Submitted:
25 December 2025
Posted:
26 December 2025
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Materials and Methods
2.1. Study Design, Setting and Subjects
2.2. NACT Regimen
2.3. Criteria for NACT Response
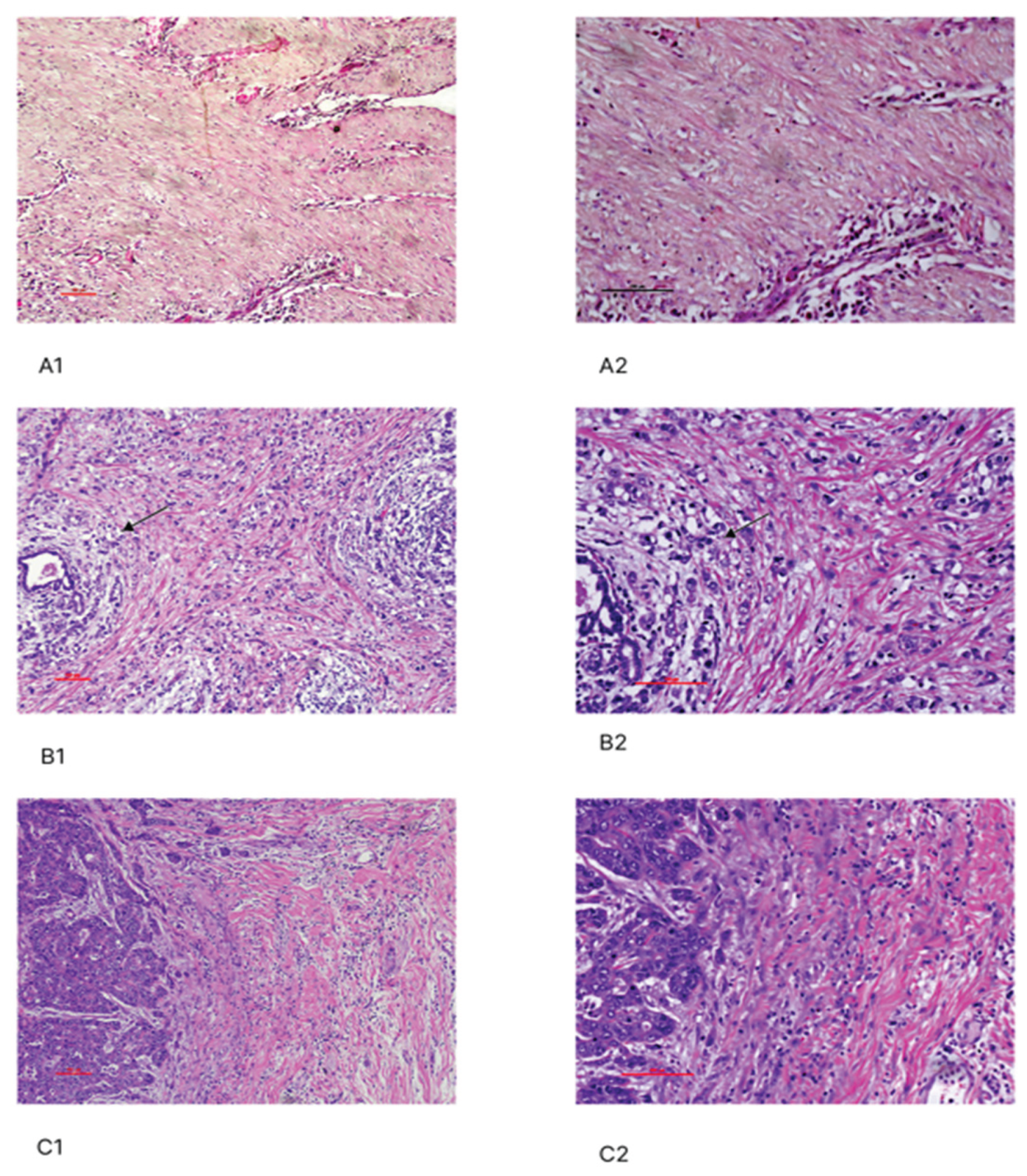
2.4. Histopathological Evaluation of Tumor-Infiltrating Lymphocytes (TIL)
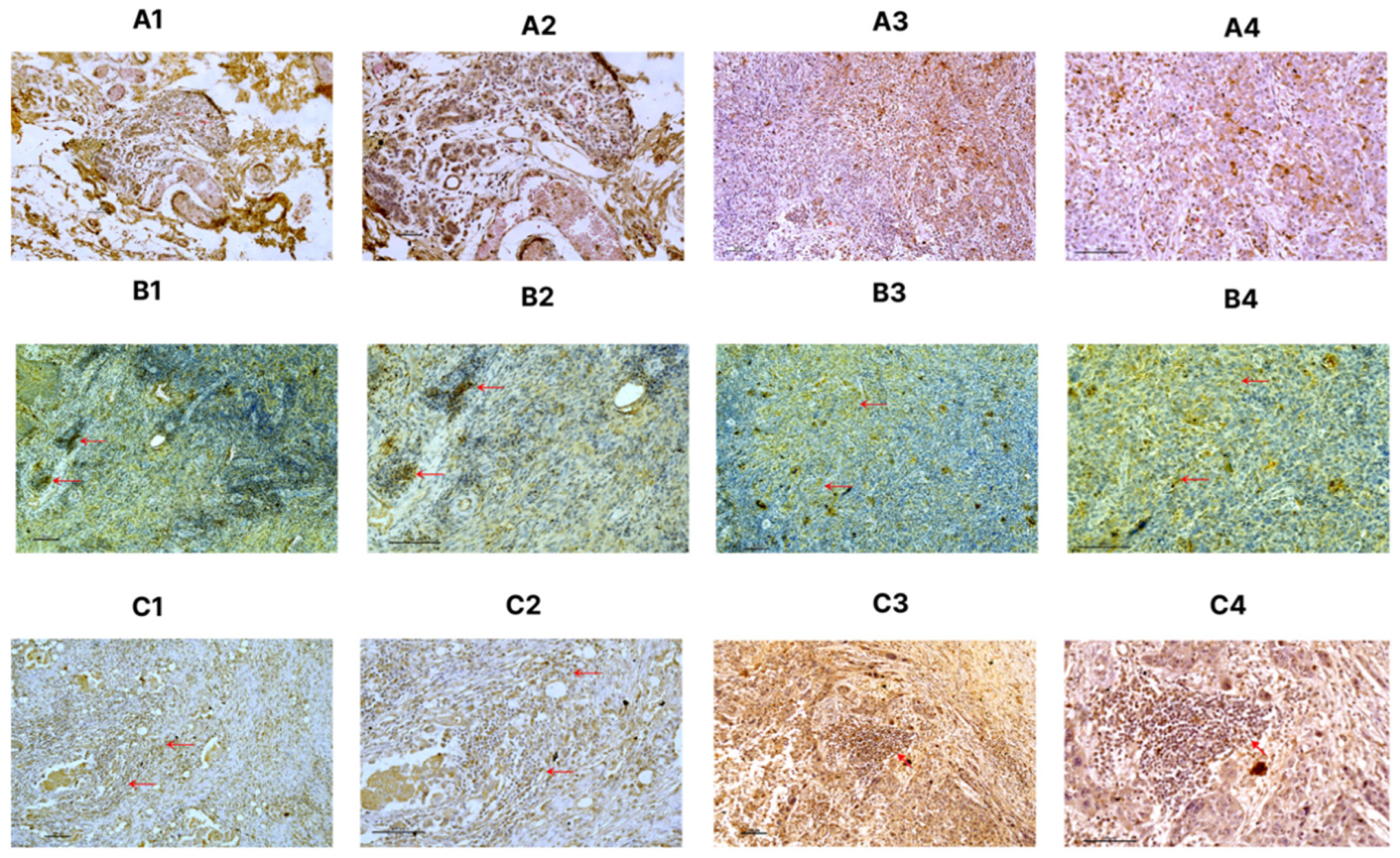
2.5. Immunohistochemical Evaluation of PD-1, PD-L1 and LAG3
2.6. Statistical Analysis
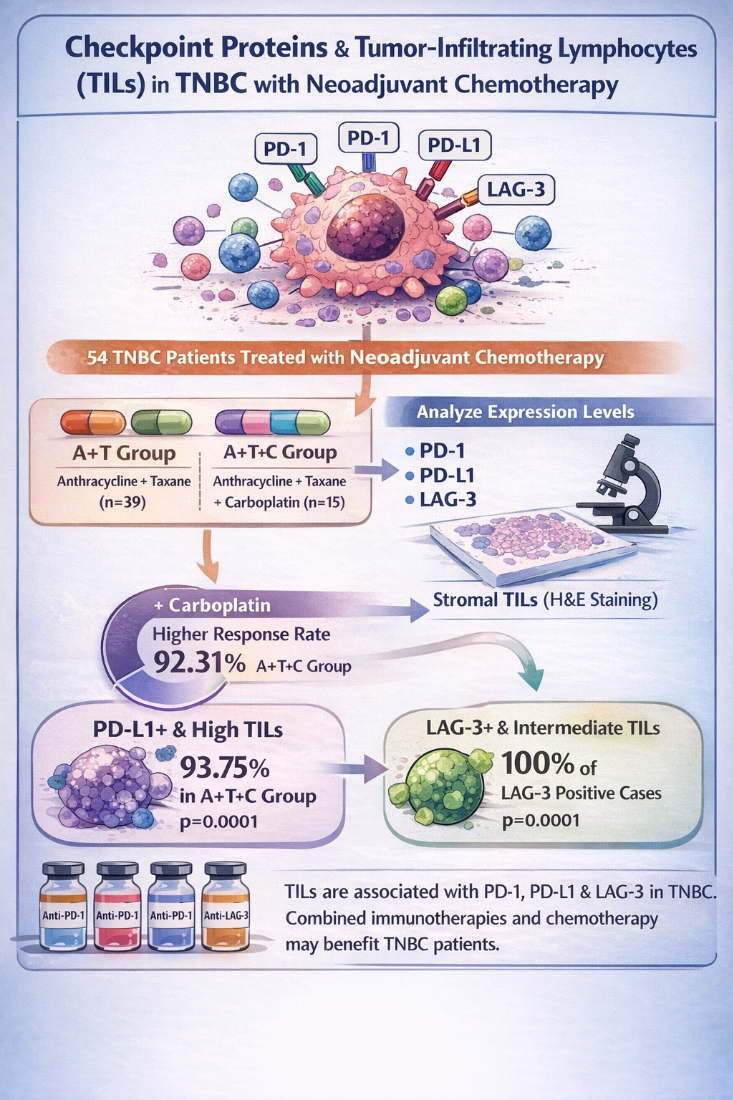
3. Results
3.1. Clinical and Pathological Findings
3.2. Clinical Response
3.3. Assessment of Stromal TIL in TNBC Patients and Their Association with Clinicopathological Parameters
3.4. Immunohistochemical Staining of PD1 and PD-L1 and Their Association with Clinicopathological Parameters
3.5. Assessment of Stromal TIL on PD-L1 and PD-1 Positive Cells
3.6. Immunohistochemical Staining of LAG-3 and Its Association with Clinicopathological Parameters
3.7. Assessment of Stromal TILs LAG-3 Positive Cells
4. Discussion
Conclusion
Supplementary Materials
Funding
Author contributions
Data availability
Acknowledgment
Declarations
Ethics approval
Informed consents
Competing interests
References
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; et al. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef]
- Foulkes, W.D.; Smith, I.E.; Reis-Filho, J.S. Triple-negative breast cancer. N Engl J Med. 2010, 363, 1938–48. [Google Scholar] [CrossRef]
- Mao, Y.; Qu, Q.; Chen, X.; Huang, O.; Wu, J.; Shen, K. The prognostic value of tumor-infiltrating lymphocytes in breast cancer: a systematic review and meta-analysis. PloS one. 2016, 11, e0152500. [Google Scholar] [CrossRef]
- Teijido, P.G.; Cabal, M.L.; Fernández, I.P.; Pérez, Y.F. Tumor-infiltrating lymphocytes in triple negative breast cancer: the future of immune targeting. Clin Med Insights Oncol. 2016, 10, CMO–S34540. [Google Scholar]
- Gao, G.; Wang, Z.; Qu, X.; Zhang, Z. Prognostic value of tumor-infiltrating lymphocytes in patients with triple-negative breast cancer: a systematic review and meta-analysis. BMC cancer 2020, 20, 179. [Google Scholar] [CrossRef]
- Gao, Z.H.; Li, C.X.; Liu, M.; Jiang, J.Y. Predictive and prognostic role of tumour-infiltrating lymphocytes in breast cancer patients with different molecular subtypes: a meta-analysis. BMC Cancer 2020, 20, 1150. [Google Scholar] [CrossRef]
- Eno, J. Immunotherapy through the years. J Adv Pract Oncol. 2017, 8, 747. [Google Scholar]
- 8; Powles, T.; Eder, J.P.; Fine, G.D.; Braiteh, F.S.; Loriot, Y.; Cruz, C.; et al. MPDL3280A (anti-PD-L1) treatment leads to clinical activity in metastatic bladder cancer. Nature 2014, 515, 558–562. [Google Scholar]
- Rizvi, NA.; Mazières, J.; Planchard, D.; Stinchcombe, T.E.; Dy, G.K.; Antonia, S.J.; et al. Activity and safety of nivolumab, an anti-PD-1 immune checkpoint inhibitor, for patients with advanced, refractory squamous non-small-cell lung cancer (CheckMate 063): a phase 2, single arm trial. Lancet Oncol. 2015, 16, 257–265. [Google Scholar] [CrossRef] [PubMed]
- Yang, T.; Li, W.; Huang, T.; Zhou, J. Immunotherapy Targeting PD-1/PD-L1 in Early-Stage Triple-Negative Breast Cancer. J Pers Med. 2023, 13, 526. [Google Scholar] [CrossRef] [PubMed]
- Gonzalez-Ericsson, P.I.; Stovgaard, E.S.; Sua, L.F.; Reisenbichler, E.; Kos, Z.; Carter, J.M.; et al. International Immuno-Oncology Biomarker Working Group. The path to a better biomarker: application of a risk management framework for the implementation of PD-L1 and TILs as immuno-oncology biomarkers in breast cancer clinical trials and daily practice. J Pathol. 2020, 250, 667–684. [Google Scholar] [CrossRef]
- Zhao, X.; Subramanian, S. Intrinsic Resistance of Solid Tumors to Immune Checkpoint Blockade Therapy Resistance to Immune Checkpoint Blockade Therapy. Cancer Res. 2017, 77, 817–822. [Google Scholar] [CrossRef]
- Kisielow, M.; Kisielow, J.; Capoferri-Sollami, G.; Karjalainen, K. Expression of lymphocyte activation gene 3 (LAG-3) on B cells is induced by T cells. Eur J Immunol. 2005, 35, 2081–2088. [Google Scholar] [CrossRef] [PubMed]
- Andrews, L.P.; Marciscano, A.E.; Drake, C.G.; Vignali, D.A.A. LAG 3 (CD 223) as a cancer immunotherapy target. Immunol Rev. 2017, 276, 80–96. [Google Scholar] [CrossRef]
- Takaya, S.; Saito, H.; Ikeguchi, M. Upregulation of immune checkpoint molecules, PD-1 and LAG-3, on CD4+ and CD8+ T cells after gastric cancer surgery. Yonago Acta Med. 2015, 58, 39. [Google Scholar]
- 16; Thomassin-Naggara, I.; Tardivon, A.; Chopier, J. Standardized diagnosis and reporting of breast cancer. Diagn Interv Imaging. 2014, 95, 759–766. [Google Scholar]
- Kazuhiro, K.; Yasuo, M.; Toshiko, Y.; Soichi, O.; Tomoko, H.; Koichiro, Y. Assessment of tumor response to neoadjuvant chemotherapy in patients with breast cancer using MRI and FDG-PET/CT-RECIST 1.1 vs. PERCIST 1.0. Nagoya J Med Sci. 2018, 80, 183–197. [Google Scholar]
- Salgado, R.; Denkert, C.; Demaria, S.; Sirtaine, N.; Klauschen, F.; Pruneri, G.; et al. The evaluation of tumor-infiltrating lymphocytes (TILs) in breast cancer: recommendations by an International TILs Working Group 2014. 2014. Ann Oncol. 2015, 26, 259–71. [Google Scholar] [CrossRef] [PubMed]
- Dieci, M.V.; Radosevic-Robin, N.; Fineberg, S.; van den Eynden, G.; Ternes, N.; Penault-Llorca, F.; et al. Update on tumor-infiltrating lymphocytes (TILs) in breast cancer, including recommendations to assess TILs in residual disease after neoadjuvant therapy and in carcinoma in situ: A report of the International Immuno-Oncology Biomarker Working Group on Breast Cancer. Semin Cancer Biol. 2018, 52, 16–25. [Google Scholar]
- Vennapusa, B.; Baker, B.; owanetz, M.; Boone, J.; Menzl, I.; Bruey, J.M.; et al. Development of a PD-L1 complementary diagnostic immunohistochemistry assay (SP142) for atezolizumab. Appl Immunohistochem Mol Morphol 2019, 27, 92e100. [Google Scholar] [CrossRef]
- Kulangara, K.; Zhang, N.; Corigliano, E.; Guerrero, L.; Waldroup, S.; Jaiswal, D.; et al. Clinical utility of the combined positive score for programmed death ligand-1 expression and the approval of pembrolizumab for treatment of gastric cancer. Arch Pathol Lab Med 2019, 143, 330e7. [Google Scholar] [CrossRef]
- Huang, R.Y.; Francois, A.; McGray, A.R.; Miliotto, A.; Odunsi, K. Compensatory upregulation of PD-1, LAG-3, and CTLA-4 limits the efficacy of single-agent checkpoint blockade in metastatic ovarian cancer. Oncoimmunology 2017, 6, e1249561. [Google Scholar] [CrossRef]
- Loibl, S.; Weber, K.E.; Timms, K.M.; Elkin, E.P.; Hahnen, E.; Fasching, P.A.; et al. Survival analysis of carboplatin added to an anthracycline/taxane-based neoadjuvant chemotherapy and HRD score as predictor of response-final results from GeparSixto. Ann Oncol. 2018, 1, 2341–2347. [Google Scholar] [CrossRef]
- Prihantono; Faruk, M. Neoadjuvant chemotherapy response, disease-free survival, and overall survival of breast cancer in a single institution. Surg Open Sci. 2023, 15, 19–25. [Google Scholar] [CrossRef]
- Kunnuru, S.K.R.; Thiyagarajan, M.; Daniel, J.M.; Singh, K. B. A Study on Clinical and Pathological Responses to Neoadjuvant Chemotherapy in Breast Carcinoma. Breast Cancer. 2020, 12, 259–266. [Google Scholar] [CrossRef] [PubMed]
- Teng, M.W.; Ngiow, S.F.; Ribas, A.; Smyth, M.J. Classifying cancers based on T-cell infiltration and PD-L1. Cancer Res. 2015, 75, 2139–2145. [Google Scholar] [CrossRef] [PubMed]
- Denkert, C.; Von. Minckwitz, G.; Darb-Esfahani, S.; Lederer, B.; Heppner, B.I.; Weber, K.E.; et al. Tumour-infiltrating lymphocytes and prognosis in different subtypes of breast cancer: A pooled analysis of 3771 patients treated with neoadjuvant therapy. Lancet Oncol. 2018, 19, 40–50. [Google Scholar] [CrossRef]
- Stanton, S.E; Adams, S.; Disis, M.L. Variation in the incidence and magnitude of tumor-infiltrating lymphocytes in breast cancer subtypes: A systematic review. JAMA Oncol. 2016, 2, 1354–1360. [Google Scholar] [CrossRef]
- Karn, T.; Jiang, T.; Hatzis, C.; Sänger, N.; El-Balat, A.; Rody, A.; et al. Association between genomic metrics and immune infiltration in triple-negative breast cancer. JAMA Oncol. 2019, 3, 1707–1711. [Google Scholar] [CrossRef]
- Muenst, S.; Schaerli, A.R.; Gao, F.; Däster, S.; Trella, E.; Droeser, R.A.; et al. Expression of programmed death ligand 1 (PD-L1) is associated with poor prognosis in human breast cancer. Breast Cancer Res Treat. 2014, 146, 15–24. [Google Scholar] [CrossRef] [PubMed]
- Schalper, K.A.; Velcheti, V.; Carvajal, D.; Wimberly, H.; Brown, J.; Pusztai, L.; et al. In Situ Tumor PD-L1 mRNA Expression Is Associated with Increased TILs and Better Outcome in Breast Carcinomas PD-L1 mRNA Positivity and Outcome in Breast Cancer. Clin Cancer Res. 2014, 20, 2773–2782. [Google Scholar] [CrossRef]
- Berckelaer, C.V.; Rypens, C.; van Dam, P.; Pouillon, L.; Parizel, M.; Schats, K.A.; et al. Infiltrating stromal immune cells in inflammatory breast cancer are associated with an improved outcome and increased PD-L1 expression. Breast Cancer Res. 2019, 21, 1–12. [Google Scholar] [CrossRef]
- Amin, N.H.; Abou-Bakr, A.A.; Eissa, S.; Nassar, H.R.; Eissa, T.S.; Mohamed, G. Expression of PD-L1 in Early-Stage Invasive Breast Carcinoma and Its Relation to Tumor-Infiltrating Lymphocytes. Asian Pac J Cancer Prev. 2022, 23, 1091. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Liu, Y. PD-L1 expression in tumor infiltrated lymphocytes predicts survival in triple-negative breast cancer. Pathol Res Pract. 2019, 216, 152802. [Google Scholar] [CrossRef]
- Mittendorf, E.A.; Philips, A.V.; Meric-Bernstam, F.; Qiao, N.; Wu, Y.; Harrington, S.; et al. PD-L1 expression in triple-negative breast cancer. Cancer Immunol Res. 2014, 2, 361–370. [Google Scholar] [CrossRef]
- Alkhayyal, N.; Elemam, N.M.; Hussein, A.; Magdub, S.; Jundi, M.; Maghazachi, A.A.; et al. Expression of immune checkpoint (PD-L1 and IDO) and tumour-infiltrating lymphocytes in breast cancer. Heliyon 2022, 8, e10482. [Google Scholar] [CrossRef]
- Beckers, R.K.; Selinger, C.I.; Vilain, R.; Madore, J.; Wilmott, Js.; Harvey, K.; et al. Programmed death ligand 1 expression in triple-negative breast cancer is associated with tumour-infiltrating lymphocytes and improved outcome. Histopathology 2016, 69, 25–34. [Google Scholar] [CrossRef]
- 38; Oner, G.; Önder, S.; Karatay, H.; Ak, N.; Tükenmez, M.; Müslümanoğlu, M.; et al. Clinical impact of PD-L1 expression in triple-negative breast cancer patients with residual tumor burden after neoadjuvant chemotherapy. World J Surg Oncol. 2021, 19, 264. [Google Scholar]
- Ren, X.; Wu, H.; Lu, J.; Zhang, Y.; Luo, Y.; Xu, Q.; et al. PD1 protein expression in tumor infiltrated lymphocytes rather than PDL1 in tumor cells predicts survival in triple-negative breast cancer. Cancer Biol. Ther. 2018, 19, 373–380. [Google Scholar] [CrossRef] [PubMed]
- Bottai, G.; Carlotta, R.; Agnese, L.; Tommaso, L.D.; Tinterri, C.; Torrisi, R.; et al. An immune stratification reveals a subset of PD-1/LAG-3 double-positive triple-negative breast cancers. Breast Cancer Res. 2016, 18, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Tieying, D.; Qijia, X.; Zhao, H.; Qin, L.; Zhang, Q. Lymphocyte-Activation Gene-3 Expression and Prognostic Value in Neoadjuvant-Treated Triple-Negative Breast Cancer. J Breast Cancer 2018, 21, 124–133. [Google Scholar] [CrossRef] [PubMed]
- Ibrahim, R.; Saleh, K.; Chahine, C.; Khoury, R.; Khalife, N.; Le Cesne, A. LAG-3 Inhibitors: Novel Immune Checkpoint Inhibitors Changing the Landscape of Immunotherapy. Biomedicines 2023, 11, 1878. [Google Scholar] [CrossRef] [PubMed]
| Characteristics |
N=54 (%) |
| Average age (Years) | |
| 24- 40 | 37(68.51) |
| 41 - 45 | 17(31.48) |
| T prior to NACT | |
| cT1 | 1(1.86) |
| cT2 | 18(33.33) |
| cT3 | 29(53.70) |
| cT4 | 6(11.11) |
| N prior to NACT | |
| cN0 | 5(9.25) |
| cN1 | 43(79.6) |
| cN2 | 4(7.40) |
| cN3 | 2(3.7) |
| T Stage after surgery | |
| ypT0 | 9(16.67) |
| ypT1 | 07(12.96) |
| ypT2 | 35(64.81) |
| ypT3 | 3(5.56) |
| N Stage after surgery | |
| ypN0 | 7(12.96) |
| ypN1 | 44(81.48) |
| ypN2 | 1(1.86) |
| ypN3 | 2(3.70) |
| M Stage | |
| M0 | 51(94.44) |
| M1 | 3(5.55) |
| Tumor subtype | |
| IDC | 54(100) |
| Histological grade | |
| I | 1(1.85) |
| II | 11(20.37) |
| III | 42(77.77) |
| Surgery | |
| MRM | 50(92.59) |
| BCS | 4(7.40) |
| Family History | |
| Yes | 4(7.40) |
| No | 50(92.59) |
| NACT group | |
| A+T | 15(27.78) |
| A+T+C | 39(77.22) |
| NACT regimen | N |
RECIST Evaluation |
Frequency (%) |
Response (%) |
p-Value |
| A +T | 15 | CR | 1(6.67%) | Positive (60) |
0.01* |
| PR | 8(53.33%) | ||||
| SD | 2(13.33%) | Negative (40) |
|||
| PD | 4(26.67%) | ||||
| A+T+C | 39 | CR | 10(25.64%) | Positive (92.31) |
0.01* |
| PR | 26(66.67%) | ||||
| SD | 2(5.13%) | Negative (7.69) |
|||
| PD | 1(2.56%) |
| NACT regimen |
Tumor size (cm) before NACT Mean ±SD |
Tumor size (cm) after NACT Mean ±SD |
Mean difference of size after NACT |
p- Value |
| A +T | 4.76±1.28 | 3.56±1.53 | 1.2 | 0.001* |
| A+T+C | 5.2±1.43 | 2.53±1.65 | 2.67 | 0.001* |
| Characteristics | TILs | |||
| Low | Intermediate | High | p-Value | |
| N (%) | N (%) | N (%) | ||
|
Age Years | ||||
| 24 - 40 | 6(16.21) | 15(40.54) | 16(43.24) | 0.536 |
| 41 - 45 | 1(5.88) | 9(52.94) | 7(41.18) | |
| Tumor Subtype | ||||
| IDC | 9(16.66) | 23(42.59) | 22(40.74) | 0.613 |
| Histological grade | ||||
| I | 0(0) | 0(0) | 1(100) | 0.536 |
| II | 0(0) | 3(27.27) | 8(72.73) | |
| III | 7(16.67) | 18(42.86) | 17(40.48) | |
| T Stage | ||||
| T1 | 0(0) | 0(0) | 1(100) | 0.049* |
| T2 | 0(0) | 5(41.67) | 7(58.33) | |
| T3 | 4(13.33) | 10(33.33) | 16(53.33) | |
| T4 | 1(9.09) | 6(54.55) | 4(36.36) | |
| N Stage | ||||
| N0 | 0(0) | 3(42.86) | 4(57.14) | 0.538 |
| N1 | 6(13.64) | 16(36.36) | 22(50) | |
| N2 | 0(0) | 1(100) | 0(0) | |
| N3 | 1(50) | 0(0) | 1(50) | |
| M Stage | ||||
| M0 | 6(11.76) | 23(45.09) | 22(43.13) | 0.499 |
| M1 | 1(33.33) | 1(33.33) | 1(33.33) | |
|
CHARACTE- RISTICS |
PD-L1_TC | PD-L1_IC | PD-1_TC | PD-1_IC | ||||||||
| Negative | Positive | p Value | Negative | Positive | P Value | Negative | Positive | P Value | Negative | Positive | p- Value | |
| N (%) | N (%) | N (%) | N (%) | N (%) | N (%) | N (%) | N (%) | |||||
| AGE (YEARS) | ||||||||||||
| 24 - 40 | 14 (37.84) | 23 (62.16) |
0.563 |
28 (75.68) | 9 (24.32) |
0.745 |
20 (54.05) | 17 (45.95) |
0.56 |
11 (29.73) | 26 (70.27) | 0.912 |
| 41 - 45 | 8 (47.06) | 9 (52.94) | 12 (70.59) | 5 (29.41) | 11 (64.71) | 6(35.29) | 5 (29.41) | 12 (70.59) | ||||
| TUMOR SUBTYPE | ||||||||||||
| IDC | 21 (38.88) | 33 (61.11) | 1.00 | 37 (68.51) | 17 (31.48) | 1.00 | 39 (72.22) | 15 (27.77) | 1.00 | 9 (16.66) | 45 (83.33) | 1.00 |
| HISTOLOGICAL GRADE | ||||||||||||
| I | 0 (0) | 1(100) |
0.849 |
0 (0) | 1(100) |
0.359 |
0 (0) | 1 (100) |
0.227 |
1 (100) | 0 (0) | 0.174 |
| II | 4 (36.36) | 7 (63.63) | 5 (45.45) | 6(54.54) | 8 (72.73) | 3 (27.27) | 2 (18.18) | 9 (81.82) | ||||
| III | 18 (42.85) | 24 (57.14) | 32 (76.19) | 10 (23.80) | 34 (80.95) | 8 (19.05) | 6 (14.28) | 36 (85.71) | ||||
| T STAGE | ||||||||||||
| T1 | 0 (0) | 1(100) | 0.036* | 1(100) | 0 (0) | 0.203 | 1 (100) | 0 (0) | 0.928 | 0 (0) | 1 (100) | 0.073 |
| T2 | 9 (75) | 3 (25) | 11 (91.67) | 1 (8.33) | 10 (83.33) | 2 (16.67) | 2 (16.66) | 10 (83.33) | ||||
| T3 | 10 (33.33) | 20 (66.67) | 22 (73.33) | 8 (26.67) | 23 (76.66) | 7 (23.33) | 2 (6.66) | 28 (93.33) | ||||
| T4 | 3 (27.27) | 8(72.72) | 6 (54.54) | 5 (45.45) | 2(18.18) | 9(81.81) | 4 (36.36) | 7 (63.63) | ||||
| N STAGE | ||||||||||||
| N0 | 1 (14.29) | 6 (85.71) |
0.375 |
5 (71.42) | 2 (28.57) |
0.864 |
4 (57.14) | 3 (42.85) | 1.00 | 0 (0) | 7 (100) | 0.381 |
| N1 | 20 (45.45) | 24 (54.55) | 31 (70.45) | 13 (29.55) | 33 (75) | 11 (25) | 8 (18.18) | 36 (81.81) | ||||
| N2 | 0 (0) | 1(100) | 1(100) | 0 (0) | 1 (100) | 0 (0) | 0 (0) | 1 (100) | ||||
| N3 | 1 (50) | 1 (50) | 2 (100) | 0 (0) | 2 (100) | 0 (0) | 1 (50) | 1 (50) | ||||
| METASTASIS | ||||||||||||
| M0 | 21 (41.18) | 30 (58.82) | 1.00 | 37 (72.55) | 14 (27.45) | 0.56 | 39 (76.47) | 12 (24) | 1.00 | 8 (15.69) | 43 (84.31) | 0.428 |
| M1 | 1 (33.33) | 2 (66.67) | 3 (100) | 0 (0) | 3 (100) | 0 (0%) | 1 (33.33) | 2 (66.67) | ||||
| NACT | ||||||||||||
| A+T (N=15) | 6(40) | 9 (60) | 0.598 | 12(80) | 3(20) | 0.404 | 10(66.66) | 5(33.33) | 0.560 | 1 (6.66) | 14 (93.33) | 0.483 |
| A+T+C (N=39) | 16 (41.03) | 23 (58.97) | 28(71.79) | 11(28.21) | 30(76.92) | 9(23.08) | 6 (15.38) | 33(84.61) | ||||
|
TIL A+T (N=15) | ||||||||||||
| LOW | 3(100) | 0(0) |
0.005* |
3(100) | 0(0) |
1.000 |
2(66.67) | 1(33.33) |
0.385 |
2(66.67) | 1(33.33) |
0.103 |
| INTERMEDIATE | 3(60) | 2(40) | 4(80) | 1(20) | 5(100) | 0(0) | 0(0) | 5(100) | ||||
| HIGH | 0(0) | 7(100) | 5(71.43) | 2(28.57) | 5(71.43)28. | 2(57.57) | 1(14.29) | 6(85.71) | ||||
|
TIL A+T+C (N=39) | ||||||||||||
| LOW | 4(100) | 0(0) |
0.0001* |
4(100) | 0(0) |
0.412 |
2(50) | 2(50) |
0.001* |
2(50) | 2(50) |
0.123 |
| INTERMEDIATE | 11(57.89) | 8(42.11) | 14(73.68) | 5(26.32) | 19(100) | 0(0) | 3(15.79 | 16(84.21) | ||||
| HIGH | 1(6.25) | 15(93.75) | 10(62.50) | 6(37.5) | 9(56.25) | 7(43.75) | 1(6.25) | 15(93.75) | ||||
| CHARACTERISTICS | TC | IC | ||||
| Negative | Positive | p Value | Negative | Positive | p-Value | |
| N (%) | N (%) | N (%) | N (%) | |||
| AGE (YEARS) | ||||||
| 24 - 40 | 30 (81,08) | 7 (18.92) | 1 | 8 (21.62) | 29 (78.38) |
0.244 |
| 41 - 45 | 13 (76.47) | 4 (23.53) | 1 (5.88) | 16 (94.12) | ||
| TUMOR SUBTYPE | ||||||
| IDC | 30 (55.55) | 24 (44.44) | 0.157 | 17 (31.48) | 37 (68.51) | 0.356 |
| HISTOLOGICAL GRADE | ||||||
| I | 1 (100) | 0 (0) | 0.503 | 0 (0) | 1 (100) | 0.542 |
| II | 5 (45.45) | 6 (54.55) | 3 (27.27) | 8 (73.73) | ||
| III | 25 (59.52) | 17 (40.48) | 14 (33.33) | 28 (66.67) | ||
| T STAGE | ||||||
| T1 | 0 (0) | 1 (100) |
0.444 |
0 (0) | 1 (100) | 0.721 |
| T 2 | 6 (50) | 6 (50) | 4 (33) | 8 (67) | ||
| T 3 | 17 (56.67) | 13 (43.33) | 10 (33) | 20 (67) | ||
| T 4 | 8 (72.73) | 3 (27.27) | 2 (27) | 9 (73) | ||
| N STAGE | ||||||
| N0 | 5 (71.43) | 2 (28.57) |
0.809 |
1 (14.29) | 6 (85.71) |
0.633 |
| N1 | 24 (54.55) | 20 (45.45) | 15 (34.09) | 29 (65.91) | ||
| N2 | 1 (100) | 0 (0) | 0 (0) | 1 (100) | ||
| N3 | 1 (50) | 1 (50) | 1 (50) | 1 (50) | ||
| METASTASIS | ||||||
| M0 | 29 (56.86) | 22 (43.14) | 1 | 16 (31.37) | 35 (68.63) | 0.891 |
| M1 | 2 (66.67) | 1 (33.33) | 1 (33.33) | 2 (66.67) | ||
| NACT | ||||||
| A+T (N=15) | 7(46.67) | 8(53.33) | 0.247 | 5(33.33) | 10(66.67) | 0.550 |
| A+T+C (N=39) | 24(61.54) | 15(38.46) | 12(30.77) | 27(69.23) | ||
|
TIL A+T (N=15) |
||||||
| LOW | 2(66.67) | 1(33.33) | 0.510 | 3(100) | 0(0) | 0.050* |
| INTERMEDIATE | 3(60) | 2(40) | 1(20) | 4(80) | ||
| HIGH | 2(28.57) | 5(71.43) | 1(14.29) | 6(85.71) | ||
|
TIL A+T+C (N=39) | ||||||
| LOW | 1( 2.56) | 3 (7.69 ) | 0.403 | 4(100) | 0(0) | 0.0001* |
| INTERMEDIATE | 14(35.89) | 5(12.82) | 0(0) | 19(100) | ||
| HIGH | 4(10.25 ) | 12(30.76 ) | 6(15.38) | 10(25.64) | ||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





