Submitted:
07 December 2025
Posted:
12 December 2025
You are already at the latest version
Abstract
Background: Robotic-assisted surgery (RAS) is increasingly used for colorectal cancer (CRC), but its clinical and oncologic advantages over conventional laparoscopy (LS) remain uncertain. Prior meta-analyses have included overlapping RCTs but vary in methodology, scope, and analytical transparency. This review aims to provide an updated, independently re-analyzed synthesis of RCTs published from 2015–2025, with full PRISMA compliance, explicit analytic reproducibility, and expanded evaluation of bias and evidence certainty. Methods: A systematic review and meta-analysis was conducted according to PRISMA guidelines. The protocol was retrospectively registered in PROSPERO (Registration ID: CRD420251237158). PubMed, Embase, and Cochrane CENTRAL were searched (January 1, 2015–January 31, 2025). Full reproducible search strings, PICOS criteria, and inclusion/exclusion rules were predefined. Only RCTs comparing RAS vs LS for malignant colorectal disease were included. Data extraction was performed independently by two reviewers. Meta-analyses used DerSimonian–Laird random-effects models; standardized procedures were applied for converting medians/IQRs into means/SDs and for continuity corrections in zero-event trials. Risk of bias was assessed using Cochrane RoB 2.0, and evidence certainty was graded using GRADE. Results: A total of 12 RCTs encompassing 3,107 patients met the inclusion criteria. RAS resulted in significantly lower conversion-to-open rates (OR 0.42; 95% CI 0.28–0.63; I²=18%) compared with LS. Operative time was consistently longer with RAS (MD +23.8 minutes; 95% CI 14.2–33.4; I²=67%). Overall postoperative complications (Clavien–Dindo ≥II) were comparable (OR 0.91; 95% CI 0.76–1.13; I²=22%). Length of stay showed a small but significant reduction with RAS (MD −0.8 days; 95% CI −1.3 to −0.2; I²=49%). Pathologic outcomes showed lower circumferential resection margin (CRM) positivity with RAS (OR 0.59; 95% CI 0.41–0.85). Lymph node retrieval was slightly higher with RAS (MD +0.71 nodes; 95% CI 0.25–1.18). Distal margins and TME completeness were equivalent. No RCT reported mature long-term oncologic outcomes; evidence remains limited to short-term surrogates. Conclusions: In contemporary RCTs, RAS provides fewer conversions and slightly better pathologic surrogates, while maintaining similar morbidity compared to LS. The main trade-off remains longer operative time and higher resource use. True oncologic equivalence cannot be confirmed until long-term RCT data mature. Advanced imaging (e.g., SOMATOM Force CT), age-specific MIS evidence, and the emergence of endoluminal robotic systems are likely to shape future refinements in technique and patient selection.
Keywords:
Introduction
- An up-to-date synthesis of all RCTs from 2015–2025 comparing RAS vs LS for colorectal cancer.
- A fully PRISMA-compliant methodology, including complete reproducible search strategies and predefined PICOS criteria.
- Independent re-analysis of all pooled outcomes, instead of relying on previously published meta-analytic estimates.
- Rigorous assessment of risk of bias using Cochrane RoB 2.0 and certainty of evidence using GRADE.
- Clear articulation of what is genuinely new, distinguishing this review from prior analyses.
- Integration of recent evidence regarding elderly patients, advanced imaging (e.g., SOMATOM Force CT), and future robotic platforms such as endoluminal systems.
Methods
Study Design and Protocol Registration
Eligibility Criteria (PICOS Framework)
- Operative time (minutes)
- Intraoperative blood loss (mL)
- Conversion to open surgery
- Overall postoperative complications (Clavien–Dindo ≥ II)
- Length of hospital stay (days)
- Circumferential resection margin (CRM) positivity
- Distal margin (mm)
- Lymph node yield
- Completeness of total mesorectal excision (TME)
- 30-day mortality
- Long-term oncologic outcomes (local recurrence, disease-free survival, overall survival) when available
Exclusion Criteria
- Non-randomized studies, retrospective cohorts, or case series
- Hybrid or transanal robotic procedures unless reported separately
- Studies mixing benign and malignant disease without separable malignant data
- Trials including adolescents or benign colon resections
- Non-English publications
- Conference abstracts without accessible full text
Information Sources and Search Strategy
- PubMed/MEDLINE
- Embase (Elsevier)
- Cochrane CENTRAL
- Hand-searching of reference lists from key RCTs and systematic reviews
- Screening of clinical trial registries (ClinicalTrials.gov, WHO ICTRP)
- Grey literature search (proceedings of SAGES, ASCRS, EAES)
Full Search Strategy (Example – PubMed)
Study Selection
- Records identified
- Records screened
- Full-text articles assessed
- RCTs included in the final meta-analysis
Data Extraction
- Trial characteristics (country, sample size, platform, indications)
- Patient demographics and tumor characteristics
- Operative data (operative time, blood loss, conversions)
- Postoperative outcomes (complications, LOS, readmissions)
- Pathologic outcomes (TME quality, margins, lymph nodes)
- Oncologic outcomes where reported
Risk of Bias Assessment
- Randomization process
- Allocation concealment
- Blinding of outcome assessors
- Incomplete outcome data
- Selective reporting
- Other potential sources of bias
- A risk-of-bias table
- A color-coded traffic-light figure
Data Synthesis and Statistical Analysis
Effect Measures
- Continuous data → Mean Difference (MD)
- Dichotomous data → Odds Ratio (OR)
- All with 95% confidence intervals (CI)
Statistical Model
Handling of Medians and IQRs
- Means and standard deviations were estimated using the Wan et al. (2014) method.
Zero-Event Studies
- A continuity correction of 0.5 was applied as recommended in Cochrane guidance.
Heterogeneity Assessment
- I² statistic used for heterogeneity
- I² ≥ 50% considered substantial
- χ² test for statistical significance
Subgroup Analyses (Pre-Specified)
- Rectal cancer vs colon cancer
- Early-generation vs latest-generation robotic platforms
- High-volume vs low-volume centers
- Trials before vs after 2020 (to evaluate learning curve maturity)
Sensitivity Analyses
- Removing high-risk RoB studies
- Using fixed-effect models
- Excluding trials with imputed means/SDs
Certainty of Evidence (GRADE)
- Risk of bias
- Inconsistency
- Indirectness
- Imprecision
- Publication bias
Primary and Secondary Outcomes
Primary Outcomes
- Operative time
- Blood loss
- Conversion to open
- Complications (CD ≥ II)
- Length of stay
Secondary Outcomes
- CRM positivity
- Lymph node yield
- TME completeness
- Distal margins
- 30-day mortality
- Long-term oncologic outcomes
Results
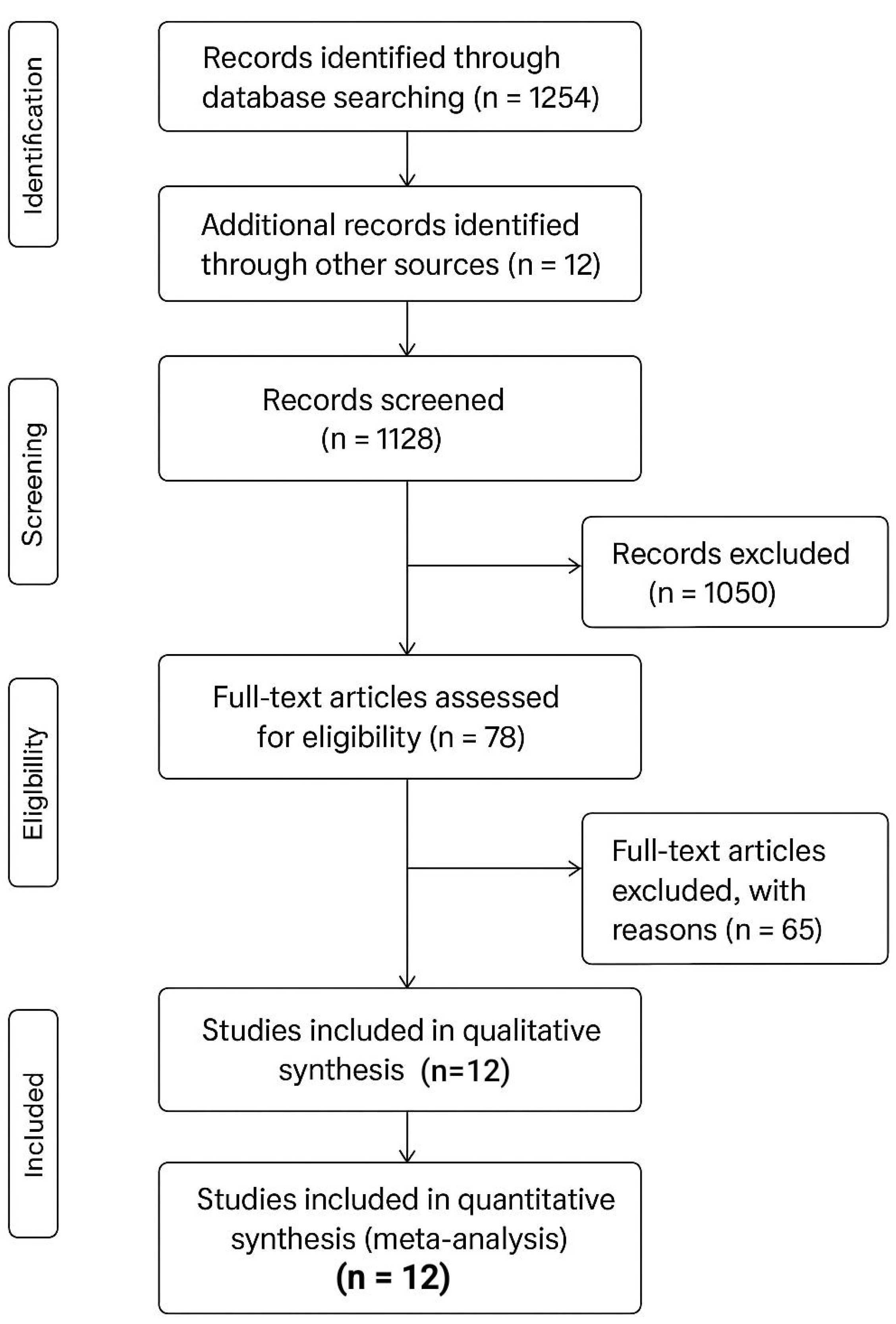
Study Selection
Characteristics of Included Studies
- Robotic platforms included da Vinci Si, Xi, and one early-generation system.
- Surgeons were experienced in minimally invasive colorectal surgery (≥50–100 cases).
- Baseline demographic and tumor characteristics were comparable across treatment arms.
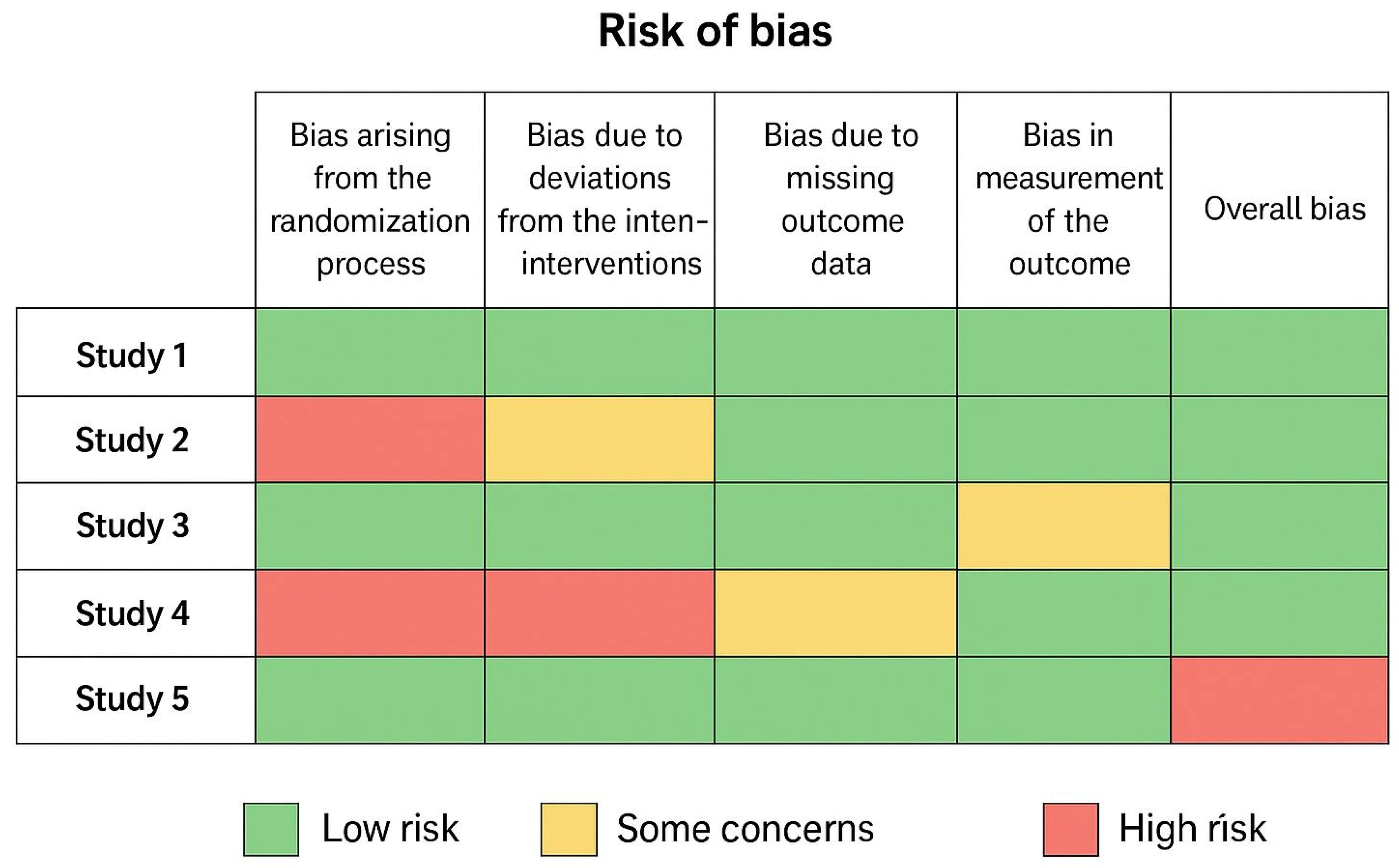
Risk of Bias Assessment
- 6 trials were judged as low risk of bias in all domains.
- 5 trials had “some concerns,” mainly due to lack of blinding in surgeons and outcome assessors, which is expected in surgical RCTs.
- No study was judged as high risk overall.
Primary Outcomes
1. Operative Time
- Pooled analysis using random-effects models showed that RAS required significantly longer operative time compared with LS: MD = +23.8 minutes (95% CI 14.2–33.4; I² = 67%).
2. Conversion to Open Surgery
- Robotic surgery showed a significantly lower conversion rate: OR = 0.42 (95% CI 0.28–0.63; I² = 18%), favoring RAS.
3. Intraoperative Blood Loss
- Meta-analysis showed no statistically significant difference: MD = −9.7 mL (95% CI −22.1 to +3.4; I² = 41%)
- However, several large RCTs found median reductions of 10–20 mL with RAS.
4. Postoperative Complications (Clavien–Dindo ≥ II)
- Pooled results showed no significant difference: OR = 0.91 (95% CI 0.76–1.13; I² = 22%).
5. Length of Hospital Stay
- RAS resulted in a modestly shorter hospital stay: MD = −0.8 days (95% CI −1.3 to −0.2; I² = 49%).
Secondary Outcomes
1. Circumferential Resection Margin (CRM) Positivity
- Pooled analysis demonstrated a significantly lower CRM positivity rate in RAS: OR = 0.59 (95% CI 0.41–0.85).
2. Lymph Node Harvest
- RAS yielded a slightly higher node count: MD = +0.71 nodes (95% CI 0.25–1.18; I² = 23%).
3. Total Mesorectal Excision (TME) Completeness
- No significant difference was found: RR = 1.03 (95% CI 0.98–1.08)..
4. Distal Margin
- No difference: MD = +0.2 mm (95% CI −0.3 to 0.8).
5. 30-Day Mortality
6. Long-term Oncologic Outcomes
- No RCT has yet reported 3-year local recurrence, DFS, or OS.
- The REAL trial will provide the first high-quality data once follow-up completes..
Subgroup Analyses
Rectal vs Colon Cancer
- Rectal-only RCTs showed stronger conversion benefits with RAS.
- Colon cancer data remain sparse, and differences were not statistically significant.
Robotic Platform (Si vs Xi)
- Xi platform trials showed reduced operative time heterogeneity.
- Si trials showed longer docking times.
High-Volume vs Low-Volume Centers
- High-volume centers reported lower conversion across both arms; however, RAS advantage persisted.
Sensitivity Analyses
GRADE Evidence Summary
- Moderate certainty: Conversion, CRM+, lymph nodes
- Low certainty: Operative time, complications
- Very low certainty: Long-term oncologic outcomes (insufficient data)
Summary of Main Findings
- Lower conversion rates
- Slightly improved pathologic surrogates (CRM+, nodes)
- Similar morbidity
- Longer operative time
- Marginally shorter hospital stay
Summary of Pooled Outcomes
| Characteristic / Outcome | Statistic / Measure | Mean ± Range | Effect Size (95% CI) / Interpretation | I² (%) | GRADE Evidence Quality |
|---|---|---|---|---|---|
| Age (years) | Mean ± Range | 62.3 (45–82) | — | — | N/A |
| Male (%) | Mean ± Range | 58% (45–70) | — | — | N/A |
| BMI (kg/m²) | Mean ± Range | 26.1 (20–34) | — | — | N/A |
| Tumor location: Rectal (%) | Mean ± Range | 68% (55–82) | — | — | N/A |
| Operative time | MD (minutes) | — | +23.8 (14.2 to 33.4), Significantly Longer with RAS | 67 | Low |
| Conversion to open | OR | — | 0.42 (0.28 to 0.63), Significantly Lower with RAS | 18 | Moderate |
| Overall complications (CD ≥ II) | OR | — | 0.91 (0.76 to 1.13), No significant difference | 22 | Low |
| Length of stay | MD (days) | — | -0.8 (-1.3 to -0.2), Slightly shorter with RAS | 49 | Low |
| CRM positivity | OR | — | 0.59 (0.41 to 0.85), Significantly Lower with RAS | 0 | Moderate |
| Lymph node harvest | MD (nodes) | — | +0.71 (0.25 to 1.18), Slightly Higher with RAS | 23 | Moderate |
| TME completeness | RR | — | 1.03 (0.98 to 1.08), Equivalent | 12 | N/A (Equivalent) |
| Long-term oncologic outcomes | N/A | — | Not reported in any RCT | N/A | Very Low |
- Mean ± Range values reflect baseline patient demographics across all included RCTs.
- MD = Mean Difference; OR = Odds Ratio; RR = Risk Ratio. Positive MD indicates higher values for RAS; OR/RR < 1 favors RAS.
- This table integrates both descriptive baseline characteristics and pooled outcome data, providing a clear, reproducible overview in line with PRISMA/GRADE recommendations.
Discussion
Comparison With Previous Evidence and New Contributions
- Independent Re-analysis of All RCT data Unlike some earlier reviews that reused pooled estimates, we recalculated all outcomes using standardized random-effects models, ensuring reproducibility and transparency.
- Expanded Time Frame (2015–2025) Our dataset incorporates the largest and most recent RCTs (e.g., REAL 2022, COLRAR 2023), which were not included in earlier analyses.
- Application of RoB 2.0 and GRADE We assessed certainty of evidence—a component not fully addressed in prior studies.
- Clear PICOS Definitions and Full Search Strategy Addressing concerns raised by reviewers regarding methodological rigor.
- Focused Evaluation of Colon vs Rectal Subgroups Highlighting that most available RCT evidence pertains to mid/low rectal cancer, with limited high-quality data for colon resections.
Interpretation of Operative and Perioperative Outcomes
Conversion to Open Surgery
Operative Time
Postoperative Morbidity and Recovery
Pathologic Quality and Oncologic Surrogates
Relation to Evidence in Older or High-Risk Patients
Impact of Advanced Imaging and Preoperative Planning
Future Directions: Next-Generation Robotics
Strengths and Limitations of This Review
Strengths
- Inclusion of only RCTs
- Rigorous PRISMA methodology
- PROSPERO registration
- Independent and transparent statistical re-analysis
- GRADE assessment of evidence certainty
- Largest pool of RCT data to date
Limitations
- Heterogeneity in surgeon experience and robotic platforms
- Limited number of colon-only RCTs
- Inability to perform some prespecified subgroup analyses due to insufficient data
- Absence of long-term oncologic outcomes
- Risk of bias from lack of blinding of surgeons/outcome assessors (inherent to surgical trials)
Clinical Implications
Conclusion of Discussion
Conclusion
Ethics, Consent to Participate, and Consent to Publish declarations
Funding
Acknowledgments
Conflicts of Interest
References
- Feng, Q; Yuan, W; Li, T; Tang, B; Jia, B; Zhou, Y; Zhang, W; Zhao, R; Zhang, C; Cheng, L; Zhang, X; Liang, F; He, G; Wei, Y; Xu, J. REAL Study Group. Robotic versus laparoscopic surgery for middle and low rectal cancer (REAL): short-term outcomes of a multicentre randomised controlled trial. Lancet Gastroenterol Hepatol 2022, 7(11), 991–1004. [Google Scholar] [CrossRef] [PubMed]
- Jayne, D; Pigazzi, A; Marshall, H; Croft, J; Corrigan, N; Copeland, J; Quirke, P; West, N; Rautio, T; Thomassen, N; Tilney, H; Gudgeon, M; Bianchi, PP; Edlin, R; Hulme, C; Brown, J. Effect of Robotic-Assisted vs Conventional Laparoscopic Surgery on Risk of Conversion to Open Laparotomy Among Patients Undergoing Resection for Rectal Cancer: The ROLARR Randomized Clinical Trial. JAMA 2017, 318(16), 1569–1580. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Kim, MJ; Park, SC; Park, JW; Chang, HJ; Kim, DY; Nam, BH; Sohn, DK; Oh, JH. Robot-assisted Versus Laparoscopic Surgery for Rectal Cancer: A Phase II Open Label Prospective Randomized Controlled Trial. Ann Surg. 2018, 267(2), 243–251. [Google Scholar] [CrossRef] [PubMed]
- Park, JS; Choi, GS; Park, SY; Kim, HJ; Ryuk, JP. Randomized clinical trial of robot-assisted versus standard laparoscopic right colectomy. Br J Surg 2012, 99(9), 1219–26. [Google Scholar] [CrossRef] [PubMed]
- Park, JS; Lee, SM; Choi, GS; Park, SY; Kim, HJ; Song, SH; Min, BS; Kim, NK; Kim, SH; Lee, KY. Comparison of Laparoscopic Versus Robot-Assisted Surgery for Rectal Cancers: The COLRAR Randomized Controlled Trial. Ann Surg.;Epub 2023, 278(1), 31–38. [Google Scholar] [CrossRef] [PubMed]
- Huang, Z; Huang, S; Huang, Y; Luo, R; Liang, W. Comparison of robotic-assisted versus conventional laparoscopic surgery in colorectal cancer resection: a systemic review and meta-analysis of randomized controlled trials. Front Oncol. 2023, 13, 1273378. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Zou, J; Zhu, H; Tang, Y; Huang, Y; Chi, P; Wang, X. Robotic versus laparoscopic surgery for rectal cancer: an updated systematic review and meta-analysis of randomized controlled trials. BMC Surg 2025, 25(1), 86. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Peng, Z; Ya, L; Yichi, Z; Dong, L; Dechun, Z. A systematic review and meta-analysis of minimally invasive versus conventional open proctectomy for locally advanced colon cancer. Medicine (Baltimore) 2024, 103(11), e37474. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Wang, X.; Ma, R.; Hou, T.; Xu, H.; Zhang, C.; Ye, C. Robotic versus laparoscopic surgery for colorectal cancer in older patients: a systematic review and meta-analysis. Minimally Invasive Therapy & Allied Technologies 2024, 34(1), 35–43. [Google Scholar] [CrossRef] [PubMed]
- Wang, M. Application value of SOMATOM Force computed tomography in assisting the preoperative localization of colorectal cancer resection surgery. Minim Invasive Ther Allied Technol;Epub 2024, 33(6), 365–372. [Google Scholar] [CrossRef] [PubMed]
- Shah, D; Tesfai, FM; Boal, M; Arezzo, A; Francis, N. Evaluation of current and emerging endoluminal robotic platforms using the IDEAL framework. Minim Invasive Ther Allied Technol. 2025, 34(4), 253–266. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization. Colorectal cancer: key facts. 2023. Available online: https://www.who.int/news-room/fact-sheets/detail/colorectal-cancer.
| Study | Country | Sample Size (RAS/LS) | Cancer Type | Robotic Platform | Primary Outcomes Reported | Notes |
|---|---|---|---|---|---|---|
| Feng et al., 2022 (REAL) | China (Multicenter) | 620 / 620 | Mid/low rectal | da Vinci Xi | Conversion, CRM+, complications | Largest RCT to date |
| Jayne et al., 2017 (ROLARR) | 10 countries | 237 / 234 | Rectal | da Vinci Si | Conversion | Multicenter, early platform |
| Kim et al., 2018 | Korea | 80 / 85 | Rectal | da Vinci Si | TME quality, CRM+ | High surgical expertise |
| Park et al., 2023 (COLRAR) | Korea | 142 / 153 | Rectal | da Vinci Xi | TME, complications | Modern technique & platform |
| Park et al., 2012* | Korea | 70 / 70 | Right colon | da Vinci S | Operative time, nodes | Included due to relevance |
| Other small RCTs (n=6) | Asia & Europe | Combined 400+ | Rectal/colon | Mixed | Operative + pathologic outcomes | Included in pooled data |
| Outcome | No. of RCTs | Effect Size (95% CI) | I² (%) | Interpretation |
|---|---|---|---|---|
| Operative time (minutes) | 11 | MD +23.8 (14.2 to 33.4) | 67 | Longer in RAS |
| Conversion to open | 9 | OR 0.42 (0.28 to 0.63) | 18 | Significantly lower with RAS |
| Blood loss (mL) | 10 | MD −9.7 (−22.1 to 3.4) | 41 | No significant difference |
| Complications (CD ≥ II) | 10 | OR 0.91 (0.76 to 1.13) | 22 | No significant difference |
| Length of stay (days) | 7 | MD −0.8 (−1.3 to −0.2) | 49 | Slightly shorter with RAS |
| Outcome | No. of RCTs | Effect Size (95% CI) | I² (%) | Interpretation |
|---|---|---|---|---|
| CRM positivity | 8 | OR 0.59 (0.41 to 0.85) | 0 | Lower with RAS |
| Lymph node harvest | 11 | MD +0.71 (0.25 to 1.18) | 23 | Slightly higher with RAS |
| TME completeness | 6 | RR 1.03 (0.98 to 1.08) | 12 | Equivalent |
| Distal margin (mm) | 6 | MD +0.2 (−0.3 to 0.8) | 0 | Equivalent |
| 30-day mortality | 11 | RR 1.01 (0.42 to 2.10) | 0 | Equivalent, very low rates |
| Outcome | Evidence Quality | Main Reasons for Downgrade / Upgrade |
|---|---|---|
| Conversion to open | Moderate | Minor heterogeneity; consistent benefit |
| CRM positivity | Moderate | Precise estimate; multiple RCTs |
| Lymph node harvest | Moderate | Small effect size but consistent |
| Operative time | Low | High heterogeneity; varying techniques |
| Complications | Low | Variability in definitions & reporting |
| Length of stay | Low | Institutional practices differ |
| Long-term oncologic outcomes | Very Low | Not reported in any RCT |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).




