Introduction
The optic nerve, part of the central nervous system (CNS), is responsible for transmitting visual information from the retina to the brain. Oligodendrocytes (OLs), the myelinating glia of the CNS, along with microglia and astrocytes play key roles in maintaining the structural and functional integrity of the retinal ganglion cell (RGC) axons of the optic nerve. Dysregulation or loss of OLs can lead to demyelination in the optic pathway and contribute to optic nerve disorders such as optic neuritis, which is commonly associated with multiple sclerosis and other demyelinating diseases [
1]. Developing tools that enables precise and specific genetic manipulation of optic nerve OLs and other glia populations in the optic nerve is important for advancing our understanding of visual system development, injury, and repair.
One approach to targeting genetic manipulation in the optic nerve is employing the Cre/LoxP DNA recombination system. The Cre/loxP system has been extensively used for gene manipulation in research and its usefulness has been advanced by using spatial- and temporal-regulation of Cre activity; making it an efficient technique for tissue-specific gene manipulation at specific times in development and/or under different disease-associated conditions. One strategy for the inducible regulation of Cre activity is the use of CreERT, a fusion protein combining Cre recombinase and a modified estrogen receptor (ERT), which makes Cre inactive in the absence of the tamoxifen (Tam) or 4-Hydroxytemxifen (4-OHT) ligand. In addition, Cre expression under a tissue specific promoter can provide tissue-specificity with the Cre/loxP system. The temporal administration of 4-OHT can provide complementary temporal specificity [
2,
3,
4]. The CreERT system has been used to genetically modify OLs using mouse models in which CreERT expression is driven by the lineage-specific promoter proteolipid protein 1 (
Plp1); which is expressed only in some glia, more specifically in Schwann cells (SCs) (peripheral nervous system) and oligodendrocytes (CNS) [
5,
6]. We previously, used this model and showed that when Tam is applied to the mouse’s skin, the treated skin Schawn cells express CreERT, promoting DNA recombination of loxP-flanked DNA while leaving other tissues genetically unedited [
6]. This model can also be used to study gene function specifically in the OLs of central nervous system in the context of the visual system [
5,
7].
Systemic delivery of Tam resulted in
Plp1-CreERT/Rosa26-Lacz mediated β-gal staining in the CNS [
5]. However, Tam treatment has been associated with significant drawbacks such as off-target recombination, hormonal side effects, cardiomyopathy, nutritional frailty and increased morbidity in mice [
8,
9,
10,
11,
12]. These challenges are particularly limiting when the goal is to restrict genetic manipulation to the optic pathway without affecting surrounding or distal CNS regions or other tissues. Importantly, topical Tam eye drops have been shown to be both safe and effective for use within the visual pathway, and their use does not affect the structure and function of retinal neurons and glial reactivity in the mouse [
13]. It also has been shown that Tam eyedrops induce Cre mediated recombination in the entire eye and beyond the retina, indicates that Tam eyedrops may be a safe route to induce Cre mediated genetic manipulation in the optic pathways [
14]. In addition, localized injection-based treatment with a Tam analogue and active metabolite, 4-OHT, showed higher recombination efficiency compared to Tam in the calvaria bone, without evidence of significant systemic recombination, enhancing the utility and specificity of this system [
15].
To investigate the potential of a topical, non-invasive and localized approach for CreERT induction in the optic nerve, we delivered 4-OHT via eyedrops to
Plp1-creERT/tdTomato
f/f mice [
6]. In the
Plp1-creERT/tdTomato
f/f mice, 4-OHT activates CreERT recombinase which removes the stop codon between the CAG promoter (a ubiquitously expressing CMV early enhancer/chicken β-actin hybrid promoter) and the open reading frame for the fluorescent protein tdTomato. We have previously showed CreERT mediated tdTomato expression in the SCs of many organs including skin, peripheral neurons, and many other organs [
6]. In this study, we administered topical 4-OHT eyedrops to
Plp1-CreERT/tdTomato
f/f mice to induce Cre-mediated recombination specifically in OLs along the visual pathway and assessed the efficiency and specificity of Cre-recombination by quantifying the extent of tdTomato expression. Potential 4-OHT-associated retinal toxicity was measured by assessing retina function (ERG) and through fundus imaging analyses. Our approach capitalizes on the anatomical proximity of the ocular surface to the optic nerve head, offering a direct route to modulate gene expression in the optic nerve while minimizing systemic effects.
Methods
Animal
The
Plp1-creERT/tdTomato
f/f mouse was generated in the Dr. Yuri Bunimovich lab by crossing B6.Cg-Tg(Plp1-cre/ERT)3Pop/J mice with B6.Cg-Gt(ROSA)26Sor
tm14(CAG-tdTomato)Hze/J mice (Stock No: 007914 and 005975, respectively), originally purchased from the Jackson Laboratory. Detailed genotyping information and primer sequences are provided in the original publication describing these mice [
6]. All animal studies were conducted in accordance with the NIH guidelines for the Care and Use of Laboratory Animals and approved by the Institutional Animal Care and Use Committee of the University of Pittsburgh (Pittsburgh, PA). The
Plp1-creERT/tdTomato
f/f mouse were transferred and housed in the Wilmer Wood animal facility at the Johns Hopkins University School of Medicine and maintained under approved animal protocol (MO23M113).
4-Hydroxytamoxifen (4-OHT)
4-hydroxytamoxifen (4-OHT) (4-OHT; Sigma, H6278) was used instead of Tam due to its greater potency and solubility in a DMSO/ethanol/PBS mixture. Briefly, 10 mg of 4-OHT (Sigma, H6278) was reconstituted with 200 µL of DMSO to make a 50 µg/ul stock solution that was stored at -80 ºC. 500 µg (10 µL) of 50 µg/µL 4-OHT stock in DMSO was mixed with 30 µL of 100% ethyl alcohol to make a 12.5 µg/µL solution. This DMSO/ethanol diluted 4-OHT (3.75 µL) was further diluted in sterile PBS (96.25 µL) to achieve final concentration 0.4875 µg/µL (0.93% DMSO and 2.81% ethyl alcohol). During PBS addition, the 4-OHT starts precipitate which was dissolved by vigorous vortex and heating at 37 °C for 30 minutes in dark. Mice received topical eye drops of the diluted 4-OHT: 11 µL (approximately 5 µg), once a day for OD (8 doses) and twice a day for OS (13 doses). All the 4-OHT treated mouse was 2 female and one male while vehicle treated mouse was one male. The age of the mice at the time of treatment was ~12 months.
Pattern ERG (pERG)
Mice were dark-adapted overnight and anesthetized the following day using Ketamine (10 mg/ml) / Xylazine (1 mg/ml) and their pupils were dilated using tropicamide (0.5%). Pattern-reversal visual evoked potential (VEP) measurements were recorded using a visual pattern stimulator (CELERIS, Diagnosys LLC). Briefly, GenTeel lubricant was applied to eyes to keep them moist and set up all respective electrodes (tail needle electrodes (ground) were inserted at the base of the tail, a reference needle electrode was inserted at the snout, a VEP needle electrode was inserted above the visual cortex, a pERG stimulator electrode was placed on one eye (touching the center of the cornea), and the flash ERG stimulator electrode was placed on the other eye. Recordings were sampled with a black and white bars pattern reversal with 50 cd/m2 luminescence with 100% contrast while a frequency cutoff between 1 to 50 Hz for pERG and 1 to 80 Hz for VEP signals was fixed. A digital 50/60 Hz notch filter was applied and total 300 sweep were recorded per measurement. pERG-VEP were recorded pre and 7 days post 4-OHT treatment.
Anesthesia, Perfusion and Cryosection
All animals were anesthetized using a Ketamine (10 mg/ml) / Xylazine (1 mg/ml) mixture followed by perfusion with 25 ml of sterile PBS followed by 25 ml of 4% paraformaldehyde (PFA) in PBS. Animal heads were carefully dissected to enucleate the eyes and keeping their optic nerves intact and attached with the eyes; the brain was harvested separately. Harvested eyes attached optic nerves and brains were post fixed in 4% PFA at 4
°
C for 2 hours and overnight, respectively, and then incubated in 30% sucrose overnight. Dehydrated tissues were fixed in OCT blocks and optic nerve transverse and cross sections (12 µm) and coronal brain sections (30 µm) were prepared for immunohistochemistry.
Immunohistochemistry and Confocal Imaging
Mice were euthanized after 25 days post 4-OHT treatment start day. Frozen cryosections were incubated in PBS at room temperature for 10 minutes followed by PBS + Triton-X (0.1%) for 30 minutes and blocked in 10% Normal Donkey Serum (NDS) in PBST for blocking for 1 hour. Cryosections were incubated overnight with the primary antibody, Olig 2 (AF2418, R&D Systems) (10 µg/mL) diluted in 5% NDS and 1.5% BSA. After 3 PBST (0.5% tween-20) washes, cryosections were incubated with Donkey derived Anti-Goat 488 and Hoechst (2 μg/mL) for 1 hour. Slides were washed with PBST and mounted with prolong gold antifade reagent (Invitrogen, Cat# P36930). Tiled images were captured and stitched with a Leica 710 confocal microscope using a 20x objective for optic nerve and a 10x objective for brain sections. Images were processed using Leica software and scale bars are as described in the respective images and figure legends. The number of Olig-2 and tdTomato positive cells were manually counted using Fiji ImageJ, and the percentage of tdTomato positive cells was calculated by considering Olig-2 positive cells as 100 percent.
Results
4-OHT Eye Drops Have No Detectable Effect on Retinal Ganglion Cell Function
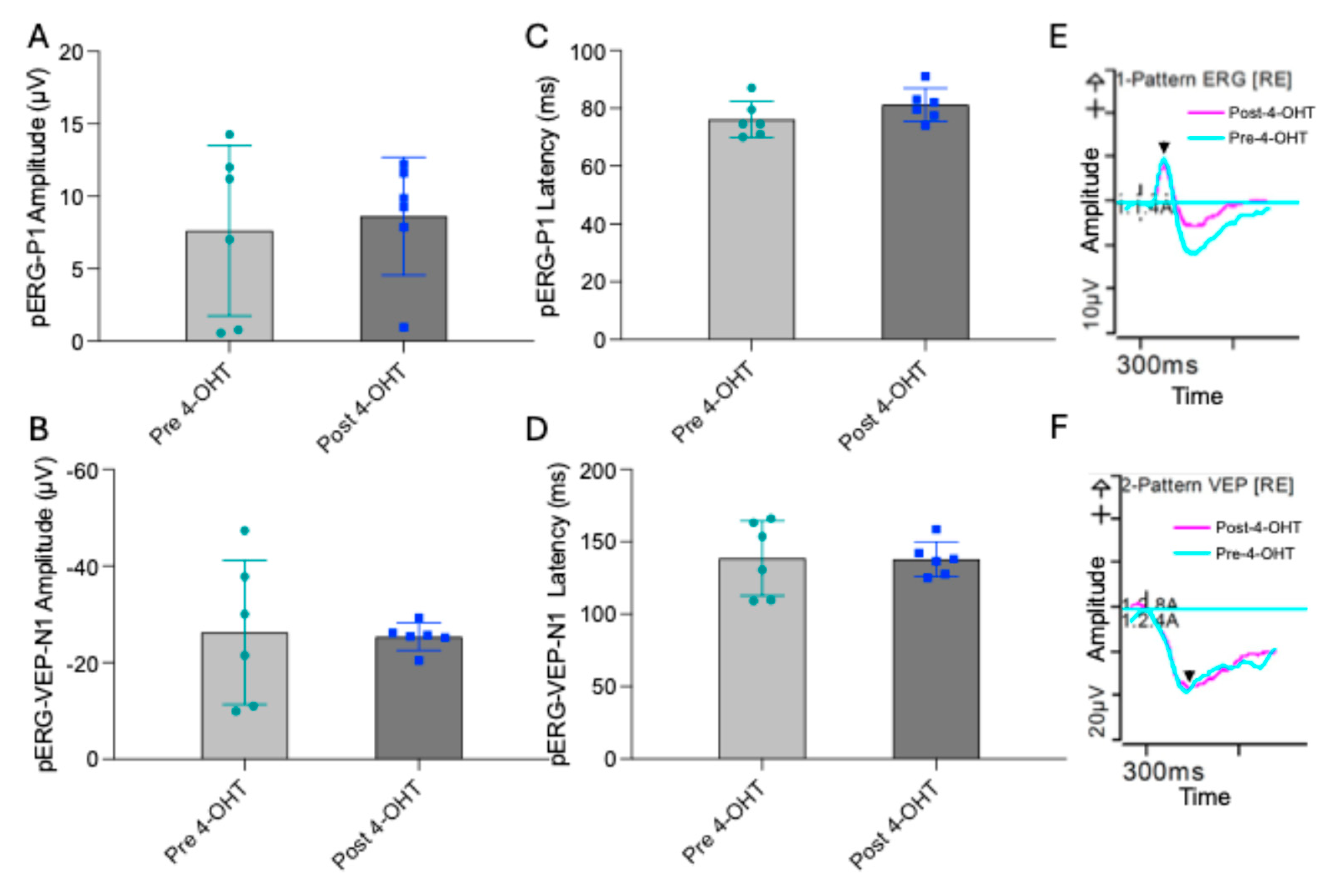
Pattern electroretinography (pERG) measures the functional response of RGCs to alternating light–dark visual patterns. These signals can also be recorded from the visual cortex as pattern visual evoked potentials (pERG-VEP). To assess whether topical administration of 4-OHT affects retinal or cortical electrophysiology, pERG and pERG-VEP recordings were obtained from mice before and after 4-OHT eye drop treatment. We did not observe any significant differences in the major electrophysiological parameters, including the pERG P1-wave amplitude (Pre 4-OHT: 7.6±5.8, post 4-OHT: 8.6±4.0) and the pERG-VEP N1-wave amplitude (Pre 4-OHT: -26.2±14.9, post 4-OHT: -25.3±2.8) (
Figure 1). Similarly, the latencies of both pERG P1 (Pre 4-OHT 76.0±6.3, Post 4-OHT: 81.1±5.8) and pERG-VEP N1 components remained unchanged following 4-OHT administration (Pre 4-OHT 138.5±25.9, Post 4-OHT: 137.9±11.9). These findings indicate that topical 4-OHT treatment does not alter RGC function or visual pathway activity in this experimental context.
Topical 4-OHT Induced OLs Specific Recombination in the Optic Nerve of PLP1-CreERT Mice
To evaluate Cre-mediated recombination following topical 4-OHT treatment, we performed immunohistochemical (IHC) analysis of optic nerves from
Plp1-creERT/tdTomato
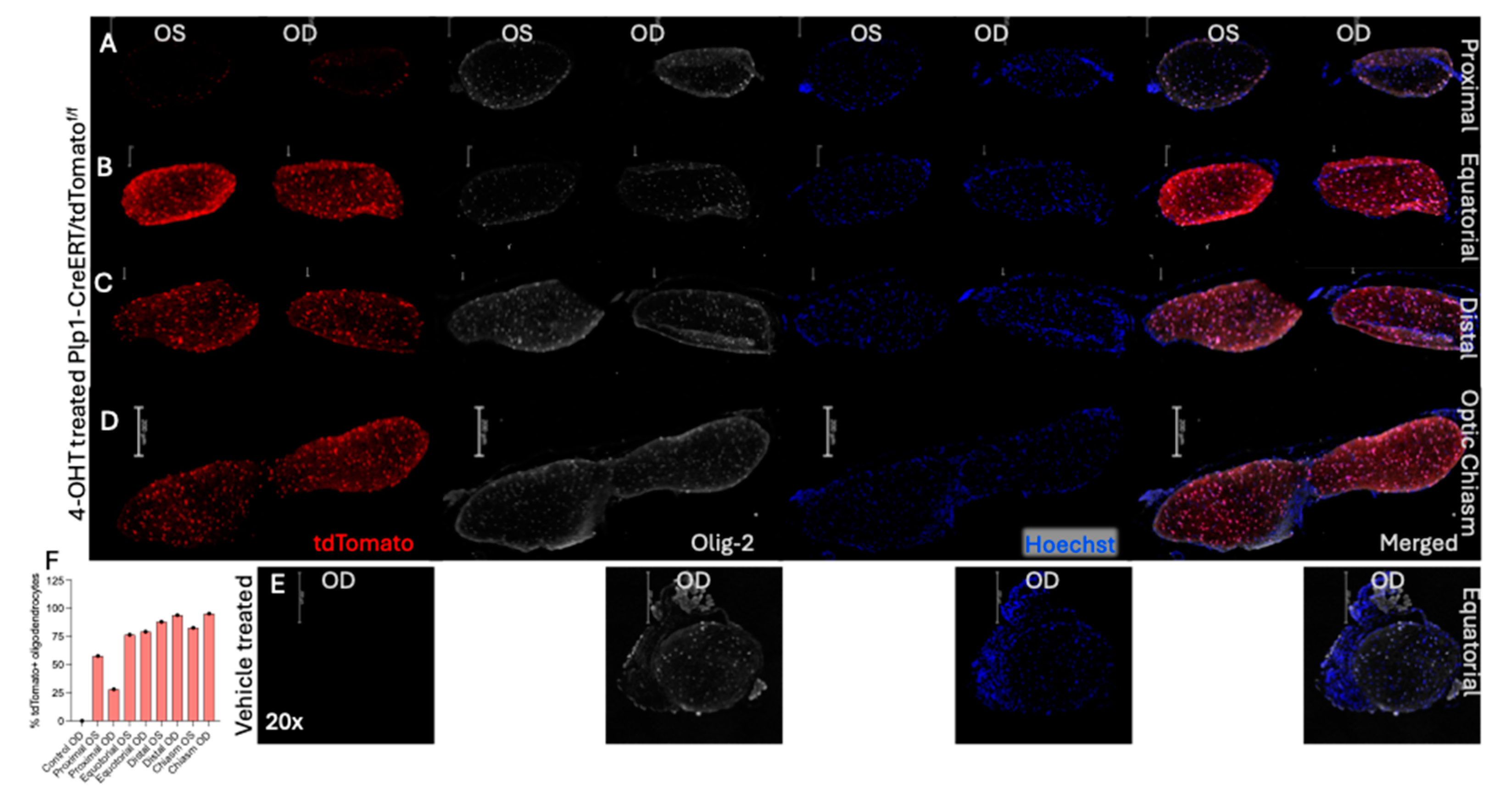
f/f mice. No tdTomato expression was observed in the mice treated with PBS-eyedrops while transverse optic nerve sections of the 4-OHT-eyedrop treated mice revealed robust tdTomato fluorescence that colocalized with Olig2 (oligodendrocyte transcription factor 2)-stained cells, confirming recombination specifically in OLs. The tdTomato expression was observed in the whole optic nerve including the optic chiasm area with approximately 80% of Olig2-positive cells expressing tdTomato with no significance differences between OS (left) and OD (right) eye-localized expression (
Figure 2). We observed that all tdTomato+ cells are co-stained with Olig-2, but all Olig-2 positive cells do not express tdTomato (
Figure 2 A-I and P-S).
The tdTomato expression was not visible in the retina and other optic nerve glia of 4-OHT treated mice, further indicating OL-specific Cre expression (
Figure 2). Similarly, cross-sections of 4-OHT-treated optic nerves show almost no tdTomato expression in the proximal ON, while OL-specific tdTomato expression was prominent in the equatorial ON, distal ON, and optic chiasm. Similar to the transverse section, cross-sections also showed more than 80% of theolig2 stained cells expressed tdTomato while again no tdTomato expression was observed in the optic-nerve cross-sections of vehicle treated mice (
Figure 3). This result suggests that the topical eyedrop administration of 4-OHT induces Cre-mediated OL-specific recombination in the optic track of
Plp1-CreERT/tdTomato
f/f mice.
Discussion
In this study, we demonstrate that topical administration of 4-OHT via eyedrops induces CreERT activation, mediating DNA recombination in OLs of the optic nerve and visual pathway in
Plp1-CreERT/tdTomato
f/f mice. Importantly, this localized method of 4-OHT administration does not adversely affect the functional integrity of RGCs or visual signal transmission, as confirmed by electrophysiological assessments. Preserving visual function and integrity is critical for experiments assessing the effects of gene KO in the optic nerve. After 4-OHT eyedrop treatment there was no indication of retinal degeneration, assessed by fundus imaging (Suppl.
Figure 1), and pERG-P1 and pERG-VEP-N1 wave amplitude measurements (
Figure 1A, 1B and 1E). Additionally, pERG-VEP-N1 wave implicit time (latency), a sign of demyelination, was not delayed post 4-OHT treatment (
Figure 1D and 1F). Taken together, these data suggest that the topical route of 4-OHT administration does not impair RGC function or retinal structural integrity, making this approach a promising strategy for inducible ON gene-KO. Our findings are in agreement with previous studies showing that Tam does not affect the retinal structure and function [
13].
We observed that more than 80% of the olig2 expressing cells (OLs) in the ON co-express tdTomato, indicating efficient Cre activation and DNA recombination (
Figure 2 and
Figure 3). The 20% of Olig2-staining cells that do not show tdTomato expression could represent a subset of OLs; perhaps a subset less prone to DNA recombination or OLs that are newly recruited to the region and have less exposure to the 4-OHT treatment. The cells that do not show Olig-2 or tdTomato expression but stain with Hoechst are possibly other glial cells (
Figure 2P-S, green arrow). Prolonged 4-OHT dosing may allow us to distinguish this, and if there is a particular functional OLs subtype, profiling these cells may provide insight into OLs biology. These findings align with the previous finding that showed CreERT mediated recombination in OLs and SCs after systemic Tam treatment [
5]. We also observed that tdTomato fluorescence extends to the optic chiasm with a similar percentage of OLs showing expression (~80%), while no tdTomato was detected in the retina and lamina cribrosa, likely due to the absence of OLs in these tissues (
Figure 2 and
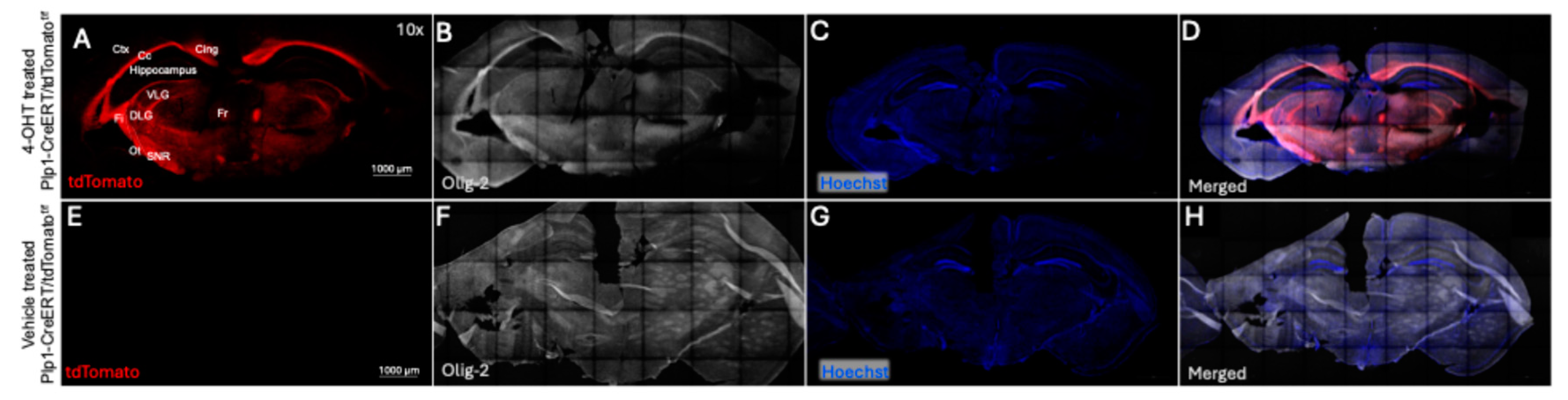
Figure 3). The finding that 4-OHT eyedrops do not impair retinal function- or structural-integrity make this approach a useful strategy for inducing Cre-mediated gene knock out (KO) in the ON. However, it should be noted that beyond the optic nerve several additional CNS regions, including the optic tract, hippocampus, and corpus callosum, also showed 4-OHT-eyedrop dependent tdTomato expression, suggesting that 4-OHT eyedrops can penetrate beyond the optic nerve and induce Cre-mediated recombination in more distal CNS areas (
Figure 4). Although unexpected, and in some circumstances a potential issue, this broad distribution of CreERT activation raises the possibility of using this method to study gene KO not only in the visual pathway but also in certain brain regions involved in visual processing.
This study established localized 4-OHT eyedrops as a method to induce CreERT activity in the optic pathway using the
Plp1-CreERT/tdTomato
f/f mouse model. We showed induction of CreERT-mediated DNA recombination specifically in OLs. A surprising finding, however, with the dosing paradigm we used, was that the effect of 4-OHT eye drops extended into the brain, as we detected tdTomato expressing OLs in multiple brain regions, in addition to the optic nerve. Given this finding, the suitability of the protocol we describe for a particular experiment will depend on the specific research question being addressed, and whether knock-out in the brain will be an issue. For some experiments, the promoter driving CreERT and titration of 4-OHT concentration will have to be carefully considered to minimize recombination in CNS regions not being targeted [
15,
16].
In conclusion, the findings presented here establish an easy and non-invasive method for activating CreERT mediated gene recombination along the visual pathway and in multiple brain regions using a simple topical eye drop approach. Using this strategy in the Plp1-CreER model provides a platform for gene manipulation of optic nerve OLs, enabling mechanistic studies of glial biology in the visual system. It provides an initial indication that topical eye drops can reach and affect ON OLs, suggesting a potential route of therapeutic delivery for demyelinating or neurodegenerative visual disorders, while minimizing systemic exposure. We suggest this approach could be used to perform genetic manipulation studies in mouse models of a variety of optic nerve conditions such as optic neuritis, Leber’s Hereditary Optic Neuropathy, Glaucoma, and Multiple Sclerosis in order to investigate myelin repair and RGC axonal preservation strategies in the optic nerve.
Author Contributions
A.V., K.V., Y.B., and D.Z. conceptualized and designed the study. A.V. participated in most of the experiments (4-OHT treatment, Fundus imaging, ERG, and immunohistochemistry. K.V. and Y.B. generated the mouse model. K.S. bred the animals, maintained them, and shipped them. A.S. contributed to data processing and image analysis. C.Q. helped in mouse tissue processing. A. V., C.B., and D.Z. constructed the manuscript, writing and reviewing the manuscript. Y.B. and D.Z. for fundings. All authors participated in manuscript editing.
Acknowledgments
A.V., A.S., and C.B. acknowledge the Writing Accountability Groups (WAGs) of Wilmer Eye Institute. A.V. acknowledge Athanasios S. Alexandris (neuropathology, JHU) for frequent scientific discussion about the study. A.V. acknowledge the Knights Templar Eye Foundation Career Starter Grant. We also thank the Guerrieri Family Foundation and the Gilbert Family Foundation for research funding and P30 Core Grant EY001765/EY/NEI NIH. Y. B. acknowledge NIH R01 CA266529.
List of Abbreviations
4-OHT: 4-hydroxytamoxifen
Tam: Tamoxifen
CNS: Central Nervous System
SCs: Schwann cells
ON: Optic Nerve
OLs: Oligodendrocytes
RGCs: Retinal Ganglion Cells
OD: Oculus Dexter (Right Eye)
OS: Oculus Sinister (Left Eye)
NDS: Normal Donkey Serum (NDS
PFA: Paraformaldehyde
PBS: Phosphate Buffer Saline
Hz: Hertz
cd/m2: Candelas/square meter
ms: Mile Second
References
- Ciapa, MA; Salaru, DL; Statescu, C; Sascau, RA; Bogdanici, CM. Optic Neuritis in Multiple Sclerosis-A Review of Molecular Mechanisms Involved in the Degenerative Process. Curr Issues Mol Biol. 2022, 44(9), 3959–79. [Google Scholar] [CrossRef] [PubMed]
- Cazzulino, AS; Martinez, R; Tomm, NK; Denny, CA. Improved specificity of hippocampal memory trace labeling. Hippocampus 2016, 26(6), 752–62. [Google Scholar] [CrossRef] [PubMed]
- Guenthner, CJ; Miyamichi, K; Yang, HH; Heller, HC; Luo, L. Permanent genetic access to transiently active neurons via TRAP: targeted recombination in active populations. Neuron 2013, 78(5), 773–84. [Google Scholar] [CrossRef] [PubMed]
- Hans, S; Kaslin, J; Freudenreich, D; Brand, M. Temporally-controlled site-specific recombination in zebrafish. PLoS One 2009, 4(2), e4640. [Google Scholar] [CrossRef] [PubMed]
- Leone, DP; Genoud, S; Atanasoski, S; Grausenburger, R; Berger, P; Metzger, D; et al. Tamoxifen-inducible glia-specific Cre mice for somatic mutagenesis in oligodendrocytes and Schwann cells. Mol Cell Neurosci. 2003, 22(4), 430–40. [Google Scholar] [CrossRef] [PubMed]
- Kruglov, O; Vats, K; Soman, V; Tyurin, VA; Tyurina, YY; Wang, J; et al. Melanoma-associated repair-like Schwann cells suppress anti-tumor T-cells via 12/15-LOX/COX2-associated eicosanoid production. Oncoimmunology 2023, 12(1), 2192098. [Google Scholar] [CrossRef] [PubMed]
- Doerflinger, NH; Macklin, WB; Popko, B. Inducible site-specific recombination in myelinating cells. Genesis 2003, 35(1), 63–72. [Google Scholar] [CrossRef] [PubMed]
- Halpage, J; DaSilva Pantoja, P; Mancarella, S. Prolonged tamoxifen-enriched diet is associated with cardiomyopathy and nutritional frailty in mice. Exp Physiol. 2024, 109(4), 513–23. [Google Scholar] [CrossRef] [PubMed]
- Ilchuk, LA; Stavskaya, NI; Varlamova, EA; Khamidullina, AI; Tatarskiy, VV; Mogila, VA; et al. Limitations of Tamoxifen Application for In Vivo Genome Editing Using Cre/ER(T2) System. Int J Mol Sci. 2022, 23(22). [Google Scholar] [CrossRef] [PubMed]
- Willems, A; De Gendt, K; Deboel, L; Swinnen, JV; Verhoeven, G. The development of an inducible androgen receptor knockout model in mouse to study the postmeiotic effects of androgens on germ cell development. Spermatogenesis 2011, 1(4), 341–53. [Google Scholar] [CrossRef] [PubMed]
- Patel, SH; O’Hara, L; Atanassova, N; Smith, SE; Curley, MK; Rebourcet, D; et al. Low-dose tamoxifen treatment in juvenile males has long-term adverse effects on the reproductive system: implications for inducible transgenics. Sci Rep. 2017, 7(1), 8991. [Google Scholar] [CrossRef] [PubMed]
- Huh, WJ; Khurana, SS; Geahlen, JH; Kohli, K; Waller, RA; Mills, JC. Tamoxifen induces rapid, reversible atrophy, and metaplasia in mouse stomach. Gastroenterology 2012, 142(1), 21–4 e7. [Google Scholar] [CrossRef] [PubMed]
- Boneva, SK; Gross, TR; Schlecht, A; Schmitt, SI; Sippl, C; Jagle, H; et al. Cre recombinase expression or topical tamoxifen treatment do not affect retinal structure and function, neuronal vulnerability or glial reactivity in the mouse eye. Neuroscience 2016, 325, 188–201. [Google Scholar] [CrossRef] [PubMed]
- Schlecht, A; Leimbeck, SV; Tamm, ER; Braunger, BM. Tamoxifen-Containing Eye Drops Successfully Trigger Cre-Mediated Recombination in the Entire Eye. Adv Exp Med Biol. 2016, 854, 495–500. [Google Scholar] [PubMed]
- Hou, J; Lin, CP; Intini, G. Activation of creER recombinase in the mouse calvaria induces local recombination without effects on distant skeletal segments. Sci Rep. 2021, 11(1), 8214. [Google Scholar] [CrossRef] [PubMed]
- Hayashi, S; McMahon, AP. Efficient recombination in diverse tissues by a tamoxifen-inducible form of Cre: a tool for temporally regulated gene activation/inactivation in the mouse. Dev Biol. 2002, 244(2), 305–18. [Google Scholar] [CrossRef] [PubMed]
Figure 1.
Pattern ERG-VEP of mice before and after 4-OHT-eyedrop treatment. A and B. Bar graphs show pERG-P1 wave amplitude (A) and pERG-N1 wave amplitude (B). C and D. bar graph shows the pERG-P1 wave latency (C) and pERF-Vep-N1 wave latency. E is the pERG-P1 wave form, F is the pERG-VEP-N1, (y-axis is amplitude (µV) and x-axis are latency in mile seconds (ms) against time wave form. (N=3 males, for 4-OHT treated mice).
Figure 1.
Pattern ERG-VEP of mice before and after 4-OHT-eyedrop treatment. A and B. Bar graphs show pERG-P1 wave amplitude (A) and pERG-N1 wave amplitude (B). C and D. bar graph shows the pERG-P1 wave latency (C) and pERF-Vep-N1 wave latency. E is the pERG-P1 wave form, F is the pERG-VEP-N1, (y-axis is amplitude (µV) and x-axis are latency in mile seconds (ms) against time wave form. (N=3 males, for 4-OHT treated mice).
Figure 2.
Transverse section of vehicle- and 4-OHT- treated optic nerves of Plp1-CreERT/tdTomatof/f mice. A. Immunofluorescence of 4-OHT treated optic nerve of OS (B-E) and OD (F-I) attached with the retina eye cup. B-I is the magnified white box shown in 4-OHT treated optic nerve of OS (B-E) and OD (F-I), showing the CreERT mediated tdTomato expression (red, B&F) and Olig 2 stain (white, C&G), and Hoechst (blue, D&H), and merged images (E&I) across the entire optic nerve including the optic chiasm. J. vehicle treated optic nerve of left eye (OS) with retina eye cup. K-N. are the magnified white box of OS optic nerve shown in image J, not showing tdTomato expression (no visible red, K) and Olig 2 stain (white, L), and Hoechst (M), and merged images (N). Scale bar is 1000 µM. O. Bar graph shows the average percentage of tdTomato expressing cells calculated by considering total Olig-2 positive cells as 100%. P-S. Magnified (40x) proximal area of optic nerve transverse section of 4-OHT- treated optic nerves showing Cre mediated tdTomato expression (red, P) and Olig 2 stain (white, Q), and Hoechst (blue, R), and merged images (S). Red arrow with white border indicates the tdTomato and Olig-2 positive cells, white arrow with red border shows only the Olig-2 positive cells, green arrow with white border shows tdTomato and Olig-2, both negative (possibly other glia’s). Scale bar is 100 µM. N=2 for 4-OHT treated and N=1 for vehicle treated mouse. Error bars are standard deviation. The cells were counted by manual marking using Fiji ImageJ.
Figure 2.
Transverse section of vehicle- and 4-OHT- treated optic nerves of Plp1-CreERT/tdTomatof/f mice. A. Immunofluorescence of 4-OHT treated optic nerve of OS (B-E) and OD (F-I) attached with the retina eye cup. B-I is the magnified white box shown in 4-OHT treated optic nerve of OS (B-E) and OD (F-I), showing the CreERT mediated tdTomato expression (red, B&F) and Olig 2 stain (white, C&G), and Hoechst (blue, D&H), and merged images (E&I) across the entire optic nerve including the optic chiasm. J. vehicle treated optic nerve of left eye (OS) with retina eye cup. K-N. are the magnified white box of OS optic nerve shown in image J, not showing tdTomato expression (no visible red, K) and Olig 2 stain (white, L), and Hoechst (M), and merged images (N). Scale bar is 1000 µM. O. Bar graph shows the average percentage of tdTomato expressing cells calculated by considering total Olig-2 positive cells as 100%. P-S. Magnified (40x) proximal area of optic nerve transverse section of 4-OHT- treated optic nerves showing Cre mediated tdTomato expression (red, P) and Olig 2 stain (white, Q), and Hoechst (blue, R), and merged images (S). Red arrow with white border indicates the tdTomato and Olig-2 positive cells, white arrow with red border shows only the Olig-2 positive cells, green arrow with white border shows tdTomato and Olig-2, both negative (possibly other glia’s). Scale bar is 100 µM. N=2 for 4-OHT treated and N=1 for vehicle treated mouse. Error bars are standard deviation. The cells were counted by manual marking using Fiji ImageJ.
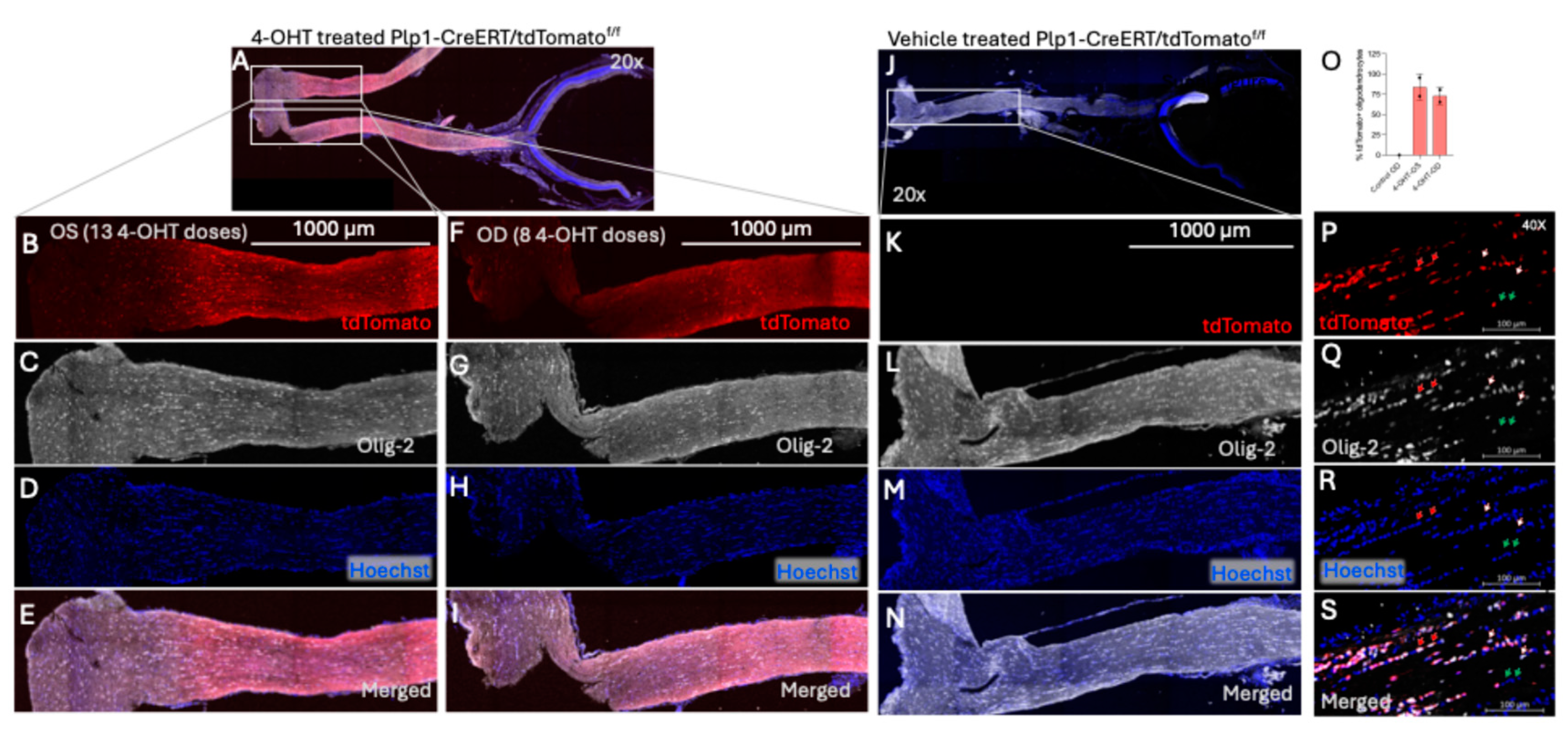
Figure 3.
Cross-section of vehicle- and 4-OHT treated optic nerve of Plp1-CreERT/tdTomatof/f mice. A-D. Immunofluorescence sections of 4-OHT treated optic nerve taken at proximal (A), equatorial (B), and distal (C), and optic chiasm (D) regions of the optic nerve showing the CreERT mediated tdTomato expression (red) and Olig 2 stain (white), and Hoechst (blue), and merged images. E. Vehicle treated optic nerve of OD not showing the CreERT mediated tdTomato expression (no visible red) and Olig 2 stain (white), and Hoechst (blue), and merged images. Scale bar is 200 µM. F. Bar graph shows the average percentage of tdTomato expressing cells calculated by considering total Olig-2 positive cells as 100%. N=1 for 4-OHT treated and N=1 for vehicle treated mouse. Only one optic nerve in each group, hence no error bars visible. The cells were counted by manual marking using Fiji ImageJ.
Figure 3.
Cross-section of vehicle- and 4-OHT treated optic nerve of Plp1-CreERT/tdTomatof/f mice. A-D. Immunofluorescence sections of 4-OHT treated optic nerve taken at proximal (A), equatorial (B), and distal (C), and optic chiasm (D) regions of the optic nerve showing the CreERT mediated tdTomato expression (red) and Olig 2 stain (white), and Hoechst (blue), and merged images. E. Vehicle treated optic nerve of OD not showing the CreERT mediated tdTomato expression (no visible red) and Olig 2 stain (white), and Hoechst (blue), and merged images. Scale bar is 200 µM. F. Bar graph shows the average percentage of tdTomato expressing cells calculated by considering total Olig-2 positive cells as 100%. N=1 for 4-OHT treated and N=1 for vehicle treated mouse. Only one optic nerve in each group, hence no error bars visible. The cells were counted by manual marking using Fiji ImageJ.
Figure 4.
Coronal plane section of brain of Plp1-CreERT/tdTomatof/f mice. A-D. Immunofluorescence of coronal brain sections of 4-OHT treated mouse showing the CreERT mediated tdTomato expression (red, A) and Olig 2 stain (white, B), and Hoechst (blue, C), and merged images (D) in different regions of brain including hippocampus, cortex and optic tract. E-H. vehicle treated mouse brain not showing tdTomato expression (no visible red, E) and Olig 2 stain (white, F), Hoechst (G), and merged images (H). Annotation in A is as follows; (Ctx) Cerebral cortex, (Cg) Cingulum, (Fr) Fasciculus retroflexus, (Fi) fimbria, (Cc) Corpus callosum, (Ot) Optic tract, (VLG) Ventral lateral geniculus, (DLG) Dorsal lateral geniculus, (SNR) Substantia nigra. Scale bar is 1000 µM. N=2 for 4-OHT treated and N=1 for vehicle treated mouse.
Figure 4.
Coronal plane section of brain of Plp1-CreERT/tdTomatof/f mice. A-D. Immunofluorescence of coronal brain sections of 4-OHT treated mouse showing the CreERT mediated tdTomato expression (red, A) and Olig 2 stain (white, B), and Hoechst (blue, C), and merged images (D) in different regions of brain including hippocampus, cortex and optic tract. E-H. vehicle treated mouse brain not showing tdTomato expression (no visible red, E) and Olig 2 stain (white, F), Hoechst (G), and merged images (H). Annotation in A is as follows; (Ctx) Cerebral cortex, (Cg) Cingulum, (Fr) Fasciculus retroflexus, (Fi) fimbria, (Cc) Corpus callosum, (Ot) Optic tract, (VLG) Ventral lateral geniculus, (DLG) Dorsal lateral geniculus, (SNR) Substantia nigra. Scale bar is 1000 µM. N=2 for 4-OHT treated and N=1 for vehicle treated mouse.
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |