Submitted:
23 December 2025
Posted:
24 December 2025
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Materials and Methods
3. Results
3.1. Metal Complexes with Schiff Bases Derived from Salicylaldehyde
| Comp. | Ligand | Metal ion | IC50 MCF-7 | other tested cell lines | other tests | Ref. |
|---|---|---|---|---|---|---|
| 1 | 4(N,N)-diethylaminosalicylaldehyde-4(N)-thiosemicarbazone [H2-DEAsal-tsc] | Ni(II) | 5.37 ± 0.21 μM | A549 HeLa |
ctDNA, BSA | 38 |
| 2 | 4(N,N)-diethylaminosalicylaldehyde-4(N)-methylthiosemicarbazone [H2-DEAsal-mtsc] | 4.91 ± 0.18 μM | ||||
| 3 | 4(N,N)-diethylaminosalicylaldehyde-4(N)-ethylthiosemicarbazone [H2-DEAsal-etsc] | 4.66 ± 0.22 μM | ||||
| 4 | 4(N,N)diethylaminosalicylaldehyde-4(N)-phenylthiosemicarbazone [H2-DEAsal-ptsc] | 5.69 ± 0.17 μM | ||||
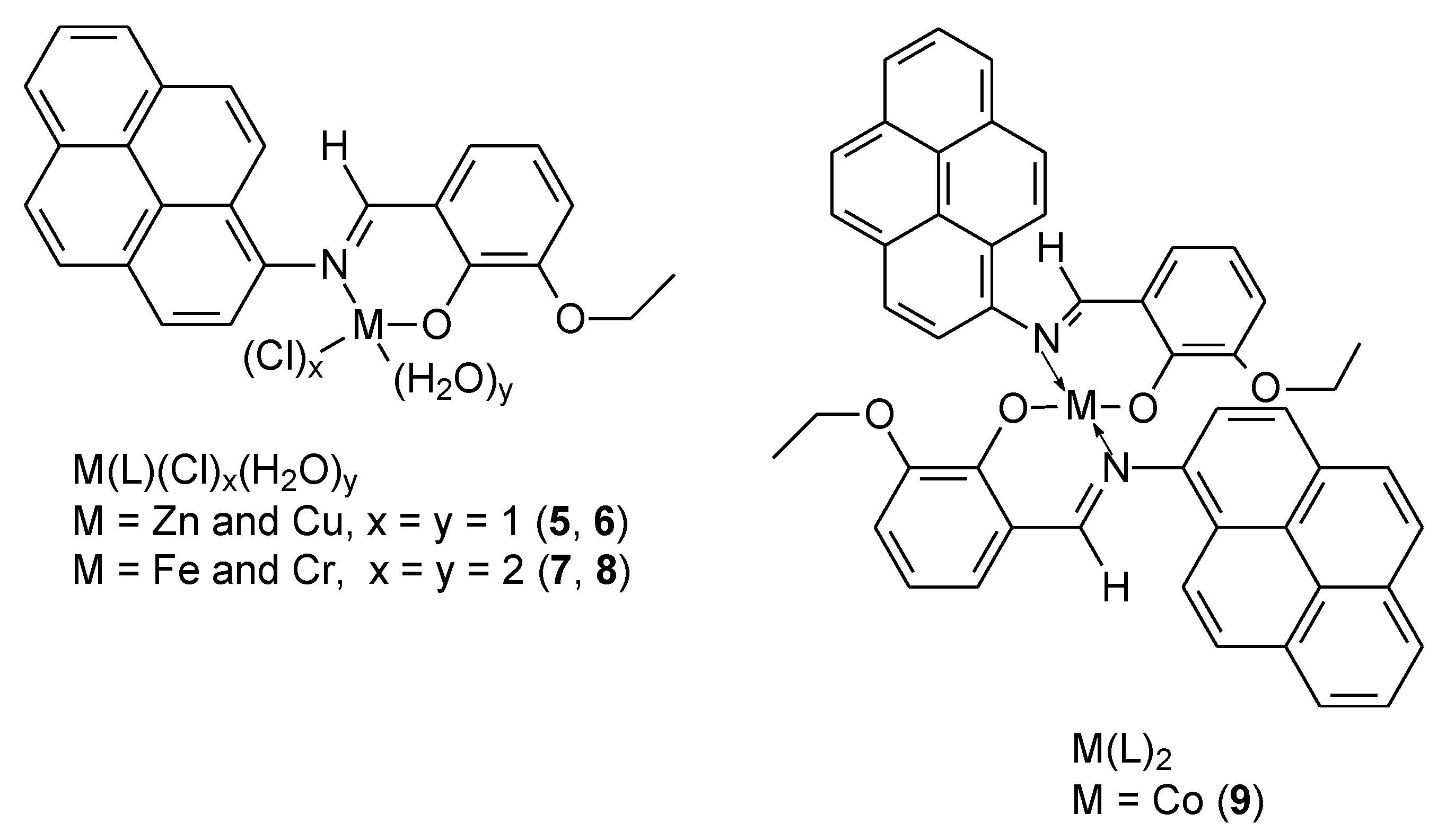
| 5 | ((E)-2-ethoxy-6((pyren-1-ylimino)methyl)phenol) | Zn(II) | 12.742 ± 0.73 μg/ml | 39 | ||
| 6 | Cu(II) | 5.661 ± 0.33 μg/ml | ||||
| 7 | Fe(III) | 58.708 ± 3.37 μg/ml | ||||
| 8 | Cr(III) | 16.895 ± 0.98 μg/ml | ||||
| 9 | Co(II) | 21.141 ± 1.21 μg/ml | ||||
| 10 | salicylidene carbohydrazide | Cu(II) | 2.22 ± 0.08 μM | MDA-MB-231 | ctDNA, ROS, MTT | 40 |
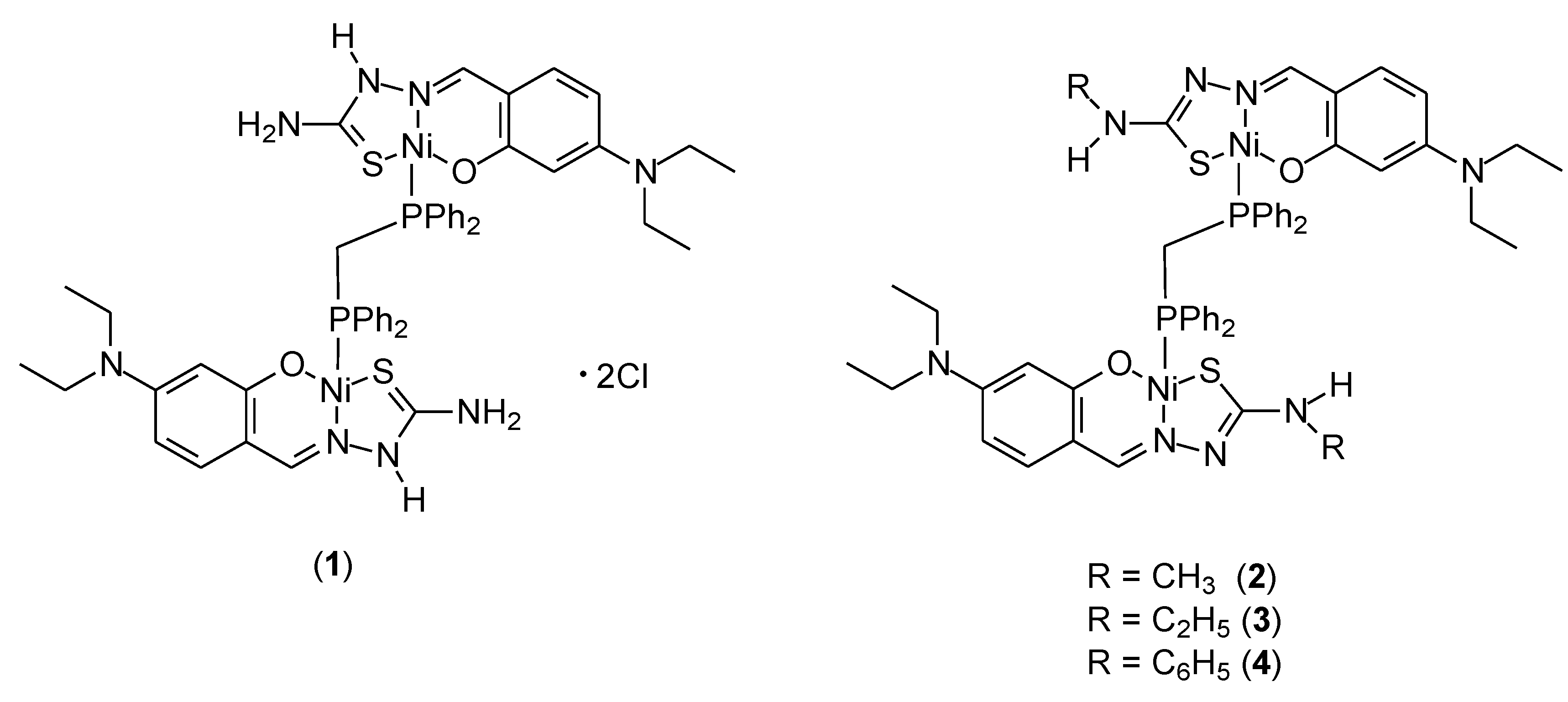
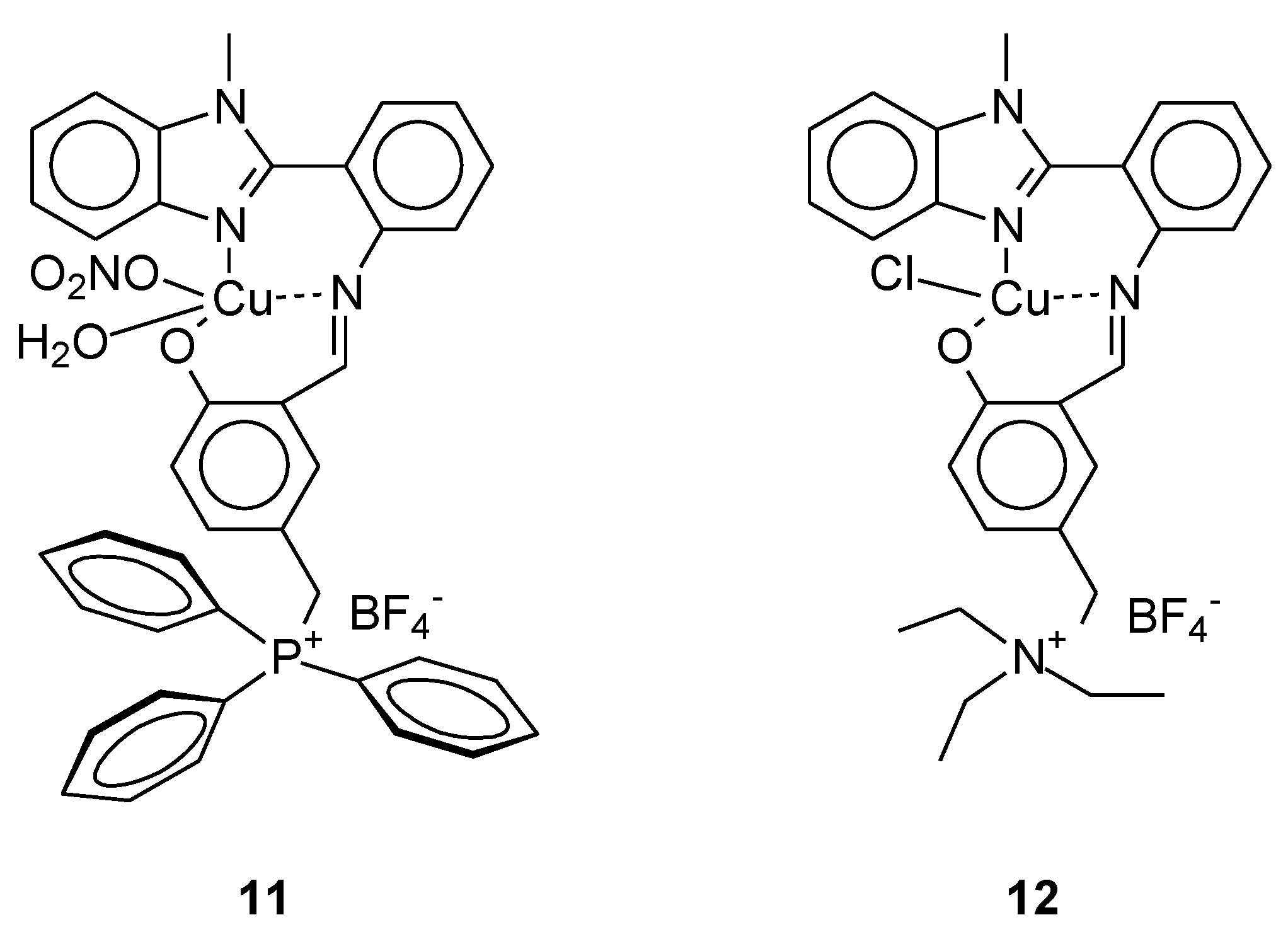
| 11 | 2-(1-methyl-1H-benzo[d]imidazol-2-yl)aniline with (3-formyl-4-hydroxybenzyl)triphenylphosphonium chloride | Cu(II) | 25.00 ± 1.17 μM | A-549 HeLa | ctDNA, ROS, AO/EB, MTT | 41 |
| 12 | 2-(1-methyl-1H-benzo[d]imidazol-2-yl)aniline with N,N-diethyl-N-(3-formyl-4-hydroxybenzyl)ethanaminium chloride | 80.12 ± 0.016 μM | HaCaT | Molecular docking with DNA | ||
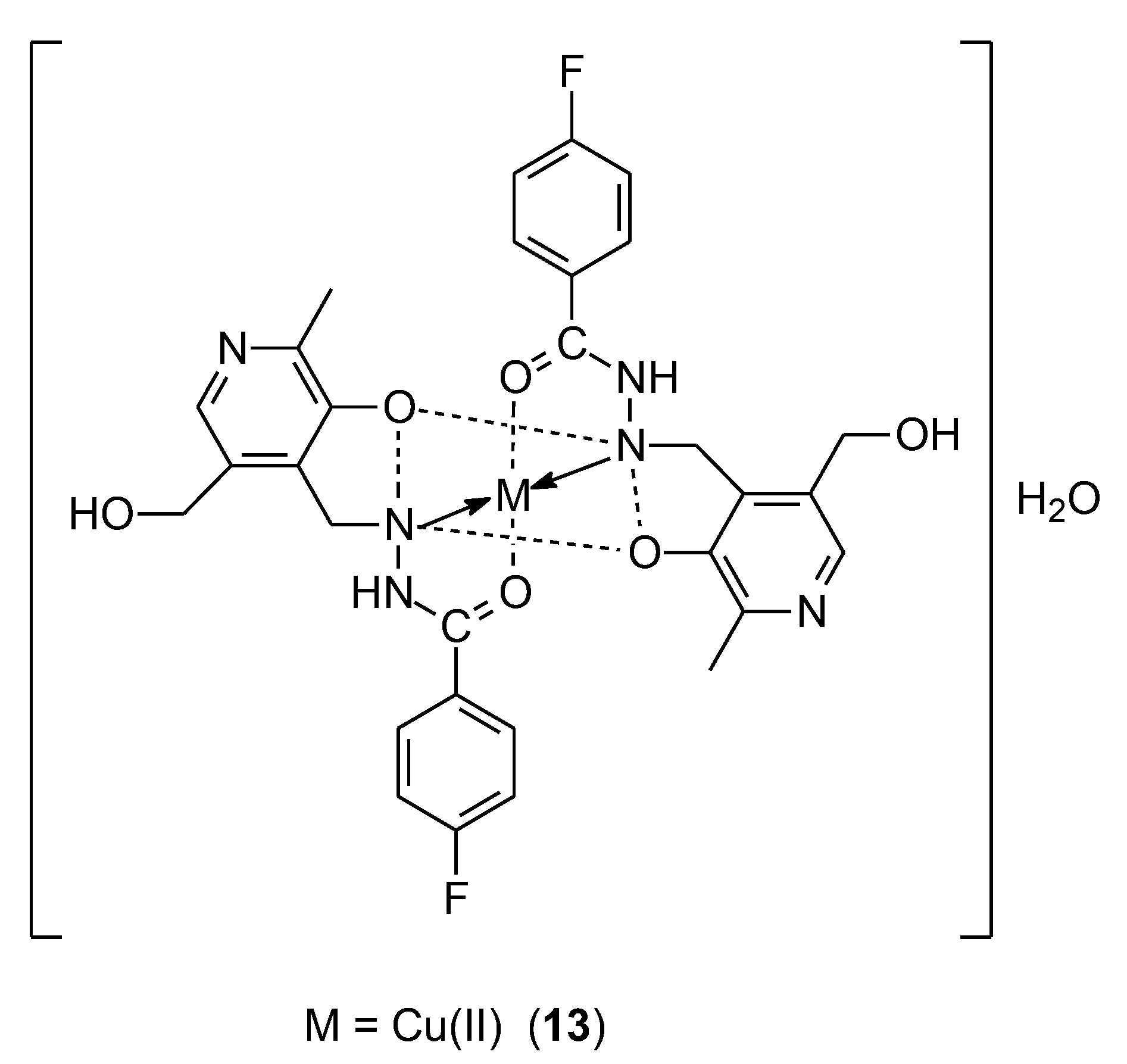
| 13 | 4-fluoro-N-((3-hydroxy-5-(hydroxymethyl)-2-methylpyridin-4-yl)methylene)benzohydrazide (PLFBH) | Cu(II) | 15.30 ± 0.55 μM | HelLa A549 | ctDNA | 42 |
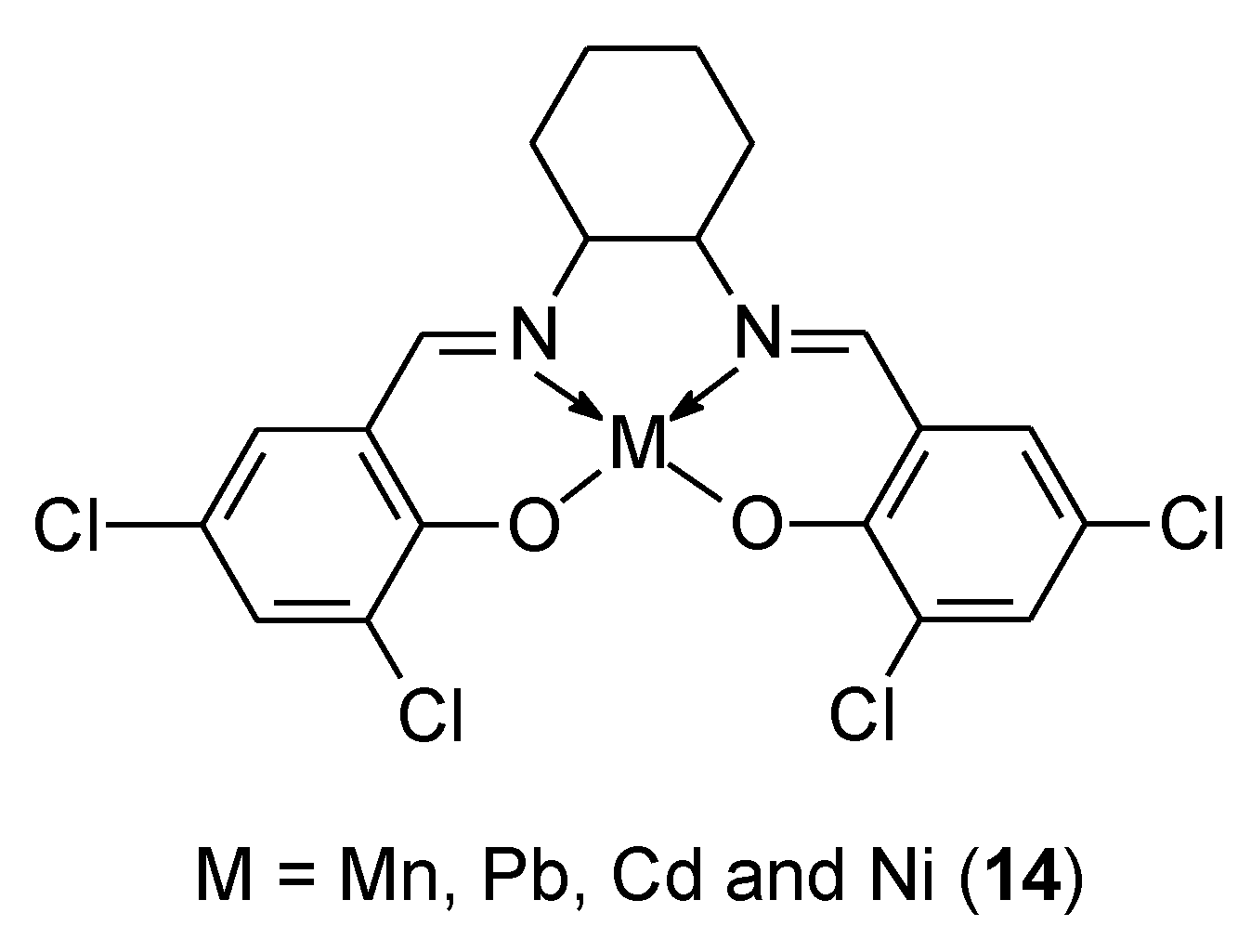
| 14 | 3,5-dichlorosalicylaldehyde and trans-1,2-diaminocyclohexane | Ni(II) | 108.1 µg/ml | DPPH Docking with BSA Docking DNA, MTT test PAINS | 43 | |
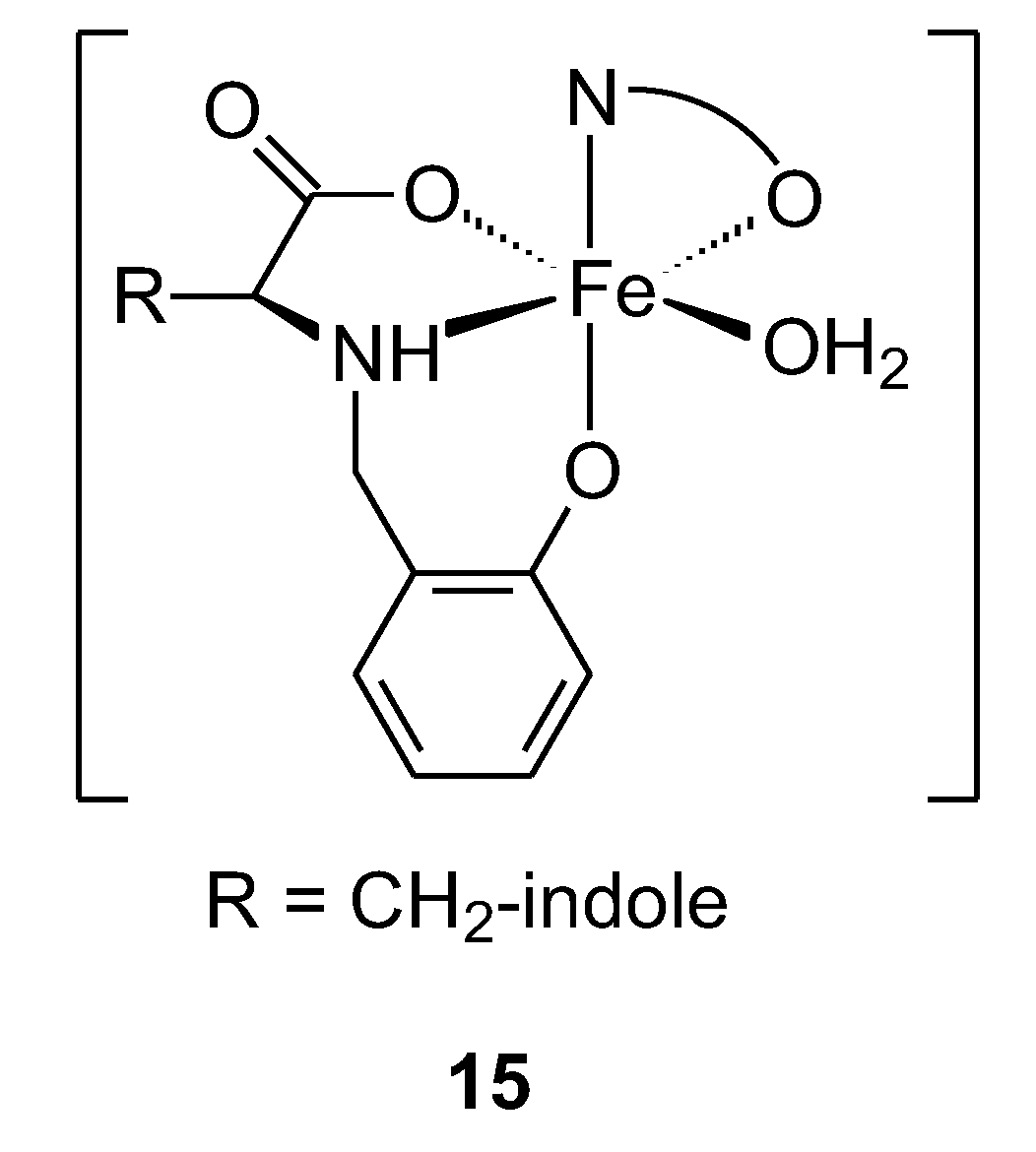
| 15 | N-salicylyl-L-tryptophan sodium salt | Fe(III) | 4.3 ± 0.2 μM | MG-63 HT-29 L929 | BSA, ctDNA, MTT test ROS | 44 |
| 16 | 10.7 ± 2.5 μM | |||||
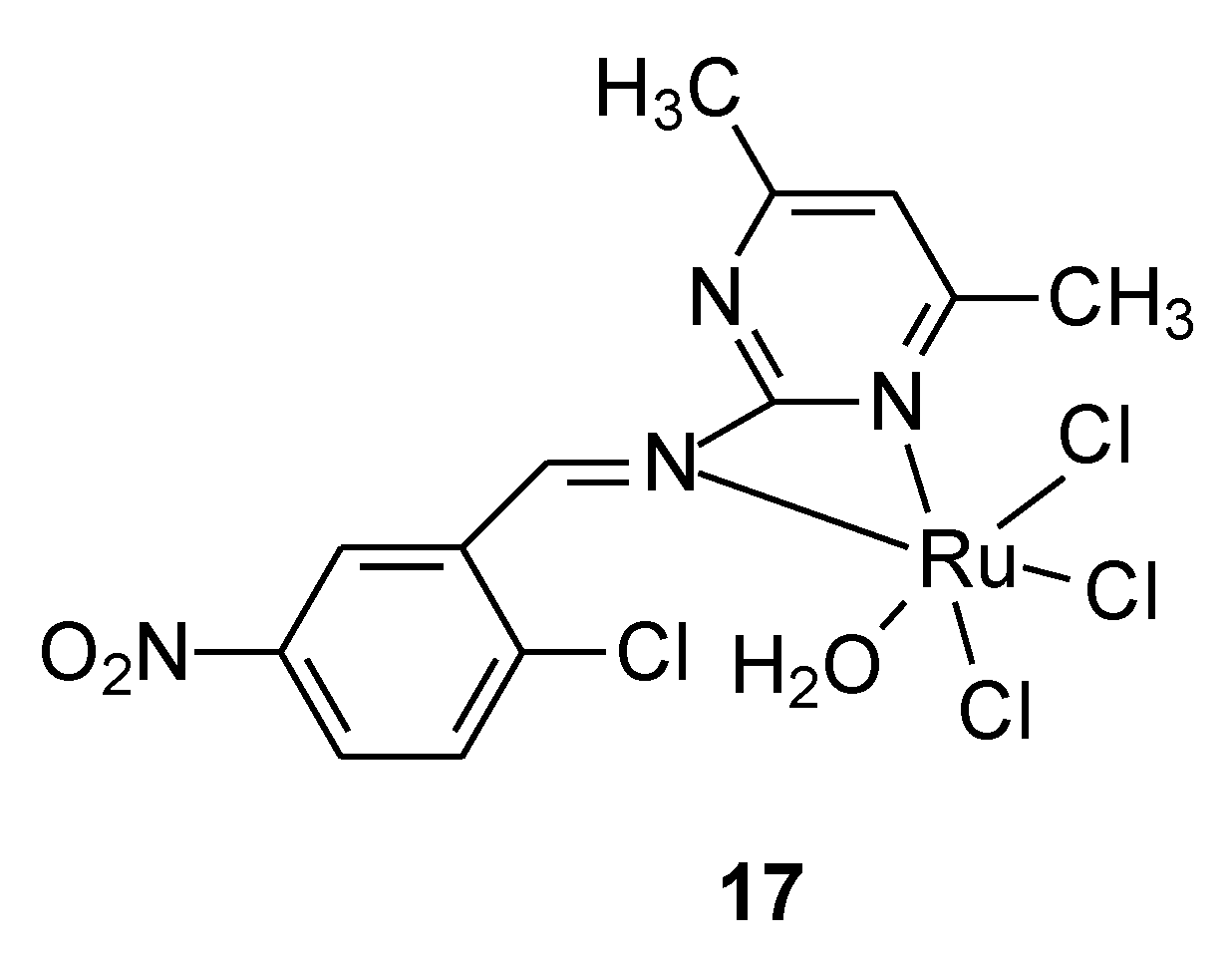
| 17 | 2-Chloro-5-Nitrophenyl-(4,6-Dimethylpyrimidinyl)methanimine Schiff Base | Ru(III) | 46.7 µM | T47D HCT116 HepG2 | Annexin V/Propidium Iodide Staining for Apoptosis Assessment Expression Levels of Caspase 3, VEGF-A, mTOR, NF-kB, and SND1 by RT-PCR | 45 |
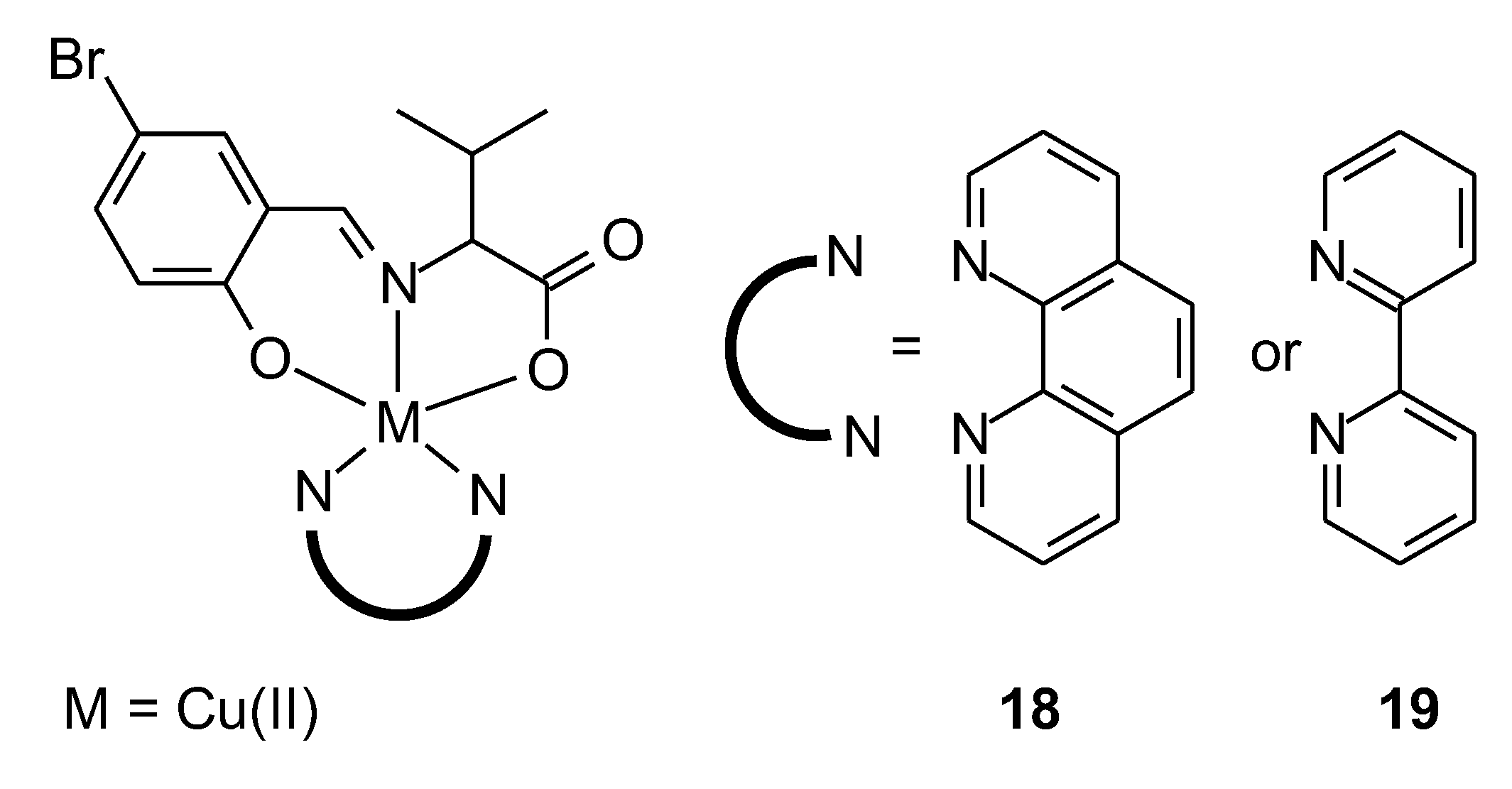
| 18 | potassium(E)-2-((5-bromo-2-hydroxybenzylidene)amino) 3-methylbutanoate | Cu(II) | 17.13 ± 0.74 μM | A549 HeLa | MTT test DPPH | 46 |
| 19 | 33.18 ± 1.14 μM | |||||
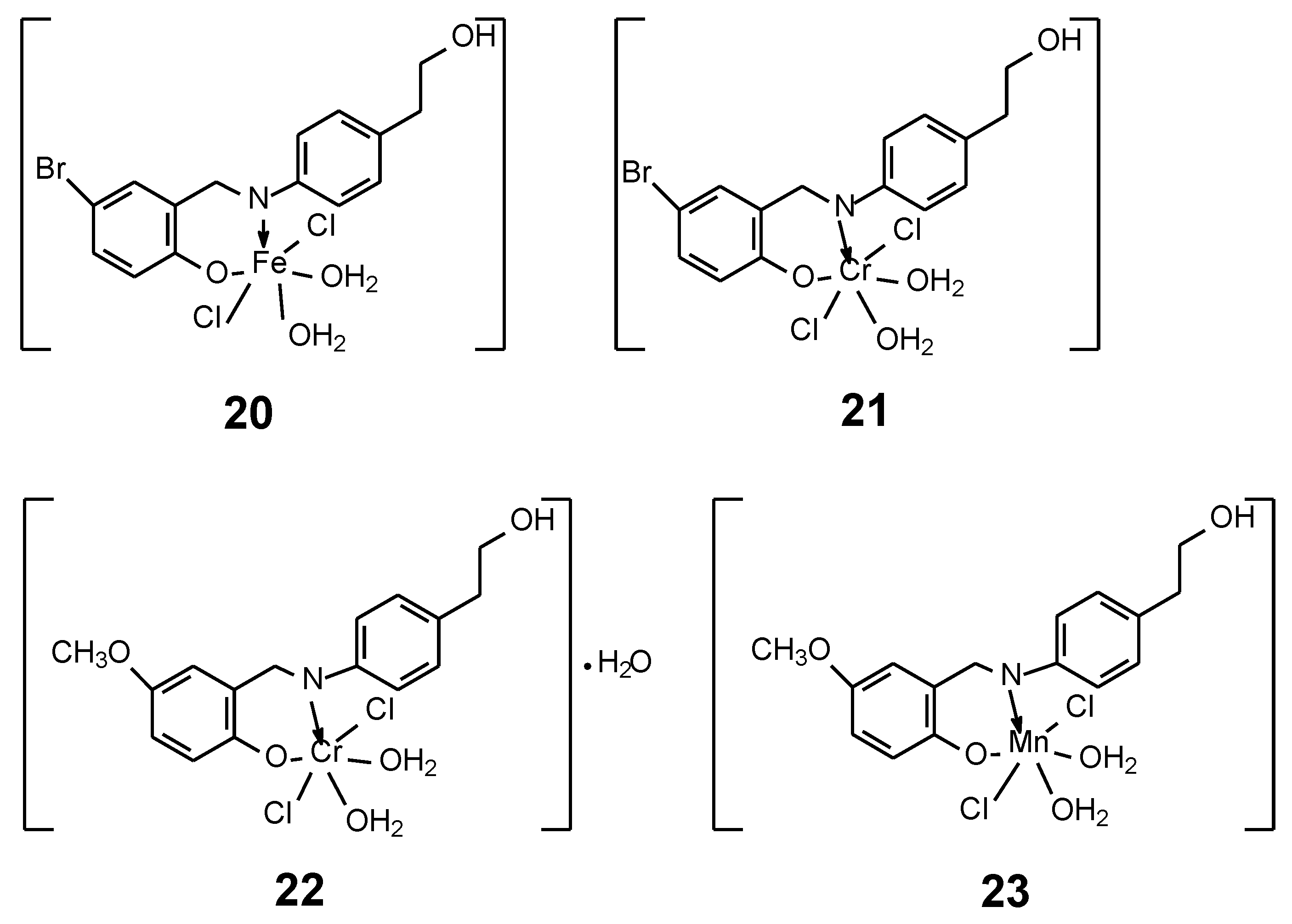
| 20 | 4-bromo-2-[(E)-{[4-(2-hydroxyethyl)phenyl]imino}methyl]phenol | Fe(III) | 60.00 µg/µl | HepG2 | SAR ctDNA ROS | 47 |
| 21 | Cr(III) | 37.00 µg/µl | ||||
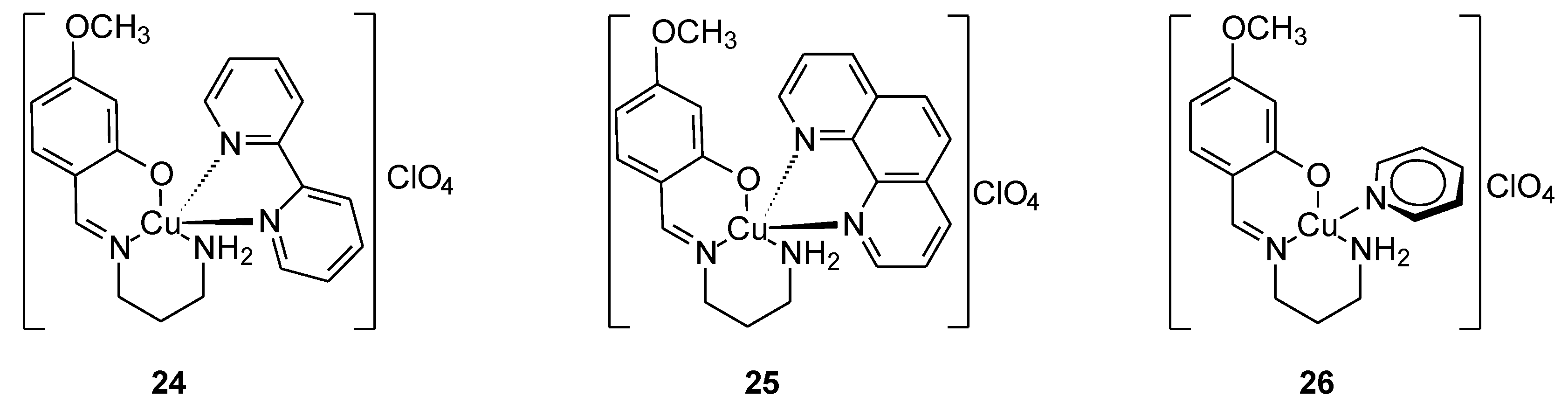
| 22 | 2-[(E)-{[4-(2-hydroxyethyl) phenyl]imino}methyl]-4-methoxy phenol | Cr(III) | 37.00 µg/µl | |||
| 23 | Mn(II) | 3.00 µg/µl | ||||
| 4 | 1,3-propanediamine with 2-hydroxy-4-methoxybenzaldehyde | Cu(II) | 15.87 µM | HCT116 A549 | Molecular docking | 48 |
| 25 | 4.97 µM | |||||
| 26 | 21.75 µM | |||||
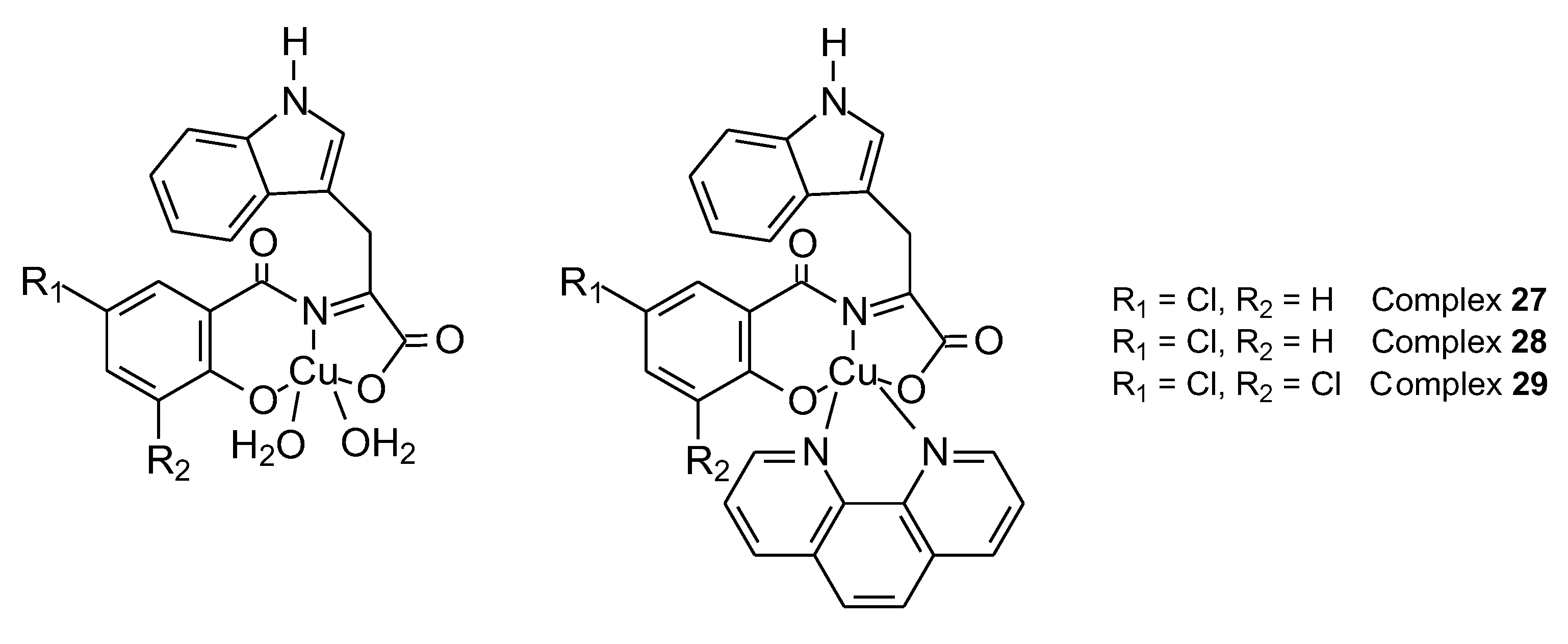
| 27 | Condensation L-tryptophan with 5-chlorosalicyladehyde | Cu(II) | MDA-MB 231 MCF-10A |
ctDNA, BSA, ROS, SRB test | 49 | |
| 28 | 4.31 ± 1.1 µM | |||||
| 29 | Condensation L-tryptophan with 3,5-chlorosalicyladehyde | 2.38 ± 0.3 µM | ||||
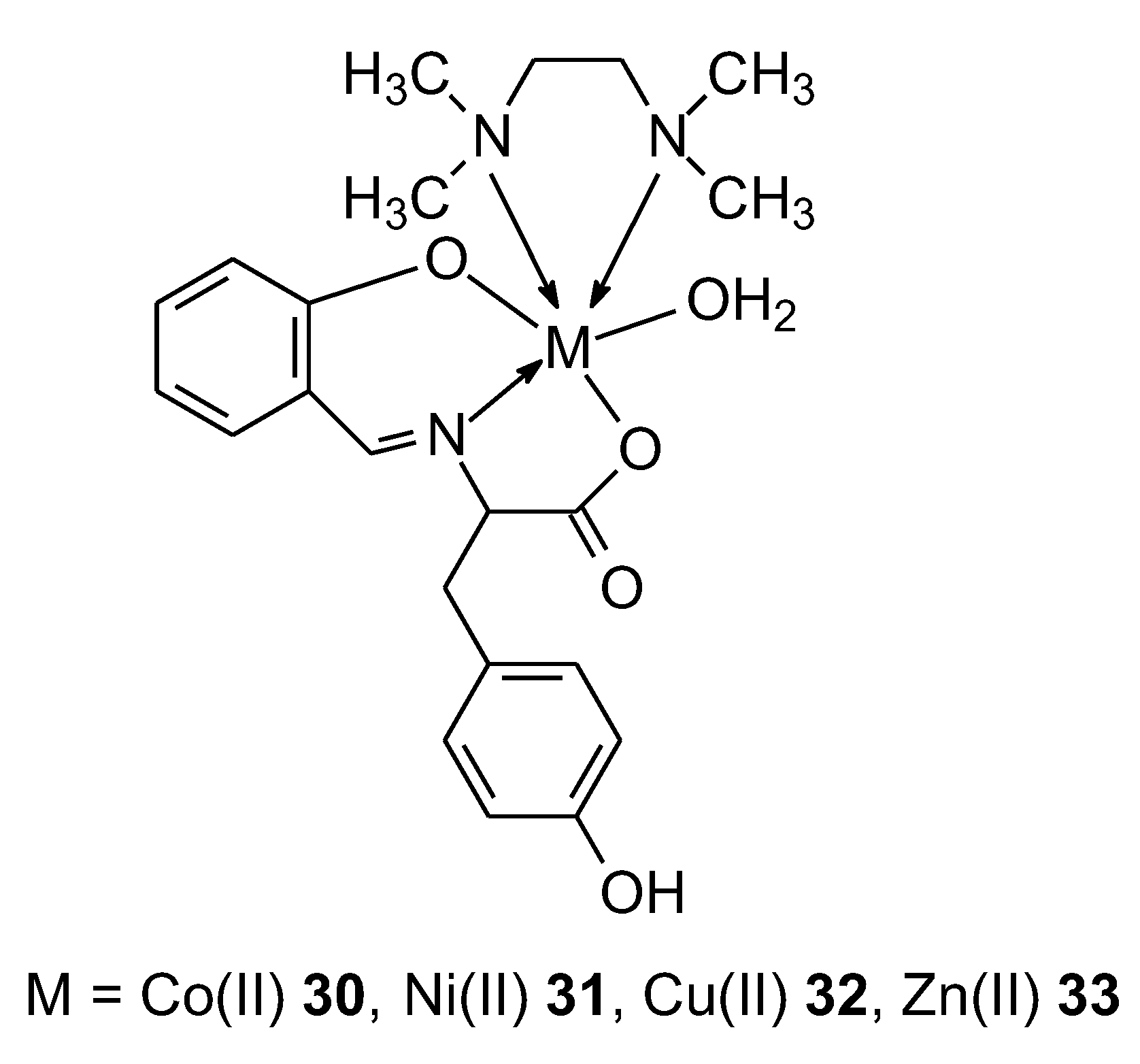
| 30 |
Ligang as Schiff bases – derivative of reaction L-tyrosine and salicylaldehyde |
Co(II) | 8.8 µg/ml | ctDNA DPPH radical scavenging activity |
50 | |
| 31 | Ni(II) | 2.8µg/ml | ||||
| 32 | Zn(II) | 2.5µg/ml | ||||
| 33 | Cu(II) | 4.2 µg/ml | ||||
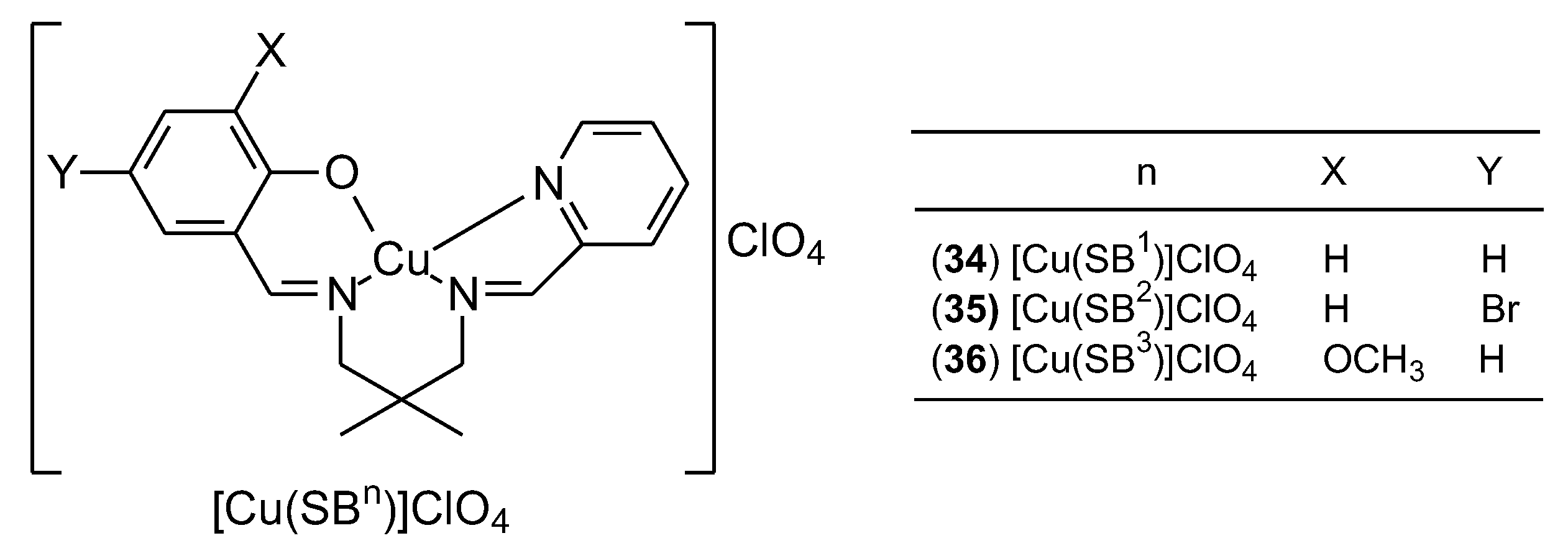
| 34 | Condensation of 2,2-dimethyl-1,3-propanediamine with salicylaldehyde | Cu(II) | 90 μM | HCT-116 A549 | Molecular docking with DNA | 51 |
| 35 | Condensation of 2,2-dimethyl-1,3-propanediamine with5-bromosalicylaldehyde | 147.4 μM | ||||
| 36 | Condensation of 2,2-dimethyl-1,3-propanediamine with 3-methoxysalicylaldehyde | 21.7 μM | ||||
| 37 | (E)-N′-(5-bromo-2-hydroxybenzylidene)isonicotinohydrazide | Co(II) | 7.26 μg/ml | DPPH radical scavenging activity Antioxidant activity |
52 | |
| 38 | Ni(II) | 70.93 μg/ml | ||||
| 39 | Cu(II) | 128.32 μg/ml | ||||
| 40 | Zn(II) | 2.73 μg/ml |
3.2. Examples of Metal Complexes with Sulfur-Containing Schiff Bases
| Comp. | Ligand | Metal ion | IC50 MCF-7 | other tested cell lines | other tests | Ref. |
|---|---|---|---|---|---|---|
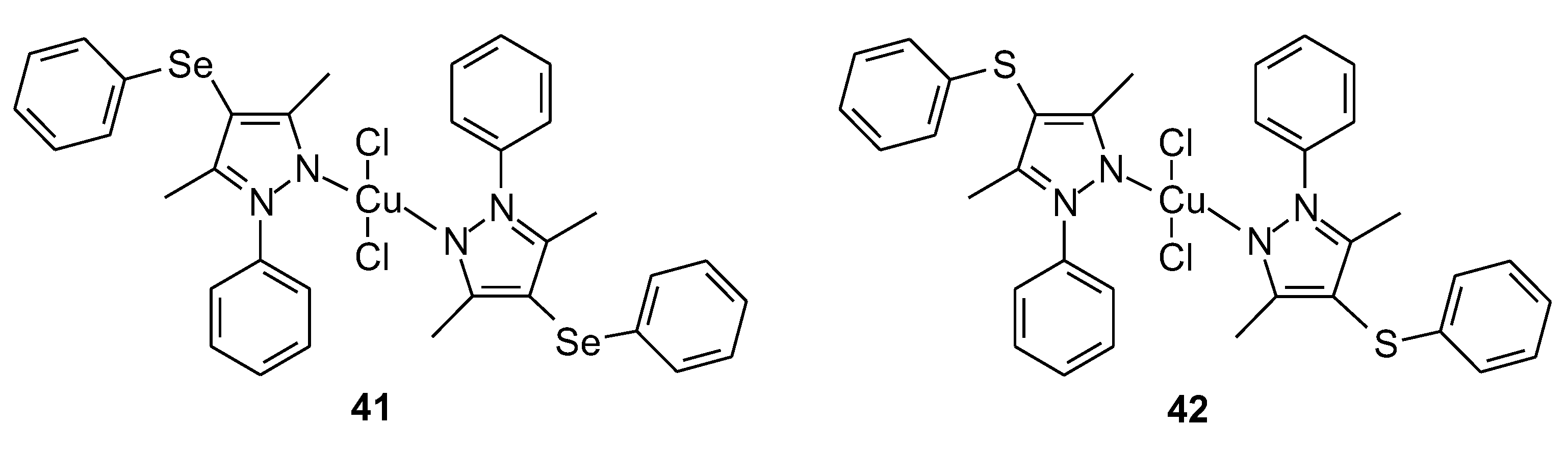
| 41 | [3,5-dimethyl-1-phenyl-4-(phenylselanyl)-1H-pyrazole] | Cu(II) | 44 ± 11 (SI = 1.4) μM | V79 MRC-5 U2OS HepG2 | DPPH | 53 |
| 42 | [3,5-dimethyl-1-phenyl-4-(phenylsulfur)-1H-pyrazole] | 59 ± 2 μM | ||||
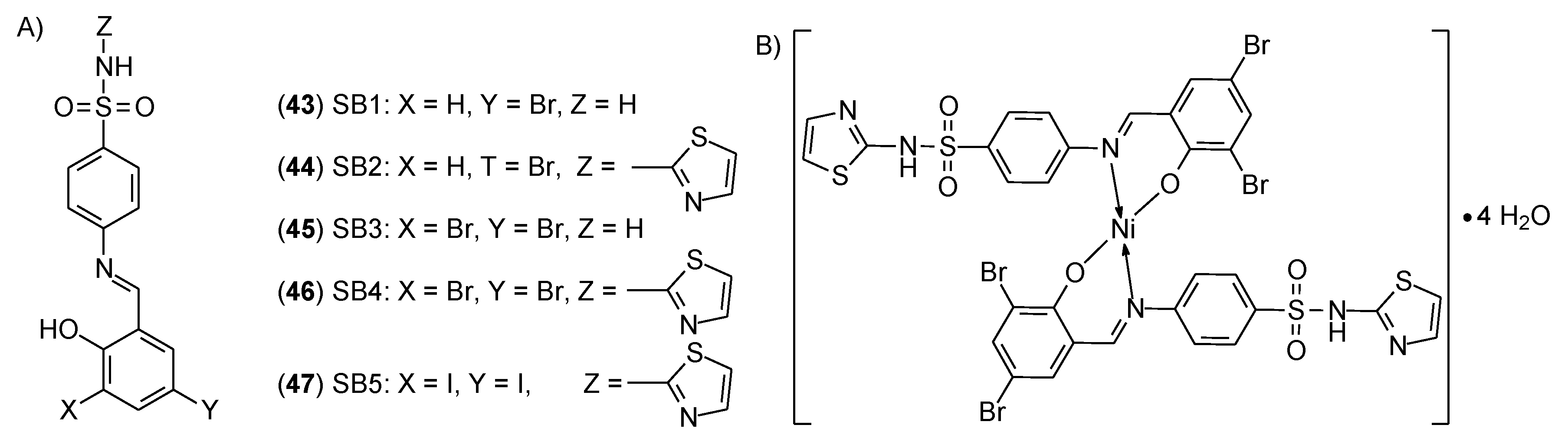
| 43 | 4-((5-Bromo-2-hydroxybenzylidene)amino)-benzenesulfonamide | Ni(II) | - | OEC | 55 | |
| 44 | 4-((5-Bromo-2-hydroxybenzylidene)amino)-N-(1,3-thiazol-2-yl)benzenesulfonamide | 11.2 ± 0.9 μM | ||||
| 45 | 4-((3,5-Dibromo-2-hydroxybenzylidene)amino)-benzenesulfonamide | - | ||||
| 46 | 4-((3,5-Dibromo-2-hydroxybenzylidene)amino)-N-(1,3-thiazol-2-yl)benzenesulfonamide | 4.33 ± 0.5 μM | ||||
| 47 | 4-((3,5-Diiodo-2-hydroxybenzylidene)amino)-N-(1,3-thiazol-2-yl)benzenesulfonamide | > 100 μM | ||||
| 48 | 2-((E)-(6-Ethoxybenzo[d]thiazol-2-ylimino)methyl)-4-chlorophenol | Zn(II) | 37.67 μM | HeLa | ctDNA, Inhibition of RS formation, Antioxidant activity |
56 |
| 49 | Ni(II) | 51.32 μM | ||||
| 50 | Co(II) | 58.41 μM | ||||
| 51 | Cu(II) | 67.59 μM | ||||
| 52 | SMDTC-glyoxal | Cu(II) | 1.7 ± 0.1 µM | MDA-MB-231 |
|
57 |
| 53 | SBDTC–glyoxal | >50 µM | ||||
| 54 | SMDTC–Butanedione | 46 ± 1.0 µM | ||||
| 55 | SBDTC–Butanedione | 11 ± 1.9 µM | ||||
| 56 | SMDTC–Pentadione | 14 ± 2.1 µM | ||||
| 57 | SBDTC–Pentadione | >50 µM | ||||
| 58 | SMDTC–Hexadione | 45 ± 2.3 µM | ||||
| 59 | SBDTC–Hexadione | 7.3 ± 2.8 µM | ||||
| 60 | SMDTC–Heptadione | 20 ± 1.5 µM | ||||
| 61 | SBDTC–Heptadione | >50 µM | ||||
| 62 | Sodium 2-hydroxy-3-methoxy-5-sulfonate-benzaldehyde-3-thiosemicarbazone | Cu(II) | 4.1 ± 1.0 μM |
HCT-15 LoVo HEK293 2008 MDA-MB-231 A431 PSN-1 | 63 | |
| 63 | Sodium 2-hydroxy-3-methoxy-5-sulfonate-benzaldehyde-4-ethyl-3-thiosemicarbazone | 8.3 ± 0.2 μM |
||||
| 64 | Sodium 2-hydroxy-3-methoxy-5-sulfonate-benzaldehyde-3-methyl-thiosemicarbazone | 8.9 ± 0.4 μM |
||||
| 65 | Sodium 2,3-dihydroxy -5-sulfonate-benzaldehyde-3-thiosemicarbazone | 1.3 ± 0.4 μM |
||||
| 66 | Sodium 2,3-dihydroxy-3-methoxy-5-sulfonate-benzaldehyde-4-ethyl-3-thiosemicarbazone | 1.7 ± 1.0 μM |
||||
| 67 | Sodium 2-hydroxy-3-methoxy-5-sulfonate-benzaldehyde-3-thiosemicarbazone | Ni(II) | >100 μM | |||
| 68 | Sodium 2-hydroxy-3-methoxy-5-sulfonate-benzaldehyde-3-thiosemicarbazone | Zn(II) | >100 μM | |||
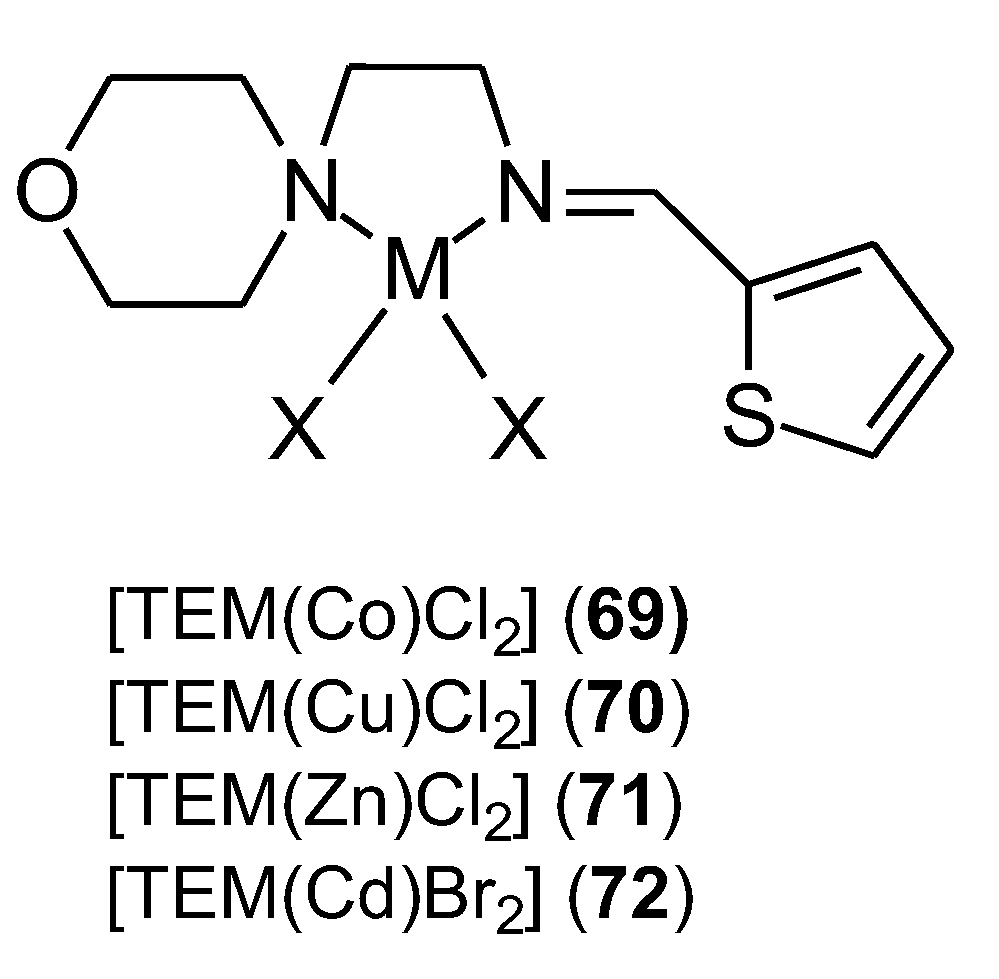
| 69 | (E)-2-morpholino-N-(thiophen-2-ylmethylene)ethanamine | Co(II) | 4.0 ± 1.06 μM | 68 | ||
| 70 | Cu(II) | 5.9 ± 0.23 μM | ||||
| 71 | Zn(II) | 3.3 ± 0.01 μM | ||||
| 72 | Cd(II) | 4.0 ± 1.06 μM | ||||
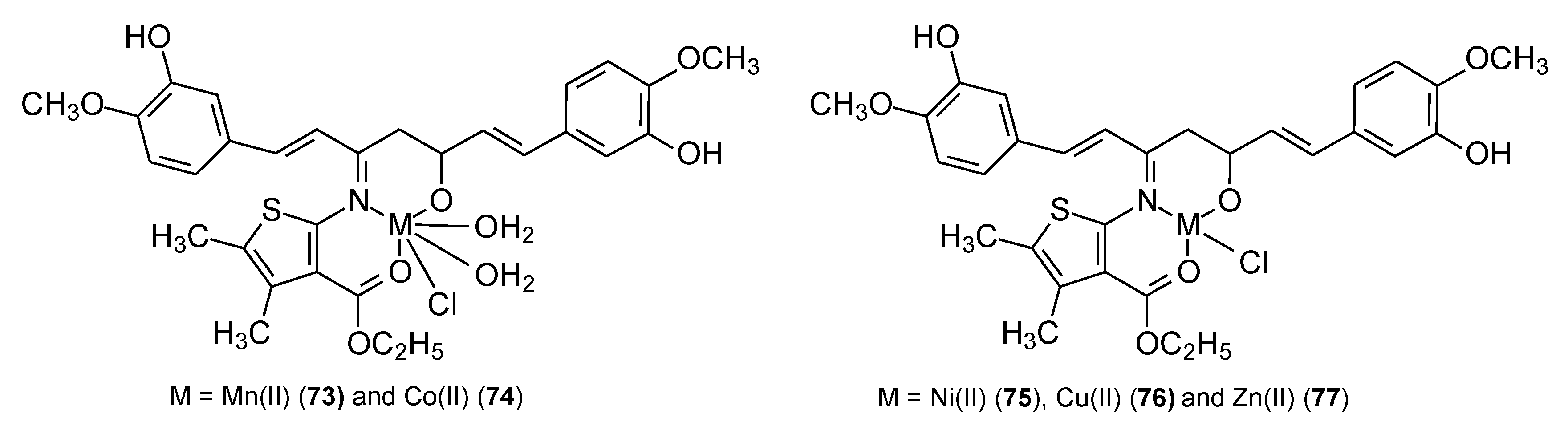
| 73 | curcumin and synthesized 2-amino-3-carboxyethyl-4,5-dimethylthiophene | Mn(II) | < 10 μg/ml | K-562 | DPPH, Antioxidant activity |
69 |
| 74 | Co(II) | < 10 μg/ml | ||||
| 75 | Ni(II) | < 10 μg/ml | ||||
| 76 | Cu(II) | >80 10 μg/ml | ||||
| 77 | Zn(II) | < 10 μg/ml | ||||
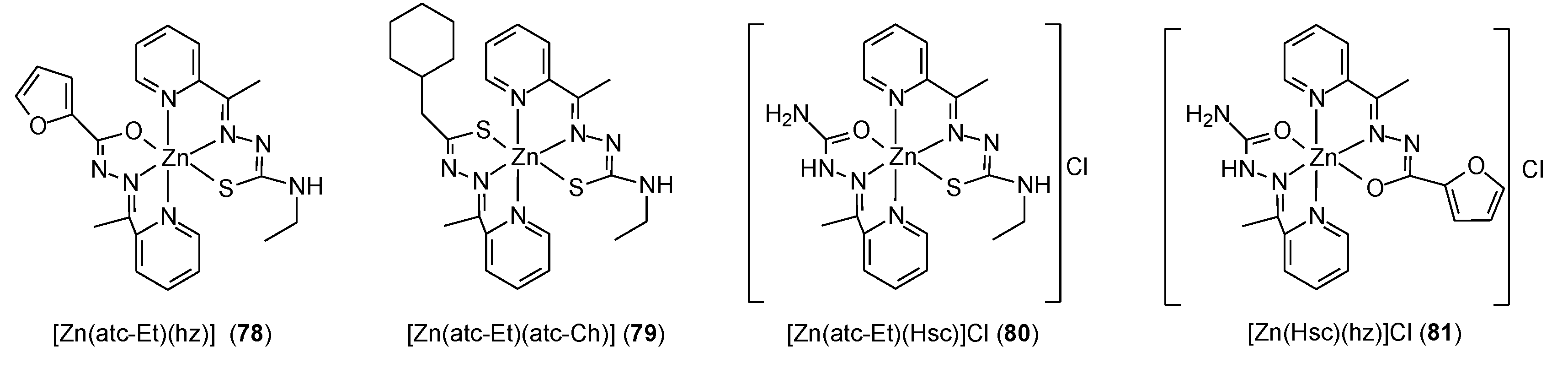
| 78 | Hatc-Ch: 2-Acetylpyridine-4-cyclohexyl-3-thiosemicarbazone | Zn(II) | 9.43 µM | MDA-MB-453 MDA-MB-231 MCF10A HUVEC HFF MCF10A HUVEC HFF | 71 | |
| 79 | Hatc-Et: 2-Acetylpyridine-4-ethyl-3-thiosemicarbazone | 18.49 µM | ||||
| 80 | Hsc: 2-Acetylpyridine-semicarbazone | 19.34 µM | ||||
| 81 | Hhz: 2-Acetylpyridine-furanoylhidrazone | 10.41 µM | ||||
| 82 | (Z)-2-((E)-1-(2-(4-chlorophenyl)hydrazinylidene)propan-2-ylidene)-N-phenylhydrazine-1-carbothioamide | Fe(III) | 20 µg/ml | 72 | ||
| 83 | Co(II) | 23 µg/ml | ||||
| 84 | Cu(II) | 10.5 µg/ml | ||||
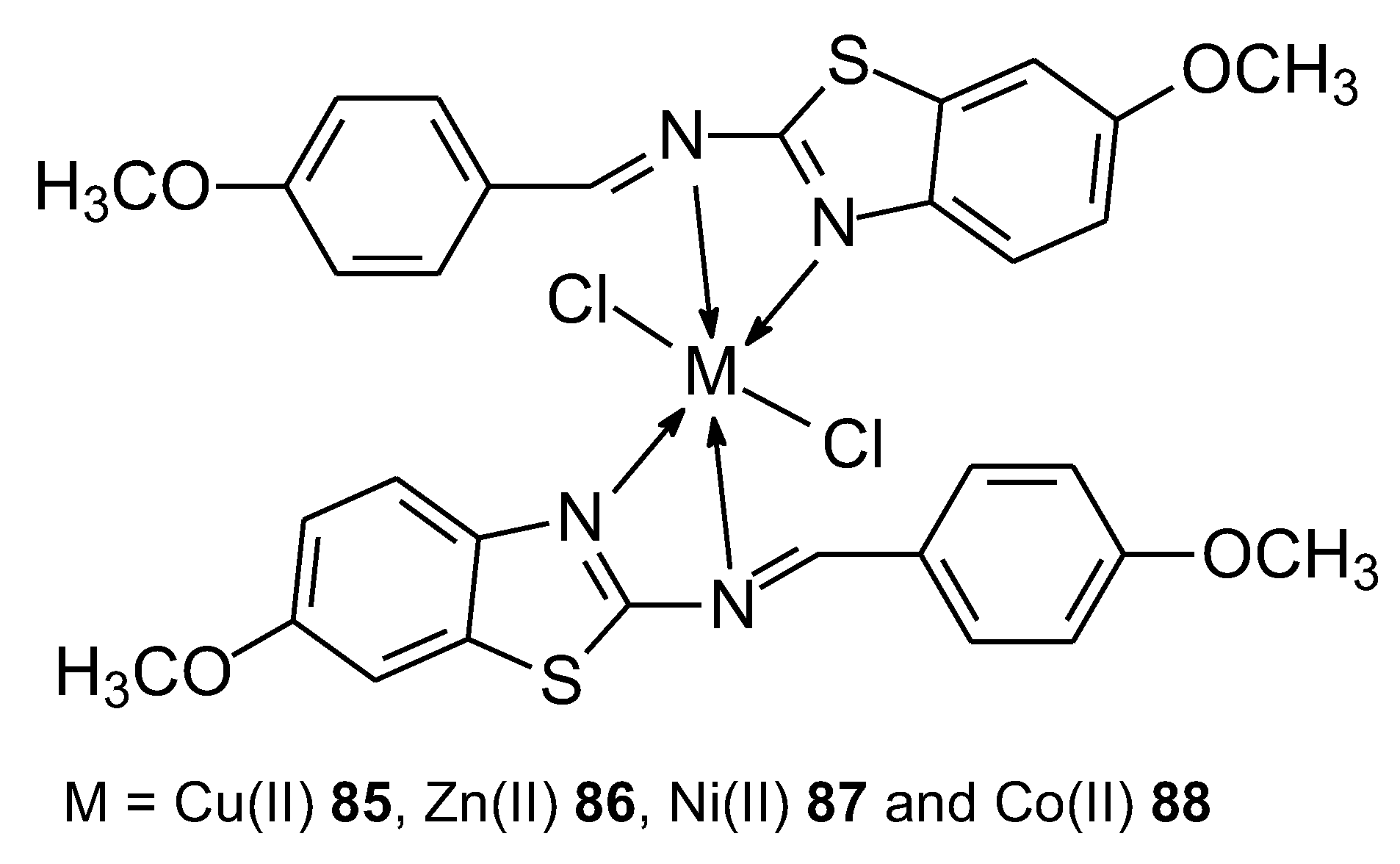
| 85 | (E)-7-methoxy-N-(4-methoxybenzylidene)benzo[d]-thiazol-2-amine | Cu(II) | 12 ± 0.03 (μg ± SD) | Hela Hep2 HepG2 | Antioxidant activity by DPPH method, DFT calculation, Molecular docking |
76 |
| 86 | Zn(II) | 24 ± 0.15 (μg ± SD) | ||||
| 87 | Ni(II) | 37 ± 0.05 (μg ± SD) | ||||
| 88 | Co(II) | 43 ± 0.06 (μg ± SD) | ||||
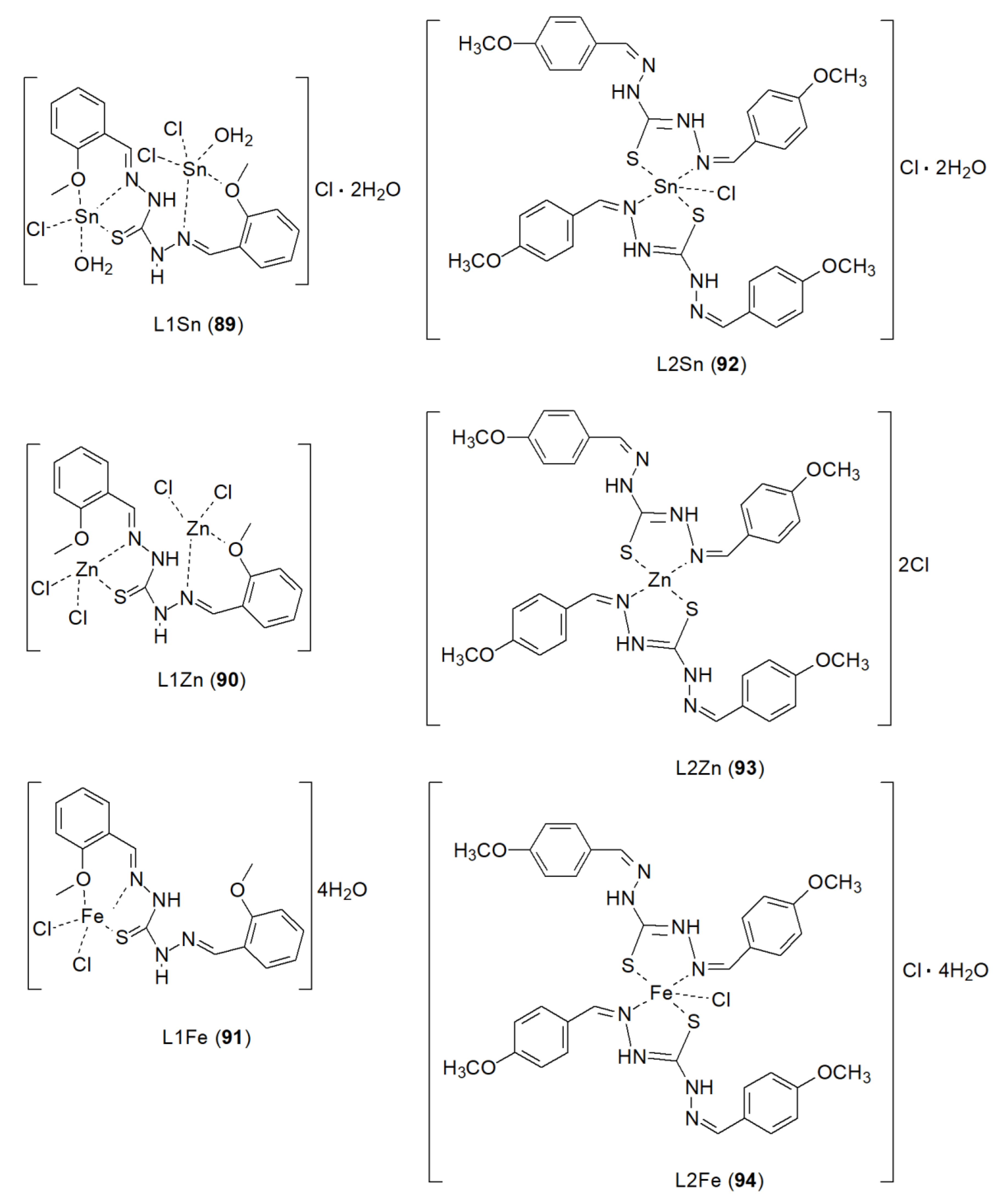
| 89 | 1, 5-bis(2-methoxyanisaldehyde) thiocarbohydrazine | Sn(II) | 263.50 ± 38.89 µM | A549 HeLa U87 T47D MDA-MB-231 MDA-MB-453 BT-549 PANC1 HT-29 HCT116 SW480 SW620 CACO2 RAW | SRB, DPPH |
77 |
| 90 | Zn(II) | 47.69 ± 3.32 µM | ||||
| 91 | Fe(II) | 183.20 ± 6.72 µM | ||||
| 92 | 1, 5-bis (4-methoxyanisaldehyde) thiocarbohydrazine | Sn(II) | 434.64 ± 35.44 µM | |||
| 93 | Zn(II) | 157.17 ± 7.74 µM | ||||
| 94 | Fe(II) | 135.06 ± 6.84 µM |
3.3. Metal Complexes with Other Types of Schiff Bases
| Comp. | Ligand | Metal ion | IC50 MCF-7 | other tested cell lines | other tests | Ref. |
|---|---|---|---|---|---|---|
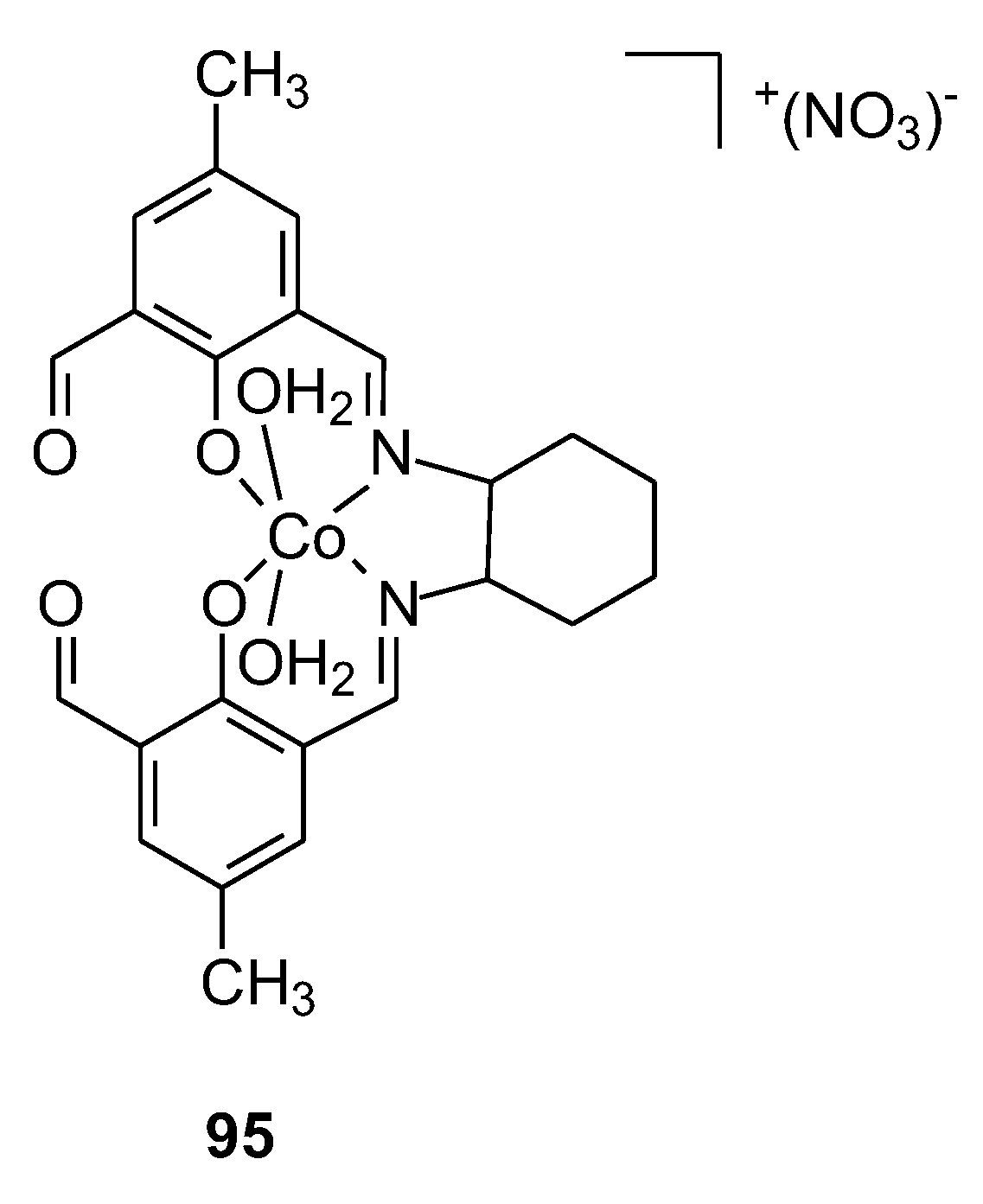
| 95 | Cyclohexane-1,2-diamine, 2,6-diformyl-4-methylphenol | Co(II) | 16.81 ± 1.33 μM | LS-174 MCR-5 | 80 | |
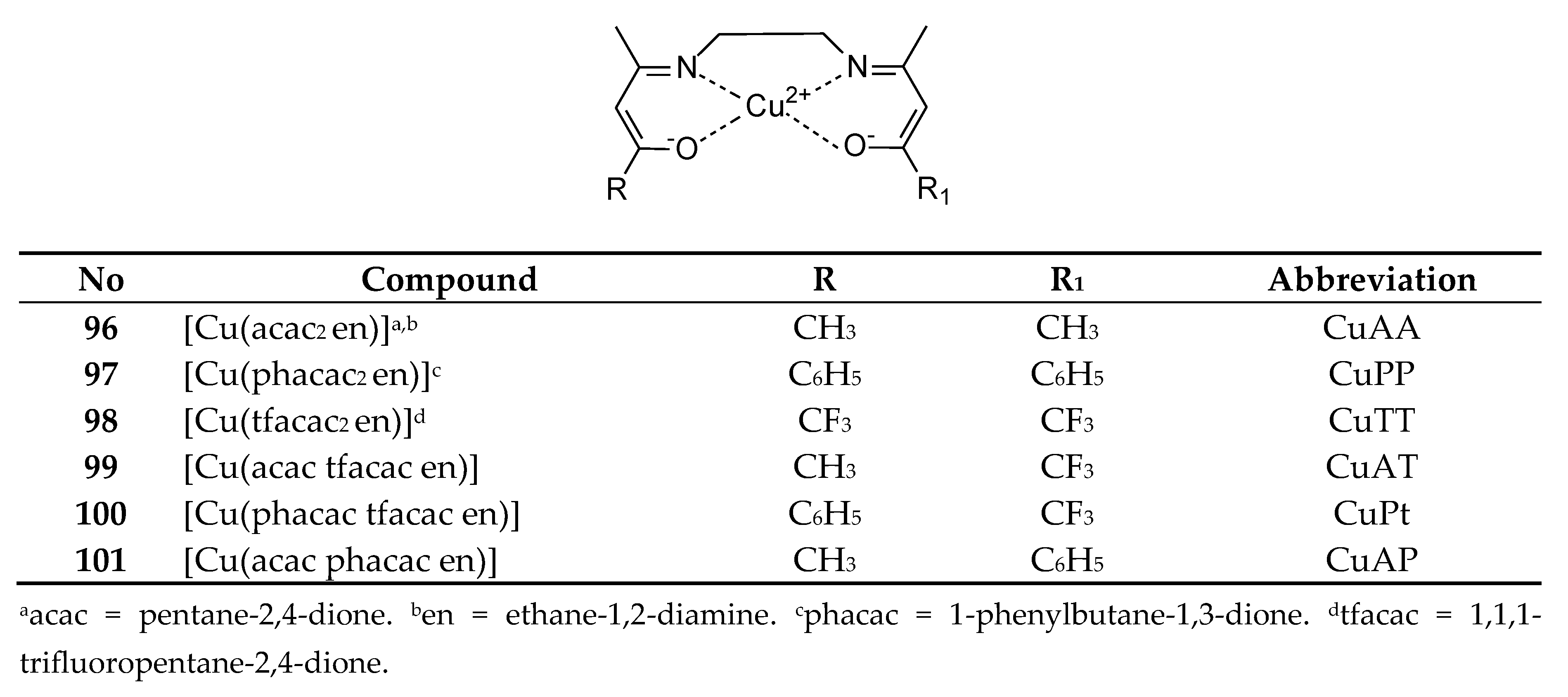
| 96 | [Cu(acac2 en)] | Cu(II) | 17.53 ± 2.83 μM | ROS, Molecular docking interaction with HSA | 81 | |
| 97 | [Cu(phacac2 en)] | |||||
| 98 | [Cu(tfacac2 en)] | 21.29 ± 2.55 μM | ||||
| 99 | [Cu(acac tfacac en)] | |||||
| 100 | [Cu(acac tfacac en)] | 30.02 ± 2.05 μM | ||||
| 101 | [Cu(acac phacac en)] | |||||
| 102 | E)-2-((4-(1H-benzo[d]imidazol-2-yl)phenylimino)methyl)-6-bromo-4-chlorophenol (L1) | Cu(II) | Molecular docking | 82 | ||
| 103 | Ni(II) | 1.89 LD50 mg/ml | ||||
| 104 | Pd(II) | |||||
| 105 | Zn(II) | |||||
| 106 | (E)-1-((4-(1H-benzo[d]imidazol-2-yl)phenylimino)methyl)naphthalen-2-ol (L2) | Cu(II) | 0.129 LD50 mg/ml | |||
| 107 | Ni(II) | |||||
| 108 | Pd(II) | 3.09 LD50 mg/ml | ||||
| 109 | Zn(II) | |||||
| 110 | Condensation of aldehyde (3-(3-formyl-4-hydroxybenzyl)-1-methyl-1H-imidazol-3-ium chloride) and 4-(1-naphthyl)-3-thiosemicarbazide | Mn(II) | 257.1±2.90 μM 193.4±2.57 μM 79.14±1.01 μM 127.6 ± 5.69 μM 206.9 ± 5.61 μM |
SW-872 | 83 | |
| 111 | Fe(III) | |||||
| 112 | Ni(II) | |||||
| 113 | Cu(II) | |||||
| 114 | Zn(II) | |||||
| 115 | N4MacL1 | Zn(II) | 10.23±0.41 µM 9.78±0.32 µM 7.40±0.45 µM |
A549 HT-29 |
86 | |
| 116 | N4MacL2 | |||||
| 117 | N4MacL3 | |||||
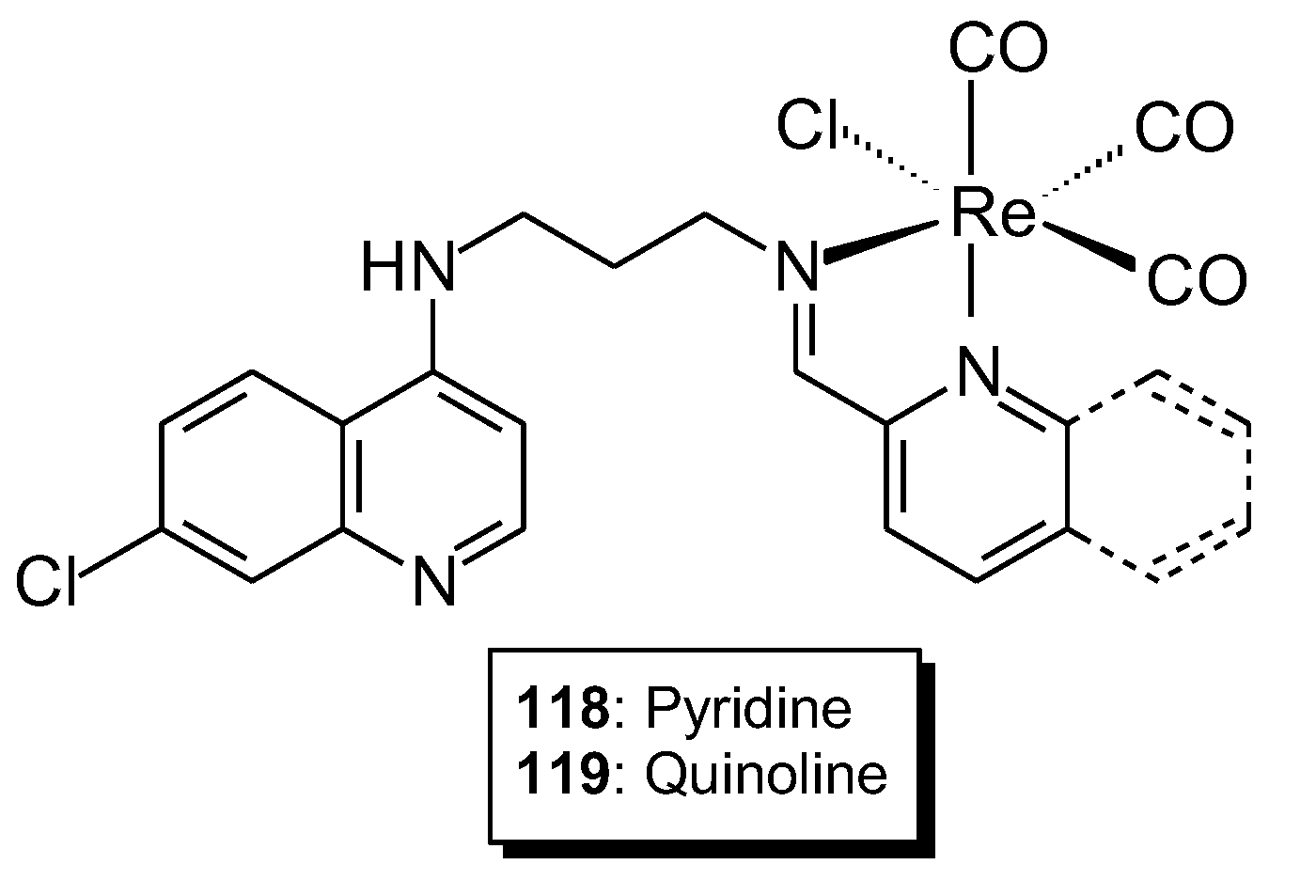
| 118 | N1-(7-chloroquinolin-4-yl)propane-1,3-diamine | Re(I) | 8.55 ± 1.08 μM | MDA-MB-231 FG-0 | Molecular docking | 92 |
| 119 | 7-Chloro-N-(3-((quinolin-2-ylmethylene)amino)propyl)quinolin-4-amine | 6.82 ± 1.03 μM | ||||
| 120 | functionalized at the 2-position with 1-(3-aminopropyl)imidazole (HL1) | Zn(II) | 7.3 ± 2.4 µM | 95 | ||
| 121 | functionalized at the 1-(3-aminopropyl)-2-methyl-1H-imidazole (HL2). | 6.7 ± 1.0 µM | ||||
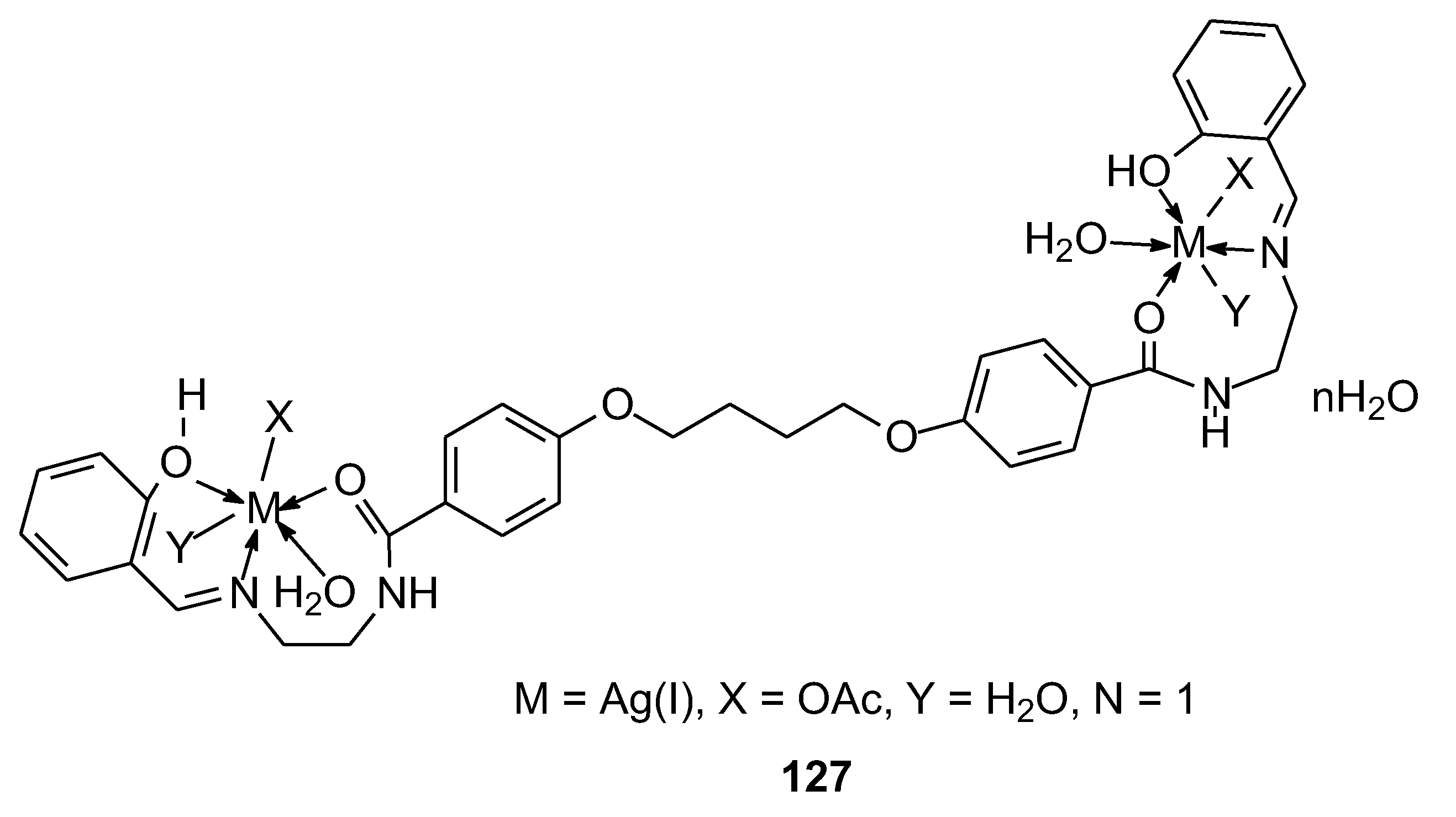
| 122 | combination of two moles of salysaldehyde 4,4′-(butane-1,4-diylbis(oxy))bis(N-(2-aminoethyl) benzamide) |
Mn(II) | data on the chart | HepG-2 | Molecular docking | 96 |
| 123 | Ni(II) | |||||
| 124 | Cu(II) | |||||
| 125 | Zn(II) | |||||
| 126 | Hg(II) | |||||
| 127 | Ag(I) | |||||
| 128 | (cyclopenta-2,4-dien-1-yl)(cyclopenta-2,4-dien-1-yl) (1-((8-aminonaphthalen-1-yl)imino)ethyl) |
Cr(II) | 19.1 mg/ml | Molecular docking | 97 | |
| 129 | Fe(III) | 13.3 mg/ml | ||||
| 130 | Mn(II) | 15.9 mg/ml | ||||
| 131 | Cu(II) | 12.0 mg/ml | ||||
| 132 | Cd(II) | 17.6 mg/ml | ||||
| 133 | Co(II) | 14.7 mg/ml | ||||
| 134 | Zn(II) | 14.0 mg/ml | ||||
| 135 | Ni(II) | 20.3 mg/ml | ||||
| 136 | 1-(1H-benzimidazol-2-yliminomethyl)naphthalen-2-ol |
([Cu(L)(H₂O)]ClO₄) | 78.1 ± 1.7 µM | 100 | ||
| 137 | [Cu(L)(OAc)] | 63.9 ± 1.8 µM | ||||
| 138 | [Cu(L)(NO₃)] | 56.5 ± 1.8 µM | ||||
| 139 | new Schiff base ligands by reacting 3-nitrobenzaldehyde with thiourea | Cu(II) | data on the chart | SW620 A549 | Antioxidant activity | 101 |
| 140 | Zn(II) |
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
List of Abbreviations
| bpy | 2,2′-Bipyridine |
| BSA Cl8HQ |
Bovine Serum Albumin 5-chloro-8-quinolinol |
| ctDNA dppm DEAsal |
circulating tumor DNA 1,1′-bis(diphenylphosphino)methane 4(N,N)-diethylaminosalicylaldehyde |
| DFT | Density Functional Theory |
| DPPH etsc |
2,2-diphenylo-1-picrylhydrazyl ethylthiosemicarbazone |
| FDA 8HQ |
Food and Drug Administration 8-hydroxyquinoline |
| IC50 | IC50 is defined as the concentration of a drug required for 50% inhibition of a biological or biochemical function |
| LDH mtsc |
Lactate dehydrogenase methylthiosemicarbazone |
| MTT | colorimetric assay for assessing cell metabolic activity |
| phen ptsc |
1,10-phenanthroline phenylthiosemicarbazone |
| py | pyridine |
| ROS sal |
Reactive Oxygen Species salicylaldehyde |
| SI | Selectivity index |
| SRB tsc |
sulfate-reducing bacteria thiosemicarbazone |
| WHO | World Health Organization |
References
- Breast Cancer. Available online: https://www.who.int/news-room/fact-sheets/detail/breast-cancer (accessed on 6 November 2025).
- Breast Cancer - Recent Advances in Biology, Imaging and Therapeutics; Done, S., Ed.; IntechOpen: Erscheinungsort nicht ermittelbar: Rijeka, Croatia, 2011; ISBN 978-953-307-730-7. [Google Scholar] [CrossRef]
- Soule, H.D.; Vazquez, J.; Long, A.; Albert, S.; Brennan, M. A Human Cell Line From a Pleural Effusion Derived From a Breast Carcinoma2. J Natl Cancer Inst 1973, 51, 1409–1416. [Google Scholar] [CrossRef] [PubMed]
- Kauffman, G.B.; Pentimalli, R.; Doldi (1908–2001), S.; Hall, M.D. Michele Peyrone (1813–1883), Discoverer of Cisplatin. Platinum Metals Review 2010, 54, 250–256. [Google Scholar] [CrossRef]
- Rosenberg, B.; Vancamp, L.; Trosko, J.E.; Mansour, V.H. Platinum Compounds: A New Class of Potent Antitumour Agents. Nature 1969, 222, 385–386. [Google Scholar] [CrossRef]
- Shen, D.-W.; Pouliot, L.M.; Hall, M.D.; Gottesman, M.M. Cisplatin Resistance: A Cellular Self-Defense Mechanism Resulting from Multiple Epigenetic and Genetic Changes. Pharmacological Reviews 2012, 64, 706–721. [Google Scholar] [CrossRef] [PubMed]
- Johnstone, T.C.; Park, G.Y.; Lippard, S.J. Understanding and Improving Platinum Anticancer Drugs – Phenanthriplatin. Anticancer Res 2014, 34, 471–476. [Google Scholar]
- Galanski, M.S.; Jakupec, M.A.; Keppler, B.K. Update of the Preclinical Situation of Anticancer Platinum Complexes: Novel Design Strategies and Innovative Analytical Approaches. Current Medicinal Chemistry 2005, 12, 2075–2094. [Google Scholar] [CrossRef]
- Ndagi, U.; Mhlongo, N.; Soliman, M.E. Metal Complexes in Cancer Therapy - an Update from Drug Design Perspective. DDDT 2017, 11, 599–616. [Google Scholar] [CrossRef] [PubMed]
- Nath, P.; Datta, A.; Adhikari, S. Recent Advances of Metal-Based Anticancer Agents and Their In Vivo Potential Against Various Types of Malignancies. In Handbook of Animal Models and its Uses in Cancer Research; Springer: Singapore, 2022; pp. 1–28. ISBN 978-981-19-1282-5. [Google Scholar]
- Bhattacharjee, T.; Adhikari, S.; Bhattacharjee, S.; Debnath, S.; Das, A.; Daniliuc, C.G.; Thirumoorthy, K.; Malayaperumal, S.; Banerjee, A.; Pathak, S.; Frontera, A. Exploring Dithiolate-Amine Binary Ligand Systems for the Supramolecular Assemblies of Ni(II) Coordination Compounds: Crystal Structures, Theoretical Studies, Cytotoxicity Studies, and Molecular Docking Studies. Inorganica Chimica Acta 2022, 543, 121157. [Google Scholar] [CrossRef]
- Yu, M.; Zhang, Y.; Fang, M.; Jehan, S.; Zhou, W. Current Advances of Nanomedicines Delivering Arsenic Trioxide for Enhanced Tumor Therapy. Pharmaceutics 2022, 14, 743. [Google Scholar] [CrossRef] [PubMed]
- Varol, M.; Koparal, A.T.; Benkli, K.; Bostancioglu, R.B. Anti-Lung Cancer and Anti-Angiogenic Activities of New Designed Boronated Phenylalanine Metal Complexes. Current Drug Delivery 2018, 15, 1417–1425. [Google Scholar] [CrossRef]
- Raczuk, E.; Dmochowska, B.; Samaszko-Fiertek, J.; Madaj, J. Different Schiff Bases—Structure, Importance and Classification. Molecules 2022, 27, 787. [Google Scholar] [CrossRef]
- Santini, C.; Pellei, M.; Gandin, V.; Porchia, M.; Tisato, F.; Marzano, C. Advances in Copper Complexes as Anticancer Agents. Chem. Rev. 2013, 114, 815–862. [Google Scholar] [CrossRef]
- Levina, A.; Crans, D.C.; Lay, P.A. Advantageous Reactivity of Unstable Metal Complexes: Potential Applications of Metal-Based Anticancer Drugs for Intratumoral Injections. Pharmaceutics 2022, 14, 790. [Google Scholar] [CrossRef]
- Matela, G. Schiff Bases and Complexes: A Review on Anti-Cancer Activity. Anti-Cancer Agents in Medicinal Chemistry 2020, 20, 1908–1917. [Google Scholar] [CrossRef] [PubMed]
- Sathiyanarayanan, V.; Prasath, P.V.; Sekhar, P.C.; Ravichandran, K.; Easwaramoorthy, D.; Mohammad, F.; Al-Lohedan, H.A.; Oh, W.C.; Sagadevan, S. Docking and in Vitro Molecular Biology Studies of P-Anisidine-Appended 1-Hydroxy-2-Acetonapthanone Schiff Base Lanthanum(III) Complexes. RSC Adv. 2020, 10, 16457–16472. [Google Scholar] [CrossRef] [PubMed]
- Singh, N.K.; Kumbhar, A.A.; Pokharel, Y.R.; Yadav, P.N. Anticancer Potency of Copper(II) Complexes of Thiosemicarbazones. Journal of Inorganic Biochemistry 2020, 210, 111134. [Google Scholar] [CrossRef]
- Tadele, K.T.; Tsega, T.W. Schiff Bases and Their Metal Complexes as Potential Anticancer Candidates: A Review of Recent Works. AntiCancer Agents in Medicinal Chemistry 2019, 19, 1786–1795. [Google Scholar] [CrossRef] [PubMed]
- Malik, M.A.; Dar, O.A.; Gull, P.; Wani, M.Y.; Hashmi, A.A. Heterocyclic Schiff Base Transition Metal Complexes in Antimicrobial and Anticancer Chemotherapy. Med. Chem. Commun. 2018, 9, 409–436. [Google Scholar] [CrossRef]
- Deo, K.M.; Pages, B.J.; Ang, D.L.; Gordon, C.P.; Aldrich-Wright, J.R. Transition Metal Intercalators as Anticancer Agents—Recent Advances. International Journal of Molecular Sciences 2016, 17, 1818. [Google Scholar] [CrossRef]
- Zafar, W.; Sumrra, S.H.; Chohan, Z.H. A Review: Pharmacological Aspects of Metal Based 1,2,4-Triazole Derived Schiff Bases. European Journal of Medicinal Chemistry 2021, 222, 113602. [Google Scholar] [CrossRef]
- Jiang, M.; Yan, Q.; Fu, Y.; Meng, L.; Gai, S.; Pan, X.; Qin, Y.; Jiang, C. Development of Cu(II) 4-Hydroxybenzoylhydrazone Complexes That Induce Mitochondrial DNA Damage and Mitochondria-Mediated Apoptosis in Liver Cancer. Journal of Inorganic Biochemistry 2024, 256, 112550. [Google Scholar] [CrossRef]
- Masood, S.; Jamshaid, M.; Zafar, M.N.; Mughal, E.U.; Ashfaq, M.; Tahir, M.N. ONO-Pincer Zn(II) & Cd(II) Complexes: Synthesis, Structural Characterization, Hirshfeld Surface Analysis and CTDNA Interactions. Journal of Molecular Structure 2024, 1295, 136571. [Google Scholar] [CrossRef]
- Cao, Q.; Li, Y.; Freisinger, E.; Qin, P.Z.; Sigel, R.K.O.; Mao, Z.-W. G-Quadruplex DNA Targeted Metal Complexes Acting as Potential Anticancer Drugs. Inorg. Chem. Front. 2017, 4, 10–32. [Google Scholar] [CrossRef]
- Podolski-Renić, A.; Čipak Gašparović, A.; Valente, A.; López, Ó.; Bormio Nunes, J.H.; Kowol, C.R.; Heffeter, P.; Filipović, N.R. Schiff Bases and Their Metal Complexes to Target and Overcome (Multidrug) Resistance in Cancer. European Journal of Medicinal Chemistry 2024, 270, 116363. [Google Scholar] [CrossRef]
- Abu-Dief, A.M.; Mohamed, I.M.A. A Review on Versatile Applications of Transition Metal Complexes Incorporating Schiff Bases. Beni-Suef University Journal of Basic and Applied Sciences 2015, 4, 119–133. [Google Scholar] [CrossRef] [PubMed]
- More, M.S.; Joshi, P.G.; Mishra, Y.K.; Khanna, P.K. Metal Complexes Driven from Schiff Bases and Semicarbazones for Biomedical and Allied Applications: A Review. Materials Today Chemistry 2019, 14, 100195. [Google Scholar] [CrossRef]
- Prakash, A.; Adhikari, D. Application of Schiff Bases and Their Metal Complexes-A Review. International Journal of ChemTech Research 2011, 3, 1891–1896. [Google Scholar]
- Boulechfar, C.; Ferkous, H.; Delimi, A.; Djedouani, A.; Kahlouche, A.; Boublia, A.; Darwish, A.S.; Lemaoui, T.; Verma, R.; Benguerba, Y. Schiff Bases and Their Metal Complexes: A Review on the History, Synthesis, and Applications. Inorganic Chemistry Communications 2023, 150, 110451. [Google Scholar] [CrossRef]
- Uddin, M.N.; Ahmed, S.S.; Alam, S.M.R. REVIEW: Biomedical Applications of Schiff Base Metal Complexes. Journal of Coordination Chemistry 2020, 73, 3109–3149. [Google Scholar] [CrossRef]
- Juyal, V.K.; Pathak, A.; Panwar, M.; Thakuri, S.C.; Prakash, O.; Agrwal, A.; Nand, V. Schiff Base Metal Complexes as a Versatile Catalyst: A Review. Journal of Organometallic Chemistry 2023, 999, 122825. [Google Scholar] [CrossRef]
- Ebosie, N.P.; Ogwuegbu, M.O.C.; Onyedika, G.O.; Onwumere, F.C. Biological and Analytical Applications of Schiff Base Metal Complexes Derived from Salicylidene-4-Aminoantipyrine and Its Derivatives: A Review. J IRAN CHEM SOC 2021, 18, 3145–3175. [Google Scholar] [CrossRef]
- Berhanu, A.L.; Gaurav; Mohiuddin, I.; Malik, A.K.; Aulakh, J.S.; Kumar, V.; Kim, K.-H. A Review of the Applications of Schiff Bases as Optical Chemical Sensors. TrAC Trends in Analytical Chemistry 2019, 116, 74–91. [Google Scholar] [CrossRef]
- Sandhu, Q.-U.-A.; Pervaiz, M.; Majid, A.; Younas, U.; Saeed, Z.; Ashraf, A.; Khan, R.R.M.; Ullah, S.; Ali, F.; Jelani, S. Review: Schiff Base Metal Complexes as Anti-Inflammatory Agents. Journal of Coordination Chemistry 2023, 76, 1094–1118. [Google Scholar] [CrossRef]
- Sonawane, H.R.; Vibhute, B.T.; Aghav, B.D.; Deore, J.V.; Patil, S.K. Versatile Applications of Transition Metal Incorporating Quinoline Schiff Base Metal Complexes: An Overview. European Journal of Medicinal Chemistry 2023, 258, 115549. [Google Scholar] [CrossRef] [PubMed]
- Kalaiarasi, G.; Dharani, S.; Rajkumar, S.R.J.; Lynch, V.M.; Prabhakaran, R. Binuclear Ni(II) Complexes Containing ONS Donor Schiff Base Ligands: Preparation, Spectral Characterization, X-Ray Crystallography and Biological Exploration. Journal of Inorganic Biochemistry 2020, 211, 111176. [Google Scholar] [CrossRef]
- Aazam, E.S.; Majrashi, M.A. Novel Schiff Base Derived from Amino Pyrene: Synthesis, Characterization, Crystal Structure Determination, and Anticancer Applications of the Ligand and Its Metal Complexes. Molecules 2023, 28, 7352. [Google Scholar] [CrossRef]
- Paliwal, K.; Swain, A.; Mishra, D.P.; Antharjanam, P.K.S.; Kumar, M. A Novel Copper(II) Complex with a Salicylidene Carbohydrazide Ligand That Promotes Oxidative Stress and Apoptosis in Triple Negative Breast Cancer Cells. Dalton Trans. 2024, 53, 17702–17720. [Google Scholar] [CrossRef]
- Paul, A.; Singh, P.; Kuznetsov, M.L.; Karmakar, A.; da Silva, M.F.C.G.; Koch, B.; Pombeiro, A.J.L. Influence of Anchoring Moieties on New Benzimidazole-Based Schiff Base Copper(II) Complexes towards Estrogen Dependent Breast Cancer Cells. Dalton Trans. 2021, 50, 3701–3716. [Google Scholar] [CrossRef] [PubMed]
- Kavitha, R.; Reddy, Ch.V.R.; Sireesha, B. Synthesis, Spectroscopic and Biological Activity Evaluation of Ni(II), Cu(II) and Zn(II) Complexes of Schiff Base Derived from Pyridoxal and 4-Fluorobenzohydrazide. Nucleosides, Nucleotides & Nucleic Acids 2021, 40, 845–866. [Google Scholar] [CrossRef]
- Priya, J.; Madheswari, D. Biomolecular Docking Interactions, Cytotoxicity and Antioxidant Property Evaluations with Novel Mn(II), Ni(II), Cd(II) and Pb(II) Schiff Base Ligand Complexes: Synthesis and Characterization. J Biosci 2022, 47, 29. [Google Scholar] [CrossRef]
- Ferretti, V.; Matos, C.P.; Canelas, C.; Pessoa, J.C.; Tomaz, A.I.; Starosta, R.; Correia, I.; León, I.E. New Ternary Fe(III)-8-Hydroxyquinoline–Reduced Schiff Base Complexes as Selective Anticancer Drug Candidates. Journal of Inorganic Biochemistry 2022, 236, 111961. [Google Scholar] [CrossRef] [PubMed]
- Noureldeen, A.F.H.; Aziz, S.W.; Shouman, S.A.; Mohamed, M.M.; Attia, Y.M.; Ramadan, R.M.; Elhady, M.M. Molecular Design, Spectroscopic, DFT, Pharmacological, and Molecular Docking Studies of Novel Ruthenium(III)–Schiff Base Complex: An Inhibitor of Progression in HepG2 Cells. Int J Environ Res Public Health 2022, 19, 13624. [Google Scholar] [CrossRef] [PubMed]
- Sasikumar, G.; Subramani, A.; Tamilarasan, R.; Rajesh, P.; Sasikumar, P.; Albukhaty, S.; Mohammed, M.K.A.; Karthikeyan, S.; Al-aqbi, Z.T.; Al-Doghachi, F.A.J.; Taufiq-Yap, Y.H. Catalytic, Theoretical, and Biological Investigations of Ternary Metal (II) Complexes Derived from L-Valine-Based Schiff Bases and Heterocyclic Bases. Molecules 2023, 28, 2931. [Google Scholar] [CrossRef] [PubMed]
- Abdel-Rahman, L.H.; Abdelghani, A.A.; AlObaid, A.A.; El-ezz, D.A.; Warad, I.; Shehata, M.R.; Abdalla, E.M. Novel Bromo and Methoxy Substituted Schiff Base Complexes of Mn(II), Fe(III), and Cr(III) for Anticancer, Antimicrobial, Docking, and ADMET Studies. Sci Rep 2023, 13, 3199. [Google Scholar] [CrossRef]
- Ghasemi, L.; Esfahani, M.H.; Sahebi, U.; Divsalar, A.; Abbasi, A.; Behzad, M. Experimental and Molecular Docking Investigation of Anticancer Activity of New Mixed-Ligand Schiff Base Complexes against Human Colorectal (HCT116), Lung (A549) and Breast (MCF7) Carcinoma Cell Lines. Journal of Molecular Structure 2023, 1294, 136568. [Google Scholar] [CrossRef]
- Gültekin, B.; Özbağcı, D.I.; Aydın, İ.; Aydın, R.; Arı, F.; Zorlu, Y. New Copper(II) Complexes Containing Tryptophan Based Schiff Bases as Promising Antiproliferative Agents on Breast Cancer Cells. Journal of Molecular Structure 2024, 1301, 137273. [Google Scholar] [CrossRef]
- Mahadevi, P.; Sumathi, S.; Mehta, A. Ternary Schiff Base Metal (II) Complexes with N,N,N′,N′-Tetramethylethylenediamine: Influence of Secondary Chelates on Antimicrobial, Scavenging Activity, DNA Interaction and Cytotoxicity. Polyhedron 2024, 255, 116951. [Google Scholar] [CrossRef]
- Behzad, M.; Ghasemi, L.; Hossieni, S.B.; Esfahani, M.H.; Divsalar, A.; Kučeráková, M.; Dusek, M. Cu(II) Complexes with Unsymmetrical N3O-Type Schiff Base Ligands: Synthesis, Crystal Structures, in Vitro Antiproliferative Studies against the Human Breast (MCF-7), Colon (HCT-116) and Lung (A549) Cancer Cell Lines, and Molecular Docking Studies. Inorganic Chemistry Communications 2025, 177, 114367. [Google Scholar] [CrossRef]
- Devraye, S.; Jadhav, S.; Zangade, S. Preparation, Structure Elucidation, Free Radical Scavenging, in-Vitro Antimicrobial and Cytotoxic Proficiency of Isoniazid Incorporated Tridentate Co(II), Ni(II), Cu(II) and Zn(II) Schiff Base-Metal Complexes. Results in Chemistry 2025, 16, 102407. [Google Scholar] [CrossRef]
- Mansour, M.S.A.; Abdelkarim, A.T.; El-Sherif, A.A.; Mahmoud, W.H. Metal Complexes Featuring a Quinazoline Schiff Base Ligand and Glycine: Synthesis, Characterization, DFT and Molecular Docking Analyses Revealing Their Potent Antibacterial, Anti-Helicobacter Pylori, and Anti-COVID-19 Activities. BMC Chemistry 2024, 18, 150. [Google Scholar] [CrossRef]
- Asran, A.M.; Khalid Aldhalmi, A.; Nassar Ali Musa, E.; Fayek, A.A.; Mansour, M.S.A.; El-Sherif, A.A. Tridentate N-Donor Schiff Base Metal Complexes: Synthesis, Characterization, Computational Studies, and Assessment of Biomedical Applications in Cancer Therapy, Helicobacter Pylori Eradication, and COVID-19 Treatment. Inorganic Chemistry Communications 2025, 171, 113371. [Google Scholar] [CrossRef]
- Oliveira, D.H.; Aquino, T.B.; Nascimento, J.E.R.; Perin, G.; Jacob, R.G.; Alves, D. Direct Synthesis of 4-Organylselanylpyrazoles by Copper- Catalyzed One-Pot Cyclocondensation and CH Bond Selenylation Reactions. Advanced Synthesis & Catalysis 2015, 357, 4041–4049. [Google Scholar] [CrossRef]
- Pinheiro, A.C.; Busatto, F.F.; Schaefer, B.T.; Tomasini, P.P.; Nunes, I.J.; Machado, T.D.S.; Cargnelutti, R.; de Aquino, T.F.B.; Ferreira, K.D.Q.; Casaril, A.M.; Jacob, R.G.; Savegnago, L.; Hartwig, D.; Saffi, J. Synthesis, Characterization, Antioxidant Potential, and Cytotoxicity Screening of New Cu(II) Complexes with 4-(Arylchalcogenyl)-1H-Pyrazoles Ligands. Journal of Inorganic Biochemistry 2022, 237, 112013. [Google Scholar] [CrossRef]
- Elsamra, R.M.I.; Masoud, M.S.; Ramadan, A.M. Designing Metal Chelates of Halogenated Sulfonamide Schiff Bases as Potent Nonplatinum Anticancer Drugs Using Spectroscopic, Molecular Docking and Biological Studies. Sci Rep 2022, 12, 20192. [Google Scholar] [CrossRef]
- Gopichand, K.; Mahipal, V.; Nageswara Rao, N.; Majeed Ganai, A.; Venkateswar Rao, P. Co(II), Ni(II), Cu(II), and Zn(II) Complexes with Benzothiazole Schiff Base Ligand: Preparation, Spectral Characterization, DNA Binding, and In Vitro Cytotoxic Activities. Results in Chemistry 2023, 5, 100868. [Google Scholar] [CrossRef]
- Break, M.K.B.; Fung, T.Y.; Koh, M.Z.; Ho, W.Y.; Tahir, M.I.M.; Elfar, O.A.; Syed, R.U.; Khojali, W.M.A.; Alluhaibi, T.M.; Huwaimel, B.; Wiart, Ch.; Khoo, T.-J. Synthesis, Crystal Structure, Antibacterial and In Vitro Anticancer Activity of Novel Macroacyclic Schiff Bases and Their Cu (II) Complexes Derived from S-Methyl and S-Benzyl Dithiocarbazate. Molecules 2023, 28, 5009. [Google Scholar] [CrossRef]
- Samy, F.; Omar, F.M. Synthesis, Characterization, Antitumor Activity, Molecular Modeling and Docking of New Ligand, (2,5-Pyrrole)-Bis(5,6-Diphenyl-[1,2,4]-Triazin-3-Yl)Hydrazone and Its Complexes. Journal of Molecular Structure 2020, 1222, 128910. [Google Scholar] [CrossRef]
- Lima, F.C.; Só, Y.A.O.; Gargano, R.; de Oliveira, D.M.; Gatto, C.C. Structural, Theoretical and Biological Activity of Mono and Binuclear Nickel(II) Complexes with Symmetrical and Asymmetrical 4,6-Diacetylresorcinol-Dithiocarbazate Ligands. Journal of Inorganic Biochemistry 2021, 224, 111559. [Google Scholar] [CrossRef] [PubMed]
- Cavalcante, C. de Q.O.; da Mota, T.H.A.; de Oliveira, D.M.; Nascimento, É.C.M.; Martins, J.B.L.; Pittella-Silva, F.; Gatto, C.C. Dithiocarbazate Ligands and Their Ni(II) Complexes with Potential Biological Activity: Structural, Antitumor and Molecular Docking Study. Front. Mol. Biosci. 2023, 10, 1146820. [Google Scholar] [CrossRef] [PubMed]
- Priya Gogoi, H.; Singh, A.; Barman, P.; Choudhury, D. A New Potential ONO Schiff-Base Ligand and Its Cu(II), Zn(II) and Cd(II) Complexes: Synthesis, Structural Elucidation, Theoretical and Bioactivity Studies. Inorganic Chemistry Communications 2022, 146, 110153. [Google Scholar] [CrossRef]
- Khoo, T.-J.; Break, M.K. bin; Crouse, K.A.; Tahir, M.I.M.; Ali, A.M.; Cowley, A.R.; Watkin, D.J.; Tarafder, M.T.H. Synthesis, Characterization and Biological Activity of Two Schiff Base Ligands and Their Nickel(II), Copper(II), Zinc(II) and Cadmium(II) Complexes Derived from S-4-Picolyldithiocarbazate and X-Ray Crystal Structure of Cadmium(II) Complex Derived from Pyridine-2-Carboxaldehyde. Inorganica Chimica Acta 2014, 413, 68–76. [Google Scholar] [CrossRef]
- Miglioli, F.; De Franco, M.; Bartoli, J.; Scaccaglia, M.; Pelosi, G.; Marzano, C.; Rogolino, D.; Gandin, V.; Carcelli, M. Anticancer Activity of New Water-Soluble Sulfonated Thiosemicarbazone Copper(II) Complexes Targeting Disulfide Isomerase. European Journal of Medicinal Chemistry 2024, 276, 116697. [Google Scholar] [CrossRef]
- Jansson, P.J.; Kalinowski, D.S.; Lane, D.J.R.; Kovacevic, Z.; Seebacher, N.A.; Fouani, L.; Sahni, S.; Merlot, A.M.; Richardson, D.R. The Renaissance of Polypharmacology in the Development of Anti-Cancer Therapeutics: Inhibition of the “Triad of Death” in Cancer by Di-2-Pyridylketone Thiosemicarbazones. Pharmacological Research 2015, 100, 255–260. [Google Scholar] [CrossRef]
- Yu, Y.; Kalinowski, D.S.; Kovacevic, Z.; Siafakas, A.R.; Jansson, P.J.; Stefani, C.; Lovejoy, D.B.; Sharpe, P.C.; Bernhardt, P.V.; Richardson, D.R. Thiosemicarbazones from the Old to New: Iron Chelators That Are More Than Just Ribonucleotide Reductase Inhibitors. J. Med. Chem. 2009, 52, 5271–5294. [Google Scholar] [CrossRef] [PubMed]
- Nayab, S.; Jan, K.; Kim, S.-H.; Kim, S.-H.; Shams, D.F.; Son, Y.; Yoon, M.; Lee, H. Insight into the Inhibitory Potential of Metal Complexes Supported by (E)-2-Morpholino-N-(Thiophen-2-Ylmethylene)Ethanamine: Synthesis, Structural Properties, Biological Evaluation and Docking Studies. Dalton Trans. 2024, 53, 11295–11309. [Google Scholar] [CrossRef] [PubMed]
- Sumi, M.; Nevaditha, N.T.; Sindhu Kumari, B. Synthesis, Structural Evaluation, Antioxidant, DNA Cleavage, Anticancer Activities and Molecular Docking Study of Metal Complexes of 2-Amino Thiophene Derivative. Journal of Molecular Structure 2023, 1272, 134091. [Google Scholar] [CrossRef]
- Tweedy, B.G. Plant Extracts with Metal Ions as Potential Antimicrobial Agents. Phytopathology 1964, 55, 910–914. [Google Scholar]
- Machado, R.A.D.S.; Siqueira, R.P.; da Silva, F.C.; de Matos, A.C.P.; Borges, D.S.; Rocha, G.G.; de Souza, T.C.P.; Souza, R.A.C.; de Oliveira, C.R.; Ferreira, A.G.; da Silva Maia, P.I.; Deflon, V.M.; Oliviera, C.G.; Araujo, T.G. A New Heteroleptic Zn(II) Complex with Schiff Bases Sensitizes Triple-Negative Breast Cancer Cells to Doxorubicin and Paclitaxel. Pharmaceutics 2024, 16, 1610. [Google Scholar] [CrossRef]
- Alghabban, H.M.; Nass, N.M.; Alaeq, R.A.; Alghabban, D.M.; Alrahimi, J.; Jambi, R.J.; Abd El-Karim, A.T.; Mansour, M.S.A.; Fayek, A.A.; El-Sherif, A.A. Synthesis, Characterization, DFT Analysis, and Molecular Docking Studies of Carbothioamide Schiff Base-Phenanthroline Ternary Metal Complexes: Multi-Targeted Therapeutic Agents against Leukemia, and Biomedical Application. Med Oncol 2025, 42, 336. [Google Scholar] [CrossRef]
- Richa; Kumar, V.; Kataria, R. Phenanthroline and Schiff Base Associated Cu(II)-Coordinated Compounds Containing N, O as Donor Atoms for Potent Anticancer Activity. Journal of Inorganic Biochemistry 2024, 251, 112440. [Google Scholar] [CrossRef]
- Kinthada, P.M.M.S.; Prabhakar, L.D.; Venkata Reddy, D. Synthesis and Characterization of Complexes of (4-Methoxybenzaldehyde)-4-Phenyl-3-Thiosemicarbazone. Inorganica Chimica Acta 1988, 141, 179–183. [Google Scholar] [CrossRef]
- İlkimen, H.; Yenikaya, C.; Sarı, M.; Bülbül, M.; Aslan, M.; Süzen, Y. Synthesis and Characterization of Some Metal Complexes of a Proton Transfer Salt, and Their Inhibition Studies on Carbonic Anhydrase Isozymes and the Evaluation of the Results by Statistical Analysis. Journal of Enzyme Inhibition and Medicinal Chemistry 2014, 29, 695–701. [Google Scholar] [CrossRef]
- Michael, S.; Jeyaraman, P.; Jancy, J.V.; Muniyandi, V.; Raman, N. Exploring the Enzyme Inhibitor Potential and Therapeutic Applications of Transition Metal Complexes of Methoxy-Schiff Base via Triangular Investigation. International Journal of Biological Macromolecules 2025, 306, 141760. [Google Scholar] [CrossRef]
- Mahdy, A.; Zahra, J.A.; Haddadin, R.N.; Al-Hiari, Y.; Kasabri, V. Multi-Biological Activity Evaluation of Sn(П), Zn(П) and Fe(П) Complexes Based on Thiocarbohydrazide Schiff Bases: Synthesis, Spectroscopic Investigations and Fluorescence Studies. Sci Rep 2025, 15, 31757. [Google Scholar] [CrossRef]
- Lugasi, S.O. New Synthetic Pathways for Thiocarbohydrazide and Salicylaldehyde Azine Compounds. Asian Journal of Chemical Sciences 2017, 1–8. [Google Scholar] [CrossRef]
- Nwosu-Obieogu, K.; Kalu, U.C. In Situ Epoxidation of Sesame Seed Oil for Synthesis of a Bio-Based Resin. EUROPEAN J SUSTAINAB DEV 2020, 4, em0121. [Google Scholar] [CrossRef] [PubMed]
- Dasgupta, S.; Kar, K.; Barua, A.; Ghosh, D.; Kabi, B.; Dewan, K.; Chandra, A. A Significantly Non-Toxic Novel Cobalt(III) Schiff Base Complex Induces Apoptosis via G2-M Cell Cycle Arrest in Human Breast Cancer Cell Line MCF-7. Life Sciences 2022, 308, 120963. [Google Scholar] [CrossRef]
- Mijatović, A.; Gligorijević, N.; Ćoćić, D.; Spasić, S.; Lolić, A.; Aranđelović, S.; Nikolić, M.; Baošić, R. In Vitro and in Silico Study of the Biological Activity of Tetradentate Schiff Base Copper(II) Complexes with Ethylenediamine-Bridge. Journal of Inorganic Biochemistry 2023, 244, 112224. [Google Scholar] [CrossRef] [PubMed]
- Mahmood, K.; Akhter, Z.; Perveen, F.; Aisha; Bibi, M.; Ismail, H.; Tabassum, N.; Yousuf, S.; Ashraf, A.R.; Qayyum, M.A. Synthesis, DNA Binding and Biological Evaluation of Benzimidazole Schiff Base Ligands and Their Metal(Ii) Complexes. RSC Adv 2023, 13, 11982–11999. [Google Scholar] [CrossRef] [PubMed]
- Feizpour, S.; Hosseini-Yazdi, S.A.; Safarzadeh, E.; Baradaran, B.; Dusek, M.; Poupon, M. A Novel Water-Soluble Thiosemicarbazone Schiff Base Ligand and Its Complexes as Potential Anticancer Agents and Cellular Fluorescence Imaging. J Biol Inorg Chem 2023, 28, 457–472. [Google Scholar] [CrossRef]
- Mirzaahmadi, A.; Hosseini-Yazdi, S.A.; Safarzadeh, E.; Baradaran, B.; Samolova, E.; Dusek, M. New Series of Water-Soluble Thiosemicarbazones and Their Copper(II) Complexes as Potentially Promising Anticancer Compounds. Journal of Molecular Liquids 2019, 293, 111412. [Google Scholar] [CrossRef]
- Neamțu, M.; Macaev, F.; Boldescu, V.; Hodoroaba, V.-D.; Nădejde, C.; Schneider, R.J.; Paul, A.; Ababei, G.; Panne, U. Removal of Pollutants by the New Fenton-like Highly Active Catalysts Containing an Imidazolium Salt and a Schiff Base. Applied Catalysis B: Environmental 2016, 183, 335–342. [Google Scholar] [CrossRef]
- Mamta; Chaudhary, A. Novel Tetraaza Macrocyclic Schiff Base Complexes of Bivalent Zinc: Microwave-Assisted Green Synthesis, Spectroscopic Characterization, Density Functional Theory Calculations, Molecular Docking Studies, in Vitro Antimicrobial and Anticancer Activities. Biometals 2024, 37, 1431–1456. [Google Scholar] [CrossRef]
- Mamta; Pinki; Chaudhary, A. Synthesis, Spectroscopic Elucidation, Density Functional Theory Calculation, and Molecular Docking Studies of a Novel Series of Tetradentate Macrocyclic Schiff Base Ligands and Their Zn(II) Complexes and Investigations of Their Antimicrobial, Anti-Inflammatory, and Anticancer Activities. Applied Organometallic Chemistry 2024, 38, e7330. [Google Scholar] [CrossRef]
- Mamta; Chaudhary, A. Synthesis, DFT Calculation, Molecular Docking Studies and Biological Evaluation of a Novel Series of Schiff Base Tetradentate Macrocyclic Ligands and Their Zn(II) Complexes as Antimicrobial, Anti-Inflammatory and Anticancer Agents. Res Chem Intermed 2023, 49, 4671–4712. [Google Scholar] [CrossRef]
- Mamta; Chaudhary, A. Synthesis, Spectroscopic Characterization, in Vitro Antimicrobial Activity, Antioxidant Study and Theoretical Approaches towards DNA Gyrase, DHFR Enzyme, NADPH Enzyme of N8-Tetraoxomacrocyclic Complexes of Zn(II). Journal of Molecular Structure 2024, 1295, 136743. [Google Scholar] [CrossRef]
- Manjare, S.B.; Mahadik, R.K.; Manval, K.S.; More, P.P.; Dalvi, S.S. Microwave-Assisted Rapid and Green Synthesis of Schiff Bases Using Cashew Shell Extract as a Natural Acid Catalyst. ACS Omega 2023, 8, 473–479. [Google Scholar] [CrossRef]
- Kumar, A.; Kuang, Y.; Liang, Z.; Sun, X. Microwave Chemistry, Recent Advancements, and Eco-Friendly Microwave-Assisted Synthesis of Nanoarchitectures and Their Applications: A Review. Materials Today Nano 2020, 11, 100076. [Google Scholar] [CrossRef]
- Zinman, P.S.; Welsh, A.; Omondi, R.O.; Khan, S.; Prince, S.; Nordlander, E.; Smith, G.S. Aminoquinoline-Based Re(I) Tricarbonyl Complexes: Insights into Their Antiproliferative Activity and Mechanisms of Action. European Journal of Medicinal Chemistry 2024, 266, 116094. [Google Scholar] [CrossRef]
- Maurya, S.S.; Bahuguna, A.; Khan, S.I.; Kumar, D.; Kholiya, R.; Rawat, D.S. N-Substituted Aminoquinoline-Pyrimidine Hybrids: Synthesis, in Vitro Antimalarial Activity Evaluation and Docking Studies. European Journal of Medicinal Chemistry 2019, 162, 277–289. [Google Scholar] [CrossRef]
- Musonda, C.C.; Gut, J.; Rosenthal, P.J.; Yardley, V.; Carvalho de Souza, R.C.; Chibale, K. Application of Multicomponent Reactions to Antimalarial Drug Discovery. Part 2: New Antiplasmodial and Antitrypanosomal 4-Aminoquinoline γ- and δ-Lactams via a ‘Catch and Release’ Protocol. Bioorganic & Medicinal Chemistry 2006, 14, 5605–5615. [Google Scholar] [CrossRef]
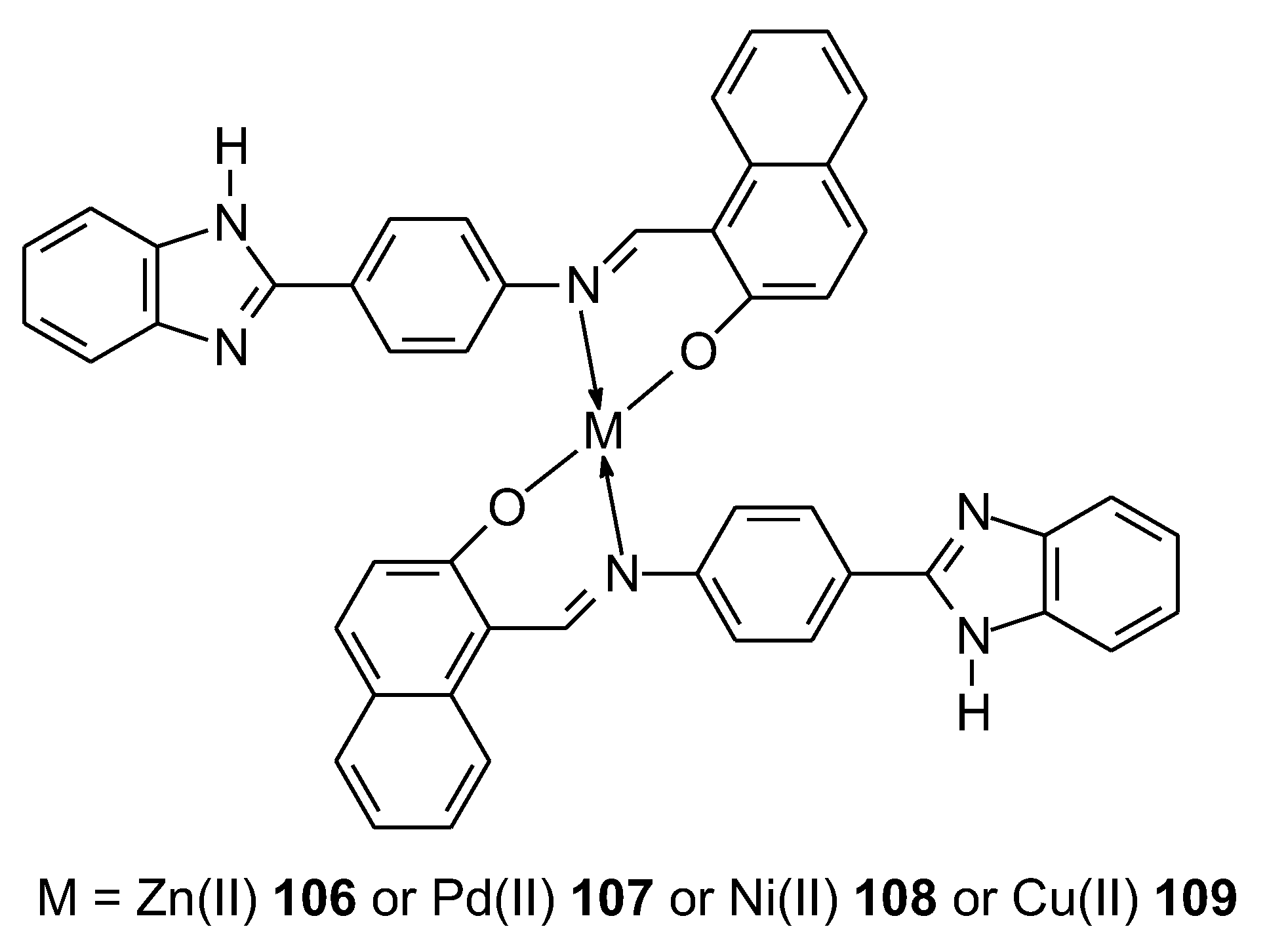
- Côrte-Real, L.; Sergi, B.; Yildirim, B.; Colucas, R.; Starosta, R.; Fontrodona, X.; Romero, I.; André, V.; Acilan, C.; Correia, I. Enhanced Selectivity towards Melanoma Cells with Zinc(II)-Schiff Bases Containing Imidazole Derivatives. Dalton Trans. 2024, 53, 9416–9432. [Google Scholar] [CrossRef] [PubMed]
- Faheem, S.M.; Osman, H.M.; El-Tabl, A.S.; Abd-El Wahed, M.M.; Younes, S.M. New Nano-Complexes Targeting Protein 3S7S in Breast Cancer and Protein 4OO6 in Liver Cancer Investigated in Cell Line. Sci Rep 2024, 14, 16891. [Google Scholar] [CrossRef] [PubMed]
- Derafa, W.; Moustafa, B.S.; Mohamed, G.G.; Taha, R.H.; Farhana, A. Design and Synthesis of a Novel Isoleucine-Derived Schiff Base Ligand: Structural Characterization, Molecular Docking, and in Vitro Biological Activity Evaluation. Int J Health Sci (Qassim) 2024, 18, 31–47. [Google Scholar] [PubMed]
- Yun, J.Y.; Kim, A.; Hwang, S.M.; Yun, D.; Lee, H.; Kim, K.-T.; Kim, C. A Novel Benzimidazole-Based Fluorescence Probe for Detecting Zinc Ion in Aqueous Solution and Zebrafish. bull. Chem. Soc. Jpn. 2019, 92, 961–966. [Google Scholar] [CrossRef]
- Yarkandi, N.H.; El-Ghamry, H.A.; Gaber, M. Synthesis, Spectroscopic and DNA Binding Ability of CoII, NiII, CuII and ZnII Complexes of Schiff Base Ligand (E)-1-(((1H-Benzo[d]Imidazol-2-Yl)Methylimino)Methyl)Naphthalen-2-Ol. X-Ray Crystal Structure Determination of Cobalt (II) Complex. Materials Science and Engineering: C 2017, 75, 1059–1067. [Google Scholar] [CrossRef]
- Ferreira, W.V.; Ráice, F.R.; da Silva, A.F.; Nunes, I.J.; Cervo, R.; Cargnelutti, R.; Saffi, J.; Milani, J.L.S.; Casagrande, O. de L.; Pinheiro, A.C. Synthesis, Characterization, Electronic Properties, and Cytotoxic Activities on Cancer Cells Line of Novel Cu(II) Complexes with Benzimidazole-Schiff Base Tridentate Ligand. Journal of Molecular Structure 2025, 1327, 141239. [Google Scholar] [CrossRef]
- Ibrahim, S.S.; Al-darwesh, M.Y.; Arkawazi, S.W.; Babakr, K.A.; Qader, I.N. Synthesis, Characterization, Antioxidant, Antibacterial Activity, and Molecular Docking Studies of Zn (II) and Cu (II) Complexes Nanoparticles. Journal of Molecular Structure 2025, 1326, 141120. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





