Submitted:
22 December 2025
Posted:
23 December 2025
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Materials and Methods
2.1. Biological Material
2.2. Phytochemical Analysis
2.3. Antioxidant Activity by DPPH
2.4. Statistical Analysis
3. Results and Discussion
3.1. Extract Yield
3.2. Phytochemical Profile
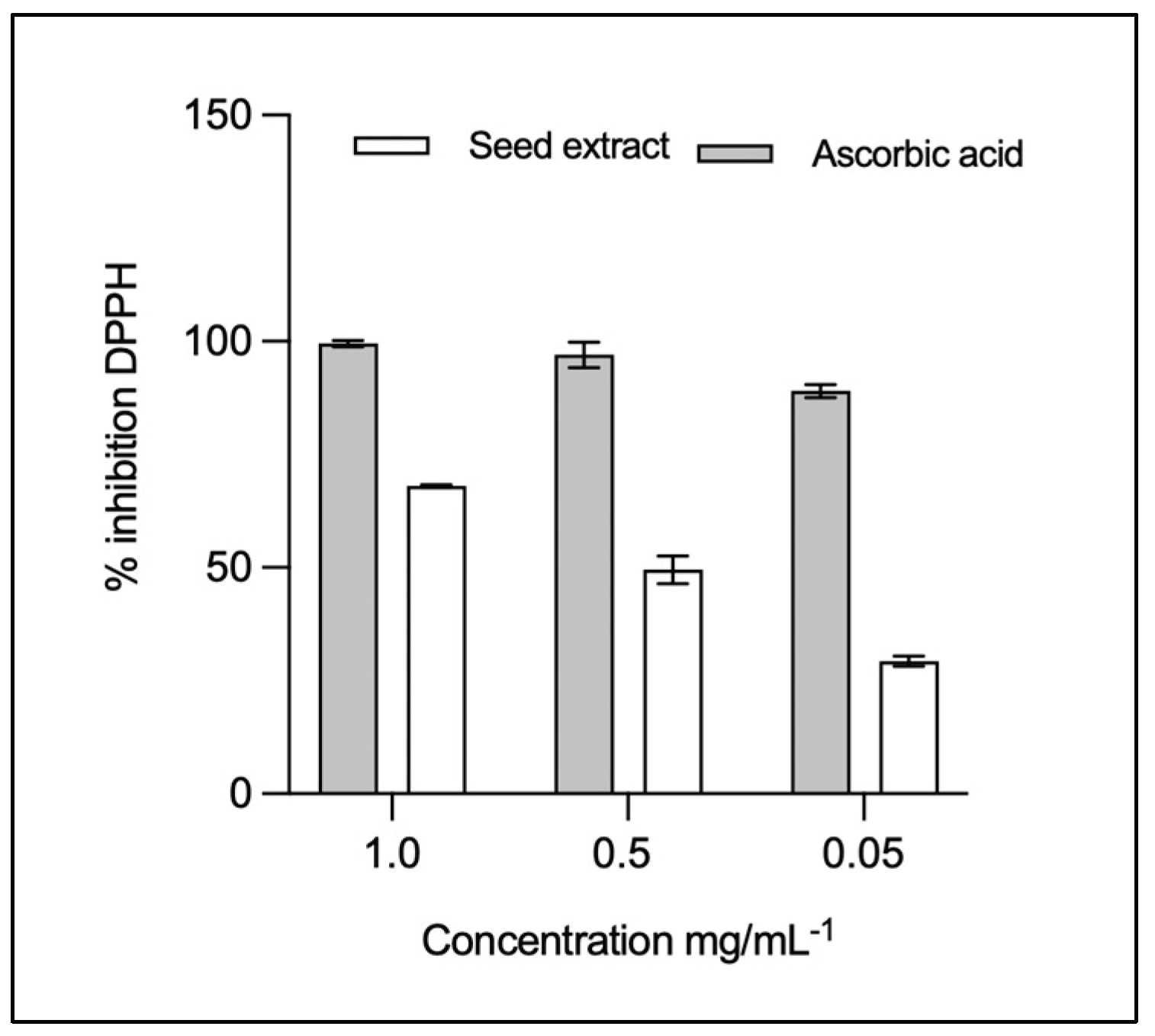
3.3. Antioxidant Activity
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Acknowledgments
Conflicts of Interest
References
- Viada Pupo, Esther, Gómez Robles, Lisvelt, & Campaña Marrero, Ibel Reyna. (2017). Estrés oxidativo. Correo Científico Médico, 21(1), 171-186. http://scielo.sld.cu/scielo.php?script=sci_arttext&pid=S1560-43812017000100014&lng=es&tlng=es.
- Guija-Poma, Emilio, Inocente-Camones, Miguel Ángel, Ponce-Pardo, John, & Zarzosa-Norabuena, Edwin. (2015). Evaluación de la técnica 2,2-Difenil-1-Picrilhidrazilo (DPPH) para determinar capacidad antioxidante. Horizonte Médico (Lima), 15(1), 57-60. http://www.scielo.org.pe/scielo.php?script=sci_arttext&pid=S1727-558X2015000100008&lng=es&tlng=es.
- Charlton, N. C., Mastyugin, M., Török, B., & Török, M. (2023). Structural Features of Small Molecule Antioxidants and Strategic Modifications to Improve Potential Bioactivity. Molecules, 28(3), 1057. [CrossRef]
- Rajendran, P.; Nandakumar, N.; Rengarajan, T.; Palaniswami, R.; Gnanadhas, E.N.; Lakshminarasaiah, U.; Gopas, J.; Nishigaki, I. (2014). Antioxidants and human diseases. Clin. Chim. Acta, 436, 332–347.
- Kumar, Vinay, Khan; Khan, Abdullah; Tripathi, Anu; Praveen, Dixit; Bajaj, U.K. (2015). Role of oxidative stress in various diseases: Relevance of dietary antioxidants. The Journal of Phytopharmacology, 4(2): 126-132.
- Aouache, R.; Biquard, L.; Vaiman, D.; Miralles, F. (2018). Oxidative Stress in Preeclampsia and Placental Diseases. Int. J. Mol. Sci., 19(5), 1496.
- Echavarría, A., D’Armas, H., Matute-L., N., Jaramillo, C., Rojas-de-Astudillo, L., & Benítez, R. (2016). Evaluación de la capacidad antioxidante y metabolitos secundarios de extractos de dieciséis plantas medicinales. Revista Ciencia Unemi, 9 (20), 29-35.
- Salas-Pérez, Lilia, Moncayo-Lujan, María del Rosario, Borroel-García, Victoria Jared, Guzmán-Silos, Tania Lizzeth, & Ramírez-Aragón, Mercedes Georgina. (2022). Composición fitoquímica y actividad antioxidante en tres variedades de albahaca por efecto de distintos solventes. Revista mexicana de ciencias agrícolas, 13(28), 113-123. [CrossRef]
- Gallegos Zurita, Maritza, Castro Posligua, Aída Águeda, Salazar Carranza, Luz Angélica, Mazacon Mora, Maite Cecilia, Orellana Villegas, Margarita, & Guija Poma, Emilio Teodoro. (2023). Metabolitos secundarios y capacidad antioxidante de especies vegetales en Ecuador. Salud(i)Ciencia, 25(7), 410-419. [CrossRef]
- Vilela, Alejandra E, González-Paleo, Luciana, & Ravetta, Damián A. (2011). Metabolismo secundario de plantas leñosas de zonas áridas: mecanismos de producción, funciones y posibilidades de aprovechamiento. Ecología austral, 21(3), 317-327. https://www.scielo.org.ar/scielo.php?script=sci_arttext&pid=S1667-782X2011000300007&lng=es&tlng=pt.
- Ramírez R., Dranguet A., Morales L. (2020). Actividad antiiflamatoria de plantas medicinales. Revista Granmnese de Desarrollo Rural. 16: 320-332.
- Nyonje, W.A., Owino, W. & Abong, G.O. Biochemical evaluation of spent Bixa (Bixa Orellana) seeds for potential application in the food industry. Discov Food 5, 193 (2025). [CrossRef]
- Hirko B., Getu A. (2022). Bixa orellana (Annatto Bixa): a review on use, structure, extraction methods and analysis. Journal of Agronomy, Technology and Engineering Management, 5(1): 687-696.
- De Oliveira, J. R. G.; Bonnet, A.; Braconnier, E.; Groult, H.; Prunier, G.; Beaugeard, L.; Grougnet, R.; Guedes, J. R. S.; Alves, C. A. F.; Picot, L. (2019). Bixin, an apocarotenoid isolated from Bixa orellana L., sensitizes human melanoma cells to dacarbazine-induced apoptosis through ROS-mediated cytotoxicity. Food and Chemical Toxicology, 125,549-561. doi: ff10.1016/j.fct.2019.02.013ff. ffhal-02074546f.
- Cuong Tran, V. and Chin Koo, B. (2016). Effects of annatto (Bixa orellana L.) seeds powder on physicochemical properties, antioxidant and antimicrobial activities of pork patties during refrigerated storage. Korean J. Food Sci. An., 36 (4), 476~486.
- Zarza-García, A. L.; Sauri-Duch, E.; Raddatz-Mota, D.; Cuevas-Glory, L. F.; Pinzón-López, L., L.; Rivera-Cabrera, F.; Mendoza-Espinoza, J. A. (2017). Pharmacological, phytochemical and morphological study of three Mayan accessions of Bixa orellana L. leaves. Emirates Journal of Food and Agriculture, 29(3), 163-169.
- Kusmita, L.; Franyoto Y.D.; Mutmainah M.; Puspitaningrum I.; Nurcahyanti A.D. (2022). Bixa orellana L. carotenoids: antiproliferative activity on human lung cancer, breast cancer, and cervical cancer cells in vitro. Nat Prod Res., 36(24):6421-6427. [CrossRef]
- Tarkany, B. R.; Barreto, de A. P. M.; de Oliveira S. I. M.; de Carvalho J. E; Nogueira, C. P. R., y Ann, F. M. (2020). Bixa orellana L. by-products’ fractions from an industrial process: antiproliferative activity on tumor cells and chemical profile. Nat Prod Res, 85(4):431-40. DOI.10.1080/14786419.2020.1826482.
- Valencia, D.; Aguilar González, D. I.; Ortega Gacía, J.; Godoy Hernández, G.; Leyva Peralta, M. A.; Moo Huchin, V. M.; Clarenc Aarland, R.; Quintero Vargas, J.; Mendoza Espinoza, J. A.; Zarza García, A. L. (2023). Phytochemical profile, antioxidant and antiproliferative activity from leaves and seeds of Bixa orellana L. from the Yucatán Peninsula, Mexico. Pharmacognosy Magazine, 19(2): 482–490. [CrossRef]
- Technologies, Inc. (2012). Mass Spectrometry Software. https://www.agilent.com/en/product/software-informatics/mass-spectrometry-software?Campaign_Source=PAN_PSM_Brand_G&gclsrc=aw.ds&gad_source=1&gad_campaignid=21857819690&gbraid=0AAAAADSHcWcc-Jpyzc19xKtqEx2nHE9be&gclid=Cj0KCQiAiqDJBhCXARIsABk2kSmnt7No7uSJF7pAtz-4MaZQnFrw9YqFfT5pB8gWRpsqafAO-Jk8lIcaAskKEALw_wcB.
- National Institute of Standars and Technology. (2025). https://www.nist.gov/.
- Liu L, Y Sun, T Laura, X Liang, H Ye, X Zeng (2009) Determination of polyphenolic content and antioxidant activity of kudingcha made from Ilex kudingcha C. J. Tseng. Food Chem. 112:35-41.
- Ibarra Estrada E., Pacheco Sánchez M., García Mateos R., San Miguel Chávez R., Ramírez Valverde G., & Soto Hernández M. (2011). Actividad antioxidante de alcaloides de Erythrina americana Miller. Revista fitotecnia mexicana, 34(4), 241-246. http://www.scielo.org.mx/scielo.php?script=sci_arttext&pid=S0187-73802011000400005&lng=es&tlng=es.
- GraphPad Prism. (2025). Versión 10.0. Consultado el 10 de noviembre de 2025. https://www.graphpad.com/.
- Valarezo, E.; Torres Torres, S.; Pineda Guamizo, N.; Jaramillo Fierro, X.; Cartuche, L.; Morocho, V.; Meneses, M. A. (2023). Study of essential oil Isolated from achiote (Bixa orellana) leaves: chemical composition, enantiomeric distribution and antimicrobial, antioxidant and anticholinesterase activities. Antibiotics. 12(4), 710. [CrossRef]
- Raju SK, Chandrasekar S, Vengadhajalapathy P, Sundaram R, Periyasamy S, Chinnaraj T, Sekar P, Kumar S. (2022). Revisión de la composición fitoquímica y las actividades farmacológicas de Bixa orellana L. J Pharm Biol Sci, 10(2):57-67. [CrossRef]
- Raddatz-Mota D, Pérez-Flores LJ, Carrari F, Mendoza-Espinoza JA, de León-Sánchez FD, Pinzón-López LL, Godoy-Hernández G, Rivera-Cabrera F. (2017). Achiote (Bixa orellana L.): a natural source of pigment and vitamin E. J Food Sci Technol., 54(6):1729-1741. [CrossRef]

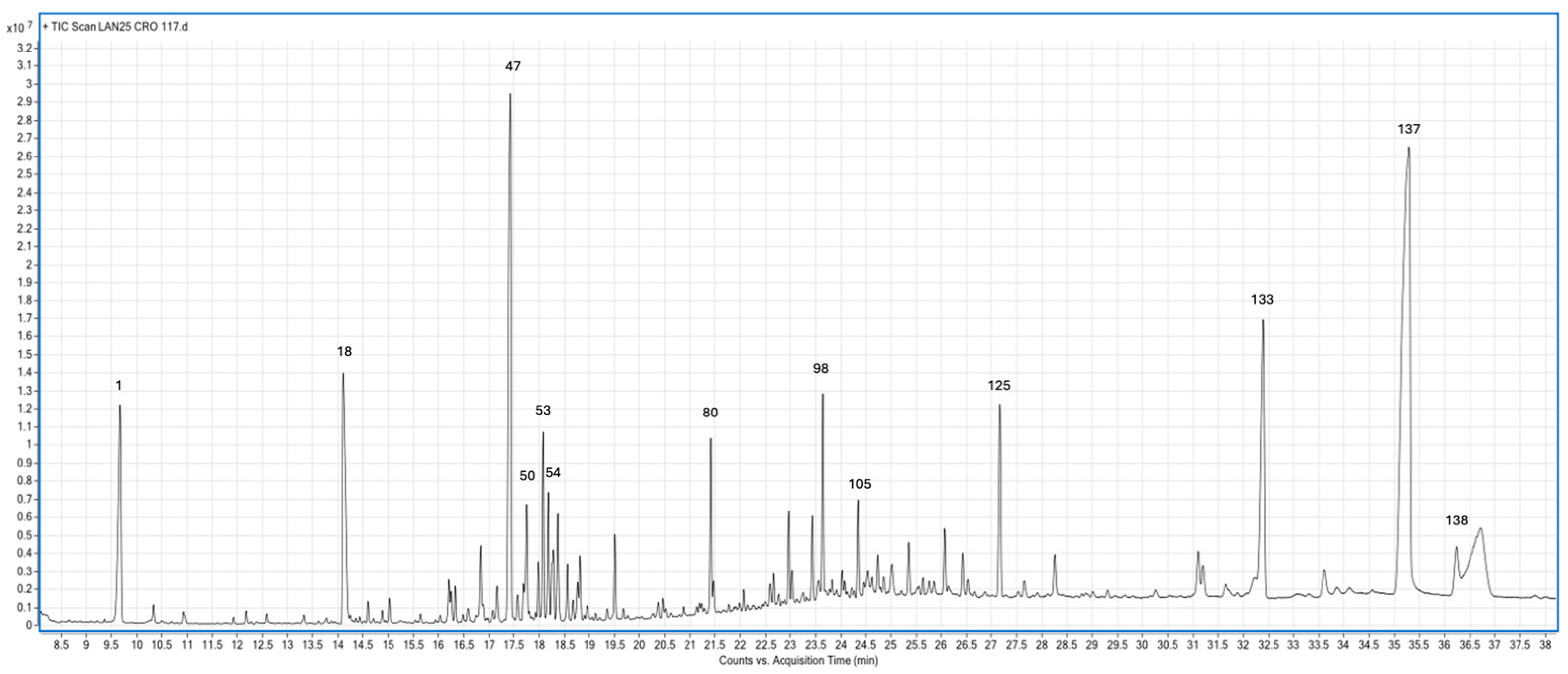
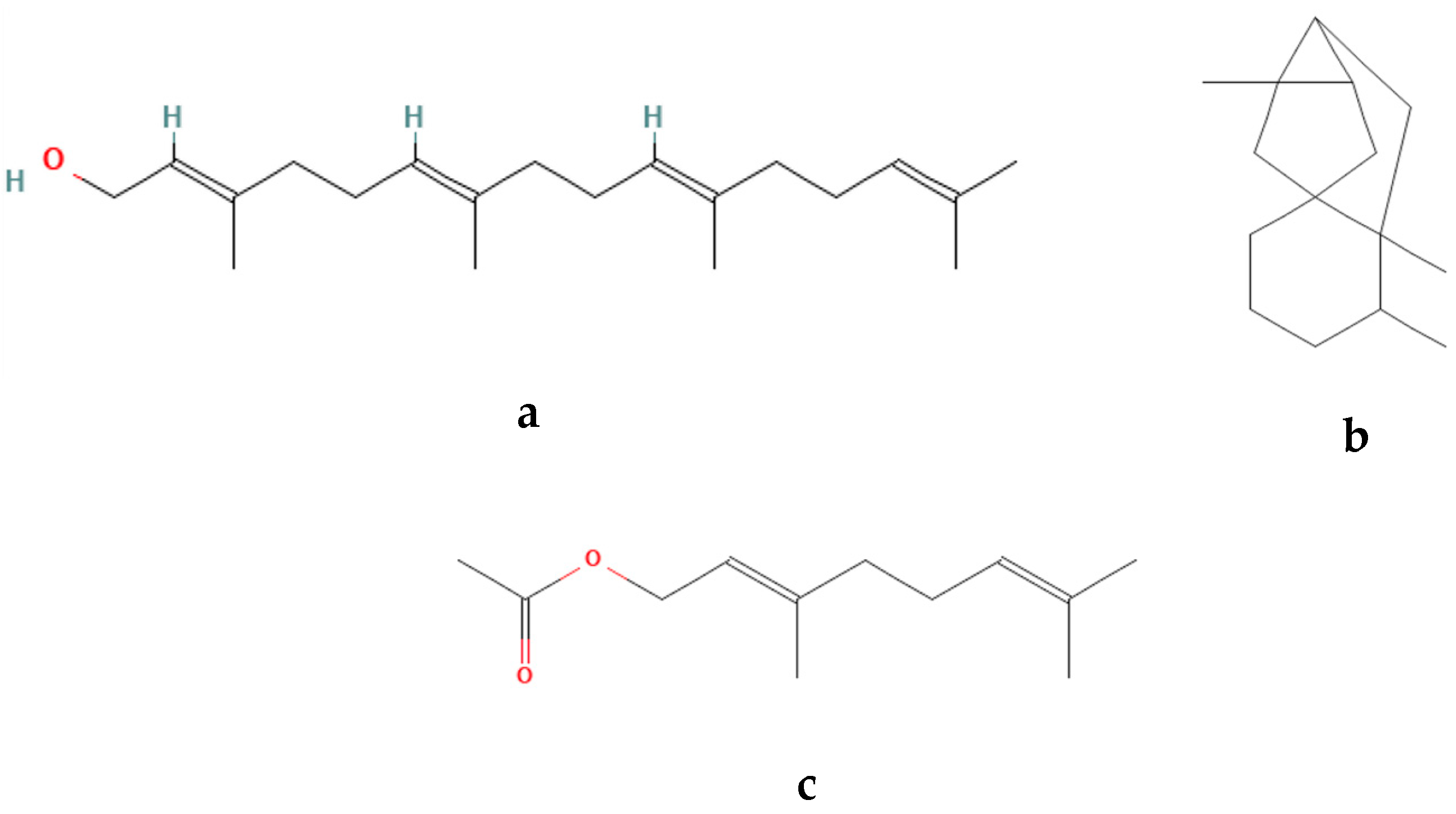
| Number | Name | RT Min | Percentage % | Chemical group |
| 1 | Isobutyl acetate | 8.656 | 0.0189 | Ester |
| 2 | 2,2,5-trimetilhexano | 8.88 | 0.0129 | Alkano |
| 3 | 2-Butenal, 2-methyl- | 9.087 | 0.0112 | Aldehyde |
| 4 | Benzene, 1,3-dimethyl- | 9.675 | 4.9286 | Aromatic hydrocarbon |
| 5 | Dodecane | 10.337 | 0.2762 | Alkane |
| 6 | 2-Hexanol | 10.696 | 0.0162 | Alcohol |
| 7 | Cyclopentanol, 1-methyl- | 10.927 | 0.1195 | Alcohol |
| 8 | 2-Propanone, 1-hydroxy- | 12.179 | 0.1542 | Hydroxyacetona |
| 9 | Propanoic acid, 2-hydroxy-, methyl ester, (.+/-.)- | 12.281 | 0.0195 | Fatty acid ester |
| 10 | Acetaldehyde, hydroxy- | 12.389 | 0.08592 | Hydroxyaldehyde |
| 11 | 5-Hepten-2-one, 6-methyl- | 12.584 | 0.09831 | Ketone |
| 12 | Acetic acid, hydroxy-, methyl ester | 13.302 | 0.0256 | Carboxylic ester |
| 13 | Tetradecane | 13.333 | 0.0894 | Alkane |
| 14 | Ethanol, 2-butoxy- | 13.621 | 0.0261 | Alcohol |
| 15 | 2,2’-Bioxirane | 13.774 | 0.0663 | Epoxide |
| 16 | Benzene, 1,3-bis(1,1-dimethylethyl)- | 13.874 | 0.0233 | Alkylbenzene |
| 17 | Dodecane, 1-iodo- | 13.938 | 0.0215 | Alkane |
| 18 | Acetic acid | 14.107 | 6.0109 | Carboxylic acid |
| 19 | 2(3H)-Furanone, 5-methyl- | 14.166 | 0.068 | Lactone |
| 20 | Propanoic acid, 2-oxo-, methyl ester | 14.25 | 0.0758 | Fatty acid |
| 21 | α-Cubebene | 14.435 | 0.0571 | Sesquiterpene |
| 22 | σ-Elemene | 14.599 | 0.2261 | Sesquiterpene |
| 23 | 2-Ethyl-1-hexanol | 14.705 | 0.0575 | Alcohol |
| 24 | Ylangene | 14.882 | 0.1443 | Sesquiterpene |
| 25 | α-Copaene | 15.023 | 0.2872 | Sesquiterpene |
| 26 | 2,4-Dihydroxy-2,5-dimethyl-3(2H)-furan-3-one | 15.256 | 0.0613 | Furano |
| 27 | cis-Muurola-4(15),5-diene | 15.644 | 0.1055 | Sesquiterpene |
| 28 | Furan, 2-ethyl-5-methyl- | 15.76 | 0.0133 | Furan |
| 29 | 2-Furancarboxaldehyde, 5-methyl- | 15.88 | 0.0092 | Furan |
| 30 | 1,2-Propanediol, 1-acetate | 15.943 | 0.0351 | Ester |
| 31 | Pentanoic acid, 4-oxo-, methyl ester | 16.037 | 0.0772 | Fatty acid |
| 32 | Hexadecane | 16.181 | 0.0417 | Alkane |
| 33 | (-)-Aristolene | 16.208 | 0.5095 | Sesquiterpene |
| 34 | Methyl 4-oxo-2-pentenoate | 16.253 | 0.3422 | Ester |
| 35 | β-Elemene | 16.334 | 0.3993 | Sesquiterpene |
| 36 | B-copaene | 16.487 | 0.0888 | Sesquiterpene |
| 37 | Cyclooctasiloxane, hexadecamethyl- | 16.571 | 0.029 | Xylosane |
| 38 | Caryophyllene | 16.589 | 0.1648 | Sesquiterpene |
| 39 | 1,2-Ethanediol, monoacetate | 16.809 | 0.099 | Ester |
| 40 | Naphthalene | 16.835 | 0.9478 | Sesquiterpene |
| 41 | Isoledene | 16.887 | 0.1224 | Sesquiterpene |
| 42 | Benzaldehyde, 4-methyl- | 16.941 | 0.0451 | Aldehyde |
| 43 | Elixene | 16.976 | 0.0253 | Sesquiterpene |
| 44 | Isoledene | 17.087 | 0.1501 | Sesquiterpene |
| 45 | Cyclosativene | 17.17 | 0.4759 | Sesquiterpene |
| 46 | Glyoxal, 4-methylphenyl- | 17.31 | 0.0259 | Aldehyde |
| 47 | Ishwarane | 17.433 | 11.0164 | Sesquiterpene |
| 48 | Humulene | 17.579 | 0.3048 | Sesquiterpene |
| 49 | 2-Furancarboxylic acid, 3-methyl-, methyl ester | 17.639 | 0.0112 | Ester |
| 50 | α-Amorphene | 17.755 | 1.7063 | Sesquiterpene |
| 51 | δ-Selinene | 17.928 | 0.0724 | Sesquiterpene |
| 52 | Aristolochene | 17.984 | 0.6452 | Sesquiterpene |
| 53 | β-Cubebene | 18.082 | 2.2405 | Sesquiterpene |
| 54 | Valencene | 18.185 | 1.4967 | Sesquiterpene |
| 55 | 2-Isopropenyl-4a,8-dimethyl-1,2,3,4,4a,5,6,7-octahydronaphthalene | 18.285 | 0.974 | Sesquiterpene |
| 56 | Benzoic acid, 3-methyl-, methyl ester | 18.353 | 0.2178 | Carboxylic acid |
| 57 | Naphthalene | 18.373 | 1.1754 | Aromatic hydrocarbon |
| 58 | δ-Cadinene | 18.563 | 0.6178 | Sesquiterpene |
| 59 | γ-Cadinene | 18.675 | 0.1652 | Sesquiterpene |
| 60 | 1,2-Cyclopentanedione | 18.754 | 0.3006 | Ketone |
| 61 | (-)-.α-Panasinsen | 18.808 | 0.775 | Sesquiterpene |
| 62 | Valencen | 18.895 | 0.0481 | Sesquiterpene |
| 63 | Ethanone, 1-(4-methylphenyl)- | 18.956 | 0.1712 | Ketone |
| 64 | 2-Hexanone, 6-(acetyloxy)- | 19.127 | 0.0699 | Ketone/ester |
| 65 | 4-Methylphenyl acetone | 19.355 | 0.1071 | Ketone |
| 66 | Acetic acid | 19.464 | 0.0477 | Aromatic hydrocarbon |
| 67 | 1H-Cyclopropa[a]naphthalene | 19.508 | 0.9378 | Sesquiterpene |
| 68 | Calamenene | 19.577 | 0.0111 | Sesquiterpene |
| 69 | 5,9-Undecadien-2-one, 6,10-dimethyl-, (Z)- | 19.676 | 0.1084 | Monoterpene |
| 70 | 2,5-Dihydroxyheptane | 19.763 | 0.0511 | Alcohol |
| 71 | Epicubebol | 20.245 | 0.0349 | Alcohol |
| 72 | 2,6-Bis(1,1-dimethylethyl)-4-(1-oxopropyl)phenol | 20.322 | 0.0108 | Phenol |
| 73 | 2,5-Dihydroxyheptane | 20.368 | 0.2035 | Alcohol |
| 74 | Furan, tetrahydro-2,5-dimethyl- | 20.458 | 0.1935 | Ester |
| 75 | Naphthalene | 20.625 | 0.0329 | Aromatic hydrocarbon |
| 76 | Carotol | 20.864 | 0.0966 | Sesquiterpene |
| 77 | β-Calacorene | 21.124 | 0.0448 | Aromatic hydrocarbon |
| 78 | Cadina-1(10),6,8-triene | 21.191 | 0.1192 | Sesquiterpene |
| 79 | 1,5-Hexanediol | 21.234 | 0.1271 | Alcohol |
| 80 | Benzaldehyde | 21.415 | 2.0419 | Aldehyde |
| 81 | γ-Gurjunene | 21.468 | 0.3336 | Sesquiterpene |
| 82 | 1,1,4,7-Tetramethyldecahydro-1H-cyclopropa[e]azulene-4,7-diol | 21.771 | 0.0685 | Sesquiterpene/alcohol |
| 83 | Geranil hexanoato | 21.873 | 0.0463 | Ester |
| 84 | Cyclododecasiloxane | 21.901 | 0.0158 | Xylosane |
| 85 | Cyclohexylideneacetone | 21.981 | 0.0852 | Ketone |
| 86 | 1,3,6,10-Cyclotetradecatetraene | 22.071 | 0.2154 | Diterpene |
| 87 | 1,3,6,10-Cyclotetradecatetraene | 22.487 | 0.0918 | Diterpene |
| 88 | Dihydroxyacetone | 22.584 | 0.3637 | Ketone |
| 89 | 1,2,4-Trimethoxybenzene | 22.655 | 0.3557 | Benzene |
| 90 | 5-Cyclodecen-1-ol | 22.759 | 0.0946 | Sesquiterpene |
| 91 | Piperazine, 1,4-dimethyl- | 22.826 | 0.053 | Ester |
| 92 | Espatulenol | 22.965 | 1.0143 | Sesquiterpene |
| 93 | γ-Costol | 23.027 | 0.3133 | Alcohol |
| 94 | 1-Nonanol | 23.125 | 0.0319 | Alcohol |
| 95 | Cembrene | 23.252 | 0.1186 | Diterpene |
| 96 | Selin-6-en-4.alpha.-ol | 23.431 | 0.9214 | Sesquiterpene |
| 97 | Germacreno-δ-4-ol | 23.558 | 0.2542 | Alcohol |
| 98 | Palmitic acid | 23.637 | 2.3622 | Fatty acid |
| 99 | Glycerol α-monoacetate | 23.826 | 0.1558 | Alcohol |
| 100 | α-Springene | 23.917 | 0.0507 | Diterpene |
| 101 | Ethyl palmitate | 24.02 | 0.2777 | Fatty acid ester |
| 102 | 4(15),5,10(14)-Germacratrien-1-ol | 24.075 | 0.1772 | Sesquiterpene |
| 103 | Isospathulenol | 24.222 | 0.1035 | Sesquiterpene |
| 104 | 1-Naphthalenol | 24.271 | 0.0543 | Sesquiterpene |
| 105 | Isospathulenol | 24.35 | 1.131 | Sesquiterpene |
| 106 | 1-Naphthalenol | 24.459 | 0.1421 | Sesquiterpene |
| 107 | Glycerol 1-monoacetate | 24.539 | 0.3332 | Ester |
| 108 | 4H-Pyran-4-one, 2,3-dihydro-3,5-dihydroxy-6-methyl- | 24.621 | 0.1979 | Ketone |
| 109 | Humulane-1,6-dien-3-ol | 24.736 | 0.5011 | Sesquiterpene |
| 110 | α-Muurolene-14-ol | 24.865 | 0.2078 | Alcohol |
| 111 | Glycerin | 25.004 | 0.2592 | Alcohol |
| 112 | Sobrerol | 25.024 | 0.216 | Alcohol |
| 113 | β-Selinene | 25.035 | 0.2466 | Sesquiterpene |
| 114 | Viridiflorol | 25.358 | 0.7184 | Sesquiterpene |
| 115 | Farnesyl acetone | 25.559 | 0.12 | Ketone |
| 116 | 7R,8R-8-Hydroxy-4-isopropylidene-7-methylbicyclo [5.3.1]undec-1-ene | 25.641 | 0.1988 | Alkene |
| 117 | 2-Decenoic acid | 25.763 | 0.1744 | Fatty acid |
| 118 | 2-Naphthalenol, | 25.865 | 0.2292 | Sesquiterpene |
| 119 | Methyl stearate | 26.074 | 0.8862 | Fatty acid ester |
| 120 | Isobutyl palmitate | 26.195 | 0.0468 | Ester |
| 121 | Elaidic acid | 26.427 | 0.5943 | Fatty acid |
| 122 | Elaidic acid | 26.527 | 0.1887 | Fatty acid |
| 123 | Dehydrofukinone | 26.537 | 0.1071 | Ketone/sesquiterpene |
| 124 | Cyclododecasiloxane | 26.953 | 0.014 | Xylosane |
| 125 | Linoleic acid | 27.169 | 3.0674 | Fatty acid |
| 126 | 5-Hydroxymethylfurfural | 27.527 | 0.0946 | Aldehyde/alcohol |
| 127 | Linoleic acid | 27.649 | 0.2297 | Fatty acid |
| 128 | Linolenic acid | 28.261 | 0.7189 | Fatty acid |
| 129 | trans-2-Dodecenoic acid | 29.014 | 0.0979 | Fatty acid |
| 130 | Eicosanoic acid, methyl ester | 29.306 | 0.1185 | Fatty acid |
| 131 | Geranyl-.α.-terpinene | 31.203 | 0.6396 | Diterpene |
| 132 | Geranylgeranyl formate | 31.656 | 0.2687 | Terpenoid ester |
| 133 | Gernylgeraniol acetate | 32.397 | 7.7694 | Acetate ester |
| 134 | Acetic acid, 2-(1-buten-3-yl)-2-nitro-, ethyl ester | 33.614 | 0.7082 | Ester |
| 135 | Farnesyl propionate | 33.856 | 0.17078 | Sesquiterpene |
| 136 | Adipic acid | 34.102 | 0.1543 | Carboxylic acid |
| 137 | Geranylgeraniol | 35.293 | 27.5022 | Diterpene |
| 138 | Palmitic acid | 36.239 | 1.3027 | Fatty acid |
| 139 | Tyrosol, acetate | 37.8 | 0.0942 | Ester |
| 140 | Tyrosol, acetate | 37.8 | 0.0941 | Ester |
| 141 | Phthalic acid, butyl 2-pentyl ester | 37.998 | 0.0365 | Ester |
| Extract or compound | Concentration (mg/mL-1) | DPPH radical inhibition (%) | IC50 ± DE (mg mL-1) |
| Ascorbic acid | 1.0 0.5 0.05 |
99.50 97.00 89.00 |
0.2266 ± 0.0303 |
| Seed | 1.0 0.5 0.05 |
59.70 47.50 31.45 |
0.5108 ± 0.1891 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license.





