+ As co-first author
Summary
Cenchrus fungigraminus has strong potential for feed production, and silage technology can effectively reduce the hardness of forage fiber and increase the nutrient content. By studying the effects of different heights and treatment methods on the quality of C. fungigraminus silage, this study provides feasible methodological guidance for the research on the feed production of C. fungigraminus.
1. Introduction
Feed consumption for ruminants currently accounts for approximately 60% of total production costs. At present, primary feed ingredients such as corn and soybeans are edible for both humans and livestock, which exacerbates the “food-feed competition” issue [
1]. Consequently, identifying high-yield, low-cost forage resources has become a priority in ruminant nutrition research.
Cenchrus fungigraminus (Juncao) is a perennial grass widely cultivated in the tropical regions of Asia and Africa; it possesses a favorable nutritional profile and demonstrates significant potential as a forage crop [
2].
Utilizing
Juncao through ensiling technology can effectively optimize its nutritional structure by increasing crude protein and NH
3-N content while reducing fiber levels [
3], thereby enhancing its palatability for ruminants. Due to its high-yield characteristics,
Juncao can effectively alleviate the competition for grain between humans and livestock and reduce feeding costs, presenting immense prospects for large-scale feed utilization. Current research indicates that appropriate ensiling methods can mitigate feed losses caused by adverse weather and storage conditions, subsequently improving the voluntary intake rate of cattle and sheep [
4]. However, the harvesting height, moisture content, and the application of silage additives all exert significant influences on fermentation quality and feed characteristics [
5].
Regarding harvesting height, corn harvested at a greater height exhibits significantly higher dry matter, crude protein, and starch content compared to lower harvest heights [
6]. Similarly,
Pennisetum glaucifolium harvested at 90–110 cm shows a significantly improved fermentation effect and higher intake rates in cattle compared to those harvested at 70 cm [
7]. In terms of additives, the application of lactic acid bacteria (LAB) before ensiling, compared to natural fermentation, optimizes the microbial community structure. This promotes the utilization of water-soluble carbohydrates to produce lactic and acetic acids [
8], which enhances fermentation stability, inhibits the proliferation of pathogenic microorganisms [
9], and reduces both the spoilage rate and butyric acid production [
10].
In specific studies on
Juncao silage, the addition of compound probiotics (
Lactobacillus plantarum,
Bifidobacterium animalis, and
Bacillus coagulans) has been shown to effectively lower the pH, increase crude protein and water-soluble carbohydrate content, and inhibit butyric acid formation [
11]. Furthermore, feeding
Juncao silage to sheep can significantly increase average daily gain and reduce muscle water loss [
12]. Despite these advancements, as
Juncao is a relatively new forage species, research on its systematic feed application remains incomplete. Specifically, there are no definitive reports regarding the optimal harvesting height or standardized additive protocols, leading to a fragmented landscape of ensiling techniques without unified standards. Therefore, by collecting fermented feed from farmers in Southwest China and conducting controlled laboratory experiments, this study investigates the effects of different harvesting heights and treatments on
Juncao silage and identifies the optimal process for cellulose degradation. The goal is to provide a scientifically robust theoretical framework and technical demonstration for
Juncao ensiling.
2. Materials and Methods
2.1. Experimental Materials 2.1. Experimental Sites
The ensiling experiments involving different growth heights and processing methods were conducted in Xundian County. Additionally, 25 samples of silage produced under practical farming conditions were collected from five distinct regions in Southwest China: Jianshui County (23°37′N 102°50′E; hereinafter referred to as
J), Yanshan County (23°36′47″N 104°20′28″E;
Y), Simao District (22°46′39″N 100°58′35″E;
S), Changning County (24°49′38″N 99°36′36″E;
C), and Xundian County (25°33′48″N 103°15′21″E;
X). Details of the collected samples and the specific sampling locations are presented in
Table 1 and
Figure 1, respectively.
2.2. Experimental Materials
The Juncao (Cenchrus fungigraminus) used in this experiment was planted on June 2, 2022, in Changning County, Baoshan City, Yunnan Province, China, with a plant and row spacing of 60 cm × 80 cm. The second-year growth of Juncao was harvested on July 25, 2023.
The silage additive used was a Chr. Hansen silage inoculant provided by Wuhan Kelibo Animal Husbandry Technology Co., Ltd. The primary active ingredients included Lactococcus lactis (≥ 6.2×1010 cfu/g and Lactobacillus buchneri (≥ 6.2×1010 cfu/g). For application, the inoculant was prepared as a 0.002‰ (w/v) aqueous solution.
Vacuum packaging was performed using a single-chamber vacuum machine (Model: DZ-600/700/800 2E) manufactured by Shanghai Jiahe Packaging Machinery Co., Ltd. The fermentation bags used were 1 kg capacity bags equipped with one-way valves (23×40 cm), provided by Wenzhou Huaguan Packaging Co., Ltd.
2.3. Experimental Design
Three growth heights were selected for the ensiling experiments: 100–150 cm (Low), 150–200 cm (Mid), and 200–250 cm (High). The fresh Juncao from each group was chopped into lengths of 2–3 cm and divided into three equal portions, with three replicates for each height. The samples were packed into silage bags, evacuated and sealed using a vacuum machine, and then subjected to natural fermentation at room temperature for 60 days. After fermentation, the conventional nutritional components and volatile fatty acid (VFA) contents of the silage samples were measured to determine the optimal harvesting height and evaluate the impact of different heights on cellulose degradation.
Furthermore, Juncao at a height of 2.5–3.0 m (Tall), which possessed the highest fiber content, was harvested and assigned to two treatment groups: the control group (T1), with no additives, and the microbial group (T2), with the addition of 0.002‰ (w/v) microbial inoculant. Each treatment was performed in triplicate to evaluate the effects of the silage additive on the silage quality and fiber content of Juncao.
By comparing the 25 samples collected from five regions in Southwest China and their respective field processing methods, the effects of varying moisture levels, carbon sources, and air-drying degrees on silage quality and cellulose degradation were investigated. The nutritional compositions of
Juncao at different growth heights used in this study are summarized in
Table 2.
2.4. Determination of Indices and Methods
2.4.1. Sensory Quality Evaluation
The sensory quality of the silage was evaluated based on the sensory evaluation methods and grading standards established by the German Agricultural Society (Deutsche Landwirtschafts-Gesellschaft, DLG) [
13]. The evaluation focused on three primary parameters: odor (smell), color, and texture (structure) to determine the overall quality grade of the silage mixtures.
Table 4.
Sensory Evaluation Standards for Silage Quality.
Table 4.
Sensory Evaluation Standards for Silage Quality.
| Sensory indicators |
Scoring Criteria |
Score |
| odor |
It has a strong butyric or ammonia odor, or almost no sour taste.
It has a strong butyric acid taste, or a pungent, burnt, or musty smell.
It has a weak butyric acid taste, or a strong sour taste and a weak aromatic taste.
It has a strong or distinct aroma of bread, without any butyric acid odor. |
2
4
10
14 |
| Structure |
Stem and leaf rot or severe pollution
The stem and leaf structure is visibly damaged, or there is mild contamination.
Slight damage to stem and leaf structure
The stem and leaf structure is intact and clearly visible. |
01
2
4 |
| Color |
Severe discoloration, turning dark green or brown.
Slight discoloration, turning light yellow or yellowish-green.
It closely resembles the color of the raw material, turning light brown after drying. |
01
2 |
| Total Score |
16-20 |
10-15 |
5-9 |
0-4 |
| grade |
Level 1 Excellent |
Level 2 is acceptable |
Level 3 Intermediate |
Level 4 corruption |
2.4.2. Determination of Nutrient Content
The nutrient contents were determined according to the following standards: Dry Matter (DM) was measured following Determination of moisture in feeds (GB/T 6435-2014); Crude Protein (CP) followed Determination of crude protein in feeds (GB/T 6432-2018); Ether Extract (EE) followed Determination of crude fat in feeds (GB/T 6433-2006); Neutral Detergent Fiber (NDF) followed Determination of neutral detergent fiber in feeds (GB/T 20806-2022); Acid Detergent Fiber (ADF) followed Determination of acid detergent fiber in feeds (NY/T 1459-2022); and Crude Fiber (CF) followed Determination of crude fiber in feeds (GB/T 6434-2022).
The concentrations of Acid Detergent Lignin (ADL), EE, Water-Soluble Carbohydrates (WSC), Ammonia Nitrogen (NH3-N), and Total Nitrogen (TN) were analyzed using Near-Infrared Spectroscopy (NIRS) based on the CVAS (Cumberland Valley Analytical Services) detection and data analysis model. The Relative Feed Value (RFV) was calculated based on the NDF and ADF contents using the following formula:
RFV=(88.9-0.779×ADF)×(120/NDF)/1.29
2.4.3. Determination of Fermentation Quality
The pH of the silage extract was measured using a portable pH meter (Model: PH-30, Shanghai Yueping Scientific Instrument Co., Ltd.). The ammonia nitrogen (NH3-N) concentration was determined using the phenol-hypochlorite colorimetric method (also known as the indophenol blue method). The concentrations of volatile fatty acids (VFAs), specifically acetic acid, propionic acid, and butyric acid, along with lactic acid, were quantified using gas chromatography (GC).
2.4.4. Gray Relational Analysis Method for Evaluating Nutritional Value
The nutritional evaluation of elephant grass silage was performed using the Grey Relational Analysis (GRA) method; the specific formula can be found in the references.
2.4.5. Statistical Analysis
The experimental data were initially organized using Excel 2016 and subsequently subjected to statistical analysis using SPSS 26.0. One-way analysis of variance (ANOVA) was performed, followed by Duncan’s multiple range test for mean comparisons. Statistical significance was defined at P < 0.05, and extreme significance was defined at P < 0.01, while P > 0.05 indicated no significant difference. Correlation analysis and data visualization were conducted using GraphPad Prism and R software.
3. Results and Analysis
3.1. Effects of Growth Height on Ensiling Quality and Cellulose Degradation of Juncao
As shown in Table 4, the contents of ADF, CF, and ADL in Juncao silage increased significantly (P < 0.01) as the growth height increased. Conversely, the CP, EE, and TDN contents, as well as the pH values, decreased significantly (P < 0.01). Regarding the RFV index, the Low group was significantly higher than both the Mid and High groups (P < 0.01). The Starch content in the Mid group was significantly higher than that in the Low and High groups (P < 0.01), while the DM content in the High group was significantly higher than the other two groups (P < 0.01). No significant differences were observed among the groups for the remaining indicators (P > 0.05).
Table 4.
Effects of different growth height treatments on the nutritional value and silage quality of Cenchrus fungigraminus.
Table 4.
Effects of different growth height treatments on the nutritional value and silage quality of Cenchrus fungigraminus.
| Item |
Group |
SEM |
P-vaule
|
| Low |
Mid |
High |
| DM |
12.90Aa
|
12.65Aa
|
14.98Bb
|
0.34 |
<0.01 |
| CP |
13.93Cc
|
10.18Bb
|
7.85Aa
|
0.76 |
<0.01 |
| ADF |
40.76Aa
|
43.40Bb
|
45.40Cc
|
0.64 |
<0.01 |
| NDF |
62.98 |
64.33 |
65.25 |
0.42 |
0.67 |
| CF |
36.75Aa
|
39.40Bb
|
40.83Cc
|
0.56 |
<0.01 |
| ADL |
3.50Aa
|
3.95Bb
|
5.01Cc
|
0.20 |
<0.01 |
| Starch |
0.40Aa
|
0.90Bb
|
0.40Aa
|
0.09 |
0.02 |
| EE |
3.89Cc
|
2.83Bb
|
2.22Aa
|
0.21 |
<0.01 |
| TDN |
59.58Cc
|
57.58Bb
|
55.88Aa
|
0.50 |
<0.01 |
| RFV |
84.50Bb
|
79.75Aa
|
76.00Aa
|
1.21 |
<0.01 |
| WSC |
1.64 |
1.50 |
1.23 |
0.10 |
0.27 |
| pH |
4.41Cc
|
4.00Bb
|
3.32Aa
|
0.14 |
<0.01 |
As illustrated in
Figure 2, the NH
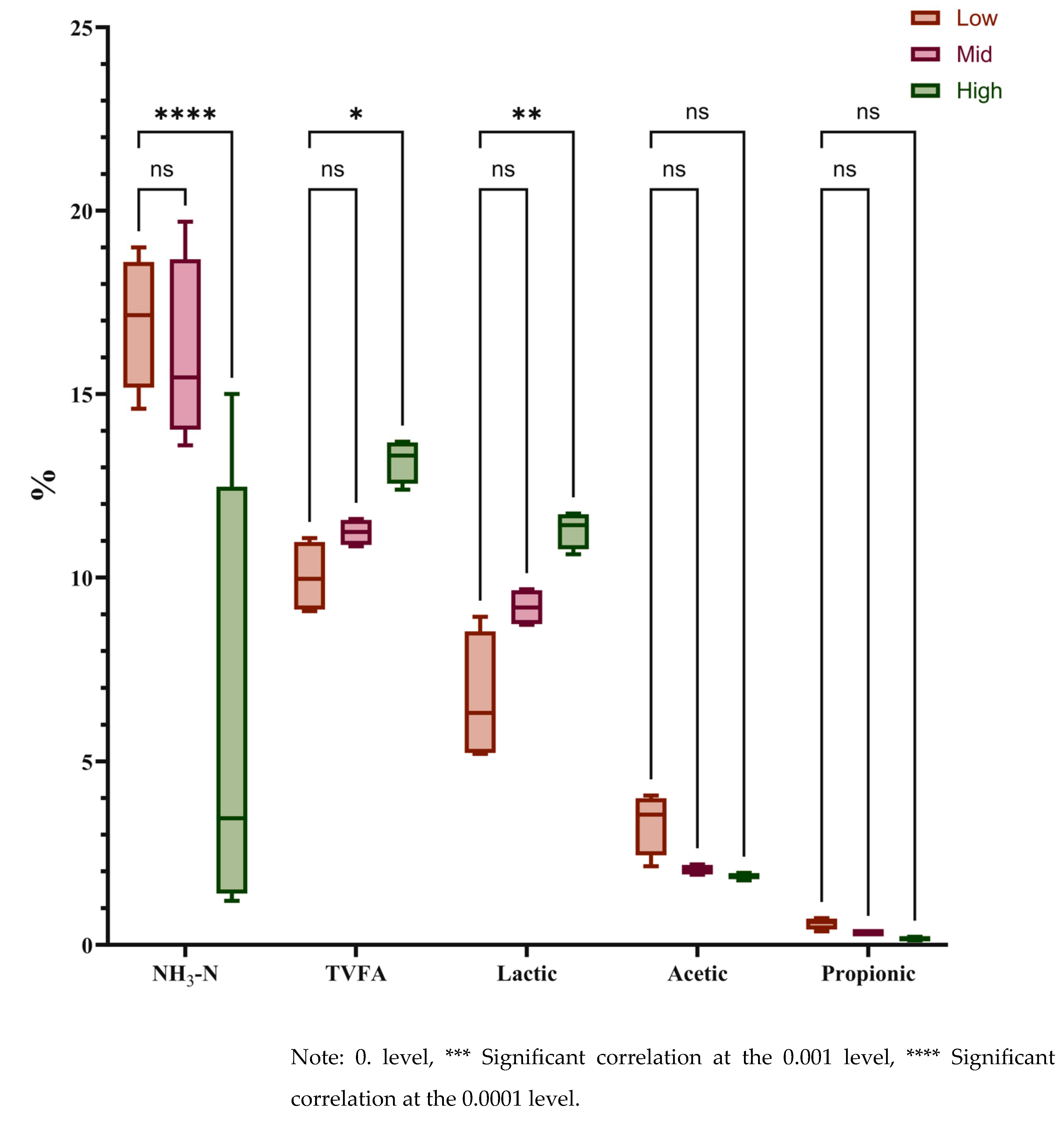
3-N ratio in the Low group was significantly higher than that in the High group (P < 0.01), while no significant difference was observed between the Low and Mid groups (P > 0.05). The Lactic acid content in the High group was significantly higher than that in the Low group (P < 0.05), with no significant difference found between the Low and Mid groups (P > 0.05). Similarly, the TVFA (Total Volatile Fatty Acid) content in the High group was significantly higher than in the Low group (P < 0.05), whereas the difference between the Low and Mid groups was not significant (P > 0.05). No significant differences were detected among the three groups regarding Acetic acid and Propionic acid contents (P > 0.05).
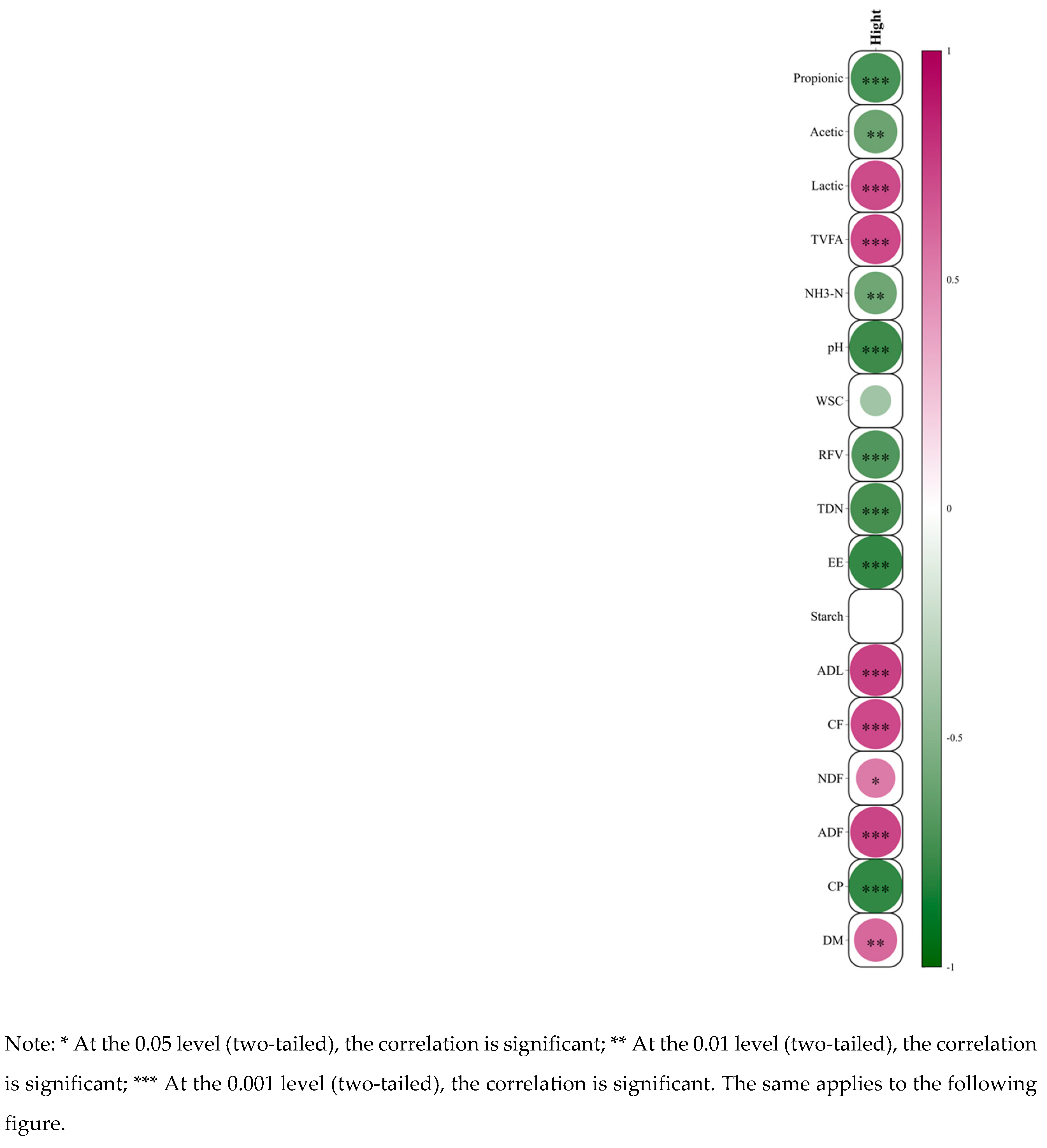
As shown in
Figure 3, the growth height of
Juncao exhibited an extremely significant positive correlation (P < 0.01) with DM, ADF, CF, ADL, TVFA, and Lactic acid content. A significant positive correlation (P < 0.05) was observed between growth height and NDF. Conversely, growth height was extremely significantly negatively correlated (P < 0.01) with CP, EE, TDN, RFV, pH, NH
3-N ratio, and the contents of Acetic acid and Propionic acid.
3.2. Effects of Silage Additives on Silage Quality and Cellulose Degradation of Juncao
As shown in
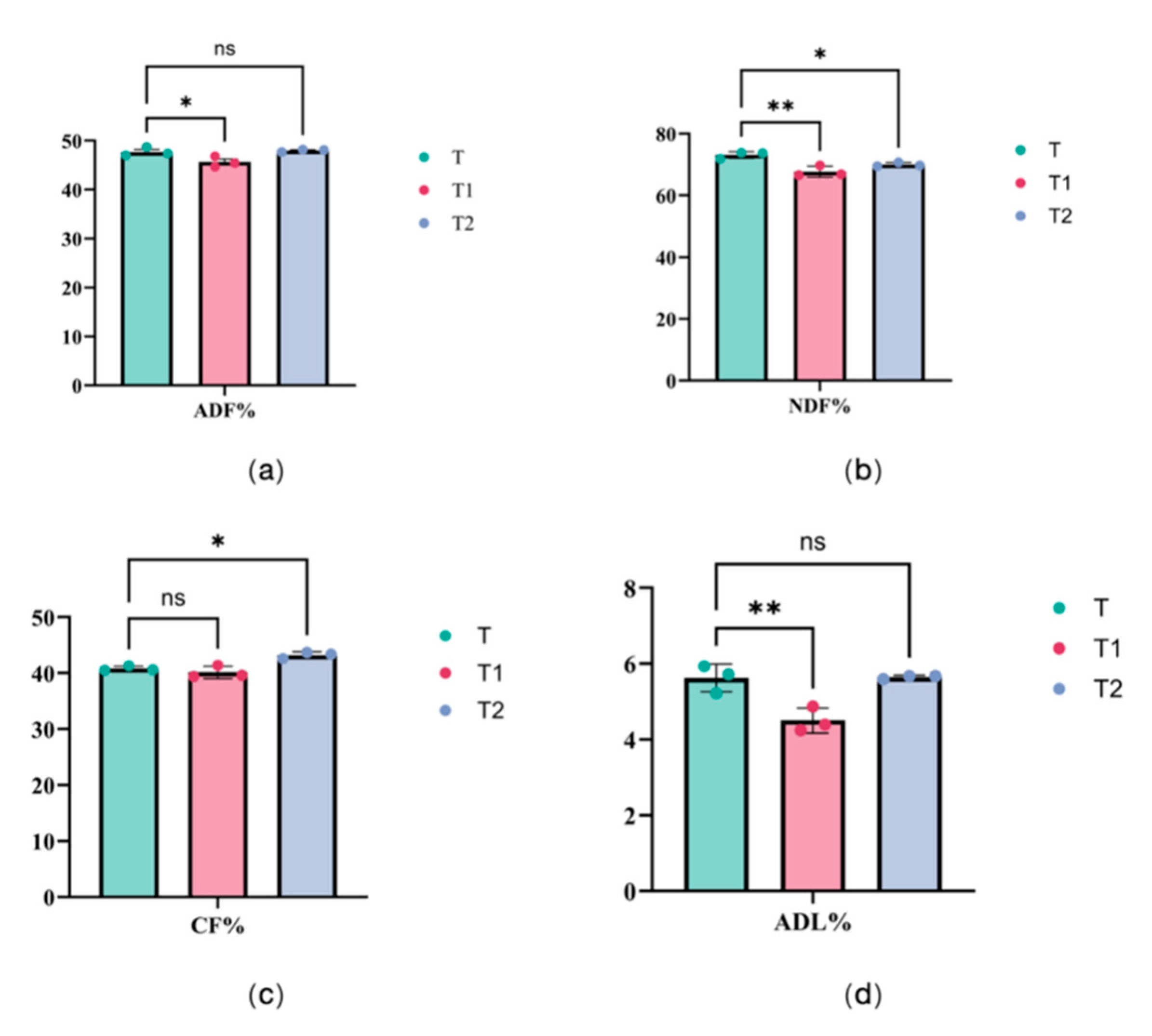
Table 5, the CP (Crude Protein) and EE (Ether Extract) contents in the T1 group were significantly higher than those in the T2 group (P < 0.05), and the TDN (Total Digestible Nutrients) index in the T1 group was extremely significantly higher than that in the T2 group (P < 0.01). Conversely, the EE, ADL (Lignin) contents, and WSC (Water-Soluble Carbohydrate) index in the T1 group were significantly lower than those in the T2 group (P < 0.05), while the pH value in the T1 group was extremely significantly lower than that in the T2 group (P < 0.01).
As illustrated in Figure 4, by comparing the cellulose degradation effects of the T1 and T2 groups against the fresh forage, it was observed that the ADF content in the natural fermentation group (T1) was significantly reduced (P < 0.05). Furthermore, the NDF and ADL contents in the T1 group decreased extremely significantly (P < 0.01). These results indicate that natural fermentation exhibits a superior cellulose degradation effect compared to the addition of silage inoculants.
Figure 3.
Effect of adding silage agents on the fiber content of Juncao.
Figure 3.
Effect of adding silage agents on the fiber content of Juncao.
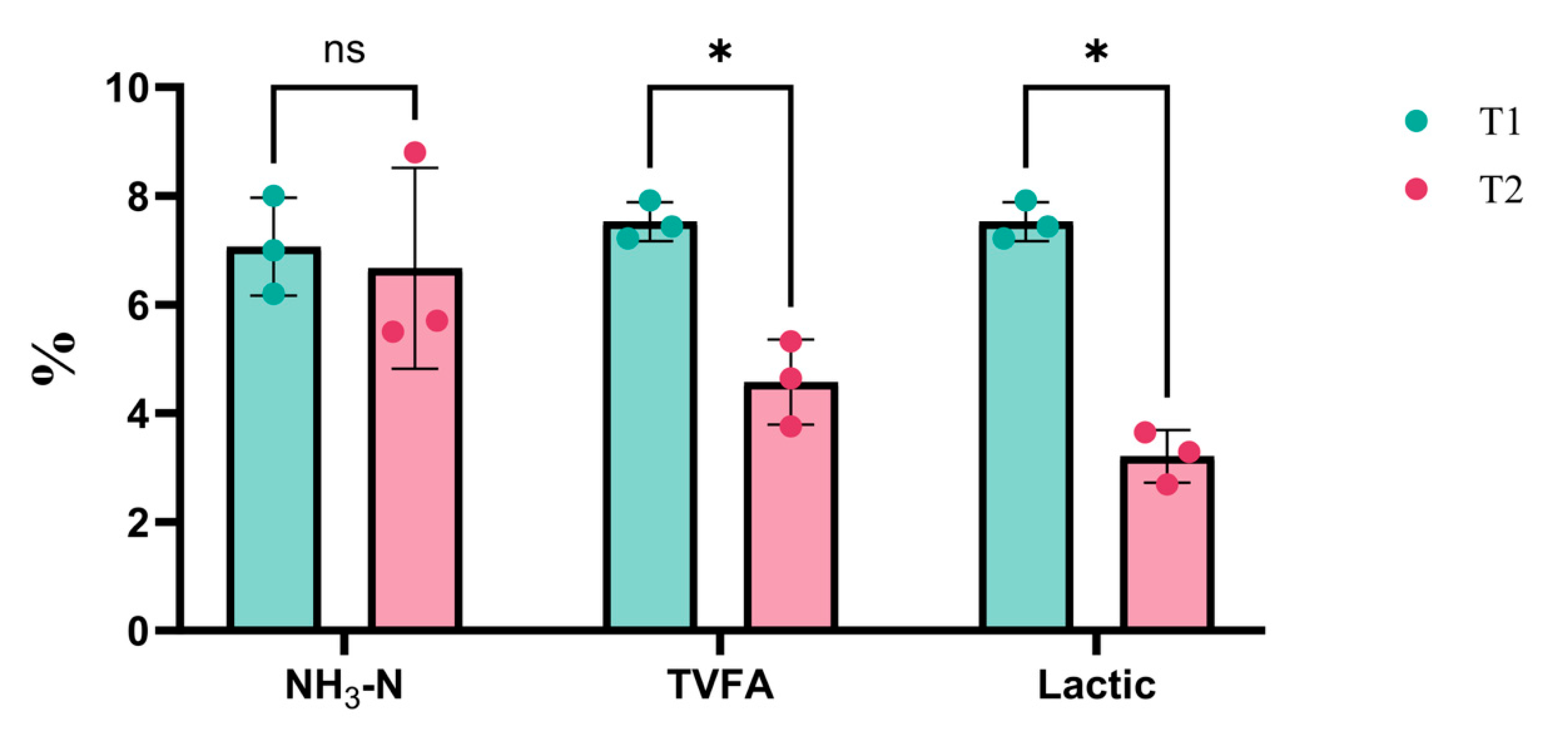
As illustrated in Figure 5, the concentrations of total volatile fatty acids (TVFA) and lactic acid in the T1 group were significantly higher than those in the T2 group (P < 0.05).
3.3. Impact of Microbial Inoculants on Silage Quality and Cellulose Degradation of Juncao
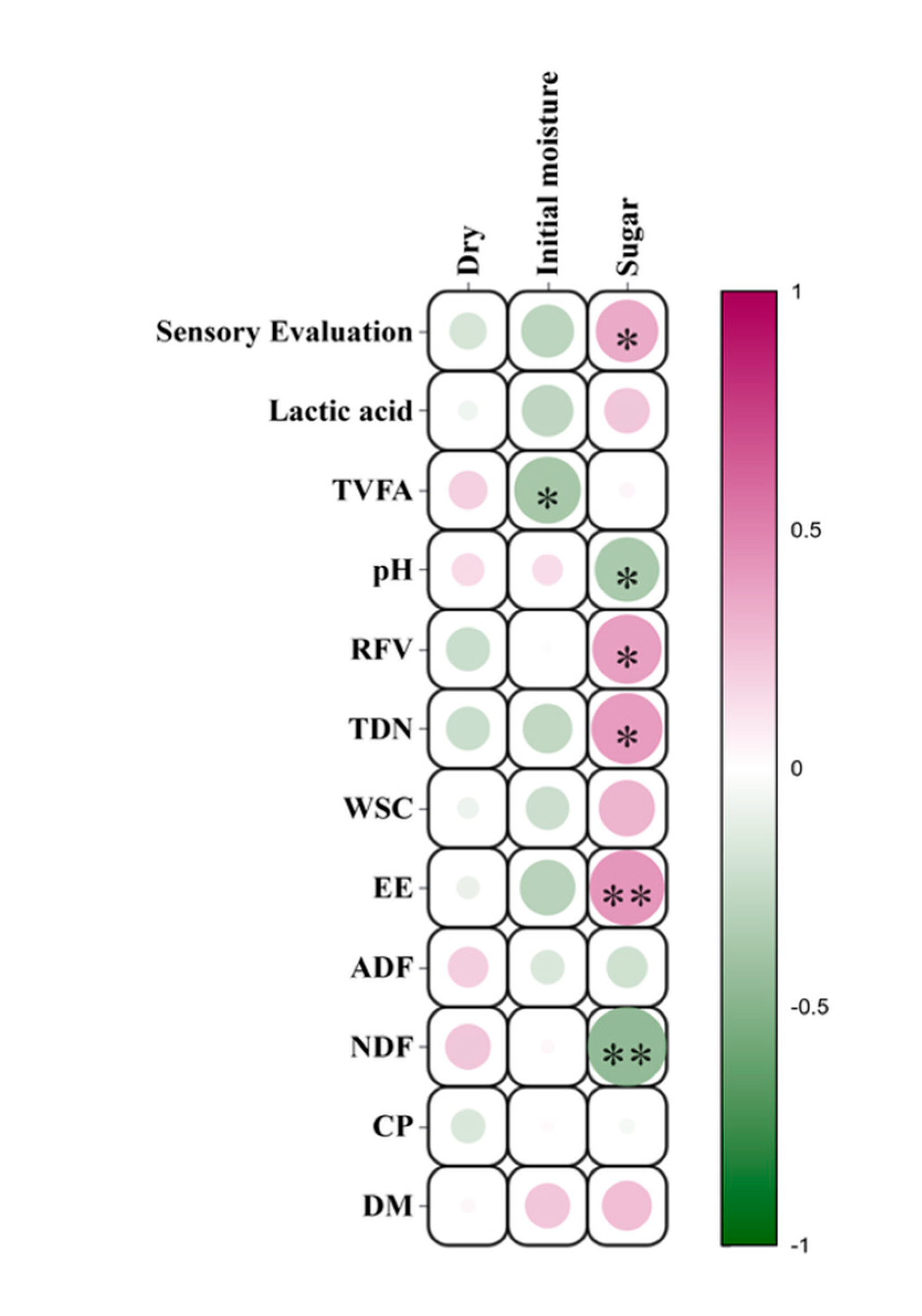
As shown in
Table 6 and
Figure 6, the moisture content of Juncao silage was significantly negatively correlated with total volatile fatty acids (TVFA). The addition of carbon sources exhibited a significant negative correlation with pH, while showing significant positive correlations with sensory indicators, RFV, and TDN. Furthermore, it was extremely significantly positively correlated with EE and extremely significantly negatively correlated with NDF.
In this study, a Grey Relational Analysis (GRA) was employed to conduct a comprehensive evaluation of 25 different Juncao silage treatments across five regions based on 12 indicators. According to
Table 7, the weighting order of the evaluation indicators was as follows: Sensory Evaluation > TDN > pH > TVFA > EE > NDF > CP > DM > ADF > Lactic Acid > RFV > WSC.
Based on both equal-weight and weighted relational degrees (
Table 7), the top ten treatments were identified as J2, J3, J6, X6, J5, J4, J1, X7, C4, and S1. The weighted relational degree analysis revealed several key findings:
Harvest Timing: The silage quality of the second ratoon (second crop) was superior to the first crop (e.g., Y2 > Y1, C4 > C2, and X6 > X1).
Additives: Treatments with added materials such as corn flour or rice bran outperformed those without additives (e.g., J2 [4% corn flour] > J1 [control]; J5 [10% rice bran] > J4 [control]).
Storage Method: The quality of bag silage was superior to pit silage (trench silage) (e.g., S1 > S2).
Moisture Regulation: Reducing moisture through wilting after chopping was detrimental to fermentation (e.g., X2 [wilted 24h] and X3 [wilted 48h] ranked low).
Inclusion of Dry Forage: While adding dry hay successfully reduced the moisture content of Juncao, it negatively impacted silage quality due to the poor nutritional value of the hay itself (e.g., X4, X5, X9, and X10 ranked between 17th and 24th, whereas the corresponding groups without hay, X1 and X6, ranked 13th and 3rd, respectively).
4. Discussion
The pH value serves as a primary visual and biochemical indicator for evaluating silage fermentation quality. In this study, the pH of Juncao silage decreased as the harvesting height increased. Furthermore, during the fermentation process, the pH of the natural fermentation group was significantly lower than that of the group with silage additives. These findings suggest that harvesting Juncao at a height of 1.5–2.5 m and utilizing natural fermentation allows the pH to stabilize at an optimal level (approximately 4.0).
Current research generally identifies lactic acid as the predominant factor influencing silage pH [
14], which is consistent with the lactic acid results observed in this study (
Figure 2, Figure 5). This indicates that higher levels of lactic acid are produced during the ensiling of
Juncao at the 1.5–2.5 m height interval and under natural fermentation conditions. The low pH environment created by lactic acid stabilizes fermentation by inhibiting acid-intolerant microorganisms [
15].
Regarding the effect of harvest height on pH, previous studies on buffalograss silage reported that higher harvest heights resulted in significantly higher pH values compared to lower heights; however, similar to our findings, pH showed a linear decrease as height increased [
16]. This phenomenon could be attributed to the linear increase in dry matter (DM) content with crop height. In our experiment, the starch content of
Juncao at 1.5–2.0 m was significantly higher than in the other two groups. Higher carbohydrate content typically increases microbial abundance in silage, allowing lactic acid bacteria (LAB) to utilize water-soluble carbohydrates (WSC) to produce substantial amounts of lactic acid [
17], thereby promoting a rapid decline in pH [
18].
Ammonia nitrogen (NH
3-N) is considered the most reliable variable for detecting the fermentation quality of silage. Our results demonstrated that the NH
3-N concentration in 2.0–2.5 m
Juncao silage was extremely significantly lower than in the other two groups (
Figure 2), while no significant difference was observed between natural fermentation and the addition of silage additives (Figure 5). A lower proportion of NH
3-N indicates reduced protein degradation, which enhances the apparent digestibility and overall quality of the silage [
19].
Silage fermentation is generally divided into six distinct stages: aerobic phase, acetic acid fermentation, initiation of lactic acid fermentation, completion of lactic acid fermentation, storage, and feeding [
20]. Existing research suggests that acetic acid concentrations are typically higher in low-dry-matter (DM) silage [
21], where acetic acid serves as a crucial precursor for milk fat synthesis in ruminants [
22]. However, our findings diverge from this pattern. In this study, although DM content increased with harvesting height, correlation analysis indicated a significant negative correlation between acetic acid content and height. This discrepancy may be attributed to the specific types of lactic acid bacteria (LAB) present.
Mainstream theory classifies LAB into homofermentative and heterofermentative strains [
23]. Certain heterofermentative LAB can degrade lactic acid into acetic acid and propionic acid [
24]. In our results, the trends for propionic and acetic acids were similar, while the lactic acid trend was the opposite. This suggests that during
Juncao ensiling, the present LAB did not degrade lactic acid into other organic acids but instead facilitated lactic acid accumulation, further depressing the pH.
Furthermore, while adding inoculants is generally expected to increase protein content and minimize nutrient loss [
25], the protein content in our inoculant-treated group was significantly lower than that of the natural fermentation group. This might be due to the abundance of epiphytic (endogenous) bacteria naturally present on
Juncao, which may have exhibited an antagonistic effect when supplemented with exogenous LAB strains.
Regarding fiber degradation, natural fermentation significantly reduced the contents of NDF, ADF, and ADL, whereas the addition of silage inoculants showed no significant impact. This contradicts several mainstream studies where additives are typically used to degrade cellulose. For instance, Dong [
26] found that adding
Lactobacillus plantarum and cellulase significantly reduced fiber content in king grass silage. Similarly, Silva [
27] reported a linear decrease in NDF with increasing cellulase levels, achieving the lowest ADF at a 4.5% inclusion rate. The inverse results observed in our study may be explained by the higher lactic acid levels in the natural fermentation group; the sustained low pH likely facilitated the acid hydrolysis of the plant cell walls, leading to the observed reduction in NDF and ADF values.
In this study, silage samples collected from local farmers and herdsmen were also evaluated to assess the impact of various fermentation conditions on the quality of
Juncao silage. The results indicated that initial moisture content was significantly negatively correlated with total volatile fatty acids (TVFA). This could be attributed to the fact that higher moisture levels often lead to contamination in silage, which subsequently reduces the concentration of volatile fatty acids [
28].
Furthermore, the addition of exogenous carbon sources showed a significant positive correlation with sensory quality and nutritional components. Current research generally maintains that incorporating exogenous carbon sources—such as molasses or brown sugar—during the ensiling process can accelerate fermentation and enhance silage quality [
29]. These additives not only provide sufficient substrates for the rapid accumulation of lactic acid and a sharp decline in pH but also promote the synthesis of microbial proteins, thereby improving the overall nutritional profile [
30]. These findings are consistent with our results, demonstrating that the inclusion of appropriate exogenous carbon sources can effectively improve the fermentation quality of
Juncao silage.
Although this study collected and analyzed multiple samples from various regions in Southwest China, certain limitations remain. The selection of silage inoculants was not exhaustive; instead, a mainstream commercial inoculant was utilized. Given that Juncao is a tropical plant rich in endogenous microbiota, future research should prioritize comprehensive microbial profiling and strain identification to develop specialized inoculants tailored to this species. Nevertheless, this study indirectly demonstrates that compared to conventional commercial additives, natural fermentation of Juncao can achieve excellent results. This finding not only suggests a potential reduction in input and labor costs but also provides a theoretical foundation and new perspectives for subsequent research on high-quality Juncao silage production.
In subsequent studies, our team will conduct in vivo animal trials to validate the fermentation effects observed in this experiment. These trials aim to provide a rigorous scientific basis for the utilization of Juncao silage in ruminant diets. By further refining the feed processing and utilization techniques, we seek to advance the strategy of “replacing grain with forage” in livestock production, ultimately offering scientific support for sustainable animal husbandry.
5. Conclusions
Based on the comparative study of the fermentation effects of Juncao silage at different growth heights and under various treatments, this research demonstrates that harvesting Juncao at a height of 2.0–2.5 m, combined with natural fermentation and the addition of exogenous carbon sources, yields high-quality silage. This approach effectively maximizes the reduction of fiber content in Juncao silage. However, some limitations remain in the current study. Future research should systematically evaluate the efficacy of various microbial inoculants across different growth heights to further optimize the fermentation process and identify the most suitable silage additives for Juncao.
Author: Contributions:Conceptualization, H.H.; data curation, H.H., Z.W., and T.L.; formal analysis, H.H., Z.W., T.L., F.H. and B.M.; funding acquisi-tion, J.D., M.Z., Z.L., and D.L.; investigation, H.H., J.D., M.Z., P.H., and J.L.; methodology, H.H., Z.W., S.S., D.L., and T.L.; project administration, H.H., J.D., M.Z., P.H., and D.L.; resources, H.H., and D.L.; software, H.H., Z.W., T.L. F.H. and B.M.; supervision, J.D., and Z.L.; validation, H.H., Z.W., and T.L.; visualization, Z.W., and H.H.; writing-original draft, H.H., Z.W., and T.L.; writing-review and editing, D.L., and T.L. All authors have read and agreed to the published version of the manuscript.
Funding
This study was financially supported by National Key Research and Development Program Project “Key Technologies for High-Efficiency Cultivation of Edible and Medicinal Fungi and Feed Conversion Using Juncao” (2023YFD1600502) and 2022 - 2024 Central Agricultural Production Development Fund “Yunnan Province JUJUNCAO Planting Experiment, Demonstration and Promotion Project”.
Institutional Review Board Statement
This experiment was approved by the Yunnan Forage and Feed Workstation Ethics Committee: Number: 2022001.
Informed Consent Statement
Informed consent was obtained from all subjects involved in the study.
Data Availability Statement
The original contributions presented in this study are included in the article. Further inquiries can be directed to the corresponding author(s).
Conflicts: of Interest:The authors declare no competing financial interests or personal relationships that could have influenced the work reported in this paper.
References
- Ineichen SM, Zumwald J, Reidy B, Nemecek T. Feed-food and land use competition of lowland and mountain dairy cow farms. animal. 2023;17(12):101028. [CrossRef]
- Triveni B, Rao K, Teja A, RaviKumar M, Singh T. Hybrid napier grass a potential asset for livestock production. 2022;11:4081-4083.
- Dong-sheng Y, Yu-chi NIU, Yu-fei Y, Xin-lei J, Shui-yuan H a. O. Effect of nutrient quality in different parts, height treatments, and silage time of Cenchrus fungigraminus in Hetao area. Feed Res. 2024;47(17):128. [CrossRef]
- Coblentz WK, Akins MS. Silage review: Recent advances and future technologies for baled silages. J Dairy Sci. 2018;101(5):4075-4092. [CrossRef]
- Muck RE, Kung Jr. L, Collins M. Silage Production. In: Forages. John Wiley & Sons, Ltd; 2020:767-787. [CrossRef]
- Cardoso FF, Kemp SE, Schmidt RJ, Cardoso FC. Effects of cut height and inoculant application on yield, nutrient composition, fermentative profile, and in vitro degradability of brown midrib whole-plant corn silage. Appl Anim Sci. 2023;39(3):117-124. [CrossRef]
- Tarekegn A, Nurfeta A, Bayssa M. Height at harvest and additives influence desho grass (Pennisetum glaucifolium) silage fermentation quality, animal preference and nutritional value.
- Kim D, Lee K, Choi K. Role of LAB in Silage Fermentation: Effect on Nutritional Quality and Organic Acid Production—An Overview. Cent Res Environ Dis Fac Publ. Published online January 6, 2021. [CrossRef]
- Gonda H, Nikodinoska I, Le Cocq K, Moran CA. Efficacy of six lactic acid bacteria strains as silage inoculants in forages with different dry matter and water-soluble carbohydrate content. Grass Forage Sci. 2023;78(4):636-647. [CrossRef]
- Okoye CO, Wang Y, Gao L, et al. The performance of lactic acid bacteria in silage production: A review of modern biotechnology for silage improvement. Microbiol Res. 2023;266:127212. [CrossRef]
- Zhao Qingfeng, Wang Zeren, Li Yong, Hu Fuli, Han Lu, Zhu Pingjun. Effects of compound probiotics on the quality of fermented giant reed feed. Feed Res. 2022;45(22):116. [CrossRef]
- Wang Zeren, Li Yong, Zhao Qingfeng, Zhu Pingjun, Hu Fuli. Effects of fermented giant reed feed on growth performance, slaughter performance, meat quality and serum biochemical indicators in sheep. Feed Res. 2022;45(24):22. [CrossRef]
- Pauly T, Wyss U. Efficacy testing of silage additives—Methodology and existing schemes. Grass Forage Sci. 2019;74(2):201-210. [CrossRef]
- Kung L, Shaver RD, Grant RJ, Schmidt RJ. Silage review: Interpretation of chemical, microbial, and organoleptic components of silages. J Dairy Sci. 2018;101(5):4020-4033. [CrossRef]
- Muck: Effects of silage additives on ensiling - Google. Accessed December 12, 2025. https://scholar.google.com/scholar_lookup?title=Effect%20of%20silage%20additives%20on%20ensiling&publication_year=1997&author=R.E.%20Muck&author=L.%20Kung%20Jr.
- Pinho RMA, Santos EM, Carvalho GGP de, et al. Microbial and fermentation profiles, losses and chemical composition of silages of buffel grass harvested at different cutting heights. Rev Bras Zootec. 2013;42:850-856. [CrossRef]
- RE M. Inoculation of silage and its effects on silage quality. In: Informational Conference with Dairy and Forage Industries. US Dairy Forage Res Center; 1996:43-51. Accessed December 12, 2025. https://cir.nii.ac.jp/crid/1572824500598991488.
- Santos EM, Pereira OG, Garcia R, et al. Microbial populations, fermentative profile and chemical composition of signalgrass silages at different regrowth ages. Rev Bras Zootec. 2011;40:747-755. [CrossRef]
- Du E, Mao N, Liu S, et al. Effects of different wet distillers’ grains ratios on fermentation quality, nitrogen fractions and bacterial communities of total mixed ration silage. BMC Microbiol. 2025;25(1):31. [CrossRef]
- Ouwehand AC. Silage Fermentation. In: Lactic Acid Bacteria. 6th ed. CRC Press; 2024.
- Franco M, Rinne M. Dry Matter Content and Additives with Different Modes of Action Modify the Preservation Characteristics of Grass Silage. Fermentation. 2023;9(7):640. [CrossRef]
- Cui Y, Liu H, Gao Z, et al. Whole-plant corn silage improves rumen fermentation and growth performance of beef cattle by altering rumen microbiota. Appl Microbiol Biotechnol. 2022;106(11):4187-4198. [CrossRef]
- Leite GM, Santos EM, de Oliveira JS, et al. Isolation of Acetic Acid-Producing Bacterial Strains and Utilization as Microbial Inoculants in Sorghum Silages. Agriculture. 2025;15(3):241. [CrossRef]
- Zheng J, Wittouck S, Salvetti E, et al. A taxonomic note on the genus Lactobacillus: Description of 23 novel genera, emended description of the genus Lactobacillus Beijerinck 1901, and union of Lactobacillaceae and Leuconostocaceae. Int J Syst Evol Microbiol. 2020;70(4):2782-2858. [CrossRef]
- Oskoueian E, Jahromi MF, Jafari S, Shakeri M, Le HH, Ebrahimi M. Manipulation of Rice Straw Silage Fermentation with Different Types of Lactic Acid Bacteria Inoculant Affects Rumen Microbial Fermentation Characteristics and Methane Production. Vet Sci. 2021;8(6):100. [CrossRef]
- Dong D, Zhang L, Zhao J, Dong Z, Li J, Shao T. Synergistic Effects of Exogenous Lactobacillus plantarum and Fibrolytic Enzymes on Fermentation Quality, Fiber Degradation, and In Vitro Digestibility of Napiergrass (Pennisetum purpureum) Silage. Agronomy. 2025;15(2):340. [CrossRef]
- Lemos MF, Andrade AP, Silva PHF da, et al. Nutritional value, fermentation losses and aerobic stability of elephant grass (Pennisetum purpureum Schum.) silage treated with exogenous fibrolytic enzymes. Acta Sci Anim Sci. 2020;42:e48272. [CrossRef]
- Muhammad SA, Abdullahi S, Tinat PI, Taha M. CHEMICAL COMPOSITION, FERMENTATION CHARACTERISTICS AND ANTI-NUTRITIONAL CONTENT OF ENSILED MAIZE COB - SWEET POTATO VINE MIXTURE. Sci World J. 2025;20(3):1236-1240.
- Luo R, Zhang Y, Wang F, et al. Effects of Sugar Cane Molasses Addition on the Fermentation Quality, Microbial Community, and Tastes of Alfalfa Silage. Animals. 2021;11(2):355. [CrossRef]
- Application of condensed molasses fermentation solubles and lactic acid bacteria in corn silage production - Chen - 2020 - Journal of the Science of Food and Agriculture - Wiley Online Library. Accessed December 12, 2025. https://scijournals.onlinelibrary.wiley.com/doi/abs/10.1002/jsfa.10304.
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2026 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).