1. Introduction
Harnessing solar energy through photovoltaic technologies has been a major focus of research for decades. Among these, organic solar cells (OSCs) have attracted considerable attention due to their unique advantages, including lightweight construction, mechanical flexibility, low-cost solution processability, and potential for large-area fabrication [
1,
2,
3] A particularly promising OSC architecture is the bulk heterojunction (BHJ), which forms a nanoscale interpenetrating network between electron-donating polymers and electron-accepting fullerene derivatives, facilitating efficient exciton dissociation and charge collection. Poly(3-hexylthiophene-2,5-diyl) (P3HT) and the fullerene derivative [
6,
6]-phenyl-C61-butyric acid methyl ester (PCBM) are among the most widely used materials for BHJ-OSCs. In a typical P3HT:PCBM device, the active layer is sandwiched between a transparent front electrode, such as indium tin oxide (ITO), and a metallic back electrode, such as aluminum (Al) [
4,
5,
6]. Despite their advantages, P3HT:PCBM OSCs are generally limited to power conversion efficiencies below ~6% [
7,
8], constrained by factors such as interfacial chemical incompatibility [
9,
10], low open-circuit voltage [
11,
12,
13], active layer degradation [
14], and limitations in light absorption and layer thickness [
4,
15,
16,
17].
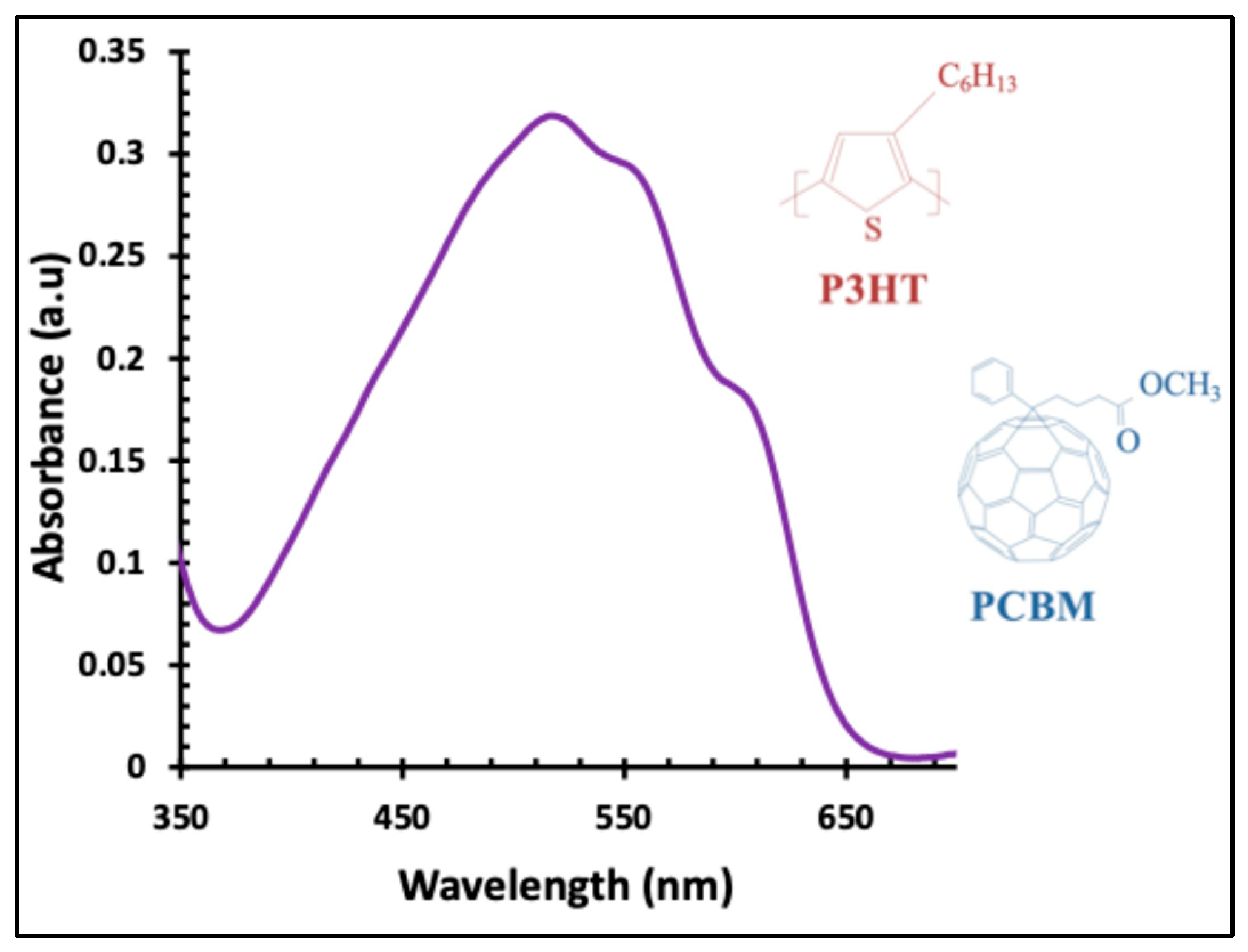
Figure 1 shows the chemical structures of P3HT:PCBM, and its absorption spectrum, which exhibits a main absorption band from 420 to 620 nm, with a maximum at 520 nm and shoulders at 560 and 600 nm.
Various strategies have been explored to enhance OSC performance. These include introducing noble metal nanoparticles to improve light absorption [
18,
19,
20,
21,
22], modifying cathodic interfaces to facilitate electron extraction [
6,
23,
24,
25], optimizing annealing conditions to enhance polymer:fullerene morphology [
4,
5,
26], and engineering anodic interfacial layers to improve hole collection [
27,
28,
29].
A major limitation in P3HT:PCBM OSCs is the restricted light absorption of the active layer. Increasing the thickness beyond ~250 nm to boost absorption is not practical, as it promotes charge recombination and increases series resistance (R
S), both of which lower device efficiency [
2,
4,
15,
16,
17,
26,
30,
31]. Consequently, enhancing absorption without increasing thickness is critical, for example by integrating noble metal nanoparticles or ultrathin metallic films to exploit plasmonic effects [
18,
19,
20,
21,
22].
Optimizing electrode interfaces is another key factor. Reducing R
S and facilitating charge extraction enhances PCE [
9]. Techniques such as oxygen plasma treatment of ITO [
32,
33] and deposition of hole-transporting buffer layers like poly(3,4-ethylenedioxythiophene):poly(styrenesulfonate) (PEDOT:PSS) [
34,
35] improve interfacial quality. Deposition of noble metal or metal oxide thin films on the ITO surface can further tune the work function to promote efficient hole collection [
1,
9,
19].
Among these materials, Ag/AgO thin films are promising for anodic interface modification due to their high transparency, conductivity, and absorptivity [
36]. Prior studies have shown that annealing Ag nanofilms can significantly enhance their light absorption, which overlaps the P3HT:PCBM active layer spectrum [
37,
38,
39]. However, there remains a research gap: while plasmonic and metal/metal-oxide interlayers have been explored for enhancing OSCs, the
combined effect of Ag film thickness and annealing on both optical absorption and device performance in P3HT:PCBM OSCs has not been systematically investigated. Specifically, the relationship between ultrathin Ag/AgO interfacial layers, their LSPR-enhanced absorption, energy-level alignment, and charge transport has not been fully elucidated.
In this work, we address this gap by studying the effect of annealing Ag nanofilms of varying thicknesses (1–6 nm) at 300 °C on their optical properties. We also examine how deposition and annealing of Ag films with thicknesses of 1, 2, and 6 nm influence the optical absorption, charge transport, and overall photovoltaic performance of P3HT:PCBM OSCs. This study provides insight into optimizing ultrathin Ag/AgO interfacial layers to enhance hole collection and device efficiency.
2. Materials and Fabrication
2.1. Chemical Materials
All chemicals and materials were used as received, with no additional purification. Regioregular poly(3-hexylthiophene) (P3HT, 98%) was sourced from Rieke Metals. The fullerene derivative [
6,
6]-phenyl-C₆₁-butyric acid methyl ester (PCBM, >99.5%) and 1,2-dichlorobenzene (anhydrous, 99%) were purchased from Sigma-Aldrich. Poly(3,4-ethylenedioxythiophene):poly(styrenesulfonate) (PEDOT:PSS, CLEVIOS™ P VP AI 4083), was obtained from HC Starck. Silver (Ag), aluminum (Al), and lithium fluoride (LiF), each with a purity of 99.99% (metals basis), were supplied by Alfa Aesar.
2.2. Instrumentation
UV–visible absorption spectra were recorded using a double-beam spectrophotometer (PerkinElmer LAMBDA 650, PerkinElmer, Shelton, CT, USA) over the wavelength range of approximately 300–850 nm. The photovoltaic performance of the organic solar cell devices was evaluated under simulated solar illumination using a xenon-lamp solar simulator (Oriel Instruments) equipped with an AM 1.5G filter and operated under ambient conditions. The light intensity was calibrated to 100 mW cm⁻² using a calibrated light meter (LI-250, LI-COR Biosciences, Lincoln, NE, USA). Current–voltage (J–V) characteristics were measured with a source-measure unit/electrometer (Keithley 2400, Keithley Instruments, Solon, OH, USA). Thin films of the active layer (P3HT:PCBM) were deposited using a spin coater (Laurell WS-650Mz-23NPPB, Laurell Technologies Corporation, Lansdale, PA, USA). Metal electrodes (Ag, Li, and Al) were deposited by thermal evaporation using a vacuum evaporator (Tecuum VCM 600 V1, Tecuum AG, Winterthur, Switzerland). In addition, a hotplate, ultrasonic bath, and plasma etcher were employed during the fabrication of the organic solar cells.
3. Fabrication and Deposition
3.1. Fabrication of OSC Device
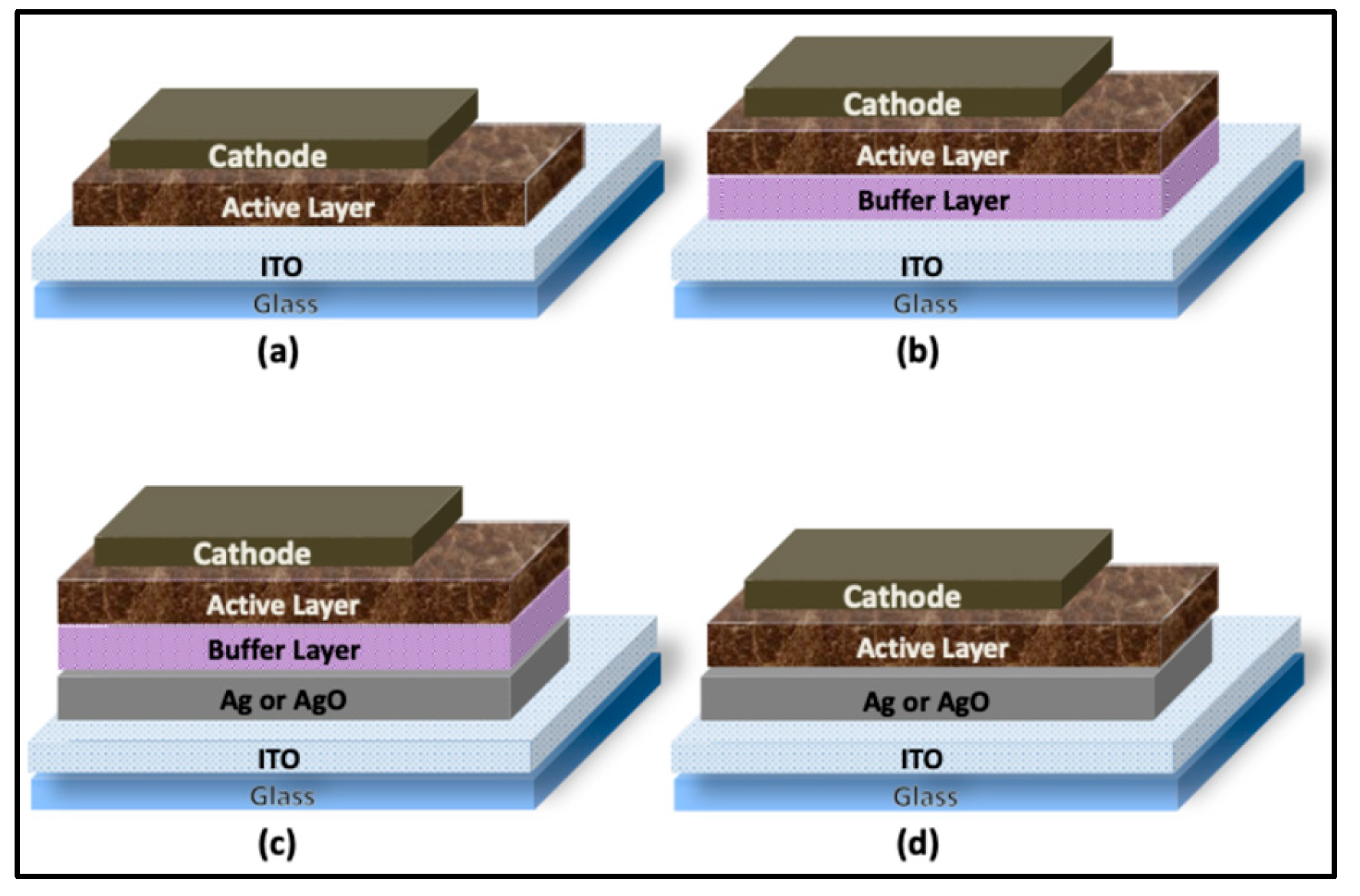
To prepare the device substrates, indium tin oxide (ITO)–coated glass was cut to the desired dimensions, patterned, and initially cleaned with a laboratory detergent. The substrates were subsequently sonicated in acetone, isopropanol, and deionized (DI) water for 10 min each. After cleaning, the substrates were dried under a nitrogen stream, thermally treated at 150 °C for 20 min, and then exposed to oxygen plasma to improve surface cleanliness and wettability. In all devices, the ITO layer served as the anodic electrode. Standard devices with the architecture Glass/Anode/BufferLayer/ActiveLayer/Cathode, as illustrated in
Figure 1(b), were fabricated as follows: An aqueous dispersion of the buffer layer material, PEDOT:PSS, was filtered through a 0.45 µm PVDF membrane and spin-coated onto the pretreated ITO substrates at 4000 rpm for 30 s, using an acceleration time of 10 s to reach 1100 rpm. The resulting films were annealed at 120 °C for 1 h to ensure complete solvent removal and subsequently transferred into a nitrogen-filled glove box for deposition of the active layer. The bulk heterojunction active layer was prepared by dissolving P3HT and PCBM (20 mg mL
-1, 1:0.8 w/w) in 1,2-dichlorobenzene, followed by filtration through a 0.45 µm PTFE membrane. The solution was deposited onto the ITO/PEDOT:PSS stack using a three-step spin-coating process (200 rpm for 5 s, 500 rpm for 5 s, and 1000 rpm for 60 s) to form a uniform active layer. The as-cast films were placed in covered Petri dishes inside the glove box and allowed to dry under ambient conditions for 30 min.
Finally, the cathode was deposited by thermal evaporation under high-vacuum conditions (<10⁻⁶ Torr), forming a bilayer consisting of lithium fluoride (LiF, ~6 nm) and aluminum (Al, ~90 nm). Deposition rates were maintained at approximately 1 Å s⁻¹ for LiF and 2.5–5 Å s⁻¹ for Al. A shadow mask was employed to define the electrode geometry. Each substrate contained eight identical devices, each with an active area of 0.16 cm². The final device configurations are summarized in
Figure 2.
For devices incorporating an Ag nanofilm, silver was thermally evaporated onto pre-cleaned ITO-coated glass substrates. The deposition was carried out at a controlled rate of approximately 2.5–5 Å s⁻¹. Following deposition, the substrates were placed on a hotplate and thermally annealed at 300 °C for either 1 or 2 h prior to completing the deposition of the remaining device layers. Ag nanofilms with nominal thicknesses ranging from 1 to 6 nm were fabricated. The optical absorption characteristics of the deposited Ag nanofilms were subsequently examined using a UV–visible spectrophotometer.
4. Measurements and Discussion
4.1. Optical Absorption of Ag Nanofilms
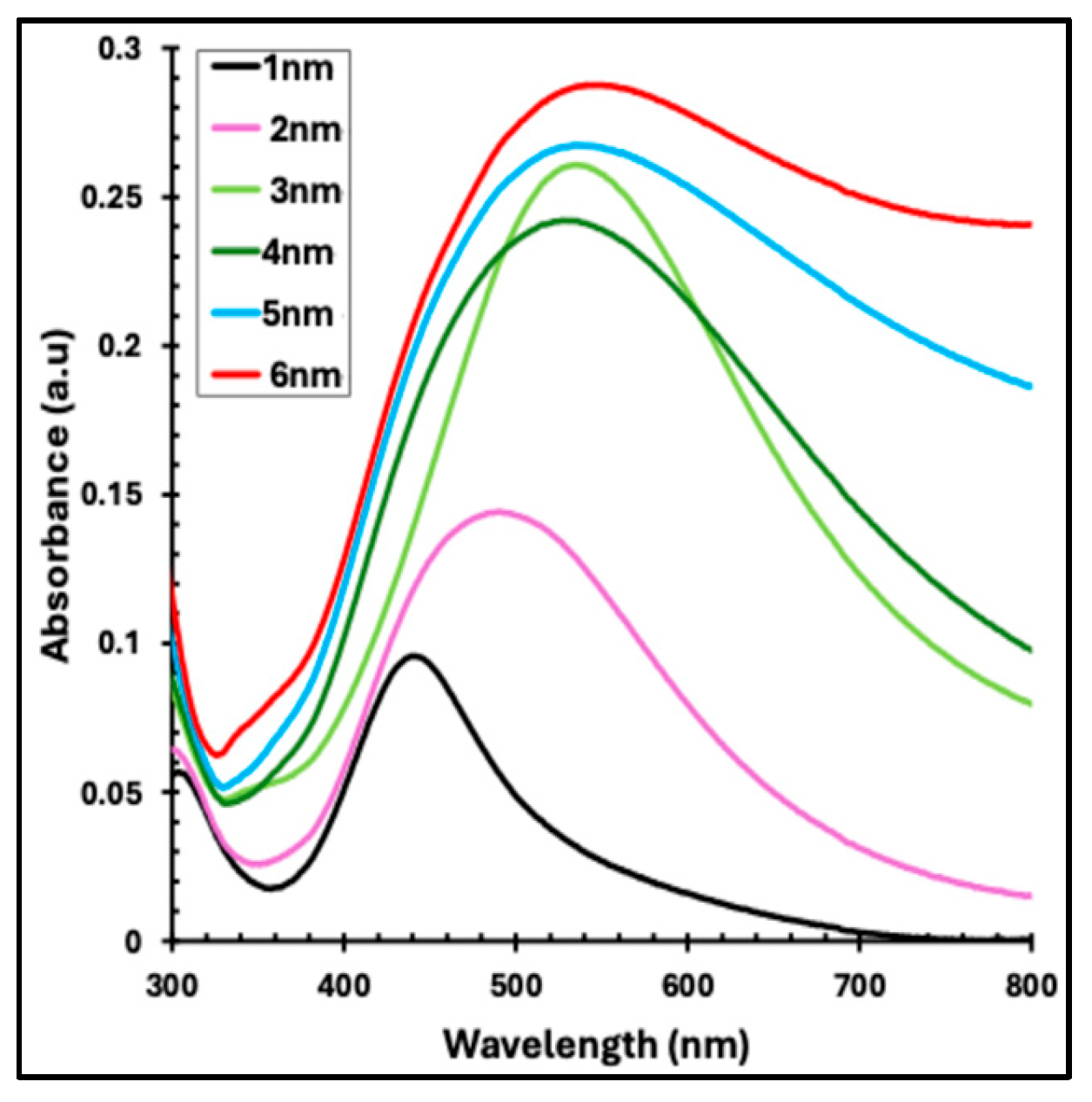
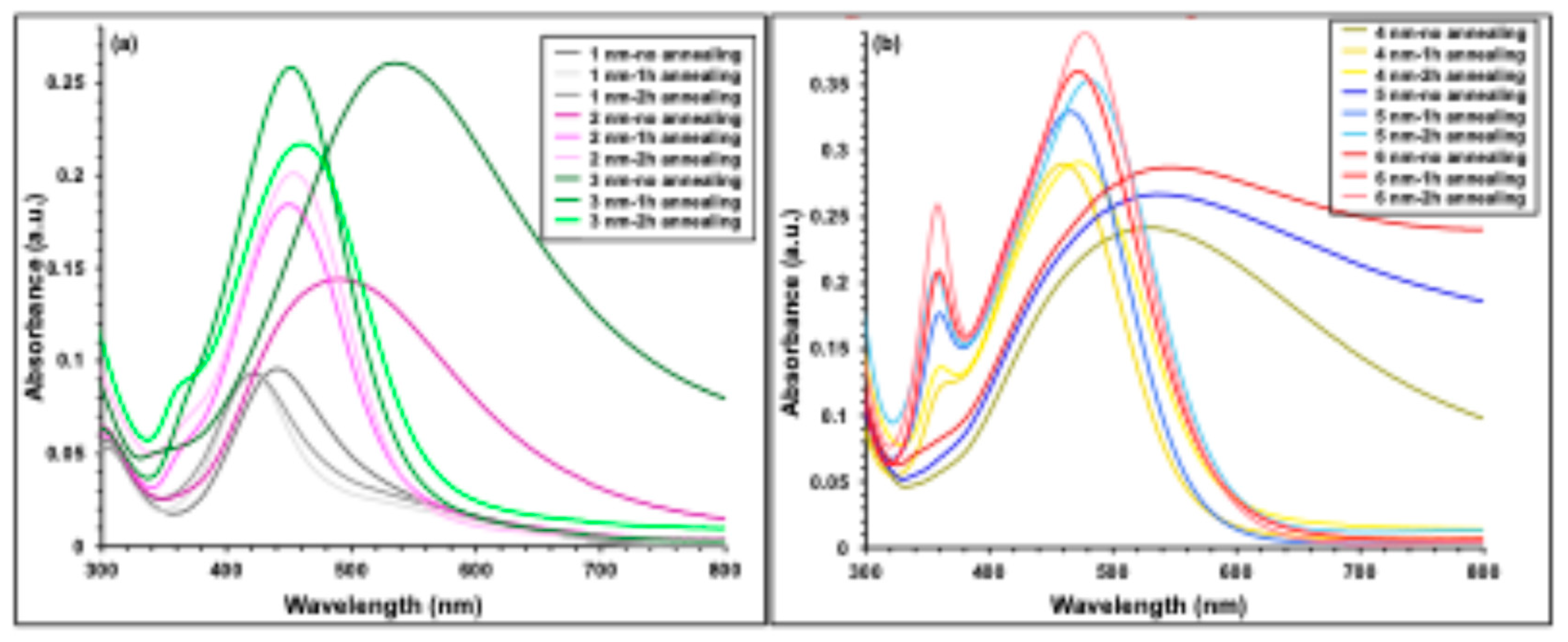
Figure 3 presents the UV–visible absorbance spectra of thermally evaporated Ag nanofilms with thicknesses ranging from 1 to 6 nm. As the Ag nanofilm thickness increases, a pronounced enhancement in absorbance intensity is observed. Concurrently, the characteristic absorption band becomes progressively broader and exhibits a red shift, moving from approximately 443 nm for the 1 nm Ag nanofilm to about 546 nm for the 6 nm film. These absorption features are attributed to localized surface plasmon resonance (LSPR), which arises from the collective oscillation of free electrons at the metal–dielectric interface, leading to strong light absorption and scattering in the visible region of the spectrum [
40,
41]. Notably, the spectral range of Ag nanofilm absorption significantly overlaps with that of the P3HT:PCBM active layer which is illustrated in
Figure 1, indicating potential optical interaction between the plasmonic layer and the photoactive material.
4.2. Optical Absorption of Annealed Ag Nanofilms
Figure 4 compares the UV–visible absorption spectra of Ag nanofilms before and after thermal annealing at 300 °C for durations of 1 and 2 h. For the 1 nm Ag film, represented by the grey curves in
Figure 4(a), annealing did not significantly alter the absorption intensity; however, a noticeable blue shift of the plasmonic peak from approximately 445 nm to 421 nm was observed after annealing. In the case of the 2 nm Ag film (pink curves in
Figure 4a), thermal treatment led to a clear enhancement in absorption intensity, particularly after 2 h of annealing, accompanied by a pronounced blue shift from about 500 nm to 421 nm. For the 3 nm Ag film (green curves in
Figure 4a), annealing for 2 h resulted in a reduction in absorption intensity, while still inducing a blue shift of the absorption peak from approximately 540 nm to 450 nm. In contrast, thicker Ag nanofilms with nominal thicknesses of 4, 5, and 6 nm (yellow, blue, and red curves in
Figure 4(b), respectively) exhibited an overall increase in absorption intensity after annealing, with the most pronounced enhancement observed for the 2 h annealed samples. For these films, the main absorption peak shifted from around 550 nm to approximately 480 nm following annealing. In addition, secondary absorption peaks near 360 nm emerged for the 4–6 nm films after thermal treatment.
These results demonstrate that the optical absorption of Ag nanofilms is strongly influenced by annealing temperature, annealing duration, and initial film thickness. The enhanced absorption observed after annealing is attributed to the transformation of the continuous Ag nanofilm into discrete or quasi-spherical Ag nanoparticles, which support localized surface plasmon resonance (LSPR). Both the annealing temperature and time play critical roles in tuning the nanoparticle size and crystallinity, thereby modifying the position and intensity of the plasmonic absorption peaks [
36,
39]. The observed blue shifts in the absorption maxima after annealing are commonly associated with quantum confinement effects, suggesting a reduction in the effective particle size [
38,
42]. Furthermore, the appearance of secondary absorption peaks in the annealed 4–6 nm films may be related to particle shape anisotropy or elongation, which enables plasmon excitation along multiple axes [
1]. It should also be noted that annealing Ag nanofilms under ambient conditions can promote partial oxidation, leading to the formation of AgO due to increased atomic mobility and oxygen availability during air annealing [
37,
43].
4.3. Effect of 1 nm Ag/AgO Film on OSC Parameters
A 1 nm Ag layer was thermally evaporated onto pre-cleaned ITO-coated glass substrates, which were subsequently annealed at 300 °C for 2 h prior to deposition of the remaining device layers. For comparison, four sets of devices, corresponding to the architectures shown in
Figure 2, were fabricated simultaneously: (1) ITO/Active Layer/Cathode, (2) ITO/BufferLayer/ActiveLayer/Cathode, (3) ITO/1nm AgO(2h-annealing)/BufferLayer/ActiveLayer/Cathode, (4) ITO/1nmAgO(2 h-annealing)/ActiveLayer/Cathode. To evaluate the effect of the Ag/AgO layer on hole collection efficiency and overall device performance, the influence of the Ag/AgO films on both the optical and electrical properties of the devices was investigated.
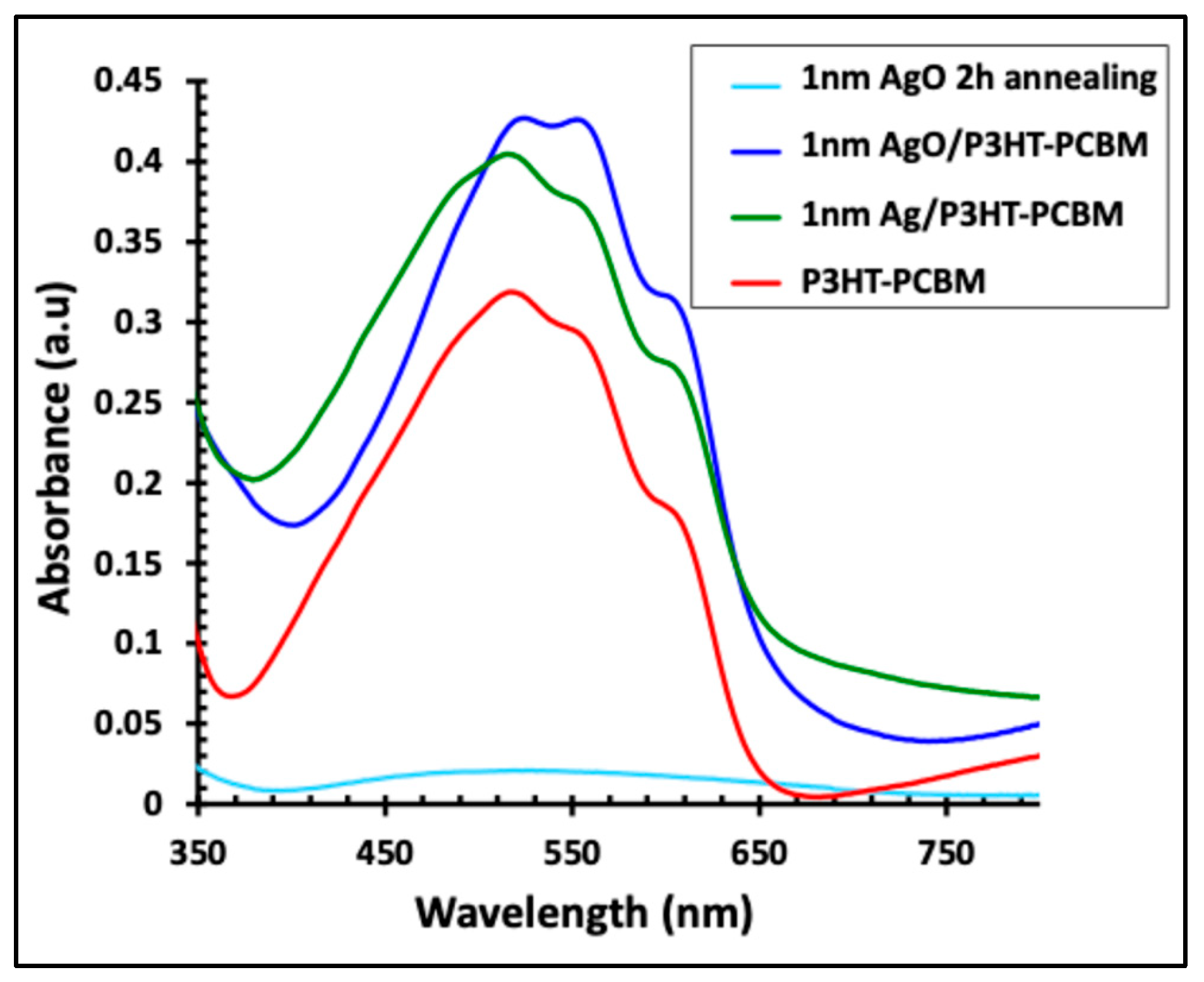
Figure 5 displays the absorption spectra of the P3HT:PCBM active layer alone and in combination with a 1 nm Ag film, before and after 2 h of annealing. The faint blue line represents the absorption of the AgO film after annealing. Incorporation of the Ag nanofilm into the P3HT:PCBM layer (green line) enhanced the light absorption intensity by approximately 25% and broadened the absorption range from 420–620 nm to 380–650 nm. Following 2 h of annealing, the resulting AgO/P3HT:PCBM composite exhibited a further increase in absorption intensity (~34%) and a red shift of the absorption peak, indicating improved light-harvesting capabilities.
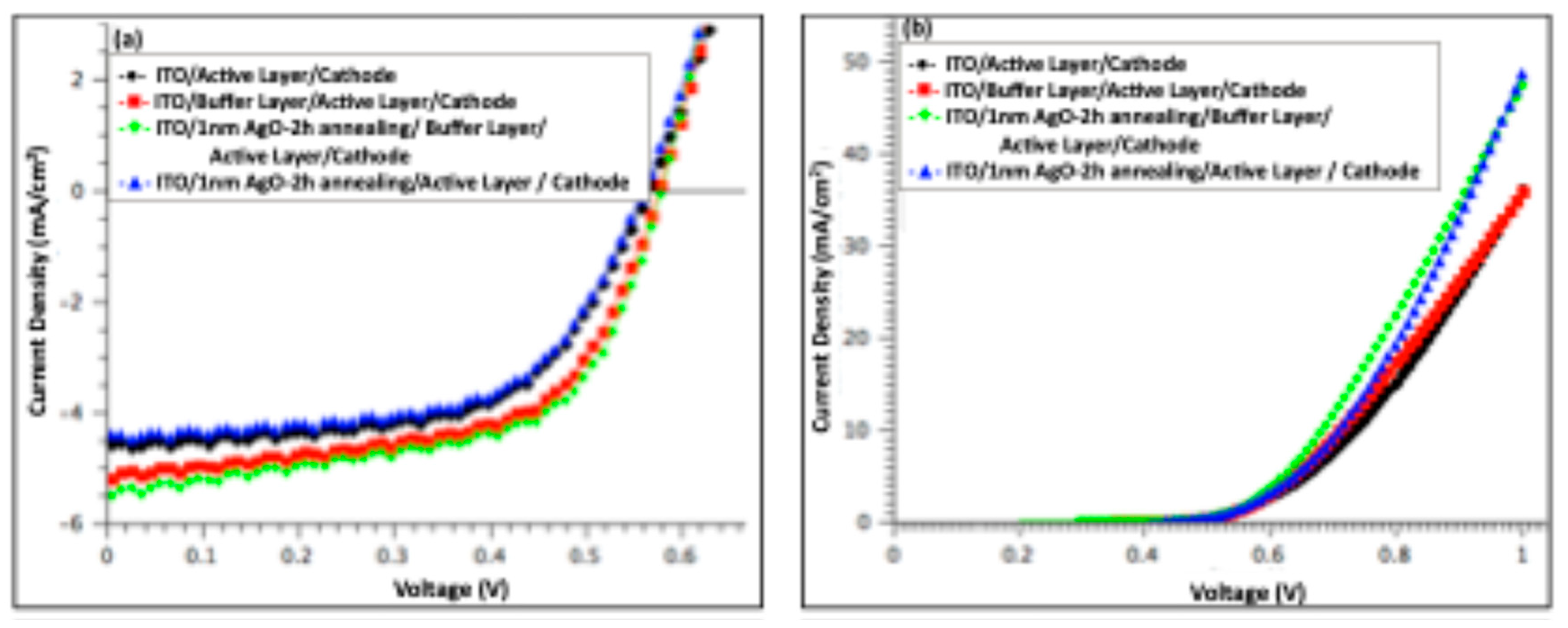
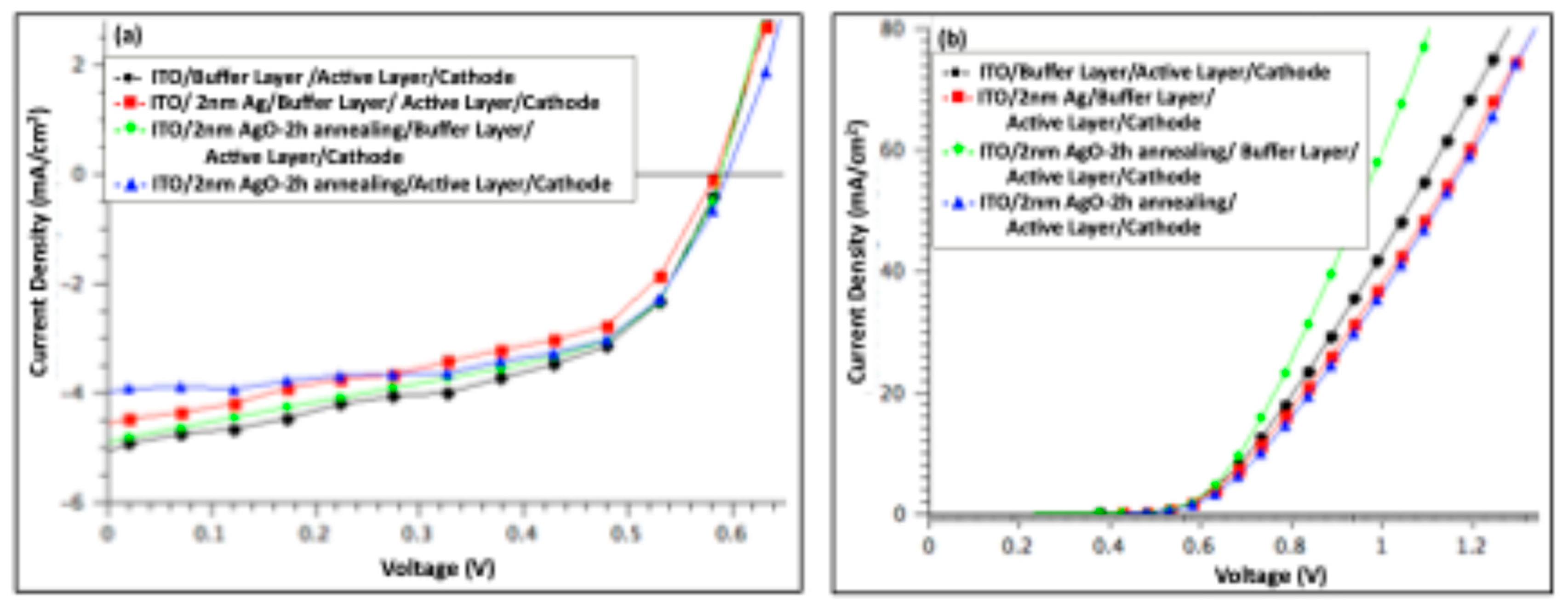
Figure 6 shows the J–V characteristics of organic solar cell devices measured under illumination (a) and in the dark (b), comparing devices before and after incorporation of a 1 nm Ag/AgO film (pristine and after 2 h of annealing). And
Table 1 summarizes the key photovoltaic parameters, including open-circuit voltage (V
OC), short-circuit current density (J
SC), fill factor (FF), and power conversion efficiency (PCE). The series resistance (R
S) was estimated from the slope of the dark I–V curves at 0.9 V.
The photovoltaic performance was optimized in devices featuring a modified anode consisting of a buffer layer combined with a 1 nm AgO film annealed at 300 °C for 2 h. These devices exhibited PCE of 1.9%, representing a 6% improvement compared to the reference. This enhancement primarily arose from a 6% increase in the JSC, rising from 5.20 to 5.51 mA cm⁻², and an 11% reduction in RS. The VOC remained unchanged relative to the pristine device. The simultaneous increase in JSC and decrease in RS indicate improved charge collection and overall device performance.
4.4. Effect of 2 nm Ag Film on OSC Parameters
In this set of experiments, a 2 nm Ag layer was thermally evaporated onto pre-cleaned ITO substrates and subsequently annealed at 300 °C for 2 h prior to deposition of the remaining device layers. Four sets of devices were fabricated simultaneously to evaluate the impact of the Ag/AgO layer on device performance: (1) ITO/BufferLayer/ActiveLayer/Cathode, (2) ITO/2nmAg/BufferLayer/ActiveLayer/ Cathode, (3) ITO/2nmAgO(2h-annealing)/BufferLayer/ActiveLayer/Cathode, (4) ITO/2nmAgO(2h-annealing)/ActiveLayer/Cathode
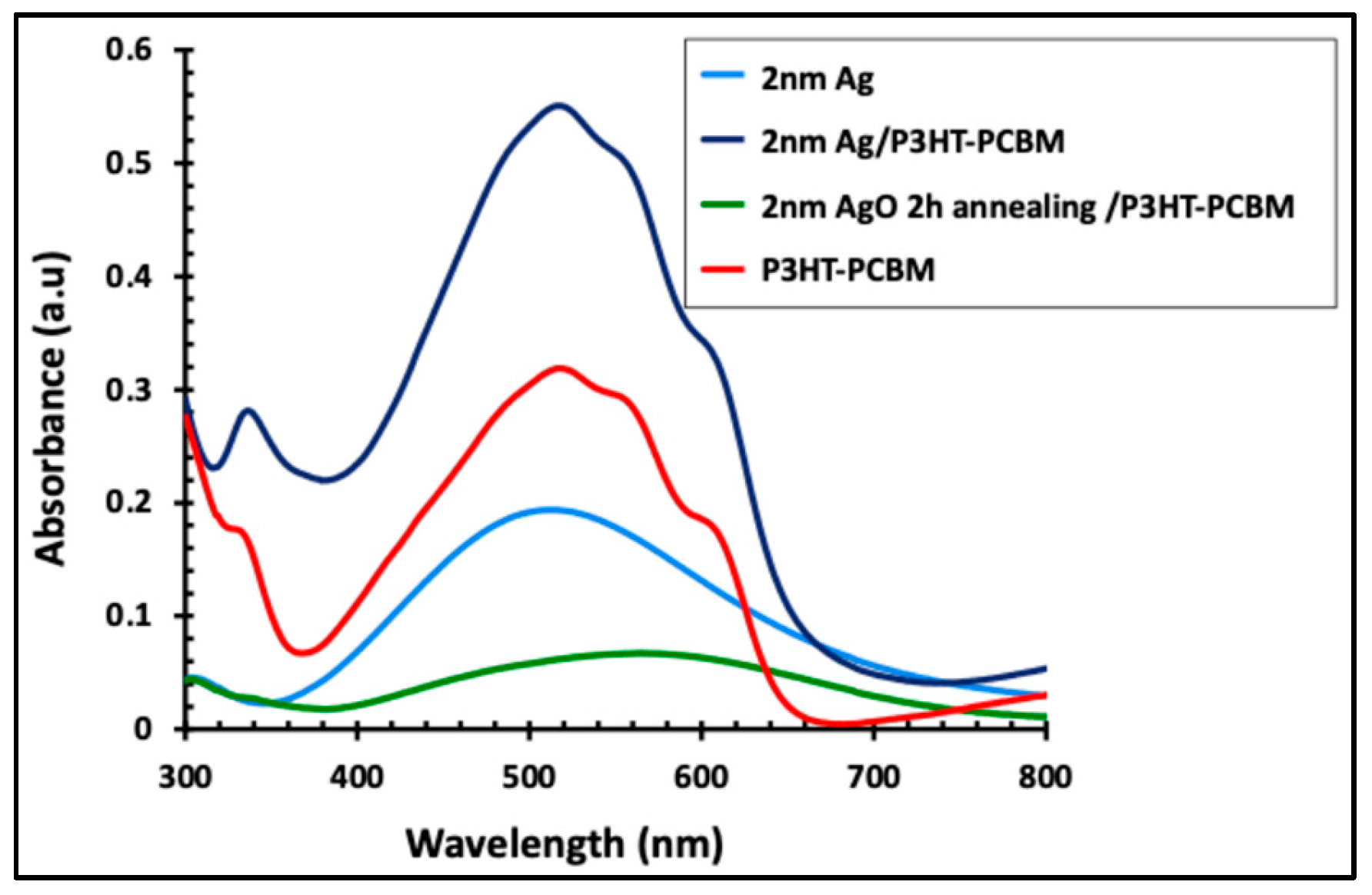
Figure 7 displays the absorption spectra of the P3HT:PCBM active layer alone and in combination with the 2 nm Ag film, both before and after 2 h of annealing. The blue line represents the as-deposited 2 nm Ag film without annealing. Incorporation of the Ag nanofilm into the P3HT:PCBM layer, shown by the dark line, significantly enhanced the light absorption intensity by approximately 72% and extended the absorption range from 420–620 nm to 420–670 nm. In contrast, annealing the 2 nm Ag film for 2 h produced an AgO/P3HT:PCBM composite with markedly reduced absorption intensity and a red-shifted peak, altering the original absorption profile of the active layer.
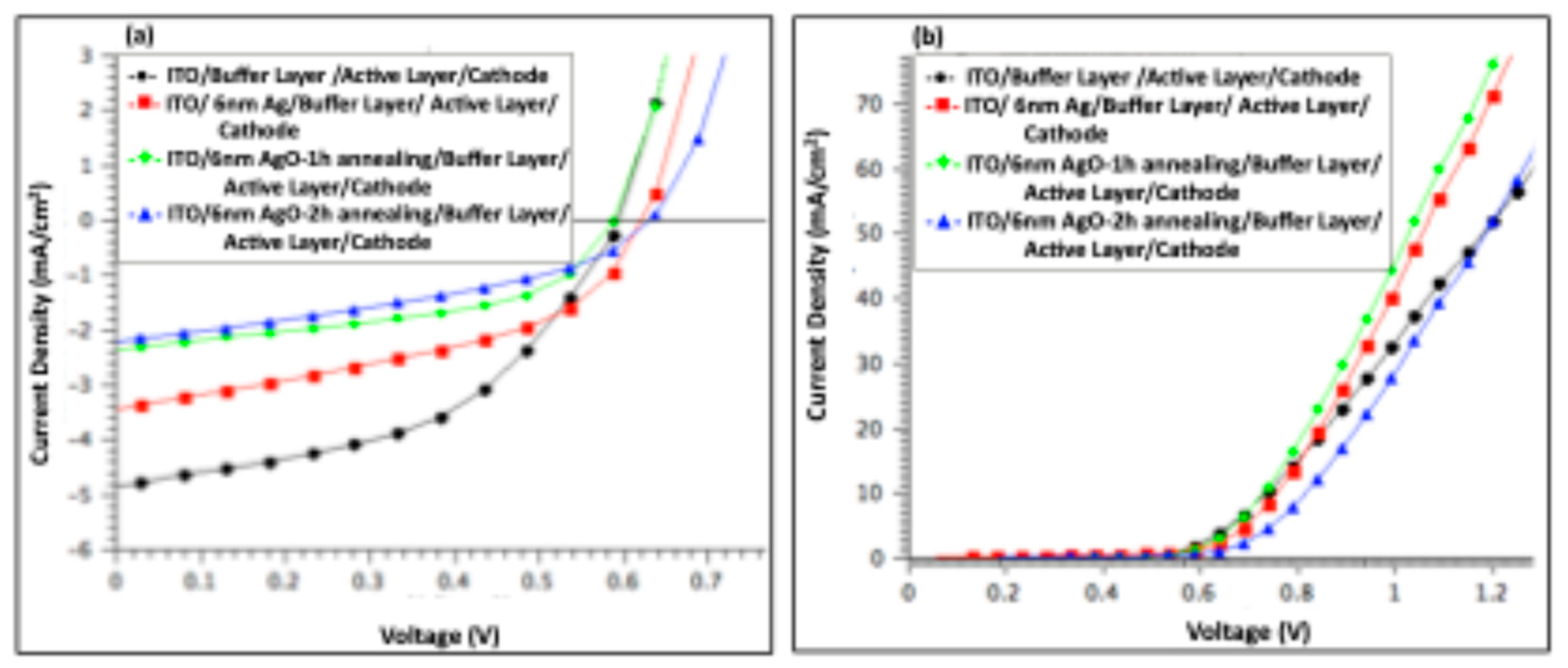
Figure 8 presents the J–V characteristics of organic solar cell devices measured (a) under illumination and (b) in the dark, comparing devices before and after incorporation of a 2 nm Ag/AgO film (pristine and after 2 h of annealing).
Table 2 summarizes the key photovoltaic parameters, including V
OC, J
SC, FF, and PCE. The R
S was determined from the slope of the dark I–V curves at 0.9 V.
The data in
Table 2 indicate that adding a 2 nm Ag or AgO layer to the anodic interface negatively affected overall device performance. While the V
OC remained largely unchanged across all devices, the J
SC decreased by 2.2% for devices containing the 2 h annealed AgO film and by 9.3% for devices with the non-annealed Ag film. The highest performance was observed in devices without any modifications to the anodic buffer layer, suggesting that the absence of Ag or AgO nanofilms facilitates more efficient hole collection.
4.5. Effect of 6 nm Ag Film on OSC Parameters
In this experiment, a 6 nm Ag layer was thermally evaporated onto pre-cleaned ITO substrates and subsequently annealed at 300 °C for 1 and 2 h prior to deposition of the remaining device layers. For comparison, four sets of devices, corresponding to the architectures illustrated in
Figure 2, were fabricated simultaneously: (1) ITO/BufferLayer/ActiveLayer/Cathode, (2) ITO/6nmAg/BufferLayer/ActiveLayer /Cathode, (3) ITO/6nmAgO(1h-annealing)/BufferLayer/ActiveLayer/Cathode, (4) ITO/6nmAgO (2h-annealing)/BufferLayer/ActiveLayer/Cathode. The impact of the Ag/AgO layer on hole collection efficiency was assessed by evaluating the overall performance of the devices.
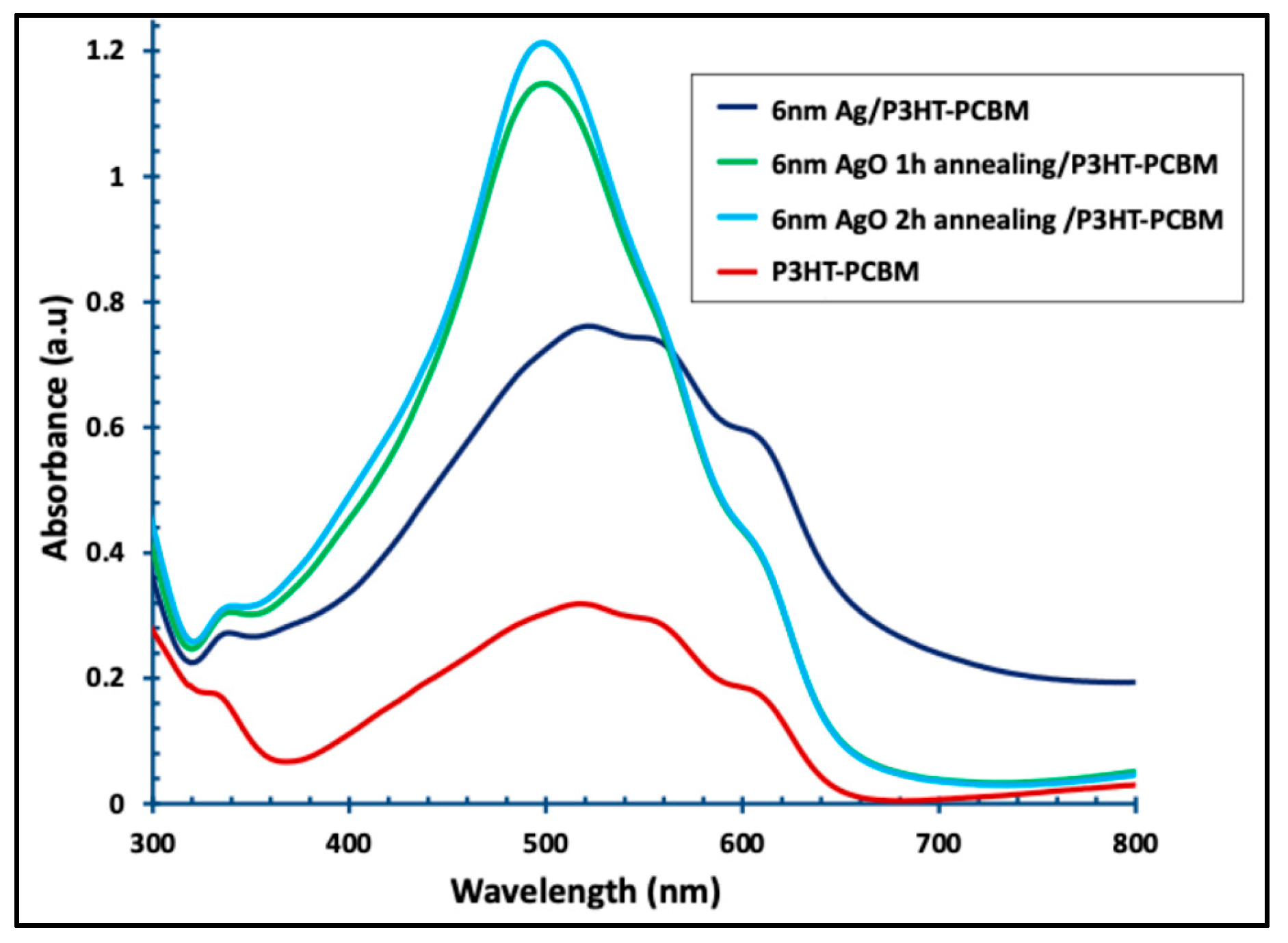
Figure 9 shows the UV–visible absorption spectra of the pristine P3HT:PCBM layer and the P3HT:PCBM layer combined with a 6 nm Ag film, both before and after annealing for 1 and 2 h. Incorporation of the 6 nm Ag film into the P3HT:PCBM layer (dark blue line) enhanced the absorption intensity by approximately 137% and broadened and red-shifted the absorption band from 420–620 nm to 420–670 nm. Thermal annealing of the Ag films for 1 and 2 h (green and faint blue lines) further modified the absorption profile of the P3HT:PCBM layer, resulting in a single, intense peak with higher absorptivity.
The photovoltaic parameters of devices incorporating a 6 nm Ag or AgO film are summarized in
Table 3. The highest performance was achieved by the pristine device with the architecture ITO/Buffer Layer/Active Layer/Cathode. Introduction of the 6 nm Ag film led to a significant reduction in overall device performance, with a 47% decrease in PCE, primarily due to a 37% drop in the J
SC. This indicates less efficient hole extraction by the anode compared to the pristine device. Annealing the Ag film further exacerbated this effect, resulting in a 60% reduction in J
SC, likely due to the formation of energy-quenching states that limit charge carrier collection at the electrodes [
44].
The dark J–V characteristics of all fabricated devices, shown in
Figure 6(b),
Figure 8(b) and
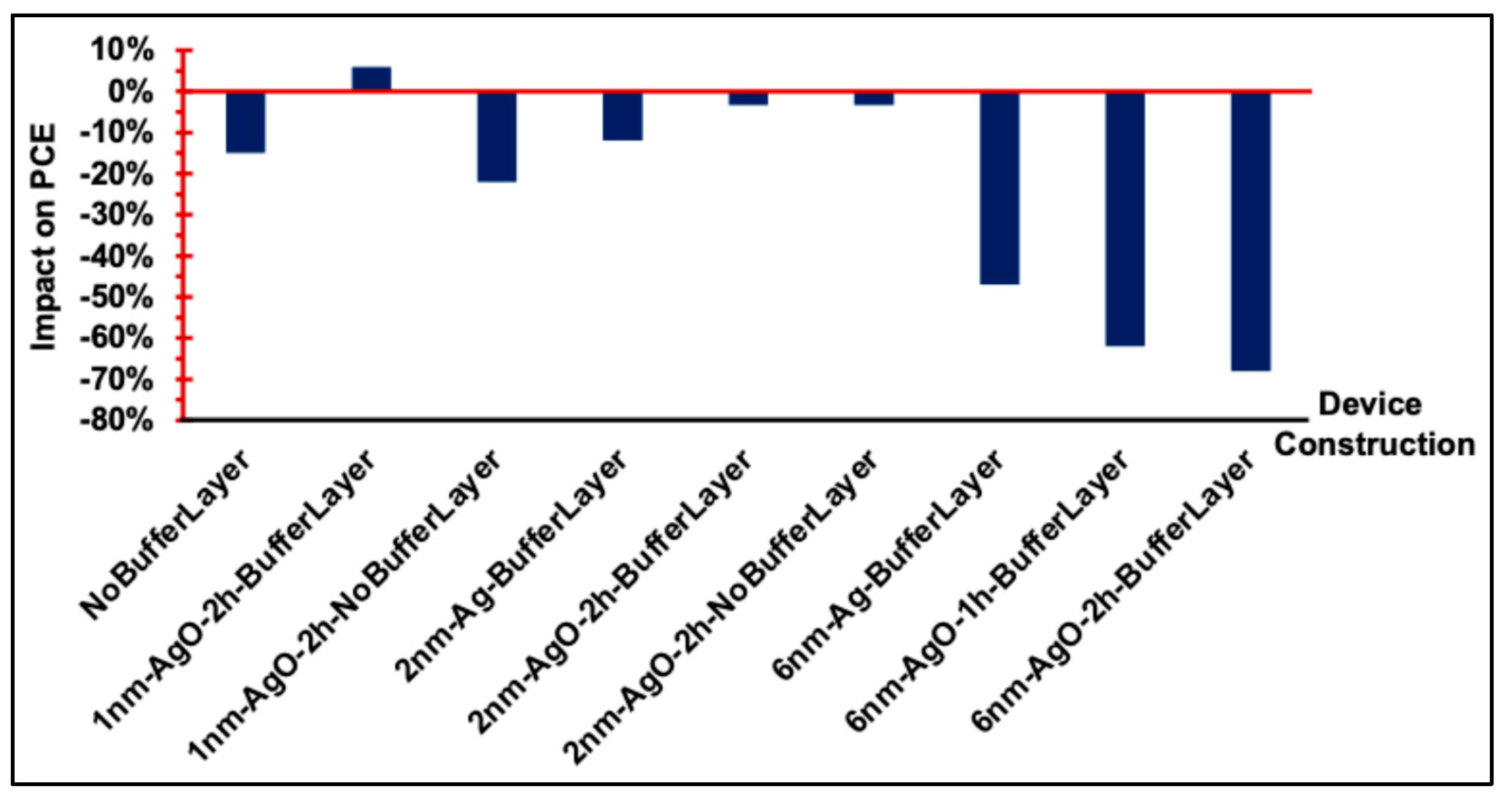
Figure 10(b), demonstrate good diode behavior. Under illumination, the performance of OSC devices with and without a PEDOT:PSS buffer layer and Ag/AgO interfacial layers is summarized in
Figure 11. The results indicate that devices incorporating a PEDOT:PSS buffer layer consistently exhibited the best overall performance, serving as the standard configuration. Introducing an interfacial Ag or AgO layer to modify the anodic buffer primarily influenced the J
SC and R
S, while the V
OC remained largely unaffected. Among the various modifications, a 1 nm AgO layer annealed at 300 °C for 2 h provided the most effective enhancement of the anodic buffer, improving charge collection and device performance.
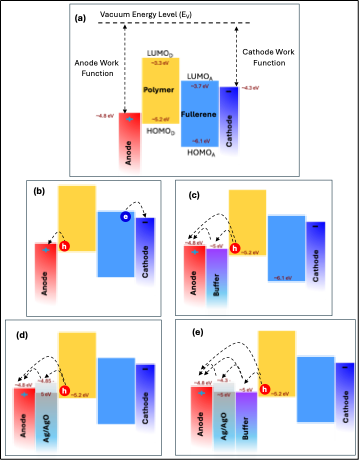
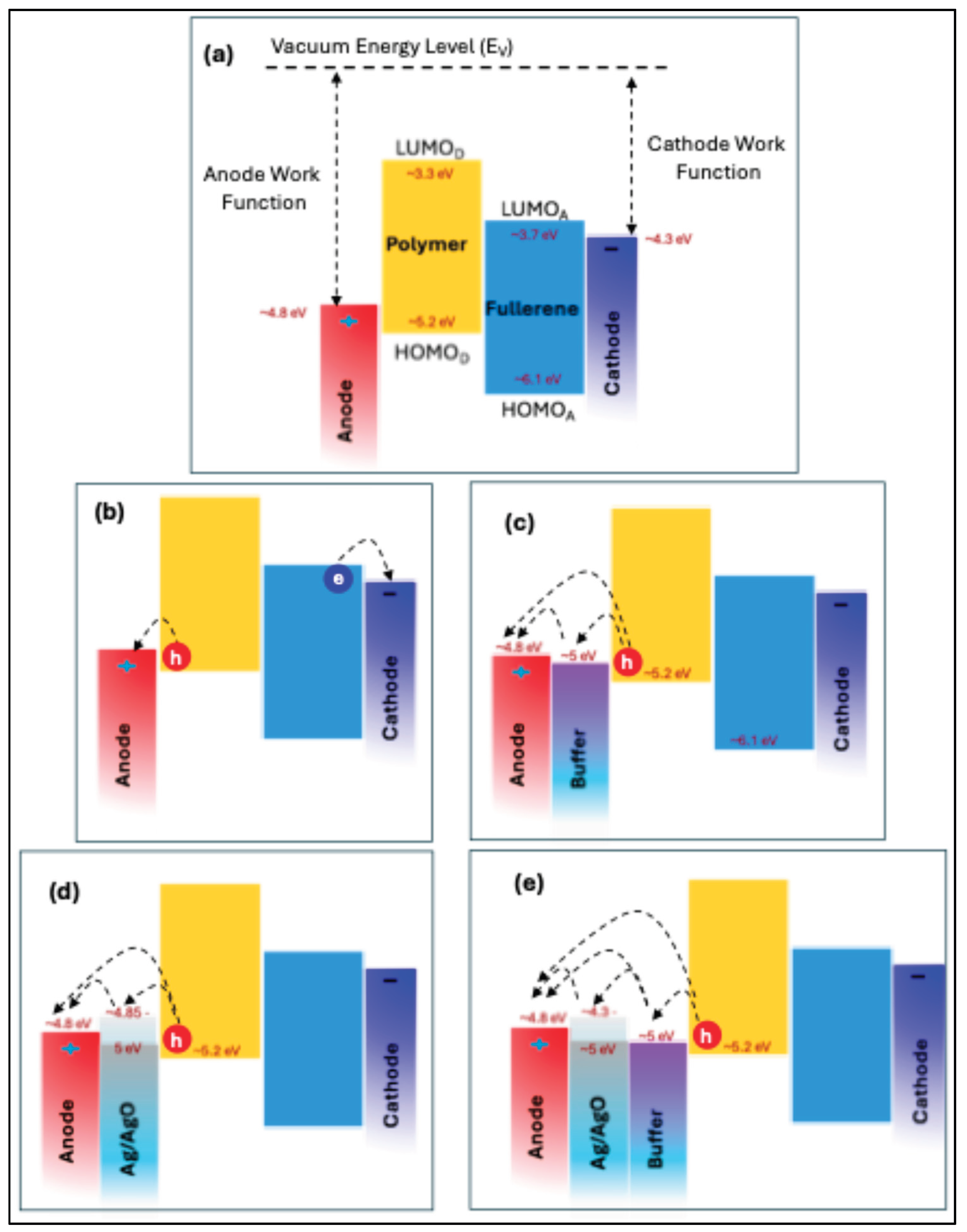
To explain these results,
Figure 12 presents the energy band diagram of the materials employed in this study. The work functions of the electrodes, along with the HOMO and LUMO energy levels of both the polymer and fullerene, were obtained from reference [
1]. The work function of Ag/AgO is dependent on both film thickness and annealing temperature, ranging from 4.3 to 5.0 eV [
45,
46,
47]. As illustrated in
Figure 12(c), (d), and (e), the Ag/AgO work function aligns well with the HOMO level of the polymer and the work function of the ITO anode, establishing an efficient hole-collecting interface. Notably, a 1 nm AgO film annealed at 300 °C for 2 h resulted in a 6% increase in PCE. This improvement can be attributed to several factors: (1) SPR of the Ag thin film enhances the light absorption of the P3HT:PCBM active layer (
Figure 5), generating more charge carriers, increasing J_SC, and boosting the PCE [
1,
48]; (2) Energy-level alignment between the AgO work function, the polymer HOMO, and the anode work function facilitates efficient hole transport to the anode, enhancing device performance; (3) Reduced sheet resistance and improved surface morphology of the AgO-modified layer—by approximately 11%—further support effective charge collection and transport. Together, these factors synergistically improve hole transport efficiency and overall photovoltaic performance in the OSC devices.
Increasing the thickness of the AgO layer and/or the annealing temperature resulted in devices with significantly reduced performance. This decline can be attributed to the oxidation behavior of silver, which is highly dependent on both annealing time and temperature. Excessive oxidation alters the grain size of the silver layer, adversely affecting charge transport and collection. Furthermore, the formation of an AgO oxide layer introduces energy-quenching states for charge carriers, limiting their mobility and reducing the photocurrent, ultimately diminishing the overall efficiency of the device [
44].
5. Conclusion and Future Work
In this study, we systematically investigated the effect of thermally annealed Ag films of varying thicknesses (1–6 nm) at 300 °C on their optical absorption, as well as their influence on the performance of P3HT:PCBM-based organic solar cells. Silver thin films exhibited high transparency and conductivity, with absorption features overlapping the P3HT:PCBM active layer. Increasing the Ag film thickness led to enhanced absorption intensity, peak broadening, and a red shift, with the absorption maximum shifting from 443 nm for a 1 nm film to 546 nm for a 6 nm film. The improvement in light absorption after annealing was attributed to the formation of spherical Ag nanoparticles that support localized surface plasmon resonance. The annealing temperature and duration modulate the nanoparticle size, enhance crystallinity, and influence both absorption intensity and peak position.
The dark J–V curves of all fabricated devices indicated good diode behavior. Under illumination, devices incorporating a PEDOT:PSS buffer layer consistently exhibited the best performance. Among the various Ag/AgO interfacial modifications, a 1 nm AgO film annealed at 300 °C for 2 h provided the most effective enhancement of the anodic buffer layer, increasing the power conversion efficiency (PCE) by 6%. This improvement is attributed to the optimal alignment of the AgO work function with the polymer HOMO and the anode work function, which facilitates efficient hole transport to the electrode. In addition, the 1 nm AgO layer reduced the series resistance by 11% and improved surface morphology, both of which contribute to enhanced device performance. The LSPR effect of the Ag thin film further enhanced the active layer light absorption by ~25%, generating more charge carriers, increasing JSC, and boosting PCE.
However, increasing the AgO thickness or annealing time led to a deterioration in device performance. This decline is associated with accelerated silver oxidation, which alters grain size and reduces charge transport efficiency, and the formation of energy-quenching states that hinder free carrier collection, ultimately limiting photocurrent and overall device efficiency. Collectively, these findings demonstrate that ultrathin, optimally annealed AgO layers can serve as an effective anodic interfacial modification to improve OSC performance, while excessive thickness or annealing is detrimental.
Future research will focus on optimizing Ag/AgO nanostructures through precise deposition techniques to maximize LSPR effects while minimizing quenching. Alternative plasmonic materials, such as Au and hybrid interfacial layers combining metals with conductive polymers, could be explored to further enhance hole collection and reduce series resistance. Long-term stability studies under illumination and thermal stress are needed to assess practical device applicability.
Funding
This research received no external funding.
Data Availability Statement
The original contributions presented in this study are included in the article/Supplementary Material. Further inquiries can be directed to the corresponding author.
Acknowledgments
The author acknowledges with thanks the University of Jeddah for its research facilities. The author used Grammarly for grammar correction and wording revision, and occasionally supplemented it with ChatGPT.
Conflicts of Interest
The author reports no conflicts of interest. The author alone is responsible for the content and writing of this article.
References
- Mahmoud, A.Y.; Zhang, J.; Ma, D.; Izquierdo, R.; Truong, V.-V. Optically-enhanced performance of polymer solar cells with low concentration of gold nanorods in the anodic buffer layer. Organic Electronics 2012, vol. 13(no. 12), 3102–3107. [Google Scholar] [CrossRef]
- Mahmoud, A.Y.; Zhang, J.; Ma, D.; Izquierdo, R.; Truong, V.-V. Thickness dependent enhanced efficiency of polymer solar cells with gold nanorods embedded in the photoactive layer. Solar Energy Materials & Solar Cells 2013, vol. 116(no. 1), 1–8. [Google Scholar]
- Mahmoud, A.Y.; Izquierdo, R.; Truong, V.-V. Gold Nanorods Incorporated Cathode for Better Performance of Polymer Solar Cells. Journal of Nanomaterials 2014, vol. 2014(no. 1), 1–7. [Google Scholar] [CrossRef]
- Mohamed Shaban, Mohamed Benghanem, Abdullah Almohammedi and Mohamed Rabia, “Optimization of the Active Layer P3HT:PCBM for Organic Solar Cell,” . Coatings 2021, vol. 11(no. 7), 1–15. [CrossRef]
- oungkyoo Kim, Stelios A. Choulis, Jenny Nelson, Donal D. C. Bradley, Steffan Cook, James R. Durrant Y., Device annealing effect in organic solar cells with blends of regioregular poly(3-hexylthiophene) and soluble fullerene. Applied Physics Letters 2002, vol. 86(no. 6), 063502–063505. [CrossRef]
- D. Darwis1 E. Sesa, D. Farhamza, Iqbal, “The Fabrication of Bulk Heterojunction P3HT: PCBM Organic Photovoltaics,” IOP Conf. Series: Materials Science and Engineering, vol. 367, no. 012029, pp. 1-6, 2018. [CrossRef]
- Brabec, C.J.; Gowrisanker, S.; Halls, J.J.M.; Laird, D.; Jia, S.; Williams, S.P. Polymer–Fullerene Bulk-Heterojunction Solar Cells. Advanced Materials 2010, vol. 22(no. 34), 3839–3856. [Google Scholar] [CrossRef]
- Shaban, M.; Benghanem, M.; Almohammedi, A.; Rabia, M. Optimization of the Active Layer P3HT:PCBM for Organic Solar Cell. Coating 2021, vol. 11(no. 7), 1–15. [Google Scholar] [CrossRef]
- Lei, H.; Qin, P.; Ke, W.; Guo, Y.; Dai, X.; Chen, Z.; Wang, H.; Li, B.; Zheng, Q.; Fang, G. Performance enhancement of polymer solar cells with high work function CuS modified ITO as anodes. Organic Electronics Volume 2015, vol. 22(no. 28), 173–179. [Google Scholar] [CrossRef]
- SHOFIQUL ISLAM, MD. Investigation of the Current of P3HT: PCBM-Based Organic Solar Cell Extracting the Spatial Recombination Coefficient of the Active Laye. IEEE Access 2021, vol. 9, 130502–130518. [Google Scholar] [CrossRef]
- Lai, Y.-Y.; Cheng, Y.-J.; Hsu, C.-S. Applications of functional fullerene materials in polymer solar cells. Energy & Environmental Science 2014, vol. 7(no. 6), 1866–1883. [Google Scholar] [CrossRef]
- Chen, H.-Y.; Hou, J.; Zhang, S.; Liang, Y.; Yang, G.; Yang, Y.; Yu, L.; Wu, Yue; Li, Gang. Polymer solar cells with enhanced open-circuit voltage and efficiency. Nature Photonics 2009, vol. 3, pages 649–653. [Google Scholar] [CrossRef]
- Luo, J.; Wu, H.; He, C.; Li, A.; Yang, W.; Cao, Y. Enhanced open-circuit voltage in polymer solar cells. Applied Physics Letters 2009, vol. 95, 043301. [Google Scholar] [CrossRef]
- Fallata, A.A.; Bahabry, R.R.; Mahmoud, A.Y. Performance Degradation of Polymer Solar Cells Measured in High Humidity Environment. Journal of Nanoelectronics and Optoelectronics 2021, vol. 16, 1–6. [Google Scholar] [CrossRef]
- Sharma, N.; Gupta, S.K.; Negi, Chandra Mohan Singh. Influence of active layer thickness on photovoltaic performance of PTB7:PC70BM bulk heterojunction solar cell. Superlattices and Microstructures 2019, vol. 135, 106278. [Google Scholar] [CrossRef]
- Zang, Y.; XinJufeng, Q.; Lin, Z. Effect of Active Layer Thickness on the Performance of Polymer Solar Cells Based on a Highly Efficient Donor Material of PTB7-Th. The Journal of Physical Chemistry C 2018, vol. 122(no. 29), 16532–16539. [Google Scholar] [CrossRef]
- Scharber, M.C. On the Effi ciency Limit of Conjugated Polymer:Fullerene-Based Bulk Heterojunction Solar Cells. Advanced Materials 2016, vol. 28(no. 10), 1994–2001. [Google Scholar] [CrossRef]
- Benaya, i.; Taouti, M.M.; Bougnina, K.; Deghfel, B.; Zoukel, A. Gold nanoparticles in P3HT: PCBM active layer: A simulation of new organic solar cell designs. Solid-State Electronics Volume 2025, vol. 225(no. 3), 1–11. [Google Scholar] [CrossRef]
- Wang, H.P.; Pigeon, S.; Izquierdo, R.; Martel, R. Electrical bistability by self-assembled gold nanoparticles in organic diodes. Applied Physic Letters 2006, vol. 89(no. 183502), 1–3. [Google Scholar] [CrossRef]
- Singh, C.R.; Honold, T.; Gujar, T.P.; Retsch, M.; Fery, A.; Karg, M.; Thelakkat, M. The role of colloidal plasmonic nanostructures in organic solar cells. Physical Chemistry Chemical Physics 2016, vol. 18(no. 33), 23255–23163. [Google Scholar] [CrossRef]
- Diukman, I.; Tzabari, L.; Berkovitch, N.; Tessler, N.; Orenstein, M. Controlling absorption enhancement in organic photovoltaic cells by patterning Au nano disks within the active layer. Optics Express 2011, vol. 19(no. 1), 1–8. [Google Scholar] [CrossRef] [PubMed]
- Das, P.K.; Dhawan, A. Plasmonic enhancement of photovoltaic characteristics of organic solar cells by employing parabola nanostructures at the back of the solar cell. RSC Advances 2023, vol. 13(no. 38), 26780–26792. [Google Scholar] [CrossRef] [PubMed]
- Chi, D.; Qu, S.; Wang, Z.; Wang, J. High efficiency P3HT:PCBM solar cells with an inserted PCBM layer. Journal of Materials Chemistry 2014, vol. 2(no. 22), 4383–4387. [Google Scholar] [CrossRef]
- Tremolet de Villers, Bertrand; Tassone, Christopher J.; Tolbert, Sarah H.; Schwartz, Benjamin J. Improving the Reproducibility of P3HT:PCBM Solar Cells by Controlling the PCBM/Cathode Interface. The Journal of Physical Chemistry C 2009, vol. 113(no. 44), 18978–18982. [Google Scholar] [CrossRef]
- Yao, K.; Chen, L.; Chen, Y.; Li, F.; Wanga, P. Influence of water-soluble polythiophene as an interfacial layer on the P3HT/PCBM bulk heterojunction organic photovoltaics. Journal of Materials chemistry 2011, vol. 21(no. 36), 13780–13784. [Google Scholar] [CrossRef]
- Zidan, M.N.; Ismail, T.; Fahim, I.S. Effect of thickness and temperature on solar cell performance. Materials Research Express 2021, vol. 8(no. 095508), 1–12. [Google Scholar] [CrossRef]
- Irwin, M.D.; Liu, J.; Leever, B.J.; Servaites, J.D.; Hersam, M.C.; Durstock, M.F.; Marks, T.J. Consequences of Anode Interfacial Layer Deletion. HCl-Treated ITO in P3HT:PCBM-Based Bulk-Heterojunction Organic Photovoltaic Devices. Langmuir 2010, vol. 26(no. 4), 2584–2591. [Google Scholar] [CrossRef]
- Sayantan Das; T. L. Alford, “Improved efficiency of P3HT:PCBM solar cells by incorporation of silver oxide interfacial layer,” . Journal of Applied Physics 2014, vol. 116(no. 044905), 1–3. [CrossRef]
- Zu-Liang, Z.; Yong-Sheng, W.; Da-Wei, H.; Ming, F. Improved performance of P3HT:PCBM solar cells by both anode modification and short-wavelength energy utilization using Tb(aca)3phen. Chinese Physics B 2014, vol. 23(no. 098802), 1–13. [Google Scholar] [CrossRef]
- Peet, J. ; Wen, L. ; Byrne, P. ; Rodman, S. ; Forberich, K. ; Shao, Y. ; Drolet, N. ; Gaudiana, R. ; Dennler, G. ; Waller, D. , “Bulk heterojunction solar cells with thick active layers and high fill factors enabled by a bithiophene-co-thiazolothiazole push-pull copolymer,” Applied Physics Letters, vol. 98, no. 4, p. 043301 https://ui.adsabs.harvard.edu/link_gateway/2011ApPhL..98d3301P/doi:10.1063/1.3544940, 2011. [CrossRef]
- Shen, Y.; Li, K.; Majumdar, N.; Campbell, J.C.; Gupta, M.C. Bulk and contact resistance in P3HT:PCBM heterojunction solar cells. Solar Energy Materials and Solar Cells 2011, vol. 95(no. 8), 2314–2317. [Google Scholar] [CrossRef]
- Wu, C.C.; Wu, C.I.; Sturm, J.C.; Kahn, A. the methods to modify ITO anode surface are listed below: (1) oxygen plasma treatment or UV ozone treatment [13]. Applied Physics Letters 1997, vol. 70(no. 11), 1–3. [Google Scholar]
- Cheng, W.; He, L.; Fan, X.; Ou, Q. Surface modification of indium tin oxide by oxygen plasma immersion ion implantation. Science China Technological Sciences 2013, vol. 56(no. 10), 925–929. [Google Scholar] [CrossRef]
- Fan, X.; Stott, N.E.; Zeng, J.; Li, Y.; Ouyang, J.; Chu, L.; Song, W. PEDOT:PSS materials for optoelectronics, thermoelectrics, and exible and stretchable electronics. Journal of Materials Chemistry A 2023, vol. 11(no. 18561), 1–31. [Google Scholar] [CrossRef]
- Ke, Q.B.; Wu, J.-R.; Lin, C.-C.; Chang, S.H. Understanding the PEDOT:PSS, PTAA and P3CT-X Hole-Transport-Layer-Based Inverted Perovskite Solar Cells. Polymers 2022, vol. 14(no. 823), 1–22. [Google Scholar] [CrossRef] [PubMed]
- Pandey, P.; Kunwar, S.; Sui, M.; Li, M.-Y.; Zhang, Q.; Lee, J. Effect of Annealing Temperature on Morphological and Optical Transition of Silver Nanoparticles on c-Plane Sapphire. Journal of Nanoscience and Nanotechnology 2018, vol. 18(no. 5), 3466–3477. [Google Scholar] [CrossRef]
- Pal, A.K.; Mohan, D.B. Structural, Morphological and Optical Properties of Ag–AgO Thin Films with the Effect of Increasing Film Thickness and Annealing Temperature. Optical Materials 2015, vol. 48(no. 20), 121–132. [Google Scholar] [CrossRef]
- Lee, K.J.; Huang, T.; Nallathamby, P.D.; Xu, X.-H.N. Wavelength Dependent Specific Plasmon Resonance Coupling of Single Silver Nanoparticles with EGFP. Nanoscale 2015, vol. 7(no. 42), 17623–17630. [Google Scholar] [CrossRef]
- Chokboribal, J.; Vanidshow, W.; Yuwasonth, W.; Chananonnawathorn, C.; Waiwijit, U.; Hincheeranun, W.; Dhanasiwawong, K.; Horprathum, M.; Rattana, T.; Sujinnapram, S.; Phae-ngam, W. Annealed plasmonic Ag nanoparticle films for surface enhanced fluorescence substrate. Materials Today: Proceedings 2021, vol. 47(no. 12), 3492–3495. [Google Scholar] [CrossRef]
- Waitkus, J.; Chang, Y.; Liu, L.; Puttaswamy, S.V.; Chung, T.; Vargas, A.M.M.; Dollery, S.J.; O’Connell, M.R.; Cai, H.; Tobin, G.J.; Bhalla, N.; Du, K. Gold Nanoparticle Enabled Localized Surface Plasmon Resonance on Unique Gold Nanomushroom Structures for On-Chip CRISPR-Cas13a Sensing. Advanced Materials 2023, vol. 10(no. 1), 1–9. [Google Scholar] [CrossRef]
- Alebrahim, M.A.; Ahmad, A.A.; Migdadi, A.B.; Al-Bataineh, Q.M. Localize surface plasmon resonance of gold nanoparticles and their effect on the polyethylene oxide nanocomposite films. Physica B: Condensed Matter 2024, vol. 679(no. 22), 1–10. [Google Scholar] [CrossRef]
- Peng, S.; McMahon, J.M.; Schatz, G.C.; Gray, S.K.; Sun, Y. Reversing the size-dependence of surface plasmon resonances. PNAS 2010, vol. 107(no. 33), 14530–14534. [Google Scholar] [CrossRef]
- Kim, M.; Kimb, K.-B. Rapid Thermal Annealing at the Temperature of 650°C Ag Films on SiO2 Deposited STS Substrates. pplied Science and Convergence Technology 2017, vol. 26(no. 6), 208–213. [Google Scholar] [CrossRef]
- Jong Bok Kim; Chang Su Kim; Youn Sang Kim; Yueh-Lin Loo, “Oxidation of silver electrodes induces transition from conventional to inverted photovoltaic characteristics in polymer solar cells,” . Applied Physics Letters 2009, vol. 95(no. 183301), 1–3. [CrossRef]
- Akaike, K.; Hosokai, T.; Ono, Y.; Tsuruta, R.; Yamada, Y. Increasing Electrode Work Function Using a Natural Molecule. Advanced Materials Interfaces 2023, vol. 10(no. 2201800), 1–7. [Google Scholar] [CrossRef]
- Jong Bok Kim; Chang Su Kim; Youn Sang Kim; Yueh-Lin Loo, “Oxidation of silver electrodes induces transition from conventional to inverted photovoltaic characteristics in polymer solar cells,” . Applied Physics Letters 2009, vol. 95(no. 183301), 1–3. [CrossRef]
- Akbi, M.; Lefort, A. Work function measurements of contact materials for industrial use. Journal of Physics D: Applied Physics 1998, vol. 31(no. 11), 1301–1308. [Google Scholar] [CrossRef]
- Liu, X.-H.; Hou, L.-X.; Wang, J.-F.; Liu, B.; Yu, Z.-S.; Ma, L.-Q.; Yang, S.-P.; Fu, G.-S. Plasmonic-enhanced polymer solar cells with high efficiency by addition of silver nanoparticles of different sizes in different layers. Solar Energy 2014, vol. 110, 627–635. [Google Scholar] [CrossRef]
Figure 1.
UV–visible absorbance spectrum of the P3HT:PCBM active layer, along with the chemical structures of P3HT and PCBM.
Figure 1.
UV–visible absorbance spectrum of the P3HT:PCBM active layer, along with the chemical structures of P3HT and PCBM.
Figure 2.
Schematic representations of the final device architectures: (a) device without an Ag nanofilm and without a buffer layer; (b) device without an Ag nanofilm but with a buffer layer; (c) device incorporating both an Ag nanofilm and a buffer layer; and (d) device incorporating an Ag nanofilm without a buffer layer.
Figure 2.
Schematic representations of the final device architectures: (a) device without an Ag nanofilm and without a buffer layer; (b) device without an Ag nanofilm but with a buffer layer; (c) device incorporating both an Ag nanofilm and a buffer layer; and (d) device incorporating an Ag nanofilm without a buffer layer.
Figure 3.
UV–visible absorbance spectra of thermally evaporated Ag nanofilms with nominal thicknesses ranging from 1 to 6 nm.
Figure 3.
UV–visible absorbance spectra of thermally evaporated Ag nanofilms with nominal thicknesses ranging from 1 to 6 nm.
Figure 4.
UV–visible absorbance spectra of thermally evaporated Ag nanofilms with thicknesses ranging from 1 to 6 nm, measured before and after annealing at 300 °C for 1 and 2 h.
Figure 4.
UV–visible absorbance spectra of thermally evaporated Ag nanofilms with thicknesses ranging from 1 to 6 nm, measured before and after annealing at 300 °C for 1 and 2 h.
Figure 5.
UV–visible absorbance spectra of the 1 nm Ag/P3HT:PCBM film before and after annealing at 300 °C for 2 h, compared with the pristine P3HT:PCBM layer and the 1 nm AgO film after 2 h of annealing.
Figure 5.
UV–visible absorbance spectra of the 1 nm Ag/P3HT:PCBM film before and after annealing at 300 °C for 2 h, compared with the pristine P3HT:PCBM layer and the 1 nm AgO film after 2 h of annealing.
Figure 6.
J–V characteristics of organic solar cell devices: comparison between devices with a 1 nm Ag film annealed at 300 °C for 2 h, devices without Ag, and devices without both Ag and buffer layers, measured (a) under illumination and (b) in the dark.
Figure 6.
J–V characteristics of organic solar cell devices: comparison between devices with a 1 nm Ag film annealed at 300 °C for 2 h, devices without Ag, and devices without both Ag and buffer layers, measured (a) under illumination and (b) in the dark.
Figure 7.
UV–visible absorbance spectra of the 2 nm Ag/P3HT:PCBM film before and after annealing at 300 °C for 2 h, compared with the pristine P3HT:PCBM active layer and the 2 nm Ag film alone.
Figure 7.
UV–visible absorbance spectra of the 2 nm Ag/P3HT:PCBM film before and after annealing at 300 °C for 2 h, compared with the pristine P3HT:PCBM active layer and the 2 nm Ag film alone.
Figure 8.
The J–V characteristics for OSC devices with and without 2 nm Ag annealed at 300°C for 2 hours, along with devices without a buffer layer or devices without annealing of Ag (a) under light and (b) in dark.
Figure 8.
The J–V characteristics for OSC devices with and without 2 nm Ag annealed at 300°C for 2 hours, along with devices without a buffer layer or devices without annealing of Ag (a) under light and (b) in dark.
Figure 9.
UV–visible absorbance spectra of the 6 nm Ag/P3HT:PCBM film before and after annealing at 300 °C for 1 and 2 h, compared with the pristine P3HT:PCBM active layer.
Figure 9.
UV–visible absorbance spectra of the 6 nm Ag/P3HT:PCBM film before and after annealing at 300 °C for 1 and 2 h, compared with the pristine P3HT:PCBM active layer.
Figure 10.
J–V characteristics of OSC devices with and without a 6 nm Ag film, including films annealed at 300 °C for 1 and 2 h, as well as devices without Ag or without annealing, measured (a) under illumination and (b) in the dark.
Figure 10.
J–V characteristics of OSC devices with and without a 6 nm Ag film, including films annealed at 300 °C for 1 and 2 h, as well as devices without Ag or without annealing, measured (a) under illumination and (b) in the dark.
Figure 11.
Comparison of the relative changes in PCE resulting from the incorporation of Ag or AgO interfacial layers in OSC devices.
Figure 11.
Comparison of the relative changes in PCE resulting from the incorporation of Ag or AgO interfacial layers in OSC devices.
Figure 12.
(a) Energy band diagram of the materials used in this study. (b) Schematic of charge carrier (electron and hole) transport to the respective electrodes. (c) Role of the buffer layer in facilitating hole collection. (d) Illustration of the Ag/AgO interfacial layer with varying work functions, showing optimal alignment with the polymer HOMO and the anode work function. (e) Energy band diagram and hole transport pathway for the OSC configuration exhibiting the best performance.
Figure 12.
(a) Energy band diagram of the materials used in this study. (b) Schematic of charge carrier (electron and hole) transport to the respective electrodes. (c) Role of the buffer layer in facilitating hole collection. (d) Illustration of the Ag/AgO interfacial layer with varying work functions, showing optimal alignment with the polymer HOMO and the anode work function. (e) Energy band diagram and hole transport pathway for the OSC configuration exhibiting the best performance.
Table 1.
Photovoltaic parameters of OSC devices, for devices with a 1 nm Ag/AgO film annealed at 300 °C for 2 h, as well as for devices without Ag or buffer layers.
Table 1.
Photovoltaic parameters of OSC devices, for devices with a 1 nm Ag/AgO film annealed at 300 °C for 2 h, as well as for devices without Ag or buffer layers.
| Device configuration |
VOC
(V) |
JSC (mA/cm2) |
FF
% |
RS
(Ωcm2) |
PCE
% |
± Δ % PCE |
| ITO/BufferLayer/ActiveLayer/Cathode |
0.58 |
-5.2 |
58.65 |
0.063 |
1.8 |
- |
| ITO/ActiveLayer/Cathode |
0.57 |
-4.55 |
59.10 |
0.063 |
1.53 |
- 15 % |
ITO/1nmAgO-2hAnnealing
/BufferLayer/ActiveLayer/Cathode
|
0.58 |
-5.51 |
58.48 |
0.056 |
1.9 |
+ 6 % |
| ITO/1nmAgO-2hAnnealing/Active Layer/Cathode |
0.54 |
-4.25 |
59.19 |
0.063 |
1.4 |
- 22 % |
Table 2.
Photovoltaic parameters of OSC devices with and without a 2 nm Ag film (pristine and annealed at 300 °C for 2 h), including devices without a buffer layer.
Table 2.
Photovoltaic parameters of OSC devices with and without a 2 nm Ag film (pristine and annealed at 300 °C for 2 h), including devices without a buffer layer.
| Device configuration |
VOC
(V) |
JSC (mA/cm2) |
FF
% |
RS
(Ωcm2) |
PCE
% |
± Δ % PCE |
| ITO/BufferLayer/ActiveLayer/Cathode |
0.58 |
-4.92 |
52.67 |
0.056 |
1.51 |
- |
| ITO/2nmAg/BufferLaye/ActiveLayer/Cathode |
0.58 |
-4.46 |
51.19 |
0.056 |
1.33 |
- 12 % |
| ITO/2nmAgO-2hAnnealing/BufferLayer/Active Layer/Cathode |
0.58 |
-4.81 |
52.21 |
0.038 |
1.46 |
- 3.3 % |
| ITO/2nmAgO-2hAnnealing/ActiveLayer/Cathode |
0.58 |
-3.93 |
63.75 |
0.056 |
1.46 |
- 3.3 % |
Table 3.
Photovoltaic parameters of OSC devices with and without a 6 nm Ag/AgO film, including films annealed at 300 °C for 1 and 2 h, as well as devices without Ag annealing. RS was determined from the slope of the dark I–V curve at 1.0 V.
Table 3.
Photovoltaic parameters of OSC devices with and without a 6 nm Ag/AgO film, including films annealed at 300 °C for 1 and 2 h, as well as devices without Ag annealing. RS was determined from the slope of the dark I–V curve at 1.0 V.
| Device configuration |
VOC
(V) |
JSC (mA/cm2) |
FF
% |
RS
(Ωcm2) |
PCE
% |
± Δ % PCE |
| ITO/BufferLayer/ActiveLayer/Cathode |
0.59 |
-5.59 |
54.82 |
0.069 |
1.8 |
- |
| ITO/6nmAg/BufferLayer/Active layer/Cathode |
0.59 |
-3.51 |
46.25 |
0.044 |
0.95 |
- 47 % |
| ITO/6nm AgO-1hAnnealing/BufferLayer /ActiveLayer/cathode |
0.59 |
-2.37 |
48.73 |
0.041 |
0.68 |
- 62 % |
| ITO/6nm AgO-2hAnnealing/BufferLayer /ActiveLayer/Cathode |
0.64 |
-2.26 |
39.46 |
0.056 |
0.57 |
- 68 % |
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).