1. Introduction
LL-37 is the sole human cathelicidin, encoded by the CAMP gene, comprising 37 amino acids beginning with two leucines (LL). It is expressed by a wide range of cells, including neutrophils, monocytes/macrophages, keratinocytes, synoviocytes, and epithelial cells, highlighting its fundamental role in innate immunity and host defense [
1,
2,
3,
4,
5,
6]. Beyond its antimicrobial activity, LL-37 functions as a pleiotropic immunomodulator, bridging innate and adaptive immunity and participating in inflammation and autoimmunity [
7,
8,
9,
10].
Physiologically, LL-37 exhibits broad-spectrum antimicrobial activity against Gram-positive and Gram-negative bacteria, viruses, and fungi. Its primary mechanism involves disruption of microbial membranes, leading to cell lysis [
11,
12]. Beyond this direct microbicidal effect, LL-37 modulates the host immune response through interactions with pattern recognition receptors such as TLR2, TLR4, and TLR9[11р 12]. These interactions trigger intracellular signaling pathways that stimulate the production of pro-inflammatory cytokines including IL-1β, IL-6, IL-8, and IL-23, and promote the recruitment of immune cells, such as neutrophils, monocytes, and lymphocytes, to sites of infection or tissue injury [
11,
12,
13,
14].
Recent studies have highlighted the dual nature of LL-37, revealing that under certain conditions it can exert pro-inflammatory effects. This occurs via the formation of complexes with self-DNA or RNA, which activate plasmacytoid dendritic cells, leading to IFN-α production. This interferon-driven response is a central pathogenic mechanism in immune-mediated inflammatory diseases such as psoriasis and systemic lupus erythematosus (SLE), where chronic immune activation and tissue damage are hallmarks [
15,
16,
17].
In psoriasis and psoriatic arthritis (PsA), LL-37 is overexpressed in lesional skin and synovial tissue. It plays a key role in amplifying the IL-23/Th17 axis, promoting IL-17 secretion, and driving the recruitment and activation of pathogenic T-cell subsets. Importantly, patients with psoriasis and PsA develop circulating anti-LL-37 antibodies, and their levels correlate with disease activity, suggesting a direct link between LL-37–mediated immune activation and autoimmunity [
17,
18,
19,
32]. LL-37 has therefore been proposed as a potential autoantigen capable of breaking immune tolerance and sustaining chronic inflammation.
In rheumatoid arthritis (RA), LL-37 is also detectable in synovial fluid and tissue, where it is associated with neutrophil activation and synovial inflammation. However, the levels of anti-LL-37 antibodies in RA are lower compared to PsA, suggesting disease-specific differences in LL-37–mediated immune modulation [
20,
21,
22,
33]. Similarly, in knee osteoarthritis (OA), LL-37 expression appears to be primarily related to mechanical stress and low-grade inflammation. While anti-LL-37 antibodies have been detected in OA, their levels are generally low, indicating a limited role for adaptive immune activation in this degenerative disorder [
17,
23].
The development of anti-LL-37 antibodies typically requires prolonged exposure to the peptide in the context of chronic inflammation. These antibodies serve as markers of immune dysregulation and impaired tolerance, reflecting a state of heightened autoimmune susceptibility. They are best characterized in conditions such as psoriasis, PsA, and SLE [
14,
16,
20,
21,
22]. The study of these antibodies is therefore of clinic interest, as they may not only reflect disease activity but also contribute to pathogenesis by neutralizing LL-37’s immunoregulatory functions.
Beyond its role in skin and joint diseases, LL-37 has been implicated in broader immunological processes. It acts as an endogenous danger signal, modulating immune responses under inflammatory conditions, including sepsis and systemic infections. LL-37’s ability to activate and recruit immune cells, induce chemokines, and influence cytokine networks positions it as a key regulator of inflammation and tissue homeostasis. Understanding its pleiotropic functions may inform the development of novel therapeutic strategies targeting antimicrobial peptides or their stable analogs in inflammatory and infectious diseases [
24].
LL-37 exerts diverse biological effects, including pro- and anti-inflammatory actions, chemokine induction, modulation of immune cell functions, and pro-apoptotic activity. It exhibits antimicrobial properties against bacteria, fungi, and parasites and may contribute to carcinogenesis through modulation of the tumor microenvironment [
25,
26,
27,
28]. Mechanistically, LL-37 activates natural killer (NK) cells and CD4+ type 1 T lymphocytes, promotes endothelial cell migration, facilitates microthrombus formation, and enhances wound healing and re-epithelialization in vivo [
29,
30]. The presence of anti-LL-37 antibodies could inhibit these protective immune responses, potentially stabilizing pathological inflammation in rheumatic diseases [
31].
Historical studies further support LL-37’s disease-specific roles. In PsA, early work (2018) demonstrated LL-37’s pro-inflammatory function in psoriatic skin lesions and proposed its role as an autoantigen for Th1-Th17/CD8+ T cells. Anti-LL-37 antibodies correlated with inflammatory mediators in PsA but not in OA, suggesting their involvement in autoreactive T-cell activation and sustained neutrophil-driven inflammation [
32]. In RA, immunohistochemical analysis of synovial biopsies (2012) revealed LL-37 expression near blood vessels and throughout inflamed tissue, highlighting its potential contribution to synovial inflammation and immune cell recruitment [
33].
Despite these insights, knowledge of LL-37 and anti-LL-37 antibodies in the local joint microenvironment remains limited. Most studies have focused on circulating levels, leaving a gap in understanding their synovial presence and clinical relevance. Investigating these antibodies in synovial fluid may provide important clues about disease-specific immune mechanisms, help distinguish inflammatory from degenerative arthropathies, and identify biomarkers with potential prognostic or therapeutic value.
Aim of the Study
The present study aims to investigate anti-LL-37 antibodies in the synovial fluid of patients with psoriatic arthritis, rheumatoid arthritis, and knee osteoarthritis. This work may inform the development of diagnostic and therapeutic strategies targeting LL-37–mediated pathways in inflammatory and degenerative joint diseases.
2. Materials and Methods
2.1. Study Design and Participants
This cross-sectional study enrolled 90 patients (35–65 years) and 30 age- and gender-matched healthy controls from the University Rheumatology Clinics “St. George” and “Kaspela,” Plovdiv, Bulgaria. Participants were classified according to ACP/EULAR criteria [
34] into PsA (n=30), RA (n=30), and knee osteoarthritis (GoA, n=30). Data were collected between March and November 2025.
Only patients with peripheral polyarticular asymmetric PsA were included, as this represented the majority of cases during recruitment. Patients with other PsA subtypes were excluded due to insufficient numbers for subgroup analysis. Inclusion criteria for PsA were: confirmed diagnosis with synovial effusion, no prior biological therapy (TNF-α or IL-12/23, IL-17, IL-23 inhibitors), absence of psychiatric comorbidities, and written informed consent. RA patients were included if they had confirmed synovial effusion, were biologic-naïve, and provided consent. GoA inclusion required ≥5 years of knee pain in patients >50 years, stiffness <30 min, crepitus, joint deformity/enlargement without local warmth, radiographic osteophytes, ESR <40 mm/h, negative rheumatoid factor, presence of effusion, and consent.
Exclusion criteria across all groups included refusal to participate, diagnosis of a rheumatic disease other than PsA, RA, or GoA, current or previous biologic therapy, decompensated cardiovascular, pulmonary, or renal disease, and hematologic disorders.
2.2. Ethical Approval
The study complied with the Declaration of Helsinki (1964) and its revised version (Edinburgh, 2000). All procedures—including patient data analysis, blood collection, synovial fluid aspiration, and informed consent—were approved by the Ethics Committee of the Medical University of Plovdiv (Protocol No. 5/5.04.2025). The study was conducted under Project No. NO-07/2025.
2.3. Sample Size Calculation
Sample size was determined using the EPITOOLS calculator (
https://epitools.ausvet.com.au/casecontrols) with α=0.05 and 95% CI. A total of 120 participants were required: 60 inflammatory joint disease patients (PsA+RA), 30 GoA patients, and 30 healthy controls, maintaining a 2:1 ratio between inflammatory and degenerative groups.
2.4. Synovial Fluid Collection
Arthrocentesis was performed under ultrasound guidance by an experienced rheumatologist, strictly adhering to antiseptic protocols. Only the knee joint was aspirated, yielding 10–15 mL of clear, pale yellow synovial fluid with good viscosity. Samples were examined for crystals using polarized light microscopy; patients with crystals were excluded.
2.5. Biomarker Measurement by ELISA
Synovial fluid samples were centrifuged at 3,000 rpm for 10 min and stored at −80°C until analysis. Concentrations of IL-1β (ng/mL), IL-6 (pg/mL), IL-23 (pg/mL), and anti-LL-37 antibodies were measured using ELISA (eBioscience, San Diego, CA, USA) according to manufacturer instructions. Measurements were performed in duplicate, and mean values were used for analysis. Intra- and inter-assay coefficients of variation were <10%. Standard curves and quality control samples were included on each plate. Values below the detection limit were assigned half the minimum detectable concentration.
2.6. Statistical Analysis
Data were analyzed using SPSS v26 (IBM Corp., Armonk, NY, USA) and Excel 2019. Normality was assessed prior to analysis. Comparative analyses included chi-square tests, one-way ANOVA, and post hoc LSD tests. Pearson correlation coefficients were used for normally distributed variables. ROC curve analysis evaluated the discriminative capacity of anti-LL-37 antibodies between groups, with AUC values of 0.7–0.8 indicating acceptable diagnostic accuracy. All tests were two-tailed; p<0.05 was considered statistically significant. Graphical representations were generated in SPSS.
3. Results
The mean age of participants was 47.78 ± 5.65 years in the PsA group, 46.41 ± 6.31 years in the RA group, 48.81 ± 5.11 years in the GoA group, and 46.54 ± 6.54 years in the healthy control group. One-way ANOVA analysis revealed no statistically significant difference in age between the groups (p = 0.741).
Key demographic and clinical characteristics of the study participants are summarized in
Table 1.
Analysis of cytokine concentrations in synovial fluid revealed significant differences between the study groups. Mean ± standard deviation (SD) levels of IL-1β and IL-6 were highest in RA patients, followed by PsA, GoA, and healthy controls (p < 0.01).
Mean ± SD levels of IL-23 and anti-LL-37 antibodies were highest in PsA patients, followed by RA, GoA, and healthy controls (p < 0.01) (
Table 2).
3.1. Anti-LL-37 Antibody Levels and Correlation Analysis
A detailed comparative analysis of anti-LL-37 levels (pg/mL) across the study groups, using the least significant difference (LSD) test, revealed statistically significant differences between specific pairwise comparisons. The mean difference in anti-LL-37 levels between PsA and RA patients was 0.01765 (p = 0.001), between PsA and GoA patients 0.01329 (p = 0.001), and between PsA and healthy controls 0.01198 (p = 0.001). No significant difference was observed between GoA patients and healthy controls (p = 0.804).
A strong positive correlation was identified between IL-1β, IL-6, IL-23 levels and anti-LL-37 concentrations in both PsA and RA patients (
Table 3).
3.2. Diagnostic Performance of Anti-LL-37 Antibodies
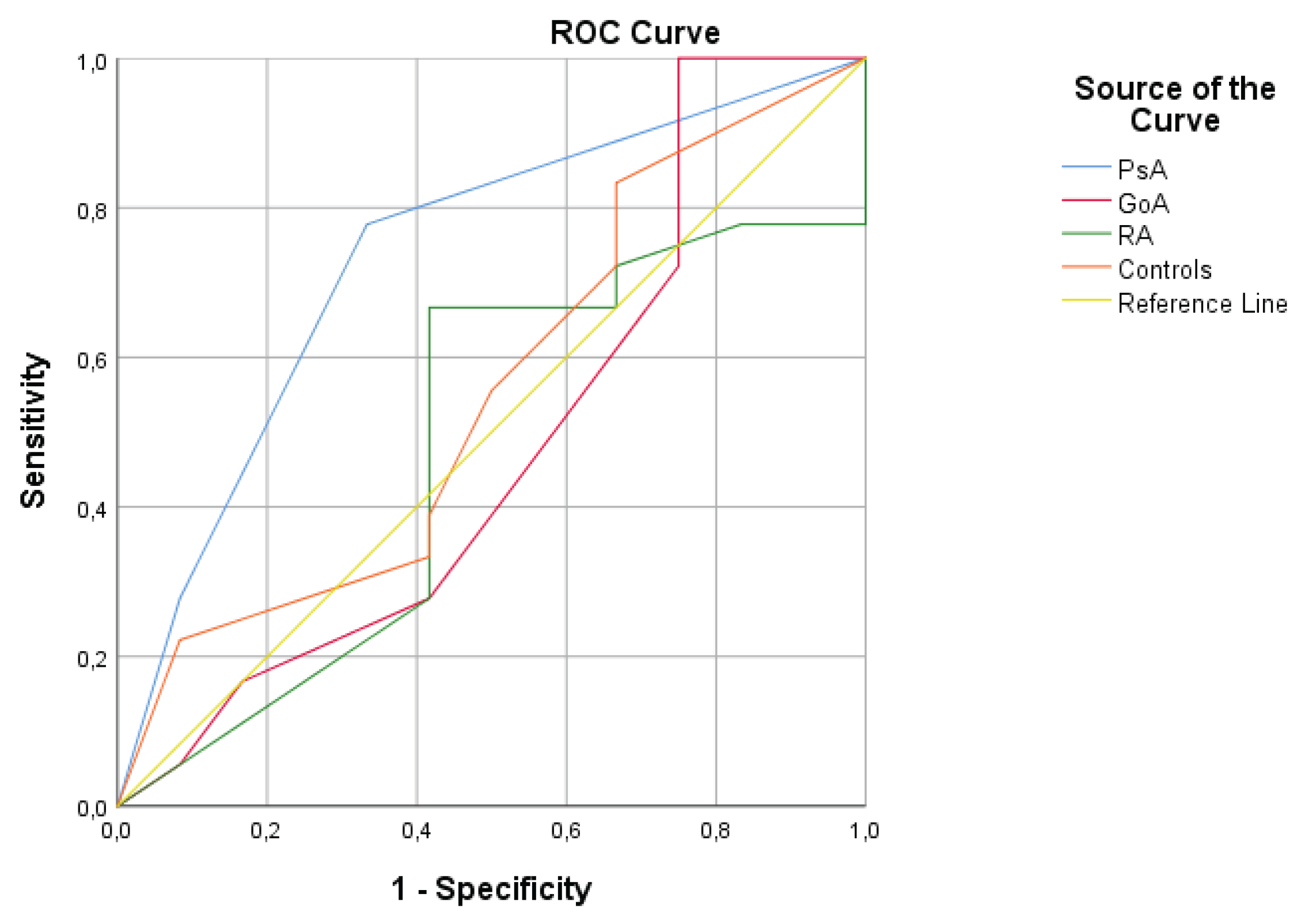
The diagnostic performance of anti-LL-37 levels in distinguishing between study groups was evaluated using receiver operating characteristic (ROC) curve analysis:
In PsA patients, the area under the curve (AUC) was 0.736, indicating reasonable diagnostic accuracy in differentiating PsA from healthy controls. The AUC was statistically significant (p < 0.001) with a 95% confidence interval (CI) of 0.549–0.924.
In GoA patients, the AUC was 0.484, suggesting limited diagnostic utility of the biomarker in distinguishing GoA from controls. The AUC was statistically significant (p < 0.001) with a 95% CI of 0.257–0.711.
In RA patients, the AUC was 0.479, also indicating limited diagnostic value for differentiating RA from healthy controls. The AUC was statistically significant (p < 0.001) with a 95% CI of 0.337–0.769 (
Table 4).
Figure 1.
ROC curves for examined anti-LL37 to differentiate between patients witt PsA and control, GoA and control, and RA and control.
Figure 1.
ROC curves for examined anti-LL37 to differentiate between patients witt PsA and control, GoA and control, and RA and control.
4. Discussion
The present study provides novel evidence supporting a disease-specific role of anti-LL-37 antibodies in the synovial microenvironment of inflammatory arthritides, with a particular emphasis on psoriatic arthritis (PsA). By directly analyzing synovial fluid rather than systemic circulation, our results offer mechanistic insights into local immune dysregulation at the site of joint inflammation [
28,
29,
30,
31].
The most prominent finding is the significantly elevated concentration of anti-LL-37 antibodies in the synovial fluid of PsA patients compared with rheumatoid arthritis (RA), knee osteoarthritis (GoA), and healthy controls. This observation aligns with accumulating evidence identifying LL-37 as a bona fide autoantigen in psoriasis and PsA, capable of breaking immune tolerance and perpetuating chronic inflammation through Th1/Th17-driven pathways. The markedly higher synovial levels of anti-LL-37 antibodies in PsA suggest an active, localized autoimmune response rather than passive diffusion from the circulation [
13,
17].
Mechanistically, LL-37 has been shown to form complexes with self-DNA and RNA, facilitating their uptake by plasmacytoid and myeloid dendritic cells and triggering Toll-like receptor (TLR) signaling. This process leads to sustained production of IL-23, a pivotal cytokine in PsA pathogenesis. The exceptionally strong correlation between anti-LL-37 antibodies and IL-23 levels observed in our PsA cohort strongly supports the involvement of the LL-37–IL-23/Th17 axis in maintaining synovial inflammation. These findings suggest that anti-LL-37 antibodies may not merely represent epiphenomena but may actively participate in immune amplification loops within the joint [
4,
13].
All authors agree that LL-37 has attracted considerable research attention over the past decade due to its broad-spectrum immunomodulatory and antimicrobial activity. Despite extensive study, many questions regarding its efficacy and safety in vivo remain unanswered. LL-37 may offer new avenues for the prevention and treatment of certain carcinomas and persistent infections. Conversely, targeting or blocking LL-37 could potentially have therapeutic benefits in PsA that is resistant to conventional anti-cytokine therapies. In recent years, there has been a persistent search for novel biomolecules that could help differentiate PsA from rheumatoid arthritis (RA) and degenerative joint diseases, as well as for innovative therapeutic options for rheumatologic conditions.
Our findings are consistent with those reported by Frasca et al. According to Frasca, antibodies against LL-37 correlate with clinical inflammatory markers in PsA. Specifically, antibodies to carbamylated or citrullinated LL-37 are present in synovial fluid and plasma from PsA patients, and to a lesser extent in plasma from patients with psoriasis, but are absent in healthy controls [
32]. Moreover, plasma antibodies to carbamylated LL-37 correlate with PsA disease activity, as measured by DAS44, but do not correlate with psoriasis disease activity (PASI). Elevated levels of anti-LL-37 antibodies were observed in PsA patients but not in synovial tissues or synovial fluid of patients with osteoarthritis. These findings suggest that LL-37 may serve as a novel autoantigenic target in PsA, and that anti-LL-37 antibodies may play a role in the pathogenesis of the disease [
32].
In 2019
Tianfu Wu et al. developed a system of typical autoantigens in rheumatic diseases. According to the authors, IgG autoantibodies against two new antigens, one of which is LL37, are significantly increased in patients with psoriasis and psoriatic arthritis, respectively, compared to healthy controls, which is why the authors assume that LL37 may be involved in the pathogenesis of psoriatic arthritis [
36].
Tianfu Wu and co-authors conclude that these autoantibodies may be useful biomarkers and indicative of therapeutic targets for psoriasis and psoriatic arthritis [
36].
2021 O'Neil et al. found that elevated levels of cathelicidin LL37 have been documented in the synovium of patients with RA, but the cellular source remains unclear [
20]. They studied anti-carLL37 antibodies in synovial fluid from people with RA and found that elevated levels of carLL37 were found in plasma and synovial fluid from patients with RA compared to healthy controls and that they correlated with radiological findings of bone erosion in the hands and feet of patients with RA. According to the authors, CarLL37-IgG immune complexes enhance the ability of monocytes to differentiate into osteoclasts and potentiate osteoclast-mediated extracellular matrix resorption [
20]. A shortcoming of this study is that the authors did not compare the results of RA patients with those of other rheumatological diseases such as PsA or gonarthrosis, as we did in our study.
In contrast, patients with RA exhibited significantly lower synovial levels of anti-LL-37 antibodies despite markedly elevated concentrations of IL-1β and IL-6. This dissociation indicates that, although LL-37 is present in RA synovium and contributes to neutrophil activation and innate immune responses, it is less likely to function as a dominant autoantigen in RA. Instead, RA appears to be characterized by broader, less antigen-specific inflammatory cascades driven predominantly by classical pro-inflammatory cytokines. Our findings are consistent with previous reports describing anti-LL-37 antibodies in RA at substantially lower levels than in PsA.
In patients with GoA, synovial anti-LL-37 antibody levels were low and comparable to those of healthy controls, reinforcing the concept that LL-37 plays a limited role in degenerative joint disease. In this context, LL-37 is more likely associated with mechanical stress, low-grade innate immune activation, and tissue remodeling rather than adaptive autoimmunity. The absence of a significant autoimmune response against LL-37 further differentiates GoA from inflammatory arthritides at the molecular level.
The ROC curve analysis demonstrated moderate diagnostic accuracy of synovial anti-LL-37 antibodies for distinguishing PsA from healthy controls, while showing poor discriminatory value in RA and GoA. These findings highlight the potential utility of anti-LL-37 antibodies as an adjunctive biomarker in PsA, particularly in challenging differential diagnostic scenarios. However, their limited performance in RA and GoA underscores the disease-specific nature of LL-37–driven autoimmunity.
Several limitations should be acknowledged. The cross-sectional design precludes assessment of temporal relationships and treatment-related dynamics of anti-LL-37 antibodies. Additionally, the relatively small sample size and restriction to peripheral polyarticular PsA may limit generalizability. Nevertheless, the study’s major strength lies in its focus on the synovial compartment, providing direct insight into local immune mechanisms rather than relying solely on systemic biomarkers.
In conclusion, our findings support a central pathogenic role for LL-37 and anti-LL-37 antibodies in PsA, closely linked to IL-23–mediated immune activation within the joint. These data reinforce the concept of PsA as a disease driven by antigen-specific autoimmune responses at the interface of innate and adaptive immunity. Future longitudinal and multicenter studies are warranted to explore the prognostic value of anti-LL-37 antibodies and to evaluate their potential as therapeutic targets in psoriatic arthritis.
References
- Ridyard, K.E.; Overhage, J. The Potential of Human Peptide LL-37 as an Antimicrobial and Anti-Biofilm Agent. Antibiotics 2021, 10, 650. [Google Scholar] [CrossRef]
- Vera-Cruz, A.; Tanphaichitr, N.; Angel, J.B. Antimicrobial Peptide, LL-37, And Its Potential As An Anti-HIV Agent. Clin. Investig. Med. 2021, 44, E64–E71. [Google Scholar] [CrossRef] [PubMed]
- E Bowdish, D.M.; Davidson, D.J.; Lau, Y.E.; Lee, K.; Scott, M.G.; Hancock, R.E.W. Impact of LL-37 on anti-infective immunity. J. Leukoc. Biol. 2004, 77, 451–459. [Google Scholar] [CrossRef] [PubMed]
- Hemshekhar, M.; Choi, K.-Y.G.; Mookherjee, N. Host Defense Peptide LL-37-Mediated Chemoattractant Properties, but Not Anti-Inflammatory Cytokine IL-1RA Production, Is Selectively Controlled by Cdc42 Rho GTPase via G Protein-Coupled Receptors and JNK Mitogen-Activated Protein Kinase. Front. Immunol. 2018, 9, 1871. [Google Scholar] [CrossRef] [PubMed]
- Son, G.-H.; Lee, J.-J.; Kim, Y.; Lee, K.-Y. The Role of Antimicrobial Peptides in Preterm Birth. Int. J. Mol. Sci. 2021, 22, 8905. [Google Scholar] [CrossRef]
- Marissen, J.; Reichert, L.; Härtel, C.; Fortmann, M.I.; Faust, K.; Msanga, D.; Harder, J.; Zemlin, M.; de Agüero, M.G.; Masjosthusmann, K.; et al. Antimicrobial Peptides (AMPs) and the Microbiome in Preterm Infants: Consequences and Opportunities for Future Therapeutics. Int. J. Mol. Sci. 2024, 25, 6684. [Google Scholar] [CrossRef]
- Chieosilapatham, P.; Ikeda, S.; Ogawa, H.; Niyonsaba, F. Tissue-specific Regulation of Innate Immune Responses by Human Cathelicidin LL-37. Curr. Pharm. Des. 2018, 24, 1079–1091. [Google Scholar] [CrossRef]
- Leite, M.L.; Duque, H.M.; Rodrigues, G.R.; da Cunha, N.B.; Franco, O.L. The LL-37 domain: A clue to cathelicidin immunomodulatory response? Peptides 2023, 165, 171011. [Google Scholar] [CrossRef]
- Méndez-Samperio, P. The human cathelicidin hCAP18/LL-37: A multifunctional peptide involved in mycobacterial infections. Peptides 2010, 31, 1791–1798. [Google Scholar] [CrossRef]
- Kim, S.H.; Min, Y.-H.; Park, M.C. Antimicrobial Peptides: Current Status, Mechanisms of Action, and Strategies to Overcome Therapeutic Limitations. Microorganisms 2025, 13, 2574. [Google Scholar] [CrossRef]
- Tokajuk, J.; Deptuła, P.; Piktel, E.; Daniluk, T.; Chmielewska, S.; Wollny, T.; Wolak, P.; Fiedoruk, K.; Bucki, R. Cathelicidin LL-37 in Health and Diseases of the Oral Cavity. Biomedicines 2022, 10, 1086. [Google Scholar] [CrossRef] [PubMed]
- Shih, C.-C.; Liao, W.-C.; Ke, H.-Y.; Kuo, C.-W.; Tsao, C.-M.; Tsai, W.-C.; Chiu, Y.-L.; Huang, H.-C.; Wu, C.-C. Antimicrobial peptide cathelicidin LL-37 preserves intestinal barrier and organ function in rats with heat stroke. Biomed. Pharmacother. 2023, 161, 114565. [Google Scholar] [CrossRef] [PubMed]
- Lappin, M.; Dellett, M.; Mills, K.; Lundy, F.; Irwin, C. The neutralising and stimulatory effects of antimicrobial peptide LL-37 in human gingival fibroblasts. Arch. Oral Biol. 2023, 148, 105634. [Google Scholar] [CrossRef]
- Derradjia, A.; Alanazi, H.; Park, H.J.; Djeribi, R.; Semlali, A.; Rouabhia, M. α-tocopherol decreases interleukin-1β and -6 and increases human β-defensin-1 and -2 secretion in human gingival fibroblasts stimulated with Porphyromonas gingivalis lipopolysaccharide. J. Periodontal Res. 2015, 51, 295–303. [Google Scholar] [CrossRef] [PubMed]
- Inomata, M.; Horie, T.; Into, T. Effect of the Antimicrobial Peptide LL-37 on Gene Expression of Chemokines and 29 Toll-like Receptor-Associated Proteins in Human Gingival Fibroblasts Under Stimulation with Porphyromonas gingivalis Lipopolysaccharide. Probiotics Antimicrob. Proteins 2019, 12, 64–72. [Google Scholar] [CrossRef]
- White, J.K.; Muhammad, T.; Alsheim, E.; Mohanty, S.; Blasi-Romero, A.; Gunasekera, S.; Strömstedt, A.A.; Ferraz, N.; Göransson, U.; Brauner, A. A stable cyclized antimicrobial peptide derived from LL-37 with host immunomodulatory effects and activity against uropathogens. Cell. Mol. Life Sci. 2022, 79, 1–17. [Google Scholar] [CrossRef]
- Bierkarre, H.; Harder, J.; Cuthbert, R.; Emery, P.; Leuschner, I.; Mrowietz, U.; Hedderich, J.; McGonagle, D.; Gläser, R. Differential expression of antimicrobial peptides in psoriasis and psoriatic arthritis as a novel contributory mechanism for skin and joint disease heterogeneity. Scand. J. Rheumatol. 2015, 45, 188–196. [Google Scholar] [CrossRef]
- Harder, J.; Dressel, S.; Wittersheim, M.; Cordes, J.; Meyer-Hoffert, U.; Mrowietz, U.; Fölster-Holst, R.; Proksch, E.; Schröder, J.-M.; Schwarz, T.; et al. Enhanced Expression and Secretion of Antimicrobial Peptides in Atopic Dermatitis and after Superficial Skin Injury. J. Investig. Dermatol. 2010, 130, 1355–1364. [Google Scholar] [CrossRef]
- Yuan, Y.; Qiu, J.; Lin, Z.; Li, W.; Haley, C.; Mui, U.N.; Ning, J.; Tyring, S.K.; Wu, T. Identification of Novel Autoantibodies Associated With Psoriatic Arthritis. Arthritis Rheumatol. 2019, 71, 941–951. [Google Scholar] [CrossRef]
- O’neil, L.J.; Oliveira, C.B.; Sandoval-Heglund, D.; Barrera-Vargas, A.; Merayo-Chalico, J.; Aguirre-Aguilar, E.; Kaplan, M.J.; Carmona-Rivera, C. Anti-Carbamylated LL37 Antibodies Promote Pathogenic Bone Resorption in Rheumatoid Arthritis. Front. Immunol. 2021, 12. [Google Scholar] [CrossRef]
- Brevet, P.; Lattard, C.; Guillou, C.; Rottenberg, P.; Fardellone, P.; Le-Loët, X.; Lequerré, T.; Cosette, P.; Boyer, O.; Fréret, M.; et al. Anti-Carbamylated Fibrinogen Antibodies Might Be Associated With a Specific Rheumatoid Phenotype and Include a Subset Recognizing In Vivo Epitopes of Its γ Chain One of Which Is Not Cross Reactive With Anti-Citrullinated Protein Antibodies. Front. Immunol. 2021, 12. [Google Scholar] [CrossRef] [PubMed]
- Wu, C.-Y.; Yang, H.-Y.; Luo, S.-F.; Lai, J.-H. From Rheumatoid Factor to Anti-Citrullinated Protein Antibodies and Anti-Carbamylated Protein Antibodies for Diagnosis and Prognosis Prediction in Patients with Rheumatoid Arthritis. Int. J. Mol. Sci. 2021, 22, 686. [Google Scholar] [CrossRef] [PubMed]
- Formigo-Couceiro, J.; Castillo-Martín, J.; Cordero-García, C.; Rueda, A.F.-B.; Samitier-Pastor, B.; Soriano-Guillén, A. Recomendaciones para el tratamiento intervencionista de la gonartrosis: consenso de expertos y algoritmos. 2025, 59, 100880. [Google Scholar] [CrossRef] [PubMed]
- Mańkowska, A.; Paprocka, P.; Król, G.; Lesiak, A.; Spałek, J.; Piktel, E.; Okła, S.; Bijak, P.; Niklińska, W.; Durnaś, B.; et al. Significance of the LL-37 Peptide Delivered from Human Cathelicidin in the Pathogenesis, Treatment, and Diagnosis of Sepsis. Arch. Immunol. et Ther. Exp. 2025, 73. [Google Scholar] [CrossRef]
- Alagarasu, K.; Patil, P.; Shil, P.; Seervi, M.; Kakade, M.; Tillu, H.; Salunke, A. In-vitro effect of human cathelicidin antimicrobial peptide LL-37 on dengue virus type 2. Peptides 2017, 92, 23–30. [Google Scholar] [CrossRef]
- López-González, M.; Meza-Sánchez, D.; García-Cordero, J.; Bustos-Arriaga, J.; Valle, C.V.-D.; Marsch-Moreno, M.; Castro-Jiménez, T.; Flores-Romo, L.; Santos-Argumedo, L.; Gutiérrez-Castañeda, B.; et al. Human keratinocyte cultures (HaCaT) can be infected by DENV, triggering innate immune responses that include IFNλ and LL37. Immunobiology 2018, 223, 608–617. [Google Scholar] [CrossRef]
- Castillo, J.A.; Giraldo, D.M.; Smit, J.M.; A Rodenhuis-Zybert, I.; Urcuqui-Inchima, S. Vitamin D-induced LL-37 modulates innate immune responses of human primary macrophages during DENV-2 infection. Pathog. Dis. 2022, 80. [Google Scholar] [CrossRef]
- Ren, S.X.; Cheng, A.S.; To, K.F.; Tong, J.H.; Li, M.S.; Shen, J.; Wong, C.C.; Zhang, L.; Chan, R.L.; Wang, X.J.; et al. Host Immune Defense Peptide LL-37 Activates Caspase-Independent Apoptosis and Suppresses Colon Cancer. Cancer Res. 2012, 72, 6512–6523. [Google Scholar] [CrossRef]
- Currie, S.M.; Findlay, E.G.; McFarlane, A.J.; Fitch, P.M.; Böttcher, B.; Colegrave, N.; Paras, A.; Jozwik, A.; Chiu, C.; Schwarze, J.; et al. Cathelicidins Have Direct Antiviral Activity against Respiratory Syncytial Virus In Vitro and Protective Function In Vivo in Mice and Humans. J. Immunol. 2016, 196, 2699–2710. [Google Scholar] [CrossRef]
- Li, K.; Tao, N.; Zheng, L.; Sun, T. LL-37 restored glucocorticoid sensitivity impaired by virus dsRNA in lung. Int. Immunopharmacol. 2020, 79, 106057. [Google Scholar] [CrossRef]
- Sigurgrímsdóttir, H.; Eysteinsdóttir, J.H.; Kristjánsson, Á.K.; Agnarsson, B.A.; Freysdottir, J.; Lúðvíksson, B.R. Skin-Homing Potential of Peripheral Immune Cells From Psoriasis Patients and the Effects of LL -37 on Their Secretion of Chemokines During Psoriasis-Mimicking Stimulation. Scand. J. Immunol. 2025, 102, e70066. [Google Scholar] [CrossRef]
- Frasca, L.; Palazzo, R.; Chimenti, M.S.; Alivernini, S.; Tolusso, B.; Bui, L.; Botti, E.; Giunta, A.; Bianchi, L.; Petricca, L.; et al. Anti-LL37 Antibodies Are Present in Psoriatic Arthritis (PsA) Patients: New Biomarkers in PsA. Front. Immunol. 2018, 9. [Google Scholar] [CrossRef]
- Neregård, P.; Engström, M.; Agerberth, B.; Catrina, A.I. LL-37 is expressed in the inflamed synovium in patients with rheumatoid arthritis and downregulated by TNF inhibitors. Ann. Rheum. Dis. 2012, 71, A12–A12. [Google Scholar] [CrossRef]
- Aringer, M.; Baerwald, C.; Bergner, R.; Feuchtenberger, M.; Gebhardt, C.; Hagen, M.; Keyßer, G.; Lorenz, H.-M.; Witte, T. Rheumatologie im Medizinstudium. Z. fur Rheumatol. 2020, 80, 2–8. [Google Scholar] [CrossRef]
- Memariani, H.; Memariani, M. Antibiofilm properties of cathelicidin LL-37: an in-depth review. World J. Microbiol. Biotechnol. 2023, 39, 1–28. [Google Scholar] [CrossRef]
- Wu, T.; Yuan, Y.; Tyring, S.K. Discovery of Autoantibodies Associated with Psoriatic Arthritis. J. Immunol. 2019, 202, 179.2–179.2. [Google Scholar] [CrossRef]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |