1. Introduction
Obesity is a major global health challenge and a well-established risk factor for dyslipidemia, insulin resistance, type 2 diabetes mellitus, and cardiovascular disease (1). The clustering of elevated triglycerides, reduced high-density lipoprotein cholesterol (HDL-C), increased low-density lipoprotein cholesterol (LDL-C), and central adiposity contributes substantially to atherogenesis and cardiometabolic morbidity (2). Traditional lipid parameters, while clinically useful, may not fully capture the complexity of cardiovascular risk associated with obesity-related metabolic dysfunction (3).
In recent years, composite lipid-derived indices, such as the triglyceride-to-HDL cholesterol ratio (TG/HDL), the atherogenic indices, like Castelli indices, have emerged as robust predictors of insulin resistance, small dense LDL particles, and cardiovascular events (4). Similarly, indices integrating lipid and glucose parameters, including the triglyceride–glucose body mass index (TyG-BMI) and metabolic syndrome–related insulin resistance (MTS-IR), have gained relevance as practical and cost-effective tools for assessing metabolic risk in clinical settings (5).
Bariatric surgery is currently the most effective therapeutic strategy for achieving sustained weight loss in individuals with severe obesity (6). Beyond its effects on body weight, Roux-en-Y gastric bypass (RYGB) induces profound metabolic changes mediated by alterations in gut hormone secretion, bile acid metabolism, insulin sensitivity, and adipokine profiles. These mechanisms contribute to improvements in glucose homeostasis and lipid metabolism that often precede substantial weight loss (7).
Although numerous studies have reported improvements in lipid profile following bariatric surgery, fewer investigations have examined the longitudinal evolution of atherogenic and insulin resistance indices in parallel with traditional lipid parameters over extended follow-up periods (8,9). Understanding these integrated metabolic changes is essential for accurately assessing postoperative cardiovascular risk and optimizing long-term patient management (10).
Therefore, this study aimed to evaluate the temporal effects of Roux-en-Y gastric bypass on lipid profile parameters, atherogenic indices, and insulin resistance–related markers over an 18-month follow-up period. By integrating conventional and composite metabolic index, this work seeks to provide a comprehensive assessment of the cardiometabolic benefits of bariatric surgery (11–13).
2. Materials and Methods
2.1. Study Design and Participants
This longitudinal observational study included adult patients with obesity undergoing RYGB at an obesity and metabolic center. A total of 40 patients were consecutively enrolled. The mean age of the cohort was 46.6 years, and 82.5% of participants were female. All participants were evaluated at four time points: preoperative baseline (E1) and at 6 months (E3), 12 months (E4), and 18 months (E5) after surgery.
Inclusion criteria comprised adults aged ≥18 years who underwent primary RYGB and had complete biochemical and anthropometric data available at all follow-up time points. Patients with revisional bariatric procedures, active inflammatory or infectious diseases, chronic liver disease, malignant disease, or incomplete follow-up data were excluded from the analysis.
2.2. Surgical Procedure
All patients underwent standardized Roux-en-Y gastric bypass performed by an experienced bariatric surgical team, following established clinical protocols. The procedure consisted of the creation of a small gastric pouch with a Roux limb and biliopancreatic limb according to institutional standards. Postoperative care followed a multidisciplinary approach, including nutritional counseling and routine clinical follow-up.
2.3. Data Collection and Anthropometric Assessment
Anthropometric measurements were obtained at each study visit by trained healthcare professionals. Body weight was measured using a calibrated scale, and height was measured using a stadiometer, with participants wearing light clothing and no shoes. Waist circumference was measured at the midpoint between the lower margin of the last rib and the iliac crest. Percentage of total weight loss (%TWL) was calculated relative to baseline body weight.
The body roundness index (BRI) was calculated to assess central adiposity and body fat distribution using established equations (14).
2.4. Biochemical Measurements
Fasting blood samples were collected after an overnight fast of at least 8 hours at each time point. Serum concentrations of total cholesterol, triglycerides, HDL cholesterol, LDL cholesterol, and glucose were measured using standard automated enzymatic methods in the hospital’s certified clinical laboratory.
2.5. Atherogenic and Insulin Resistance Indices
Atherogenic and insulin resistance–related indices were calculated using established formulas. The triglyceride-to-HDL cholesterol ratio (TG/HDL) was computed as the ratio between fasting triglycerides and HDL cholesterol. The atherogenic indices, the Castelli I and Castelli II index, were calculated to assess lipid-related cardiovascular risk (15).
Insulin resistance was evaluated using the triglyceride–glucose body mass index (TyG-BMI) (16,17), which integrates fasting triglycerides, fasting glucose, and body mass index, and the metabolic syndrome–related insulin resistance (MTS-IR) index (18). These indices were selected due to their validated association with insulin resistance, metabolic syndrome, and cardiovascular risk.
2.6. Ethical Considerations
The study was conducted in accordance with the Declaration of Helsinki. Ethical approval was obtained from the institutional Ethics Committee (approval code: XXX). Written informed consent was obtained from all participants prior to inclusion in the study.
2.7. Statistical Analysis
Statistical analyses were performed using jamovi (version 2.3) based on the R statistical environment (R Core Team, version 4.1). Continuous variables were assessed for normality using visual inspection of histograms and Q–Q plots. Descriptive statistics are presented as mean ± standard deviation (SD) for all continuous variables. Longitudinal changes in lipid profile parameters, atherogenic indices, insulin resistance–related indices, anthropometric measures, and weight loss outcomes were analyzed descriptively across the four time points: preoperative baseline (E1) and 6 (E3), 12 (E4), and 18 (E5) months after surgery. Graphical representations were used to illustrate temporal trends in metabolic parameters. A two-tailed significance level of 0.05 was considered for all analyses.
3. Results
3.1. Study Population Characteristics
A total of 40 patients undergoing RYGB were included in the analysis. The mean age of the cohort was 46.6 years, and 82.5% of participants were female. All patients were evaluated preoperatively (E1) and at 6 months (E2), 12 months (E3), and 18 months (E4) postoperatively. Complete longitudinal data were available for all analyzed variables at each time point.
3.2. Changes in Lipid Profile After Bariatric Surgery
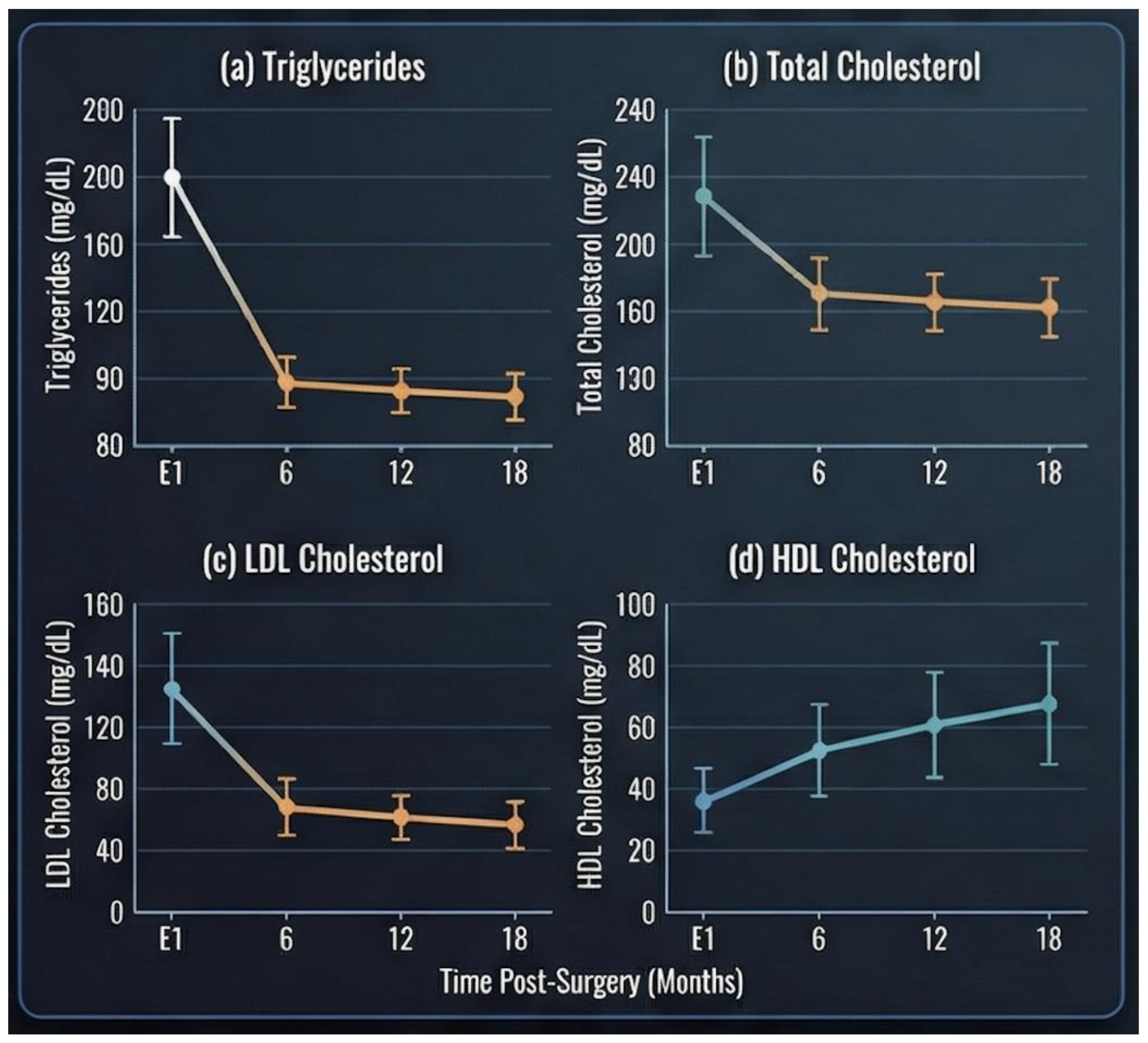
Significant improvements in lipid profile parameters were observed following surgery (
Figure 1). Mean triglyceride levels decreased progressively from 137 ± 56.8 mg/dL at baseline to 109 ± 40.3 mg/dL at 6 months, 95.6 ± 27.8 mg/dL at 12 months, and 91.6 ± 32.6 mg/dL at 18 months postoperatively.
Total cholesterol levels showed a gradual reduction over time, declining from 172 ± 34.5 mg/dL at baseline to 162 ± 37.0 mg/dL at 6 months, 157 ± 23.8 mg/dL at 12 months, and 160 ± 29.8 mg/dL at 18 months. A similar pattern was observed for LDL cholesterol, which decreased from 97.6 ± 34.0 mg/dL preoperatively to 92.9 ± 32.2 mg/dL at 6 months, reaching the lowest mean value at 12 months (86.8 ± 22.1 mg/dL), with slight stabilization at 18 months (89.0 ± 26.7 mg/dL).
In contrast, HDL cholesterol levels exhibited a progressive increase over follow-up, rising from 47.3 ± 10.5 mg/dL at baseline to 47.4 ± 11.7 mg/dL at 6 months, 50.6 ± 11.1 mg/dL at 12 months, and 52.5 ± 11.8 mg/dL at 18 months post-surgery.
3.3. Evolution of Atherogenic Lipid Indices
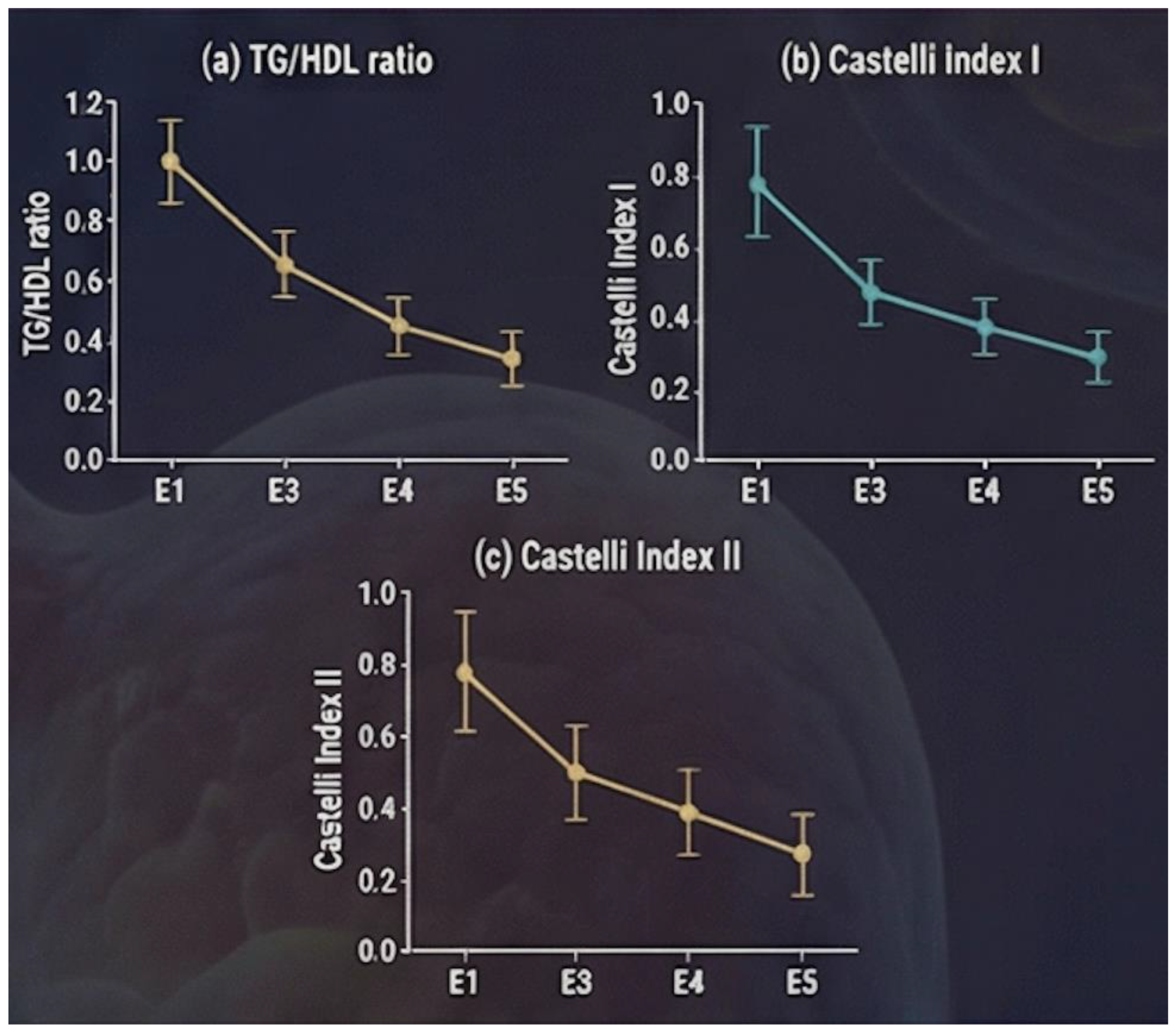
A consistent postoperative improvement was observed across all evaluated atherogenic indices (
Figure 2). The triglyceride-to-HDL cholesterol ratio (TG/HDL) decreased from 3.03 ± 1.52 at baseline to 2.54 ± 1.61 at 6 months, 2.02 ± 0.86 at 12 months, and 1.84 ± 0.81 at 18 months.
The atherogenic index Castelli I also showed a progressive reduction, with mean values declining from 3.78 ± 0.98 preoperatively to 3.62 ± 1.40 at 6 months, 3.23 ± 0.90 at 12 months, and 3.15 ± 0.79 at 18 months postoperatively. Similarly, the Castelli II index decreased steadily over time, from 2.18 ± 0.91 at baseline to 2.12 ± 1.13 at 6 months and 1.83 ± 0.79 at 12 months, indicating a less atherogenic lipid profile during follow-up.
3.4. Insulin Resistance–Related Indices
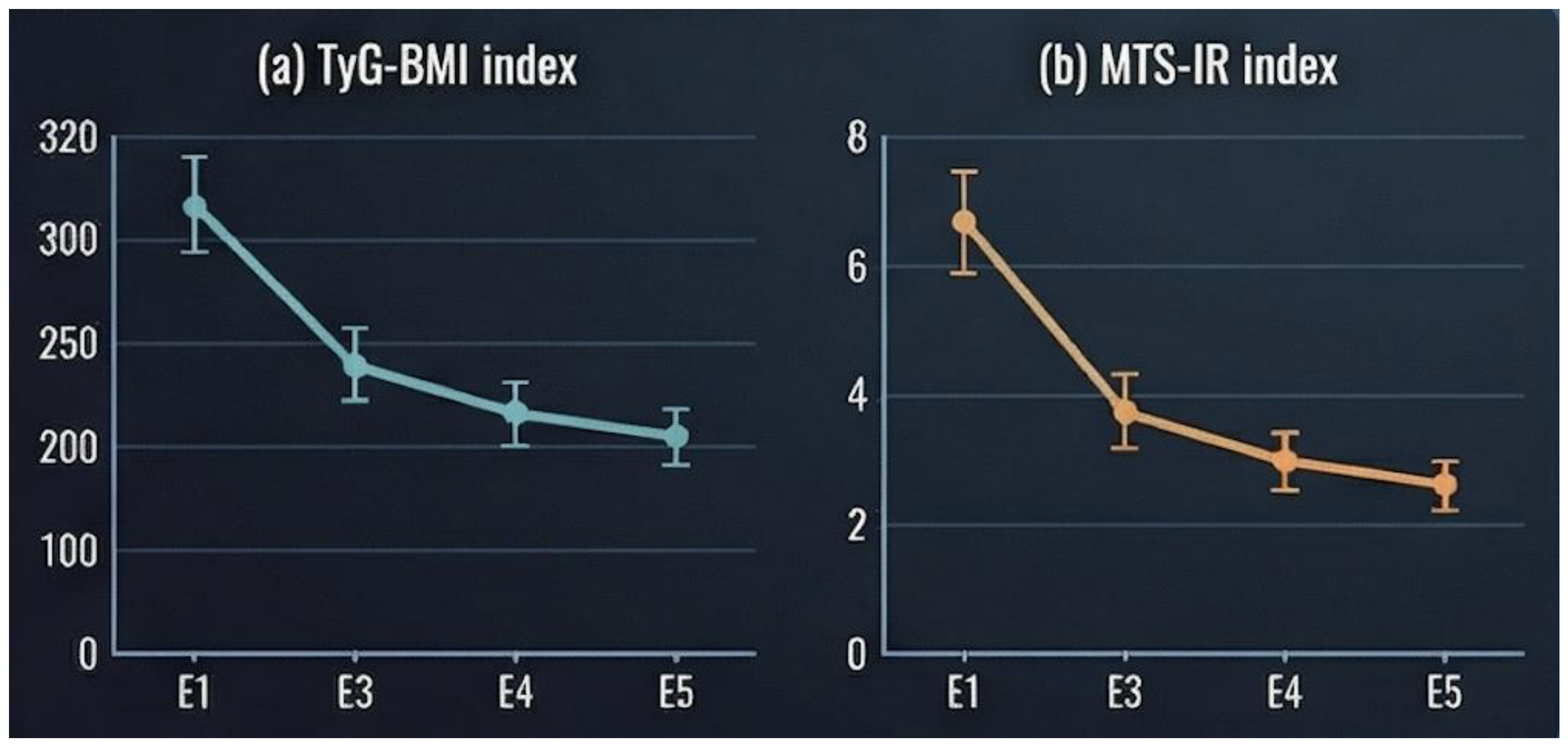
Indices reflecting insulin resistance demonstrated marked postoperative improvement. The TyG-BMI index decreased substantially from 374 ± 49.1 at baseline to 253 ± 38.8 at 6 months, followed by further reductions at 12 months (229 ± 38.4) and stabilization at 18 months (229 ± 42.1) (
Figure 3a).
Likewise, the metabolic syndrome–related insulin resistance (MTS-IR) index declined from 64.9 ± 9.32 at baseline to 44.5 ± 7.34 at 6 months and 39.8 ± 6.87 at 12 months, reflecting a significant attenuation of insulin resistance following surgery (
Figure 3b).
3.5. Weight Loss and Central Adiposity Parameters
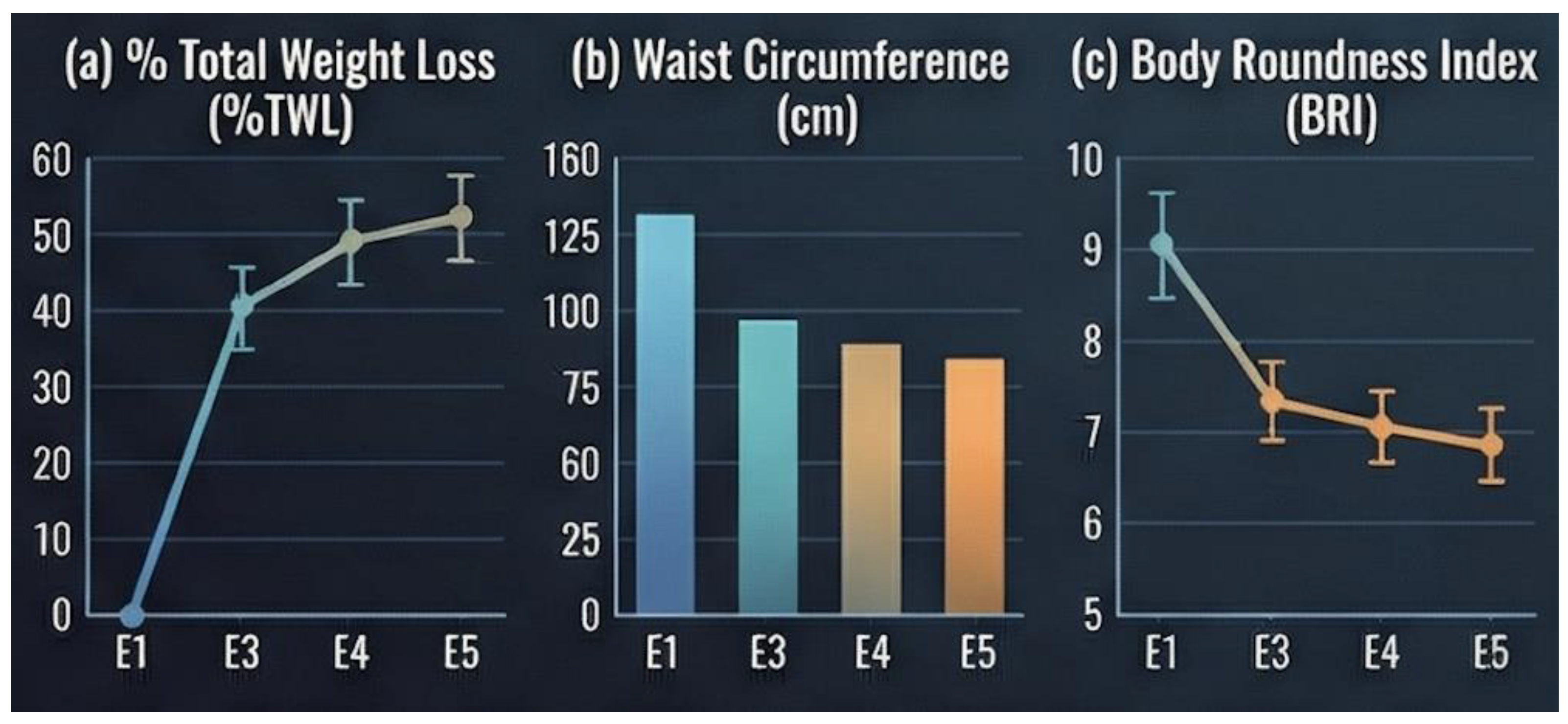
Substantial and sustained weight loss was observed throughout follow-up (
Table 1). Mean percentage of total weight loss (%TWL) increased progressively from the early postoperative period to 18 months. In parallel, waist circumference decreased from 124 ± 10.8 cm at baseline to 110 ± 10.5 cm at 6 months, 97.2 ± 11.0 cm at 12 months, and 91.0 ± 10.6 cm at 18 months postoperatively.
The body roundness index (BRI) followed a similar downward trend, decreasing from 17.6 ± 2.27 at baseline to 13.7 ± 2.08 at 6 months, 13.1 ± 2.07 at 12 months, and 13.0 ± 2.06 at 18 months (
Figure 4c). These reductions in central adiposity coincided with the observed improvements in lipid parameters and indices of insulin resistance.
4. Discussion
The present study demonstrates that Roux-en-Y gastric bypass (RYGB) induces a marked and sustained improvement in lipid metabolism, atherogenic risk indices, and insulin resistance over an 18-month follow-up period in adults with obesity. Beyond weight loss, our findings highlight the profound metabolic benefits of bariatric surgery, reinforcing its role as a cardiometabolic intervention rather than solely a restrictive or malabsorptive procedure.
A significant and progressive reduction in serum triglyceride concentrations was observed as early as six months after surgery, with continued improvement up to 18 months. Hypertriglyceridemia is closely associated with insulin resistance, hepatic overproduction of very-low-density lipoproteins, and increased cardiovascular risk (4,6). The early decline in triglyceride levels observed in this cohort likely reflects rapid postoperative improvements in insulin sensitivity, reduced free fatty acid flux to the liver, and favorable changes in gut-derived hormones, particularly glucagon-like peptide-1 (GLP-1). These mechanisms collectively contribute to decreased hepatic lipogenesis and enhanced peripheral lipid utilization (19,20).
Total cholesterol and low-density lipoprotein cholesterol (LDL-C) levels also showed a consistent reduction throughout follow-up. Although LDL-C reductions after bariatric surgery may vary across studies, the sustained decrease observed here suggests long-term modulation of lipid absorption, bile acid metabolism, and hepatic cholesterol synthesis. Importantly, these changes were maintained at 18 months, indicating metabolic stabilization rather than transient postoperative effects.
High-density lipoprotein cholesterol (HDL-C) demonstrated a delayed but clinically relevant increase, becoming more evident at 12 and 18 months postoperatively (11). This temporal pattern aligns with previous evidence indicating that HDL-C improvements typically follow sustained weight loss and increased insulin sensitivity rather than early postoperative caloric restriction alone. The rise in HDL-C contributes meaningfully to cardiovascular risk reduction, particularly when interpreted in conjunction with triglyceride lowering and improved lipid ratios.
A key strength of this study lies in the evaluation of multiple atherogenic indices, which provide superior cardiovascular risk stratification compared with isolated lipid parameters. The triglyceride-to-HDL cholesterol ratio (TG/HDL), a robust surrogate marker of insulin resistance and small dense LDL particles, decreased substantially over time, reaching values associated with lower cardiometabolic risk. Similarly, both the atherogenic index, Castelli I and Castelli II index exhibited consistent postoperative reductions, reflecting a shift toward a less atherogenic lipid profile (7,21). These findings emphasize that RYGB favorably alters lipid interactions and ratios that are directly implicated in atherosclerotic progression.
In parallel, indices of insulin resistance demonstrated marked improvement. The TyG-BMI index, which integrates fasting triglycerides, glucose, and adiposity, showed a pronounced reduction from baseline to 18 months, highlighting a significant attenuation of insulin resistance (15). This improvement was corroborated by the decrease in the metabolic syndrome–related insulin resistance (MTS-IR) index (18). Together, these findings suggest that bariatric surgery induces a comprehensive metabolic reprogramming, linking adiposity reduction to improved glucose–lipid homeostasis.
The reduction in waist circumference and body roundness index (BRI) further supports the central role of visceral fat loss in mediating these metabolic benefits. Visceral adiposity is strongly associated with dyslipidemia, systemic inflammation, and insulin resistance (22,23). Its reduction following RYGB likely contributes to improved adipokine secretion profiles, including increased adiponectin and decreased leptin levels, thereby enhancing insulin sensitivity and lipid metabolism.
Clinically, these results have important implications. The sustained improvement in atherogenic and insulin resistance indices suggests a meaningful reduction in long-term cardiovascular risk among patients undergoing RYGB. Moreover, the use of composite indices such as TG/HDL and TyG-BMI offers clinicians practical and cost-effective tools for postoperative metabolic monitoring, allowing early identification of patients who may require intensified lifestyle or pharmacological interventions (10,17).
Despite its strengths, this study has limitations. The relatively small sample size and predominance of female participants may limit generalizability. Additionally, the absence of a non-surgical control group precludes direct comparison with conservative management. Nevertheless, the longitudinal design and comprehensive metabolic assessment strengthen the robustness of the observed trends.
In conclusion, Roux-en-Y gastric bypass leads to sustained improvements in lipid profile, atherogenic indices, and insulin resistance up to 18 months post-surgery. These findings reinforce the role of bariatric surgery as an effective strategy for reducing cardiometabolic risk and highlight the clinical value of integrating lipid-derived indices into routine postoperative assessment. Future studies with larger cohorts and longer follow-up are warranted to confirm whether these metabolic improvements translate into reduced cardiovascular events and mortality
5. Conclusions
Roux-en-Y gastric bypass is associated with sustained and clinically meaningful improvements in lipid profile, atherogenic indices, and insulin resistance up to 18 months after surgery. The observed reductions in triglycerides, LDL cholesterol, and composite atherogenic indices, together with increases in HDL cholesterol, indicate a shift toward a less atherogenic lipid phenotype. Concurrent improvements in insulin resistance indices highlight the broad metabolic impact of bariatric surgery beyond weight loss alone.
The use of lipid-derived and insulin resistance indices, such as TG/HDL and TyG-BMI, offers valuable and accessible tools for monitoring cardiometabolic risk in the postoperative setting. These findings support the role of Roux-en-Y gastric bypass as an effective strategy for long-term cardiovascular risk reduction and underscore the importance of comprehensive metabolic assessment in patients with obesity undergoing bariatric surgery.
Author Contributions
For research articles with several authors, a short paragraph specifying their individual contributions must be provided. The following statements should be used: “Conceptualization, C.M. and M.C.; methodology, C.M.; software, C.M.; validation, M.C., A.A. and J.G.; formal analysis, C.M.; investigation, C.M. and A.A.; resources, M.C.; data curation, A.A.; writing—original draft preparation, C.M.; writing—review and editing, M.C.; visualization, J.G.; supervision, J.G.; project administration, C.M.; All authors have read and agreed to the published version of the manuscript.”.
Funding
This research received no external funding.
Institutional Review Board Statement
The study was conducted in accordance with the Declaration of Helsinki and approved by the Institutional Ethics Committee of Hospital do Espirito Santo Évora (protocol code 062/22 and 03/11/2022).
Informed Consent Statement
Informed consent was obtained from all subjects involved in the study.
Data Availability Statement
We encourage all authors of articles published in MDPI journals to share their research data. In this section, please provide details regarding where data supporting reported results can be found, including links to publicly archived datasets analyzed or generated during the study. Where no new data were created, or where data is unavailable due to privacy or ethical restrictions, a statement is still required. Suggested Data Availability Statements are available in section “MDPI Research Data Policies” at
https://www.mdpi.com/ethics.
Conflicts of Interest
The authors declare no conflicts of interest.
Abbreviations
The following abbreviations are used in this manuscript:
| RYGB |
Roux-en-Y gastric bypass |
| LDL-C |
lipoprotein cholesterol |
| TG/HDL |
Triglyceride-to-HDL cholesterol ratio |
| TyG-BMI |
Triglyceride–glucose body mass index |
| HDL-C |
lipoprotein cholesterol |
| BRI |
body roundness index |
| %TWL |
percentage of total weight loss |
| GLP-1 |
glucagon-like peptide-1 |
References
- Phelps, N.H.; Singleton, R.K.; Zhou, B.; Heap, R.A.; Mishra, A.; Bennett, J.E.; et al. Worldwide trends in underweight and obesity from 1990 to 2022: a pooled analysis of 3663 population-representative studies with 222 million children, adolescents, and adults. Lancet 2024, 403, 1027–1050. [Google Scholar] [CrossRef] [PubMed]
- Grundy, S.M. Metabolic syndrome update. Trends Cardiovasc Med. 2016, 26, pp. 364–373. Available online: https://www.sciencedirect.com/science/article/abs/pii/S1050173815002492?via%3Dihub.
- Lavie, C.J.; Milani, R.V.; Ventura, H.O. Obesity and Cardiovascular Disease. Risk Factor, Paradox, and Impact of Weight Loss. J Am Coll Cardiol 2009, 53, 1925–1932. Available online: https://pubmed.ncbi.nlm.nih.gov/19460605/. [CrossRef] [PubMed]
- Goldenberg, I.; Benderly, M.; Sidi, R.; Boyko, V.; Tenenbaum, A.; Tanne, D.; et al. Relation of Clinical Benefit of Raising High-Density Lipoprotein Cholesterol to Serum Levels of Low-Density Lipoprotein Cholesterol in Patients With Coronary Heart Disease (from the Bezafibrate Infarction Prevention Trial). American Journal of Cardiology 2009, 103, 41–45. [Google Scholar] [CrossRef] [PubMed]
- Kones, R. Molecular sources of residual cardiovascular risk, clinical signals, and innovative solutions: Relationship with subclinical disease, undertreatment, and poor adherence: Implications of new evidence upon optimizing cardiovascular patient outcomes. Vasc Health Risk Manag. 2013, 9, 617–670. [Google Scholar] [CrossRef] [PubMed]
- Sharma, R.K.; Singh, V.N.; Reddy, H.K. Thinking beyond low-density lipoprotein cholesterol: Strategies to further reduce cardiovascular risk. Vasc Health Risk Manag. 2009, 5, 793–799. [Google Scholar] [CrossRef] [PubMed]
- Dobiášová, M.; Frohlich, J. The plasma parameter log (TG/HDL-C) as an atherogenic index: correlation with lipoprotein particle size and esterification rate inapob-lipoprotein-depleted plasma (FERHDL). Clin Biochem 2001, 34, 583–588. Available online: https://www.sciencedirect.com/science/article/abs/pii/S0009912001002636?via%3Dihub. [CrossRef] [PubMed]
- Guerrero-Romero, F.; Simental-Mendía, L.E.; González-Ortiz, M.; Martínez-Abundis, E.; Ramos-Zavala, M.G.; Hernández-González, S.O.; et al. The product of triglycerides and glucose, a simple measure of insulin sensitivity. Comparison with the euglycemic-hyperinsulinemic clamp. J Clin Endocrinol Metab. 2010, 95, pp. 3347–3351. Available online: https://pubmed.ncbi.nlm.nih.gov/20484475/.
- Da Luz, P.L.; Favarato, D.; Faria-Neto, J.R.; Lemos, P.; Chagas, A.C.P. High Ratio of Triglycerides to HDL-Cholesterol Predicts Extensive Coronary Disease. Clinics. 2008, 63, pp. 427–432. Available online: https://www.sciencedirect.com/science/article/pii/S1807593222027600?via%3Dihub.
- Xiao, S.; Zhang, Q.; Yang, H.Y.; Tong, J.Y.; Yang, R.Q. The association between triglyceride glucose-body mass index and all-cause and cardiovascular mortality in diabetes patients: a retrospective study from NHANES database. Scientific Reports 2024, 14, 13884. Available online: https://www.nature.com/articles/s41598-024-63886-z. [CrossRef] [PubMed]
- Ashrafian, H.; Le Roux, C.W.; Darzi, A.; Athanasiou, T. Effects of bariatric surgery on cardiovascular function. Circulation. 2008, 118, pp. 2091–2102. Available online: https://pubmed.ncbi.nlm.nih.gov/19001033/.
- Mingrone, G.; Panunzi, S.; De Gaetano, A.; Guidone, C.; Iaconelli, A.; Leccesi, L.; et al. Bariatric surgery versus conventional medical therapy for type 2 diabetes. N Engl J Med 2012, 366, 1577–1585. Available online: https://pubmed.ncbi.nlm.nih.gov/22449317/. [CrossRef] [PubMed]
- Schauer, P.R.; Bhatt, D.L.; Kirwan, J.P.; Wolski, K.; Aminian, A.; Brethauer, S.A.; et al. Bariatric Surgery versus Intensive Medical Therapy for Diabetes — 5-Year Outcomes. New England Journal of Medicine 2017, 376, 641–651. Available online: https://www.nejm.org/doi/pdf/10.1056/NEJMoa1600869. [CrossRef] [PubMed]
- Thomas, D.M.; Bredlau, C.; Bosy-Westphal, A.; Mueller, M.; Shen, W.; Gallagher, D.; et al. Relationships between body roundness with body fat and visceral adipose tissue emerging from a new geometrical model. Obesity 2013, 21, 2264–2271. [Google Scholar] [CrossRef] [PubMed]
- Cui, L.; Zhou, F.; Chen, Y.; Liu, R.; Xing, Y. The lipid ratio castelli’s risk index II is a novel biomarker for intraplaque neovascularization in patients with carotid stenosis. Lipids in Health and Disease. 2025. Available online: https://link.springer.com/article/10.1186/s12944-025-02821-1.
- Tao, L.C.; Xu Jni Wang Tting; Hua, F.; Li, J.J. Triglyceride-glucose index as a marker in cardiovascular diseases: landscape and limitations. Cardiovasc Diabetol. 2022, 21. Available online: https://pubmed.ncbi.nlm.nih.gov/35524263/.
- Song, B.; Zhao, X.; Yao, T.; Lu, W.; Zhang, H.; Liu, T.; et al. Triglyceride Glucose-Body Mass Index and Risk of Incident Type 2 Diabetes Mellitus in Japanese People With Normal Glycemic Level: A Population-Based Longitudinal Cohort Study. Front Endocrinol (Lausanne) 2022, 13, 907973. Available online: https://pmc.ncbi.nlm.nih.gov/articles/PMC9336540/. [CrossRef] [PubMed]
- Yu, L.; Liu, Y.; Guo, R.; Yang, T.; Pan, G.; He, Y.; et al. The metabolic syndrome-insulin resistance index: a tool for identifying dyslipidemia across varied glucose metabolic score in patients with cardiovascular disease. Front Endocrinol (Lausanne) 2025, 16, 1473308. [Google Scholar] [CrossRef] [PubMed]
- Sjöström, L. Review of the key results from the Swedish Obese Subjects (SOS) trial – a prospective controlled intervention study of bariatric surgery. J Intern Med 2013, 273, 219–234. Available online: https://onlinelibrary.wiley.com/doi/full/10.1111/joim.12012. [CrossRef] [PubMed]
- Gloy, V.L.; Briel, M.; Bhatt, D.L.; Kashyap, S.R.; Schauer, P.R.; Mingrone, G.; et al. Bariatric surgery versus non-surgical treatment for obesity: a systematic review and meta-analysis of randomised controlled trials. BMJ. 2013, p. 347. Available online: https://www.bmj.com/content/347/bmj.f5934.
- da Luz, F.Q.; Hay, P.; Touyz, S.; Sainsbury, A. Obesity with Comorbid Eating Disorders: Associated Health Risks and Treatment Approaches. Nutrients 2018, 10. [Google Scholar] [CrossRef] [PubMed]
- Chang, Y.; Guo, X.; Chen, Y.; Guo, L.; Li, Z.; Yu, S.; et al. A body shape index and body roundness index: Two new body indices to identify diabetes mellitus among rural populations in northeast China. BMC Public Health 2015, 15. [Google Scholar] [CrossRef] [PubMed]
- Maessen, M.F.H.; Eijsvogels, T.M.H.; Verheggen, R.J.H.M.; Hopman, M.T.E.; Verbeek, A.L.M.; De Vegt, F. Entering a new era of body indices: The feasibility of a body shape index and body roundness index to identify cardiovascular health status. PLoS ONE 2014, 9, 69. [Google Scholar] [CrossRef] [PubMed]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |