Submitted:
18 August 2025
Posted:
19 August 2025
You are already at the latest version
Abstract
Keywords:
1. Introduction
1.1. The Current State of Research
1.2. Specific Aims of the Review
- To evaluate the effectiveness of the compounded semaglutide for weight loss.
- To study the effects of the use of compounded semaglutide in healthy people who are normal or overweight (BMI less than 29.9).
- To evaluate safety, efficacy, extent of weight loss with likely consequential health benefits.
- To propose a novel method to declare the ideal or target weight which bridges the difference in body composition, bone structure and sex. Achieving a target weight is also proposed to measure the success of the weight loss program. The 'success zone' is defined as within 75% of the target weight.
1.3. The Primary Review Question
2. Materials and Methods
2.1. Retrospective Cohort Study Design
2.2. Intervention Which Was Assessed
2.3. Success Zone and Weight Goals
2.4. Inclusion and Exclusion Criteria
2.5. Sample Size and Recruitment
2.6. Informed Consent
2.7. Data Collection and Follow-Up
2.7.a. How Patients Were Identified
2.7.b. Who Will Collect Data and from Where
2.8. Monitoring and Safety Measures
2.9. Data Management and Confidentiality
2.10. Risk and Benefit Analysis
2.11. Statistical Analysis
2.12. Cost to Subjects
3. Results
3.1. Outcome
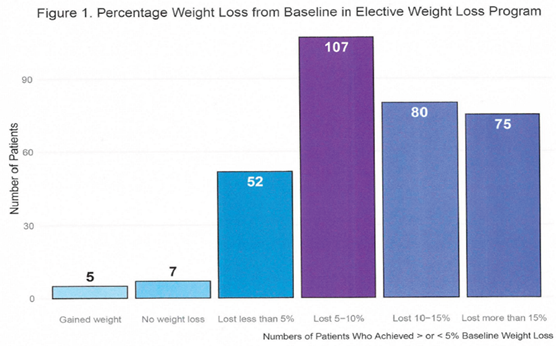
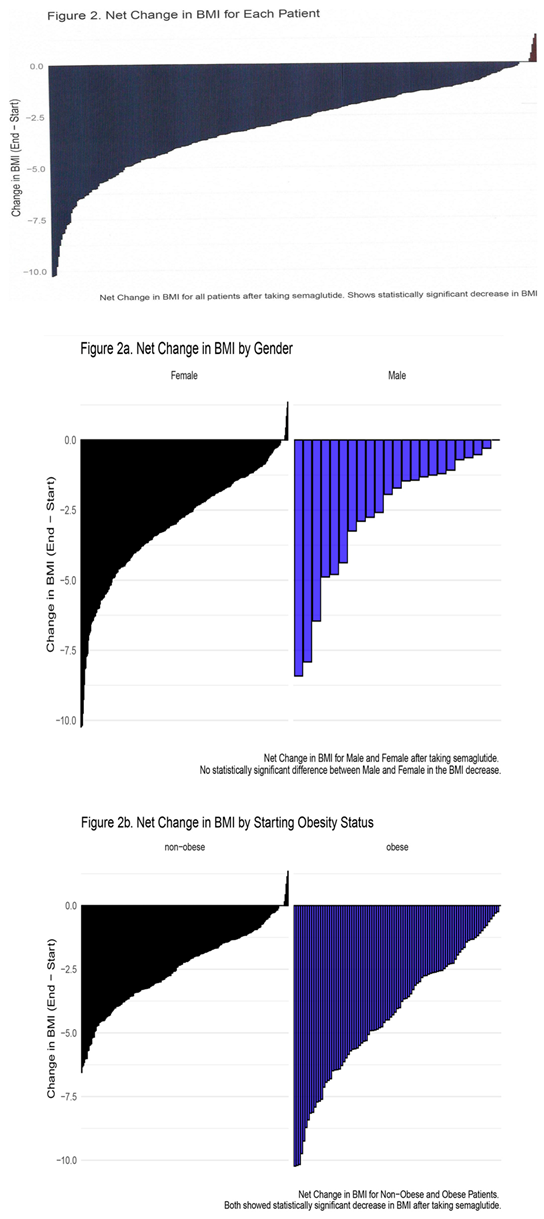
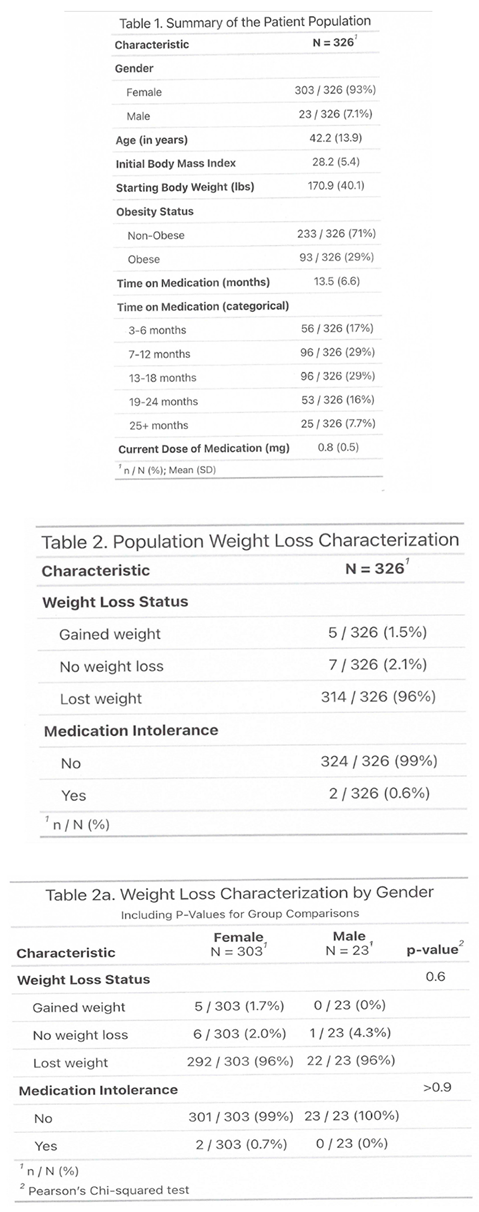
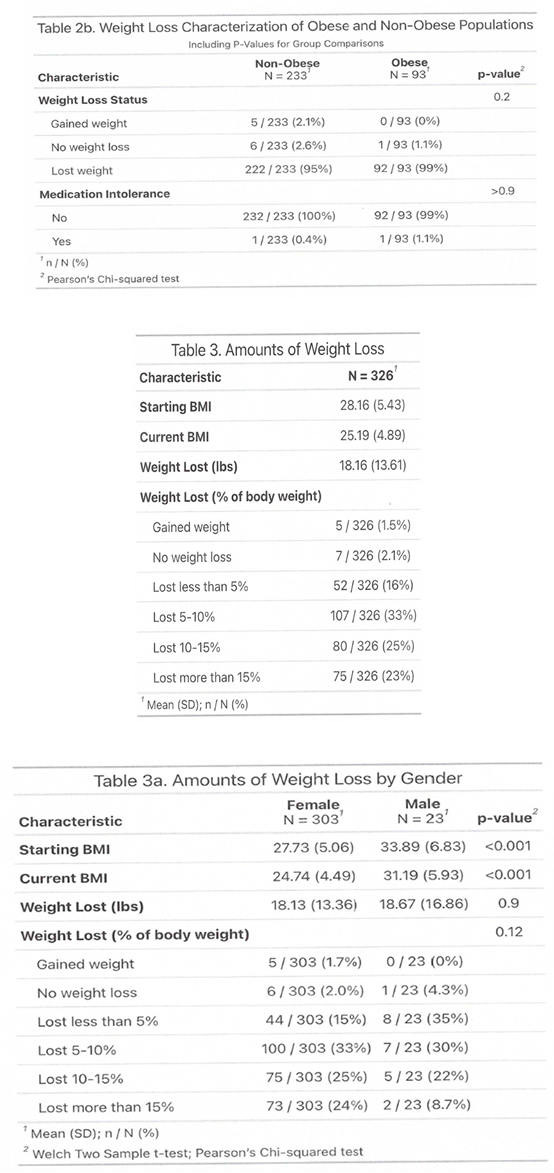
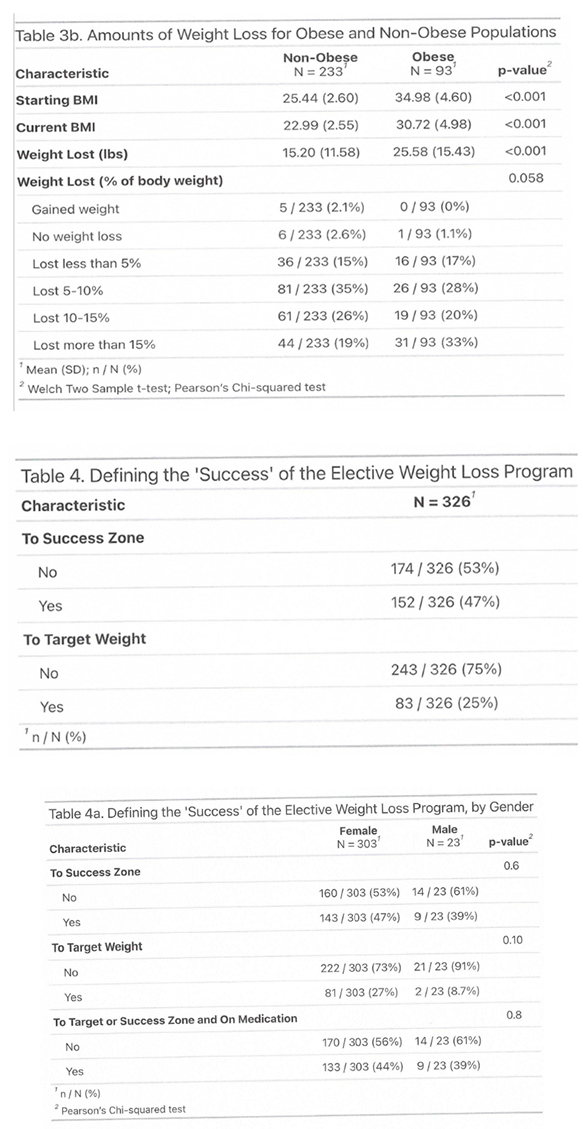
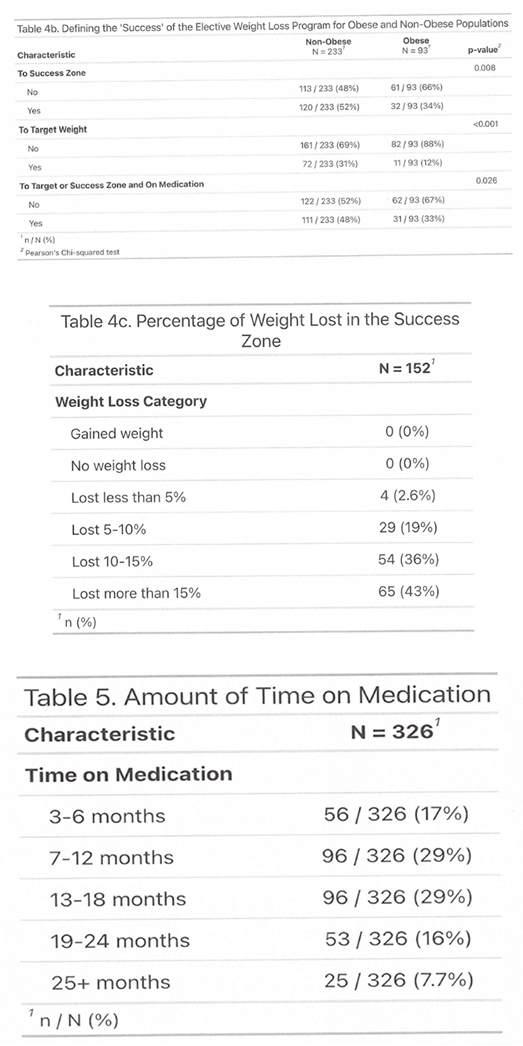
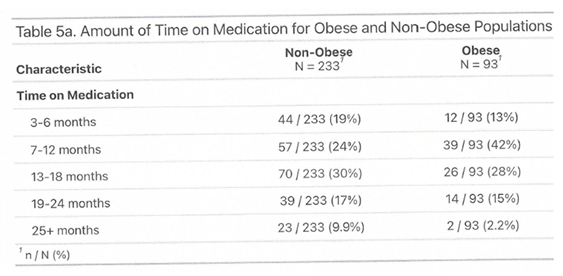
3.2. Side Effects
4. Discussion
5. Conclusions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| EWL | Elective Weight Loss |
References
- Ward, Z. J., Bleich, S. N., Cradock, A. L., Barrett, J. L., Giles, C. M., Flax, C., ... & Gortmaker, S. L. Projected US state-level prevalence of adult obesity and severe obesity. New England Journal of Medicine 2019, 381, 2440–2450. [Google Scholar] [CrossRef] [PubMed]
- Kraus, W. E., Bhapkar, M., Huffman, K. M., Pieper, C. F., Das, S. K., Redman, L. M., ... & Fontana, L. 2 years of calorie restriction and cardiometabolic risk (CALERIE): exploratory outcomes of a multicentre, phase 2, randomised controlled trial. The Lancet Diabetes & Endocrinology 2019, 7, 673–683. [Google Scholar] [CrossRef] [PubMed]
- Centers for Disease Control and Prevention. CDC twenty-four seven. Saving lives, protecting people: heart disease. USA, Published , 2024. Available online: https://www.cdc.gov/heart-disease/data-research/facts-stats/index.html.
- Adeva-Andany, M.M.; Domínguez-Montero, A.; Adeva-Contreras, L.; Fernández-Fernández, C.; Carneiro-Freire, N.; González-Lucán, M. Body Fat Distribution Contributes to Defining the Relationship between Insulin Resistance and Obesity in Human Diseases. Current Diabetes Reviews 2024, 20, 66–96. [Google Scholar] [CrossRef] [PubMed]
- Fontana, L.; Meyer, T.E.; Klein, S.; Holloszy, J.O. Long-term calorie restriction is highly effective in reducing the risk of atherosclerosis in humans. Proceedings of the National Academy of Sciences 2004, 101, 6659–6663. Available online: https://www.pnas.org/doi/abs/10.1073/pnas.0308291101. [CrossRef]
- Speakman, J.R.; Mitchell, S.E. (2011). Caloric restriction. Molecular Aspects of Medicine 2011, 32, 159–221. Available online: https://www.sciencedirect.com/science/article/pii/S009829971100032X. [CrossRef]
- Waziry, R., Ryan, C. P., Corcoran, D. L., Huffman, K. M., Kobor, M. S., Kothari, M., ... & Belsky, D.W. Effect of long-term caloric restriction on DNA methylation measures of biological aging in healthy adults from the CALERIE trial. Nature Aging 2023, 3, 248–257. Available online: https://www.nature.com/articles/s43587-022-00357-y.
- Napoleão, A.; Fernandes, L.; Miranda, C.; Marum, A.P. Effects of calorie restriction on health span and insulin resistance: Classic calorie restriction diet vs. ketosis-inducing diet. Nutrients 2021, 13, 1302. Available online: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC8071299. [CrossRef]
- Ravussin, E.; Redman, L.M.; Rochon, J.; Das, S.K.; Fontana, L.; Kraus, W.E. .. & CALERIE Study Group. A 2-year randomized controlled trial of human caloric restriction: feasibility and effects on predictors of health span and longevity. Journals of Gerontology Series A: Biomedical Sciences and Medical Sciences 2015, 70, 1097–1104. Available online: https://pubmed.ncbi.nlm.nih.gov/26187233/.
- Ryan, D.H.; Yockey, S.R. Weight loss and improvement in comorbidity: differences at 5%, 10%, 15%, and over. Current Obesity Reports 2017, 6, 187–194. [Google Scholar] [CrossRef]
- Guh, D.P.; Zhang, W.; Bansback, N.; Amarsi, Z.; Birmingham, C.L.; Anis, A.H. The incidence of co-morbidities related to obesity and overweight: a systematic review and meta-analysis. BMC Public Health 2009, 9, 1–20. [Google Scholar] [CrossRef]
- Wilding, J. P., Batterham, R. L., Calanna, S., Davies, M., Van Gaal, L. F., Lingvay, I., ... & Kushner, R.F. Once-weekly semaglutide in adults with overweight or obesity. New England Journal of Medicine 2021, 384, 989–1002. [Google Scholar] [CrossRef]
- Ryan, D.H.; Lingvay, I.; Colhoun, H.M.; Deanfield, J.; Emerson, S.S.; Kahn, S.E.; Kushner, R.F.; Marso, S.; Plutzky, J.; Brown-Frandsen, K.; Gronning, M.O.; Hovingh, G.K.; Holst, A.G.; Ravn, H.; Lincoff, A.M. Semaglutide effects on cardiovascular outcomes in people with overweight or obesity (SELECT) rationale and design. Am Heart J. 2020, 229, 75–85. [Google Scholar] [CrossRef] [PubMed]
- Wali S, University of California, Los Angeles. Financial Incentives for Weight Reduction Study (FIReWoRk) [Internet]. ClinicalTrials.gov; 2023 [cited 2025 Jan 10]. Available online: https://clinicaltrials.gov/ct2/show/NCT03157713.
- Davies, M.; Færch, L.; Jeppesen, O.K.; Pakseresht, A.; Pedersen, S.D.; Perreault, L.; Rosenstock, J.; Shimomura, I.; Viljoen, A.; Wadden, T.A.; Lingvay, I.; STEP 2 Study Group. Semaglutide 2·4 mg once a week in adults with overweight or obesity, and type 2 diabetes (STEP 2): a randomised, double-blind, double-dummy, placebo-controlled, phase 3 trial. Lancet. 2021, 397, 971–984. [Google Scholar] [CrossRef] [PubMed]
- Wadden, T.A.; Bailey, T.S.; Billings, L.K.; Davies, M.; Frias, J.P.; Koroleva, A.; Lingvay, I.; O'Neil, P.M.; Rubino, D.M.; Skovgaard, D.; Wallenstein, S.O.; Garvey, W.T.; STEP 3 Investigators. Effect of subcutaneous semaglutide vs placebo as an adjunct to intensive behavioral therapy on body weight in adults with overweight or obesity: The STEP 3 randomized clinical trial. JAMA. 2021, 325, 1403–1413. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Rubino, D.; Abrahamsson, N.; Davies, M.; Hesse, D.; Greenway, F.L.; Jensen, C.; Lingvay, I.; Mosenzon, O.; Rosenstock, J.; Rubio, M.A.; Rudofsky, G.; Tadayon, S.; Wadden, T.A.; Dicker, D.; STEP 4 Investigators. Effect of continued weekly subcutaneous semaglutide vs placebo on weight loss maintenance in adults with overweight or obesity: The STEP 4 randomized clinical trial. JAMA 2021, 325, 1414–1425. [Google Scholar] [CrossRef] [PubMed]
- Garvey, W.T.; Batterham, R.L.; Bhatta, M.; Buscemi, S.; Christensen, L.N.; Frias, J.P.; Jódar, E.; Kandler, K.; Rigas, G.; Wadden, T.A.; Wharton, S.; STEP 5 Study Group. Two-year effects of semaglutide in adults with overweight or obesity: the STEP 5 trial. Nat Med. 2022, 28, 2083–2091. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Kadowaki, T.; Isendahl, J.; Khalid, U.; Lee, S.Y.; Nishida, T.; Ogawa, W.; Tobe, K.; Yamauchi, T.; Lim, S.; STEP 6 investigators. Semaglutide once a week in adults with overweight or obesity, with or without type 2 diabetes in an East Asian population (STEP 6): a randomised, double-blind, double-dummy, placebo-controlled, phase 3a trial. Lancet Diabetes Endocrinol. 2022, 10, 193–206. [Google Scholar] [CrossRef] [PubMed]
- Rubino, D.M.; Greenway, F.L.; Khalid, U.; O'Neil, P.M.; Rosenstock, J.; Sørrig, R.; Wadden, T.A.; Wizert, A.; Garvey, W.T.; STEP 8 Investigators. Effect of weekly subcutaneous semaglutide vs daily liraglutide on body weight in adults with overweight or obesity without diabetes: The STEP 8 randomized clinical trial. JAMA. 2022, 327, 138–150. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Humphreys, S. (2010). The unethical use of BMI in contemporary general practice. British Journal of General Practice 2010, 60, 696–697. [Google Scholar] [CrossRef]
- Pétré, B., Scheen, A., Ziegler, O., Donneau, A. F., Dardenne, N., Husson, E., ...& Guillaume, M. Weight loss expectations and determinants in a large community-based sample. Preventive Medicine Reports 2018, 12, 12–19. [Google Scholar] [CrossRef]
- Flore, G.; Preti, A.; Carta, M.G.; Deledda, A.; Fosci, M.; Nardi, A.E. . & Velluzzi, F. Weight maintenance after dietary weight loss: systematic review and meta-analysis on the effectiveness of behavioural intensive intervention. Nutrients 2022, 14, 1259. [Google Scholar]
- Lowe, M.R.; Kral, T.V.; Miller-Kovach, K. Weight-loss maintenance 1, 2 and 5 years after successful completion of a weight-loss programme. British Journal of Nutrition 2008, 99, 925–930. [Google Scholar] [CrossRef]
- Hintze, L.J.; Mahmoodianfard, S.; Auguste, C.B.; Doucet, É. (2017). Weight loss and appetite control in women. Current Obesity Reports 2017, 6, 334–351. [Google Scholar] [CrossRef] [PubMed]
- Miller-Kovach, K.; Hermann, M.; Winick, M. The psychological ramifications of weight management. Journal of Women's Health & Gender-Based Medicine 1999, 8, 477–482. [Google Scholar] [CrossRef]
- Emmerich, S.D.; Fryar, C.D.; Stierman, B.; Ogden, C.L. Obesity and severe obesity prevalence in adults: United States, 21–23. NCHS Data Brief. 2024 Sep;(508). Available online: https://www.cdc.gov/nchs/data/databriefs/db508.pdf.
- Maegawa, S.; Lu, Y.; Tahara, T.; Lee, J.T.; Madzo, J.; Liang, S.; Jelinek, J.; Colman, R.J.; Issa, J.P.J. Caloric restriction delays age-related methylation drift. Nat Commun. 2017, 8, 539. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Hahn, O.; Grönke, S.; Stubbs, T.M.; Ficz, G.; Hendrich, O.; Krueger, F.; Andrews, S.; Zhang, Q.; Wakelam, M.J.; Beyer, A.; Reik, W.; Partridge, L. Dietary restriction protects from age-associated DNA methylation and induces epigenetic reprogramming of lipid metabolism. Genome Biol. 2017, 18, 56. [Google Scholar] [CrossRef]
- Petkovich, D.A.; Podolskiy, D.I.; Lobanov, A.V.; Lee, S.G.; Miller, R.A.; Gladyshev, V.N. Using DNA Methylation Profiling to Evaluate Biological Age and Longevity Interventions. Cell Metab. 2017, 25, 954–960.e6. [Google Scholar] [CrossRef]
- Fontana, L. The scientific basis of caloric restriction leading to longer life. Curr Opin Gastroenterol. 2009, 25, 144–50. Available online: https://pubmed.ncbi.nlm.nih.gov/19262201/. [CrossRef] [PubMed]
- Dutton, G.R.; Perri, M.G.; Dancer-Brown, M.; Goble, M.; Van Vessem, N. Weight loss goals of patients in a health maintenance organization. Eat Behav. 2010, 11, 74–78. Available online: https://pubmed.ncbi.nlm.nih.gov/20188289/. [CrossRef]
- Fildes, A.; Charlton, J.; Rudisill, C.; Littlejohns, P.; Prevost, A.T.; Gulliford, M.C. Probability of an Obese Person Attaining Normal Body Weight: Cohort Study Using Electronic Health Records. Am J Public Health. 2015, 105, e54–9. Available online: https://pubmed.ncbi.nlm.nih.gov/23747584/. [CrossRef] [PubMed]
- Han, S.H.; Safeek, R.; Ockerman, K.; Trieu, N.; Mars, P.; Klenke, A.; Furnas, H.; Sorice-Virk, S. Public interest in the off-label use of glucagon-like peptide 1 agonists (Ozempic) for cosmetic weight loss: A Google Trends analysis. Aesthet Surg J. 2024, 44, 60–7. [Google Scholar] [CrossRef]
- Montero, A.; Sparks, G.; Presiado, M.; Hamel, L. KFF Health Tracking Poll 24: The public’s use and views of GLP-1 drugs. Published May 10, 2024. 2024. Available online: https://www.kff.org/health-costs/poll-finding/kff-health-tracking-poll-may-2024-the-publics-use-and-views-of-glp-1-drugs/.
- Sochovsky, N.; Miles, H. Global Equity Observer GLP-1: The Weight of Speculation. Invest Insight. Morgan Stanley Investment Management. 2023 Dec. Available online: https://www.morganstanley.com/im/publication/insights/articles/article_geoglp1_us.pdf.
- Colman, R.J.; Beasley, T.M.; Kemnitz, J.W.; Johnson, S.C.; Weindruch, R.; Anderson, R.M. Caloric restriction reduces age-related and all-cause mortality in rhesus monkeys. Nat Commun. 2014, 5, 3557. Available online: https://pubmed.ncbi.nlm.nih.gov/24691430/. [CrossRef]
- Mattison, J.A.; Roth, G.S.; Beasley, T.M.; Tilmont, E.M.; Handy, A.M.; Herbert, R.L.; Longo, D.L.; Allison, D.B.; Young, J.E.; Bryant, M.; Barnard, D.; Ward, W.F.; Qi, W.; Ingram, D.K.; de Cabo, R. Impact of caloric restriction on health and survival in rhesus monkeys from the NIA study. Nature 2012, 489, 318–21. [Google Scholar] [CrossRef]
- Das, J.K.; Banskota, N.; Candia, J.; Griswold, M.E.; Orenduff, M.; de Cabo, R.; Corcoran, D.L.; Das, S.K.; De, S.; Huffman, K.M.; Kraus, V.B.; Kraus, W.E.; Martin, C.K.; Racette, S.B.; Redman, L.M.; Schilling, B.; Belsky, D.W.; Ferrucci, L. Calorie restriction modulates the transcription of genes related to stress response and longevity in human muscle: The CALERIE study. Aging Cell. 2023, 22, e13963. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Gutin, I. In BMI We Trust: Reframing the Body Mass Index as a Measure of Health. Soc Theory Health. 2018, 16, 256–71. Available online: https://pubmed.ncbi.nlm.nih.gov/31007613/. [CrossRef]
- McEvedy, S.M.; Sullivan-Mort, G.; McLean, S.A.; Pascoe, M.C.; Paxton, S.J. Ineffectiveness of commercial weight-loss programs for achieving modest but meaningful weight loss: Systematic review and meta-analysis. J Health Psychol. 2017, 22, 1614–27. Available online: https://pubmed.ncbi.nlm.nih.gov/28810454/. [CrossRef] [PubMed]
- Hayashi, D.; Edwards, C.; Emond, J.A.; Gilbert-Diamond, D.; Butt, M.; Rigby, A.; et al. What is food noise? A conceptual model of food cue reactivity. Nutrients 2023, 15, 4809. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Blaine, B.E.; Rodman, J.; Newman, J.M. Weight loss treatment and psychological well-being: a review and meta-analysis. J Health Psychol. 2007, 12, 66–82. Available online: https://pubmed.ncbi.nlm.nih.gov/22888821/. [CrossRef]
- Jackson, S.E.; Steptoe, A.; Beeken, R.J.; Kivimaki, M.; Wardle, J. Psychological changes following weight loss in overweight and obese adults: a prospective cohort study. PLoS One 2014, 9, e104552. Available online: https://pubmed.ncbi.nlm.nih.gov/30326501/. [CrossRef]
- Swencionis, C.; Wylie-Rosett, J.; Lent, M.R.; Ginsberg, M.; Cimino, C.; Wassertheil-Smoller, S.; et al. Weight change, psychological well-being, and vitality in adults participating in a cognitive-behavioral weight loss program. Health Psychol. 2013, 32, 439–46. Available online: https://pubmed.ncbi.nlm.nih.gov/25098417/. [CrossRef] [PubMed]
- Chlabicz, M.; Dubatówka, M.; Jamiołkowski, J.; Sowa, P.; Łapińska, M.; Raczkowski, A.; et al. Subjective well-being in non-obese individuals depends strongly on body composition. Sci Rep. 2021, 11, 21797. Available online: https://www.nature.com/articles/s41598-021-01205-6. [CrossRef] [PubMed]
- Tahrani, A.A.; Morton, J. Benefits of weight loss of 10% or more in patients with overweight or obesity: A review. Obesity (Silver Spring). 2022. [Google Scholar] [CrossRef]
- Ryan, D.H.; Lingvay, I.; Deanfield, J.; et al. Long-term weight loss effects of semaglutide in obesity without diabetes in the SELECT trial. Nat Med. 2024, 30, 2049–2057. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).



