Submitted:
11 December 2025
Posted:
12 December 2025
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Methods
2.2.1. Surface Tension
2.2.2. Critical Micelle Concentration
2.2.3. Protein Denaturation
2.3. Foam Generation and Stability
3. Results and Discussion
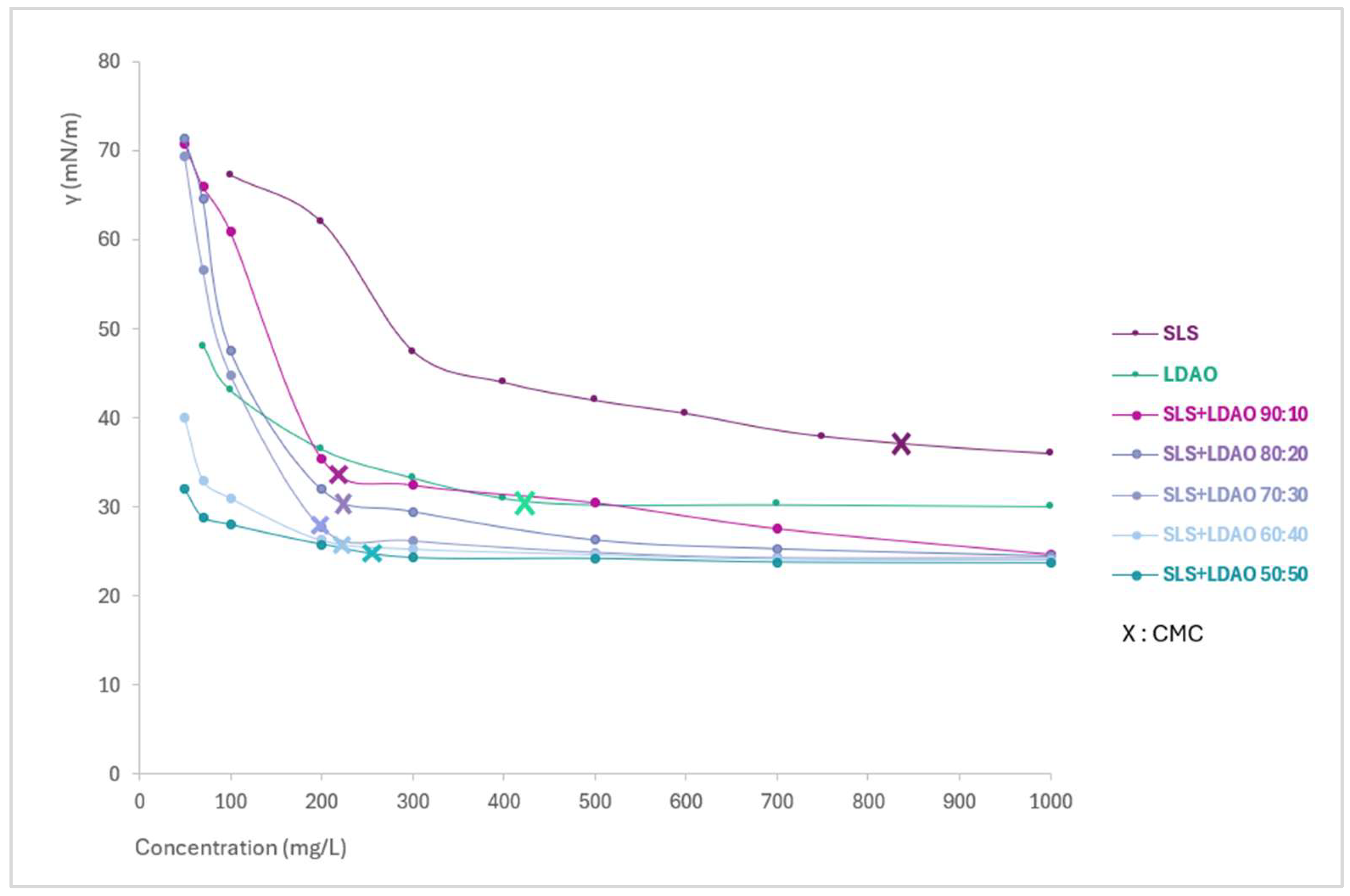
3.1. Determination of Surface Tension and CMC
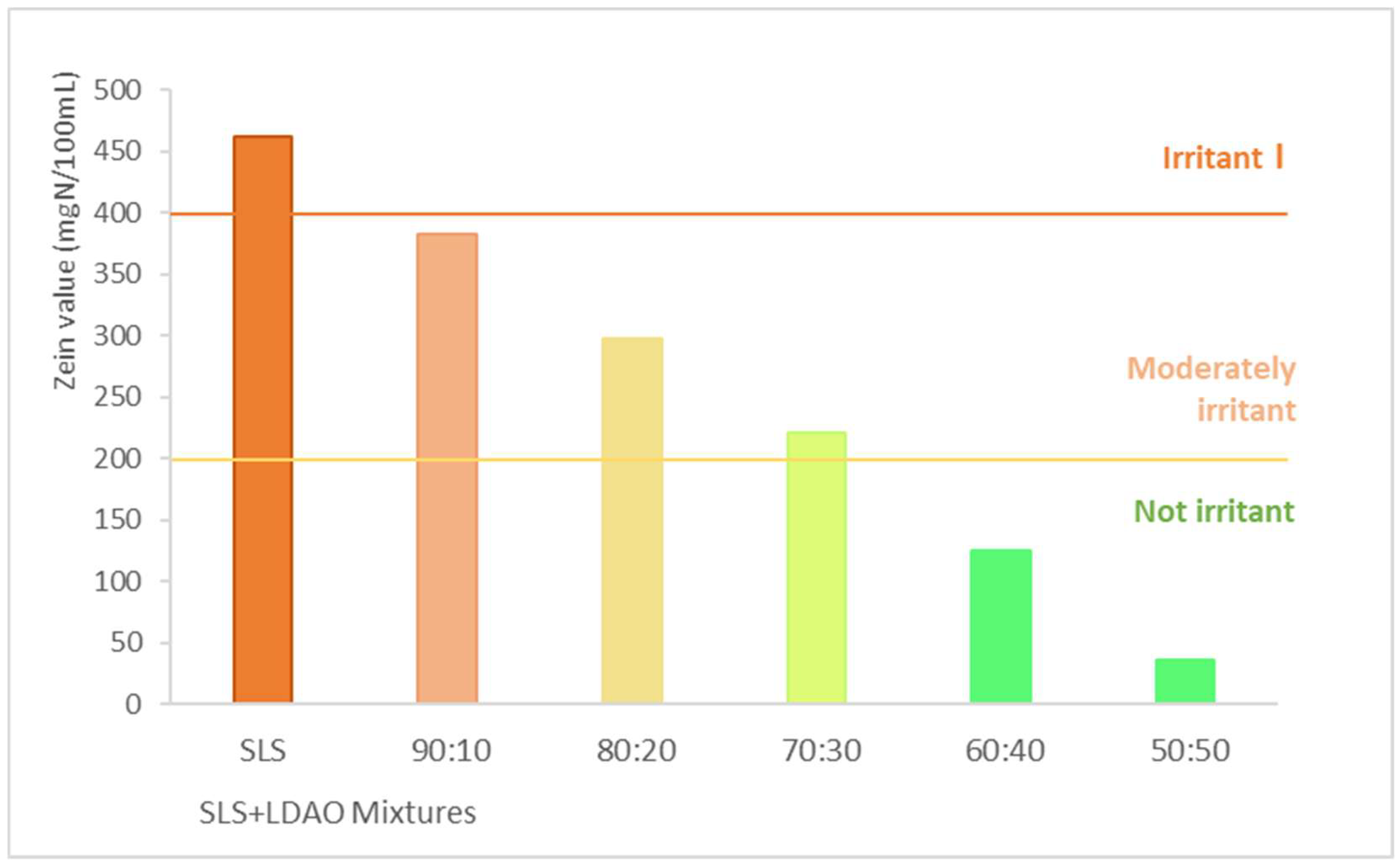
3.2. Protein Denaturation
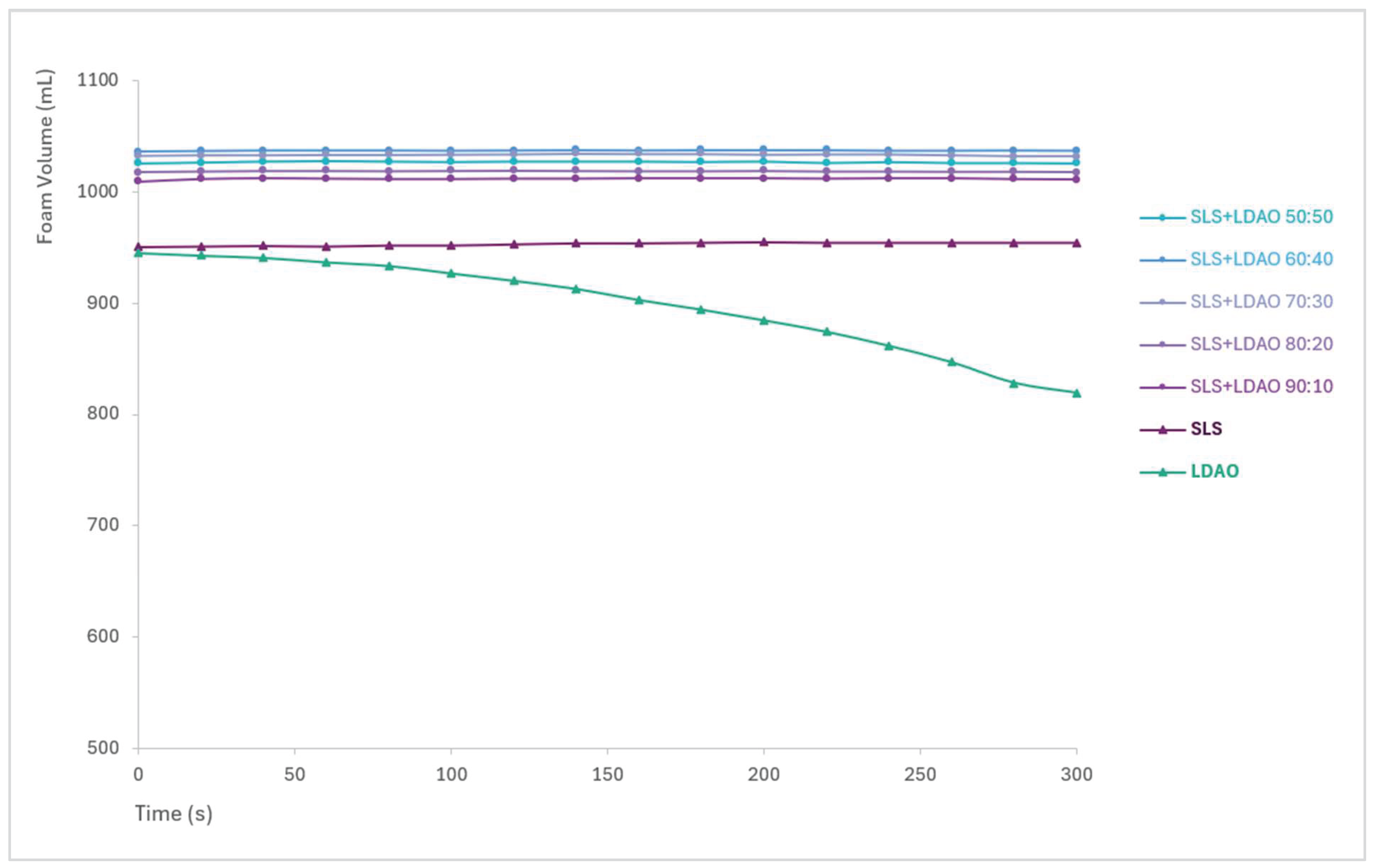
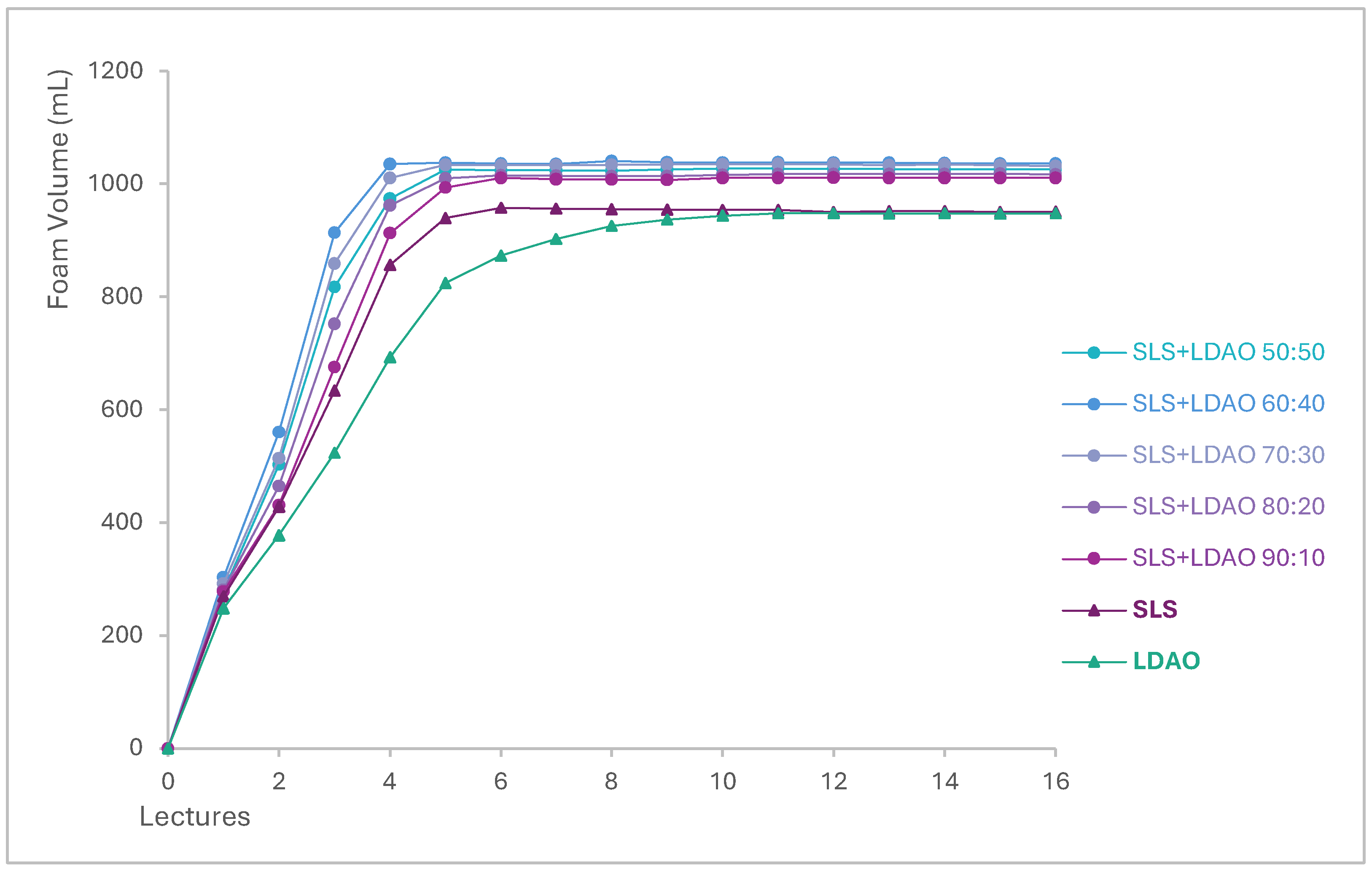
3.3. Foam Generation and Stability
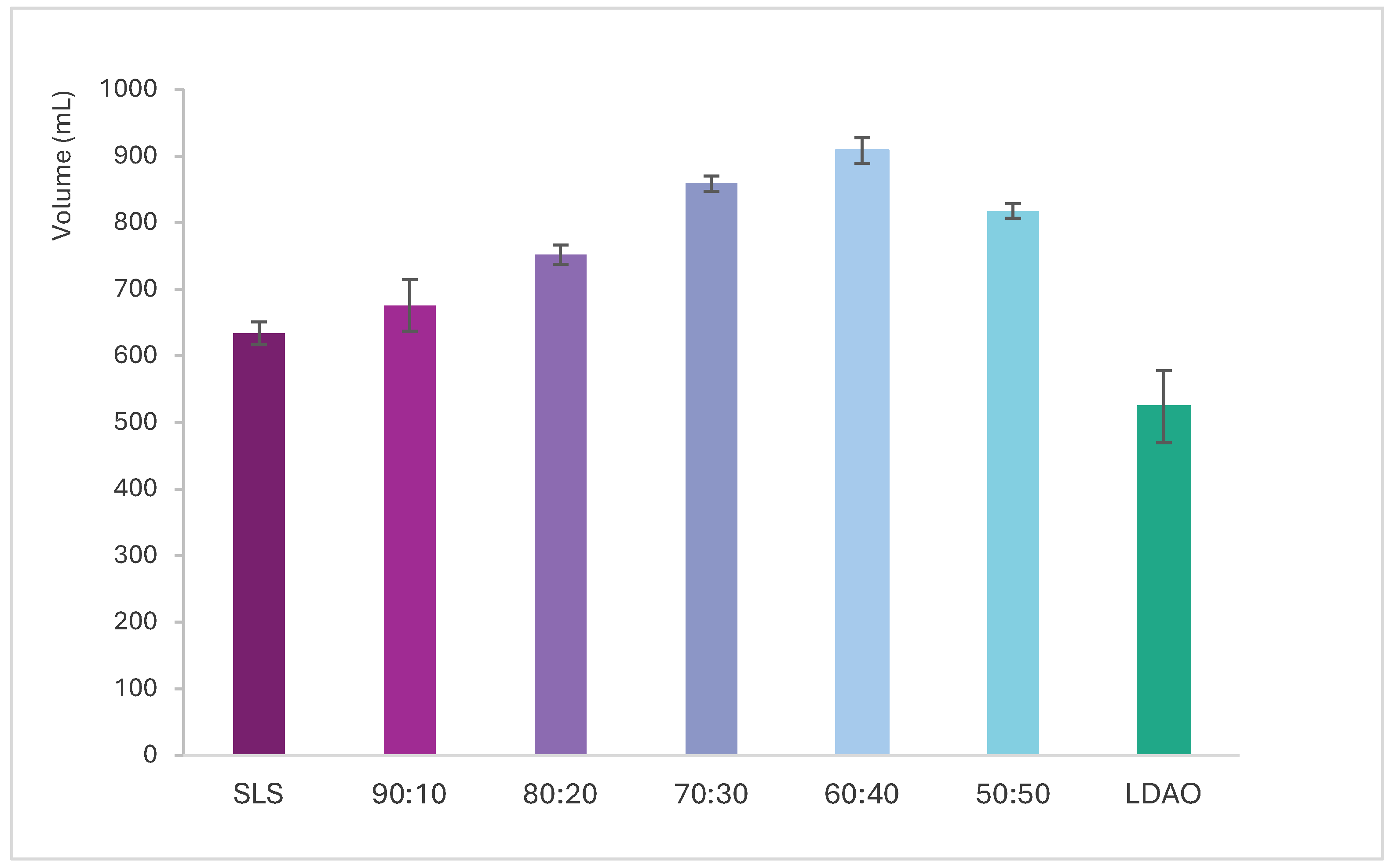
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| SLS | Sodium Lauryl Sulfate |
| LDAO | Lauryl Dimethyl Amine Oxide |
| CMC | Critical Micelle Concentration |
| HLB | Hydrophilic-Lipophilic Balance |
| INCI | International nomenclature of cosmetics ingredients |
| Zn | Zein |
References
- Rosen, M.J.; Kunjappu, J.T. Surfactants and interfacial phenomena; 2012.
- Myers, D. Surfactant Science and Technology; John Wiley & Sons, 2005.
- Lai, K.-Y. Liquid detergents; CRC Press, 2005.
- Tadros, T.F. Applied Surfactants: Principles and applications; 2005.
- Klein, K. Evaluating Shampoo Foam. Cosmetics & Toiletries 2004, 119 (10), 67–72.
- Blagojević, S.M.; Pejić, N.D.; Blagojević, S.N. Synergism and Physicochemical Properties of Anionic/Amphoteric Surfactant Mixtures with Nonionic Surfactant of Amine Oxide Type. Russian Journal of Physical Chemistry A 2017, 91 (13), 2690–2695. [CrossRef]
- Salager, J.L. Surfactants – Types and Uses; Lab. FIRP, Booklet 300A; Laboratorio FIRP: Mérida, 2019.
- Ranji, H.; Babajanzadeh, B.; Sherizadeh, S. Detergents and surfactants: a brief review. Open Access Journal of Science 2019, 3 (2). [CrossRef]
- Salomon, G.; Giordano-Labadie, F. Surfactant irritations and allergies. European Journal of Dermatology 2022, 32 (6), 677–681. [CrossRef]
- Horita, K.; Horita, D.; Tomita, H.; Yasoshima, M.; Yagami, A.; Matsunaga, K. Effects of different base agents on prediction of skin irritation by sodium lauryl sulfate using patch testing and repeated application test. Toxicology 2017, 382, 10–15. [CrossRef]
- Singh, S.K.; Bajpai, M.; Tyagi, V.K. Amine Oxides: A review. Journal of Oleo Science 2006, 55 (3), 99–119. [CrossRef]
- Zhang, H.; Miller, C.A.; Garrett, P.R.; Raney, K.H. Lauryl alcohol and amine oxide as foam stabilizers in the presence of hardness and oily soil. Journal of Surfactants and Detergents 2005, 8 (1), 99–107. [CrossRef]
- Ríos, F.; Lechuga, M.; Fernández-Serrano, M.; Fernández-Arteaga, A. Aerobic biodegradation of amphoteric amine-oxide-based surfactants: Effect of molecular structure, initial surfactant concentration and pH. Chemosphere 2016, 171, 324–331. [CrossRef]
- Joshi, T.; Mata, J.; Bahadur, P. Micellization and interaction of anionic and nonionic mixed surfactant systems in water. Colloids and Surfaces a Physicochemical and Engineering Aspects 2005, 260 (1–3), 209–215. [CrossRef]
- Kotsi, K.; Dong, T.; Kobayashi, T.; Robbie, I. M.; Striolo, A.; Angeli, P. Synergistic effects between a non-ionic and an anionic surfactant on the micellization process and the adsorption at liquid/air surfaces. Soft Matter 2023, 20 (3), 523–534. [CrossRef]
- Jadidi, N.; Adib, B.; Malihi, F. B. Synergism and performance optimization in liquid detergents containing binary mixtures of Anionic–Nonionic, and Anionic–Cationic surfactants. Journal of Surfactants and Detergents 2012, 16 (1), 115–121. [CrossRef]
- Vilaplana, J.; Lecha, M.; Trullas, C.; Coll, J.; Comelles, F.; Romaguera, C.; Pelejero, C. A physicochemical approach to minimize the irritant capacity of anionic surfactants. Exogenous Dermatology 2002, 1 (1), 22–26. [CrossRef]
- Grady, B. P. Surfactant mixtures: A short review. Journal of Surfactants and Detergents 2022, 26 (3), 237–250. [CrossRef]
- Abdel-Rahem, R. Synergism in mixed Anionic–Amphoteric surfactant solutions: Influence of anionic surfactant chain length. Tenside Surfactants Detergents 2009, 46 (5), 298–305. [CrossRef]
- Seweryn, A. Interactions between surfactants and the skin – Theory and practice. Advances in Colloid and Interface Science 2018, 256, 242–255. [CrossRef]
- Martín, J. F. G.; Herrera-Márquez, O.; Vicaria, J. M.; Jurado, E. Synergistic effect on wettability of mixtures of amine oxides, alkylpolyglucosides, and ethoxylated fatty alcohols. Journal of Surfactants and Detergents 2014, 17 (5), 1035–1042. [CrossRef]
- Alves, L.; Magalhães, S.; Esteves, C.; Sebastião, M.; Antunes, F. Synergisms between Surfactants, Polymers, and Alcohols to Improve the Foamability of Mixed Systems. J — Multidisciplinary Scientific Journal 2024, 7 (2), 169–182. [CrossRef]
- De Jongh, C.M.; Jakasa, I.; Verberk, M.M.; Kezic, S. Variation in barrier impairment and inflammation of human skin as determined by sodium lauryl sulphate penetration rate. British Journal of Dermatology 2005, 154 (4), 651–657. [CrossRef]
- Paye, M.; Block, C.; Hamaide, N.; Hüttmann, G. -e.; Kirkwood, S.; Lally, C.; Lloyd, P. H.; Makela, P.; Razenberg, H.; Young, R. Antagonisms between Surfactants: The Case of Laundry Detergents. Tenside Surfactants Detergents 2006, 43 (6), 290–294. [CrossRef]
- Löffler, H.; Happle, R. Profile of irritant patch testing with detergents: sodium lauryl sulfate, sodium laureth sulfate and alkyl polyglucoside. Contact Dermatitis 2003, 48 (1), 26–32. [CrossRef]
- Berry, J.D.; Neeson, M.J.; Dagastine, R.R.; Chan, D.Y.C.; Tabor, R.F. Measurement of surface and interfacial tension using pendant drop tensiometry. Journal of Colloid and Interface Science 2015, 454, 226–237. [CrossRef]
- DB-ALM Protocol n° 26. The Zein Test: Skin Irritation and Corrosivity; European Commission, Joint Research Centre. 26_P_The Zein Test.pdf (accessed Nov 21, 2025).
- Lechuga, M.; Avila-Sierra, A.; Lobato-Guarnido, I.; GarcíaLópez, A. I.; Ríos, F.; Fernández-Serrano, M. Mitigating the Skin Irritation Potential of Mixtures of Anionic and Non-Ionic Surfactants by Incorporating Low-Toxicity Silica Nanoparticles. J Journal of Molecular Liquids 2023, 383, 122021. [CrossRef]
- Muherei, M.A.; Junin, R. Equilibrium adsorption isotherms of anionic, nonionic surfactants and their mixtures to shale and sandstone. Modern Applied Science 2009, 3 (2). [CrossRef]
- Paye, M. Mechanism of Skin Irritation by Surfactants and Anti-Irritants for Surfactant-Based Products. In Handbook of Cosmetic Science and Technology, 4th ed.; Barel, A.; Paye, M.; Maibach, H., Eds.; CRC Press, 2014; pp 353–366. [CrossRef]
- Lechuga, M.; García, P.A.; García-López, A. I.; Tapia-Navarro, C.; Ríos, F. Development of a Predictive Classification Model for Surfactant-Induced Skin Irritation. ACS Omega 2025, 10 (46) 55868–55878. [CrossRef]
- Shah, S.K.; Chakraborty, G.; Bhattarai, A.; De, R. Synergistic and antagonistic effects in micellization of mixed surfactants. Journal of Molecular Liquids 2022, 368, 120678. [CrossRef]
- Bera, A.; Ojha, K.; Mandal, A. Synergistic effect of mixed surfactant systems on foam behavior and surface tension. Journal of Surfactants and Detergents 2013, 16 (4), 621–630. [CrossRef]
| Chemical name | Abbreviation | INCI | % Active matter | Length alkyl chain | Chemical structure depiction |
| Sulfuric acid, mono-C12-14-alkyl esters, sodium salts | SLS | Sodium lauryl sulfate | 30 | 12 | 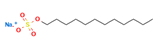 |
| Dodecyldimethylamine oxide | LDAO | Lauramine Oxide | 30 | 12 |  |
| Zein Number (mgN/100mL) | Irritation Category |
| 0-200 | Not irritant |
| 200-400 | Moderately irritant |
| 400-500 | Irritant Type I |
| >500 | Irritant Type II |
| Sample | CMC (mg/L) | Lowest γ (mN/m) | C1-2 (mg/L) *31mN/m |
| SLS | 840 | 32.05 | 1155,0 |
| LDAO | 418 | 30.1 | 363.6 |
| SLS+LDAO (90:10) | 208 | 24.7 | 217,6 |
| SLS+LDAO (80:20) | 212 | 24.5 | 206,4 |
| SLS+LDAO (70:30) | 202 | 24.3 | 180,8 |
| SLS+LDAO (60:40) | 219 | 24.1 | 109,1 |
| SLS+LDAO (50:50) | 263 | 23.7 | 56,3 |
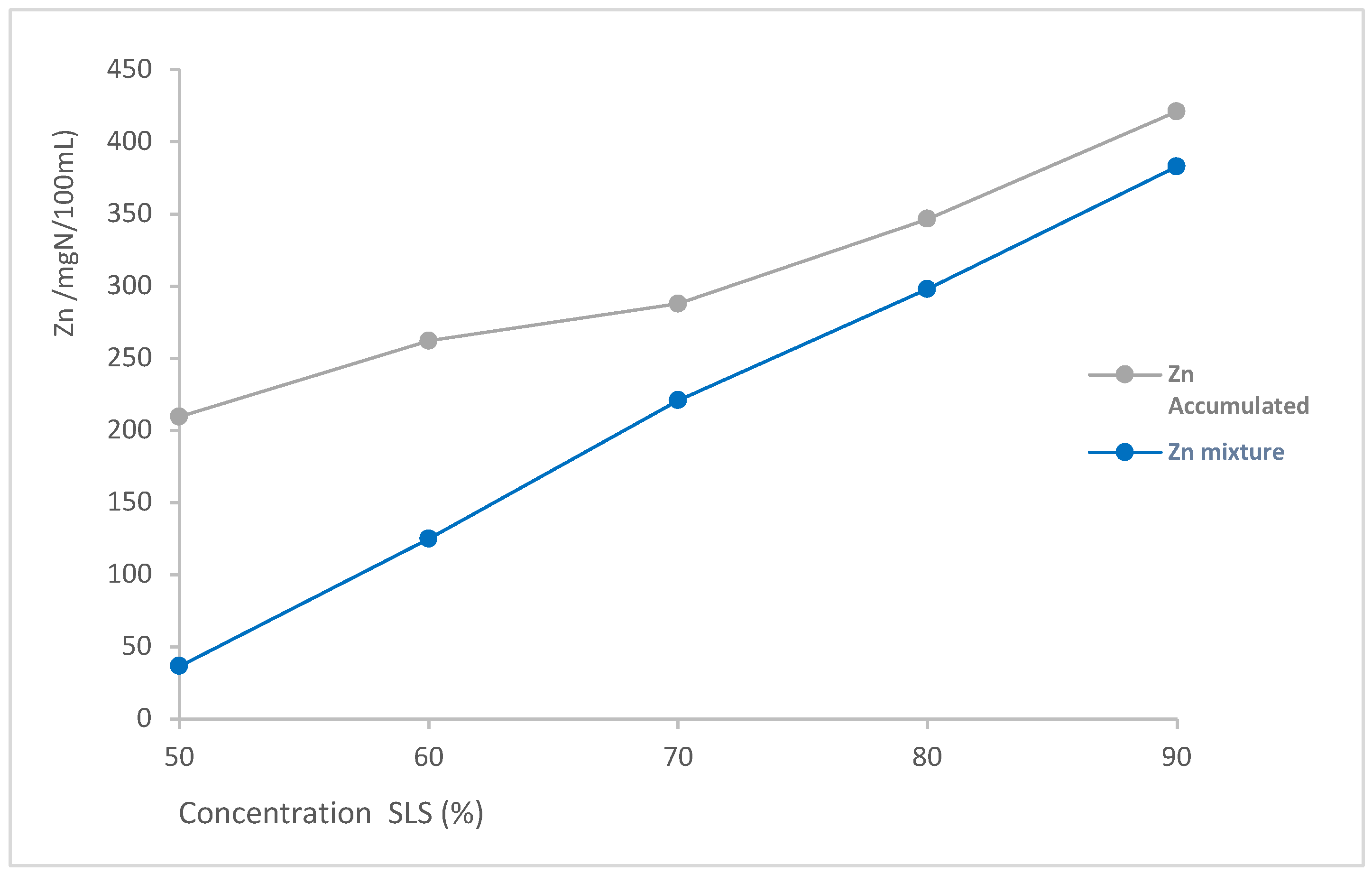
| Surfactant mixtures SLS+LDAO | Zn individual (mgN/100mL) | Zn Accumulated (mgN/100mL) | Zn mixture (mgN/100mL) | % increase |
| 90:10 | 412 | 421.13 | 383.0 | 9.95 |
| 9.13 | ||||
| 80:20 | 341.6 | 346.36 | 297.8 | 16.32 |
| 4.76 | ||||
| 70:30 | 285.3 | 287.59 | 221.0 | 30.14 |
| 2.29 | ||||
| 60:40 | 258.8 | 262.12 | 124.9 | 109.83 |
| 2.32 | ||||
| 50:50 | 208 | 209.55 | 36.5 | 474.90 |
| 1.55 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





