1. Introduction
Aggregation-induced emission (AIE) active polymers have gained significant attention due to their unique photophysical properties and applications in chemosensing, bioimaging, and optoelectronics.1-3 Unlike traditional fluorophores that suffer from aggregation-caused quenching (ACQ), AIE-active materials exhibit enhanced emission in the aggregated state, making them ideal for solid-state applications.4 Herein, we report the synthesis of a novel amide-containing polymer derived from pyrazine-2,5-dicarboxylic acid and naphthalene-1,5-diamine, which demonstrates prominent AIE characteristics. The rigid aromatic backbone and amide linkages facilitate restricted intramolecular rotation (RIR), a key mechanism for AIE behavior.
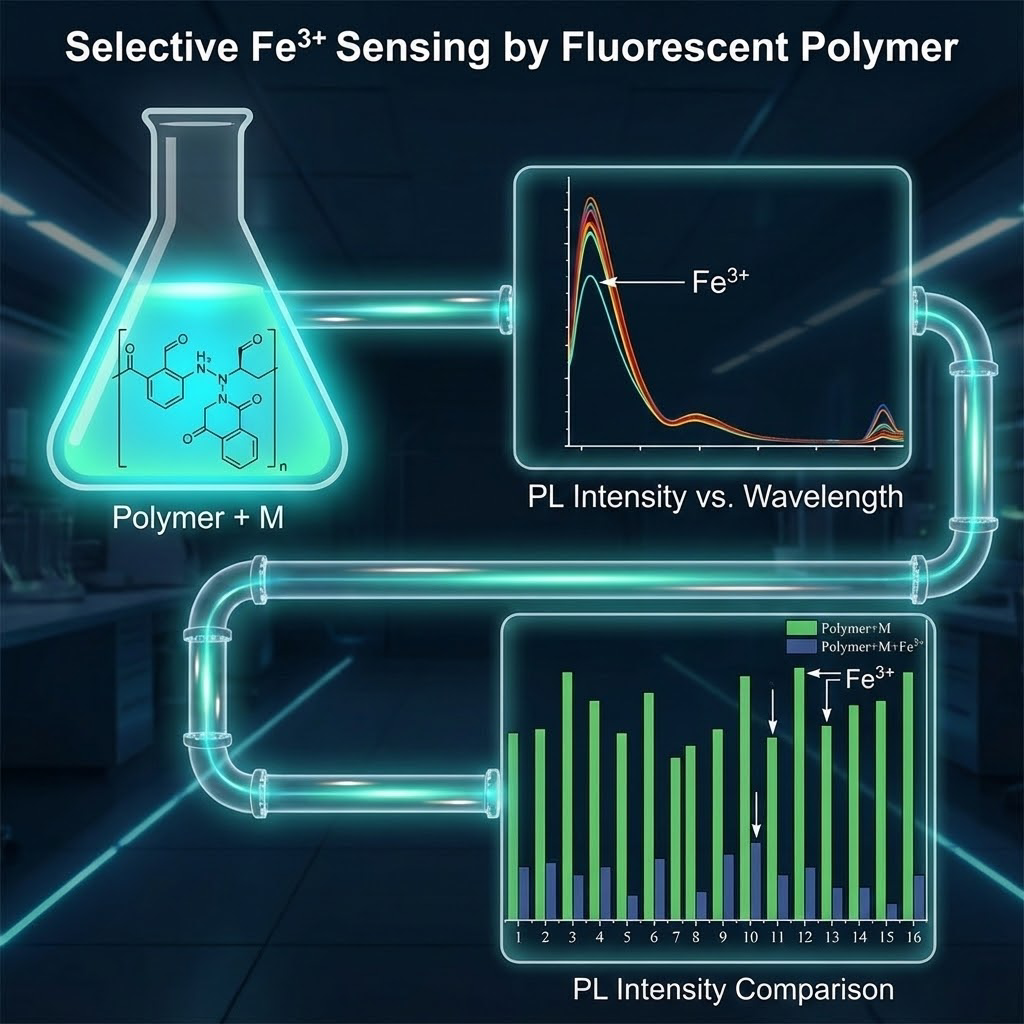
Iron (Fe³⁺) plays a crucial role in biological and environmental systems, but its excessive levels can lead to severe health and ecological risks.5-7 Thus, developing sensitive and selective probes for Fe³⁺ detection is essential. The synthesized polymer exhibits strong fluorescence emission in aggregated states, which is selectively quenched upon interaction with Fe³⁺, enabling its use as a turn-off sensor. This selective response is attributed to the coordination between Fe³⁺ and the polymer’s pyrazine and amide moieties. The proposed sensor offers a simple, cost-effective, and highly sensitive platform for Fe³⁺ detection, with potential applications in environmental monitoring and biomedical diagnostics.
1. Experimental Section
2.1. Materials and Instrumentation
All reactions were conducted under an inert atmosphere using anhydrous solvents in oven-dried glassware. Solvents were transferred via syringe or cannula, with cooling provided by ice/water or dry ice/acetone baths. Products were purified by rotary evaporation and column chromatography. Characterization included ¹H NMR (400 MHz, TMS as internal standard), GPC (TOSOH EcoSEC), and photoluminescence spectroscopy (Shanghai Lengguang F98). Particle size distribution was determined using a dynamic light scattering analyzer (Shandong Winner802).
2.2. Synthetic Procedure
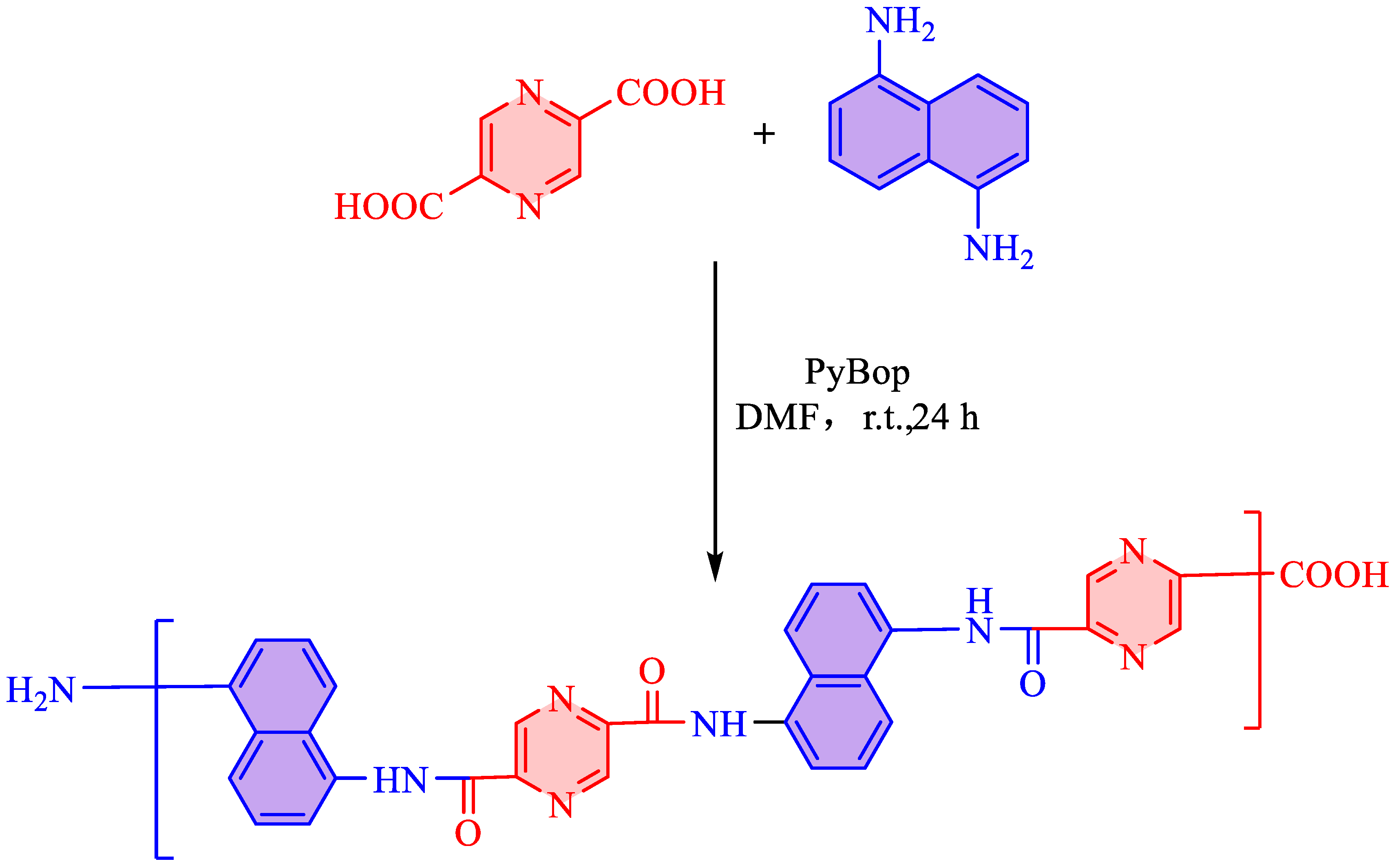
A mixture of pyrazine-2,5-dicarboxylic acid (1.0 equiv, 1 mmol, 168 mg) and naphthalene-1,5-diamine (1.0 equiv, 1 mmol, 158 mg) was dissolved in 20 mL anhydrous N,N-dimethylformamide (DMF) under a nitrogen atmosphere. To this solution, PyBOP (benzotriazol-1-yl-oxytripyrrolidinophosphonium hexafluorophosphate, 3 equiv, 3 mmol, 1.56 g) was added in one portion. The reaction mixture was stirred at room temperature for 24 hours. Upon completion, After cooling to room temperature and removal of the solvent under decreased pressure, residue was obtained. This residue was washed with methanol and HCl at a concentration of 6.0 M to produce product Polymer: Dark yellow solid. M
n=4278, M
w=4368, PDI=1.021 (
Figure 1).
2. Results and Discussions
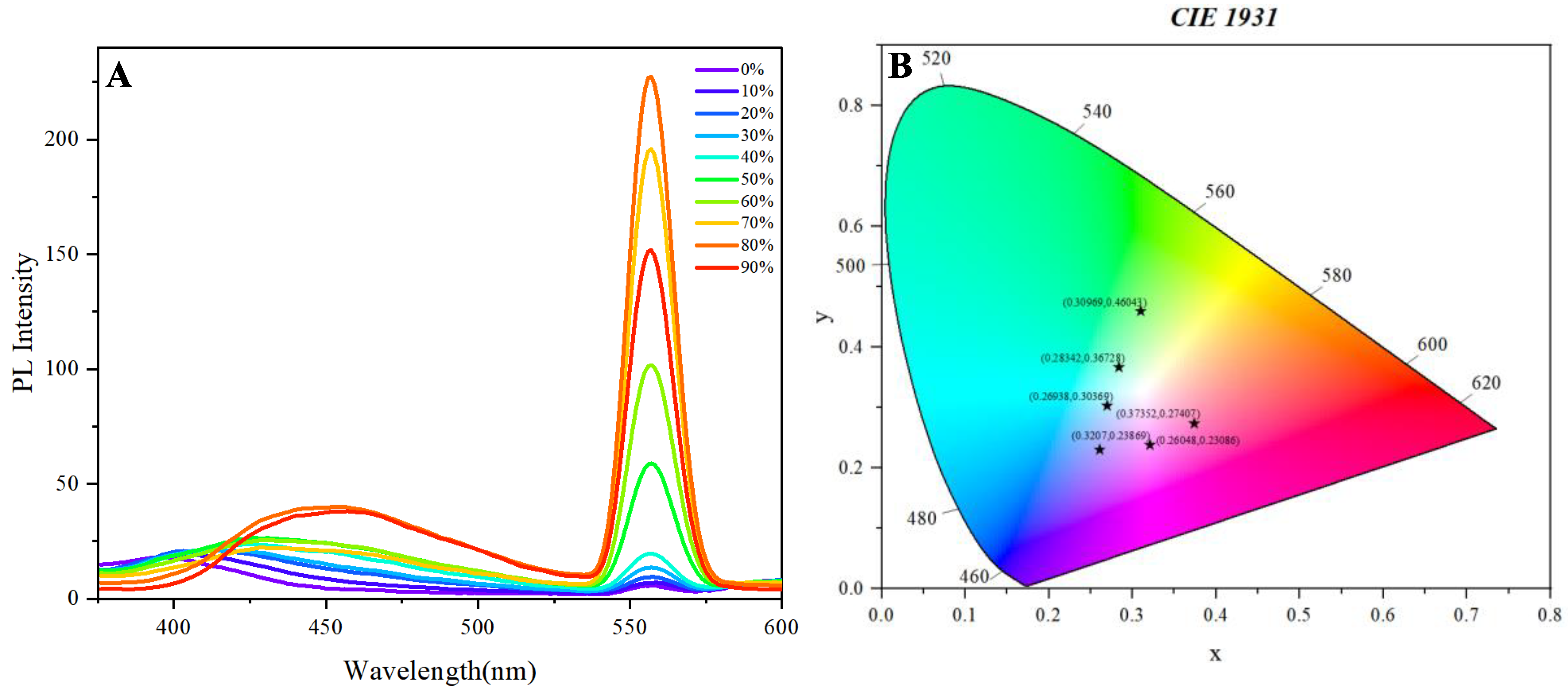
Figure 2A demonstrates the aggregation-induced emission (AIE) behavior of the polymer in THF/water mixtures with increasing water fractions (
fw). In pure THF (0%
fw), the polymer exhibits weak fluorescence due to intramolecular rotation dissipating excited-state energy. As f_w increases (10–70%), the emission intensity rises significantly, peaking at 60–70%
fw, which confirms AIE activity (
Figure 2A). This enhancement arises from restricted intramolecular rotation (RIR) in the aggregated state, a hallmark of AIEgens. Notably, the emission maximum undergoes a slight red-shift (e.g., from 450 nm to 470 nm), suggesting J-aggregate formation or planarization of the polymer backbone upon aggregation.
Figure 2B quantifies the accompanying chromaticity shift using CIE 1931 coordinates. The polymer’s emission color transitions from blue (0%
fw: 0.373, 0.274) to cyan (60%
fw: 0.283, 0.367) and finally to greenish-yellow (70%
fw: 0.309, 0.460), as water-induced aggregation alters the electronic environment (
Figure 2B). This shift correlates with changes in dipole-dipole interactions and conjugation length in the aggregated state. The nonlinear trajectory of coordinates (e.g., 50%
fw: 0.269, 0.303) further implies a multi-stage aggregation process, possibly involving micelle formation or π-stacking reorganization.
Collectively, these results validate the polymer’s dual response to aggregation: (1) fluorescence enhancement (AIE) and (2) tunable emission color, underscoring its potential for multi-parameter sensing or optoelectronic applications.
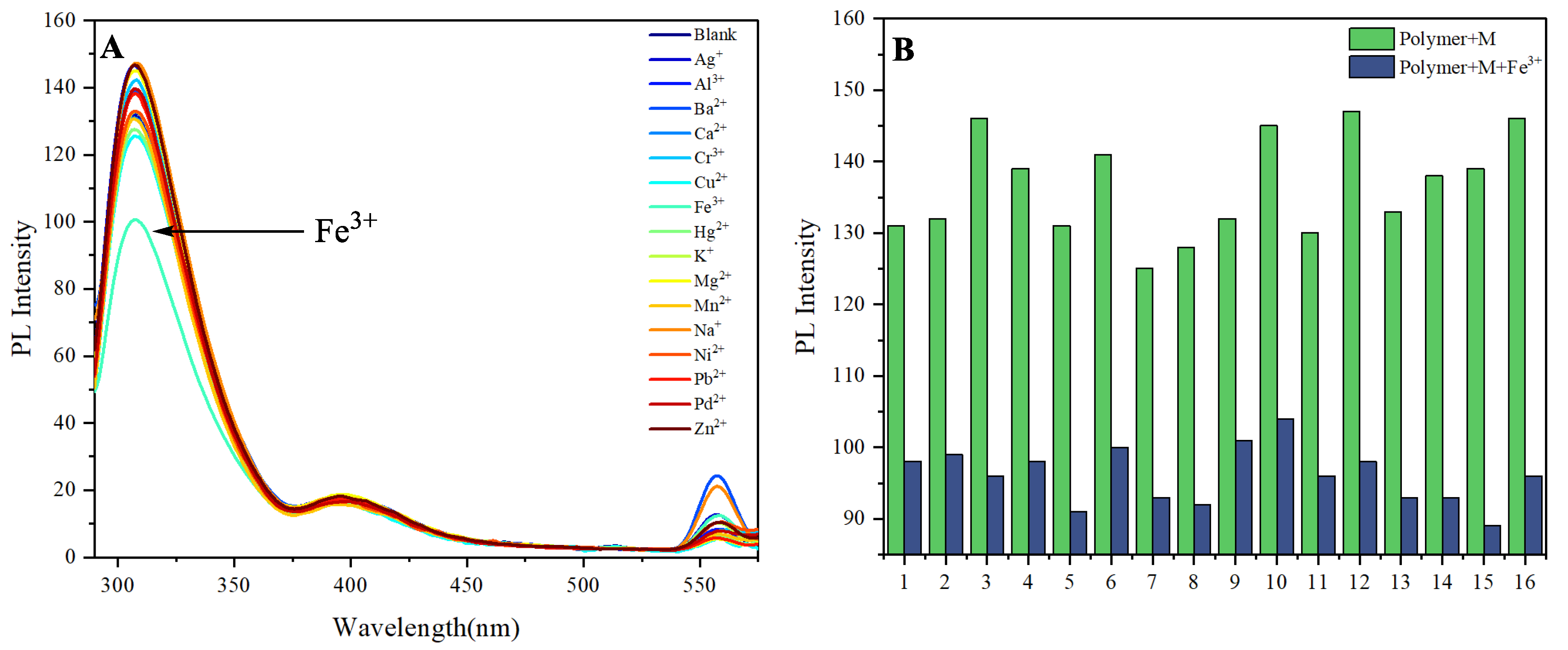
Figure 3 illustrates the photoluminescence (PL) response of the Pyrazine-Naphthalene-based AIE-active polymer to the introduction of various metal ions, providing critical insights into its chemosensory capabilities. A striking observation from the spectral data is the pronounced quenching of the polymer's characteristic emission upon the addition of Fe
3+ ions, a phenomenon that significantly dwarfs the impact of other metal ions tested. The pristine polymer solution exhibits strong fluorescence at its characteristic emission maximum, consistent with its aggregation-induced emission (AIE) properties, which typically arise from the restriction of intramolecular motion in the aggregated state. However, the introduction of Fe
3+ ions leads to a dramatic decrease in this emission intensity (
Figure 3A), suggesting a highly effective quenching mechanism. This substantial quenching by Fe3+ is indicative of a strong interaction between the metal ion and the polymer's functional groups.
Several mechanisms could underpin this selective and efficient quenching. The pyrazine and naphthalene units within the polymer backbone are rich in nitrogen and oxygen heteroatoms and electron-rich π-systems, respectively, providing multiple potential binding sites (e.g., nitrogen lone pairs, π-electron clouds) for metal coordination. Fe3+ being a hard Lewis acid, possesses a high charge density and strong oxidizing capabilities, making it particularly prone to interact with electron-rich moieties. The quenching could arise from a combination of processes such as electron transfer from the excited state of the polymer to Fe3+, chelation-enhanced quenching (chelation leading to a more efficient non-radiative decay pathway), or the formation of an aggregate between the polymer and Fe3+ that suppresses fluorescence.
In stark contrast, other metal ions show relatively minor or negligible effects on the polymer's fluorescence. This high specificity towards Fe3+ highlights the unique binding affinity and subsequent quenching mechanism triggered by its presence. This preferential sensing capability positions the Pyrazine-Naphthalene-based AIE polymer as a highly promising candidate for the selective and sensitive detection of Fe3+ ions in various applications, from environmental monitoring to biological sensing. Further investigation into the exact coordination geometry and electron transfer pathways would elucidate the precise molecular mechanism behind this remarkable selectivity (
Figure 3B).
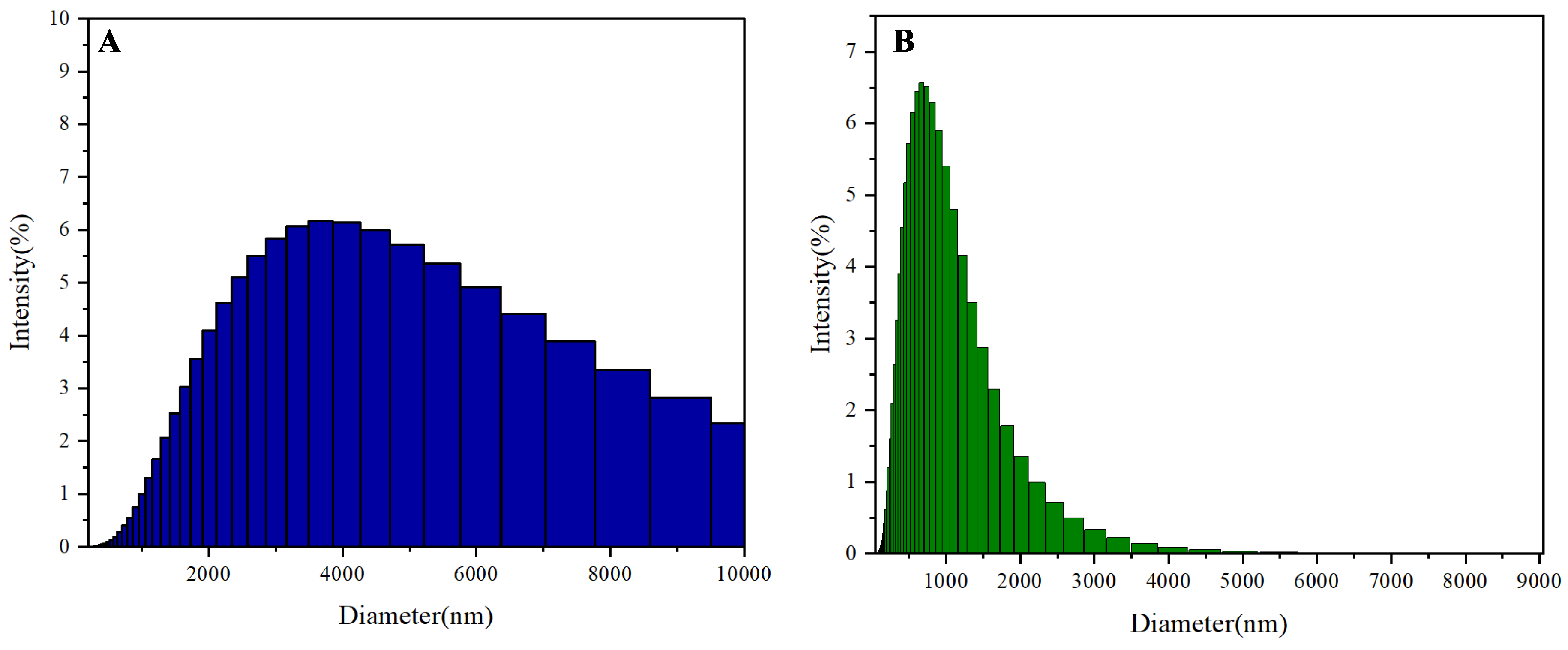
Dynamic light scattering (DLS) analysis (
Figure 4) reveals a significant reduction in the polymer's particle size upon Fe³⁺ addition, from 3674 nm to 740 nm. This sharp decrease aligns with the polymer's aggregation-induced emission (AIE) behavior and its role as a Fe³⁺ fluorescence probe. In the absence of Fe³⁺, the polymer forms large aggregates in THF/water mixtures, restricting intramolecular rotation (RIR) and enhancing fluorescence (
Figure 4A). The introduction of Fe³⁺ disrupts these aggregates due to strong coordination between Fe³⁺ and the polymer's pyrazine/amide moieties, fragmenting the particles into smaller complexes (
Figure 4B).
The size reduction correlates with the observed fluorescence quenching (
Figure 3), as disaggregation diminishes the AIE effect. Additionally, Fe³⁺-polymer chelation may promote non-radiative decay pathways (e.g., electron transfer), further suppressing emission. The specificity of this response—unchanged particle sizes with other metal ions—underscores the polymer's selective binding affinity for Fe³⁺. These findings support a dual mechanism: (1) Fe³⁺-induced aggregate disruption and (2) chelation-enhanced quenching, synergistically enabling sensitive Fe³⁺ detection. This structural insight reinforces the polymer's potential as a robust Fe³⁺ probe for environmental or biological applications.
Conclusion
In conclusion, a novel amide-containing AIE-active polymer, synthesized from pyrazine-2,5-dicarboxylic acid and naphthalene-1,5-diamine, exhibited pronounced fluorescence in aggregated states. This polymer demonstrated remarkable selectivity and sensitivity as a fluorescence probe for Fe³⁺ ions, showing significant quenching. Mechanistic investigations elucidated a chelation-enhanced quenching effect as the primary sensing mechanism. This straightforward synthesis strategy offers a facile route to develop efficient polymeric AIE probes, holding substantial promise for applications in new multilayer chirality8-11 and biological sensing where selective Fe³⁺ detection is crucial.
References
- R. Hu, A. Qin, B.Z. Tang, AIE polymers: Synthesis and applications, Prog. Polym. Sci. 100 (2020) 101176. [CrossRef]
- P. Chowdhury, A. Banerjee, B. Saha, K. Bauri, P. De, Stimuli-responsive aggregation-induced emission (AIE)-active polymers for biomedical applications, ACS Biomater. Sci. Eng. 8 (2022) 4207–4229. [CrossRef] [PubMed]
- H. Chen, M.-H. Li, Recent progress in fluorescent vesicles with aggregation-induced emission, Chin. J. Polym. Sci. 37 (2019) 352–371. [CrossRef]
- S. Liu, J. Liu, X. Li, X. Du, C. Yin, Y. Luo, C. Li, Fluorescent particles based on aggregation-induced emission for optical diagnostics of the central nervous system, Research (Wash. D.C.) 8 (2025) 0564. [CrossRef] [PubMed]
- N. Xu, Q. Zhang, B. Hou, Q. Cheng, G. Zhang, A novel magnesium metal–organic framework as a multiresponsive luminescent sensor for Fe(III) ions, pesticides, and antibiotics with high selectivity and sensitivity, Inorg. Chem. 57 (2018) 13330–13340. [CrossRef] [PubMed]
- M.S. Razzaque, S.J. Wimalawansa, Minerals and human health: From deficiency to toxicity, Nutrients 17 (2025) 454. [CrossRef] [PubMed]
- J. Cho, S. Kim, Fluorescent calix[4]triazole for selective fluoride anion sensing, RSC Adv. 15 (2025) 4342–4347. [CrossRef] [PubMed]
- S. Zhang, Z. Zhang, Design and synthesis of multilayer 3D naphthalene-based polymers: A study of stokes shift and aggregation behavior, Mater. Lett. 399 (2025) 139051. [CrossRef]
- S. Zhang, Q. Yuan, G. Li, New multiple-layered 3D polymers showing aggregation-induced emission and polarization, RSC Adv. 14 (2024) 13342–13350. [CrossRef] [PubMed]
- S. Jin, Y. Wang, Y. Tang, J.-Y. Wang, T. Xu, J. Pan, S. Zhang, Q. Yuan, A.U. Rahman, J.D. McDonald, G.-Q. Wang, S. Li, G. Li, Orientational chirality, its asymmetric control, and computational study, Research (Wash. D.C.) 2022 (2022) 0012. [CrossRef] [PubMed]
- S. Zhang, Z. Zhang, Y. Zhang, G. Li, Z. Zhang, Fluorescence probes from multilayer 3D polymers: AIE phenomenon and its application in detecting barium ions, Macromol. Chem. Phys. (2025). https://doi.org/10.1002/macp.202500201. [CrossRef]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).