer. Science 2022, 375, eaay9040. [CrossRef]
- Ott, J.J.; Ullrich, A.; Miller, A.B. The importance of early symptom recognition in the context of early detection and cancer survival. Eur. J. Cancer 2009, 45, 2743–2748. [CrossRef]
- van den Bergh, R. C. N.; Loeb, S.; Roobol, M. J. Impact of Early Diagnosis of Prostate Cancer on Survival Outcomes. Eur. Urol. Focus 2015, 1(2), 137–146. [CrossRef]
- Ma, L.; Guo, H.; Zhao, Y.; Liu, Z.; Wang, C.; Bu, J.; Sun, T.; Wei, J. Liquid biopsy in cancer: current status, challenges and future prospects. Signal Transduct. Target. Ther. 2024, 9, 336. [CrossRef]
- Yang, W.; Nguyen, R.; Safri, F.; Shiddiky, M.J.A.; Warkiani, M.E.; George, J.; Qiao, L. Liquid biopsy in hepatocellular carcinoma: ctDNA as a potential biomarker for diagnosis and prognosis. Curr. Oncol. Rep. 2025, 27, 791–802. [CrossRef]
- Ge, Q.; Zhang, Z.-Y.; Li, S.-N.; Ma, J.-Q.; Zhao, Z. Liquid biopsy: comprehensive overview of circulating tumor DNA (Review). Oncol. Lett. 2024, 28(5), 548. [CrossRef]
- Lapitz, A.; Azkargorta, M.; Milkiewicz, P.; et al. Liquid biopsy-based protein biomarkers for risk prediction, early diagnosis and prognostication of cholangiocarcinoma. Journal of Hepatology 2023, 79(1), 93–108. [CrossRef]
- Jun, B.-H., Ed. Nanotechnology for Bioapplications; Springer Singapore: Singapore, 2021. [CrossRef]
- Wu, Y.; Fu, Y.; Guo, J.; Guo, J. Single-molecule immunoassay technology: recent advances. Talanta 2023, 265, 124903. [CrossRef]
- Stosic, K.; Senar, O.A.; Tarfouss, J.; Bouchart, C.; Navez, J.; Van Laethem, J.-L.; Arsenijevic, T. A comprehensive review of the potential role of liquid biopsy as a diagnostic, prognostic, and predictive biomarker in pancreatic ductal adenocarcinoma. Cells 2024, 13(1), 3. [CrossRef]
- Gawel, S.H.; Jackson, L.; Jeanblanc, N.; Davis, G.J. Current and future opportunities for liquid biopsy of circulating biomarkers to aid in early cancer detection. J. Cancer Metastasis Treat. 2022, 8, 26. [CrossRef]
- Seyhan, A.A. Circulating liquid biopsy biomarkers in glioblastoma: advances and challenges. Int. J. Mol. Sci. 2024, 25(14), 7974. [CrossRef]
- Kulasingam, V.; Diamandis, E.P. Strategies for discovering novel cancer biomarkers through utilization of emerging technologies. Nat. Clin. Pract. Oncol. 2008, 5, 588–599. [CrossRef]
- Tighe, P.J.; Ryder, R.R.; Todd, I.; Fairclough, L.C. ELISA in the multiplex era: potentials and pitfalls. Proteomics Clin. Appl. 2015, 9(3–4), 406–422. [CrossRef]
- Topkaya, S. N.; Azimzadeh, M.; Ozsoz, M. Electrochemical biosensors for cancer biomarkers detection: recent advances and challenges. Electroanalysis 2016, 28(7), 1402–1419. [CrossRef]
- Kinnamon, D. S.; Heggestad, J. T.; Liu, J.; Chilkoti, A. Technologies for Frugal and Sensitive Point-of-Care Immunoassays. Annu. Rev. Anal. Chem. 2022, 15, 123–149. [CrossRef]
- Sachdeva, S.; Davis, R.W.; Saha, A.K. Microfluidic point-of-care testing: commercial landscape and future directions. Front. Bioeng. Biotechnol. 2021, 8, 602659. [CrossRef]
- Koczula, K.M.; Gallotta, A. Lateral flow assays. Essays in Biochemistry 2016, 60(1), 111–120. [CrossRef]
- Kim, J.; Shin, M.-S.; Shin, J.; Kim, H.-M.; Pham, X.-H.; Park, S.-M.; Kim, D.-E.; Kim, Y. J.; Jun, B.-H. Recent trends in lateral flow immunoassays with optical nanoparticles. Int. J. Mol. Sci. 2023, 24(11), 9600. [CrossRef]
- Anfossi, L.; Di Nardo, F.; Cavalera, S.; Giovannoli, C.; Baggiani, C. Multiplex lateral flow immunoassay: an overview of strategies towards high-throughput point-of-need testing. Biosensors 2019, 9(1), 2. [CrossRef]
- Nuntawong, P.; Putalun, W.; Tanaka, H.; Morimoto, S.; Sakamoto, S. Lateral flow immunoassay for small-molecules detection in phytoproducts: a review. Journal of Natural Medicines 2022, 76, 521–545. [CrossRef]
- Kakkar, S.; Gupta, P.; Singh Yadav, S.P.; Raj, D.; Singh, G.; Chauhan, S.; Mishra, M.K.; Martín-Ortega, E.; Chiussi, S.; Kant, K. Lateral flow assays: Progress and evolution of recent trends in point-of-care applications. Mater. Today Bio 2024, 28, 101188. [CrossRef]
- Mirica, A.-C.; Stan, D.; Chelcea, I.-C.; Mihailescu, C. M.; Ofiteru, A.; Bocancia-Mateescu, L.-A. Latest Trends in Lateral Flow Immunoassay (LFIA) Detection Labels and Conjugation Process. Front. Bioeng. Biotechnol. 2022, 10, 922772. [CrossRef]
- Liu, Y.; Zhan, L.; Qin, Z.; Sackrison, J.; Bischof, J. C. Ultrasensitive and Highly Specific Lateral Flow Assays for Point-of-Care Diagnosis. ACS Nano 2021, 15(3), 3593–3611. [CrossRef]
- Sajid, M.; Kawde, A.-N.; Daud, M. Designs, formats and applications of lateral flow assay: A literature review. J. Saudi Chem. Soc. 2015, 19, 689–705. [CrossRef]
- Wen, C.; Dou, Y.; Liu, Y.; Jiang, X.; Tu, X.; Zhang, R. Au nanoshell-based lateral flow immunoassay for colorimetric and photothermal dual-mode detection of interleukin-6. Molecules 2024, 29(15), 3683. [CrossRef]
- Chen, X.; Ding, L.; Huang, X.; Xiong, Y. Tailoring noble metal nanoparticle designs to enable sensitive lateral flow immunoassay. Theranostics 2022, 12(2), 574–602. [CrossRef]
- Yin, X.; Liu, S.; Kukkar, D.; Wang, J.; Zhang, D.; Kim, K.-H. Performance enhancement of the lateral flow immunoassay by use of composite nanoparticles as signal labels. TrAC Trends Anal. Chem. 2024, 170, 117441. [CrossRef]
- Quesada-González, D.; Merkoçi, A. Nanoparticle-based lateral flow biosensors. Biosens. Bioelectron. 2015, 73, 47–63. [CrossRef]
- Bahadır, E.B.; Sezgintürk, M.K. Lateral flow assays: principles, designs and labels. TrAC Trends Anal. Chem. 2016, 82, 286–306. [CrossRef]
- Atta, S.; Zhao, Y.; Sanchez, S.; Seedial, D.; Devadhasan, J. P.; et al. Plasmonic-Enhanced Colorimetric Lateral Flow Immunoassays Using Bimetallic Silver-Coated Gold Nanostars. ACS Appl. Mater. Interfaces 2024, 16(40), 54907–54918. [CrossRef]
- Oh, H.-K.; Kim, K.; Park, J.; Im, H.; Maher, S.; et al. Plasmon color-preserved gold nanoparticle clusters for high sensitivity detection of SARS-CoV-2 based on lateral flow immunoassay. Biosens. Bioelectron. 2022, 205, 114094. [CrossRef]
- Shin, M.; Kim, W.; Yoo, K.; Cho, H. S.; Jang, S.; et al. Highly sensitive multiplexed colorimetric lateral flow immunoassay by plasmon-controlled metal–silica isoform nanocomposites: PINs. Nano Converg. 2024, 11, 42. [CrossRef]
- Fu, X.; Cheng, Z.; Yu, J.; Choo, P.; Chen, L.; et al. A SERS-based lateral flow assay biosensor for highly sensitive detection of HIV-1 DNA. Biosens. Bioelectron. 2016, 78, 530–537. [CrossRef]
- Wang, Y.; Schlücker, S. Rational design and synthesis of SERS labels. Analyst 2013, 138, 2224–2238. [CrossRef]
- Verzijl, D.; Riedl, T.; Parren, P. W. H. I.; Gerritsen, A. F. A novel label-free cell-based assay technology using biolayer interferometry. Biosens. Bioelectron. 2017, 87, 388–395. [CrossRef]
- Wang, L.; Xu, T.; Zhang, X.; et al. Multifunctional conductive hydrogel-based flexible wearable sensors. TrAC Trends Anal. Chem. 2021, 134, 116130. [CrossRef]
- Schlücker, S. Surface-enhanced Raman spectroscopy: concepts and chemical applications. Angew. Chem. Int. Ed. 2014, 53(19), 4756–4795. [CrossRef]
- Sun, B.; Wu, H.; Fang, T.; Wang, Z.; Xu, K.; et al. Dual-Mode Colorimetric/SERS Lateral Flow Immunoassay with Machine Learning-Driven Optimization for Ultrasensitive Mycotoxin Detection. Anal. Chem. 2025, 97(9), 4824–4831. [CrossRef]
- Liang, P.; Guo, Q.; Zhao, T.; Wen, C.; Tian, Z.; et al. Ag Nanoparticles with Ultrathin Au Shell-Based Lateral Flow Immunoassay for Colorimetric and SERS Dual-Mode Detection of SARS-CoV-2 IgG. Anal. Chem. 2022, 94(23), 8466–8473. [CrossRef]
- Atta, S.; Zhao, Y.; Li, J. Q.; Vo-Dinh, T. Dual-Modal Colorimetric and Surface-Enhanced Raman Scattering (SERS)-Based Lateral Flow Immunoassay for Ultrasensitive Detection of SARS-CoV-2 Using a Plasmonic Gold Nanocrown. Anal. Chem. 2024, 96(12), 4783–4790. [CrossRef]
- Yanli Tian; Xuechi Yin; Jiawei Li; Leina Dou; Shaochi Wang; et al. A dual-mode lateral flow immunoassay by ultrahigh signal-to background ratio SERS probes for nitrofurazone metabolites ultrasensitive detection. Food Chem. 2024, 441, 138374. [CrossRef]
- Fu, Y.; Zhu, J.; Weng, G.; Li, J.; Zhao, J. Construction of colorimetric-SERS dual-mode lateral flow immunoassay strips based on hollow Au-Ag garland-like nanoprobes and the application in the detection of squamous cell carcinoma antigen. Sens. Actuators B Chem. 2025, 444, 138402. [CrossRef]
- Khlebtsov, B.; Khlebtsov, N. Surface-Enhanced Raman Scattering-Based Lateral-Flow Immunoassay. Nanomaterials 2020, 10(11), 2228. [CrossRef]
- Park, S.; Shin, J.; Kim, Y.-H.; Jun, B.-H. One-Step Synthesis of Roughened Ag Nanoshells with Internal Standards for Label-Free SERS Detection of Environmental Contaminants. ACS Appl. Nano Mater. 2025, 8, 15463–15471. [CrossRef]
- Cha, M. G.; Kang, H.; Choi, Y.-S.; Cho, Y.; Lee, M.; Lee, H.-Y.; Lee, Y.-S.; Jeong, D. H. Effect of Alkylamines on Morphology Control of Silver Nanoshells for Highly Enhanced Raman Scattering. ACS Appl. Mater. Interfaces 2019, 11, 8374–8381. [CrossRef]
- Yang, J.-K.; Hwang, I.-J.; Cha, M.-G.; Kim, H.-I.; Yim, D.-B.; Jeong, D.-H.; Lee, Y.-S.; Kim, J.-H. Reaction Kinetics-Mediated Control over Silver Nanogap Shells as Surface-Enhanced Raman Scattering Nanoprobes for Detection of Alzheimer’s Disease Biomarkers. Small 2019, 15, 1900613. [CrossRef]
- Catalona, W. J.; Hudson, M. A.; Scardino, P. T.; Richie, J. P.; Ahmann, F.; et al. Selection of Optimal Prostate Specific Antigen Cutoffs for Early Detection of Prostate Cancer: Receiver Operating Characteristic Curves. J. Urol. 1994, 152, 2037–2042. [CrossRef]
- Lee, K.J.; Kim, S.J.; Lee, J.; Lee, J.S.; Kim, S.H.; Kim, S.W. Serum CA 19-9 and CEA levels as prognostic factors in pancreatic cancer: a clinical study. Yonsei Med. J. 2013, 54, 643–649. [CrossRef]




Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2026 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).




