Submitted:
16 December 2025
Posted:
17 December 2025
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Materials and Methods
2.1. Synthesis and Aging Treatment of Fe-As Complexes
2.2. Stabilization Test of Fe-As Complexes
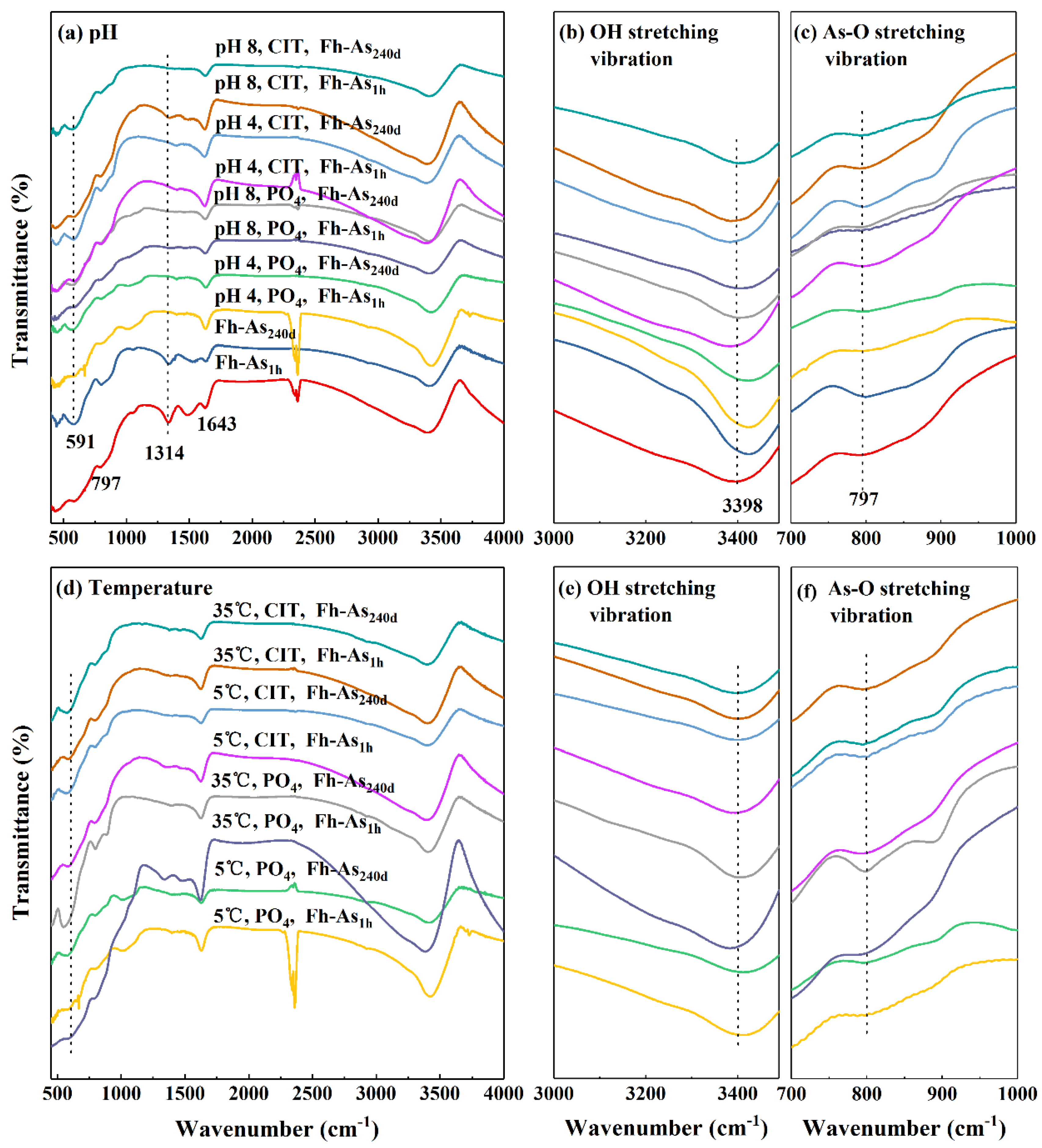
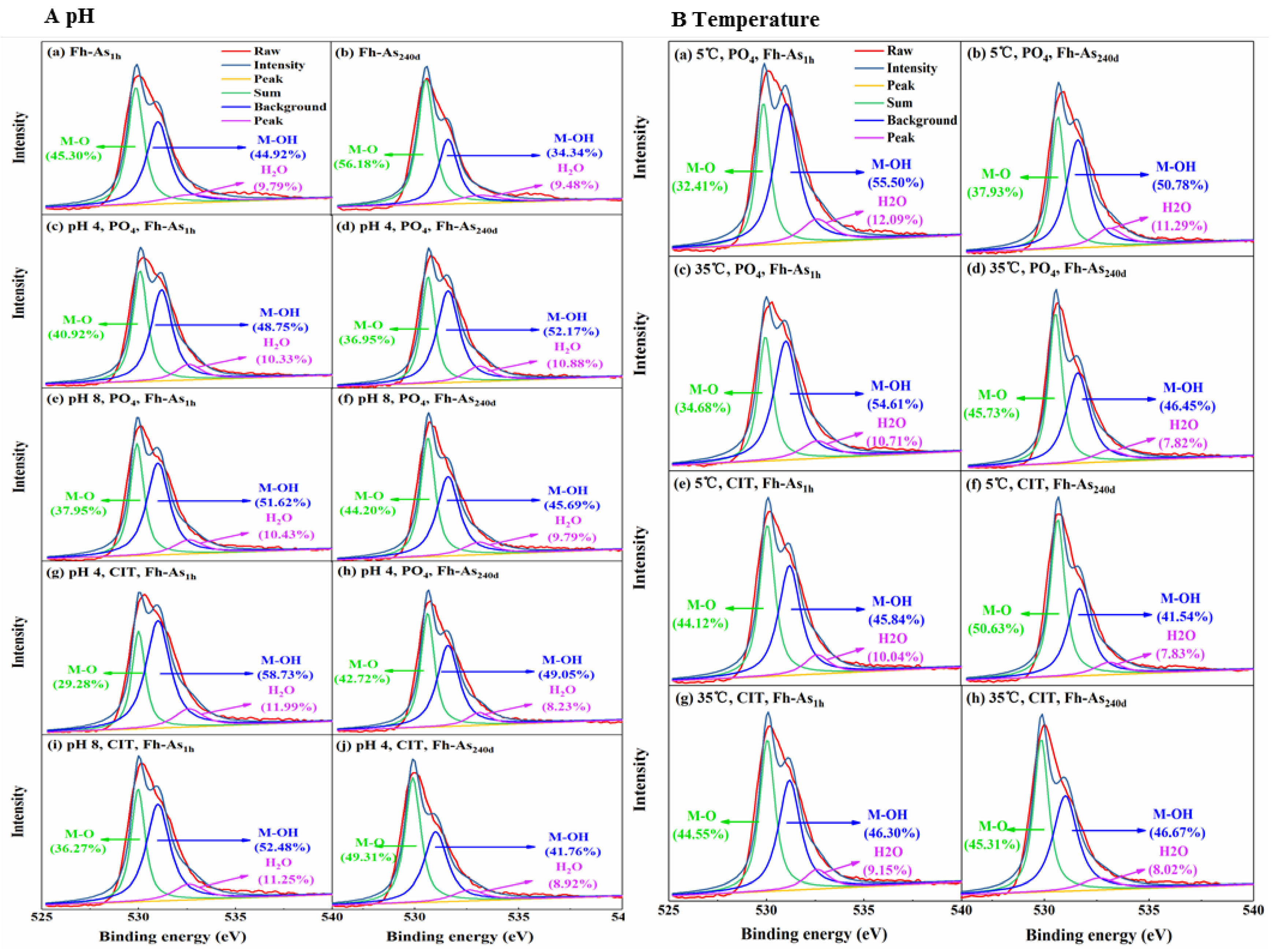
2.3. Characterization of the Fe-As Complexes Before and After Leaching
3. Results
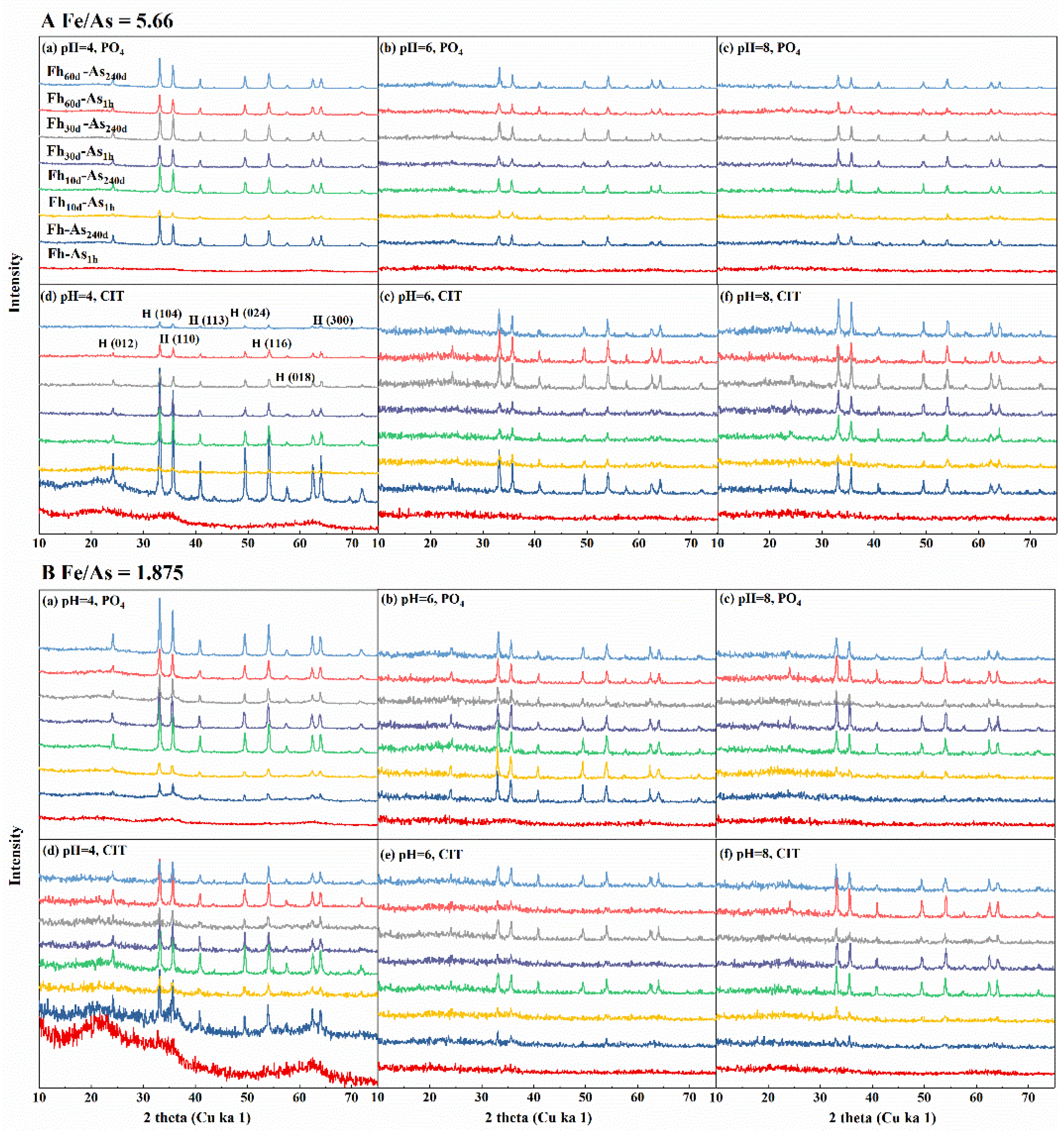
3.1. Effect of the Fe/As Molar Ratio and Aging on the Stability of Complexes
3.2. Changes in Arsenate Stability During the Adsorption of Iron Minerals with Arsenic
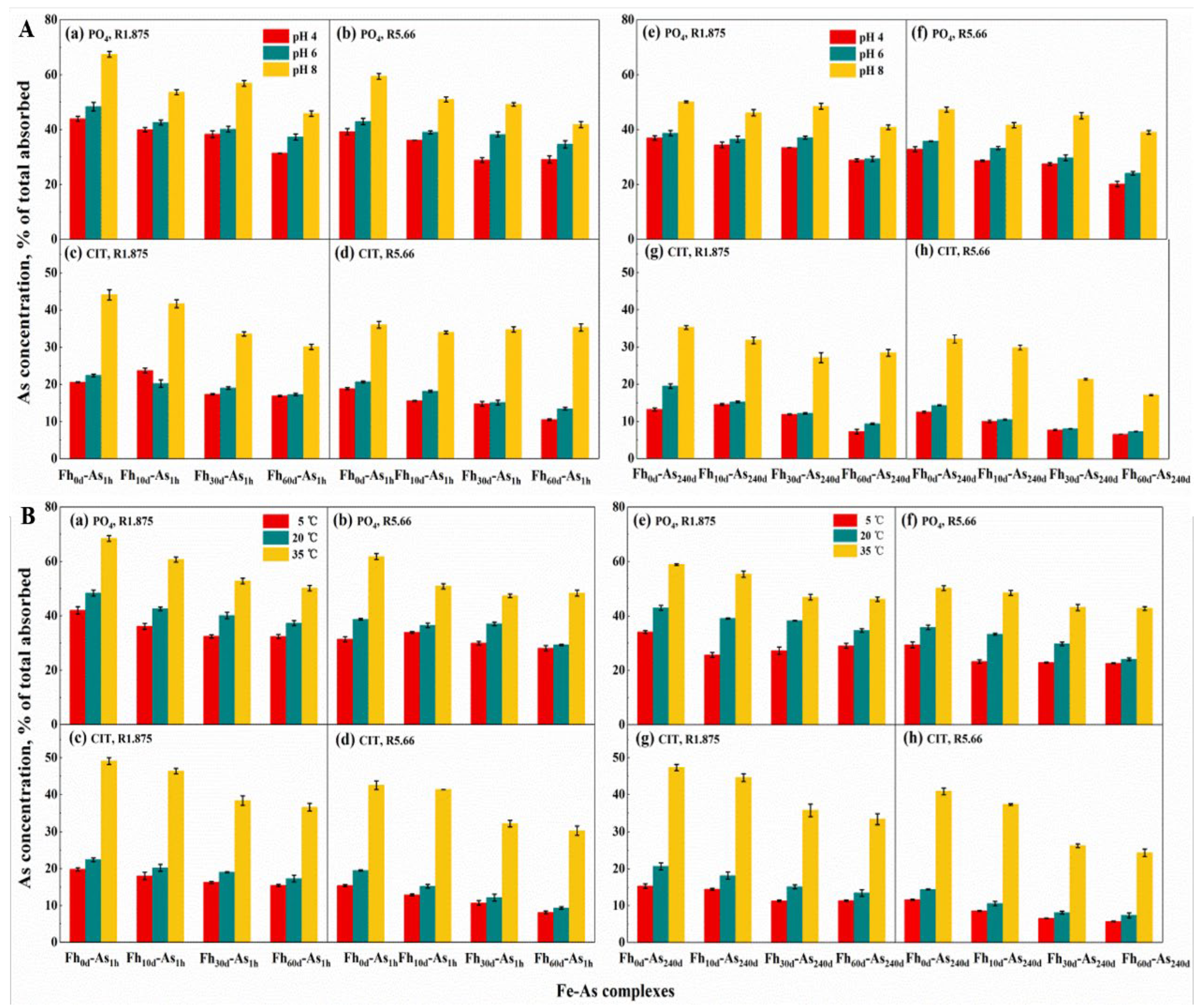
3.3. Role of the Reaction Media on the Stability of Complexes
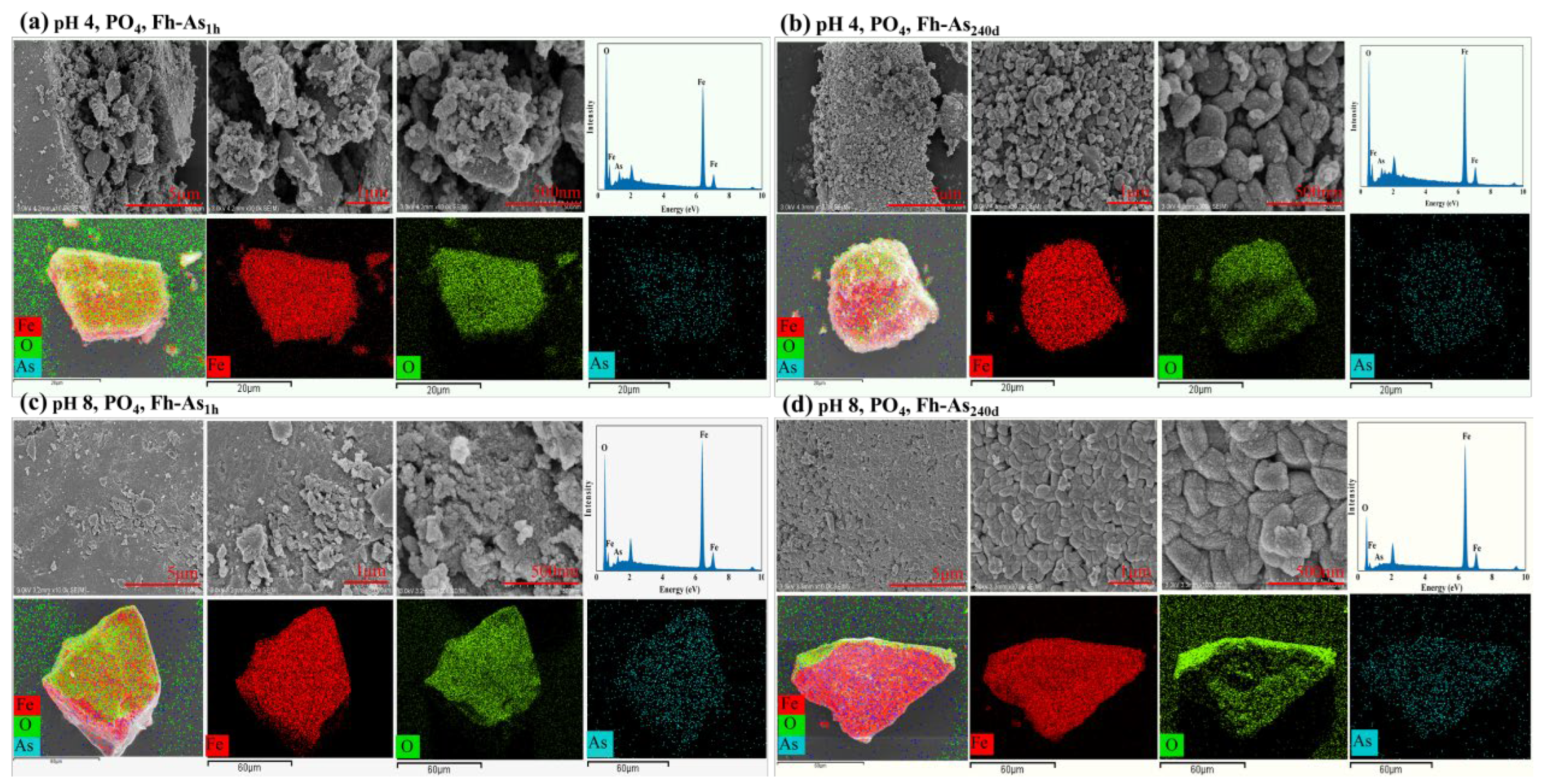
3.4. pH Dependency
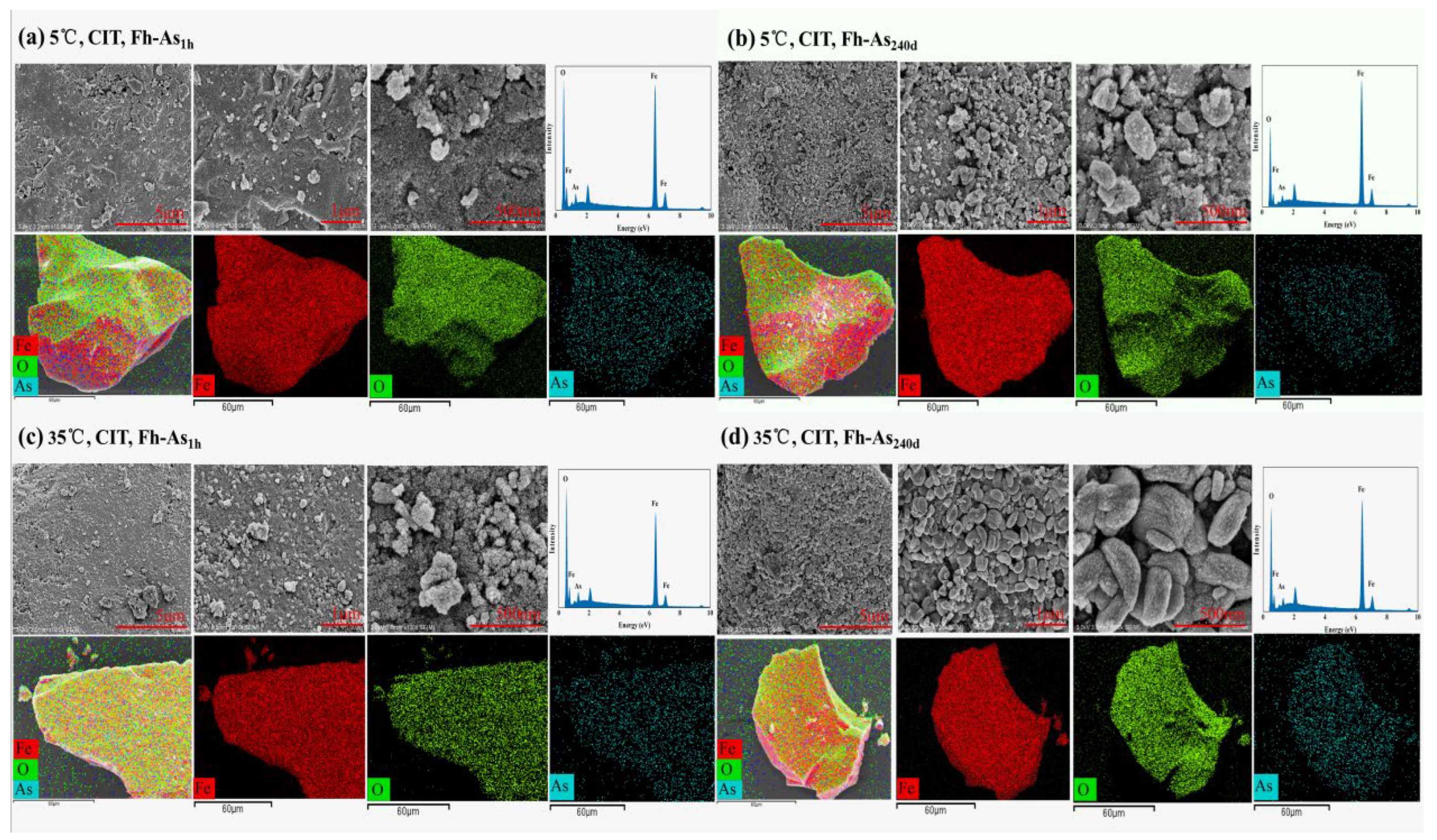
3.5. Temperature Dominance
4. Discussion
4.1. Internal Factors Influence the Fate of As in Fe-As Complexes
4.2. External Factors Influence the Fate of As in Fe-As Complexes
4.3. Stability of Fe-As Complexes Under the Interaction of Internal and External Factors
5. Conclusions
Supplementary Materials
Author Contributions
Acknowledgments
Conflicts of Interest
References
- Zhang, D.N., Cao, R., Wang, S.F., Wang, Y., Bi, R., Jia, Y.F., 2020. Fate of arsenic during up to 4.5 years of aging of FeIII-AsV complexess at acidic pH: Effect of reaction media (Nitrate vs. Sulfate), Fe/As molar ratio, and pH. Chem. Eng. J., 388(15): 124239.
- Zhang, D.; Wang, S.; Gomez, M.A.; Wang, Y.; Jia, Y. The long-term stability of FeIII-AsV coprecipitates at pH 4 and 7: Mechanisms controlling the arsenic behavior. J. Hazard. Mater. 2019, 374, 276–286. [CrossRef]
- Bolan, N.; Kunhikrishnan, A.; Thangarajan, R.; Kumpiene, J.; Park, J.; Makino, T.; Kirkham, M.B.; Scheckel, K. Remediation of heavy metal(loid)s contaminated soils – To mobilize or to immobilize?. J. Hazard. Mater. 2014, 266, 141–166. [CrossRef]
- Yoon, S.-G.; Kwak, I.-S.; Yoon, H.-O.; An, J. Adsorption Characteristics of Dimethylated Arsenicals on Iron Oxide–Modified Rice Husk Biochar. Toxics 2022, 10, 703. [CrossRef]
- Yang, X.; Huang, H.; Hu, P.; Luan, H.; Song, B.; Zheng, Z.; Zhang, C.; Yan, R.; Li, K. Assessment of Heavy Metal Pollution in Mangrove Sediments of Liusha Bay, Leizhou Peninsula, China. Toxics 2025, 13, 961. [CrossRef]
- Doerfelt, C.; Feldmann, T.; Roy, R.; Demopoulos, G.P. Stability of arsenate-bearing Fe(III)/Al(III) co-precipitates in the presence of sulfide as reducing agent under anoxic conditions. Chemosphere 2016, 151, 318–323. [CrossRef]
- Wang, Y.; Liu, H.; Wang, S.; Li, X.; Wang, X.; Jia, Y. Simultaneous removal and oxidation of arsenic from water by δ-MnO2 modified activated carbon. J. Environ. Sci. 2020, 94, 147–160. [CrossRef]
- Pérez-De-Mora, A.; Madejón, P.; Burgos, P.; Cabrera, F.; Lepp, N.W.; Madejón, E. Phytostabilization of semiarid soils residually contaminated with trace elements using by-products: Sustainability and risks. Environ. Pollut. 2011, 159, 3018–3027. [CrossRef]
- Ding, Z.; Fu, F.; Dionysiou, D.D.; Tang, B. Coadsorption and subsequent redox conversion behaviors of As(III) and Cr(VI) on Al-containing ferrihydrite. Environ. Pollut. 2018, 235, 660–669. [CrossRef]
- Liu, J.; Gao, X.; Dai, C.; Zhang, S.; Kong, S.; Wang, L.; Hu, Y. Cr(iii)-incorporated Fe(iii) hydroxides for enhanced redox conversion of As(iii) and Cr(vi) in acidic solution. Environ. Sci. Nano 2025. [CrossRef]
- Qiao, J.; Jiang, Z.; Sun, B.; Sun, Y.; Wang, Q.; Guan, X. Arsenate and arsenite removal by FeCl3: Effects of pH, As/Fe ratio, initial As concentration and co-existing solutes. Sep. Purif. Technol. 2012, 92, 106–114. [CrossRef]
- Hiemstra, T. Surface and mineral structure of ferrihydrite. Geochim. et Cosmochim. Acta 2013, 105, 316–325. [CrossRef]
- Hu, S.; Lu, Y.; Peng, L.; Wang, P.; Zhu, M.; Dohnalkova, A.C.; Chen, H.; Lin, Z.; Dang, Z.; Shi, Z. Coupled Kinetics of Ferrihydrite Transformation and As(V) Sequestration under the Effect of Humic Acids: A Mechanistic and Quantitative Study. Environ. Sci. Technol. 2018, 52, 11632–11641. [CrossRef]
- Jia, Y.; Xu, L.; Wang, X.; Demopoulos, G.P. Infrared spectroscopic and X-ray diffraction characterization of the nature of adsorbed arsenate on ferrihydrite. Geochim. et Cosmochim. Acta 2007, 71, 1643–1654. [CrossRef]
- Shi, Q.; Jing, C.; Meng, X. Competing Interactions of As Adsorption and Fe(III) Polymerization during Ferric Coprecipitation Treatment. Environ. Sci. Technol. 2018, 52, 7343–7350. [CrossRef]
- Yan, Z.; Xu, Z.; Yu, J.; Jaroniec, M. Highly Active Mesoporous Ferrihydrite Supported Pt Catalyst for Formaldehyde Removal at Room Temperature. Environ. Sci. Technol. 2015, 49, 6637–6644. [CrossRef]
- Hiemstra, T. Ferrihydrite interaction with silicate and competing oxyanions: Geometry and Hydrogen bonding of surface species. Geochim. et Cosmochim. Acta 2018, 238, 453–476. [CrossRef]
- Hiemstra, T. Surface structure controlling nanoparticle behavior: magnetism of ferrihydrite, magnetite, and maghemite. Environ. Sci. Nano 2018, 5, 752–764. [CrossRef]
- Yang, Z.; Zhang, N.; Sun, B.; Su, S.; Wang, Y.; Zhang, Y.; Wu, C.; Zeng, X. Contradictory tendency of As(V) releasing from Fe–As complexes: Influence of organic and inorganic anions. Chemosphere 2022, 286, 131469. [CrossRef]
- Zanzo, E.; Balint, R.; Prati, M.; Celi, L.; Barberis, E.; Violante, A.; Martin, M. Aging and arsenite loading control arsenic mobility from ferrihydrite-arsenite coprecipitates. Geoderma 2017, 299, 91–100. [CrossRef]
- Yuan, Z.; Zhang, D.; Wang, S.; Xu, L.; Wang, K.; Song, Y.; Xiao, F.; Jia, Y. Effect of hydroquinone-induced iron reduction on the stability of scorodite and arsenic mobilization. Hydrometallurgy 2016, 164, 228–237. [CrossRef]
- Zhang, G.; Zhang, D.; Yuan, Z.; Ma, X.; Lei, L.; Wu, X.; Lin, J.; Wang, X.; Wang, S.; Jia, Y. Fate of adsorbed arsenic during early stage sulfidization of nano-ferrihydrite. Environ. Sci. Nano 2019, 6, 2228–2240. [CrossRef]
- An, W.; Wu, C.; Xue, S.; Liu, Z.; Liu, M.; Li, W. Effects of biochar/AQDS on As(III)-adsorbed ferrihydrite reduction and arsenic (As) and iron (Fe) transformation: Abiotic and biological conditions. Chemosphere 2022, 291, 133126. [CrossRef]
- George, A.; Shen, B.; Kang, D.; Yang, J.; Luo, J. Emission control strategies of hazardous trace elements from coal-fired power plants in China. J. Environ. Sci. 2020, 93, 66–90. [CrossRef]
- Yang, Z.L., Bai, L.Y., Su, S.M., Wang, Y.N., Wu, C.X., Zeng, X.B., Sun, B.H., 2021. Stability of Fe-As complexes formed with As(V) and aged ferrihydrite. J. Environ. Sci., 100(02): 43-50.
- Zhang, P.; Yao, W.; Yuan, S. Citrate-enhanced release of arsenic during pyrite oxidation at circumneutral conditions. Water Res. 2017, 109, 245–252. [CrossRef]
- Paige, C.R.; Snodgrass, W.J.; Nicholson, R.V.; Scharer, J.M. The crystallization of arsenate-contaminated iron hydroxide solids at high pH. Water Environ. Res. 1996, 68, 981–987. [CrossRef]
- Winkler, P.; Kaiser, K.; Thompson, A.; Kalbitz, K.; Fiedler, S.; Jahn, R. Contrasting evolution of iron phase composition in soils exposed to redox fluctuations. Geochim. et Cosmochim. Acta 2018, 235, 89–102. [CrossRef]
- He, C.; Ning, Y.; Li, Y.; Guo, H.; Yang, Z.; Yang, S.; Jiang, F. The mobility of U(VI) associated with Fe(II)-induced transformation of schwertmannite and its reductive dissolution and re-precipitation in AMD environment. Appl. Geochem. 2025, 190. [CrossRef]
- Hartley, W.; Edwards, R.; Lepp, N.W. Arsenic and heavy metal mobility in iron oxide-amended contaminated soils as evaluated by short- and long-term leaching tests. Environ. Pollut. 2004, 131, 495–504. [CrossRef]
- Bolanz, R.M., Wierzbicka-Wieczorek, M., Caplovicova, M., Uhlik, P., Goettlicher, J., Steininger, R., Majzlan, J., 2013. Structural Incorporation of As5+ into Hematite. Environ. Sci. Technol., 47(16): 9140-9147.
- Zhang, T.; Zeng, X.; Zhang, H.; Lin, Q.; Su, S.; Wang, Y.; Bai, L. Investigation of synthetic ferrihydrite transformation in soils using two-step sequential extraction and the diffusive gradients in thin films (DGT) technique. Geoderma 2018, 321, 90–99. [CrossRef]
- Violante, A.; Del Gaudio, S.; Pigna, M.; Ricciardella, M.; Banerjee, D. Coprecipitation of Arsenate with Metal Oxides. 2. Nature, Mineralogy, and Reactivity of Iron(III) Precipitates. Environ. Sci. Technol. 2007, 41, 8275–8280. [CrossRef]
- Schwertmann, U., Cornell, R.M., 2000. Iron Oxides in the Laboratory: Preparation and Characterization. Weinheim, Germany: Wiley-VCH Verlag GmbH.
- Michel, F.M.; Barrón, V.; Torrent, J.; Morales, M.P.; Serna, C.J.; Boily, J.-F.; Liu, Q.; Ambrosini, A.; Cismasu, A.C.; Brown, G.E. Ordered ferrimagnetic form of ferrihydrite reveals links among structure, composition, and magnetism. Proc. Natl. Acad. Sci. 2010, 107, 2787–2792. [CrossRef]
- Filimonova, S.; Kaufhold, S.; Wagner, F.E.; Häusler, W.; Kögel-Knabner, I. The role of allophane nano-structure and Fe oxide speciation for hosting soil organic matter in an allophanic Andosol. Geochim. et Cosmochim. Acta 2016, 180, 284–302. [CrossRef]
- Hiemstra, T. Formation, stability, and solubility of metal oxide nanoparticles: Surface entropy, enthalpy, and free energy of ferrihydrite. Geochim. et Cosmochim. Acta 2015, 158, 179–198. [CrossRef]
- Russell, J.D.; Parfitt, R.L.; Fraser, A.R.; Farmer, V.C. Surface structures of gibbsite goethite and phosphated goethite. Nature 1974, 248, 220–221. [CrossRef]
- Bompoti, N.; Chrysochoou, M.; Machesky, M. Surface structure of ferrihydrite: Insights from modeling surface charge. Chem. Geol. 2017, 464, 34–45. [CrossRef]
- Wang, X.; Zhu, M.; Lan, S.; Ginder-Vogel, M.; Liu, F.; Feng, X. Formation and secondary mineralization of ferrihydrite in the presence of silicate and Mn(II). Chem. Geol. 2015, 415, 37–46. [CrossRef]
- Das, S.; Hendry, M.J.; Essilfie-Dughan, J. Effects of Adsorbed Arsenate on the Rate of Transformation of 2-Line Ferrihydrite at pH 10. Environ. Sci. Technol. 2011, 45, 5557–5563. [CrossRef]
- Ford, R.G. Rates of Hydrous Ferric Oxide Crystallization and the Influence on Coprecipitated Arsenate. Environ. Sci. Technol. 2002, 36, 2459–2463. [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).



