1. Introduction
Type 2 diabetes mellitus (T2DM) represents one of the major public health challenges in the older population, which is characterized by a high burden of comorbidities, clinical frailty, and increased cardiovascular risk [
1]. In Italy, data from the AMD Annals indicate that more than 60% of individuals with T2DM followed in diabetes clinics are aged ≥65 years, with a high prevalence of obesity, hypertension, and dyslipidaemia, resulting in a complex and cumulative cardiovascular risk profile [
2].
In older adults, glucose-lowering treatment is frequently influenced by concerns about adverse events, particularly hypoglycaemia, weight gain, and drug–drug interactions, often leading to therapeutic inertia and suboptimal glycaemic control [
3,
4]. This phenomenon is also well documented in Italian clinical practice, where a substantial proportion of older patients fail to achieve recommended glycaemic targets [
2].
Glucagon-like peptide-1 receptor agonists (GLP-1 RAs) have been shown to improve glycaemic control with a low risk of hypoglycaemia and favourable effects on body weight and cardiovascular outcomes [
5,
6]. However, their use in older patients has historically been limited by practical and organizational barriers, including the need for injectable administration and treatment complexity.
Oral semaglutide is the first GLP-1 receptor agonist available for oral administration and has demonstrated efficacy in improving glycaemic control and cardiometabolic risk factors in patients with T2DM in both randomized clinical trials and real-world settings [
7,
8,
9]. Clinical trial data have also shown a favourable safety profile, with a very low risk of hypoglycaemia when used in the absence of insulin or sulfonylureas [
7].
Despite this evidence, data specifically addressing the use of oral semaglutide in older patients remain limited, particularly with regard to the clinical heterogeneity of this population and the identification of potential predictors of treatment response [
10]. In geriatric diabetes care, it is especially relevant to determine whether the effectiveness of novel glucose-lowering therapies is influenced by factors such as advanced age, diabetes duration, body weight, renal function, or baseline metabolic profile.
Therefore, the aim of the present study was to evaluate the effectiveness and safety of oral semaglutide in a real-world population of patients with T2DM aged ≥65 years, assessing its effects on glycaemic control and major cardiometabolic parameters, and to explore the association between baseline clinical characteristics and glycaemic response.
2. Materials and Methods
2.1. Study Design and Population
This was a retrospective observational study conducted in patients with type 2 diabetes mellitus aged ≥65 years followed in an outpatient setting. Consecutive patients in whom oral semaglutide was initiated in addition to ongoing antidiabetic therapy, according to routine clinical practice and current prescribing indications, were included. Only patients with complete data available at baseline (T0) and after 12 months of follow-up (T1) were considered for the analysis.
2.2. Inclusion and Exclusion Criteria
Patients with a diagnosis of type 2 diabetes mellitus, aged ≥65 years, and eligible for treatment with oral semaglutide according to current indications were included. Patients who had modified lipid-lowering or antihypertensive therapy within 90 days before initiation of oral semaglutide, or who required addition or modification of antidiabetic therapy during follow-up, were excluded. Patients with active malignancy or end-stage chronic kidney disease were also excluded.
2.3. Data Collection
Demographic and clinical data were collected for each patient, including age, sex, diabetes duration, body weight, height, body mass index (BMI), waist circumference, systolic and diastolic blood pressure. Laboratory parameters included glycated haemoglobin (HbA1c), total cholesterol and fractions, triglycerides, serum creatinine and estimated glomerular filtration rate (eGFR). Data were collected at baseline and at 12-month follow-up.
2.4. Metabolic Indices
Indices of visceral adiposity and metabolic risk were calculated, including the Visceral Adiposity Index (VAI), the Triglyceride–Glucose index (TyG), and the Lipid Accumulation Product (LAP), according to standard formulas reported in the literature.
2.5. Study Outcomes
The primary outcome was achievement of HbA1c <7% at 12 months. Secondary outcomes included changes from baseline to follow-up in HbA1c, body weight, BMI, waist circumference, lipid profile, blood pressure, renal function and visceral adiposity indices. Associations between baseline clinical characteristics and glycaemic response were also evaluated.
2.6. Statistical Analysis
Continuous variables are reported as mean ± standard deviation. Comparisons between baseline and follow-up were performed using paired tests, parametric or non-parametric as appropriate. Associations between changes in clinical and metabolic parameters were explored using Spearman correlation analysis. A multivariable logistic regression model was constructed with achievement of HbA1c <7% at 12 months as the outcome, including baseline clinical and metabolic variables as covariates. A p value <0.05 was considered statistically significant.
2.7. Ethical Considerations
The study was conducted in accordance with the principles of the Declaration of Helsinki. As a retrospective observational study based on routine clinical data, the analysis was performed on anonymized data.
3. Results
3.1. Study Population and Changes from Baseline
A total of 81 patients aged ≥65 years treated with oral semaglutide were included in the analysis. All subjects had available HbA1c data at baseline (T0) and after 12 months of treatment (T1).
Paired comparisons between T0 and T1 showed significant improvements in glycaemic control, with a mean HbA1c reduction of −0.96 ± 1.17% (p<0.00001). A significant reduction in body weight (−4.09 ± 4.42 kg) and BMI (−1.50 ± 1.55 kg/m²) was also observed (both p<0.00001), together with a marked decrease in waist circumference.
Favourable changes were documented in the lipid profile, including reductions in total cholesterol and LDL cholesterol and an increase in HDL cholesterol. Significant improvements were also observed in metabolic–visceral indices, with reductions in VAI, TyG index and LAP index.
Renal parameters showed a small but significant decrease in serum creatinine and a corresponding increase in estimated glomerular filtration rate. Systolic and diastolic blood pressure were modestly but significantly reduced over the follow-up period.
Effect sizes were moderate to large for HbA1c, body weight, BMI and waist circumference, supporting the clinical relevance of the observed changes.
3.2. Data Completeness
The completeness of paired T0–T1 data was ≥90% for most variables, while waist circumference, VAI and LAP index showed lower completeness (58%).
3.3. Multivariable Analysis
In the multivariable logistic regression model evaluating predictors of achieving HbA1c <7% at 12 months (49 complete cases), none of the baseline clinical or metabolic variables (age, diabetes duration, BMI, baseline HbA1c, renal function or visceral adiposity indices) were independently associated with the outcome. The model showed a moderate discriminative ability (AUC 0.72), suggesting a relatively homogeneous glycaemic response to oral semaglutide within this older population.
Table 1.
Clinical and metabolic variables at baseline and after 12 months.
Table 1.
Clinical and metabolic variables at baseline and after 12 months.
| Variable |
Baseline (T0) |
12 Months (T1) |
p Value |
| Body weight (kg) |
82.96 ± 13.41 |
78.87 ± 13.40 |
<0.00001 |
| BMI (kg/m²) |
30.39 ± 4.50 |
28.88 ± 4.50 |
<0.00001 |
| Waist circumference (cm) |
105.34 ± 8.61 |
99.51 ± 9.06 |
<0.00001 |
| HbA1c (%) |
7.75 ± 1.01 |
6.80 ± 0.88 |
<0.00001 |
| Total cholesterol (mg/dL) |
168.49 ± 40.27 |
153.76 ± 35.43 |
<0.00001 |
| LDL cholesterol (mg/dL) |
89.37 ± 36.91 |
73.47 ± 31.70 |
0.00006 |
| HDL cholesterol (mg/dL) |
49.99 ± 12.04 |
53.54 ± 12.00 |
<0.00001 |
| Triglycerides (mg/dL) |
148.61 ± 66.30 |
130.54 ± 52.74 |
0.012 |
| Serum creatinine (mg/dL) |
1.05 ± 0.30 |
1.00 ± 0.29 |
0.00077 |
| eGFR (mL/min/1.73m²) |
56.26 ± 17.46 |
59.65 ± 19.67 |
0.0036 |
| Systolic BP (mmHg) |
135.56 ± 14.98 |
131.30 ± 14.68 |
0.0189 |
| Diastolic BP (mmHg) |
77.28 ± 8.26 |
74.57 ± 7.91 |
0.011 |
| VAI |
2.21 ± 1.36 |
1.62 ± 0.83 |
0.00007 |
| TyG index |
4.95 ± 0.29 |
4.80 ± 0.22 |
<0.00001 |
| LAP index |
75.11 ± 38.07 |
56.05 ± 25.32 |
0.00001 |
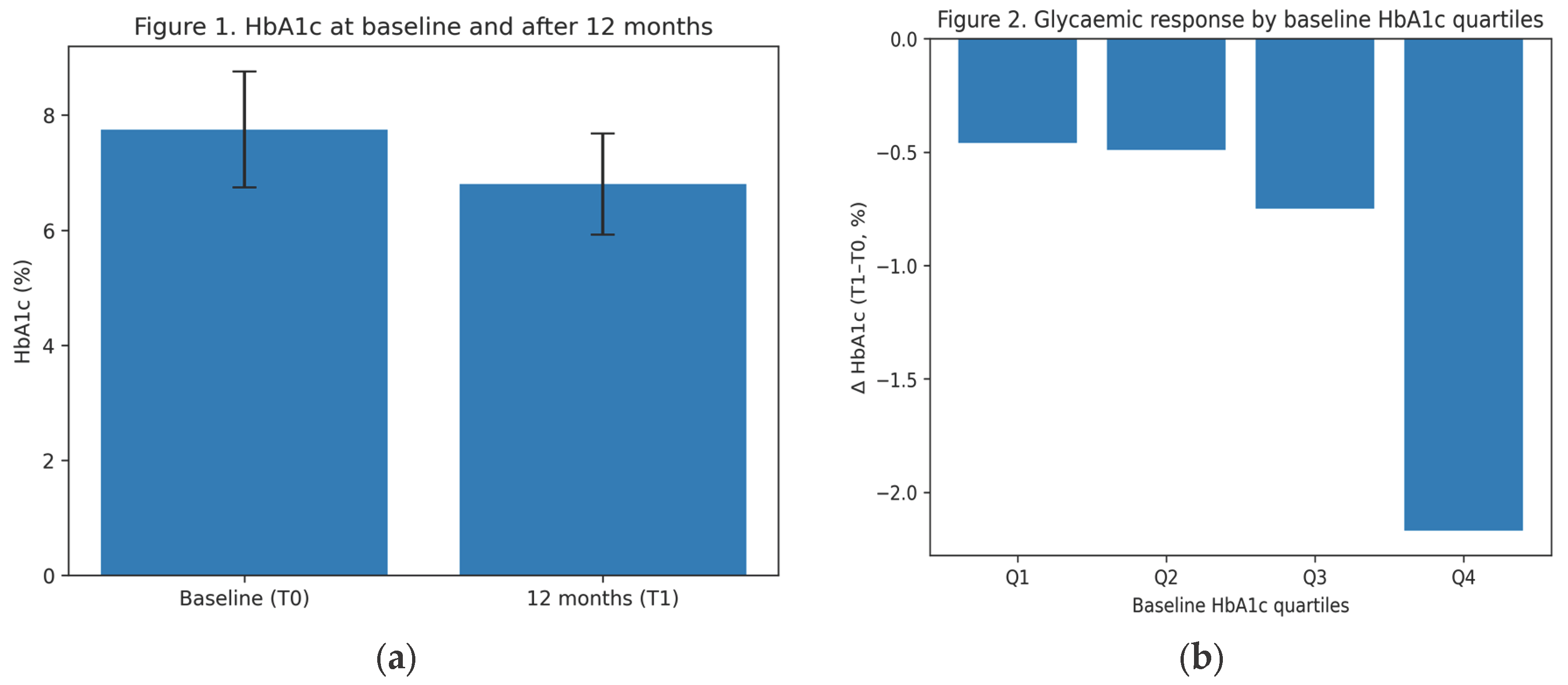
Figure 2.
(a) HbA1c levels at baseline (T0) and after 12 months (T1) of oral semaglutide treatment in patients aged ≥65 years. Bars represent mean values; error bars indicate standard deviation; (b) Mean change in HbA1c according to quartiles of baseline HbA1c after 12 months of oral semaglutide treatment, showing a greater absolute HbA1c reduction in patients with higher baseline HbA1c values.
Figure 2.
(a) HbA1c levels at baseline (T0) and after 12 months (T1) of oral semaglutide treatment in patients aged ≥65 years. Bars represent mean values; error bars indicate standard deviation; (b) Mean change in HbA1c according to quartiles of baseline HbA1c after 12 months of oral semaglutide treatment, showing a greater absolute HbA1c reduction in patients with higher baseline HbA1c values.
Table 2.
Multivariable logistic regression for achievement of HbA1c <7% at 12 months.
Table 2.
Multivariable logistic regression for achievement of HbA1c <7% at 12 months.
| Baseline Variable |
Odds Ratio |
95% CI |
p Value |
| Age (years) |
1.03 |
0.87–1.22 |
0.77 |
| Sex (male) |
1.12 |
0.54–2.31 |
0.76 |
| Diabetes duration (years) |
0.96 |
0.89–1.03 |
0.27 |
| Baseline HbA1c (%) |
0.72 |
0.31–1.68 |
0.45 |
| BMI (kg/m²) |
0.99 |
0.76–1.28 |
0.92 |
| eGFR (mL/min/1.73 m²) |
1.01 |
0.97–1.05 |
0.64 |
4. Discussion
In this observational real-world study conducted in patients with type 2 diabetes mellitus aged ≥65 years, treatment with oral semaglutide for 12 months was associated with a clinically and statistically significant improvement in glycaemic control and multiple cardiometabolic parameters, with no hypoglycaemic events reported. The main finding of the study is the demonstration of a largely homogeneous glycaemic response, independent of major baseline clinical and metabolic characteristics.
In older patients, diabetes management is frequently complicated by multimorbidity, polypharmacy, and frailty, which often lead to a cautious therapeutic approach and, in some cases, undertreatment [
3]. In this context, the risk of hypoglycaemia represents a key determinant in the choice of glucose-lowering therapy [
11]. Our findings confirm that oral semaglutide allows significant HbA1c improvement without hypoglycaemic events, reinforcing the favourable safety profile of this agent in older adults.
Another relevant finding is the absence of independent baseline predictors of achieving the glycaemic target. Multivariable analysis did not identify age, diabetes duration, body weight, renal function, or visceral adiposity indices as determinants of response, suggesting that the effectiveness of oral semaglutide is not restricted to specific clinical phenotypes. This observation is particularly relevant in geriatric care, where clinical heterogeneity is the rule rather than the exception [
12].
Analysis by baseline HbA1c quartiles showed that patients with higher initial HbA1c values achieved a greater absolute reduction in HbA1c, while reaching comparable final levels. This finding is consistent with previous clinical trials and real-world studies on GLP-1 receptor agonists and reflects a response proportional to the degree of baseline glycaemic dysregulation [
9].
Beyond glycaemic control, oral semaglutide treatment was associated with significant reductions in body weight, waist circumference, atherogenic lipid profile, and visceral adiposity indices, resulting in an overall improvement in cardiovascular risk profile. Given the high prevalence of cardiovascular disease in older patients with diabetes, these effects are of particular clinical relevance [
5].
This study has some limitations. The retrospective observational design and the lack of a control group preclude causal inference. In addition, the relatively small sample size and incomplete availability of some variables may have limited the power of certain analyses. However, the real-world nature of the population represents a major strength, reflecting routine clinical practice. In conclusion, the results of this study indicate that oral semaglutide is an effective and safe therapeutic option in older patients with type 2 diabetes mellitus, providing glycaemic and cardiometabolic benefits independent of major baseline clinical characteristics. These findings support the use of oral semaglutide also in elderly and clinically complex populations, helping to overcome traditional age-related therapeutic barriers.
5. Conclusions
The results of the present study demonstrate that oral semaglutide represents an effective and safe therapeutic option in older patients with type 2 diabetes mellitus, even in the presence of marked clinical heterogeneity. In a real-world population aged ≥65 years, treatment was associated with a significant improvement in glycaemic control and cardiometabolic profile, with no hypoglycaemic events reported.
A clinically relevant finding is the absence of independent baseline predictors of glycaemic response, suggesting that the effectiveness of oral semaglutide is not limited by advanced age, diabetes duration, body weight, renal function, or baseline metabolic profile. This aspect is particularly important in geriatric clinical practice, where patient complexity and frailty often influence therapeutic decisions [
12,
13].
The observed benefits on body weight, waist circumference, lipid profile, and visceral adiposity indices further suggest a favourable impact on overall cardiovascular risk, a primary goal in the management of diabetes in older adults [
14,
15]. In this context, the absence of hypoglycaemia is of central importance, as hypoglycaemic events are associated with relevant adverse outcomes, including functional decline, hospitalizations, and increased mortality [
16,
17].
Overall, the available evidence supports a more proactive therapeutic approach in older patients with type 2 diabetes, overcoming the traditional paradigm of age-related undertreatment. The use of oral semaglutide may help simplify therapeutic management, improve adherence, and facilitate the achievement of individualized glycaemic and cardiovascular targets [
18]. Further prospective studies with larger sample sizes are warranted to confirm these findings and to better define the role of oral semaglutide across different subgroups of older patients, including those with greater frailty or advanced comorbidities [
19].
Author Contributions
Conceptualization, Antonio Maria Labate, Provvidenza Villari; methodology, Antonio Maria Labate, Lorenzo Moretti and Provvidenza Villari; formal analysis, Antonio Maria Labate; data curation, Antonio Maria Labate, Lorenzo Moretti; writing—original draft preparation, Antonio Maria Labate, Lorenzo Moretti; writing—review and editing, Antonio Maria Labate, Lorenzo Moretti and Provvidenza Villari. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
The study was conducted in accordance with the Declaration of Helsinki. The patients included in the present analysis were enrolled within a broader institutional protocol approved by the local Ethics Committee; the present work represents a retrospective observational analysis of anonymized data collected during routine clinical practice.
Informed Consent Statement
Patient consent was waived due to the retrospective nature of the study and the use of anonymized data.
Data Availability Statement
The data presented in this study are available on reasonable request from the corresponding author.
Conflicts of Interest
The authors declare no conflict of interest.
Abbreviations
| T2DM |
Type 2 diabetes mellitus |
| DMT2 |
Diabete mellito di tipo 2 |
| HbA1c |
Glycated haemoglobin |
| BMI |
Body mass index |
| eGFR |
Estimated glomerular filtration rate |
| GLP-1 RA |
Glucagon-like peptide-1 receptor agonist |
| VAI |
Visceral Adiposity Index |
| TyG |
Triglyceride–Glucose index |
| LAP |
Lipid Accumulation Product |
| CV |
Cardiovascular |
References
- Sinclair, A.J.; Dunning, T.; Colagiuri, S. Diabetes in older adults: A consensus report. Diabetes Care 2020, 43, 1957–1970. [Google Scholar] [CrossRef]
- Associazione Medici Diabetologi (AMD). Annali AMD 2024. Quality of Diabetes Care in Italy. Rome, Italy, 2024. Available online: https://aemmedi.it/wp-content/uploads/2025/05/Annali_AMD_2024-vers.-protetta.pdf (accessed on 2 January 2026).
- Munshi, M.N.; Slyne, C.; Segal, A.R.; Sternthal, A. Hypoglycemia in older adults with diabetes. Diabetes Care 2016, 39, e53–e54. [Google Scholar]
- American Diabetes Association. Older Adults: Standards of Care in Diabetes—2024. Diabetes Care 2024, 47 Suppl. 1, S283–S295. [Google Scholar] [CrossRef]
- Gerstein, H.C.; Colhoun, H.M.; Dagenais, G.R.; et al. Dulaglutide and cardiovascular outcomes in type 2 diabetes (REWIND). Lancet 2019, 394, 121–130. [Google Scholar] [CrossRef] [PubMed]
- Marso, S.P.; Daniels, G.H.; Brown-Frandsen, K.; et al. Liraglutide and cardiovascular outcomes in type 2 diabetes (LEADER). N Engl J Med 2016, 375, 311–322. [Google Scholar] [CrossRef] [PubMed]
- Davies, M.; Bain, S.C.; Atkin, S.L.; et al. Efficacy and safety of oral semaglutide versus placebo and subcutaneous semaglutide in type 2 diabetes. N Engl J Med 2017, 377, 644–657. [Google Scholar]
- Husain, M.; Birkenfeld, A.L.; Donsmark, M.; et al. Oral semaglutide and cardiovascular outcomes in patients with type 2 diabetes (PIONEER 6). N Engl J Med 2019, 381, 841–851. [Google Scholar] [CrossRef] [PubMed]
- Pratley, R.; Amod, A.; Hoff, S.T.; et al. Oral semaglutide versus placebo and comparators in type 2 diabetes: The PIONEER program. Diabetes Care 2019, 42, 1724–1732. [Google Scholar] [CrossRef]
- Abdelhafiz, A.H.; Sinclair, A.J. Glucagon-like peptide-1 receptor agonists in older people with type 2 diabetes. Drugs Aging 2020, 37, 335–346. [Google Scholar] [CrossRef]
- Abdelhafiz, A.H.; Sinclair, A.J. Management of type 2 diabetes in older people. Diabetes Ther 2013, 4, 13–26. [Google Scholar] [CrossRef] [PubMed]
- Kirkman, M.S.; Briscoe, V.J.; Clark, N.; et al. Diabetes in older adults. Diabetes Care 2012, 35, 2650–2664. [Google Scholar] [CrossRef] [PubMed]
- Sinclair, A.J.; Abdelhafiz, A.H.; Forbes, A. Evidence-based diabetes care for older people with type 2 diabetes: A critical review. Diabet Med 2019, 36, 399–413. [Google Scholar] [CrossRef] [PubMed]
- Cosentino, F.; Grant, P.J.; Aboyans, V.; et al. 2019 ESC Guidelines on diabetes, pre-diabetes, and cardiovascular diseases. Eur Heart J 2020, 41, 255–323. [Google Scholar] [CrossRef] [PubMed]
- Rydén, L.; Grant, P.J.; Anker, S.D.; et al. ESC Guidelines on diabetes, pre-diabetes, and cardiovascular diseases. Eur Heart J 2013, 34, 3035–3087. [Google Scholar] [CrossRef] [PubMed]
- McCoy, R.G.; Lipska, K.J.; Herrin, J.; et al. Hospitalizations and mortality associated with hypoglycemia. JAMA Intern Med 2015, 175, 356–364. [Google Scholar]
- Abdelhafiz, A.H.; Rodríguez-Mañas, L.; Morley, J.E.; Sinclair, A.J. Hypoglycemia in older people. Diabetes Res Clin Pract 2015, 109, 226–234. [Google Scholar] [CrossRef]
- American Diabetes Association. Pharmacologic approaches to glycemic treatment: Standards of Care in Diabetes—2024. Diabetes Care 2024, 47 Suppl. 1, S158–S178. [Google Scholar] [CrossRef] [PubMed]
- Formiga, F.; Ferrer, A.; Chivite, D.; et al. Diabetes mellitus and frailty: A systematic review. Ageing Res Rev 2016, 29, 13–24. [Google Scholar] [CrossRef]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |