Submitted:
30 November 2025
Posted:
02 December 2025
You are already at the latest version
Abstract
Keywords:
Introduction
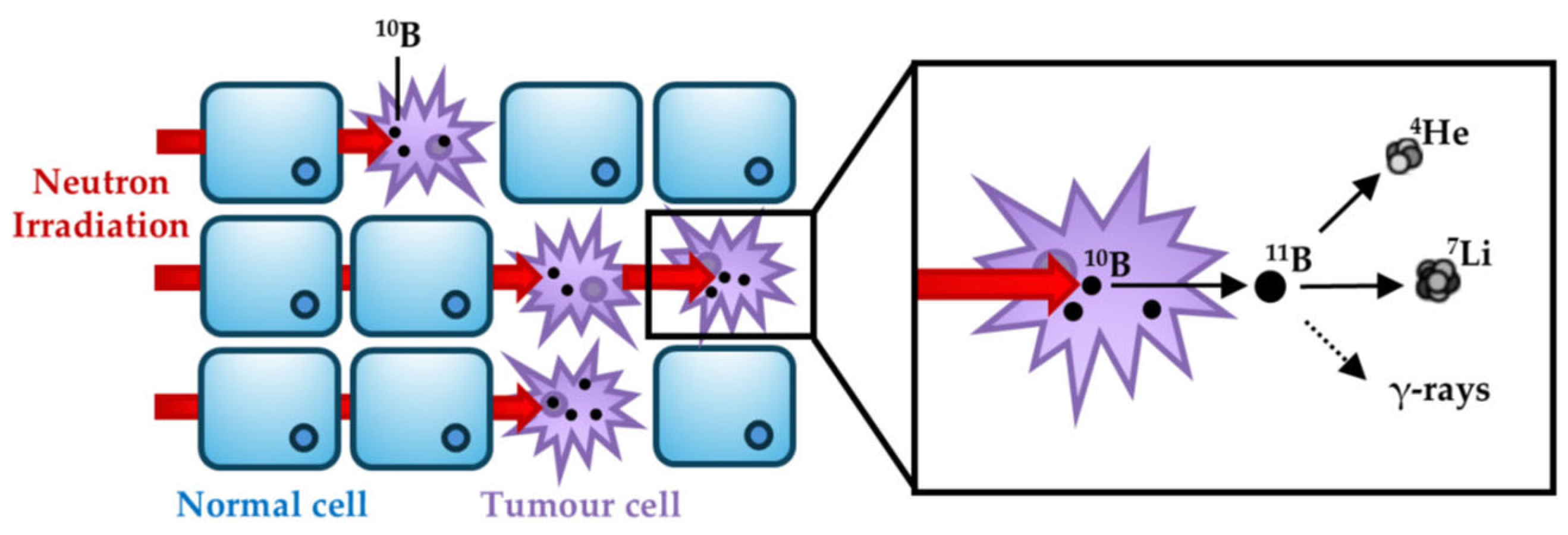
Physical and Biological Foundation of BNCT
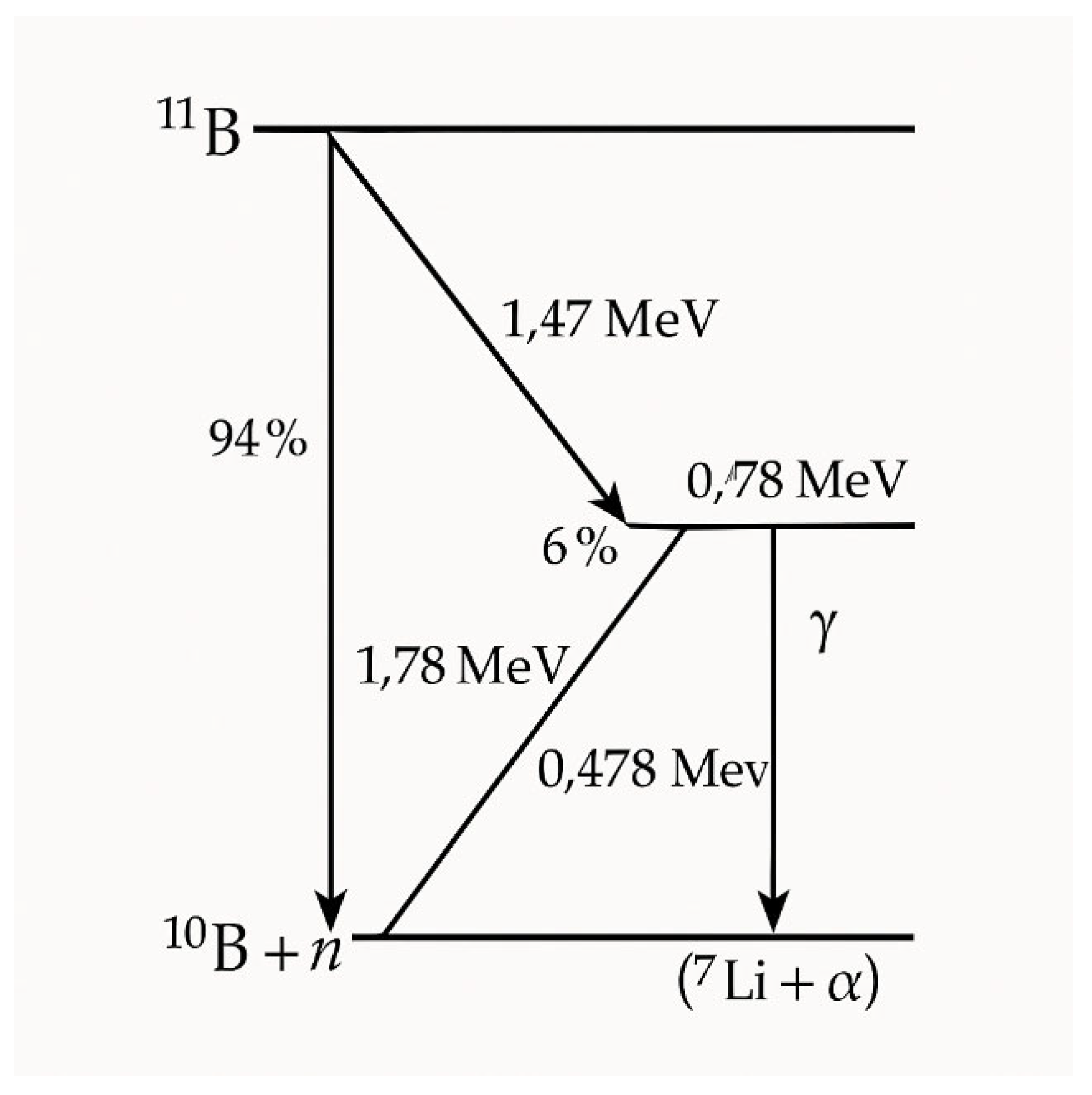
The Physical Rationale
Neutron-Tissue Interactions
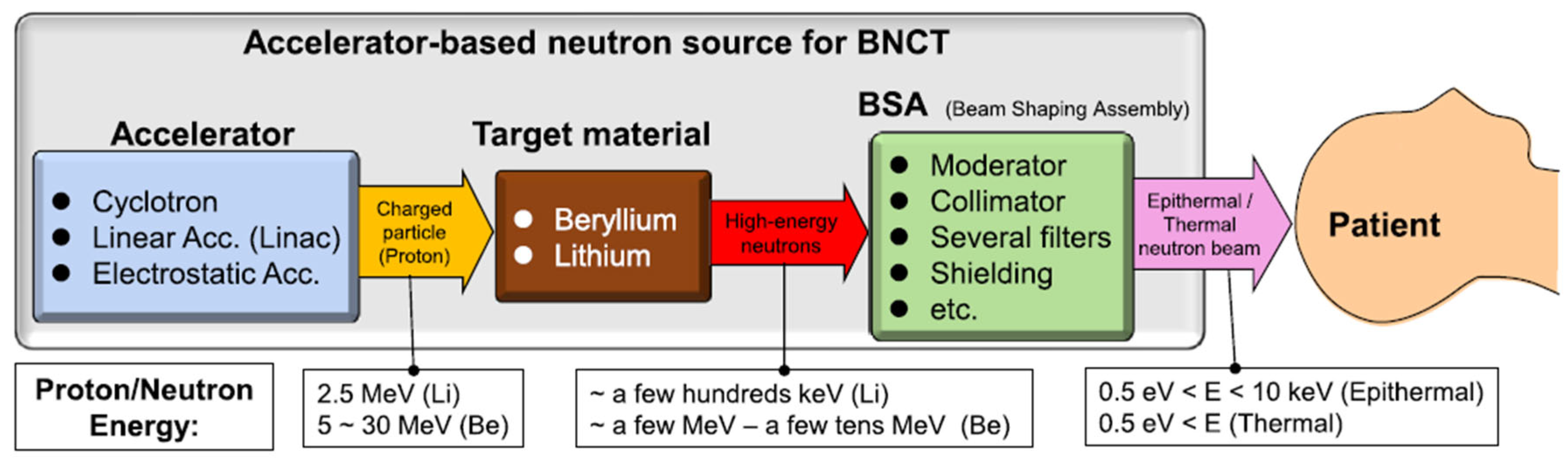
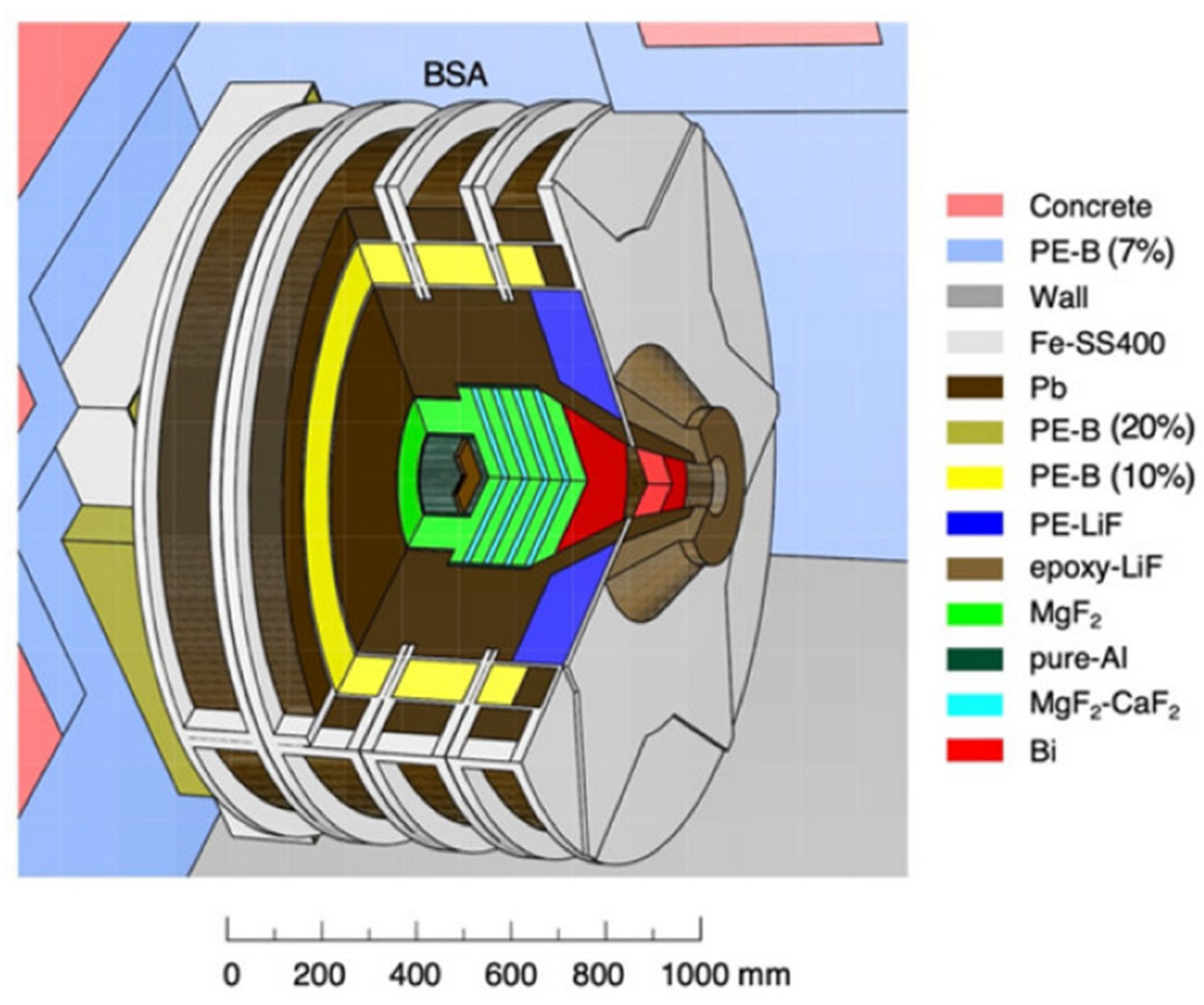
Neutron Sources and Beam Characteristics
Neutron Moderation and Energy Spectrum
Dosimetry in the BNCT radiation field
The Biological Rationale
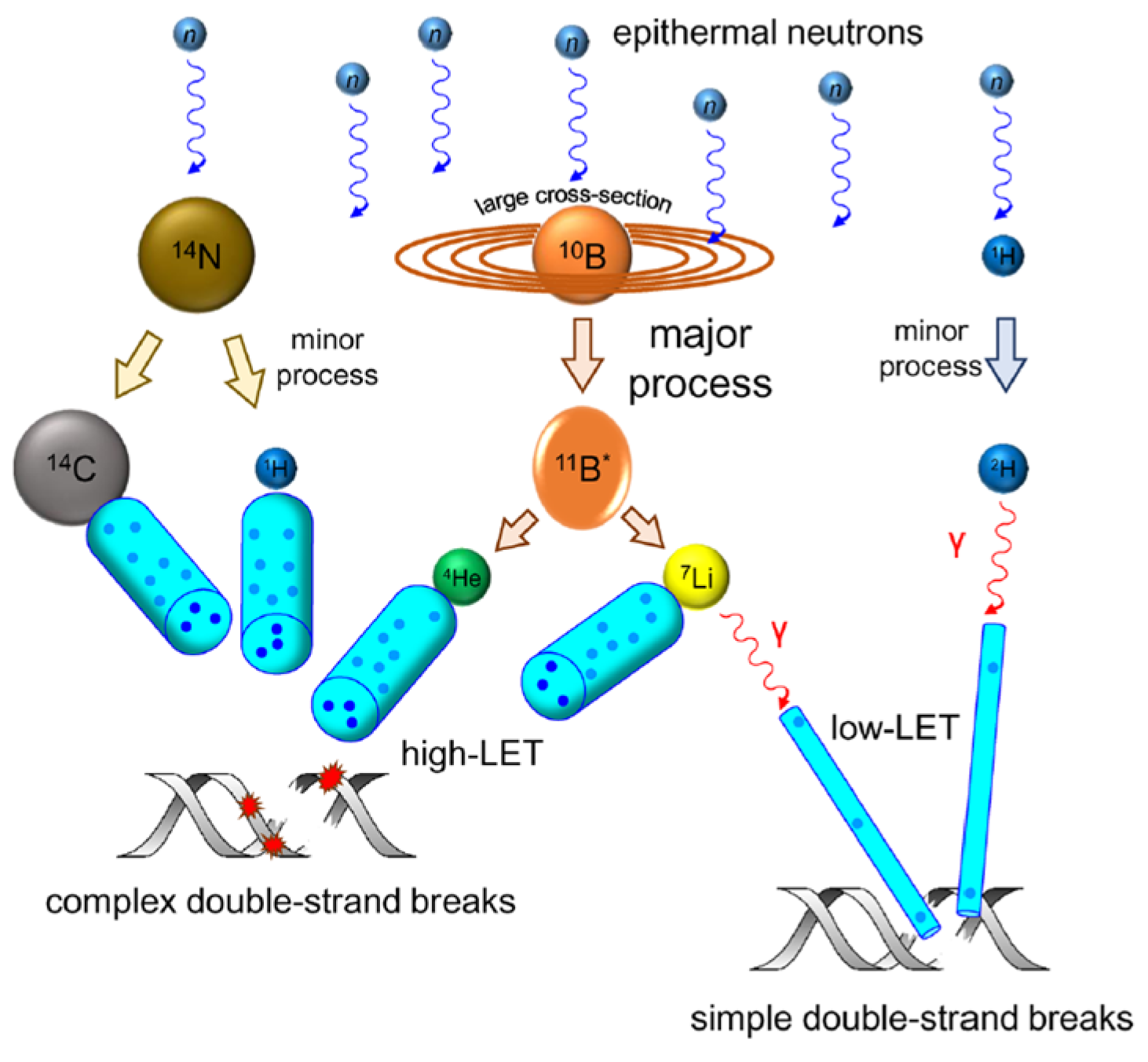
Mechanisms of DNA Damage and Cell Killing
Boron Delivering strategies
Production and Processing of Enriched ¹⁰B
Closing Remarks
References
- H. He, J. Li, P. Jiang, S. Tian, H. Wang, R. Fan, J. Liu, Y. Yang, Z. Liu, J. Wang. The basis and advances in clinical application of boron neutron capture therapy. Radiat. Oncol. 16, 216 (2021). [CrossRef]
- A. Monti Hughes, N. Hu. Optimizing boron neutron capture therapy (BNCT) to treat cancer: an updated review on the latest developments on boron compounds and strategies. Cancers 15, 4091 (2023). [CrossRef]
- L.D. Punshon, M.R. Fabbrizi, B. Phoenix, S. Green, J.L. Parsons. Current insights into the radiobiology of boron neutron capture therapy and the potential for further improving biological effectiveness. Cells 13, 2065 (2024). [CrossRef]
- G.L. Locher. Biological effects and therapeutic possibilities of neutrons. Am. J. Roentgenol. Radium Ther. Nucl. Med. 36, 1–13 (1936).
- W.H. Sweet, M. Javid. The possible use of neutron-capturing isotopes such as boron 10 in the treatment of neoplasms. I. Intracranial tumors. J. Neurosurg. 9, 200–209 (1952). [CrossRef]
- H. Hatanaka, K. Sano. A revised boron-neutron capture therapy for malignant brain tumours. I. Experience on terminally ill patients after Co-60 radiotherapy. Z. Neurol. 204, 309–332 (1973). [CrossRef]
- R.G. Fairchild, D. Gabel, B.H. Laster, D. Greenberg, W. Kiszenick, P.L. Micca. Microanalytical techniques for boron analysis using the 10B(n,α)7Li reaction. Med. Phys. 13, 50–56 (1986). [CrossRef]
- Kato, Y. Fujita, A. Maruhashi, H. Kumada, M. Ohmae, M. Kirihata, Y. Imahori, M. Suzuki, Y. Sakrai, T. Sumi, S. Iwai, M. Nakazawa, I. Murata, H. Miyamaru, K. Ono. Effectiveness of boron neutron capture therapy for recurrent head and neck malignancies. Appl. Radiat. Isot. 67, S37–S42 (2009). [CrossRef]
- R.F. Barth, M.G.H. Vicente, O.K. Harling, W.S. Kiger, et al. Current status of boron neutron capture therapy of high grade gliomas and recurrent head and neck cancer. Radiat. Oncol. 7, 146 (2012).
- T.D. Malouff, D.S. Seneviratne, D.K. Ebner, W.C. Stross, M.R. Waddle, D.M. Trifiletti, S. Krishnan. Boron neutron capture therapy: a review of clinical applications. Front. Oncol. 11, 601820 (2021). [CrossRef]
- ClinicalTrials.gov. Boron neutron capture therapy using CICS-1 and SPM-011 for malignant melanoma and angiosarcoma. Identifier: NCT04293289. Updated 2023-02-08. Accessed 2025-10-15.
- T. Zhou, K. Igawa, T. Kasai, T. Sadahira, W. Wang, T. Watanabe, P. Huang, et al. The current status and novel advances of boron neutron capture therapy clinical trials. Am. J. Cancer Res. 14, 429–447 (2024).
- P. Karihtala. The current status and future perspectives of clinical boron neutron capture therapy trials. Health Technol. 14, 1001–1005 (2024). [CrossRef]
- N. Farha, D. Dima, F. Ullah, S. Kamath. Precision oncology targets in biliary tract cancer. Cancers 15, 2105 (2023). [CrossRef]
- International Atomic Energy Agency. Advances in Boron Neutron Capture Therapy. Vienna: IAEA (2023). ISBN 978-92-0-132723-9.
- B. Hamermesh, G.R. Ringo, S. Wexler. The thermal neutron capture cross section of hydrogen. Phys. Rev. 90, 603–606 (1953). [CrossRef]
- S.F. Mughabghab. Atlas of Neutron Resonances: Resonance Parameters and Thermal Cross Sections Z = 1–100, 6th ed.; Elsevier: Amsterdam, 2018. ISBN 978-0-444-63744-2.
- M.B. Chadwick, et al. ENDF/B-VII.1 nuclear data files for neutron-induced reactions and advanced applications. Nucl. Data Sheets 112, 2887–2996 (2011).
- M. Bikchurina, T. Bykov, I. Ibrahim, et al. Dosimetry for boron neutron capture therapy developed and verified at the accelerator based neutron source VITA. Front. Nucl. Eng. 2, 1266562 (2023). [CrossRef]
- H. Kumada, T. Sakae, H. Sakurai. Current development status of accelerator based neutron source for boron neutron capture therapy. EPJ Techn. Instrum. 10, 18 (2023). [CrossRef]
- S. Altieri, N. Protti. A brief review on reactor based neutron sources for boron neutron capture therapy. Ther. Radiol. Oncol. 2, 1–6 (2018). [CrossRef]
- K. Hirose, A. Konno, J. Hiratsuka, et al. Boron neutron capture therapy using cyclotron-based epithermal neutron source and borofalan (10B) for recurrent or locally advanced head and neck cancer (JHN002): an open-label phase II trial. Radiother. Oncol. 155, 182–187 (2021). [CrossRef]
- A.A. Ivanov, A.N. Smirnov, S.Yu. Taskaev, et al. Accelerator-based neutron source for boron neutron capture therapy. Uspekhi Fiz. Nauk 192, 893–912 (2022).
- Y. Matsuya, T. Kai, T. Sato, et al. Track-structure modes in particle and heavy ion transport code system (PHITS): application to radiobiological research. Int. J. Radiat. Biol. 98, 148–157 (2022). [CrossRef]
- S. Jing, H. Guo, Y. Qi, G. Yang, Y. Huang. A portable fast neutron irradiation system for tumor therapy. Appl. Radiat. Isot. 160, 109138 (2020). [CrossRef]
- H. Kumada, K. Takada, S. Tanaka, et al. Evaluation of the characteristics of the neutron beam of a linac-based neutron source for boron neutron capture therapy. Appl. Radiat. Isot. 165, 109246 (2020). [CrossRef]
- M.E. Capoulat, D. Cartelli, M. Baldo, et al. Accelerator based-BNCT facilities worldwide and an update of the Buenos Aires project. Appl. Radiat. Isot. 219, 111723 (2025). [CrossRef]
- G. Li, W. Jiang, L. Zhang, W. Chen, Q. Li. Design of beam shaping assemblies for accelerator-based BNCT with multi-terminals. Front. Public Health 9, 642561 (2021). [CrossRef]
- N. Hu, H. Tanaka, K. Akita, et al. Accelerator based epithermal neutron source for clinical boron neutron capture therapy. J. Neutron Res. 24, 359–366 (2022). [CrossRef]
- PR Newswire. Neutron Therapeutics and Helsinki University Hospital treat first patients in the United States in phase 1 trial of boron neutron capture therapy. PR Newswire, May 16, 2025. https://go.gale.com/ps/i.do?id=GALE%7CA839961052.
- A. Pisent, F. Grespan, C. Baltador, et al. ANTHEM Project, construction of a RFQ driven BNCT neutron source. In Proceedings of the 32nd Linear Accelerator Conference (LINAC 2024), Chicago, IL, USA, 25–30 August 2024. [CrossRef]
- M. Bikchurina, T. Bykov, E. Byambatseren, et al. VITA high flux neutron source for various applications. J. Neutron Res. 24, 273–279 (2022).
- T. Nishitani, S. Yoshihashi, Y. Tanagami, et al. Neutronics analyses of the radiation field at the accelerator-based neutron source of Nagoya University for the BNCT study. J. Nucl. Eng. 3, 222–232 (2022). [CrossRef]
- D.L. Bleuel, R.J. Donahue, B.A. Ludewigt, J. Vujic. Designing accelerator-based epithermal neutron beams for boron neutron capture therapy. Med. Phys. 25, 1725–1734 (1998).
- S. Yonai, T. Aoki, Y. Tahara, M. Manabe, H. Hatanaka, K. Nakagawa. Feasibility study on epithermal neutron field for cyclotron-based boron neutron capture therapy. Med. Phys. 30, 2021–2030 (2003). [CrossRef]
- H. Tanaka, Y. Sakurai, M. Suzuki, et al. Improvement of dose distribution in phantom by using epithermal neutron source based on the Be(p,n) reaction using a 30 MeV proton cyclotron accelerator. Appl. Radiat. Isot. 67, S258–S261 (2009). [CrossRef]
- M. Hervé, N. Sauzet, D. Santos. On the epithermal neutron energy limit for accelerator-based BNCT: study and impact of new energy limits. Phys. Med. 88, 148–157 (2021). [CrossRef]
- N. Hu, M. Suzuki, S. Masunaga, et al. Experimentally determined relative biological effectiveness of cyclotron-based epithermal neutrons designed for clinical BNCT: in vitro study. J. Radiat. Res. 64, 811–815 (2023). [CrossRef]
- H. Shuai, E. Dian, F. Mezei, P. Sipos, S. Czifrus. An accelerator-based neutron source design with a thermal neutron port and an epithermal neutron port for boron neutron capture therapy. Appl. Radiat. Isot. 217, 111647 (2025). [CrossRef]
- N.S. Schmidt, D. Shabani, J. Li, P. Zakalek, E. Mauerhofer, J. Dawidowski, T. Gutberlet. Development of an epithermal and fast neutron target station for the High Brilliance Neutron Source. Eur. Phys. J. Plus 140, 171 (2025).
- International Commission on Radiological Protection (ICRP). Relative biological effectiveness (RBE), quality factor (Q), and radiation weighting factor (wₙ). Ann. ICRP 33, 2003.
- International Commission on Radiological Protection (ICRP). The 2007 recommendations of the International Commission on Radiological Protection. ICRP Publication 103. Ann. ICRP 37, 2–4 (2007).
- J. Choi, J.O. Kang. Basics of particle therapy II: relative biological effectiveness. Radiat. Oncol. J. 30, 1–13 (2012). [CrossRef]
- M. Pedrosa-Rivera, J. Praena, I. Porras, M.P. Sabariego, U. Köster, M. Haertlein, V.T. Forsyth, J.C. Ramírez, C. Jover, D. Jimena, et al. Thermal neutron relative biological effectiveness factors for boron neutron capture therapy from in vitro irradiations. Cells 9, 2144 (2020). [CrossRef]
- J. Rassow, W. Sauerwein, A. Wittig, E. Bourhis-Martin, K. Hideghéty, R. Moss. Advantage and limitations of weighting factors and weighted dose quantities and their units in boron neutron capture therapy. Med. Phys. 31, 1128–1134 (2004). [CrossRef]
- T. Sato, S.I. Masunaga, H. Kumada, N. Hamada. Microdosimetric modeling of biological effectiveness for boron neutron capture therapy considering intra- and intercellular heterogeneity in 10B distribution. Sci. Rep. 8, 988 (2018). [CrossRef]
- H. Fukuda. Response of normal tissues to boron neutron capture therapy (BNCT) with 10B-BSH and 10B-BPA. Cells 10, 2883 (2021). [CrossRef]
- Y. Han, C. Geng, J.N.D. Kondo, M. Li, J. Ramos Méndez, S. Altieri, Y. Liu, X. Tang. Microdosimetric analysis for boron neutron capture therapy via Monte Carlo track structure simulation with modified lithium cross sections. Radiat. Phys. Chem. 209, 110956 (2023). [CrossRef]
- S.J. González, E.C.C. Pozzi, A.M. Hughes, L. Provenzano, H. Koivunoro, D.G. Carando, S.I. Thorp, M.R. Casal, S. Bortolussi, V.A. Trivillin, et al. Photon iso-effective dose for cancer treatment with mixed field radiation based on dose-response assessment from human and an animal model: clinical application to BNCT for head and neck cancer. Phys. Med. Biol. 62, 7938–7958 (2017).
- G.F. Perotti Bernardini, S. Bortolussi, H. Koivunoro, L. Provenzano, C. Ferrari, L. Cansolino, I. Postuma, D.G. Carando, L. Kankaanranta, H. Joensuu, S.J. González. Comparison of photon isoeffective dose models based on in vitro and in vivo radiobiological experiments for head and neck cancer treated with BNCT. Radiat. Res. 198, 134–144 (2022). [CrossRef]
- Postuma, C. Magni, B. Marcaccio, et al. Using the photon isoeffective dose formalism to compare and combine BNCT and CIRT in a head and neck tumour. Sci. Rep. 14, 418 (2024). [CrossRef]
- V. Conte, A. Bianchi, A. Selva. Boron neutron capture therapy: microdosimetry at different boron concentrations. Appl. Sci. 14, 216 (2024). [CrossRef]
- A. Tedjani, R. Abdi, D. Boumala, A. Belafrites, J.-E. Groetz. Boron neutron capture therapy: dosimetric evaluation of a brain tumor and surrounding healthy tissues using Monte Carlo simulation. Nucl. Instrum. Methods Phys. Res., Sect. A 1040, 167240 (2022). [CrossRef]
- M.R. Fabbrizi, C.M. Nickson, J.R. Hughes, E.A. Robinson, K. Vaidya, C.P. Rubbi, A. Kacperek, H.E. Bryant, T. Helleday, J.L. Parsons. Targeting OGG1 and PARG radiosensitises head and neck cancer cells to high-LET protons through complex DNA damage persistence. Cell Death Dis. 15, 150 (2024). [CrossRef]
- E. Melia, J.L. Parsons. DNA damage and repair dependencies of ionising radiation modalities. Biosci. Rep. 43, BSR20222586 (2023). [CrossRef]
- G.V. Mechetin, D.O. Zharkov. DNA damage response and repair in boron neutron capture therapy. Genes 14, 127 (2023). [CrossRef]
- K. Maliszewska-Olejniczak, D. Kaniowski, M.T. Araszkiewicz, K. Tyminska, A. Korgul. Molecular mechanisms of specific cellular DNA damage response and repair induced by the mixed radiation field during boron neutron capture therapy. Front. Oncol. 11, 676575 (2021). [CrossRef]
- W.H. Jin, C. Seldon, M. Butkus, W. Sauerwein, H.B. Giap. A review of boron neutron capture therapy: its history and current challenges. Int. J. Part. Ther. 9, 71–82 (2022). [CrossRef]
- X. Li, P. He, Y. Wei, C. Qu, F. Tang, Y. Li. Application and perspectives of nanomaterials in boron neutron capture therapy of tumors. Cancer Nano 16, 25 (2025). [CrossRef]
- Y. Zhang, H.G. Kang, H.-Z. Xu, H. Luo, M. Suzuki, Q. Lan, X. Chen, N. Komatsu, L. Zhao. Tumor eradication by boron neutron capture therapy with 10B-enriched hexagonal boron nitride nanoparticles grafted with poly(glycerol). Adv. Mater. 35, e2301479 (2023). [CrossRef]
- S. Xu, Y. Yu, B. Zhang, K. Zhu, Y. Cheng, T. Zhang. Boron carbide nanoparticles for boron neutron capture therapy. RSC Adv. 15, 10717–10730 (2025). [CrossRef]
- M.J. Luderer, B. Muz, K. Alhallak, J. Sun, K. Wasden, N. Guenthner, P. de la Puente, C. Federico, A.K. Azab. Thermal sensitive liposomes improve delivery of boronated agents for boron neutron capture therapy. Pharm. Res. 36, 144 (2019).
- A.-M. Caminade, F. Rodríguez, R. Gramage-Doria. Dendritic structures functionalized with boron clusters, in view of BNCT. Pharmaceutics 15, 2117 (2023).
- W.-Y. Fu, Y.-L. Chiu, S.-C. Huang, W.-Y. Huang, F.-T. Hsu, H.-Y. Lee, T.-W. Wang, P.-Y. Keng. Boron neutron capture therapy enhanced by boronate ester polymer micelles: synthesis, stability, and tumor inhibition studies. Biomacromolecules 25, 4215–4232 (2024). [CrossRef]
- J. Xiang, L. Ma, J. Tong, N. Zuo, W. Hu, Y. Luo, J. Liu, T. Liang, Q. Liu. Boron-peptide conjugates with angiopep-2 for boron neutron capture therapy. Front. Med. 10, 1199881 (2023). [CrossRef]
- V.G.S.S. Jyothi, N. Kommineni. Peptide conjugated boron neutron capture therapy for enhanced tumor targeting. Nanotheranostics 8, 458–472 (2024). [CrossRef]
- M.A. Vorobyeva, M.A. Dymova, D.S. Novopashina, E.V. Kuligina, V.V. Timoshenko, I.A. Kolesnikov, S.Y. Taskaev, V.A. Richter, A.G. Venyaminova. Tumor cell-specific 2’-fluoro RNA aptamer conjugated with closo-dodecaborate as a potential agent for boron neutron capture therapy. Int. J. Mol. Sci. 22, 7326 (2021). [CrossRef]
- A. Rudawska, B. Szermer-Olearnik, A. Szczygieł, J. Mierzejewska, K. Węgierek-Ciura, P. Żeliszewska, D. Kozień, M. Chaszczewska-Markowska, Z. Adamczyk, P. Rusiniak, K. Wątor, A. Rapak, Z. Pędzich, E. Pajtasz-Piasecka. Functionalized boron carbide nanoparticles as active boron delivery agents dedicated to boron neutron capture therapy. Int. J. Nanomed. 20, 6637–6657 (2025). [CrossRef]
- S.O. Oloo, K.M. Smith, M.D.G.H. Vicente. Multi-functional boron-delivery agents for boron neutron capture therapy of cancers. Cancers 15, 3277 (2023). [CrossRef]
- A. Lanfranco, D. Alberti, S. Parisotto, P. Renzi, V. Lecomte, S. Geninatti Crich, A. Deagostino. Biotinylation of a MRI/Gd BNCT theranostic agent to access a novel tumour-targeted delivery system. Org. Biomol. Chem. 20, 5342–5354 (2022).
- A. Vitali, M.P. Demichelis, G. Di Martino, I. Postuma, S. Bortolussi, A. Falqui, C. Milanese, C. Ferrara, P. Sommi, U. Anselmi-Tamburini. Synthesis and characterization of Gd-functionalized B₄C nanoparticles for BNCT applications. Life 3, 429 (2023).
- Y. Hattori, T. Andoh, S. Kawabata, N. Hu, H. Michiue, H. Nakamura, T. Nomoto, M. Suzuki, T. Takata, H. Tanaka, T. Watanabe, K. Ono. Proposal of recommended experimental protocols for in vitro and in vivo evaluation methods of boron agents for neutron capture therapy. J. Radiat. Res. 64, 859–869 (2023). [CrossRef]
- Y.-T. Zhou, et al. Recent progress of nano-drugs in neutron capture therapy. Theranostics 14, 3193–3212 (2024). [CrossRef]
- F.P. McCandless, R.S. Herbst. Separation of the isotopes of boron by chemical exchange reactions. U.S. Patent 5,419,887 (1995).
- S. Song, Y. Mu, X. Li, P. Bai. Advances in boron-10 isotope separation by chemical exchange distillation. Ann. Nucl. Energy 37, 1–4 (2010). [CrossRef]
- J.-P. Li. Studies on separation process and production technology of boron isotope. J. Isotopes 27, 87–92 (2014).
- Y. Tang, Y. Zheng, J. Tian, J. Sun. Process intensification of chemical exchange method for boron isotope separation using micro-channel distillation technology. Micromachines 12, 1222 (2021). [CrossRef]
- A.V. Khoroshilov. Separation of boron isotopes by chemical exchange in liquid–liquid systems. J. Phys.: Conf. Ser. 1099, 012006 (2018).
- P.I. Ivanov, A.V. Khoroshilov, N.S. Panyukova. Experimental modeling of boron-10 isotope enrichment during chemical exchange in a liquid–liquid system. Russ. J. Phys. Chem. A 98, 2891–2896 (2024). [CrossRef]
- Y. Sakuma, M. Aida, M. Okamoto, H. Kakihana. Boron isotope separation by ion exchange chromatography using weakly basic anion exchange resin. Bull. Chem. Soc. Jpn. 53, 1860–1863 (1980).
- H. Zhao, R. Gao, P. Bai. Advances in boron isotope separation by ion exchange chromatography. Asian J. Chem. 26, 2187–2190 (2014).
- J.F. Wild, H. Chen, K. Liang, J. Liu, S.E. Cox, A.N. Halliday, Y. Yang. Liquid solution centrifugation for safe, scalable, and efficient isotope separation. Sci. Adv. 9, eadg8993 (2023). [CrossRef]
- V.S. Letokhov. Laser-induced separation of isotopes. At. Energy 62, 297–311 (1987). [CrossRef]
- F. Tang, et al. Studies of irradiation products of laser boron isotope separation by infrared absorption spectroscopy. Chin. Phys. 2, 2 (1982).
- U.S. Patent 4,447,303 (1984), University of California / U.S. Department of Energy.
- G.N. Makarov. Laser separation of boron isotopes: research results and options for technological implementation. Phys.-Uspekhi 68, 452–489 (2025). [CrossRef]
- Global Times. China achieves breakthrough in low-temperature distillation of boron-10 isotopes to support nuclear safety. October 10 (2024). Available at: https://www.ecns.cn/news/sci-tech/2024-10-10/detail-ihehxcae7641285.shtml.
- China Nuclear Energy Association (CNEA). China’s breakthrough in boron-10 isotope separation technology. 2024. Available at: https://en.china-nea.cn/site/content/47860.
- W. Zhang, T. Liu, J. Xu. Preparation and characterization of 10B boric acid with high purity for nuclear industry. SpringerPlus 5, 1202 (2016).
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).




