Submitted:
15 December 2025
Posted:
16 December 2025
Read the latest preprint version here
Abstract
Keywords:
Introduction
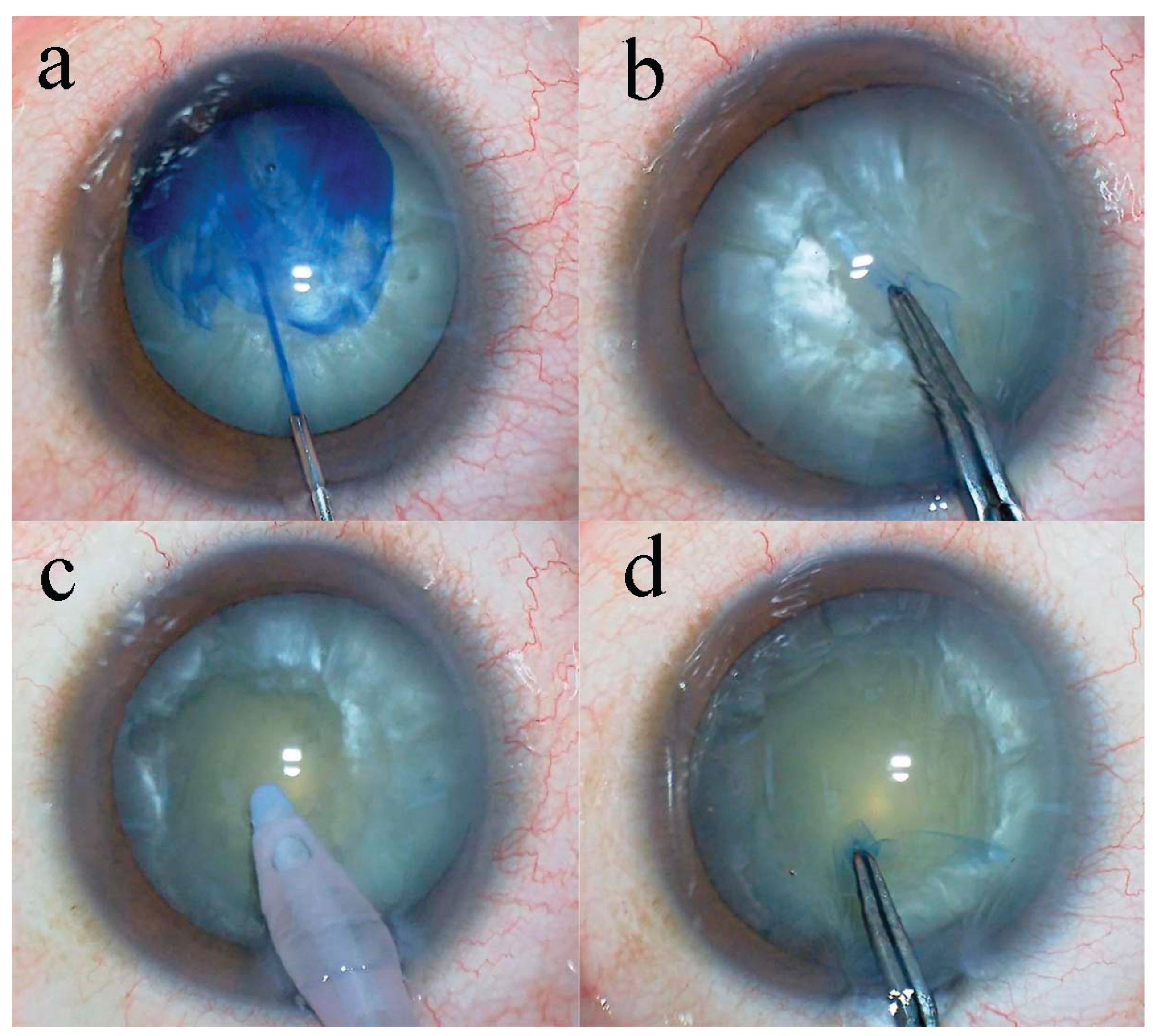
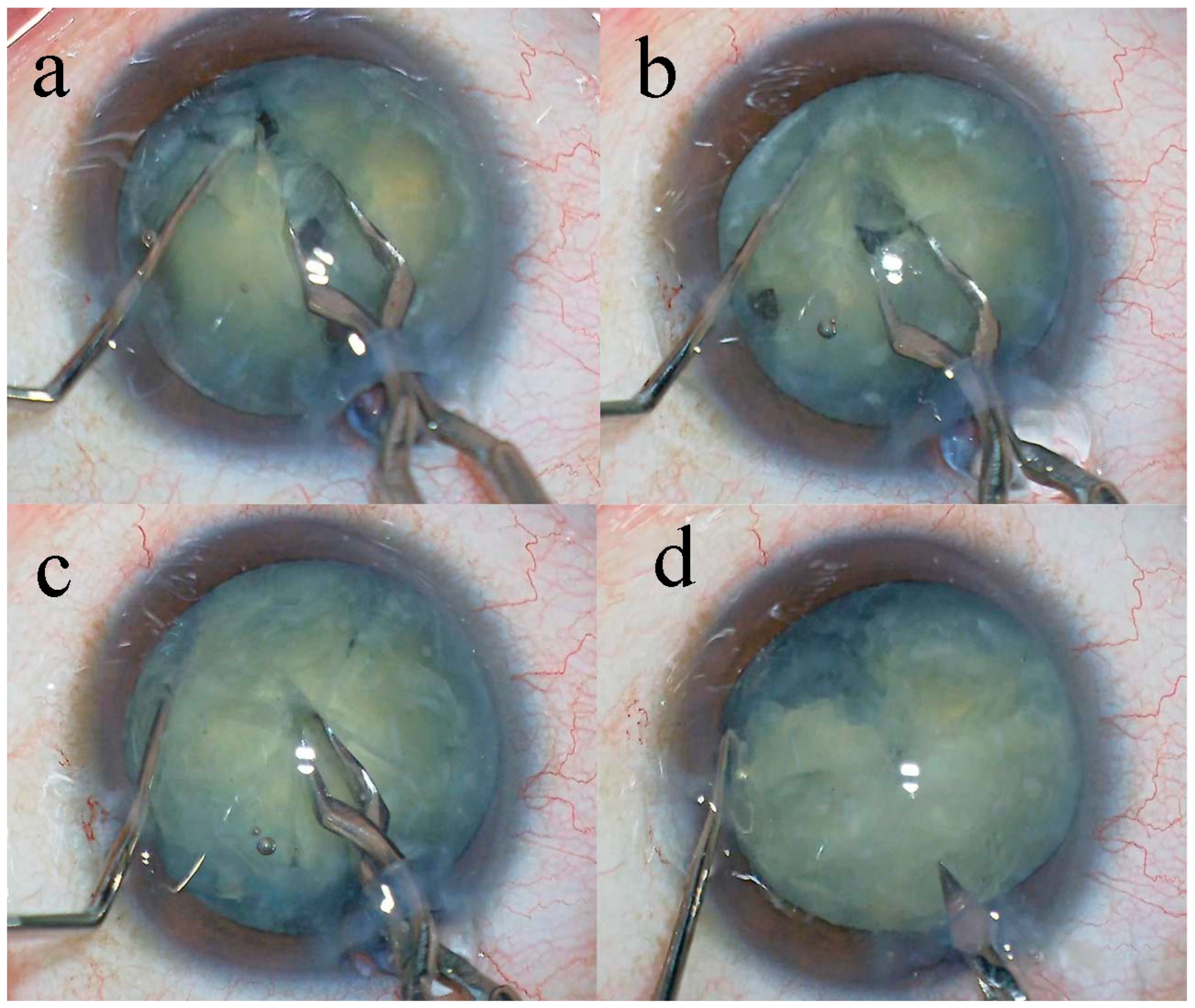
Methods
Results
Author Contributions
Funding
Data Availability Statement
Acknowledgements
Conflict of Interest
Abbreviation
References
- Akahoshi, T. Phaco Prechop. In Phaco Chop and Advanced Phaco Techniques, Second Edition ed.; Chang, D.F., Ed.; SLACK Incorporated: Thorofare, 2013; pp. 55-76.
- Akahoshi, T. Phaco Prechop: Re-evaluation of single-handed phacoemulsification using a new nucleofracture technique. Atarashii Ganka 1999, 16, 1219-1233.
- Sato, T. Efficacy and safety of the eight-chop technique in phacoemulsification for cataract patients. J Cataract Refract Surg 2023. [CrossRef]
- Sato, T. Effects of the eight-chop technique in phacoemulsification on intraocular pressure in patients with primary open-angle glaucoma and controls. EC Ophthalmology 2023, 14, 1–11.
- Jacob, S.; Agarwal, A.; Agarwal, A.; Agarwal, S.; Chowdhary, S.; Chowdhary, R.; Bagmar, A.A. Trypan blue as an adjunct for safe phacoemulsification in eyes with white cataract. J Cataract Refract Surg 2002, 28, 1819-1825. [CrossRef]
- Chakrabarti, A.; Singh, S. Phacoemulsification in eyes with white cataract. J Cataract Refract Surg 2000, 26, 1041-1047. [CrossRef]
- Hisatomi, T.; Enaida, H.; Matsumoto, H.; Kagimoto, T.; Ueno, A.; Hata, Y.; Kubota, T.; Goto, Y.; Ishibashi, T. Staining ability and biocompatibility of brilliant blue G: Preclinical study of brilliant blue G as an adjunct for capsular staining. Arch Ophthalmol 2006, 124, 514-519. [CrossRef]
- Ermisş, S.S.; Oztürk, F.; Inan, U.U. Comparing the efficacy and safety of phacoemulsification in white mature and other types of senile cataracts. Br J Ophthalmol 2003, 87, 1356-1359. [CrossRef]
- Wong, V.W.; Lai, T.Y.; Lee, G.K.; Lam, P.T.; Lam, D.S. Safety and efficacy of micro-incisional cataract surgery with bimanual phacoemulsification for white mature cataract. Ophthalmologica 2007, 221, 24-28. [CrossRef]
- Venkatesh, R.; Tan, C.S.; Sengupta, S.; Ravindran, R.D.; Krishnan, K.T.; Chang, D.F. Phacoemulsification versus manual small-incision cataract surgery for white cataract. J Cataract Refract Surg 2010, 36, 1849-1854. [CrossRef]
- Emery, J.M. Kelman phacoemulsification; patient selection. In Extracapsular Cataract Surgery, Emery, J.M., Mclyntyre, D.J., Eds.; CV Mosby: St Louis, 1983; pp. 95-100.
- Gimbel, H.V.; Willerscheidt, A.B. What to do with limited view: The intumescent cataract. J Cataract Refract Surg 1993, 19, 657-661. [CrossRef]
- Miyata, K.; Nagamoto, T.; Maruoka, S.; Tanabe, T.; Nakahara, M.; Amano, S. Efficacy and safety of the soft-shell technique in cases with a hard lens nucleus. J Cataract Refract Surg 2002, 28, 1546-1550. [CrossRef]
- Helvacioglu, F.; Yeter, C.; Tunc, Z.; Sencan, S. Outcomes of torsional microcoaxial phacoemulsification performed by 12-degree and 22-degree bent tips. J Cataract Refract Surg 2013, 39, 1219-1225. [CrossRef]
- Sato, M.; Sakata, C.; Yabe, M.; Oshika, T. Soft-shell technique using Viscoat and Healon 5: A prospective, randomized comparison between a dispersive-viscoadaptive and a dispersive-cohesive soft-shell technique. Acta Ophthalmol 2008, 86, 65-70. [CrossRef]
- Abdelmotaal, H.; Abdel-Radi, M.; Rateb, M.F.; Eldaly, Z.H.; Abdelazeem, K. Comparison of the phaco chop and drill-and-crack techniques for phacoemulsification of hard cataracts: A fellow eye study. Acta Ophthalmol 2021, 99, e378-e386. [CrossRef]
- Park, J.; Yum, H.R.; Kim, M.S.; Harrison, A.R.; Kim, E.C. Comparison of phaco-chop, divide-and-conquer, and stop-and-chop phaco techniques in microincision coaxial cataract surgery. J Cataract Refract Surg 2013, 39, 1463-1469. [CrossRef]
- Storr-Paulsen, A.; Norregaard, J.C.; Ahmed, S.; Storr-Paulsen, T.; Pedersen, T.H. Endothelial cell damage after cataract surgery: Divide-and-conquer versus phaco-chop technique. J Cataract Refract Surg 2008, 34, 996-1000. [CrossRef]
- Helvacioglu, F.; Yeter, C.; Sencan, S.; Tunc, Z.; Uyar, O.M. Comparison of two different ultrasound methods of phacoemulsification. Am J Ophthalmol 2014, 158, 221-226.e221. [CrossRef]
- Igarashi, T.; Ohsawa, I.; Kobayashi, M.; Umemoto, Y.; Arima, T.; Suzuki, H.; Igarashi, T.; Otsuka, T.; Takahashi, H. Effects of hydrogen in prevention of corneal endothelial damage during phacoemulsification: A prospective randomized clinical trial. Am J Ophthalmol 2019, 207, 10-17. [CrossRef]
- Bourne, R.R.; Minassian, D.C.; Dart, J.K.; Rosen, P.; Kaushal, S.; Wingate, N. Effect of cataract surgery on the corneal endothelium: Modern phacoemulsification compared with extracapsular cataract surgery. Ophthalmology 2004, 111, 679-685. [CrossRef]
- Upadhyay, S.; Sharma, P.; Chouhan, J.K.; Goyal, R. Comparative evaluation of modified crater (endonucleation) chop and conventional crater chop techniques during phacoemulsification of hard nuclear cataracts: A randomized study. Indian J Ophthalmol 2022, 70, 794-798. [CrossRef]
- Kosrirukvongs, P.; Slade, S.G.; Berkeley, R.G. Corneal endothelial changes after divide and conquer versus chip and flip phacoemulsification. J Cataract Refract Surg 1997, 23, 1006-1012. [CrossRef]
- Díaz-Valle, D.; Benítez del Castillo Sánchez, J.M.; Castillo, A.; Sayagués, O.; Moriche, M. Endothelial damage with cataract surgery techniques. J Cataract Refract Surg 1998, 24, 951-955. [CrossRef]
- Vasavada, A.; Singh, R.; Desai, J. Phacoemulsification of white mature cataracts. J Cataract Refract Surg 1998, 24, 270-277. [CrossRef]
- Vajpayee, R.B.; Bansal, A.; Sharma, N.; Dada, T.; Dada, V.K. Phacoemulsification of white hypermature cataract. J Cataract Refract Surg 1999, 25, 1157-1160. [CrossRef]
- Enaida, H.; Kumano, Y.; Ueno, A.; Yoshida, S.; Nakao, S.; Numa, S.; Matsui, T.; Ishibashi, T. Quantitative analysis of vitreous and plasma concentrations of brilliant blue g after use as a surgical adjuvant in chromovitrectomy. Retina 2013, 33, 2170-2174. [CrossRef]
- Ueno, A.; Hisatomi, T.; Enaida, H.; Kagimoto, T.; Mochizuki, Y.; Goto, Y.; Kubota, T.; Hata, Y.; Ishibashi, T. Biocompatibility of brilliant blue G in a rat model of subretinal injection. Retina 2007, 27, 499-504. [CrossRef]
- Baba, T.; Hagiwara, A.; Sato, E.; Arai, M.; Oshitari, T.; Yamamoto, S. Comparison of vitrectomy with brilliant blue G or indocyanine green on retinal microstructure and function of eyes with macular hole. Ophthalmology 2012, 119, 2609-2615. [CrossRef]
- Takayama, K.; Sato, T.; Karasawa, Y.; Sato, S.; Ito, M.; Takeuchi, M. Phototoxicity of indocyanine green and Brilliant Blue G under continuous fluorescent illumination on cultured human retinal pigment epithelial cells. Invest Ophthalmol Vis Sci 2012, 53, 7389-7394. [CrossRef]
- Irak-Dersu, I.; Nilson, C.; Zabriskie, N.; Durcan, J.; Spencer, H.J.; Crandall, A. Intraocular pressure change after temporal clear corneal phacoemulsification in normal eyes. Acta Ophthalmol 2010, 88, 131-134. [CrossRef]
- Steuhl, K.P.; Marahrens, P.; Frohn, C.; Frohn, A. Intraocular pressure and anterior chamber depth before and after extracapsular cataract extraction with posterior chamber lens implantation. Ophthalmic Surg 1992, 23, 233-237.
- Tennen, D.G.; Masket, S. Short-and long-term effect of clear corneal incisions on intraocular pressure. J Cataract Refract Surg 1996, 22, 568-570. [CrossRef]
- Chen, P.P.; Lin, S.C.; Junk, A.K.; Radhakrishnan, S.; Singh, K.; Chen, T.C. The effect of phacoemulsification on intraocular pressure in glaucoma patients: A report by the American Academy of Ophthalmology. Ophthalmology 2015, 122, 1294-1307. [CrossRef]
- Brazitikos, P.D.; Tsinopoulos, I.T.; Papadopoulos, N.T.; Fotiadis, K.; Stangos, N.T. Ultrasonographic classification and phacoemulsification of white senile cataracts. Ophthalmology 1999, 106, 2178-2183. [CrossRef]
| Characteristics/Parameters | Grade II | Grade III | Grade IV | Total | PValue |
| Number of eyes | 5 (6%) | 24 (30%) | 52 (64%) | 81 | |
| Mean age (years) | 50.2 ± 5.2 | 62.9 ± 10.8 | 72.2 ± 9.1 | 68.1 ± 11.3 | 0.023†, 0.001†, 0.004† |
| Sex Male | 4 (80%) | 16 (67%) | 26 (50%) | 46 | |
| Female | 1 (20%) | 8 (33%) | 26 (50%) | 35 | |
| Mean operative time (minutes) | 8.20 ± 1.77 | 9.98 ± 4.37 | 12.35 ± 4.35 | 11.40 ± 4.42 | 0.73⁎, 0.015†, 0.084⁎, |
| Mean phaco time (seconds) | 17.8 ± 21.9 | 19.3 ± 12.8 | 31.9 ± 14.0 | 31.4 ± 15.3 | 0.80⁎, <0.01†, 0.067⁎ |
| Aspiration time (minutes) | 2.37 ± 0.85 | 2.38 ± 1.77 | 2.55 ± 0.75 | 2.50 ± 0.72 | 0.63⁎ |
| CDE | 5.02 ± 6.35 | 8.04 ± 5.97 | 14.69 ± 6.29 | 12.12 ± 7.07 | 0.38⁎, <0.01†, 0.016† |
| Volume of fluid used (mL) | 53.2 ± 17.5 | 58.6 ± 13.1 | 64.3 ± 16.5 | 61.9 ± 15.8 | 0.27⁎ |
| Preoperative | 7 Weeks Postoperatively | 19 Weeks Postoperatively | PValue | |
| BCVA (logMAR) n=36 | 2.20 ± 0.35 | 0.02 ± 0.14 | 0.01 ± 0.12 | <0.01†, 1.0⁎, <0.01† |
| CECD (cells/mm2) n=14 | 2577.5 ± 221.0 | 2435.1 ± 249.2 | 2478.8 ± 214.0 | 0.25⁎⁎ |
| CECD loss in % | NA | 5.5 ± 6.0 | 3.7 ± 5.9 | NA |
| IOP (mmHg) n=40 | 13.7 ± 2.6 | 12.7 ± 2.4 | 12.4 ± 2.4 | 0.070⁎⁎⁎ |
| Study | Year | Number of Eyes | Surgical Technique | Operation Time (Minutes) |
Phaco Time (Seconds) |
CECD Loss in % | IOP Change |
| Vasavada23 | 1998 | 60 | Stop and chop | NR | NR | 18.6 | NR |
| Brazitikos33 | 1999 | 100 | D&C, Stop and chop | NR | 144 | NR | NR |
| Vajpayee24 | 1999 | 25 | Stop and chop | NR | 229.8 | 15.7 | NR |
| Chakrabarti4 | 2000 | 212 | D&C, Phaco-chop | NR | 121.8 | NR | NR |
| Jacob3 | 2002 | 52 | Karate-chop | NR | 132 | 8.5 | NR |
| Ermisş6 | 2003 | 82 | D&C | NR | 40.4 | NR | -4.2 % |
| Wong7 | 2007 | 25 | Stop and chop | 22 | 96 | 9.6 | NR |
| Venkatesh8 | 2010 | 113 | Phaco-chop | 12.2 | NR | NR | NR |
| Present | 2022 | 81 | Eight-chop | 11.4 | 31.4 | 3.7 | - 9.5 % |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





