Submitted:
18 August 2023
Posted:
22 August 2023
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Materials and Methods
2.1. Patients and treatment
2.1.1. Endpoints
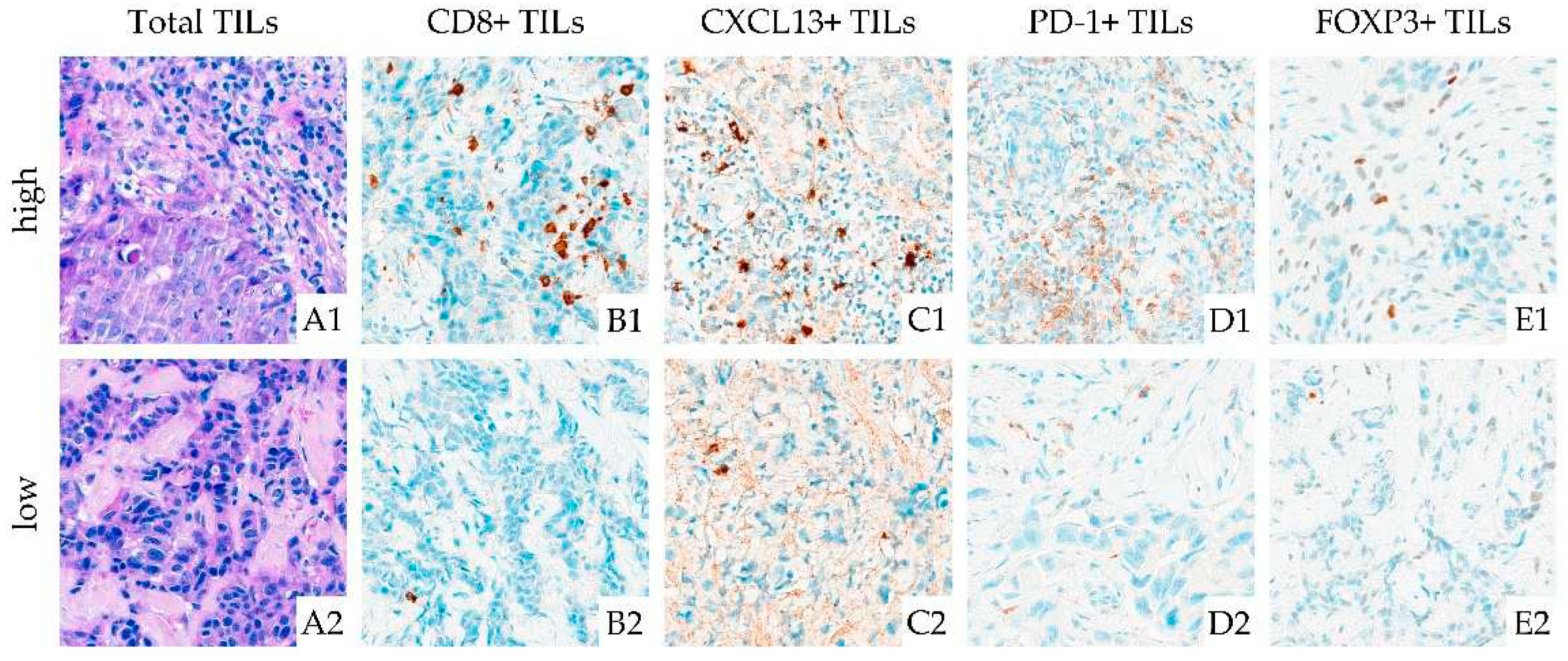
2.2. Pathohistological Examinations
2.3. Assay Methods
2.4. Statistical Analysis
2.4.1. Sample size
2.4.2. Data analysis
3. Results
3.1. Patient population
3.2. Association of TIL and its subtypes with pCR
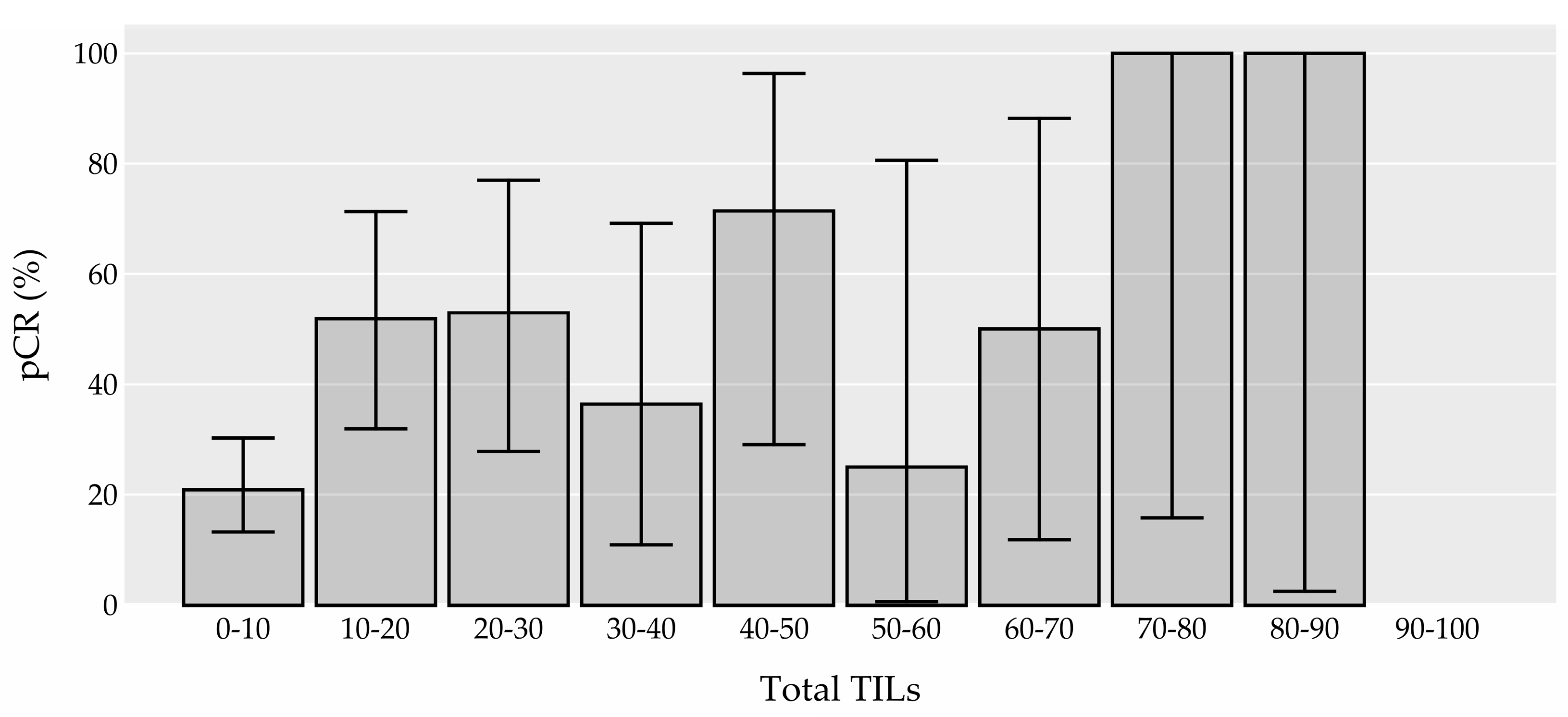
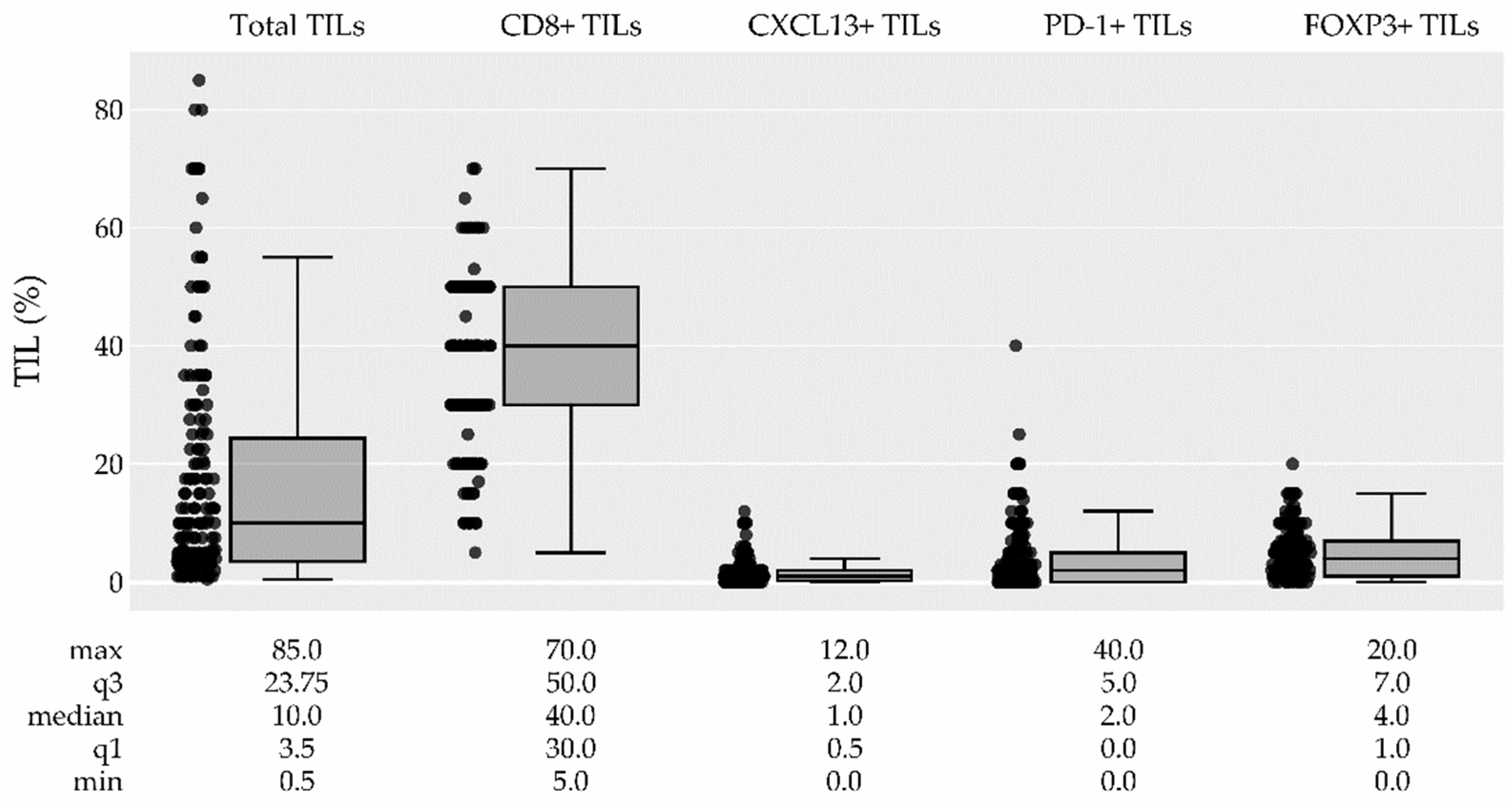
3.2.1. TIL Density and its association with pCR
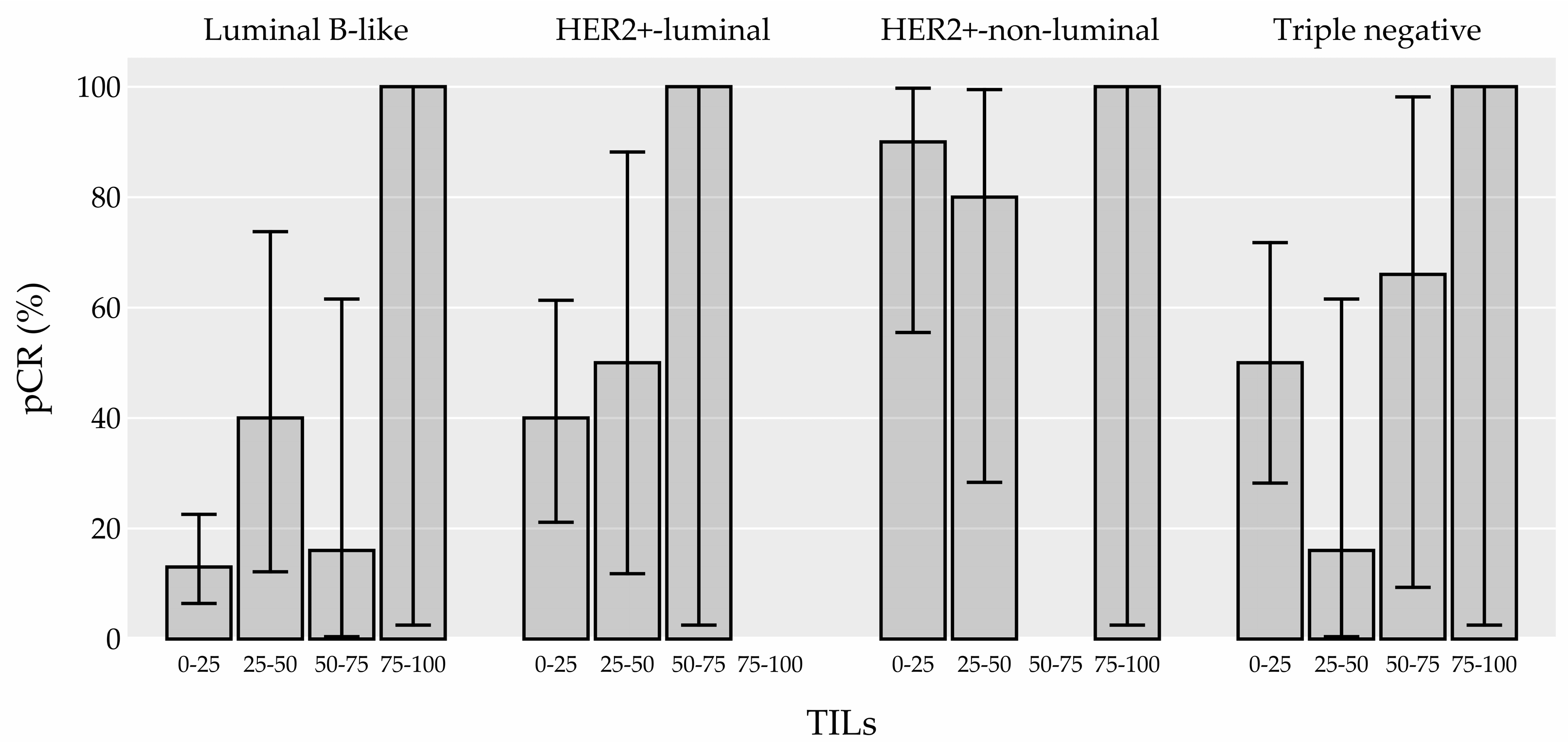
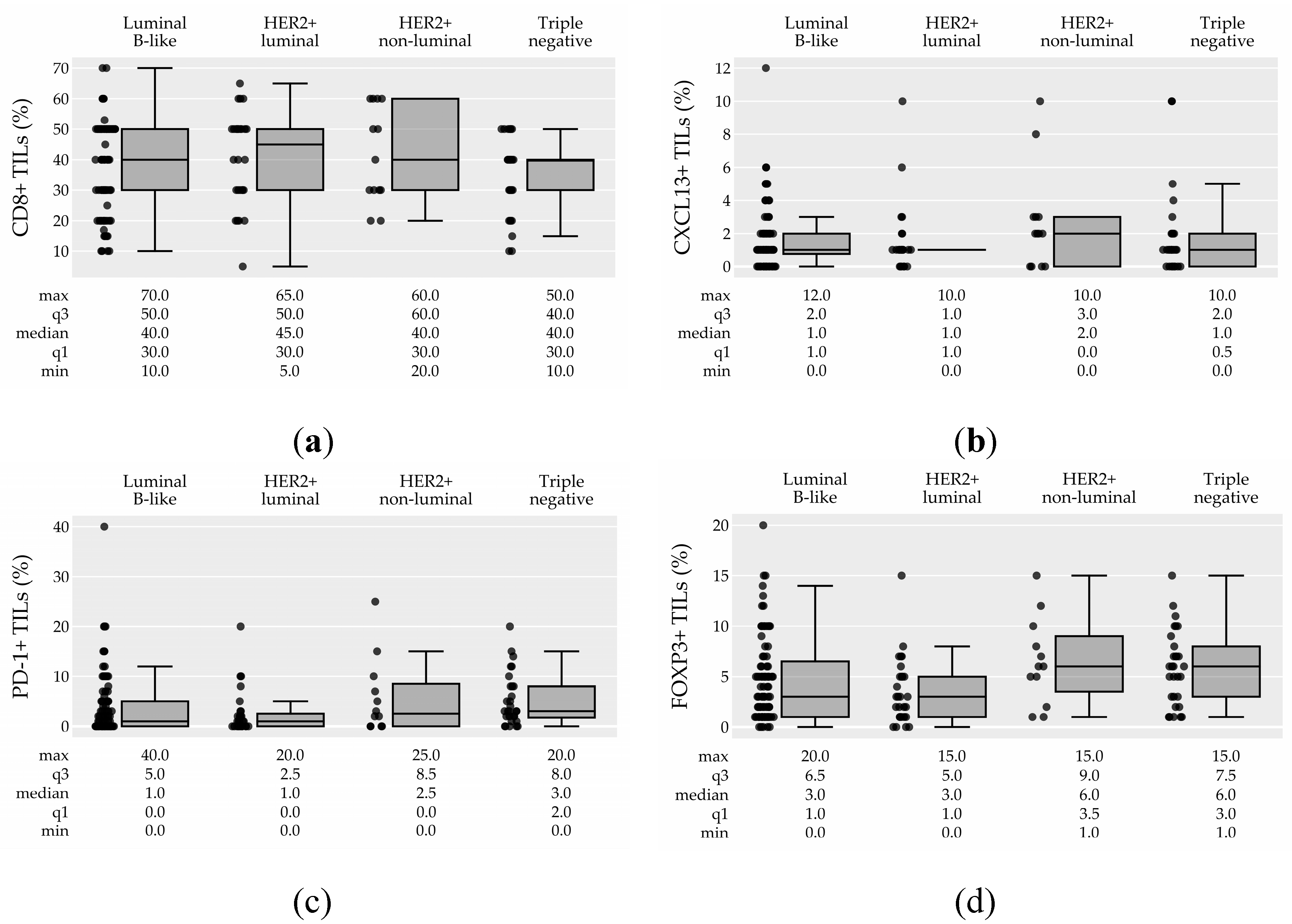
3.2.2. TIL Subtypes and their association with pCR
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Cancer Today. Available online: https://gco.iarc.fr/today/ (accessed on 23 October 2022).
- ECIS-European Cancer Information System. https://ecis.jrc.ec.europa.eu (, date last accessed). 25 March.
- Senkus, E.; Kyriakides, S.; Ohno, S.; Penault-Llorca, F.; Poortmans, P.; Rutgers, E.; Zackrisson, S.; Cardoso, F. Primary Breast Cancer: ESMO Clinical Practice Guidelines for Diagnosis, Treatment and Follow-Up. Annals of Oncology 2015, 26, v8–v30. [Google Scholar] [CrossRef] [PubMed]
- Cardoso, F.; Kyriakides, S.; Ohno, S.; Penault-Llorca, F.; Poortmans, P.; Rubio, I.T.; Zackrisson, S.; Senkus, E. Early Breast Cancer: ESMO Clinical Practice Guidelines for Diagnosis, Treatment and Follow-Up. Annals of Oncology 2019, 30, 1194–1220. [Google Scholar] [CrossRef] [PubMed]
- Goldhirsch, A.; Winer, E.P.; Coates, A.S.; Gelber, R.D.; Piccart-Gebhart, M.; Thürlimann, B.; Senn, H.-J.; Albain, K.S.; André, F.; Bergh, J.; et al. Personalizing the Treatment of Women with Early Breast Cancer: Highlights of the St Gallen International Expert Consensus on the Primary Therapy of Early Breast Cancer 2013. Annals of Oncology 2013, 24, 2206–2223. [Google Scholar] [CrossRef]
- Minckwitz, G. von; Untch, M.; Blohmer, J.-U.; Costa, S.D.; Eidtmann, H.; Fasching, P.A.; Gerber, B.; Eiermann, W.; Hilfrich, J.; Huober, J.; et al. Definition and Impact of Pathologic Complete Response on Prognosis After Neoadjuvant Chemotherapy in Various Intrinsic Breast Cancer Subtypes. Journal of Clinical Oncology 2012, 30, 1796–1804. [Google Scholar] [CrossRef] [PubMed]
- Hanahan, D.; Weinberg, R.A. Hallmarks of Cancer: The Next Generation. Cell 2011, 144, 646–674. [Google Scholar] [CrossRef]
- Nelson, M.A.; Ngamcherdtrakul, W.; Luoh, S.-W.; Yantasee, W. Prognostic and Therapeutic Role of Tumor-Infiltrating Lymphocyte Subtypes in Breast Cancer. Cancer and Metastasis Reviews 2021, 40, 519–536. [Google Scholar] [CrossRef]
- Pagès, F.; Berger, A.; Camus, M.; Sanchez-Cabo, F.; Costes, A.; Molidor, R.; Mlecnik, B.; Kirilovsky, A.; Nilsson, M.; Damotte, D.; et al. Effector Memory T Cells, Early Metastasis, and Survival in Colorectal Cancer. New England Journal of Medicine 2005, 353, 2654–2666. [Google Scholar] [CrossRef]
- Hwang, W.-T.; Adams, S.F.; Tahirovic, E.; Hagemann, I.S.; Coukos, G. Prognostic Significance of Tumor-Infiltrating T Cells in Ovarian Cancer: A Meta-Analysis. Gynecologic Oncology 2012, 124, 192–198. [Google Scholar] [CrossRef]
- Dieu-Nosjean, M.-C.; Antoine, M.; Danel, C.; Heudes, D.; Wislez, M.; Poulot, V.; Rabbe, N.; Laurans, L.; Tartour, E.; Chaisemartin, L. de; et al. Long-Term Survival for Patients With Non–Small-Cell Lung Cancer With Intratumoral Lymphoid Structures. Journal of Clinical Oncology 2008, 26, 4410–4417. [Google Scholar] [CrossRef]
- Denkert, C.; Loibl, S.; Noske, A.; Roller, M.; Müller, B.M.; Komor, M.; Budczies, J.; Darb-Esfahani, S.; Kronenwett, R.; Hanusch, C.; et al. Tumor-Associated Lymphocytes As an Independent Predictor of Response to Neoadjuvant Chemotherapy in Breast Cancer. Journal of Clinical Oncology 2010, 28, 105–113. [Google Scholar] [CrossRef]
- Denkert, C.; Minckwitz, G. von; Darb-Esfahani, S.; Lederer, B.; Heppner, B.I.; Weber, K.E.; Budczies, J.; Huober, J.; Klauschen, F.; Furlanetto, J.; et al. Tumour-Infiltrating Lymphocytes and Prognosis in Different Subtypes of Breast Cancer: a Pooled Analysis of 3771 Patients Treated with Neoadjuvant Therapy. The Lancet Oncology 2018, 19, 40–50. [Google Scholar] [CrossRef] [PubMed]
- Loi, S.; Sirtaine, N.; Piette, F.; Salgado, R.; Viale, G.; Eenoo, F.V.; Rouas, G.; Francis, P.; Crown, J.P.A.; Hitre, E.; et al. Prognostic and Predictive Value of Tumor-Infiltrating Lymphocytes in a Phase III Randomized Adjuvant Breast Cancer Trial in Node-Positive Breast Cancer Comparing the Addition of Docetaxel to Doxorubicin With Doxorubicin-Based Chemotherapy: BIG 02-98. Journal of Clinical Oncology 2013, 31, 860–867. [Google Scholar] [CrossRef] [PubMed]
- Gu-Trantien, C.; Loi, S.; Garaud, S.; Equeter, C.; Libin, M.; Wind, A. de; Ravoet, M.; Buanec, H.L.; Sibille, C.; Manfouo-Foutsop, G.; et al. CD4 Follicular Helper T Cell Infiltration Predicts Breast Cancer Survival. Journal of Clinical Investigation 2013, 123, 2873–2892. [Google Scholar] [CrossRef] [PubMed]
- Zgura, A.; Gales, L.; Bratila, E.; Anghel, R. Relationship between Tumor Infiltrating Lymphocytes and Progression in Breast Cancer. Mædica - a Journal of Clinical Medicine 2018, 13, 317–320. [Google Scholar] [CrossRef]
- Muntasell, A.; Rojo, F.; Servitja, S.; Rubio-Perez, C.; Cabo, M.; Tamborero, D.; Costa-Garcı́a Marcel; Martı́nez-Garcia Marı́a; Menéndez Sı́lvia; Vazquez, I. ; et al. NK Cell Infiltrates and HLA Class I Expression in Primary HER2 Breast Cancer Predict and Uncouple Pathological Response and Disease-Free Survival. Clinical Cancer Research 2019, 25, 1535–1545. [Google Scholar] [CrossRef] [PubMed]
- Egelston, C.A.; Avalos, C.; Tu, T.Y.; Rosario, A.; Wang, R.; Solomon, S.; Srinivasan, G.; Nelson, M.S.; Huang, Y.; Lim, M.H.; et al. Resident Memory CD8 T Cells within Cancer Islands Mediate Survival in Breast Cancer Patients. JCI Insight 2019, 4. [Google Scholar] [CrossRef]
- Ali, H.R.; Provenzano, E.; Dawson, S.-J.; Blows, F.M.; Liu, B.; Shah, M.; Earl, H.M.; Poole, C.J.; Hiller, L.; Dunn, J.A.; et al. Association between CD8 T-Cell Infiltration and Breast Cancer Survival in 12 439 Patients. Annals of Oncology 2014, 25, 1536–1543. [Google Scholar] [CrossRef]
- Ibrahim, E.M.; Al-Foheidi, M.E.; Al-Mansour, M.M.; Kazkaz, G.A. The Prognostic Value of Tumor-Infiltrating Lymphocytes in Triple-Negative Breast Cancer: a Meta-Analysis. Breast Cancer Research and Treatment 2014, 148, 467–476. [Google Scholar] [CrossRef]
- Mahmoud, S.M.A.; Paish, E.C.; Powe, D.G.; Macmillan, R.D.; Grainge, M.J.; Lee, A.H.S.; Ellis, I.O.; Green, A.R. . Tumor-infiltrating CD8+ lymphocytes predict clinical outcome in breast cancer. Journal of Clinical Oncology 2011, 29, 1949–1955. [Google Scholar] [CrossRef]
- Miyashita, M.; Sasano, H.; Tamaki, K.; Chan, M.; Hirakawa, H.; Suzuki, A.; Tada, H.; Watanabe, G.; Nemoto, N.; Nakagawa, S.; et al. Tumor-Infiltrating CD8 and FOXP3 Lymphocytes in Triple-Negative Breast Cancer: Its Correlation with Pathological Complete Response to Neoadjuvant Chemotherapy. Breast Cancer Research and Treatment 2014, 148, 525–534. [Google Scholar] [CrossRef]
- Matsumoto, H.; Thike, A.A.; Li, H.; Yeong, J.; Koo, S.-lin; Dent, R.A.; Tan, P.H.; Iqbal, J. Increased CD4 and CD8-Positive T Cell Infiltrate Signifies Good Prognosis in a Subset of Triple-Negative Breast Cancer. Breast Cancer Research and Treatment 2016, 156, 237–247. [Google Scholar] [CrossRef] [PubMed]
- Stovgaard, E.S.; Nielsen, D.; Hogdall, E.; Balslev, E. Triple Negative Breast Cancer – Prognostic Role of Immune-Related Factors: a Systematic Review. Acta Oncologica 2017, 57, 74–82. [Google Scholar] [CrossRef] [PubMed]
- Gatti-Mays, M.E.; Balko, J.M.; Gameiro, S.R.; Bear, H.D.; Prabhakaran, S.; Fukui, J.; Disis, M.L.; Nanda, R.; Gulley, J.L.; Kalinsky, K.; et al. If We Build It They Will Come: Targeting the Immune Response to Breast Cancer. npj Breast Cancer 2019, 5. [Google Scholar] [CrossRef] [PubMed]
- Kawaguchi, K.; Sakurai, M.; Yamamoto, Y.; Suzuki, E.; Tsuda, M.; Kataoka, T.R.; Hirata, M.; Nishie, M.; Nojiri, T.; Kumazoe, M.; et al. Alteration of Specific Cytokine Expression Patterns in Patients with Breast Cancer. Scientific Reports 2019, 9. [Google Scholar] [CrossRef]
- Doedens, A.L.; Stockmann, C.; Rubinstein, M.P.; Liao, D.; Zhang, N.; DeNardo, D.G.; Coussens, L.M.; Karin, M.; Goldrath, A.W.; Johnson, R.S. Macrophage Expression of Hypoxia-Inducible Factor-1 Suppresses T-Cell Function and Promotes Tumor Progression. Cancer Research 2010, 70, 7465–7475. [Google Scholar] [CrossRef]
- Ruffell, B.; Chang-Strachan, D.; Chan, V.; Rosenbusch, A.; Ho, C.M.T.; Pryer, N.; Daniel, D.; Hwang, E.S.; Rugo, H.S.; Coussens, L.M. Macrophage IL-10 Blocks CD8 T Cell-Dependent Responses to Chemotherapy by Suppressing IL-12 Expression in Intratumoral Dendritic Cells. Cancer Cell 2014, 26, 623–637. [Google Scholar] [CrossRef]
- Almand, B.; Clark, J.I.; Nikitina, E.; Beynen, J. van; English, N.R.; Knight, S.C.; Carbone, D.P.; Gabrilovich, D.I. Increased Production of Immature Myeloid Cells in Cancer Patients: A Mechanism of Immunosuppression in Cancer. The Journal of Immunology 2001, 166, 678–689. [Google Scholar] [CrossRef]
- Ngamcherdtrakul, W.; Yantasee, W. SiRNA Therapeutics for Breast Cancer: Recent Efforts in Targeting Metastasis, Drug Resistance, and Immune Evasion. Translational Research 2019, 214, 105–120. [Google Scholar] [CrossRef]
- Chen, L.; Han, X. Anti–PD-1/PD-L1 Therapy of Human Cancer: Past, Present, and Future. Journal of Clinical Investigation 2015, 125, 3384–3391. [Google Scholar] [CrossRef]
- Burugu, S.; Asleh-Aburaya, K.; Nielsen, T.O. Immune Infiltrates in the Breast Cancer Microenvironment: Detection, Characterization and Clinical Implication. Breast Cancer 2016, 24, 3–15. [Google Scholar] [CrossRef]
- Topalian, S.L.; Hodi, F.S.; Brahmer, J.R.; Gettinger, S.N.; Smith, D.C.; McDermott, D.F.; Powderly, J.D.; Carvajal, R.D.; Sosman, J.A.; Atkins, M.B.; et al. Safety, Activity, and Immune Correlates of Anti–PD-1 Antibody in Cancer. New England Journal of Medicine 2012, 366, 2443–2454. [Google Scholar] [CrossRef] [PubMed]
- Kitano, A.; Ono, M.; Yoshida, M.; Noguchi, E.; Shimomura, A.; Shimoi, T.; Kodaira, M.; Yunokawa, M.; Yonemori, K.; Shimizu, C.; et al. Tumour-Infiltrating Lymphocytes Are Correlated with Higher Expression Levels of PD-1 and PD-L1 in Early Breast Cancer. ESMO Open 2017, 2, e000150. [Google Scholar] [CrossRef] [PubMed]
- Vidula, N.; Yau, C.; Rugo, H.S. Programmed Cell Death 1 (PD-1) Receptor and Programmed Death Ligand 1 (PD-L1) Gene Expression in Primary Breast Cancer. Breast Cancer Research and Treatment 2021, 187, 387–395. [Google Scholar] [CrossRef]
- Ishigami, E.; Sakakibara, M.; Sakakibara, J.; Masuda, T.; Fujimoto, H.; Hayama, S.; Nagashima, T.; Sangai, T.; Nakagawa, A.; Nakatani, Y.; et al. Coexistence of Regulatory B Cells and Regulatory T Cells in Tumor-Infiltrating Lymphocyte Aggregates Is a Prognostic Factor in Patients with Breast Cancer. Breast Cancer 2018, 26, 180–189. [Google Scholar] [CrossRef] [PubMed]
- Takenaka M, Seki N, Toh U, Hattori S, Kawahara A, Yamaguchi T, et al. FOXP3 Expression in Tumor Cells and Tumor-Infiltrating Lymphocytes Is Associated with Breast Cancer Prognosis. Molecular and Clinical Oncology 2013, 1, 625–632. [Google Scholar] [CrossRef] [PubMed]
- Usman, A.N.; Ahmad, M.; Sinrang, A.W.; Natsir, S.; Takko, A.B.; Ariyandy, A.; Ilhamuddin, I.; Eragradini, A.R.; Hasan, I.I.; Hasyim, S. FOXP3 Regulatory T Cells on Prognosis of Breast Cancer. Breast Disease 2023, 42, 213–218. [Google Scholar] [CrossRef]
- Lal, A.; Chan, L.; DeVries, S.; Chin, K.; Scott, G.K.; Benz, C.C.; Chen, Y.-Y.; Waldman, F.M.; Hwang, E.S. FOXP3-Positive Regulatory T Lymphocytes and Epithelial FOXP3 Expression in Synchronous Normal, Ductal Carcinoma in Situ, and Invasive Cancer of the Breast. Breast Cancer Research and Treatment 2013, 139, 381–390. [Google Scholar] [CrossRef]
- Khaja, A.S.S.; Toor, S.M.; Salhat, H.E.; Faour, I.; Haq, N.U.; Ali, B.R.; Elkord, E. Preferential Accumulation of Regulatory T Cells with Highly Immunosuppressive Characteristics in Breast Tumor Microenvironment. Oncotarget 2017, 8, 33159–33171. [Google Scholar] [CrossRef]
- Bates, G.J.; Fox, S.B.; Han, C.; Leek, R.D.; Garcia, J.F.; Harris, A.L.; Banham, A.H. Quantification of Regulatory T Cells Enables the Identification of High-Risk Breast Cancer Patients and Those at Risk of Late Relapse. Journal of Clinical Oncology 2006, 24, 5373–5380. [Google Scholar] [CrossRef]
- Yeong, J.; Thike, A.A.; Lim, J.C.T.; Lee, B.; Li, H.; Wong, S.-C.; Hue, S.S.S.; Tan, P.H.; Iqbal, J. Higher Densities of Foxp3 Regulatory T Cells Are Associated with Better Prognosis in Triple-Negative Breast Cancer. Breast Cancer Research and Treatment 2017, 163, 21–35. [Google Scholar] [CrossRef]
- Sun, Y.; Wang, Y.; Lu, F.; Zhao, X.; Nie, Z.; He, B. The Prognostic Values of FOXP3 Tumor-Infiltrating T Cells in Breast Cancer: a Systematic Review and Meta-Analysis. Clinical and Translational Oncology 2023, 25, 1830–1843. [Google Scholar] [CrossRef] [PubMed]
- Martinez, L.M.; Robila, V.; Clark, N.M.; Du, W.; Idowu, M.O.; Rutkowski, M.R.; Bos, P.D. Regulatory T Cells Control the Switch From in Situ to Invasive Breast Cancer. Frontiers in Immunology 2019, 10. [Google Scholar] [CrossRef] [PubMed]
- Yazaki, S.; Shimoi, T.; Yoshida, M.; Sumiyoshi-Okuma, H.; Arakaki, M.; Saito, A.; Kita, S.; Yamamoto, K.; Kojima, Y.; Nishikawa, T.; et al. Integrative Prognostic Analysis of Tumor–Infiltrating Lymphocytes, CD8, CD20, Programmed Cell Death-Ligand 1, and Tertiary Lymphoid Structures in Patients with Early-Stage Triple-Negative Breast Cancer Who Did Not Receive Adjuvant Chemotherapy. Breast Cancer Research and Treatment 2022, 197, 287–297. [Google Scholar] [CrossRef]
- Peng, G.-L.; Li, L.; Guo, Y.-W.; Yu, P.; Yin, X.-J.; Wang, S.; Liu, C.-P. CD8 Cytotoxic and FoxP3 Regulatory T Lymphocytes Serve as Prognostic Factors in Breast Cancer. American Journal of Translational Research 2019, 11, 5039–5053. [Google Scholar] [PubMed]
- Solis-Castillo, L.A.; Garcia-Romo, G.S.; Diaz-Rodriguez, A.; Reyes-Hernandez, D.; Tellez-Rivera, E.; Rosales-Garcia, V.H.; Mendez-Cruz, A.R.; Jimenez-Flores, J.R.; Villafana-Vazquez, V.H.; Pedroza-Gonzalez, A. Tumor-Infiltrating Regulatory T Cells, CD8/Treg Ratio, and Cancer Stem Cells Are Correlated with Lymph Node Metastasis in Patients with Early Breast Cancer. Breast Cancer 2020, 27, 837–849. [Google Scholar] [CrossRef] [PubMed]
- Song, I.H.; Heo, S.-H.; Bang, W.S.; Park, H.S.; Park, I.A.; Kim, Y.-A.; Park, S.Y.; Roh, J.; Gong, G.; Lee, H.J. Predictive Value of Tertiary Lymphoid Structures Assessed by High Endothelial Venule Counts in the Neoadjuvant Setting of Triple-Negative Breast Cancer. Cancer Research and Treatment 2017, 49, 399–407. [Google Scholar] [CrossRef]
- Chen, L.; Huang, Z.; Yao, G.; Lyu, X.; Li, J.; Hu, X.; Cai, Y.; Li, W.; Li, X.; Ye, C. The Expression of CXCL13 and Its Relation to Unfavorable Clinical Characteristics in Young Breast Cancer. Journal of Translational Medicine 2015, 13. [Google Scholar] [CrossRef]
- Dangaj, D.; Bruand, M.; Grimm, A.J.; Ronet, C.; Barras, D.; Duttagupta, P.A.; Lanitis, E.; Duraiswamy, J.; Tanyi, J.L.; Benencia, F.; et al. Cooperation between Constitutive and Inducible Chemokines Enables T Cell Engraftment and Immune Attack in Solid Tumors. Cancer Cell 2019, 35, 885–900.e10. [Google Scholar] [CrossRef]
- Masuda, N.; Lee, S.-J.; Ohtani, S.; Im, Y.-H.; Lee, E.-S.; Yokota, I.; Kuroi, K.; Im, S.-A.; Park, B.-W.; Kim, S.-B.; et al. Adjuvant Capecitabine for Breast Cancer after Preoperative Chemotherapy. New England Journal of Medicine 2017, 376, 2147–2159. [Google Scholar] [CrossRef]
- Cortazar, P.; Zhang, L.; Untch, M.; Mehta, K.; Costantino, J.P.; Wolmark, N.; Bonnefoi, H.; Cameron, D.; Gianni, L.; Valagussa, P.; et al. Pathological Complete Response and Long-Term Clinical Benefit in Breast Cancer: the CTNeoBC Pooled Analysis. The Lancet 2014, 384, 164–172. [Google Scholar] [CrossRef]
- Salgado, R.; Denkert, C.; Demaria, S.; Sirtaine, N.; Klauschen, F.; Pruneri, G.; Wienert, S.; Eynden, G.V. den; Baehner, F.L.; Penault-Llorca, F.; et al. The Evaluation of Tumor-Infiltrating Lymphocytes (TILs) in Breast Cancer: Recommendations by an International TILs Working Group 2014. Annals of Oncology 2015, 26, 259–271. [Google Scholar] [CrossRef] [PubMed]
- Hendry, S.; Salgado, R.; Gevaert, T.; Russell, P.A.; John, T.; Thapa, B.; Christie, M.; Vijver, K. van de; Estrada, M.V.; Gonzalez-Ericsson, P.I.; et al. Assessing Tumor-Infiltrating Lymphocytes in Solid Tumors: A Practical Review for Pathologists and Proposal for a Standardized Method from the International Immuno-Oncology Biomarkers Working Group: Part 2: TILs in Melanoma, Gastrointestinal Tract Carcinomas, Non–Small Cell Lung Carcinoma and Mesothelioma, Endometrial and Ovarian Carcinomas, Squamous Cell Carcinoma of the Head and Neck, Genitourinary Carcinomas, and Primary Brain Tumors. Advances in Anatomic Pathology 2017, 24, 311–335. [Google Scholar] [CrossRef] [PubMed]
- Golicnik, J.P.; Gazic, B.; Ovcaricek, T.; Matos, E.; Borstnar, S. Prognostic Value of Routinely Determined Tumor Infiltrating Lymphocytes in Triple-Negative Breast Cancer. Journal of Clinical Oncology 2014, 32, 1125–1125. [Google Scholar] [CrossRef]
- Okcu, O.; Öztürk, S.D.; Öztürk, Çiğdem; Şen, B. ; Yasin, A. İrem; Bedir, R. Tumor-Infiltrating Lymphocytes (TILs)/Volume and Prognosis: The Value of TILs for Survival in HER2 and TN Breast Cancer Patients Treated with Chemotherapy. Annals of Diagnostic Pathology 2022, 58, 151930. [Google Scholar] [CrossRef]
- Jang, N.; Kwon, H.J.; Park, M.H.; Kang, S.H.; Bae, Y.K. Prognostic Value of Tumor-Infiltrating Lymphocyte Density Assessed Using a Standardized Method Based on Molecular Subtypes and Adjuvant Chemotherapy in Invasive Breast Cancer. Annals of Surgical Oncology 2018, 25, 937–946. [Google Scholar] [CrossRef]
- Mohammed, A.A.; Elsayed, F.M.; Algazar, M.; Rashed, H.E. Predictive and Prognostic Value of Tumor- Infiltrating Lymphocytes for Pathological Response to Neoadjuvant Chemotherapy in Triple Negative Breast Cancer. Gulf Journal of Oncology 2022, 1, 53–60. [Google Scholar]
- Minckwitz, G. von; Raab, G.; Caputo, A.; Schütte, M.; Hilfrich, J.; Blohmer, J.U.; Gerber, B.; Costa, S.D.; Merkle, E.; Eidtmann, H.; et al. Doxorubicin With Cyclophosphamide Followed by Docetaxel Every 21 Days Compared With Doxorubicin and Docetaxel Every 14 Days As Preoperative Treatment in Operable Breast Cancer: The GEPARDUO Study of the German Breast Group. Journal of Clinical Oncology 2005, 23, 2676–2685. [Google Scholar] [CrossRef]
- Denkert, C. Diagnostic and Therapeutic Implications of Tumor-Infiltrating Lymphocytes in Breast Cancer. Journal of Clinical Oncology 2013, 31, 836–837. [Google Scholar] [CrossRef]
- Loi, S.; Michiels, S.; Salgado, R.; Sirtaine, N.; Jose, V.; Fumagalli, D.; Kellokumpu-Lehtinen, P.-L.; Bono, P.; Kataja, V.; Desmedt, C.; et al. Tumor Infiltrating Lymphocytes Are Prognostic in Triple Negative Breast Cancer and Predictive for Trastuzumab Benefit in Early Breast Cancer: Results from the FinHER Trial. Annals of Oncology 2014, 25, 1544–1550. [Google Scholar] [CrossRef]
- Wein, L.; Savas, P.; Luen, S.J.; Virassamy, B.; Salgado, R.; Loi, S. Clinical Validity and Utility of Tumor-Infiltrating Lymphocytes in Routine Clinical Practice for Breast Cancer Patients: Current and Future Directions. Frontiers in Oncology 2017, 7. [Google Scholar] [CrossRef]
- Loi, S.; Michiels, S.; Adams, S.; Loibl, S.; Budczies, J.; Denkert, C.; Salgado, R. The Journey of Tumor-Infiltrating Lymphocytes as a Biomarker in Breast Cancer: Clinical Utility in an Era of Checkpoint Inhibition. Annals of Oncology 2021, 32, 1236–1244. [Google Scholar] [CrossRef] [PubMed]
- Pruneri, G.; Vingiani, A.; Denkert, C. Tumor Infiltrating Lymphocytes in Early Breast Cancer. The Breast 2018, 37, 207–214. [Google Scholar] [CrossRef] [PubMed]
- Denkert, C.; Minckwitz, G. von; Brase, J.C.; Sinn, B.V.; Gade, S.; Kronenwett, R.; Pfitzner, B.M.; Salat, C.; Loi, S.; Schmitt, W.D.; et al. Tumor-Infiltrating Lymphocytes and Response to Neoadjuvant Chemotherapy With or Without Carboplatin in Human Epidermal Growth Factor Receptor 2–Positive and Triple-Negative Primary Breast Cancers. Journal of Clinical Oncology 2015, 33, 983–991. [Google Scholar] [CrossRef] [PubMed]
- Issa-Nummer, Y.; Darb-Esfahani, S.; Loibl, S.; Kunz, G.; Nekljudova, V.; Schrader, I.; Sinn, B.V.; Ulmer, H.-U.; Kronenwett, R.; Just, M.; et al. Prospective Validation of Immunological Infiltrate for Prediction of Response to Neoadjuvant Chemotherapy in HER2-Negative Breast Cancer – A Substudy of the Neoadjuvant GeparQuinto Trial. PLoS ONE 2013, 8, e79775. [Google Scholar] [CrossRef] [PubMed]
- West, N.R.; Milne, K.; Truong, P.T.; Macpherson, N.; Nelson, B.H.; Watson, P.H. Tumor-Infiltrating Lymphocytes Predict Response to Anthracycline-Based Chemotherapy in Estrogen Receptor-Negative Breast Cancer. Breast Cancer Research 2011, 13. [Google Scholar] [CrossRef]
- Dieci, M.V.; Mathieu, M.C.; Guarneri, V.; Conte, P.; Delaloge, S.; Andre, F.; Goubar, A. Prognostic and Predictive Value of Tumor-Infiltrating Lymphocytes in Two Phase III Randomized Adjuvant Breast Cancer Trials. Annals of Oncology 2015, 26, 1698–1704. [Google Scholar] [CrossRef]
- Kotoula, V.; Chatzopoulos, K.; Lakis, S.; Alexopoulou, Z.; Timotheadou, E.; Zagouri, F.; Pentheroudakis, G.; Gogas, H.; Galani, E.; Efstratiou, I.; et al. Tumors with High-Density Tumor Infiltrating Lymphocytes Constitute a Favorable Entity in Breast Cancer: a Pooled Analysis of Four Prospective Adjuvant Trials. Oncotarget 2015, 7, 5074–5087. [Google Scholar] [CrossRef]
- Jong, V.M.T. de; Wang, Y.; Hoeve, N.D. ter; Opdam, M.; Stathonikos, N.; Jóźwiak, K.; Hauptmann, M.; Cornelissen, S.; Vreuls, W.; Rosenberg, E.H.; et al. Prognostic Value of Stromal Tumor-Infiltrating Lymphocytes in Young, Node-Negative, Triple-Negative Breast Cancer Patients Who Did Not Receive (Neo)Adjuvant Systemic Therapy. Journal of Clinical Oncology 2022, 40, 2361–2374. [Google Scholar] [CrossRef]
- Minckwitz, G. von; Schneeweiss, A.; Loibl, S.; Salat, C.; Denkert, C.; Rezai, M.; Blohmer, J.U.; Jackisch, C.; Paepke, S.; Gerber, B.; et al. Neoadjuvant Carboplatin in Patients with Triple-Negative and HER2-Positive Early Breast Cancer (GeparSixtoGBG 66): a Randomised Phase 2 Trial. The Lancet Oncology 2014, 15, 747–756. [Google Scholar] [CrossRef]
- Minckwitz, G. von; Rezai, M.; Loibl, S.; Fasching, P.A.; Huober, J.; Tesch, H.; Bauerfeind, I.; Hilfrich, J.; Eidtmann, H.; Gerber, B.; et al. Capecitabine in Addition to Anthracycline- and Taxane-Based Neoadjuvant Treatment in Patients With Primary Breast Cancer: Phase III GeparQuattro Study. Journal of Clinical Oncology 2010, 28, 2015–2023. [Google Scholar] [CrossRef]
- Untch, M.; Loibl, S.; Bischoff, J.; Eidtmann, H.; Kaufmann, M.; Blohmer, J.-U.; Hilfrich, J.; Strumberg, D.; Fasching, P.A.; Kreienberg, R.; et al. Lapatinib versus Trastuzumab in Combination with Neoadjuvant Anthracycline-Taxane-Based Chemotherapy (GeparQuinto, GBG 44): a Randomised Phase 3 Trial. The Lancet Oncology 2012, 13, 135–144. [Google Scholar] [CrossRef] [PubMed]
- Untch, M.; Jackisch, C.; Schneeweiss, A.; Conrad, B.; Aktas, B.; Denkert, C.; Eidtmann, H.; Wiebringhaus, H.; Kümmel, S.; Hilfrich, J.; et al. Nab-Paclitaxel versus Solvent-Based Paclitaxel in Neoadjuvant Chemotherapy for Early Breast Cancer (GeparSepto—GBG 69): a Randomised, Phase 3 Trial. The Lancet Oncology 2016, 17, 345–356. [Google Scholar] [CrossRef] [PubMed]
- Heppner, B.I.; Untch, M.; Denkert, C.; Pfitzner, B.M.; Lederer, B.; Schmitt, W.; Eidtmann, H.; Fasching, P.A.; Tesch, H.; Solbach, C.; et al. Tumor-Infiltrating Lymphocytes: A Predictive and Prognostic Biomarker in Neoadjuvant-Treated HER2-Positive Breast Cancer. Clinical Cancer Research 2016, 22, 5747–5754. [Google Scholar] [CrossRef] [PubMed]
- Bussard, K.M.; Mutkus, L.; Stumpf, K.; Gomez-Manzano, C.; Marini, F.C. Tumor-Associated Stromal Cells as Key Contributors to the Tumor Microenvironment. Breast Cancer Research 2016, 18. [Google Scholar] [CrossRef]
- Baxevanis, C.N.; Fortis, S.P.; Perez, S.A. The Balance between Breast Cancer and the Immune System: Challenges for Prognosis and Clinical Benefit from Immunotherapies. Seminars in Cancer Biology 2021, 72, 76–89. [Google Scholar] [CrossRef]
- Negro, G.; Aschenbrenner, B.; Brezar, S.K.; Cemazar, M.; Coer, A.; Gasljevic, G.; Savic, D.; Sorokin, M.; Buzdin, A.; Callari, M.; et al. Molecular Heterogeneity in Breast Carcinoma Cells with Increased Invasive Capacities. Radiology and Oncology 2020, 54, 103–118. [Google Scholar] [CrossRef]
- Baker, K.; Lachapelle, J.; Zlobec, I.; Bismar, T.A.; Terracciano, L.; Foulkes, W.D. Prognostic Significance of CD8 T Lymphocytes in Breast Cancer Depends upon Both Oestrogen Receptor Status and Histological Grade. Histopathology 2011, no–no. [Google Scholar] [CrossRef]
- Virassamy, B.; Caramia, F.; Savas, P.; Sant, S.; Wang, J.; Christo, S.N.; Byrne, A.; Clarke, K.; Brown, E.; Teo, Z.L.; et al. Intratumoral CD8 T Cells with a Tissue-Resident Memory Phenotype Mediate Local Immunity and Immune Checkpoint Responses in Breast Cancer. Cancer Cell 2023, 41, 585–601.e8. [Google Scholar] [CrossRef]
- Seo, A.N.; Lee, H.J.; Kim, E.J.; Kim, H.J.; Jang, M.H.; Lee, H.E.; Kim, Y.J.; Kim, J.H.; Park, S.Y. Tumour-Infiltrating CD8 Lymphocytes as an Independent Predictive Factor for Pathological Complete Response to Primary Systemic Therapy in Breast Cancer. British Journal of Cancer 2013, 109, 2705–2713. [Google Scholar] [CrossRef]
- Heimes, A.-S.; Madjar, K.; Edlund, K.; Battista, M.J.; Almstedt, K.; Elger, T.; Krajnak, S.; Rahnenführer, J.; Brenner, W.; Hasenburg, A.; et al. Subtype-Specific Prognostic Impact of Different Immune Signatures in Node-Negative Breast Cancer. Breast Cancer Research and Treatment 2017, 165, 293–300. [Google Scholar] [CrossRef]
- Criscitiello, C.; Bayar, M.A.; Curigliano, G.; Symmans, F.W.; Desmedt, C.; Bonnefoi, H.; Sinn, B.; Pruneri, G.; Vicier, C.; Pierga, J.Y.; et al. A Gene Signature to Predict High Tumor-Infiltrating Lymphocytes after Neoadjuvant Chemotherapy and Outcome in Patients with Triple-Negative Breast Cancer. Annals of Oncology 2018, 29, 162–169. [Google Scholar] [CrossRef] [PubMed]
- Han, M.; Li, J.; Wu, S.; Wu, C.; Yu, Y.; Liu, Y. Comparison of the Tumor Immune Microenvironment Phenotypes in Different Breast Cancers after Neoadjuvant Therapy. Cancer Medicine 2022, 12, 2906–2917. [Google Scholar] [CrossRef] [PubMed]
- Ding, Z.-C.; Zhou, G. Cytotoxic Chemotherapy and CD4 Effector T Cells: An Emerging Alliance for Durable Antitumor Effects. Clinical and Developmental Immunology 2012, 2012, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Wing, J.B.; Tanaka, A.; Sakaguchi, S. Human FOXP3 Regulatory T Cell Heterogeneity and Function in Autoimmunity and Cancer. Immunity 2019, 50, 302–316. [Google Scholar] [CrossRef] [PubMed]
- West, N.R.; Kost, S.E.; Martin, S.D.; Milne, K.; deLeeuw, R.J.; Nelson, B.H.; Watson, P.H. Tumour-Infiltrating FOXP3 Lymphocytes Are Associated with Cytotoxic Immune Responses and Good Clinical Outcome in Oestrogen Receptor-Negative Breast Cancer. British Journal of Cancer 2012, 108, 155–162. [Google Scholar] [CrossRef]
- Goda, N.; Sasada, S.; Shigematsu, H.; Masumoto, N.; Arihiro, K.; Nishikawa, H.; Sakaguchi, S.; Okada, M.; Kadoya, T. The Ratio of CD80.167em\hspace0.167emlymphocytes to Tumor-Infiltrating Suppressive FOXP30.167em\hspace0.167emeffector Regulatory T Cells Is Associated with Treatment Response in Invasive Breast Cancer. Discover Oncology 2022, 13. [Google Scholar] [CrossRef]
- Asano, Y.; Kashiwagi, S.; Goto, W.; Kurata, K.; Noda, S.; Takashima, T.; Onoda, N.; Tanaka, S.; Ohsawa, M.; Hirakawa, K. Tumour-Infiltrating CD8 to FOXP3 Lymphocyte Ratio in Predicting Treatment Responses to Neoadjuvant Chemotherapy of Aggressive Breast Cancer. British Journal of Surgery 2016, 103, 845–854. [Google Scholar] [CrossRef]
- Filion, L.G.; Izaguirre, C.A.; Garber, G.E.; Huebsh, L.; Aye, M.T. Detection of Surface and Cytoplasmic CD4 on Blood Monocytes from Normal and HIV-1 Infected Individuals. Journal of Immunological Methods 1990, 135, 59–69. [Google Scholar] [CrossRef]
| Characteristic (number of missing values) |
All patients (n=171) | Patients with pCR (n=59) | Patients without pCR (n=112) |
OR (95% CI) | p-value | Adjusted p-value† |
||
|---|---|---|---|---|---|---|---|---|
| Age median (IQR) (0) | 48.4 (41.7-57.4) | 46.4 (39.4-56.0) | 49.0 (44.7-57.5) | 0.97 (0.94-1.00) | 0.046 | 0.510 | ||
| Histology n (%) (2) | 0.014 | 0.176 | ||||||
| IBC of no special type | 161 (96.0%) | 58 (36.0%) | 103 (64.0%) | 7.35 (0.84-964.63) | 0.077 | 0.613 | ||
| ILC | 6 (4.0%) | 0 (0.0%) | 6 (100.0%) | ref. | ref. | ref. | ||
| Other | 2 (1.0%) | 0 (0.0%) | 2 (100.0%) | 2.60 (0.01-553.14) | 0.642 | 1.000 | ||
| Grade n (%) (1) | ||||||||
| Grade 3 | 124 (73.0%) | 54 (44.0%) | 70 (56.0%) | 5.83 (2.41-16.89) | <0.001 | <0.001 | ||
| Grade 2 | 46 (27.0%) | 5 (11.0%) | 41 (89.0%) | ref. | ref. | ref. | ||
| Molecular subtype n (%) (0) | <0.001 | <0.001 | ||||||
| Luminal B-like | 91 (53.0%) | 16 (18.0%) | 75 (82.0%) | ref. | ref. | |||
| Her2+-luminal | 32 (19.0%) | 14 (44.0%) | 18 (56.0%) | 3.59 (1.50-8.65) | 0.004 | 0.058 | ||
| Her2+-non-luminal | 16 (9.0%) | 14 (88.0%) | 2 (12.0%) | 26.54 (7.23-144.62) | <0.001 | < 0.001 | ||
| Triple negative | 32 (19.0%) | 15 (47.0%) | 17 (53.0%) | 4.05 (1.71-9.77) | 0.002 | 0.023 | ||
| Ki-67 median (IQR) (1) | 30.0 (20.0-50.0) | 50.0 (30.0-60.0) | 30.0 (20.0-40.0) | 1.04 (1.02-1.05) | <0.001 | < 0.001 | ||
| Ki-67 n (%) (1) | <0.001 | <0.001 | ||||||
| 10-20% | 46 (27.0%) | 3 (7.0%) | 43 (93.0%) | ref. | ref. | ref. | ||
| 20-40% | 66 (39.0%) | 25 (38.0%) | 41 (62.0%) | 7.64 (2.58-30.11) | <0.001 | 0.002 | ||
| >40% | 58 (34.0%) | 31 (53.0%) | 27 (47.0%) | 14.24 (4.78-56.53) | <0.001 | < 0.001 | ||
| Tumor stage n (%) (15) | 0.325 | 1.000 | ||||||
| T1 (≤20mm) | 25 (16.0%) | 10 (40.0%) | 15 (60.0%) | ref. | ref. | ref. | ||
| T2 (>20≤50) | 113 (72.0%) | 39 (35.0%) | 74 (65.0%) | 0.78 (0.33-1.92) | 0.584 | 1.000 | ||
| T3 (>50 mm) | 18 (12.0%) | 6 (33.0%) | 12 (67.0%) | 0.77 (0.22-2.60) | 0.670 | 1.000 | ||
| Lymph nodes n (%) (0) | ||||||||
| Positive | 125 (73.0%) | 37 (30.0%) | 88 (70.0%) | ref. | ref. | ref. | ||
| Negative | 46 (27.0%) | 22 (48.0%) | 24 (52.0%) | 2.17 (1.09-4.33) | 0.028 | 0.335 | ||
| Total TILs median (IQR) (0) | 10.0 (3.5-23.8) | 17.5 (7.5-35.0) | 5.0 (3.0-17.5) | 1.03 (1.01-1.05) | <0.001 | 0.005 | ||
| Total TILs n (%) (0) | ||||||||
| Low (<60%) | 161 (94.0%) | 53 (33.0%) | 108 (67.0%) | ref. | ref. | ref. | ||
| High (≥60%) | 10 (6.0%) | 6 (60.0%) | 4 (40.0%) | 2.93 (0.85-10.99) | 0.088 | 0.615 | ||
| CD8+ TILs median (IQR) (12) | 40.0 (30.0-50.0) | 40.0 (30.0-50.0) | 40.0 (27.5-50.0) | 1.02 (1.00-1.04) | 0.122 | 0.731 | ||
| CXCL13+ TILs median (IQR) (16) | 1.0 (0.5-2.0) | 1.0 (1.0-2.0) | 1.0 (0.0-2.0) | 1.03 (0.89-1.19) | 0.673 | 1.000 | ||
| PD-1+ TILs median (IQR) (14) | 2.0 (0.0-5.0) | 3.0 (1.0-8.0) | 1.0 (0.0-5.0) | 1.05 (1.00-1.12) | 0.053 | 0.510 | ||
| FOXP3+ TILs median (IQR) (13) | 4.0 (1.0-7.0) | 5.0 (2.0-7.0) | 3.0 (1.0-6.0) | 1.08 (1.00-1.18) | 0.051 | 0.510 | ||
| Characteristic | OR (95% CI) | p-value | |
|---|---|---|---|
| Total TILs (per 10%) | 1.27 (1.05-1.54) | 0.012 | |
| Age | 0.98 (0.95-1.01) | 0.232 | |
| Ki-67 (per 10%) | 1.38 (1.13-1.70) | 0.001 | |
| Negative lymph nodes | 2.51 (1.13-5.69) | 0.024 | |
| HER2+-luminal, HER2+-non-luminal, TN vs lumB | 5.11 (2.43-11.36) | <0.001 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





