Submitted:
28 November 2025
Posted:
03 December 2025
You are already at the latest version
Abstract
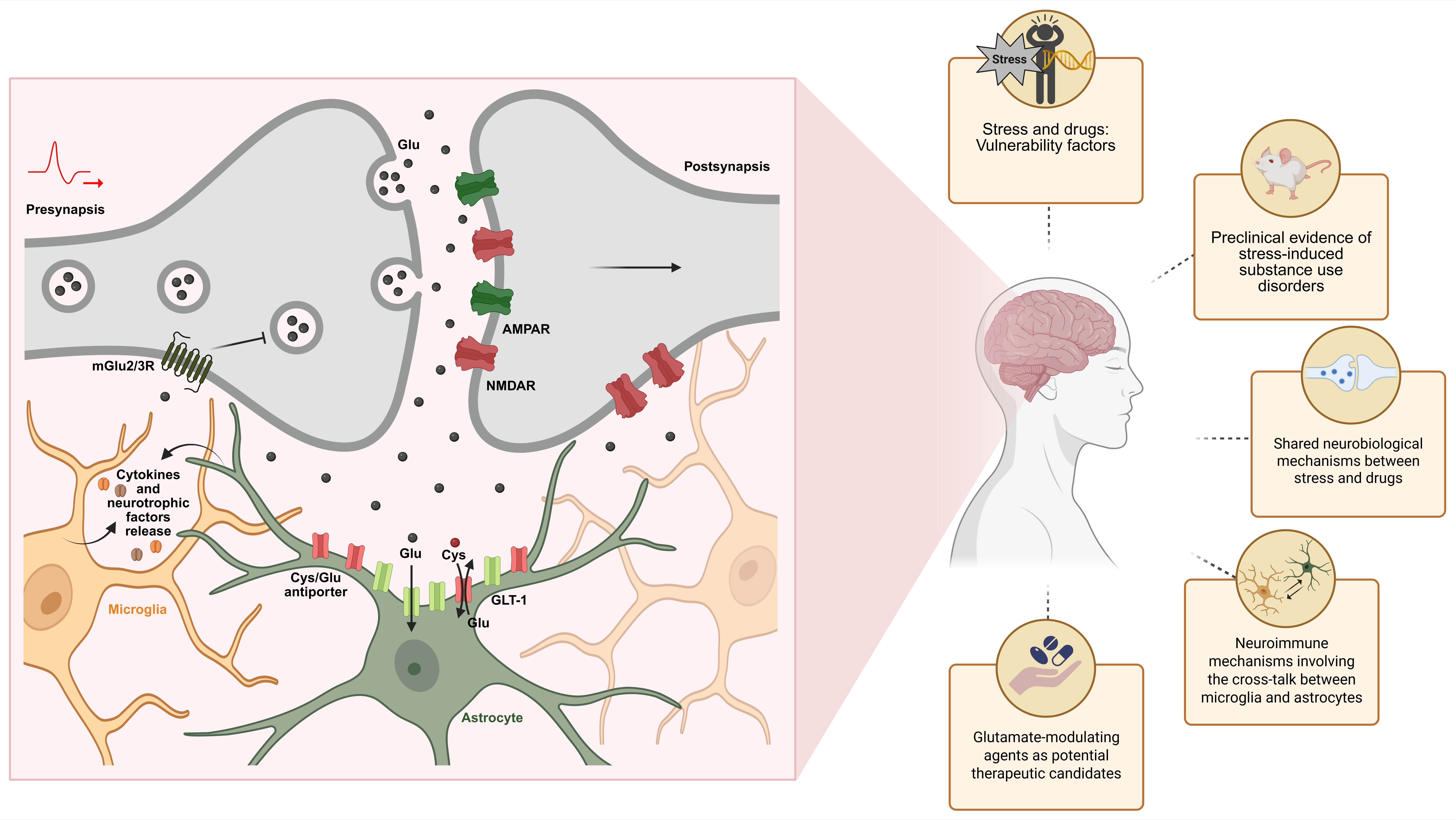
Keywords:
1. Introduction: A Neurobiological Framework for the Comorbidity of Stress and Substance Use Disorders
2. Stress as Vulnerability Factor in Substance Use Disorders: Theoretical Approaches and Interacting Risk Factors
2.1. Vulnerability Factors
2.2. Theoretical Approaches
2.3. Emergent Integrative Perspective of Substance Use Disorders
3. Motivational Neural Circuits Implicated in Stress and Substance Use Disorders Vulnerability
4. Neurobiological Mechanisms Linking Stress and Psychostimulants: Dopaminergic and Glutamatergic Interactions in the Nucleus Accumbens

5. Dysregulation of Glutamate Homeostasis in the Nucleus Accumbens Core as a Key Mechanism of Stress-Induced Cocaine Vulnerability
6. Glial Cells: Microglia and Astrocytes in Glutamate and Neuroimmune Regulation
6.1. Microglia: Characteristics, Role in Motivation Circuits, Synaptic Plasticity, Phenotypic Markers, and Activation States
6.1.1. Characteristics and Neurophysiology
6.1.2. Microglia in Motivation Circuits
6.1.3. Synaptic Activity and Plasticity
6.1.4. Phenotypic Markers
6.1.5. Activation, Priming and Morphological Changes
6.2. Astrocytes: Characteristics, Role in Motivation Circuits, Synaptic Plasticity, Neurotransmitter Regulation, and Immune Functions
6.2.1. Characteristics and Neurophysiology
6.2.2. Astrocytic Markers
6.2.3. Astrocytes in Motivation Circuits
6.2.4. Astrocytes and Synaptic Activity
7. Glial Contributions to Stress-Induced Glutamate and Neuroimmune Dysregulation in the Nucleus Accumbens Core: Microglia and Astrocyte Cross-Talk
7.1. Microglial Activation by Stress and Drugs: Neuroimmune Modulation in Mesolimbic Circuits and Peripheral Crosstalk
Stress-Induced Recruitment of Peripheral Monocytes to the Brain: Role of Corticosterone, NMDA Receptors, and IL-6 Signaling
7.2. Astrocyte Reactivity Under Stress and Drug Exposure: Glutamate and Immune Adaptations
7.3. Microglia-Astrocytes Crosstalk: Proinflammatory Signaling, GLT-1 Downregulation and Stress-Induced Vulnerability to Cocaine Use Disorder
TNF-α/NF-κB Pathway: A key Signaling Axis Driving Astrocyte–Microglia Crosstalk in Stress-Induced Cocaine Vulnerability
8. Microglia-Astrocyte Cross-Talk in the Regulation of Structural Synaptic Plasticity in the Nucleus Accumbens Core and Its Role in Stress-Induced Cocaine Vulnerability

9. Repurposing Glutamatergic Therapies for the Treatment of Substance Use Disorders Comorbidity
9.1. N-Acetylcysteine (NAC)
9.2. Ceftriaxone
9.3. Minocycline
9.4. Ampicillin/Sulbactam (AMP/SUL)
10. Conclusions
10.1. Mechanistic Synthesis
- -
- Stress-related vulnerability to cocaine use disorders arises from the interplay of HPA axis activation, neuroimmune signaling, and glial dysfunction.
- -
- Microglial TNF-α release and subsequent downregulation of astrocytic GLT-1 emerge as central pathways linking stress to glutamatergic dysregulation in the NAc core.
- -
- Glial cross-talk between microglia and astrocytes critically shapes synaptic plasticity, structural remodeling, and ultimately behavioral vulnerability to cocaine.
10.2. Weaknesses and Knowledge Gaps
- -
- Most preclinical evidence derives from male rodents; studies in females are limited despite evidence that gonadal hormones (e.g., estrogens) modulate neurobiological processes underlying stress and drug responses.
- -
- Clinical validation of glutamate-modulating drugs (minocycline, ceftriaxone, NAC, AMP/SUL) remains scarce, leaving a translational gap between promising preclinical findings and patient applications.
- -
- Integration of mechanistic rodent data with human neuroimaging and biomarker studies remains insufficient.
10.3. Future Directions
- -
- Mechanistic studies dissecting inflammatory versus non-inflammatory microglial functions, as well as peripheral–central immune crosstalk, in the comorbidity between stress and SUDs.
- -
- Systematic inclusion of females in preclinical models to capture sex-specific neuroimmune and glutamatergic adaptations.
- -
- Electrophysiological studies should determine whether the microglial and structural alterations induced by chronic stress and cocaine are accompanied by changes in neuronal excitability and synaptic transmission within the NAc, and define the temporal window and mechanisms through which microglial modulation can restore glutamatergic balance and circuit function.
- -
- Clinical trials assessing glial-targeting interventions, stratified by stress history and sex hormone status.
- -
- Early intervention strategies for stress-exposed populations aimed at modulating neuroimmune and glutamatergic mechanisms before maladaptive plasticity becomes established.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| AMP/SULB | Ampicillin/Sulbactam |
| GFAP | Glial fibrillary acidic protein |
| GLT-1 | Glutamate transporter-1 |
| IL-1β | Interleukin 1 beta |
| LTP | long-term potentiation |
| LTD | long term depression |
| mGluR2/3 | presynaptic metabotropic glutamate receptors 2/3 |
| MSNs | Medium Spiny Neurons |
| NAC | N-Acetylcysteine |
| NAc | Nucleus Accumbens |
| N-myc | N-myc proto-oncogene protein |
| PTSD | Post-Traumatic Stress Disorder |
| SUDs | Substance Use Disorders |
| TNF-α | Tumor Necrosis Factor-alpha |
References
- McEwen, B.S. Protective and Damaging Effects of the Mediators of Stress and Adaptation: Allostasis and Allostatic Load. In Allostasis, Homeostasis, and the Costs of Physiological Adaptation; Schulkin, J., Ed.; Cambridge University Press, 2004; pp. 65–98. ISBN 978-0-521-81141-5. [Google Scholar]
- McEwen, B.S. Physiology and Neurobiology of Stress and Adaptation: Central Role of the Brain. Physiological Reviews 2007, 87, 873–904. [Google Scholar] [CrossRef]
- Koob, G.F.; Volkow, N.D. Neurobiology of Addiction: A Neurocircuitry Analysis. The Lancet Psychiatry 2016, 3, 760–773. [Google Scholar] [CrossRef]
- Belin, D.; Belin-Rauscent, A.; Murray, J.E.; Everitt, B.J. Addiction: Failure of Control over Maladaptive Incentive Habits. Current Opinion in Neurobiology 2013, 23, 564–572. [Google Scholar] [CrossRef] [PubMed]
- Camí, J.; Farré, M. Drug Addiction. N Engl J Med 2003, 349, 975–986. [Google Scholar] [CrossRef] [PubMed]
- Brady, K.T.; Sinha, R. Co-Occurring Mental and Substance Use Disorders: The Neurobiological Effects of Chronic Stress. AJP 2005, 162, 1483–1493. [Google Scholar] [CrossRef]
- Breslau, N.; Davis, G.C.; Schultz, L.R. Posttraumatic Stress Disorder and the Incidence of Nicotine, Alcohol, and Other Drug Disorders in Persons Who Have Experienced Trauma. Arch Gen Psychiatry 2003, 60, 289. [Google Scholar] [CrossRef]
- Mills, K.L.; Teesson, M.; Ross, J.; Peters, L. Trauma, PTSD, and Substance Use Disorders: Findings From the Australian National Survey of Mental Health and Well-Being. AJP 2006, 163, 652–658. [Google Scholar] [CrossRef]
- Ouimette, P.; Coolhart, D.; Funderburk, J.S.; Wade, M.; Brown, P.J. Precipitants of First Substance Use in Recently Abstinent Substance Use Disorder Patients with PTSD. Addictive Behaviors 2007, 32, 1719–1727. [Google Scholar] [CrossRef]
- Piazza, P.V.; Deminiere, J.M.; Le Moal, M.; Simon, H. Stress- and Pharmacologically-Induced Behavioral Sensitization Increases Vulnerability to Acquisition of Amphetamine Self-Administration. Brain Research 1990, 514, 22–26. [Google Scholar] [CrossRef] [PubMed]
- Boyson, C.O.; Miguel, T.T.; Quadros, I.M.; DeBold, J.F.; Miczek, K.A. Prevention of Social Stress-Escalated Cocaine Self-Administration by CRF-R1 Antagonist in the Rat VTA. Psychopharmacology 2011, 218, 257–269. [Google Scholar] [CrossRef]
- Mueller, D.; Stewart, J. Cocaine-Induced Conditioned Place Preference: Reinstatement by Priming Injections of Cocaine after Extinction. Behavioural Brain Research 2000, 115, 39–47. [Google Scholar] [CrossRef] [PubMed]
- Boyson, C.O.; Holly, E.N.; Shimamoto, A.; Albrechet-Souza, L.; Weiner, L.A.; DeBold, J.F.; Miczek, K.A. Social Stress and CRF–Dopamine Interactions in the VTA: Role in Long-Term Escalation of Cocaine Self-Administration. J. Neurosci. 2014, 34, 6659–6667. [Google Scholar] [CrossRef]
- Sinha, R. How Does Stress Increase Risk of Drug Abuse and Relapse? Psychopharmacology 2001, 158, 343–359. [Google Scholar] [CrossRef]
- Sinha, R.; Garcia, M.; Paliwal, P.; Kreek, M.J.; Rounsaville, B.J. Stress-Induced Cocaine Craving and Hypothalamic-Pituitary-Adrenal Responses Are Predictive of Cocaine Relapse Outcomes. Arch Gen Psychiatry 2006, 63, 324. [Google Scholar] [CrossRef]
- Koob, G.F.; Schulkin, J. Addiction and Stress: An Allostatic View. Neuroscience & Biobehavioral Reviews 2019, 106, 245–262. [Google Scholar] [CrossRef]
- Kalivas, P.W. The Glutamate Homeostasis Hypothesis of Addiction. Nat Rev Neurosci 2009, 10, 561–572. [Google Scholar] [CrossRef]
- Myers-Schulz, B.; Koenigs, M. Functional Anatomy of Ventromedial Prefrontal Cortex: Implications for Mood and Anxiety Disorders. Mol Psychiatry 2012, 17, 132–141. [Google Scholar] [CrossRef] [PubMed]
- Woodburn, S.C.; Bollinger, J.L.; Wohleb, E.S. The Semantics of Microglia Activation: Neuroinflammation, Homeostasis, and Stress. J Neuroinflammation 2021, 18, 258. [Google Scholar] [CrossRef] [PubMed]
- Mayhew, J.; Beart, P.M.; Walker, F.R. Astrocyte and Microglial Control of Glutamatergic Signalling: A Primer on Understanding the Disruptive Role of Chronic Stress. J Neuroendocrinology 2015, 27, 498–506. [Google Scholar] [CrossRef]
- Lacagnina, M.J.; Rivera, P.D.; Bilbo, S.D. Glial and Neuroimmune Mechanisms as Critical Modulators of Drug Use and Abuse. Neuropsychopharmacol 2017, 42, 156–177. [Google Scholar] [CrossRef]
- Wang, J.; Chen, H.-S.; Li, H.-H.; Wang, H.-J.; Zou, R.-S.; Lu, X.-J.; Wang, J.; Nie, B.-B.; Wu, J.-F.; Li, S.; et al. Microglia-Dependent Excessive Synaptic Pruning Leads to Cortical Underconnectivity and Behavioral Abnormality Following Chronic Social Defeat Stress in Mice. Brain, Behavior, and Immunity 2023, 109, 23–36. [Google Scholar] [CrossRef]
- Liddelow, S.A.; Guttenplan, K.A.; Clarke, L.E.; Bennett, F.C.; Bohlen, C.J.; Schirmer, L.; Bennett, M.L.; Münch, A.E.; Chung, W.-S.; Peterson, T.C.; et al. Neurotoxic Reactive Astrocytes Are Induced by Activated Microglia. Nature 2017, 541, 481–487. [Google Scholar] [CrossRef]
- Lewitus, G.M.; Konefal, S.C.; Greenhalgh, A.D.; Pribiag, H.; Augereau, K.; Stellwagen, D. Microglial TNF-α Suppresses Cocaine-Induced Plasticity and Behavioral Sensitization. Neuron 2016, 90, 483–491. [Google Scholar] [CrossRef]
- Robinson, T. The Neural Basis of Drug Craving: An Incentive-Sensitization Theory of Addiction. Brain Research Reviews 1993, 18, 247–291. [Google Scholar] [CrossRef]
- White, F.J.; Kalivas, P.W. Neuroadaptations Involved in Amphetamine and Cocaine Addiction. Drug and Alcohol Dependence 1998, 51, 141–153. [Google Scholar] [CrossRef]
- Nestler, E.J.; Aghajanian, G.K. Molecular and Cellular Basis of Addiction. Science 1997, 278, 58–63. [Google Scholar] [CrossRef]
- Kreek, M.J.; Nielsen, D.A.; Butelman, E.R.; LaForge, K.S. Genetic Influences on Impulsivity, Risk Taking, Stress Responsivity and Vulnerability to Drug Abuse and Addiction. Nat Neurosci 2005, 8, 1450–1457. [Google Scholar] [CrossRef] [PubMed]
- Müller, D.J.; Likhodi, O.; Heinz, A. Neural Markers of Genetic Vulnerability to Drug Addiction. In Behavioral Neuroscience of Drug Addiction;Current Topics in Behavioral Neurosciences; Self, D.W., Staley Gottschalk, J.K., Eds.; Springer Berlin Heidelberg: Berlin, Heidelberg, 2010; Vol. 3, pp. 277–299. ISBN 978-3-642-03000-0. [Google Scholar]
- Sinha, R. Stress and Addiction: A Dynamic Interplay of Genes, Environment, and Drug Intake. Biological Psychiatry 2009, 66, 100–101. [Google Scholar] [CrossRef] [PubMed]
- Swendsen, J.; Conway, K.P.; Degenhardt, L.; Glantz, M.; Jin, R.; Merikangas, K.R.; Sampson, N.; Kessler, R.C. Mental Disorders as Risk Factors for Substance Use, Abuse and Dependence: Results from the 10-year Follow-up of the National Comorbidity Survey. Addiction 2010, 105, 1117–1128. [Google Scholar] [CrossRef] [PubMed]
- Boden, J.M.; Fergusson, D.M.; Horwood, L.J. Alcohol Misuse and Violent Behavior: Findings from a 30-Year Longitudinal Study. Drug and Alcohol Dependence 2012, 122, 135–141. [Google Scholar] [CrossRef]
- Clark, H.W.; Masson, C.L.; Delucchi, K.L.; Hall, S.M.; Sees, K.L. Violent Traumatic Events and Drug Abuse Severity. Journal of Substance Abuse Treatment 2001, 20, 121–127. [Google Scholar] [CrossRef]
- Montoya, I.D.; Covarrubias, L.D.; Patek, J.A.; Graves, J.A. Posttraumatic Stress Disorder Among Hispanic and African-American Drug Users. The American Journal of Drug and Alcohol Abuse 2003, 29, 729–741. [Google Scholar] [CrossRef]
- Seal, K.H. Bringing the War Back Home: Mental Health Disorders Among 103 788 US Veterans Returning From Iraq and Afghanistan Seen at Department of Veterans Affairs Facilities. Arch Intern Med 2007, 167, 476. [Google Scholar] [CrossRef] [PubMed]
- Seal, K.H.; Metzler, T.J.; Gima, K.S.; Bertenthal, D.; Maguen, S.; Marmar, C.R. Trends and Risk Factors for Mental Health Diagnoses Among Iraq and Afghanistan Veterans Using Department of Veterans Affairs Health Care, 2002–2008. Am J Public Health 2009, 99, 1651–1658. [Google Scholar] [CrossRef]
- Tuerk, P.W.; Grubaugh, A.L.; Hamner, M.B.; Foa, E.B. Diagnosis and Treatment of PTSD-Related Compulsive Checking Behaviors in Veterans of the Iraq War: The Influence of Military Context on the Expression of PTSD Symptoms. AJP 2009, 166, 762–767. [Google Scholar] [CrossRef] [PubMed]
- Ullman, S.E.; Relyea, M.; Peter-Hagene, L.; Vasquez, A.L. Trauma Histories, Substance Use Coping, PTSD, and Problem Substance Use among Sexual Assault Victims. Addictive Behaviors 2013, 38, 2219–2223. [Google Scholar] [CrossRef]
- Kalivas, P.W.; Volkow, N.D. The Neural Basis of Addiction: A Pathology of Motivation and Choice. AJP 2005, 162, 1403–1413. [Google Scholar] [CrossRef]
- O’Brien, C.P.; Childress, A.R.; Ehrman, R.; Robbins, S.J. Conditioning Factors in Drug Abuse: Can They Explain Compulsion? J Psychopharmacol 1998, 12, 15–22. [Google Scholar] [CrossRef] [PubMed]
- Nestler, E. Molecular Mechanisms of Drug Addiction [Published Erratum Appears in J Neurosci 1992 Aug;12(8):Following Table of Contents]. J. Neurosci. 1992, 12, 2439–2450. [Google Scholar] [CrossRef]
- White, N.M. Addictive Drugs as Reinforcers: Multiple Partial Actions on Memory Systems. Addiction 1996, 91, 921–949; discussion 951-965. [Google Scholar] [CrossRef]
- Nestler, E.J.; Aghajanian, G.K. Molecular and Cellular Basis of Addiction. Science 1997, 278, 58–63. [Google Scholar] [CrossRef]
- Nestler, E.J. Molecular Neurobiology of Addiction. American J Addict 2001, 10, 201–217. [Google Scholar] [CrossRef]
- Deminiere, J.M.; Piazza, P.V.; Le Moal, M.; Simon, H. Experimental Approach to Individual Vulnerability to Psychostimulant Addiction. Neuroscience & Biobehavioral Reviews 1989, 13, 141–147. [Google Scholar] [CrossRef]
- Piazza, P.V.; Le Moal, M. The Role of Stress in Drug Self-Administration. Trends in Pharmacological Sciences 1998, 19, 67–74. [Google Scholar] [CrossRef] [PubMed]
- Koob, G.F.; Le Moal, M. Plasticity of Reward Neurocircuitry and the “dark Side” of Drug Addiction. Nat Neurosci 2005, 8, 1442–1444. [Google Scholar] [CrossRef] [PubMed]
- Mews, P.; Cunningham, A.M.; Scarpa, J.; Ramakrishnan, A.; Hicks, E.M.; Bolnick, S.; Garamszegi, S.; Shen, L.; Mash, D.C.; Nestler, E.J. Convergent Abnormalities in Striatal Gene Networks in Human Cocaine Use Disorder and Mouse Cocaine Administration Models. Sci. Adv. 2023, 9, eadd8946. [Google Scholar] [CrossRef] [PubMed]
- Koob, G.F.; Buck, C.L.; Cohen, A.; Edwards, S.; Park, P.E.; Schlosburg, J.E.; Schmeichel, B.; Vendruscolo, L.F.; Wade, C.L.; Whitfield, T.W.; et al. Addiction as a Stress Surfeit Disorder. Neuropharmacology 2014, 76, 370–382. [Google Scholar] [CrossRef]
- Nestler, E.J.; Lüscher, C. The Molecular Basis of Drug Addiction: Linking Epigenetic to Synaptic and Circuit Mechanisms. Neuron 2019, 102, 48–59. [Google Scholar] [CrossRef]
- Browne, C.J.; Futamura, R.; Minier-Toribio, A.; Hicks, E.M.; Ramakrishnan, A.; Martínez-Rivera, F.J.; Estill, M.; Godino, A.; Parise, E.M.; Torres-Berrío, A.; et al. Transcriptional Signatures of Heroin Intake and Relapse throughout the Brain Reward Circuitry in Male Mice. Sci. Adv. 2023, 9, eadg8558. [Google Scholar] [CrossRef]
- Kalivas, P.W.; Stewart, J. Dopamine Transmission in the Initiation and Expression of Drug- and Stress-Induced Sensitization of Motor Activity. Brain Research Reviews 1991, 16, 223–244. [Google Scholar] [CrossRef]
- Berridge, K.C.; Robinson, T.E. What Is the Role of Dopamine in Reward: Hedonic Impact, Reward Learning, or Incentive Salience? Brain Research Reviews 1998, 28, 309–369. [Google Scholar] [CrossRef]
- Koob, G.F.; Bloom, F.E. Cellular and Molecular Mechanisms of Drug Dependence. Science 1988, 242, 715–723. [Google Scholar] [CrossRef]
- Wise, R.A. Addictive Drugs and Brain Stimulation Reward. Annu. Rev. Neurosci. 1996, 19, 319–340. [Google Scholar] [CrossRef] [PubMed]
- Pierce, R.C.; Kalivas, P.W. A Circuitry Model of the Expression of Behavioral Sensitization to Amphetamine-like Psychostimulants. Brain Research Reviews 1997, 25, 192–216. [Google Scholar] [CrossRef]
- Pierce, R.C.; Kalivas, P.W. Repeated Cocaine Modifies the Mechanism by Which Amphetamine Releases Dopamine. J. Neurosci. 1997, 17, 3254–3261. [Google Scholar] [CrossRef]
- Robinson, T. The Neural Basis of Drug Craving: An Incentive-Sensitization Theory of Addiction. Brain Research Reviews 1993, 18, 247–291. [Google Scholar] [CrossRef]
- Robinson, T.E.; Berridge, K.C. Incentive-sensitization and Addiction. Addiction 2001, 96, 103–114. [Google Scholar] [CrossRef] [PubMed]
- Marinelli, M.; Le Moal, M.; Piazza, P.V. Sensitization to the Motor Effects of Contingent Infusions of Heroin but Not of κ Agonist RU 51599. Psychopharmacology 1998, 139, 281–285. [Google Scholar] [CrossRef] [PubMed]
- Scofield, M.D.; Heinsbroek, J.A.; Gipson, C.D.; Kupchik, Y.M.; Spencer, S.; Smith, A.C.W.; Roberts-Wolfe, D.; Kalivas, P.W. The Nucleus Accumbens: Mechanisms of Addiction across Drug Classes Reflect the Importance of Glutamate Homeostasis. Pharmacological Reviews 2016, 68, 816–871. [Google Scholar] [CrossRef]
- Nall, R.W.; Heinsbroek, J.A.; Nentwig, T.B.; Kalivas, P.W.; Bobadilla, A. Circuit Selectivity in Drug versus Natural Reward Seeking Behaviors. Journal of Neurochemistry 2021, 157, 1450–1472. [Google Scholar] [CrossRef]
- Heimer, L.; Zahm, D.S.; Churchill, L.; Kalivas, P.W.; Wohltmann, C. Specificity in the Projection Patterns of Accumbal Core and Shell in the Rat. Neuroscience 1991, 41, 89–125. [Google Scholar] [CrossRef]
- Kelley, A.E. Ventral Striatal Control of Appetitive Motivation: Role in Ingestive Behavior and Reward-Related Learning. Neuroscience & Biobehavioral Reviews 2004, 27, 765–776. [Google Scholar] [CrossRef]
- Heimer, L.; Alheid, G.F.; de Olmos, J.S.; Groenewegen, H.J.; Haber, S.N.; Harlan, R.E.; Zahm, D.S. The Accumbens: Beyond the Core-Shell Dichotomy. J Neuropsychiatry Clin Neurosci 1997, 9, 354–381. [Google Scholar] [CrossRef]
- Ito, R.; Robbins, T.W.; Everitt, B.J. Differential Control over Cocaine-Seeking Behavior by Nucleus Accumbens Core and Shell. Nat Neurosci 2004, 7, 389–397. [Google Scholar] [CrossRef] [PubMed]
- Bassareo, V.; Di Chiara, G. Differential Responsiveness of Dopamine Transmission to Food-Stimuli in Nucleus Accumbens Shell/Core Compartments. Neuroscience 1999, 89, 637–641. [Google Scholar] [CrossRef] [PubMed]
- Sellings, L.H.L.; Clarke, P.B.S. Segregation of Amphetamine Reward and Locomotor Stimulation between Nucleus Accumbens Medial Shell and Core. J. Neurosci. 2003, 23, 6295–6303. [Google Scholar] [CrossRef]
- Zahm, D.S.; Brog, J.S. On the Significance of Subterritories in the “Accumbens” Part of the Rat Ventral Striatum. Neuroscience 1992, 50, 751–767. [Google Scholar] [CrossRef] [PubMed]
- Di Ciano, P.; Cardinal, R.N.; Cowell, R.A.; Little, S.J.; Everitt, B.J. Differential Involvement of NMDA, AMPA/Kainate, and Dopamine Receptors in the Nucleus Accumbens Core in the Acquisition and Performance of Pavlovian Approach Behavior. J. Neurosci. 2001, 21, 9471–9477. [Google Scholar] [CrossRef]
- Di Chiara, G. Nucleus Accumbens Shell and Core Dopamine: Differential Role in Behavior and Addiction. Behavioural Brain Research 2002, 137, 75–114. [Google Scholar] [CrossRef]
- Meredith, G.E.; Baldo, B.A.; Andrezjewski, M.E.; Kelley, A.E. The Structural Basis for Mapping Behavior onto the Ventral Striatum and Its Subdivisions. Brain Struct Funct 2008, 213, 17–27. [Google Scholar] [CrossRef]
- McFarland, K.; Lapish, C.C.; Kalivas, P.W. Prefrontal Glutamate Release into the Core of the Nucleus Accumbens Mediates Cocaine-Induced Reinstatement of Drug-Seeking Behavior. J. Neurosci. 2003, 23, 3531–3537. [Google Scholar] [CrossRef]
- Kalivas, P.W.; Churchill, L.; Klitenick, M.A. The Circuitry Mediating the Translation of Motivational Stimuli Into Adaptive Motor Responses. In Limbic Motor Circuits and Neuropsychiatry; Kalivas, P.W., Barnes, C.D., Eds.; CRC Press, 2019; pp. 237–288. ISBN 978-0-429-27441-1. [Google Scholar]
- Kalivas, P.W. Neurotransmitter Regulation of Dopamine Neurons in the Ventral Tegmental Area. Brain Research Reviews 1993, 18, 75–113. [Google Scholar] [CrossRef]
- Koob, G.F.; Nestler, E.J. The Neurobiology of Drug Addiction. J Neuropsychiatry Clin Neurosci 1997, 9, 482–497. [Google Scholar] [CrossRef]
- Koob, G.F.; Moal, M.L. Drug Abuse: Hedonic Homeostatic Dysregulation. Science 1997, 278, 52–58. [Google Scholar] [CrossRef] [PubMed]
- Nestler, E.J. Is There a Common Molecular Pathway for Addiction? Nat Neurosci 2005, 8, 1445–1449. [Google Scholar] [CrossRef] [PubMed]
- Volkow, N.D.; Wang, G.-J.; Fowler, J.S.; Tomasi, D.; Telang, F. Addiction: Beyond Dopamine Reward Circuitry. Proc. Natl. Acad. Sci. U.S.A. 2011, 108, 15037–15042. [Google Scholar] [CrossRef]
- Di Chiara, G.; Acquas, E.; Tanda, G.; Cadoni, C. Drugs of Abuse: Biochemical Surrogates of Specific Aspects of Natural Reward? Biochem Soc Symp 1993, 59, 65–81. [Google Scholar]
- Kalivas, P.W.; Duffy, P. Effect of Acute and Daily Cocaine Treatment on Extracellular Dopamine in the Nucleus Accumbens. Synapse 1990, 5, 48–58. [Google Scholar] [CrossRef]
- Sinha, R. Chronic Stress, Drug Use, and Vulnerability to Addiction. Annals of the New York Academy of Sciences 2008, 1141, 105–130. [Google Scholar] [CrossRef]
- Lu, L.; Shepard, J.D.; Scott Hall, F.; Shaham, Y. Effect of Environmental Stressors on Opiate and Psychostimulant Reinforcement, Reinstatement and Discrimination in Rats: A Review. Neuroscience & Biobehavioral Reviews 2003, 27, 457–491. [Google Scholar] [CrossRef] [PubMed]
- Nikulina, E.M.; Covington, H.E.; Ganschow, L.; Hammer, R.P.; Miczek, K.A. Long-Term Behavioral and Neuronal Cross-Sensitization to Amphetamine Induced by Repeated Brief Social Defeat Stress: Fos in the Ventral Tegmental Area and Amygdala. Neuroscience 2004, 123, 857–865. [Google Scholar] [CrossRef]
- Miczek, K.A.; Nikulina, E.M.; Shimamoto, A.; Covington, H.E. Escalated or Suppressed Cocaine Reward, Tegmental BDNF, and Accumbal Dopamine Caused by Episodic versus Continuous Social Stress in Rats. Journal of Neuroscience 2011, 31, 9848–9857. [Google Scholar] [CrossRef]
- Garcia-Keller, C.; Kupchik, Y.M.; Gipson, C.D.; Brown, R.M.; Spencer, S.; Bollati, F.; Esparza, M.A.; Roberts-Wolfe, D.J.; Heinsbroek, J.A.; Bobadilla, A.C.; et al. Glutamatergic Mechanisms of Comorbidity between Acute Stress and Cocaine Self-Administration. Molecular Psychiatry 2016, 21, 1063–1069. [Google Scholar] [CrossRef]
- Avalos, M.P.; Guzman, A.S.; Rigoni, D.; Gorostiza, E.A.; Sanchez, M.A.; Mongi-Bragato, B.; Garcia-Keller, C.; Perassi, E.M.; Virgolini, M.B.; Peralta Ramos, J.M.; et al. Minocycline Prevents Chronic Restraint Stress-Induced Vulnerability to Developing Cocaine Self-Administration and Associated Glutamatergic Mechanisms: A Potential Role of Microglia. Brain, Behavior, and Immunity 2022, 101, 359–376. [Google Scholar] [CrossRef]
- Shaham, Y.; Erb, S.; Stewart, J. Stress-Induced Relapse to Heroin and Cocaine Seeking in Rats: A Review. Brain Research Reviews 2000, 33, 13–33. [Google Scholar] [CrossRef]
- Goeders, N.E. Stress and Cocaine Addiction. Journal of Pharmacology and Experimental Therapeutics 2002, 301, 785–789. [Google Scholar] [CrossRef]
- Mantsch, J.R.; Baker, D.A.; Funk, D.; Lê, A.D.; Shaham, Y. Stress-Induced Reinstatement of Drug Seeking: 20 Years of Progress. Neuropsychopharmacol 2016, 41, 335–356. [Google Scholar] [CrossRef] [PubMed]
- Koob, G.F. Stress, Corticotropin-Releasing Factor, and Drug Addiction. Annals of the New York Academy of Sciences 1999, 897, 27–45. [Google Scholar] [CrossRef] [PubMed]
- Koob, G.F.; Zorrilla, E.P. Neurobiological Mechanisms of Addiction: Focus on Corticotropin-Releasing Factor. Curr Opin Investig Drugs 2010, 11, 63–71. [Google Scholar] [PubMed]
- Blacktop, J.M.; Seubert, C.; Baker, D.A.; Ferda, N.; Lee, G.; Graf, E.N.; Mantsch, J.R. Augmented Cocaine Seeking in Response to Stress or CRF Delivered into the Ventral Tegmental Area Following Long-Access Self-Administration Is Mediated by CRF Receptor Type 1 But Not CRF Receptor Type 2. J. Neurosci. 2011, 31, 11396–11403. [Google Scholar] [CrossRef]
- Wang, B.; Shaham, Y.; Zitzman, D.; Azari, S.; Wise, R.A.; You, Z.-B. Cocaine Experience Establishes Control of Midbrain Glutamate and Dopamine by Corticotropin-Releasing Factor: A Role in Stress-Induced Relapse to Drug Seeking. J. Neurosci. 2005, 25, 5389–5396. [Google Scholar] [CrossRef]
- Bale, T.L.; Vale, W.W. CRF and CRF Receptors: Role in Stress Responsivity and Other Behaviors. Annu. Rev. Pharmacol. Toxicol. 2004, 44, 525–557. [Google Scholar] [CrossRef]
- Swanson, L.W.; Sawchenko, P.E.; Rivier, J.; Vale, W.W. Organization of Ovine Corticotropin-Releasing Factor Immunoreactive Cells and Fibers in the Rat Brain: An Immunohistochemical Study. Neuroendocrinology 1983, 36, 165–186. [Google Scholar] [CrossRef] [PubMed]
- Sawchenko, P.E.; Imaki, T.; Potter, E.; Kovács, K.; Imaki, J.; Vale, W. The Functional Neuroanatomy of Corticotropin-Releasing Factor. Ciba Found Symp discussion 21-29. 1993, 172, 5–21. [Google Scholar] [CrossRef]
- Dunn, A.J.; Berridge, C.W.; Lai, Y.I.; Yachabach, T.L. CRF-Induced Excessive Grooming Behavior in Rats and Mice. Peptides 1987, 8, 841–844. [Google Scholar] [CrossRef] [PubMed]
- Koob, G.F.; Heinrichs, S.C. A Role for Corticotropin Releasing Factor and Urocortin in Behavioral Responses to Stressors. Brain Research 1999, 848, 141–152. [Google Scholar] [CrossRef]
- Koob, G.F.; Weiss, F. Pharmacology of Drug Self-Administration. Alcohol 1990, 7, 193–197. [Google Scholar] [CrossRef] [PubMed]
- Spanagel, R.; Weiss, F. The Dopamine Hypothesis of Reward: Past and Current Status. Trends in Neurosciences 1999, 22, 521–527. [Google Scholar] [CrossRef]
- Saal, D; Dong, Y; Bonci, A; Malenka, RC. Drugs of Abuse and Stress Trigger a Common Synaptic Adaptation in Dopamine Neurons. Neuron 2003, 37, 577–582. [Google Scholar] [CrossRef]
- Wanat, M.J.; Hopf, F.W.; Stuber, G.D.; Phillips, P.E.M.; Bonci, A. Corticotropin-releasing Factor Increases Mouse Ventral Tegmental Area Dopamine Neuron Firing through a Protein Kinase C-dependent Enhancement ofIh. The Journal of Physiology 2008, 586, 2157–2170. [Google Scholar] [CrossRef]
- Kalivas, P.W.; Duffy, P.; Latimer, L.G. Neurochemical and Behavioral Effects of Corticotropin-Releasing Factor in the Ventral Tegmental Area of the Rat. J Pharmacol Exp Ther 1987, 242, 757–763. [Google Scholar] [CrossRef] [PubMed]
- Refojo, D.; Schweizer, M.; Kuehne, C.; Ehrenberg, S.; Thoeringer, C.; Vogl, A.M.; Dedic, N.; Schumacher, M.; Von Wolff, G.; Avrabos, C.; et al. Glutamatergic and Dopaminergic Neurons Mediate Anxiogenic and Anxiolytic Effects of CRHR1. Science 2011, 333, 1903–1907. [Google Scholar] [CrossRef]
- Antelman, S.M.; Eichler, A.J.; Black, C.A.; Kocan, D. Interchangeability of Stress and Amphetamine in Sensitization. Science 1980, 207, 329–331. [Google Scholar] [CrossRef] [PubMed]
- Piazza, P.Vincenzo.; Deminière, J.-Marie.; Le Moal, Michel.; Simon, Hervé. Factors That Predict Individual Vulnerability to Amphetamine Self-Administration. Science 1989, 245, 1511–1513. [Google Scholar] [CrossRef]
- Mantsch, J.; Ho, A.; Schlussman, S.; Kreek, M. Predictable Individual Differences in the Initiation of Cocaine Self-Administration by Rats under Extended-Access Conditions Are Dose-Dependent. Psychopharmacology 2001, 157, 31–39. [Google Scholar] [CrossRef]
- Piazza, P.V.; Maccari, S.; Deminière, J.M.; Le Moal, M.; Mormède, P.; Simon, H. Corticosterone Levels Determine Individual Vulnerability to Amphetamine Self-Administration. Proc. Natl. Acad. Sci. U.S.A. 1991, 88, 2088–2092. [Google Scholar] [CrossRef]
- Deroche, V.; Piazza, P.V.; Casolini, P.; Maccari, S.; Le Moal, M.; Simon, H. Stress-Induced Sensitization to Amphetamine and Morphine Psychomotor Effects Depend on Stress-Induced Corticosterone Secretion. Brain Research 1992, 598, 343–348. [Google Scholar] [CrossRef] [PubMed]
- Deroche, V.; Piazza, P.V.; Maccari, S.; Le Moal, M.; Simon, H. Repeated Corticosterone Administration Sensitizes the Locomotor Response to Amphetamine. Brain Research 1992, 584, 309–313. [Google Scholar] [CrossRef]
- Mantsch, J.R.; Saphier, D.; Goeders, N.E. Corticosterone Facilitates the Acquisition of Cocaine Self-Administration in Rats: Opposite Effects of the Type II Glucocorticoid Receptor Agonist Dexamethasone. J Pharmacol Exp Ther 1998, 287, 72–80. [Google Scholar] [CrossRef]
- Goeders, N.E.; Guerin, G.F. Non-Contingent Electric Footshock Facilitates the Acquisition of Intravenous Cocaine Self-Administration in Rats. Psychopharmacology 1994, 114, 63–70. [Google Scholar] [CrossRef]
- Capriles, N.; Cancela, L.M. Effect of Acute and Chronic Stress Restraint on Amphetamine-Associated Place Preference: Involvement of Dopamine D1 and D2 Receptors. European Journal of Pharmacology 1999, 386, 127–134. [Google Scholar] [CrossRef]
- De Giovanni, L.N.; Guzman, A.S.; Virgolini, M.B.; Cancela, L.M. NMDA Antagonist MK 801 in Nucleus Accumbens Core but Not Shell Disrupts the Restraint Stress-Induced Reinstatement of Extinguished Cocaine-Conditioned Place Preference in Rats. Behavioural Brain Research 2016, 315, 150–159. [Google Scholar] [CrossRef]
- Guzman, A.S.; Avalos, M.P.; De Giovanni, L.N.; Euliarte, P.V.; Sanchez, M.A.; Mongi-Bragato, B.; Rigoni, D.; Bollati, F.A.; Virgolini, M.B.; Cancela, L.M. CB1R Activation in Nucleus Accumbens Core Promotes Stress-Induced Reinstatement of Cocaine Seeking by Elevating Extracellular Glutamate in a Drug-Paired Context. Sci Rep 2021, 11, 12964. [Google Scholar] [CrossRef]
- Shaham, Y.; Shalev, U.; Lu, L.; De Wit, H.; Stewart, J. The Reinstatement Model of Drug Relapse: History, Methodology and Major Findings. Psychopharmacology 2003, 168, 3–20. [Google Scholar] [CrossRef]
- Covington, H.; Miczek, K. Repeated Social-Defeat Stress, Cocaine or Morphine. Psychopharmacology 2001, 158, 388–398. [Google Scholar] [CrossRef] [PubMed]
- Miczek, K.; Yap, J.; Covingtoniii, H. Social Stress, Therapeutics and Drug Abuse: Preclinical Models of Escalated and Depressed Intake. Pharmacology & Therapeutics 2008, 120, 102–128. [Google Scholar] [CrossRef] [PubMed]
- Engeln, M.; Fox, M.E.; Lobo, M.K. Housing Conditions during Self-Administration Determine Motivation for Cocaine in Mice Following Chronic Social Defeat Stress. Psychopharmacology 2021, 238, 41–54. [Google Scholar] [CrossRef] [PubMed]
- Mantsch, J.R.; Katz, E.S. Elevation of Glucocorticoids Is Necessary but Not Sufficient for the Escalation of Cocaine Self-Administration by Chronic Electric Footshock Stress in Rats. Neuropsychopharmacol 2007, 32, 367–376. [Google Scholar] [CrossRef]
- Mantsch, J.R.; Yuferov, V.; Mathieu-Kia, A.-M.; Ho, A.; Kreek, M.J. Neuroendocrine Alterations in a High-Dose, Extended-Access Rat Self-Administration Model of Escalating Cocaine Use. Psychoneuroendocrinology 2003, 28, 836–862. [Google Scholar] [CrossRef]
- Vezina, P.; Giovino, A.A.; Wise, R.A.; Stewart, J. Environment-Specific Cross-Sensitization between the Locomotor Activating Effects of Morphine and Amphetamine. Pharmacology Biochemistry and Behavior 1989, 32, 581–584. [Google Scholar] [CrossRef]
- Borowsky, B.; Kuhn, C.M. Chronic Cocaine Administration Sensitizes Behavioral but Not Neuroendocrine Responses. Brain Research 1991, 543, 301–306. [Google Scholar] [CrossRef]
- Cadoni, C.; Di Chiara, G. Differential Changes in Accumbens Shell and Core Dopamine in Behavioral Sensitization to Nicotine. European Journal of Pharmacology 2000, 387, R23–R25. [Google Scholar] [CrossRef]
- Cadoni, C.; Pisanu, A.; Solinas, M.; Acquas, E.; Chiara, G. Behavioural Sensitization after Repeated Exposure to Δ 9 -Tetrahydrocannabinol and Cross-Sensitization with Morphine. Psychopharmacology 2001, 158, 259–266. [Google Scholar] [CrossRef]
- Quadros, P.S.; Pfau, J.L.; Goldstein, A.Y.N.; De Vries, G.J.; Wagner, C.K. Sex Differences in Progesterone Receptor Expression: A Potential Mechanism for Estradiol-Mediated Sexual Differentiation. Endocrinology 2002, 143, 3727–3739. [Google Scholar] [CrossRef]
- Paulson, P.E.; Robinson, T.E. Amphetamine-Induced Time-dependent Sensitization of Dopamine Neurotransmission in the Dorsal and Ventral Striatum: A Microdialysis Study in Behaving Rats. Synapse 1995, 19, 56–65. [Google Scholar] [CrossRef] [PubMed]
- Post, R.M.; Weiss, S.R. Psychomotor Stimulant vs. Local Anesthetic Effects of Cocaine: Role of Behavioral Sensitization and Kindling. NIDA Res Monogr 1988, 88, 217–238. [Google Scholar]
- Valjent, E.; Bertran-Gonzalez, J.; Aubier, B.; Greengard, P.; Hervé, D.; Girault, J.-A. Mechanisms of Locomotor Sensitization to Drugs of Abuse in a Two-Injection Protocol. Neuropsychopharmacol 2010, 35, 401–415. [Google Scholar] [CrossRef]
- Esparza, M.A.; Bollati, F.; Garcia-Keller, C.; Virgolini, M.B.; Lopez, L.M.; Brusco, A.; Shen, H.-W.; Kalivas, P.W.; Cancela, L.M. Stress-Induced Sensitization to Cocaine: Actin Cytoskeleton Remodeling within Mesocorticolimbic Nuclei. European Journal of Neuroscience 2012, 36. [Google Scholar] [CrossRef]
- Garcia-Keller, C.; Martinez, S.A.A.; Esparza, M.A.A.; Bollati, F.; Kalivas, P.W.W.; Cancela, L.M.M. Cross-Sensitization between Cocaine and Acute Restraint Stress Is Associated with Sensitized Dopamine but Not Glutamate Release in the Nucleus Accumbens. European Journal of Neuroscience 2013, 37, 982–995. [Google Scholar] [CrossRef] [PubMed]
- Deroche, V.; Marinelli, M.; Maccari, S.; Le Moal, M.; Simon, H.; Piazza, P. Stress-Induced Sensitization and Glucocorticoids. I. Sensitization of Dopamine-Dependent Locomotor Effects of Amphetamine and Morphine Depends on Stress-Induced Corticosterone Secretion. J. Neurosci. 1995, 15, 7181–7188. [Google Scholar] [CrossRef] [PubMed]
- Pacchioni, A.M.; Cador, M.; Bregonzio, C.; Cancela, L.M. A Glutamate–Dopamine Interaction in the Persistent Enhanced Response to Amphetamine in Nucleus Accumbens Core but Not Shell Following a Single Restraint Stress. Neuropsychopharmacol 2007, 32, 682–692. [Google Scholar] [CrossRef]
- Kõiv, K.; Vares, M.; Kroon, C.; Metelitsa, M.; Tiitsaar, K.; Laugus, K.; Jaako, K.; Harro, J. Effect of Chronic Variable Stress on Sensitization to Amphetamine in High and Low Sucrose-Consuming Rats. J Psychopharmacol 2019, 33, 1512–1523. [Google Scholar] [CrossRef] [PubMed]
- Sorg, B.A.; Kalivas, P.W. Effects of Cocaine and Footshock Stress on Extracellular Dopamine Levels in the Ventral Striatum. Brain Research 1991, 559, 29–36. [Google Scholar] [CrossRef]
- Kippin, T.E.; Szumlinski, K.K.; Kapasova, Z.; Rezner, B.; See, R.E. Prenatal Stress Enhances Responsiveness to Cocaine. Neuropsychopharmacol 2008, 33, 769–782. [Google Scholar] [CrossRef]
- Di Chiara, G. The Role of Dopamine in Drug Abuse Viewed from the Perspective of Its Role in Motivation. Drug and Alcohol Dependence 1995, 38, 95–137. [Google Scholar] [CrossRef]
- Robinson, T.E.; Jurson, P.A.; Bennett, J.A.; Bentgen, K.M. Persistent Sensitization of Dopamine Neurotransmission in Ventral Striatum (Nucleus Accumbens) Produced by Prior Experience with (+)-Amphetamine: A Microdialysis Study in Freely Moving Rats. Brain Research 1988, 462, 211–222. [Google Scholar] [CrossRef] [PubMed]
- Bonci, A.; Malenka, R.C. Properties and Plasticity of Excitatory Synapses on Dopaminergic and GABAergic Cells in the Ventral Tegmental Area. J. Neurosci. 1999, 19, 3723–3730. [Google Scholar] [CrossRef] [PubMed]
- Hahn, J.; Hopf, F.W.; Bonci, A. Chronic Cocaine Enhances Corticotropin-Releasing Factor-Dependent Potentiation of Excitatory Transmission in Ventral Tegmental Area Dopamine Neurons. Journal of Neuroscience 2009, 29, 6535–6544. [Google Scholar] [CrossRef]
- Ungless, M.A.; Singh, V.; Crowder, T.L.; Yaka, R.; Ron, D.; Bonci, A. Corticotropin-Releasing Factor Requires CRF Binding Protein to Potentiate NMDA Receptors via CRF Receptor 2 in Dopamine Neurons. Neuron 2003, 39, 401–407. [Google Scholar] [CrossRef]
- Ungless, M.A.; Argilli, E.; Bonci, A. Effects of Stress and Aversion on Dopamine Neurons: Implications for Addiction. Neuroscience and Biobehavioral Reviews 2010, 35, 151–156. [Google Scholar] [CrossRef]
- Beckstead, M.J.; Phillips, T.J. Mice Selectively Bred for High- or Low-Alcohol-Induced Locomotion Exhibit Differences in Dopamine Neuron Function. The Journal of Pharmacology and Experimental Therapeutics 2009, 329, 342–349. [Google Scholar] [CrossRef] [PubMed]
- Moghaddam, B. Stress Preferentially Increases Extraneuronal Levels of Excitatory Amino Acids in the Prefrontal Cortex: Comparison to Hippocampus and Basal Ganglia. Journal of Neurochemistry 1993, 60, 1650–1657. [Google Scholar] [CrossRef]
- Kalivas, P.W.; Duffy, P. Selective Activation of Dopamine Transmission in the Shell of the Nucleus Accumbens by Stress. Brain Research 1995, 675, 325–328. [Google Scholar] [CrossRef]
- Reid, M.S.; Berger, S.P. Evidence for Sensitization of Cocaine-Induced Nucleus Accumbens Glutamate Release. NeuroReport 1996, 7, 1325–1329. [Google Scholar] [CrossRef]
- Reid, M.S.; Hsu, K.; Berger, S.P. Cocaine and Amphetamine Preferentially Stimulate Glutamate Release in the Limbic System: Studies on the Involvement of Dopamine. Synapse 1997, 27, 95–105. [Google Scholar] [CrossRef]
- Groenewegen, H.J.; Wright, C.I.; Beijer, A.V.J.; Voorn, P. Convergence and Segregation of Ventral Striatal Inputs and Outputs. Annals of the New York Academy of Sciences 1999, 877, 49–63. [Google Scholar] [CrossRef]
- Sesack, S.R.; Deutch, A.Y.; Roth, R.H.; Bunney, B.S. Topographical Organization of the Efferent Projections of the Medial Prefrontal Cortex in the Rat: An Anterograde Tract-tracing Study with Phaseolus Vulgaris Leucoagglutinin. J of Comparative Neurology 1989, 290, 213–242. [Google Scholar] [CrossRef]
- Wright, C.I.; Groenewegen, H.J. Patterns of Overlap and Segregation between Insular Cortical, Intermediodorsal Thalamic and Basal Amygdaloid Afferents in the Nucleus Accumbens of the Rat. Neuroscience 1996, 73, 359–373. [Google Scholar] [CrossRef] [PubMed]
- Moghaddam, B. Stress Activation of Glutamate Neurotransmission in the Prefrontal Cortex: Implications for Dopamine-Associated Psychiatric Disorders. Biological Psychiatry 2002, 51, 775–787. [Google Scholar] [CrossRef] [PubMed]
- Avalos, M.P.; Guzman, A.S.; Garcia-Keller, C.; Mongi-Bragato, B.; Esparza, M.A.; Rigoni, D.; Sanchez, M.A.; Calfa, G.D.; Bollati, F.A.; Cancela, L.M. Impairment of Glutamate Homeostasis in the Nucleus Accumbens Core Underpins Cross-Sensitization to Cocaine Following Chronic Restraint Stress. Front. Physiol. 2022, 13, 896268. [Google Scholar] [CrossRef]
- Rigoni, D.; Avalos, M.P.; Boezio, M.J.; Guzmán, A.S.; Calfa, G.D.; Perassi, E.M.; Pierotti, S.M.; Bisbal, M.; Garcia-Keller, C.; Cancela, L.M.; et al. Stress-Induced Vulnerability to Develop Cocaine Addiction Depends on Cofilin Modulation. Neurobiology of Stress 2021, 15, 100349. [Google Scholar] [CrossRef]
- McFarland, K.; Davidge, S.B.; Lapish, C.C.; Kalivas, P.W. Limbic and Motor Circuitry Underlying Footshock-Induced Reinstatement of Cocaine-Seeking Behavior. J. Neurosci. 2004, 24, 1551–1560. [Google Scholar] [CrossRef]
- Campioni, M.R.; Xu, M.; McGehee, D.S. Stress-Induced Changes in Nucleus Accumbens Glutamate Synaptic Plasticity. Journal of Neurophysiology 2009, 101, 3192–3198. [Google Scholar] [CrossRef]
- Pierce, R.; Bell, K.; Duffy, P.; Kalivas, P. Repeated Cocaine Augments Excitatory Amino Acid Transmission in the Nucleus Accumbens Only in Rats Having Developed Behavioral Sensitization. J. Neurosci. 1996, 16, 1550–1560. [Google Scholar] [CrossRef]
- Kalivas, P.W.; Duffy, P. Repeated Cocaine Administration Alters Extracellular Glutamate in the Ventral Tegmental Area. Journal of Neurochemistry 1998, 70, 1497–1502. [Google Scholar] [CrossRef]
- Pendyam, S.; Mohan, A.; Kalivas, P.W.; Nair, S.S. Computational Model of Extracellular Glutamate in the Nucleus Accumbens Incorporates Neuroadaptations by Chronic Cocaine. Neuroscience 2009, 158, 1266–1276. [Google Scholar] [CrossRef]
- Baker, D.A.; Xi, Z.-X.; Shen, H.; Swanson, C.J.; Kalivas, P.W. The Origin and Neuronal Function of In Vivo Nonsynaptic Glutamate. J. Neurosci. 2002, 22, 9134–9141. [Google Scholar] [CrossRef] [PubMed]
- Baker, D.A.; McFarland, K.; Lake, R.W.; Shen, H.; Tang, X.-C.; Toda, S.; Kalivas, P.W. Neuroadaptations in Cystine-Glutamate Exchange Underlie Cocaine Relapse. Nat Neurosci 2003, 6, 743–749. [Google Scholar] [CrossRef] [PubMed]
- Knackstedt, L.A.; Moussawi, K.; Lalumiere, R.; Schwendt, M.; Klugmann, M.; Kalivas, P.W. Extinction Training after Cocaine Self-Administration Induces Glutamatergic Plasticity to Inhibit Cocaine Seeking. Journal of Neuroscience 2010, 30, 7984–7992. [Google Scholar] [CrossRef]
- Trantham-Davidson, H.; LaLumiere, R.T.; Reissner, K.J.; Kalivas, P.W.; Knackstedt, L.A. Ceftriaxone Normalizes Nucleus Accumbens Synaptic Transmission, Glutamate Transport, and Export Following Cocaine Self-Administration and Extinction Training. Journal of Neuroscience 2012, 32, 12406–12410. [Google Scholar] [CrossRef] [PubMed]
- Moussawi, K.; Zhou, W.; Shen, H.; Reichel, C.M.; See, R.E.; Carr, D.B.; Kalivas, P.W. Reversing Cocaine-Induced Synaptic Potentiation Provides Enduring Protection from Relapse. Proceedings of the National Academy of Sciences 2011, 108, 385–390. [Google Scholar] [CrossRef]
- Pow, D.V. Visualising the Activity of the Cystine-glutamate Antiporter in Glial Cells Using Antibodies to Aminoadipic Acid, a Selectively Transported Substrate. Glia 2001, 34, 27–38. [Google Scholar] [CrossRef]
- Sato, H.; Tamba, M.; Okuno, S.; Sato, K.; Keino-Masu, K.; Masu, M.; Bannai, S. Distribution of Cystine/Glutamate Exchange Transporter, System x(c)-, in the Mouse Brain. J Neurosci 2002, 22, 8028–8033. [Google Scholar] [CrossRef] [PubMed]
- Zink, M.; Vollmayr, B.; Gebicke-Haerter, P.J.; Henn, F.A. Reduced Expression of Glutamate Transporters vGluT1, EAAT2 and EAAT4 in Learned Helpless Rats, an Animal Model of Depression. Neuropharmacology 2010, 58, 465–473. [Google Scholar] [CrossRef]
- Almeida, R.F.; Thomazi, A.P.; Godinho, G.F.; Saute, J.A.M.; Wofchuk, S.T.; Souza, D.O.; Ganzella, M. Effects of Depressive-Like Behavior of Rats on Brain Glutamate Uptake. Neurochem Res 2010, 35, 1164–1171. [Google Scholar] [CrossRef]
- Rappeneau, V.; Blaker, A.; Petro, J.R.; Yamamoto, B.K.; Shimamoto, A. Disruption of the Glutamate–Glutamine Cycle Involving Astrocytes in an Animal Model of Depression for Males and Females. Front. Behav. Neurosci. 2016, 10. [Google Scholar] [CrossRef]
- Das, S.C.; Yamamoto, B.K.; Hristov, A.M.; Sari, Y. Ceftriaxone Attenuates Ethanol Drinking and Restores Extracellular Glutamate Concentration through Normalization of GLT-1 in Nucleus Accumbens of Male Alcohol-Preferring Rats. Neuropharmacology 2015, 97, 67–74. [Google Scholar] [CrossRef]
- Gipson, C.D.; Reissner, K.J.; Kupchik, Y.M.; Smith, A.C.W.; Stankeviciute, N.; Hensley-Simon, M.E.; Kalivas, P.W. Reinstatement of Nicotine Seeking Is Mediated by Glutamatergic Plasticity. Proc Natl Acad Sci U S A 2013, 110, 9124–9129. [Google Scholar] [CrossRef] [PubMed]
- Melendez, R.I.; Hicks, M.P.; Cagle, S.S.; Kalivas, P.W. Ethanol Exposure Decreases Glutamate Uptake in the Nucleus Accumbens. Alcoholism Clin & Exp Res 2005, 29, 326–333. [Google Scholar] [CrossRef]
- Reissner, K.J.; Gipson, C.D.; Tran, P.K.; Knackstedt, L.A.; Scofield, M.D.; Kalivas, P.W. Glutamate Transporter GLT-1 Mediates N-acetylcysteine Inhibition of Cocaine Reinstatement. Addiction Biology 2015, 20, 316–323. [Google Scholar] [CrossRef]
- Sari, Y.; Sreemantula, S.N. Neuroimmunophilin GPI-1046 Reduces Ethanol Consumption in Part through Activation of GLT1 in Alcohol-Preferring Rats. Neuroscience 2012, 227, 327–335. [Google Scholar] [CrossRef]
- Shen, H.; Scofield, M.D.; Boger, H.; Hensley, M.; Kalivas, P.W. Synaptic Glutamate Spillover Due to Impaired Glutamate Uptake Mediates Heroin Relapse. J. Neurosci. 2014, 34, 5649–5657. [Google Scholar] [CrossRef] [PubMed]
- Xue, C.; Ng, J.P.; Li, Y.; Wolf, M.E. Acute and Repeated Systemic Amphetamine Administration: Effects on Extracellular Glutamate, Aspartate, and Serine Levels in Rat Ventral Tegmental Area and Nucleus Accumbens. Journal of Neurochemistry 1996, 67, 352–363. [Google Scholar] [CrossRef] [PubMed]
- Fischer-Smith, K.D.; Houston, A.C.W.; Rebec, G.V. Differential Effects of Cocaine Access and Withdrawal on Glutamate Type 1 Transporter Expression in Rat Nucleus Accumbens Core and Shell. Neuroscience 2012, 210, 333–339. [Google Scholar] [CrossRef]
- Mongi-Bragato, B.; Sánchez, M.A.; Avalos, M.P.; Boezio, M.J.; Guzman, A.S.; Rigoni, D.; Perassi, E.M.; Mas, C.R.; Bisbal, M.; Bollati, F.A.; et al. Activation of Nuclear Factor-Kappa B in the Nucleus Accumbens Core Is Necessary for Chronic Stress-Induced Glutamate and Neuro-Immune Alterations That Facilitate Cocaine Self-Administration. Brain, Behavior, and Immunity 2025, 128, 1–15. [Google Scholar] [CrossRef]
- Minelli, A.; Barbaresi, P.; Reimer, R.J.; Edwards, R.H.; Conti, F. The Glial Glutamate Transporter GLT-1 Is Localized Both in the Vicinity of and at Distance from Axon Terminals in the Rat Cerebral Cortex. Neuroscience 2001, 108, 51–59. [Google Scholar] [CrossRef]
- Cholet, N. Similar Perisynaptic Glial Localization for the Na+,K+-ATPase Alpha2 Subunit and the Glutamate Transporters GLAST and GLT-1 in the Rat Somatosensory Cortex. Cerebral Cortex 2002, 12, 515–525. [Google Scholar] [CrossRef]
- Moran, M.M.; McFarland, K.; Melendez, R.I.; Kalivas, P.W.; Seamans, J.K. Cystine/Glutamate Exchange Regulates Metabotropic Glutamate Receptor Presynaptic Inhibition of Excitatory Transmission and Vulnerability to Cocaine Seeking. J. Neurosci. 2005, 25, 6389–6393. [Google Scholar] [CrossRef]
- Béchade, C.; Cantaut-Belarif, Y.; Bessis, A. Microglial Control of Neuronal Activity. Front. Cell. Neurosci. 2013, 7. [Google Scholar] [CrossRef]
- Lynch, M.A. The Multifaceted Profile of Activated Microglia. Mol Neurobiol 2009, 40, 139–156. [Google Scholar] [CrossRef] [PubMed]
- Kreutzberg, G.W. Microglia: A Sensor for Pathological Events in the CNS. Trends in Neurosciences 1996, 19, 312–318. [Google Scholar] [CrossRef]
- Mcgeer, P.; Mcgeer, E. The Inflammatory Response System of Brain: Implications for Therapy of Alzheimer and Other Neurodegenerative Diseases. Brain Research Reviews 1995, 21, 195–218. [Google Scholar] [CrossRef]
- Kettenmann, H.; Hanisch, U.-K.; Noda, M.; Verkhratsky, A. Physiology of Microglia. Physiological Reviews 2011, 91, 461–553. [Google Scholar] [CrossRef] [PubMed]
- Davalos, D.; Grutzendler, J.; Yang, G.; Kim, J.V.; Zuo, Y.; Jung, S.; Littman, D.R.; Dustin, M.L.; Gan, W.-B. ATP Mediates Rapid Microglial Response to Local Brain Injury in Vivo. Nat Neurosci 2005, 8, 752–758. [Google Scholar] [CrossRef]
- Walker, F.; Nilsson, M.; Jones, K. Acute and Chronic Stress-Induced Disturbances of Microglial Plasticity, Phenotype and Function. CDT 2013, 14, 1262–1276. [Google Scholar] [CrossRef]
- Walker, F.R.; Beynon, S.B.; Jones, K.A.; Zhao, Z.; Kongsui, R.; Cairns, M.; Nilsson, M. Dynamic Structural Remodelling of Microglia in Health and Disease: A Review of the Models, the Signals and the Mechanisms. Brain, Behavior, and Immunity 2014, 37, 1–14. [Google Scholar] [CrossRef]
- Jinno, S.; Fleischer, F.; Eckel, S.; Schmidt, V.; Kosaka, T. Spatial Arrangement of Microglia in the Mouse Hippocampus: A Stereological Study in Comparison with Astrocytes. Glia 2007, 55, 1334–1347. [Google Scholar] [CrossRef]
- Stevens, B.; Allen, N.J.; Vazquez, L.E.; Howell, G.R.; Christopherson, K.S.; Nouri, N.; Micheva, K.D.; Mehalow, A.K.; Huberman, A.D.; Stafford, B.; et al. The Classical Complement Cascade Mediates CNS Synapse Elimination. Cell 2007, 131, 1164–1178. [Google Scholar] [CrossRef] [PubMed]
- Paolicelli, R.C.; Bolasco, G.; Pagani, F.; Maggi, L.; Scianni, M.; Panzanelli, P.; Giustetto, M.; Ferreira, T.A.; Guiducci, E.; Dumas, L.; et al. Synaptic Pruning by Microglia Is Necessary for Normal Brain Development. Science 2011, 333, 1456–1458. [Google Scholar] [CrossRef] [PubMed]
- Delpech, J.-C.; Madore, C.; Nadjar, A.; Joffre, C.; Wohleb, E.S.; Layé, S. Microglia in Neuronal Plasticity: Influence of Stress. Neuropharmacology 2015, 96, 19–28. [Google Scholar] [CrossRef]
- Blank, T.; Prinz, M. Microglia as Modulators of Cognition and Neuropsychiatric Disorders. Glia 2013, 61, 62–70. [Google Scholar] [CrossRef]
- Kopec, A.M.; Smith, C.J.; Ayre, N.R.; Sweat, S.C.; Bilbo, S.D. Microglial Dopamine Receptor Elimination Defines Sex-Specific Nucleus Accumbens Development and Social Behavior in Adolescent Rats. Nat Commun 2018, 9, 3769. [Google Scholar] [CrossRef] [PubMed]
- Gongwer, M.W.; Etienne, F.; Moca, E.N.; Chappell, M.S.; Blagburn-Blanco, S.V.; Riley, J.P.; Enos, A.S.; Haratian, M.; Qi, A.; Rojo, R.; et al. Microglia Regulate Nucleus Accumbens Synaptic Development and Circuit Function Underlying Threat Avoidance Behaviors 2025.
- Tremblay, M.-È.; Lowery, R.L.; Majewska, A.K. Microglial Interactions with Synapses Are Modulated by Visual Experience. PLoS Biol 2010, 8, e1000527. [Google Scholar] [CrossRef]
- Tremblay, M.-È.; Stevens, B.; Sierra, A.; Wake, H.; Bessis, A.; Nimmerjahn, A. The Role of Microglia in the Healthy Brain: Figure 1. J. Neurosci. 2011, 31, 16064–16069. [Google Scholar] [CrossRef] [PubMed]
- Schafer, D.P.; Lehrman, E.K.; Kautzman, A.G.; Koyama, R.; Mardinly, A.R.; Yamasaki, R.; Ransohoff, R.M.; Greenberg, M.E.; Barres, B.A.; Stevens, B. Microglia Sculpt Postnatal Neural Circuits in an Activity and Complement-Dependent Manner. Neuron 2012, 74, 691–705. [Google Scholar] [CrossRef]
- Wake, H.; Moorhouse, A.J.; Jinno, S.; Kohsaka, S.; Nabekura, J. Resting Microglia Directly Monitor the Functional State of Synapses In Vivo and Determine the Fate of Ischemic Terminals. J. Neurosci. 2009, 29, 3974–3980. [Google Scholar] [CrossRef]
- Costello, D.A.; Lyons, A.; Denieffe, S.; Browne, T.C.; Cox, F.F.; Lynch, M.A. Long Term Potentiation Is Impaired in Membrane Glycoprotein CD200-Deficient Mice. Journal of Biological Chemistry 2011, 286, 34722–34732. [Google Scholar] [CrossRef]
- Kierdorf, K.; Prinz, M. Factors Regulating Microglia Activation. Front. Cell. Neurosci. 2013, 7. [Google Scholar] [CrossRef]
- Pascual, O.; Ben Achour, S.; Rostaing, P.; Triller, A.; Bessis, A. Microglia Activation Triggers Astrocyte-Mediated Modulation of Excitatory Neurotransmission. Proc. Natl. Acad. Sci. U.S.A. 2012, 109. [Google Scholar] [CrossRef]
- Piani, D.; Fontana, A. Involvement of the Cystine Transport System Xc- in the Macrophage-Induced Glutamate-Dependent Cytotoxicity to Neurons. J Immunol 1994, 152, 3578–3585. [Google Scholar] [CrossRef] [PubMed]
- Hayashi, M.K.; Ames, H.M.; Hayashi, Y. Tetrameric Hub Structure of Postsynaptic Scaffolding Protein Homer. J. Neurosci. 2006, 26, 8492–8501. [Google Scholar] [CrossRef]
- Flierl, M.A.; Rittirsch, D.; Nadeau, B.A.; Chen, A.J.; Sarma, J.V.; Zetoune, F.S.; McGuire, S.R.; List, R.P.; Day, D.E.; Hoesel, L.M.; et al. Phagocyte-Derived Catecholamines Enhance Acute Inflammatory Injury. Nature 2007, 449, 721–725. [Google Scholar] [CrossRef] [PubMed]
- Elkabes, S.; DiCicco-Bloom, E.; Black, I. Brain Microglia/Macrophages Express Neurotrophins That Selectively Regulate Microglial Proliferation and Function. J. Neurosci. 1996, 16, 2508–2521. [Google Scholar] [CrossRef]
- Chamak, B.; Morandi, V.; Mallat, M. Brain Macrophages Stimulate Neurite Growth and Regeneration by Secreting Thrombospondin. J of Neuroscience Research 1994, 38, 221–233. [Google Scholar] [CrossRef]
- Dityatev, A.; Rusakov, D.A. Molecular Signals of Plasticity at the Tetrapartite Synapse. Current Opinion in Neurobiology 2011, 21, 353–359. [Google Scholar] [CrossRef]
- Hanisch, U. Microglia as a Source and Target of Cytokines. Glia 2002, 40, 140–155. [Google Scholar] [CrossRef]
- Santello, M.; Bezzi, P.; Volterra, A. TNFα Controls Glutamatergic Gliotransmission in the Hippocampal Dentate Gyrus. Neuron 2011, 69, 988–1001. [Google Scholar] [CrossRef]
- Stellwagen, D.; Malenka, R.C. Synaptic Scaling Mediated by Glial TNF-α. Nature 2006, 440, 1054–1059. [Google Scholar] [CrossRef] [PubMed]
- Kaneko, M.; Stellwagen, D.; Malenka, R.C.; Stryker, M.P. Tumor Necrosis Factor-α Mediates One Component of Competitive, Experience-Dependent Plasticity in Developing Visual Cortex. Neuron 2008, 58, 673–680. [Google Scholar] [CrossRef]
- Carmen, J.; Rothstein, J.D.; Kerr, D.A. Tumor Necrosis Factor-α Modulates Glutamate Transport in the CNS and Is a Critical Determinant of Outcome from Viral Encephalomyelitis. Brain Research 2009, 1263, 143–154. [Google Scholar] [CrossRef]
- Tilleux, S.; Hermans, E. Neuroinflammation and Regulation of Glial Glutamate Uptake in Neurological Disorders. J of Neuroscience Research 2007, 85, 2059–2070. [Google Scholar] [CrossRef] [PubMed]
- Tolosa, L.; Caraballo-Miralles, V.; Olmos, G.; Lladó, J. TNF-α Potentiates Glutamate-Induced Spinal Cord Motoneuron Death via NF-κB. Molecular and Cellular Neuroscience 2011, 46, 176–186. [Google Scholar] [CrossRef] [PubMed]
- Barger, S.W.; Goodwin, M.E.; Porter, M.M.; Beggs, M.L. Glutamate Release from Activated Microglia Requires the Oxidative Burst and Lipid Peroxidation. Journal of Neurochemistry 2007, 101, 1205–1213. [Google Scholar] [CrossRef] [PubMed]
- Piani, D.; Spranger, M.; Frei, K.; Schaffner, A.; Fontana, A. Macrophage-induced Cytotoxicity of N-methyl-D-aspartate Receptor Positive Neurons Involves Excitatory Amino Acids Rather than Reactive Oxygen Intermediates and Cytokines. Eur J Immunol 1992, 22, 2429–2436. [Google Scholar] [CrossRef]
- Qin, S.; Colin, C.; Hinners, I.; Gervais, A.; Cheret, C.; Mallat, M. System Xc− and Apolipoprotein E Expressed by Microglia Have Opposite Effects on the Neurotoxicity of Amyloid-β Peptide 1–40. J. Neurosci. 2006, 26, 3345–3356. [Google Scholar] [CrossRef]
- Liang, J.; Takeuchi, H.; Doi, Y.; Kawanokuchi, J.; Sonobe, Y.; Jin, S.; Yawata, I.; Li, H.; Yasuoka, S.; Mizuno, T.; et al. Excitatory Amino Acid Transporter Expression by Astrocytes Is Neuroprotective against Microglial Excitotoxicity. Brain Research 2008, 1210, 11–19. [Google Scholar] [CrossRef]
- Danbolt, N.C.; Furness, D.N.; Zhou, Y. Neuronal vs Glial Glutamate Uptake: Resolving the Conundrum. Neurochemistry International 2016, 98, 29–45. [Google Scholar] [CrossRef]
- Zhang, H. Microglia-Friend or Foe. Front Biosci 2011, S3, 869. [Google Scholar] [CrossRef]
- Ito, D.; Imai, Y.; Ohsawa, K.; Nakajima, K.; Fukuuchi, Y.; Kohsaka, S. Microglia-Specific Localisation of a Novel Calcium Binding Protein, Iba1. Molecular Brain Research 1998, 57, 1–9. [Google Scholar] [CrossRef]
- Morrison, H.W.; Filosa, J.A. A Quantitative Spatiotemporal Analysis of Microglia Morphology during Ischemic Stroke and Reperfusion. J Neuroinflammation 2013, 10, 782. [Google Scholar] [CrossRef] [PubMed]
- Ito, D.; Tanaka, K.; Suzuki, S.; Dembo, T.; Fukuuchi, Y. Enhanced Expression of Iba1, Ionized Calcium-Binding Adapter Molecule 1, After Transient Focal Cerebral Ischemia In Rat Brain. Stroke 2001, 32, 1208–1215. [Google Scholar] [CrossRef]
- Walter, L.; Franklin, A.; Witting, A.; Wade, C.; Xie, Y.; Kunos, G.; Mackie, K.; Stella, N. Nonpsychotropic Cannabinoid Receptors Regulate Microglial Cell Migration. J. Neurosci. 2003, 23, 1398–1405. [Google Scholar] [CrossRef]
- Zhang, Y.; Li, H.; Li, Y.; Sun, X.; Zhu, M.; Hanley, G.; Lesage, G.; Yin, D. Essential Role of Toll-like Receptor 2 in Morphine-Induced Microglia Activation in Mice. Neurosci Lett 2011, 489, 43–47. [Google Scholar] [CrossRef]
- Northcutt, A.L.; Hutchinson, M.R.; Wang, X.; Baratta, M.V.; Hiranita, T.; Cochran, T.A.; Pomrenze, M.B.; Galer, E.L.; Kopajtic, T.A.; Li, C.M.; et al. DAT Isn’t All That: Cocaine Reward and Reinforcement Require Toll-like Receptor 4 Signaling. Mol Psychiatry 2015, 20, 1525–1537. [Google Scholar] [CrossRef]
- Frank, M.G.; Watkins, L.R.; Maier, S.F. Stress- and Glucocorticoid-Induced Priming of Neuroinflammatory Responses: Potential Mechanisms of Stress-Induced Vulnerability to Drugs of Abuse. Brain, Behavior, and Immunity 2011, 25, S21–S28. [Google Scholar] [CrossRef] [PubMed]
- Frank, M.G.; Thompson, B.M.; Watkins, L.R.; Maier, S.F. Glucocorticoids Mediate Stress-Induced Priming of Microglial pro-Inflammatory Responses. Brain, Behavior, and Immunity 2012, 26, 337–345. [Google Scholar] [CrossRef]
- Nair, A.; Bonneau, R.H. Stress-Induced Elevation of Glucocorticoids Increases Microglia Proliferation through NMDA Receptor Activation. Journal of Neuroimmunology 2006, 171, 72–85. [Google Scholar] [CrossRef] [PubMed]
- Hanisch, U.-K.; Kettenmann, H. Microglia: Active Sensor and Versatile Effector Cells in the Normal and Pathologic Brain. Nat Neurosci 2007, 10, 1387–1394. [Google Scholar] [CrossRef]
- Schwartz, M.; Butovsky, O.; Brück, W.; Hanisch, U.-K. Microglial Phenotype: Is the Commitment Reversible? Trends in Neurosciences 2006, 29, 68–74. [Google Scholar] [CrossRef]
- Streit, W.J.; Walter, S.A.; Pennell, N.A. Reactive Microgliosis. Progress in Neurobiology 1999, 57, 563–581. [Google Scholar] [CrossRef]
- Smith, J.A.; Das, A.; Ray, S.K.; Banik, N.L. Role of Pro-Inflammatory Cytokines Released from Microglia in Neurodegenerative Diseases. Brain Research Bulletin 2012, 87, 10–20. [Google Scholar] [CrossRef]
- Liu, W.; Tang, Y.; Feng, J. Cross Talk between Activation of Microglia and Astrocytes in Pathological Conditions in the Central Nervous System. Life Sciences 2011, 89, 141–146. [Google Scholar] [CrossRef] [PubMed]
- Herculano-Houzel, S. The Glia/Neuron Ratio: How It Varies Uniformly across Brain Structures and Species and What That Means for Brain Physiology and Evolution. Glia 2014, 62, 1377–1391. [Google Scholar] [CrossRef]
- Kardos, J.; Dobolyi, Á.; Szabó, Z.; Simon, Á.; Lourmet, G.; Palkovits, M.; Héja, L. Molecular Plasticity of the Nucleus Accumbens Revisited—Astrocytic Waves Shall Rise. Mol Neurobiol 2019, 56, 7950–7965. [Google Scholar] [CrossRef]
- Keller, D.; Erö, C.; Markram, H. Cell Densities in the Mouse Brain: A Systematic Review. Front. Neuroanat. 2018, 12, 83. [Google Scholar] [CrossRef] [PubMed]
- Perea, G.; Navarrete, M.; Araque, A. Tripartite Synapses: Astrocytes Process and Control Synaptic Information. Trends in Neurosciences 2009, 32, 421–431. [Google Scholar] [CrossRef]
- Bezzi, P.; Volterra, A. A Neuron–Glia Signalling Network in the Active Brain. Current Opinion in Neurobiology 2001, 11, 387–394. [Google Scholar] [CrossRef] [PubMed]
- Magistretti, P.J. Neuron–Glia Metabolic Coupling and Plasticity. Journal of Experimental Biology 2006, 209, 2304–2311. [Google Scholar] [CrossRef]
- Middeldorp, J.; Hol, E.M. GFAP in Health and Disease. Progress in Neurobiology 2011, 93, 421–443. [Google Scholar] [CrossRef]
- Perea, G.; Araque, A. Astrocytes Potentiate Transmitter Release at Single Hippocampal Synapses. Science 2007, 317, 1083–1086. [Google Scholar] [CrossRef]
- Bushong, E.A.; Martone, M.E.; Jones, Y.Z.; Ellisman, M.H. Protoplasmic Astrocytes in CA1 Stratum Radiatum Occupy Separate Anatomical Domains. J. Neurosci. 2002, 22, 183–192. [Google Scholar] [CrossRef]
- Halassa, M.M.; Fellin, T.; Takano, H.; Dong, J.-H.; Haydon, P.G. Synaptic Islands Defined by the Territory of a Single Astrocyte. Journal of Neuroscience 2007, 27, 6473–6477. [Google Scholar] [CrossRef]
- Parpura, V.; Verkhratsky, A. Astroglial Amino Acid-Based Transmitter Receptors. Amino Acids 2013, 44, 1151–1158. [Google Scholar] [CrossRef]
- Araque, A.; Parpura, V.; Sanzgiri, R.P.; Haydon, P.G. Tripartite Synapses: Glia, the Unacknowledged Partner. Trends in Neurosciences 1999, 22, 208–215. [Google Scholar] [CrossRef] [PubMed]
- Simard, M.; Arcuino, G.; Takano, T.; Liu, Q.S.; Nedergaard, M. Signaling at the Gliovascular Interface. J. Neurosci. 2003, 23, 9254–9262. [Google Scholar] [CrossRef]
- D’Ascenzo, M.; Fellin, T.; Terunuma, M.; Revilla-Sanchez, R.; Meaney, D.F.; Auberson, Y.P.; Moss, S.J.; Haydon, P.G. mGluR5 Stimulates Gliotransmission in the Nucleus Accumbens. Proc. Natl. Acad. Sci. U.S.A. 2007, 104, 1995–2000. [Google Scholar] [CrossRef]
- Anderson, C.M.; Swanson, R.A. Astrocyte Glutamate Transport: Review of Properties, Regulation, and Physiological Functions. Glia 2000, 32, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Schousboe, A. Pharmacological and Functional Characterization of Astrocytic GABA Transport: A Short Review. Neurochem Res 2000, 25, 1241–1244. [Google Scholar] [CrossRef] [PubMed]
- Corkrum, M.; Covelo, A.; Lines, J.; Bellocchio, L.; Pisansky, M.; Loke, K.; Quintana, R.; Rothwell, P.E.; Lujan, R.; Marsicano, G.; et al. Dopamine-Evoked Synaptic Regulation in the Nucleus Accumbens Requires Astrocyte Activity. Neuron 2020, 105, 1036–1047.e5. [Google Scholar] [CrossRef] [PubMed]
- Xin, W.; Schuebel, K.E.; Jair, K.; Cimbro, R.; De Biase, L.M.; Goldman, D.; Bonci, A. Ventral Midbrain Astrocytes Display Unique Physiological Features and Sensitivity to Dopamine D2 Receptor Signaling. Neuropsychopharmacol 2019, 44, 344–355. [Google Scholar] [CrossRef]
- Farina, C.; Aloisi, F.; Meinl, E. Astrocytes Are Active Players in Cerebral Innate Immunity. Trends in Immunology 2007, 28, 138–145. [Google Scholar] [CrossRef]
- Jensen, C.J.; Massie, A.; De Keyser, J. Immune Players in the CNS: The Astrocyte. J Neuroimmune Pharmacol 2013, 8, 824–839. [Google Scholar] [CrossRef]
- Lau, L.T.; Yu, A.C.-H. Astrocytes Produce and Release Interleukin-1, Interleukin-6, Tumor Necrosis Factor Alpha and Interferon-Gamma Following Traumatic and Metabolic Injury. Journal of Neurotrauma 2001, 18, 351–359. [Google Scholar] [CrossRef]
- Pekny, M.; Pekna, M. Astrocyte Reactivity and Reactive Astrogliosis: Costs and Benefits. Physiological Reviews 2014, 94, 1077–1098. [Google Scholar] [CrossRef] [PubMed]
- Tian, L.; Ma, L.; Kaarela, T.; Li, Z. Neuroimmune Crosstalk in the Central Nervous System and Its Significance for Neurological Diseases. J Neuroinflammation 2012, 9. [Google Scholar] [CrossRef] [PubMed]
- Spampinato, S.F.; Copani, A.; Nicoletti, F.; Sortino, M.A.; Caraci, F. Metabotropic Glutamate Receptors in Glial Cells: A New Potential Target for Neuroprotection? Front. Mol. Neurosci. 2018, 11. [Google Scholar] [CrossRef]
- Haydon, P.G. Glia: Listening and Talking to the Synapse. Nat Rev Neurosci 2001, 2, 185–193. [Google Scholar] [CrossRef]
- Volterra, A.; Meldolesi, J. Astrocytes, from Brain Glue to Communication Elements: The Revolution Continues. Nat Rev Neurosci 2005, 6, 626–640. [Google Scholar] [CrossRef] [PubMed]
- Halassa, M.M.; Haydon, P.G. Integrated Brain Circuits: Astrocytic Networks Modulate Neuronal Activity and Behavior. Annu. Rev. Physiol. 2010, 72, 335–355. [Google Scholar] [CrossRef]
- Volterra, A.; Magistretti, P. The Tripartite Synapse: Glia in Synaptic Transmission; Oxford University Press: Oxford, 2002; ISBN 978-0-19-850854-0. [Google Scholar]
- Poskanzer, K.E.; Yuste, R. Astrocytic Regulation of Cortical UP States. Proc. Natl. Acad. Sci. U.S.A. 2011, 108, 18453–18458. [Google Scholar] [CrossRef] [PubMed]
- Schipke, C.G.; Boucsein, C.; Ohlemeyer, C.; Kirchhoff, F.; Kettenmann, H. Astrocyte Ca2+Waves Trigger Responses in Microglial Cells in Brain Slices. FASEB j. 2002, 16, 1–16. [Google Scholar] [CrossRef] [PubMed]
- Sieger, D.; Moritz, C.; Ziegenhals, T.; Prykhozhij, S.; Peri, F. Long-Range Ca2+ Waves Transmit Brain-Damage Signals to Microglia. Developmental Cell 2012, 22, 1138–1148. [Google Scholar] [CrossRef]
- Albrecht, J.; Sidoryk-Węgrzynowicz, M.; Zielińska, M.; Aschner, M. Roles of Glutamine in Neurotransmission. Neuron Glia Biol. 2010, 6, 263–276. [Google Scholar] [CrossRef] [PubMed]
- Mahmoud, S.; Gharagozloo, M.; Simard, C.; Gris, D. Astrocytes Maintain Glutamate Homeostasis in the CNS by Controlling the Balance between Glutamate Uptake and Release. Cells 2019, 8, 184. [Google Scholar] [CrossRef]
- Petr, G.T.; Sun, Y.; Frederick, N.M.; Zhou, Y.; Dhamne, S.C.; Hameed, M.Q.; Miranda, C.; Bedoya, E.A.; Fischer, K.D.; Armsen, W.; et al. Conditional Deletion of the Glutamate Transporter GLT-1 Reveals That Astrocytic GLT-1 Protects against Fatal Epilepsy While Neuronal GLT-1 Contributes Significantly to Glutamate Uptake into Synaptosomes. J. Neurosci. 2015, 35, 5187–5201. [Google Scholar] [CrossRef]
- Rothstein, J.D.; Dykes-Hoberg, M.; Pardo, C.A.; Bristol, L.A.; Jin, L.; Kuncl, R.W.; Kanai, Y.; Hediger, M.A.; Wang, Y.; Schielke, J.P.; et al. Knockout of Glutamate Transporters Reveals a Major Role for Astroglial Transport in Excitotoxicity and Clearance of Glutamate. Neuron 1996, 16, 675–686. [Google Scholar] [CrossRef]
- Hascup, K.N.; Hascup, E.R.; Pomerleau, F.; Huettl, P.; Gerhardt, G.A. Second-by-Second Measures of L-Glutamate in the Prefrontal Cortex and Striatum of Freely Moving Mice. J Pharmacol Exp Ther 2008, 324, 725–731. [Google Scholar] [CrossRef]
- Van Der Zeyden, M.; Oldenziel, W.H.; Rea, K.; Cremers, T.I.; Westerink, B.H. Microdialysis of GABA and Glutamate: Analysis, Interpretation and Comparison with Microsensors. Pharmacology Biochemistry and Behavior 2008, 90, 135–147. [Google Scholar] [CrossRef]
- Danbolt, N.C. Glutamate Uptake. Progress in Neurobiology 2001, 65, 1–105. [Google Scholar] [CrossRef]
- Williams, S.M.; Sullivan, R.K.P.; Scott, H.L.; Finkelstein, D.I.; Colditz, P.B.; Lingwood, B.E.; Dodd, P.R.; Pow, D.V. Glial Glutamate Transporter Expression Patterns in Brains from Multiple Mammalian Species. Glia 2005, 49, 520–541. [Google Scholar] [CrossRef]
- McGrath, A.G.; Briand, L.A. A Potential Role for Microglia in Stress- and Drug-Induced Plasticity in the Nucleus Accumbens: A Mechanism for Stress-Induced Vulnerability to Substance Use Disorder. Neuroscience & Biobehavioral Reviews 2019, 107, 360–369. [Google Scholar] [CrossRef]
- Crews, F.T.; Lawrimore, C.J.; Walter, T.J.; Coleman, L.G. The Role of Neuroimmune Signaling in Alcoholism. Neuropharmacology 2017, 122, 56–73. [Google Scholar] [CrossRef] [PubMed]
- Gipson, C.D.; Rawls, S.; Scofield, M.D.; Siemsen, B.M.; Bondy, E.O.; Maher, E.E. Interactions of Neuroimmune Signaling and Glutamate Plasticity in Addiction. J Neuroinflammation 2021, 18. [Google Scholar] [CrossRef] [PubMed]
- Namba, M.D.; Kupchik, Y.M.; Spencer, S.M.; Garcia-Keller, C.; Goenaga, J.G.; Powell, G.L.; Vicino, I.A.; Hogue, I.B.; Gipson, C.D. Accumbens Neuroimmune Signaling and Dysregulation of Astrocytic Glutamate Transport Underlie Conditioned Nicotine-seeking Behavior. Addiction Biology 2020, 25, e12797. [Google Scholar] [CrossRef]
- Frank, M.G.; Weber, M.D.; Watkins, L.R.; Maier, S.F. Stress-Induced Neuroinflammatory Priming: A Liability Factor in the Etiology of Psychiatric Disorders. Neurobiology of Stress 2016, 4, 62–70. [Google Scholar] [CrossRef]
- Lo Iacono, L.; Catale, C.; Martini, A.; Valzania, A.; Viscomi, M.T.; Chiurchiù, V.; Guatteo, E.; Bussone, S.; Perrone, F.; Di Sabato, P.; et al. From Traumatic Childhood to Cocaine Abuse: The Critical Function of the Immune System. Biological Psychiatry 2018, 84, 905–916. [Google Scholar] [CrossRef]
- Boldyrev, A.A.; Carpenter, D.O.; Johnson, P. Emerging Evidence for a Similar Role of Glutamate Receptors in the Nervous and Immune Systems. Journal of Neurochemistry 2005, 95, 913–918. [Google Scholar] [CrossRef]
- Salter, M.W.; Beggs, S. Sublime Microglia: Expanding Roles for the Guardians of the CNS. Cell 2014, 158, 15–24. [Google Scholar] [CrossRef]
- Tay, T.L.; Savage, J.C.; Hui, C.W.; Bisht, K.; Tremblay, M. Microglia across the Lifespan: From Origin to Function in Brain Development, Plasticity and Cognition. The Journal of Physiology 2017, 595, 1929–1945. [Google Scholar] [CrossRef]
- Weinhard, L.; Di Bartolomei, G.; Bolasco, G.; Machado, P.; Schieber, N.L.; Neniskyte, U.; Exiga, M.; Vadisiute, A.; Raggioli, A.; Schertel, A.; et al. Microglia Remodel Synapses by Presynaptic Trogocytosis and Spine Head Filopodia Induction. Nat Commun 2018, 9, 1228. [Google Scholar] [CrossRef]
- Kreisel, T.; Frank, M.G.; Licht, T.; Reshef, R.; Ben-Menachem-Zidon, O.; Baratta, M.V.; Maier, S.F.; Yirmiya, R. Dynamic Microglial Alterations Underlie Stress-Induced Depressive-like Behavior and Suppressed Neurogenesis. Mol Psychiatry 2014, 19, 699–709. [Google Scholar] [CrossRef]
- Wang, W.; Wang, R.; Xu, J.; Qin, X.; Jiang, H.; Khalid, A.; Liu, D.; Pan, F.; Ho, C.S.H.; Ho, R.C.M. Minocycline Attenuates Stress-Induced Behavioral Changes via Its Anti-Inflammatory Effects in an Animal Model of Post-Traumatic Stress Disorder. Front. Psychiatry 2018, 9, 558. [Google Scholar] [CrossRef]
- Johnson, J.D.; O’Connor, K.A.; Watkins, L.R.; Maier, S.F. The Role of IL-1β in Stress-Induced Sensitization of Proinflammatory Cytokine and Corticosterone Responses. Neuroscience 2004, 127, 569–577. [Google Scholar] [CrossRef]
- Blandino, P.; Barnum, C.J.; Deak, T. The Involvement of Norepinephrine and Microglia in Hypothalamic and Splenic IL-1β Responses to Stress. Journal of Neuroimmunology 2006, 173, 87–95. [Google Scholar] [CrossRef] [PubMed]
- Blandino, P.; Barnum, C.J.; Solomon, L.G.; Larish, Y.; Lankow, B.S.; Deak, T. Gene Expression Changes in the Hypothalamus Provide Evidence for Regionally-Selective Changes in IL-1 and Microglial Markers after Acute Stress. Brain, Behavior, and Immunity 2009, 23, 958–968. [Google Scholar] [CrossRef] [PubMed]
- Dowlati, Y.; Herrmann, N.; Swardfager, W.; Liu, H.; Sham, L.; Reim, E.K.; Lanctôt, K.L. A Meta-Analysis of Cytokines in Major Depression. Biological Psychiatry 2010, 67, 446–457. [Google Scholar] [CrossRef] [PubMed]
- Gilman, S.E.; Trinh, N.-H.; Smoller, J.W.; Fava, M.; Murphy, J.M.; Breslau, J. Psychosocial Stressors and the Prognosis of Major Depression: A Test of Axis IV. Psychol. Med. 2013, 43, 303–316. [Google Scholar] [CrossRef]
- Kendler, K.S.; Karkowski, L.M.; Prescott, C.A. Causal Relationship Between Stressful Life Events and the Onset of Major Depression. AJP 1999, 156, 837–841. [Google Scholar] [CrossRef]
- McLaughlin, K.A.; Kubzansky, L.D.; Dunn, E.C.; Waldinger, R.; Vaillant, G.; Koenen, K.C. Childhood Social Environment, Emotional Reactivity to Stress, and Mood and Anxiety Disorders across the Life Course. Depress. Anxiety 2010, 27, 1087–1094. [Google Scholar] [CrossRef]
- Miller, G.E.; Chen, E.; Sze, J.; Marin, T.; Arevalo, J.M.G.; Doll, R.; Ma, R.; Cole, S.W. A Functional Genomic Fingerprint of Chronic Stress in Humans: Blunted Glucocorticoid and Increased NF-κB Signaling. Biological Psychiatry 2008, 64, 266–272. [Google Scholar] [CrossRef]
- Hinwood, M.; Tynan, R.J.; Charnley, J.L.; Beynon, S.B.; Day, T.A.; Walker, F.R. Chronic Stress Induced Remodeling of the Prefrontal Cortex: Structural Re-Organization of Microglia and the Inhibitory Effect of Minocycline. Cerebral Cortex 2013, 23, 1784–1797. [Google Scholar] [CrossRef]
- Tynan, R.J.; Naicker, S.; Hinwood, M.; Nalivaiko, E.; Buller, K.M.; Pow, D.V.; Day, T.A.; Walker, F.R. Chronic Stress Alters the Density and Morphology of Microglia in a Subset of Stress-Responsive Brain Regions. Brain Behav Immun 2010, 24, 1058–1068. [Google Scholar] [CrossRef]
- Hinwood, M.; Morandini, J.; Day, T.A.; Walker, F.R. Evidence That Microglia Mediate the Neurobiological Effects of Chronic Psychological Stress on the Medial Prefrontal Cortex. Cerebral Cortex 2012, 22, 1442–1454. [Google Scholar] [CrossRef]
- Gerecke, K.M.; Kolobova, A.; Allen, S.; Fawer, J.L. Exercise Protects against Chronic Restraint Stress-Induced Oxidative Stress in the Cortex and Hippocampus. Brain Research 2013, 1509, 66–78. [Google Scholar] [CrossRef]
- Wohleb, E.S.; Hanke, M.L.; Corona, A.W.; Powell, N.D.; Stiner, L.M.; Bailey, M.T.; Nelson, R.J.; Godbout, J.P.; Sheridan, J.F. β-Adrenergic Receptor Antagonism Prevents Anxiety-Like Behavior and Microglial Reactivity Induced by Repeated Social Defeat. J. Neurosci. 2011, 31, 6277–6288. [Google Scholar] [CrossRef]
- Wohleb, E.S.; Powell, N.D.; Godbout, J.P.; Sheridan, J.F. Stress-Induced Recruitment of Bone Marrow-Derived Monocytes to the Brain Promotes Anxiety-Like Behavior. J. Neurosci. 2013, 33, 13820–13833. [Google Scholar] [CrossRef] [PubMed]
- Wohleb, E.S.; Patterson, J.M.; Sharma, V.; Quan, N.; Godbout, J.P.; Sheridan, J.F. Knockdown of Interleukin-1 Receptor Type-1 on Endothelial Cells Attenuated Stress-Induced Neuroinflammation and Prevented Anxiety-Like Behavior. J. Neurosci. 2014, 34, 2583–2591. [Google Scholar] [CrossRef] [PubMed]
- Wohleb, E.S.; McKim, D.B.; Shea, D.T.; Powell, N.D.; Tarr, A.J.; Sheridan, J.F.; Godbout, J.P. Re-Establishment of Anxiety in Stress-Sensitized Mice Is Caused by Monocyte Trafficking from the Spleen to the Brain. Biological Psychiatry 2014, 75, 970–981. [Google Scholar] [CrossRef] [PubMed]
- Fontainhas, A.M.; Wang, M.; Liang, K.J.; Chen, S.; Mettu, P.; Damani, M.; Fariss, R.N.; Li, W.; Wong, W.T. Microglial Morphology and Dynamic Behavior Is Regulated by Ionotropic Glutamatergic and GABAergic Neurotransmission. PLoS ONE 2011, 6, e15973. [Google Scholar] [CrossRef]
- Silverman, M.N.; Sternberg, E.M. Glucocorticoid Regulation of Inflammation and Its Functional Correlates: From HPA Axis to Glucocorticoid Receptor Dysfunction. Annals of the New York Academy of Sciences 2012, 1261, 55–63. [Google Scholar] [CrossRef] [PubMed]
- Sorrells, S.F.; Caso, J.R.; Munhoz, C.D.; Sapolsky, R.M. The Stressed CNS: When Glucocorticoids Aggravate Inflammation. Neuron 2009, 64, 33–39. [Google Scholar] [CrossRef] [PubMed]
- Sorrells, S.F.; Sapolsky, R.M. An Inflammatory Review of Glucocorticoid Actions in the CNS. Brain, Behavior, and Immunity 2007, 21, 259–272. [Google Scholar] [CrossRef]
- Coller, J.K.; Hutchinson, M.R. Implications of Central Immune Signaling Caused by Drugs of Abuse: Mechanisms, Mediators and New Therapeutic Approaches for Prediction and Treatment of Drug Dependence. Pharmacology and Therapeutics 2012, 134, 219–245. [Google Scholar] [CrossRef]
- Liao, K.; Guo, M.; Niu, F.; Yang, L.; Callen, S.E.; Buch, S. Cocaine-Mediated Induction of Microglial Activation Involves the ER Stress-TLR2 Axis. J Neuroinflammation 2016, 13, 33. [Google Scholar] [CrossRef]
- Guo, M.-L.; Roodsari, S.K.; Cheng, Y.; Dempsey, R.E.; Hu, W. Microglia NLRP3 Inflammasome and Neuroimmune Signaling in Substance Use Disorders. Biomolecules 2023, 13, 922. [Google Scholar] [CrossRef]
- Ye, J.; Gao, S.; Liu, Z.; Chen, X.; He, J.; Hu, Z. The HMGB1–RAGE Axis in Nucleus Accumbens Facilitates Cocaine-induced Conditioned Place Preference via Modulating Microglial Activation. Brain and Behavior 2024, 14, e3457. [Google Scholar] [CrossRef]
- Wohleb, E.S.; Delpech, J.-C. Dynamic Cross-Talk between Microglia and Peripheral Monocytes Underlies Stress-Induced Neuroinflammation and Behavioral Consequences. Progress in Neuro-Psychopharmacology and Biological Psychiatry 2017, 79, 40–48. [Google Scholar] [CrossRef]
- Assis, M.A.; Hansen, C.; Lux-Lantos, V.; Cancela, L.M. Sensitization to Amphetamine Occurs Simultaneously at Immune Level and in Met-Enkephalin of the Nucleus Accumbens and Spleen: An Involved NMDA Glutamatergic Mechanism. Brain, Behavior, and Immunity 2009, 23, 464–473. [Google Scholar] [CrossRef]
- Jurga, A.M.; Paleczna, M.; Kuter, K.Z. Overview of General and Discriminating Markers of Differential Microglia Phenotypes. Front. Cell. Neurosci. 2020, 14, 198. [Google Scholar] [CrossRef] [PubMed]
- Niraula, A.; Wang, Y.; Godbout, J.P.; Sheridan, J.F. Corticosterone Production during Repeated Social Defeat Causes Monocyte Mobilization from the Bone Marrow, Glucocorticoid Resistance, and Neurovascular Adhesion Molecule Expression. J. Neurosci. 2018, 38, 2328–2340. [Google Scholar] [CrossRef]
- Moreira, F.P.; Medeiros, J.R.C.; Lhullier, A.C.; Souza, L.D.D.M.; Jansen, K.; Portela, L.V.; Lara, D.R.; Silva, R.A.D.; Wiener, C.D.; Oses, J.P. Cocaine Abuse and Effects in the Serum Levels of Cytokines IL-6 and IL-10. Drug and Alcohol Dependence 2016, 158, 181–185. [Google Scholar] [CrossRef]
- Menard, C.; Pfau, M.L.; Hodes, G.E.; Kana, V.; Wang, V.X.; Bouchard, S.; Takahashi, A.; Flanigan, M.E.; Aleyasin, H.; LeClair, K.B.; et al. Social Stress Induces Neurovascular Pathology Promoting Depression. Nat Neurosci 2017, 20, 1752–1760. [Google Scholar] [CrossRef] [PubMed]
- Banqueri, M.; Méndez, M.; Gómez-Lázaro, E.; Arias, J.L. Early Life Stress by Repeated Maternal Separation Induces Long-Term Neuroinflammatory Response in Glial Cells of Male Rats. Stress 2019, 22, 563–570. [Google Scholar] [CrossRef] [PubMed]
- Aniszewska, A.; Chłodzińska, N.; Bartkowska, K.; Winnicka, M.M.; Turlejski, K.; Djavadian, R.L. The Expression of Interleukin-6 and Its Receptor in Various Brain Regions and Their Roles in Exploratory Behavior and Stress Responses. Journal of Neuroimmunology 2015, 284, 1–9. [Google Scholar] [CrossRef]
- Shirokova, O.M.; Kuzmina, D.M.; Zaborskaya, O.G.; Shchelchkova, N.A.; Kozliaeva, E.V.; Korotchenko, S.A.; Pershin, V.I.; Vasilchikov, P.I.; Mukhina, I.V. The Long-Term Effects of Chronic Unpredictable Mild Stress Experienced During Adolescence Could Vary Depending on Biological Sex. IJMS 2025, 26, 1251. [Google Scholar] [CrossRef]
- Bodemeier Loayza Careaga, M.; Wu, T.J. Chronically Stressed Male and Female Mice Show a Similar Peripheral and Central Pro-Inflammatory Profile after an Immune Challenge. PLoS ONE 2024, 19, e0297776. [Google Scholar] [CrossRef]
- Khakh, B.S.; Sofroniew, M.V. Diversity of Astrocyte Functions and Phenotypes in Neural Circuits. Nat Neurosci 2015, 18, 942–952. [Google Scholar] [CrossRef]
- Sofroniew, M.V.; Vinters, H.V. Astrocytes: Biology and Pathology. Acta Neuropathol 2010, 119, 7–35. [Google Scholar] [CrossRef] [PubMed]
- Verkhratsky, A.; Butt, A.; Li, B.; Illes, P.; Zorec, R.; Semyanov, A.; Tang, Y.; Sofroniew, M.V. Astrocytes in Human Central Nervous System Diseases: A Frontier for New Therapies. Sig Transduct Target Ther 2023, 8, 396. [Google Scholar] [CrossRef]
- Bowers, M.S.; Kalivas, P.W. Forebrain Astroglial Plasticity Is Induced Following Withdrawal from Repeated Cocaine Administration. Eur J of Neuroscience 2003, 17, 1273–1278. [Google Scholar] [CrossRef] [PubMed]
- Narita, M.; Miyatake, M.; Shibasaki, M.; Tsuda, M.; Koizumi, S.; Narita, M.; Yajima, Y.; Inoue, K.; Suzuki, T. Long-lasting Change in Brain Dynamics Induced by Methamphetamine: Enhancement of Protein Kinase C-dependent Astrocytic Response and Behavioral Sensitization. Journal of Neurochemistry 2005, 93, 1383–1392. [Google Scholar] [CrossRef] [PubMed]
- Narita, M.; Suzuki, M.; Kuzumaki, N.; Miyatake, M.; Suzuki, T. Implication of Activated Astrocytes in the Development of Drug Dependence: Differences between Methamphetamine and Morphine. Annals of the New York Academy of Sciences 2008, 1141, 96–104. [Google Scholar] [CrossRef]
- Narita, M.; Miyatake, M.; Narita, M.; Shibasaki, M.; Shindo, K.; Nakamura, A.; Kuzumaki, N.; Nagumo, Y.; Suzuki, T. Direct Evidence of Astrocytic Modulation in the Development of Rewarding Effects Induced by Drugs of Abuse. Neuropsychopharmacol 2006, 31, 2476–2488. [Google Scholar] [CrossRef]
- Testen, A.; Sepulveda-Orengo, M.T.; Gaines, C.H.; Reissner, K.J. Region-Specific Reductions in Morphometric Properties and Synaptic Colocalization of Astrocytes Following Cocaine Self-Administration and Extinction. Front. Cell. Neurosci. 2018, 12, 246. [Google Scholar] [CrossRef] [PubMed]
- Siemsen, B.M.; Reichel, C.M.; Leong, K.C.; Garcia-Keller, C.; Gipson, C.D.; Spencer, S.; McFaddin, J.A.; Hooker, K.N.; Kalivas, P.W.; Scofield, M.D. Effects of Methamphetamine Self-Administration and Extinction on Astrocyte Structure and Function in the Nucleus Accumbens Core. Neuroscience 2019, 406, 528–541. [Google Scholar] [CrossRef]
- Tynan, R.J.; Beynon, S.B.; Hinwood, M.; Johnson, S.J.; Nilsson, M.; Woods, J.J.; Walker, F.R. Chronic Stress-Induced Disruption of the Astrocyte Network Is Driven by Structural Atrophy and Not Loss of Astrocytes. Acta Neuropathol 2013, 126, 75–91. [Google Scholar] [CrossRef]
- Çalışkan, G.; Müller, A.; Albrecht, A. Long-Term Impact of Early-Life Stress on Hippocampal Plasticity: Spotlight on Astrocytes. IJMS 2020, 21, 4999. [Google Scholar] [CrossRef]
- Leventopoulos, M.; Rüedi-Bettschen, D.; Knuesel, I.; Feldon, J.; Pryce, C.R.; Opacka-Juffry, J. Long-Term Effects of Early Life Deprivation on Brain Glia in Fischer Rats. Brain Research 2007, 1142, 119–126. [Google Scholar] [CrossRef]
- Musholt, K.; Cirillo, G.; Cavaliere, C.; Rosaria Bianco, M.; Bock, J.; Helmeke, C.; Braun, K.; Papa, M. Neonatal Separation Stress Reduces Glial Fibrillary Acidic Protein- and S100β-immunoreactive Astrocytes in the Rat Medial Precentral Cortex. Developmental Neurobiology 2009, 69, 203–211. [Google Scholar] [CrossRef]
- Woodburn, S.C.; Bollinger, J.L.; Wohleb, E.S. Synaptic and Behavioral Effects of Chronic Stress Are Linked to Dynamic and Sex-Specific Changes in Microglia Function and Astrocyte Dystrophy. Neurobiology of Stress 2021, 14, 100312. [Google Scholar] [CrossRef] [PubMed]
- Zhang, A.Y.; Elias, E.; Manners, M.T. Sex-Dependent Astrocyte Reactivity: Unveiling Chronic Stress-Induced Morphological Changes across Multiple Brain Regions. Neurobiology of Disease 2024, 200, 106610. [Google Scholar] [CrossRef]
- Berger, U.V.; Hediger, M.A. Comparative Analysis of Glutamate Transporter Expression in Rat Brain Using Differential Double in Situ Hybridization. Anatomy and Embryology 1998, 198, 13–30. [Google Scholar] [CrossRef]
- Berger, U.V.; Hediger, M.A. Distribution of the Glutamate Transporters GLAST and GLT-1 in Rat Circumventricular Organs, Meninges, and Dorsal Root Ganglia. J Comp Neurol 2000, 421, 385–399. [Google Scholar] [CrossRef]
- Berger, U.V.; Hediger, M.A. Differential Distribution of the Glutamate Transporters GLT-1 and GLAST in Tanycytes of the Third Ventricle. J of Comparative Neurology 2001, 433, 101–114. [Google Scholar] [CrossRef]
- Persson, M.; Brantefjord, M.; Hansson, E.; Rönnbäck, L. Lipopolysaccharide Increases Microglial GLT-1 Expression and Glutamate Uptake Capacity in Vitro by a Mechanism Dependent on TNF-α. Glia 2005, 51, 111–120. [Google Scholar] [CrossRef]
- Shaked, I.; Tchoresh, D.; Gersner, R.; Meiri, G.; Mordechai, S.; Xiao, X.; Hart, R.P.; Schwartz, M. Protective Autoimmunity: Interferon-γ Enables Microglia to Remove Glutamate without Evoking Inflammatory Mediators. Journal of Neurochemistry 2005, 92, 997–1009. [Google Scholar] [CrossRef]
- Lehre, K.; Levy, L.; Ottersen, O.; Storm-Mathisen, J.; Danbolt, N. Differential Expression of Two Glial Glutamate Transporters in the Rat Brain: Quantitative and Immunocytochemical Observations. J. Neurosci. 1995, 15, 1835–1853. [Google Scholar] [CrossRef] [PubMed]
- Murugan, M.; Ling, E.-A.; Kaur, C. Glutamate Receptors in Microglia. CNSNDDT 2013, 12, 773–784. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; Leak, R.K.; Hu, X. Neurotransmitter Receptors on Microglia. Stroke Vasc Neurol 2016, 1, 52–58. [Google Scholar] [CrossRef]
- Moghaddam, B.; Bolinao, M.L.; Stein-Behrens, B.; Sapolsky, R. Glucocortcoids Mediate the Stress-Induced Extracellular Accumulation of Glutamate. Brain Research 1994, 655, 251–254. [Google Scholar] [CrossRef]
- Popoli, M.; Yan, Z.; McEwen, B.S.; Sanacora, G. The Stressed Synapse: The Impact of Stress and Glucocorticoids on Glutamate Transmission. Nat Rev Neurosci 2012, 13, 22–37. [Google Scholar] [CrossRef]
- Fontainhas, A.M.; Wang, M.; Liang, K.J.; Chen, S.; Mettu, P.; Damani, M.; Fariss, R.N.; Li, W.; Wong, W.T. Microglial Morphology and Dynamic Behavior Is Regulated by Ionotropic Glutamatergic and GABAergic Neurotransmission. PLoS ONE 2011, 6, e15973. [Google Scholar] [CrossRef] [PubMed]
- Eyo, U.B.; Peng, J.; Swiatkowski, P.; Mukherjee, A.; Bispo, A.; Wu, L.-J. Neuronal Hyperactivity Recruits Microglial Processes via Neuronal NMDA Receptors and Microglial P2Y12 Receptors after Status Epilepticus. Journal of Neuroscience 2014, 34, 10528–10540. [Google Scholar] [CrossRef]
- Virgin, C.E.; Ha, T.P. -T.; Packan, D.R.; Tombaugh, G.C.; Yang, S.H.; Homer, H.C.; Sapolsky, R.M. Glucocorticoids Inhibit Glucose Transport and Glutamate Uptake in Hippocampal Astrocytes: Implications for Glucocorticoid Neurotoxicity. Journal of Neurochemistry 1991, 57, 1422–1428. [Google Scholar] [CrossRef]
- Chaparro-Huerta, V.; Rivera-Cervantes, M.C.; Torres-Mendoza, B.M.; Beas-Zárate, C. Neuronal Death and Tumor Necrosis Factor-α Response to Glutamate-Induced Excitotoxicity in the Cerebral Cortex of Neonatal Rats. Neuroscience Letters 2002, 333, 95–98. [Google Scholar] [CrossRef]
- Noda, M.; Nakanishi, H.; Nabekura, J.; Akaike, N. AMPA–Kainate Subtypes of Glutamate Receptor in Rat Cerebral Microglia. J. Neurosci. 2000, 20, 251–258. [Google Scholar] [CrossRef] [PubMed]
- Taylor, D.L.; Jones, F.; Kubota, E.S.F.C.S.; Pocock, J.M. Stimulation of Microglial Metabotropic Glutamate Receptor mGlu2 Triggers Tumor Necrosis Factor α-Induced Neurotoxicity in Concert with Microglial-Derived Fas Ligand. J. Neurosci. 2005, 25, 2952–2964. [Google Scholar] [CrossRef] [PubMed]
- Zhao, W.; Xie, W.; Le, W.; Beers, D.R.; He, Y.; Henkel, J.S.; Simpson, E.P.; Yen, A.A.; Xiao, Q.; Appel, S.H. Activated Microglia Initiate Motor Neuron Injury by a Nitric Oxide and Glutamate-Mediated Mechanism. J Neuropathol Exp Neurol 2004, 63, 964–977. [Google Scholar] [CrossRef]
- Zhu, W.; Zheng, H.; Shao, X.; Wang, W.; Yao, Q.; Li, Z. Excitotoxicity of TNFα Derived from KA Activated Microglia on Hippocampal Neurons in Vitro and in Vivo. Journal of Neurochemistry 2010, 114, 386–396. [Google Scholar] [CrossRef]
- De, A.; Krueger, J.M.; Simasko, S.M. Glutamate Induces the Expression and Release of Tumor Necrosis Factor-α in Cultured Hypothalamic Cells. Brain Research 2005, 1053, 54–61. [Google Scholar] [CrossRef]
- Cahoy, J.D.; Emery, B.; Kaushal, A.; Foo, L.C.; Zamanian, J.L.; Christopherson, K.S.; Xing, Y.; Lubischer, J.L.; Krieg, P.A.; Krupenko, S.A.; et al. A Transcriptome Database for Astrocytes, Neurons, and Oligodendrocytes: A New Resource for Understanding Brain Development and Function. J Neurosci 2008, 28, 264–278. [Google Scholar] [CrossRef]
- Doyle, J.P.; Dougherty, J.D.; Heiman, M.; Schmidt, E.F.; Stevens, T.R.; Ma, G.; Bupp, S.; Shrestha, P.; Shah, R.D.; Doughty, M.L.; et al. Application of a Translational Profiling Approach for the Comparative Analysis of CNS Cell Types. Cell 2008, 135, 749–762. [Google Scholar] [CrossRef]
- Foo, L.C.; Allen, N.J.; Bushong, E.A.; Ventura, P.B.; Chung, W.-S.; Zhou, L.; Cahoy, J.D.; Daneman, R.; Zong, H.; Ellisman, M.H.; et al. Development of a Method for the Purification and Culture of Rodent Astrocytes. Neuron 2011, 71, 799–811. [Google Scholar] [CrossRef] [PubMed]
- Meissner, A.; Mikkelsen, T.S.; Gu, H.; Wernig, M.; Hanna, J.; Sivachenko, A.; Zhang, X.; Bernstein, B.E.; Nusbaum, C.; Jaffe, D.B.; et al. Genome-Scale DNA Methylation Maps of Pluripotent and Differentiated Cells. Nature 2008, 454, 766–770. [Google Scholar] [CrossRef] [PubMed]
- Sharma, M.K.; Mansur, D.B.; Reifenberger, G.; Perry, A.; Leonard, J.R.; Aldape, K.D.; Albin, M.G.; Emnett, R.J.; Loeser, S.; Watson, M.A.; et al. Distinct Genetic Signatures among Pilocytic Astrocytomas Relate to Their Brain Region Origin. Cancer Research 2007, 67, 890–900. [Google Scholar] [CrossRef] [PubMed]
- Zamanian, J.L.; Xu, L.; Foo, L.C.; Nouri, N.; Zhou, L.; Giffard, R.G.; Barres, B.A. Genomic Analysis of Reactive Astrogliosis. J. Neurosci. 2012, 32, 6391–6410. [Google Scholar] [CrossRef]
- Clark, A.K.; Gruber-Schoffnegger, D.; Drdla-Schutting, R.; Gerhold, K.J.; Malcangio, M.; Sandkühler, J. Selective Activation of Microglia Facilitates Synaptic Strength. J. Neurosci. 2015, 35, 4552–4570. [Google Scholar] [CrossRef]
- Wohleb, E.S.; Terwilliger, R.; Duman, C.H.; Duman, R.S. Stress-Induced Neuronal Colony Stimulating Factor 1 Provokes Microglia-Mediated Neuronal Remodeling and Depressive-like Behavior. Biological Psychiatry 2018, 83, 38–49. [Google Scholar] [CrossRef]
- Bianco, F.; Pravettoni, E.; Colombo, A.; Schenk, U.; Möller, T.; Matteoli, M.; Verderio, C. Astrocyte-Derived ATP Induces Vesicle Shedding and IL-1β Release from Microglia. The Journal of Immunology 2005, 174, 7268–7277. [Google Scholar] [CrossRef]
- Jha, M.K.; Jo, M.; Kim, J.-H.; Suk, K. Microglia-Astrocyte Crosstalk: An Intimate Molecular Conversation. Neuroscientist 2019, 25, 227–240. [Google Scholar] [CrossRef] [PubMed]
- Fine, S.M.; Angel, R.A.; Perry, S.W.; Epstein, L.G.; Rothstein, J.D.; Dewhurst, S.; Gelbard, H.A. Tumor Necrosis Factor α Inhibits Glutamate Uptake by Primary Human Astrocytes. Journal of Biological Chemistry 1996, 271, 15303–15306. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Pekarskaya, O.; Bencheikh, M.; Chao, W.; Gelbard, H.A.; Ghorpade, A.; Rothstein, J.D.; Volsky, D.J. Reduced Expression of Glutamate Transporter EAAT2 and Impaired Glutamate Transport in Human Primary Astrocytes Exposed to HIV-1 or Gp120. Virology 2003, 312, 60–73. [Google Scholar] [CrossRef]
- Sitcheran, R.; Gupta, P.; Fisher, P.B.; Baldwin, A.S. Positive and Negative Regulation of EAAT2 by NF-κB: A Role for N-Myc in TNFα-Controlled Repression. EMBO J 2005, 24, 510–520. [Google Scholar] [CrossRef]
- Zou, J.Y.; Crews, F.T. TNFα Potentiates Glutamate Neurotoxicity by Inhibiting Glutamate Uptake in Organotypic Brain Slice Cultures: Neuroprotection by NFκB Inhibition. Brain Research 2005, 1034, 11–24. [Google Scholar] [CrossRef] [PubMed]
- Su, Z.; Leszczyniecka, M.; Kang, D.; Sarkar, D.; Chao, W.; Volsky, D.J.; Fisher, P.B. Insights into Glutamate Transport Regulation in Human Astrocytes: Cloning of the Promoter for Excitatory Amino Acid Transporter 2 (EAAT2). Proc. Natl. Acad. Sci. U.S.A. 2003, 100, 1955–1960. [Google Scholar] [CrossRef]
- Madrigal, J. The Increase in TNF-α Levels Is Implicated in NF-κB Activation and Inducible Nitric Oxide Synthase Expression in Brain Cortex after Immobilization Stress. Neuropsychopharmacology 2002, 26, 155–163. [Google Scholar] [CrossRef]
- Prow, N.A.; Irani, D.N. The Inflammatory Cytokine, Interleukin-1 Beta, Mediates Loss of Astroglial Glutamate Transport and Drives Excitotoxic Motor Neuron Injury in the Spinal Cord during Acute Viral Encephalomyelitis. Journal of Neurochemistry 2008, 105, 1276–1286. [Google Scholar] [CrossRef]
- Hughes, E.G.; Maguire, J.L.; McMinn, M.T.; Scholz, R.E.; Sutherland, M.L. Loss of Glial Fibrillary Acidic Protein Results in Decreased Glutamate Transport and Inhibition of PKA-Induced EAAT2 Cell Surface Trafficking. Molecular Brain Research 2004, 124, 114–123. [Google Scholar] [CrossRef]
- Yang, Y.; Gozen, O.; Watkins, A.; Lorenzini, I.; Lepore, A.; Gao, Y.; Vidensky, S.; Brennan, J.; Poulsen, D.; Won Park, J.; et al. Presynaptic Regulation of Astroglial Excitatory Neurotransmitter Transporter GLT1. Neuron 2009, 61, 880–894. [Google Scholar] [CrossRef]
- Nakagawa, T.; Otsubo, Y.; Yatani, Y.; Shirakawa, H.; Kaneko, S. Mechanisms of Substrate Transport-induced Clustering of a Glial Glutamate Transporter GLT-1 in Astroglial–Neuronal Cultures. Eur J of Neuroscience 2008, 28, 1719–1730. [Google Scholar] [CrossRef]
- Zhou, J.; Sutherland, M.L. Glutamate Transporter Cluster Formation in Astrocytic Processes Regulates Glutamate Uptake Activity. J. Neurosci. 2004, 24, 6301–6306. [Google Scholar] [CrossRef]
- Scofield, M.D.; Li, H.; Siemsen, B.M.; Healey, K.L.; Tran, P.K.; Woronoff, N.; Boger, H.A.; Kalivas, P.W.; Reissner, K.J. Cocaine Self-Administration and Extinction Leads to Reduced Glial Fibrillary Acidic Protein Expression and Morphometric Features of Astrocytes in the Nucleus Accumbens Core. Biological Psychiatry 2016, 80, 207–215. [Google Scholar] [CrossRef] [PubMed]
- Stellwagen, D.; Beattie, E.C.; Seo, J.Y.; Malenka, R.C. Differential Regulation of AMPA Receptor and GABA Receptor Trafficking by Tumor Necrosis Factor-α. J. Neurosci. 2005, 25, 3219–3228. [Google Scholar] [CrossRef]
- Pandey, G.N.; Rizavi, H.S.; Zhang, H.; Ren, X. Abnormal Gene and Protein Expression of Inflammatory Cytokines in the Postmortem Brain of Schizophrenia Patients. Schizophrenia Research 2018, 192, 247–254. [Google Scholar] [CrossRef]
- Uzzan, S.; Azab, A.N. Anti-TNF-α Compounds as a Treatment for Depression. Molecules 2021, 26, 2368. [Google Scholar] [CrossRef]
- Gupta, S.; Guleria, R.S. Involvement of Nuclear Factor-κB in Inflammation and Neuronal Plasticity Associated with Post-Traumatic Stress Disorder. Cells 2022, 11, 2034. [Google Scholar] [CrossRef] [PubMed]
- Mattson, M.P.; Meffert, M.K. Roles for NF-κB in Nerve Cell Survival, Plasticity, and Disease. Cell Death Differ 2006, 13, 852–860. [Google Scholar] [CrossRef] [PubMed]
- Singh, S.; Singh, T.G. Role of Nuclear Factor Kappa B (NF-κB) Signalling in Neurodegenerative Diseases: An Mechanistic Approach. CN 2020, 18, 918–935. [Google Scholar] [CrossRef]
- Kaltschmidt, B.; Widera, D.; Kaltschmidt, C. Signaling via NF-κB in the Nervous System. Biochimica et Biophysica Acta (BBA) - Molecular Cell Research 2005, 1745, 287–299. [Google Scholar] [CrossRef]
- Dresselhaus, E.C.; Meffert, M.K. Cellular Specificity of NF-κB Function in the Nervous System. Front. Immunol. 2019, 10, 1043. [Google Scholar] [CrossRef]
- Oeckinghaus, A.; Ghosh, S. The NF- B Family of Transcription Factors and Its Regulation. Cold Spring Harbor Perspectives in Biology 2009, 1, a000034–a000034. [Google Scholar] [CrossRef]
- Nennig, S.E.; Schank, J.R. The Role of NFkB in Drug Addiction: Beyond Inflammation. Alcohol and Alcoholism 2017, 52, 172–179. [Google Scholar] [CrossRef]
- Ang, E.; Chen, J.; Zagouras, P.; Magna, H.; Holland, J.; Schaeffer, E.; Nestler, E.J. Induction of Nuclear factor-κB in Nucleus Accumbens by Chronic Cocaine Administration. Journal of Neurochemistry 2001, 79, 221–224. [Google Scholar] [CrossRef]
- Russo, S.J.; Wilkinson, M.B.; Mazei-Robison, M.S.; Dietz, D.M.; Maze, I.; Krishnan, V.; Renthal, W.; Graham, A.; Birnbaum, S.G.; Green, T.A.; et al. Nuclear Factor κB Signaling Regulates Neuronal Morphology and Cocaine Reward. J. Neurosci. 2009, 29, 3529–3537. [Google Scholar] [CrossRef]
- Namba, M.D.; Phillips, M.N.; Neisewander, J.L.; Olive, M.F. Nuclear Factor Kappa B Signaling within the Rat Nucleus Accumbens Core Sex-Dependently Regulates Cue-Induced Cocaine Seeking and Matrix Metalloproteinase-9 Expression. Brain, Behavior, and Immunity 2022, 102, 252–265. [Google Scholar] [CrossRef]
- Wang, F.; Liu, H.; Ke, Y.; Huang, X.; Chen, S.; Zhuang, D.; Zhou, Y.; Wu, M.; Wang, Y.; Lai, M.; et al. Ibudilast-Mediated Suppression of Neuronal TLR4 in the Prefrontal Cortex Mitigates Methamphetamine-Induced Neuroinflammation and Addictive Behaviours. Addiction Biology 2025, 30, e70033. [Google Scholar] [CrossRef] [PubMed]
- Cohen, H.; Kozlovsky, N.; Matar, M.A.; Zohar, J.; Kaplan, Z. The Characteristic Long-Term Upregulation of Hippocampal NF-κB Complex in PTSD-Like Behavioral Stress Response Is Normalized by High-Dose Corticosterone and Pyrrolidine Dithiocarbamate Administered Immediately after Exposure. Neuropsychopharmacol 2011, 36, 2286–2302. [Google Scholar] [CrossRef]
- Zoppi, S.; Pérez Nievas, B.G.; Madrigal, J.L.M.; Manzanares, J.; Leza, J.C.; García-Bueno, B. Regulatory Role of Cannabinoid Receptor 1 in Stress-Induced Excitotoxicity and Neuroinflammation. Neuropsychopharmacol 2011, 36, 805–818. [Google Scholar] [CrossRef] [PubMed]
- Christoffel, D.J.; Golden, S.A.; Dumitriu, D.; Robison, A.J.; Janssen, W.G.; Ahn, H.F.; Krishnan, V.; Reyes, C.M.; Han, M.-H.; Ables, J.L.; et al. IκB Kinase Regulates Social Defeat Stress-Induced Synaptic and Behavioral Plasticity. J. Neurosci. 2011, 31, 314–321. [Google Scholar] [CrossRef] [PubMed]
- Smaga, I.; Fierro, D.; Mesa, J.; Filip, M.; Knackstedt, L.A. Molecular Changes Evoked by the Beta-Lactam Antibiotic Ceftriaxone across Rodent Models of Substance Use Disorder and Neurological Disease. Neuroscience & Biobehavioral Reviews 2020, 115, 116–130. [Google Scholar] [CrossRef] [PubMed]
- Bessa, J.M.; Morais, M.; Marques, F.; Pinto, L.; Palha, J.A.; Almeida, O.F.X.; Sousa, N. Stress-Induced Anhedonia Is Associated with Hypertrophy of Medium Spiny Neurons of the Nucleus Accumbens. Transl Psychiatry 2013, 3, e266–e266. [Google Scholar] [CrossRef]
- Loweth, J.A.; Tseng, K.Y.; Wolf, M.E. Adaptations in AMPA Receptor Transmission in the Nucleus Accumbens Contributing to Incubation of Cocaine Craving. Neuropharmacology 2014, 76, 287–300. [Google Scholar] [CrossRef] [PubMed]
- Dong, Y.; Nestler, E.J. The Neural Rejuvenation Hypothesis of Cocaine Addiction. Trends in Pharmacological Sciences 2014, 35, 374–383. [Google Scholar] [CrossRef]
- Taylor, S.B.; Anglin, J.M.; Paode, P.R.; Riggert, A.G.; Olive, M.F.; Conrad, C.D. Chronic Stress May Facilitate the Recruitment of Habit- and Addiction-Related Neurocircuitries through Neuronal Restructuring of the Striatum. Neuroscience 2014, 280, 231–242. [Google Scholar] [CrossRef]
- Brown, K.T.; Bachtell, R.K. Activation of the Immune System During a Developmental Window May Provide a Link Between Early Life Stress and Future Susceptibility to Cocaine Abuse. Biological Psychiatry 2018, 84, 865–866. [Google Scholar] [CrossRef]
- Snider, S.E.; Hendrick, E.S.; Beardsley, P.M. Glial Cell Modulators Attenuate Methamphetamine Self-Administration in Therat. European Journal of Pharmacology 2013, 701, 124–130. [Google Scholar] [CrossRef]
- Weber, M.D.; Frank, M.G.; Tracey, K.J.; Watkins, L.R.; Maier, S.F. Stress Induces the Danger-Associated Molecular Pattern HMGB-1 in the Hippocampus of Male Sprague Dawley Rats: A Priming Stimulus of Microglia and the NLRP3 Inflammasome. J. Neurosci. 2015, 35, 316–324. [Google Scholar] [CrossRef] [PubMed]
- Nimmerjahn, A.; Kirchhoff, F.; Helmchen, F. Resting Microglial Cells Are Highly Dynamic Surveillants of Brain Parenchyma in Vivo. Science 2005, 308, 1314–1318. [Google Scholar] [CrossRef]
- Kessler, R.C. THE EFFECTS OF STRESSFUL LIFE EVENTS ON DEPRESSION. Annu. Rev. Psychol. 1997, 48, 191–214. [Google Scholar] [CrossRef]
- Gaspar, R.; Soares-Cunha, C.; Domingues, A.V.; Coimbra, B.; Baptista, F.I.; Pinto, L.; Ambrósio, A.F.; Rodrigues, A.J.; Gomes, C.A. Resilience to Stress and Sex-Specific Remodeling of Microglia and Neuronal Morphology in a Rat Model of Anxiety and Anhedonia. Neurobiology of Stress 2021, 14, 100302. [Google Scholar] [CrossRef]
- Nowak, D.B.; Taborda-Bejarano, J.P.; Chaure, F.J.; Mantsch, J.R.; Garcia-Keller, C. Understanding Microglia in Mesocorticolimbic Circuits: Implications for the Study of Chronic Stress and Substance Use Disorders. Cells 2025, 14, 1014. [Google Scholar] [CrossRef]
- Reverte, I.; Marchetti, C.; Pezza, S.; Zenoni, S.F.; Scaringi, G.; Ferrucci, L.; D’Ottavio, G.; Pignataro, A.; Andolina, D.; Raspa, M.; et al. Microglia-Mediated Calcium-Permeable AMPAR Accumulation in the Nucleus Accumbens Drives Hyperlocomotion during Cocaine Withdrawal. Brain, Behavior, and Immunity 2024, 115, 535–542. [Google Scholar] [CrossRef] [PubMed]
- Spiga, S.; Mulas, G.; Piras, F.; Diana, M. The €œaddicted†Spine. Front. Neuroanat. 2014, 8. [Google Scholar] [CrossRef] [PubMed]
- Keck, T.; Mrsic-Flogel, T.D.; Vaz Afonso, M.; Eysel, U.T.; Bonhoeffer, T.; Hübener, M. Massive Restructuring of Neuronal Circuits during Functional Reorganization of Adult Visual Cortex. Nat Neurosci 2008, 11, 1162–1167. [Google Scholar] [CrossRef] [PubMed]
- Yang, G.; Pan, F.; Gan, W.-B. Stably Maintained Dendritic Spines Are Associated with Lifelong Memories. Nature 2009, 462, 920–924. [Google Scholar] [CrossRef]
- Cahill, M.E.; Walker, D.M.; Gancarz, A.M.; Wang, Z.J.; Lardner, C.K.; Bagot, R.C.; Neve, R.L.; Dietz, D.M.; Nestler, E.J. The Dendritic Spine Morphogenic Effects of Repeated Cocaine Use Occur through the Regulation of Serum Response Factor Signaling. Mol Psychiatry 2018, 23, 1474–1486. [Google Scholar] [CrossRef]
- Kim, J.; Park, B.-H.; Lee, J.H.; Park, S.K.; Kim, J.-H. Cell Type-Specific Alterations in the Nucleus Accumbens by Repeated Exposures to Cocaine. Biological Psychiatry 2011, 69, 1026–1034. [Google Scholar] [CrossRef]
- Lee, K.-W.; Kim, Y.; Kim, A.M.; Helmin, K.; Nairn, A.C.; Greengard, P. Cocaine-Induced Dendritic Spine Formation in D1 and D2 Dopamine Receptor-Containing Medium Spiny Neurons in Nucleus Accumbens. Proc. Natl. Acad. Sci. U.S.A. 2006, 103, 3399–3404. [Google Scholar] [CrossRef]
- Robinson, T.E.; Kolb, B. Alterations in the Morphology of Dendrites and Dendritic Spines in the Nucleus Accumbens and Prefrontal Cortex Following Repeated Treatment with Amphetamine or Cocaine. Eur J of Neuroscience 1999, 11, 1598–1604. [Google Scholar] [CrossRef]
- Toda, S.; Shen, H.; Kalivas, P.W. Inhibition of Actin Polymerization Prevents Cocaine-Induced Changes in Spine Morphology in the Nucleus Accumbens. Neurotox Res 2010, 18, 410–415. [Google Scholar] [CrossRef]
- Kourrich, S.; Rothwell, P.E.; Klug, J.R.; Thomas, M.J. Cocaine Experience Controls Bidirectional Synaptic Plasticity in the Nucleus Accumbens. J. Neurosci. 2007, 27, 7921–7928. [Google Scholar] [CrossRef]
- Shen, H.; Moussawi, K.; Zhou, W.; Toda, S.; Kalivas, P.W. Heroin Relapse Requires Long-Term Potentiation-like Plasticity Mediated by NMDA2b-Containing Receptors. Proceedings of the National Academy of Sciences 2011, 108, 19407–19412. [Google Scholar] [CrossRef]
- Gipson, C.D.; Kupchik, Y.M.; Kalivas, P.W. Rapid, Transient Synaptic Plasticity in Addiction. Neuropharmacology 2014, 76, 276–286. [Google Scholar] [CrossRef]
- Anderson, S.M.; Famous, K.R.; Sadri-Vakili, G.; Kumaresan, V.; Schmidt, H.D.; Bass, C.E.; Terwilliger, E.F.; Cha, J.-H.J.; Pierce, R.C. CaMKII: A Biochemical Bridge Linking Accumbens Dopamine and Glutamate Systems in Cocaine Seeking. Nat Neurosci 2008, 11, 344–353. [Google Scholar] [CrossRef]
- Boudreau, A.C.; Reimers, J.M.; Milovanovic, M.; Wolf, M.E. Cell Surface AMPA Receptors in the Rat Nucleus Accumbens Increase during Cocaine Withdrawal But Internalize after Cocaine Challenge in Association with Altered Activation of Mitogen-Activated Protein Kinases. J. Neurosci. 2007, 27, 10621–10635. [Google Scholar] [CrossRef]
- Spencer, S.; Garcia-Keller, C.; Roberts-Wolfe, D.; Heinsbroek, J.A.; Mulvaney, M.; Sorrell, A.; Kalivas, P.W. Cocaine Use Reverses Striatal Plasticity Produced During Cocaine Seeking. Biological Psychiatry 2017, 81, 616–624. [Google Scholar] [CrossRef] [PubMed]
- Kasai, H.; Fukuda, M.; Watanabe, S.; Hayashi-Takagi, A.; Noguchi, J. Structural Dynamics of Dendritic Spines in Memory and Cognition. Trends in Neurosciences 2010, 33, 121–129. [Google Scholar] [CrossRef] [PubMed]
- Fox, M.E.; Figueiredo, A.; Menken, M.S.; Lobo, M.K. Dendritic Spine Density Is Increased on Nucleus Accumbens D2 Neurons after Chronic Social Defeat. Sci Rep 2020, 10, 12393. [Google Scholar] [CrossRef] [PubMed]
- Harris, K.M.; Kater, S.B. Dendritic Spines: Cellular Specializations Imparting Both Stability and Flexibility to Synaptic Function. Annu. Rev. Neurosci. 1994, 17, 341–371. [Google Scholar] [CrossRef]
- Golden, S.A.; Russo, S.J. Mechanisms of Psychostimulant-Induced Structural Plasticity. Cold Spring Harbor Perspectives in Medicine 2012, 2, a011957–a011957. [Google Scholar] [CrossRef] [PubMed]
- Kopec, C.D.; Li, B.; Wei, W.; Boehm, J.; Malinow, R. Glutamate Receptor Exocytosis and Spine Enlargement during Chemically Induced Long-Term Potentiation. J. Neurosci. 2006, 26, 2000–2009. [Google Scholar] [CrossRef] [PubMed]
- Park, M.; Penick, E.C.; Edwards, J.G.; Kauer, J.A.; Ehlers, M.D. Recycling Endosomes Supply AMPA Receptors for LTP. Science 2004, 305, 1972–1975. [Google Scholar] [CrossRef] [PubMed]
- Park, M.; Salgado, J.M.; Ostroff, L.; Helton, T.D.; Robinson, C.G.; Harris, K.M.; Ehlers, M.D. Plasticity-Induced Growth of Dendritic Spines by Exocytic Trafficking from Recycling Endosomes. Neuron 2006, 52, 817–830. [Google Scholar] [CrossRef]
- Kasai, H. Unraveling the Mysteries of Dendritic Spine Dynamics: Five Key Principles Shaping Memory and Cognition. Proc. Jpn. Acad., Ser. B 2023, 99, 254–305. [Google Scholar] [CrossRef]
- Matsuzaki, M.; Ellis-Davies, G.C.R.; Nemoto, T.; Miyashita, Y.; Iino, M.; Kasai, H. Dendritic Spine Geometry Is Critical for AMPA Receptor Expression in Hippocampal CA1 Pyramidal Neurons. Nat Neurosci 2001, 4, 1086–1092. [Google Scholar] [CrossRef]
- Russo, S.J.; Dietz, D.M.; Dumitriu, D.; Morrison, J.H.; Malenka, R.C.; Nestler, E.J. The Addicted Synapse: Mechanisms of Synaptic and Structural Plasticity in Nucleus Accumbens. Trends in Neurosciences 2010, 33, 267–276. [Google Scholar] [CrossRef]
- De, A.; Krueger, J.M.; Simasko, S.M. Glutamate Induces the Expression and Release of Tumor Necrosis Factor-α in Cultured Hypothalamic Cells. Brain Research 2005, 1053, 54–61. [Google Scholar] [CrossRef]
- Taylor, D.L.; Jones, F.; Kubota, E.S.F.C.S.; Pocock, J.M. Stimulation of Microglial Metabotropic Glutamate Receptor mGlu2 Triggers Tumor Necrosis Factor α-Induced Neurotoxicity in Concert with Microglial-Derived Fas Ligand. J. Neurosci. 2005, 25, 2952–2964. [Google Scholar] [CrossRef]
- Beattie, E.C.; Stellwagen, D.; Morishita, W.; Bresnahan, J.C.; Ha, B.K.; Von Zastrow, M.; Beattie, M.S.; Malenka, R.C. Control of Synaptic Strength by Glial TNFα. Science 2002, 295, 2282–2285. [Google Scholar] [CrossRef]
- Albensi, B.C.; Mattson, M.P. Evidence for the Involvement of TNF and NF-?B in Hippocampal Synaptic Plasticity. Synapse 2000, 35, 151–159. [Google Scholar] [CrossRef]
- Loscher, C.E.; Mills, K.H.G.; Lynch, M.A. Interleukin-1 Receptor Antagonist Exerts Agonist Activity in the Hippocampus Independent of the Interleukin-1 Type I Receptor. Journal of Neuroimmunology 2003, 137, 117–124. [Google Scholar] [CrossRef]
- Schmid, A.W.; Lynch, M.A.; Herron, C.E. The Effects of IL-1 Receptor Antagonist on Beta Amyloid Mediated Depression of LTP in the Rat CA1 in Vivo. Hippocampus 2009, 19, 670–676. [Google Scholar] [CrossRef]
- Vereker, E.; O’Donnell, E.; Lynch, M.A. The Inhibitory Effect of Interleukin-1β on Long-Term Potentiation Is Coupled with Increased Activity of Stress-Activated Protein Kinases. J. Neurosci. 2000, 20, 6811–6819. [Google Scholar] [CrossRef] [PubMed]
- Ikegaya, Y.; Delcroix, I.; Iwakura, Y.; Matsuki, N.; Nishiyama, N. Interleukin-1β Abrogates Long-term Depression of Hippocampal CA1 Synaptic Transmission. Synapse 2003, 47, 54–57. [Google Scholar] [CrossRef]
- Lai, A.Y.; Swayze, R.D.; El-Husseini, A.; Song, C. Interleukin-1 Beta Modulates AMPA Receptor Expression and Phosphorylation in Hippocampal Neurons. Journal of Neuroimmunology 2006, 175, 97–106. [Google Scholar] [CrossRef]
- Mir, S.; Sen, T.; Sen, N. Cytokine-Induced GAPDH Sulfhydration Affects PSD95 Degradation and Memory. Molecular Cell 2014, 56, 786–795. [Google Scholar] [CrossRef]
- Dean, O.; Giorlando, F.; Berk, M. N -Acetylcysteine in Psychiatry: Current Therapeutic Evidence and Potential Mechanisms of Action. J Psychiatry Neurosci 2011, 36, 78–86. [Google Scholar] [CrossRef]
- Viña, J.; Romero, F.J.; Saez, G.T.; Pallardó, F.V. Effects of Cysteine and N-Acetyl Cysteine on GSH Content of Brain of Adult Rats. Experientia 1983, 39, 164–165. [Google Scholar] [CrossRef]
- Yin, J.; Ren, W.; Yang, G.; Duan, J.; Huang, X.; Fang, R.; Li, C.; Li, T.; Yin, Y.; Hou, Y.; et al. l -Cysteine Metabolism and Its Nutritional Implications. Molecular Nutrition Food Res 2016, 60, 134–146. [Google Scholar] [CrossRef] [PubMed]
- Pallanti, S.; Grassi, G. Pharmacologic Treatment of Obsessive-Compulsive Disorder Comorbidity. Expert Opinion on Pharmacotherapy 2014, 15, 2543–2552. [Google Scholar] [CrossRef]
- Ramkissoon, A.; Wells, P.G. Methamphetamine Oxidative Stress, Neurotoxicity, and Functional Deficits Are Modulated by Nuclear Factor-E2-Related Factor 2. Free Radical Biology and Medicine 2015, 89, 358–368. [Google Scholar] [CrossRef]
- Huang, Y.-N.; Yang, L.-Y.; Wang, J.-Y.; Lai, C.-C.; Chiu, C.-T.; Wang, J.-Y. L-Ascorbate Protects Against Methamphetamine-Induced Neurotoxicity of Cortical Cells via Inhibiting Oxidative Stress, Autophagy, and Apoptosis. Mol Neurobiol 2017, 54, 125–136. [Google Scholar] [CrossRef]
- Jang, E.Y.; Yang, C.H.; Hedges, D.M.; Kim, S.P.; Lee, J.Y.; Ekins, T.G.; Garcia, B.T.; Kim, H.Y.; Nelson, A.C.; Kim, N.J.; et al. The Role of Reactive Oxygen Species in Methamphetamine Self-Administration and Dopamine Release in the Nucleus Accumbens: ROS in METH-Taking Behavior. Addiction Biology 2017, 22, 1304–1315. [Google Scholar] [CrossRef] [PubMed]
- Madayag, A.; Lobner, D.; Kau, K.S.; Mantsch, J.R.; Abdulhameed, O.; Hearing, M.; Grier, M.D.; Baker, D.A. Repeated N -Acetylcysteine Administration Alters Plasticity-Dependent Effects of Cocaine. J. Neurosci. 2007, 27, 13968–13976. [Google Scholar] [CrossRef]
- Murray, J.E.; Lacoste, J.; Belin, D. N-Acetylcysteine as a Treatment for Addiction. In Addictions—from pathophysiology to treatment; InTech: Rijeka, Croatia, 2012; pp. 355–380. [Google Scholar]
- Ducret, E.; Puaud, M.; Lacoste, J.; Belin-Rauscent, A.; Fouyssac, M.; Dugast, E.; Murray, J.E.; Everitt, B.J.; Houeto, J.-L.; Belin, D. N-Acetylcysteine Facilitates Self-Imposed Abstinence After Escalation of Cocaine Intake. Biological Psychiatry 2016, 80, 226–234. [Google Scholar] [CrossRef] [PubMed]
- Garcia-Keller, C.; Smiley, C.; Monforton, C.; Melton, S.; Kalivas, P.W.; Gass, J. N -Acetylcysteine Treatment during Acute Stress Prevents Stress-induced Augmentation of Addictive Drug Use and Relapse. Addiction Biology 2020, 25, e12798. [Google Scholar] [CrossRef]
- Kau, K.S.; Madayag, A.; Mantsch, J.R.; Grier, M.D.; Abdulhameed, O.; Baker, D.A. Blunted Cystine–Glutamate Antiporter Function in the Nucleus Accumbens Promotes Cocaine-Induced Drug Seeking. Neuroscience 2008, 155, 530–537. [Google Scholar] [CrossRef] [PubMed]
- Moussawi, K.; Pacchioni, A.; Moran, M.; Olive, M.F.; Gass, J.T.; Lavin, A.; Kalivas, P.W. N-Acetylcysteine Reverses Cocaine-Induced Metaplasticity. Nat Neurosci 2009, 12, 182–189. [Google Scholar] [CrossRef]
- Kupchik, Y.M.; Moussawi, K.; Tang, X.-C.; Wang, X.; Kalivas, B.C.; Kolokithas, R.; Ogburn, K.B.; Kalivas, P.W. The Effect of N-Acetylcysteine in the Nucleus Accumbens on Neurotransmission and Relapse to Cocaine. Biological Psychiatry 2012, 71, 978–986. [Google Scholar] [CrossRef]
- LaRowe, S.D.; Mardikian, P.; Malcolm, R.; Myrick, H.; Kalivas, P.; McFarland, K.; Saladin, M.; McRae, A.; Brady, K. Safety and Tolerability of N-Acetylcysteine in Cocaine-Dependent Individuals. American J Addict 2006, 15, 105–110. [Google Scholar] [CrossRef]
- LaRowe, S.D.; Myrick, H.; Hedden, S.; Mardikian, P.; Saladin, M.; McRae, A.; Brady, K.; Kalivas, P.W.; Malcolm, R. Is Cocaine Desire Reduced by N -Acetylcysteine? AJP 2007, 164, 1115–1117. [Google Scholar] [CrossRef] [PubMed]
- Mardikian, P.N.; LaRowe, S.D.; Hedden, S.; Kalivas, P.W.; Malcolm, R.J. An Open-Label Trial of N-Acetylcysteine for the Treatment of Cocaine Dependence: A Pilot Study. Progress in Neuro-Psychopharmacology and Biological Psychiatry 2007, 31, 389–394. [Google Scholar] [CrossRef]
- Amen, S.L.; Piacentine, L.B.; Ahmad, M.E.; Li, S.-J.; Mantsch, J.R.; Risinger, R.C.; Baker, D.A. Repeated N-Acetyl Cysteine Reduces Cocaine Seeking in Rodents and Craving in Cocaine-Dependent Humans. Neuropsychopharmacol 2011, 36, 871–878. [Google Scholar] [CrossRef] [PubMed]
- Schmaal, L.; Veltman, D.J.; Nederveen, A.; Van Den Brink, W.; Goudriaan, A.E. N-Acetylcysteine Normalizes Glutamate Levels in Cocaine-Dependent Patients: A Randomized Crossover Magnetic Resonance Spectroscopy Study. Neuropsychopharmacol 2012, 37, 2143–2152. [Google Scholar] [CrossRef]
- LaRowe, S.D.; Kalivas, P.W.; Nicholas, J.S.; Randall, P.K.; Mardikian, P.N.; Malcolm, Robert.J. A Double-blind Placebo-controlled Trial of N-acetylcysteine in the Treatment of Cocaine Dependence. American J Addict 2013, 22, 443–452. [Google Scholar] [CrossRef]
- Engeli, E.J.E.; Preller, K.H.; Rieser, N.M.; Klar, J.; Staempfli, P.; Hulka, L.M.; Kirschner, M.; Seifritz, E.; Herdener, M. N-Acetylcysteine Reduces Prefrontal Reactivity to Cocaine Cues in Individuals with Cocaine Use Disorder. Front. Psychiatry 2025, 15, 1489194. [Google Scholar] [CrossRef] [PubMed]
- Back, S.E.; McCauley, J.L.; Korte, K.J.; Gros, D.F.; Leavitt, V.; Gray, K.M.; Hamner, M.B.; DeSantis, S.M.; Malcolm, R.; Brady, K.T.; et al. A Double-Blind, Randomized, Controlled Pilot Trial of N-Acetylcysteine in Veterans With Posttraumatic Stress Disorder and Substance Use Disorders. J. Clin. Psychiatry 2016, 77, e1439–e1446. [Google Scholar] [CrossRef]
- Palacio, J.R.; Markert, U.R.; Martínez, P. Anti-Inflammatory Properties of N-Acetylcysteine on Lipopolysaccharide-Activated Macrophages. Inflamm. Res. 2011, 60, 695–704. [Google Scholar] [CrossRef]
- Zheng, W.; Zhang, Q. -E.; Cai, D. -B.; Yang, X. -H.; Qiu, Y.; Ungvari, G.S.; Ng, C.H.; Berk, M.; Ning, Y. -P.; Xiang, Y. -T. N -acetylcysteine for Major Mental Disorders: A Systematic Review and Meta-analysis of Randomized Controlled Trials. Acta Psychiatr Scand 2018, 137, 391–400. [Google Scholar] [CrossRef]
- Möller, M.; Du Preez, J.L.; Viljoen, F.P.; Berk, M.; Harvey, B.H. N-Acetyl Cysteine Reverses Social Isolation Rearing Induced Changes in Cortico-Striatal Monoamines in Rats. Metab Brain Dis 2013, 28, 687–696. [Google Scholar] [CrossRef]
- Baker, D.G.; Nievergelt, C.M.; O’Connor, D.T. Biomarkers of PTSD: Neuropeptides and Immune Signaling. Neuropharmacology 2012, 62, 663–673. [Google Scholar] [CrossRef]
- Reiner, R.; Weiss, U.; Brombacher, U.; Lanz, P.; Montavon, M.; Furlenmeier, A.; Angehrn, P.; Probst, P.J. Ro 13-9904/001, a Novel Potent and Long-Acting Parenteral Cephalosporin. J. Antibiot. 1980, 33, 783–786. [Google Scholar] [CrossRef]
- Beskid, G.; Christenson, J.G.; Cleeland, R.; DeLorenzo, W.; Trown, P.W. In Vivo Activity of Ceftriaxone (Ro 13-9904), a New Broad-Spectrum Semisynthetic Cephalosporin. Antimicrob Agents Chemother 1981, 20, 159–167. [Google Scholar] [CrossRef] [PubMed]
- Rothstein, J.D.; Patel, S.; Regan, M.R.; Haenggeli, C.; Huang, Y.H.; Bergles, D.E.; Jin, L.; Dykes Hoberg, M.; Vidensky, S.; Chung, D.S.; et al. β-Lactam Antibiotics Offer Neuroprotection by Increasing Glutamate Transporter Expression. Nature 2005, 433, 73–77. [Google Scholar] [CrossRef] [PubMed]
- Sari, Y.; Smith, K.D.; Ali, P.K.; Rebec, G.V. Upregulation of Glt1 Attenuates Cue-Induced Reinstatement of Cocaine-Seeking Behavior in Rats. J. Neurosci. 2009, 29, 9239–9243. [Google Scholar] [CrossRef]
- Sondheimer, I.; Knackstedt, L.A. Ceftriaxone Prevents the Induction of Cocaine Sensitization and Produces Enduring Attenuation of Cue- and Cocaine-Primed Reinstatement of Cocaine-Seeking. Behavioural Brain Research 2011, 225, 252–258. [Google Scholar] [CrossRef]
- Rasmussen, B.; Unterwald, E.M.; Rawls, S.M. Glutamate Transporter Subtype 1 (GLT-1) Activator Ceftriaxone Attenuates Amphetamine-Induced Hyperactivity and Behavioral Sensitization in Rats. Drug and Alcohol Dependence 2011, 118, 484–488. [Google Scholar] [CrossRef]
- LaCrosse, A.L.; Hill, K.; Knackstedt, L.A. Ceftriaxone Attenuates Cocaine Relapse after Abstinence through Modulation of Nucleus Accumbens AMPA Subunit Expression. European Neuropsychopharmacology 2016, 26, 186–194. [Google Scholar] [CrossRef] [PubMed]
- Fischer, K.D.; Houston, A.C.W.; Rebec, G.V. Role of the Major Glutamate Transporter GLT1 in Nucleus Accumbens Core Versus Shell in Cue-Induced Cocaine-Seeking Behavior. J. Neurosci. 2013, 33, 9319–9327. [Google Scholar] [CrossRef]
- Lee, S.-G.; Su, Z.-Z.; Emdad, L.; Gupta, P.; Sarkar, D.; Borjabad, A.; Volsky, D.J.; Fisher, P.B. Mechanism of Ceftriaxone Induction of Excitatory Amino Acid Transporter-2 Expression and Glutamate Uptake in Primary Human Astrocytes. Journal of Biological Chemistry 2008, 283, 13116–13123. [Google Scholar] [CrossRef]
- Abdel-Salam, O. Drugs Used to Treat Parkinsons Disease, Present Status and Future Directions. CNSNDDT 2008, 7, 321–342. [Google Scholar] [CrossRef]
- Blum, D.; Chtarto, A.; Tenenbaum, L.; Brotchi, J.; Levivier, M. Clinical Potential of Minocycline for Neurodegenerative Disorders. Neurobiology of Disease 2004, 17, 359–366. [Google Scholar] [CrossRef]
- Buller, K.M.; Carty, M.L.; Reinebrant, H.E.; Wixey, J.A. Minocycline: A Neuroprotective Agent for Hypoxic-ischemic Brain Injury in the Neonate? J of Neuroscience Research 2009, 87, 599–608. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Ma, X.; Jiang, Y.; Pi, R.; Liu, Y.; Ma, L. The Prospects of Minocycline in Multiple Sclerosis. Journal of Neuroimmunology 2011, 235, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.-S.; Suh, Y.-H. Minocycline and Neurodegenerative Diseases. Behavioural Brain Research 2009, 196, 168–179. [Google Scholar] [CrossRef] [PubMed]
- Miyaoka, T.; Yasukawa, R.; Yasuda, H.; Hayashida, M.; Inagaki, T.; Horiguchi, J. Possible Antipsychotic Effects of Minocycline in Patients with Schizophrenia. Progress in Neuro-Psychopharmacology and Biological Psychiatry 2007, 31, 304–307. [Google Scholar] [CrossRef]
- Soczynska, J.K.; Mansur, R.B.; Brietzke, E.; Swardfager, W.; Kennedy, S.H.; Woldeyohannes, H.O.; Powell, A.M.; Manierka, M.S.; McIntyre, R.S. Novel Therapeutic Targets in Depression: Minocycline as a Candidate Treatment. Behavioural Brain Research 2012, 235, 302–317. [Google Scholar] [CrossRef]
- Stirling, D.P.; Koochesfahani, K.M.; Steeves, J.D.; Tetzlaff, W. Minocycline as a Neuroprotective Agent. Neuroscientist 2005, 11, 308–322. [Google Scholar] [CrossRef]
- Yenari, M.A.; Kauppinen, T.M.; Swanson, R.A. Microglial Activation in Stroke: Therapeutic Targets. Neurotherapeutics 2010, 7, 378–391. [Google Scholar] [CrossRef]
- Möller, T.; Bard, F.; Bhattacharya, A.; Biber, K.; Campbell, B.; Dale, E.; Eder, C.; Gan, L.; Garden, G.A.; Hughes, Z.A.; et al. Critical Data-based Re-evaluation of Minocycline as a Putative Specific Microglia Inhibitor. Glia 2016, 64, 1788–1794. [Google Scholar] [CrossRef] [PubMed]
- Kobayashi, K.; Imagama, S.; Ohgomori, T.; Hirano, K.; Uchimura, K.; Sakamoto, K.; Hirakawa, A.; Takeuchi, H.; Suzumura, A.; Ishiguro, N.; et al. Minocycline Selectively Inhibits M1 Polarization of Microglia. Cell Death Dis 2013, 4, e525–e525. [Google Scholar] [CrossRef] [PubMed]
- Moini-Zanjani, T.; Ostad, S.-N.; Labibi, F.; Ameli, H.; Mosaffa, N.; Sabetkasaei, M. Minocycline Effects on IL-6 Concentration in Macrophage and Microglial Cells in a Rat Model of Neuropathic Pain. Iran Biomed J 2016, 20, 273–279. [Google Scholar] [CrossRef]
- Switzer, J.A.; Sikora, A.; Ergul, A.; Waller, J.L.; Hess, D.C.; Fagan, S.C. Minocycline Prevents IL-6 Increase after Acute Ischemic Stroke. Transl. Stroke Res. 2012, 3, 364–368. [Google Scholar] [CrossRef]
- Wang, H.-T.; Huang, F.-L.; Hu, Z.-L.; Zhang, W.-J.; Qiao, X.-Q.; Huang, Y.-Q.; Dai, R.-P.; Li, F.; Li, C.-Q. Early-Life Social Isolation-Induced Depressive-Like Behavior in Rats Results in Microglial Activation and Neuronal Histone Methylation That Are Mitigated by Minocycline. Neurotox Res 2017, 31, 505–520. [Google Scholar] [CrossRef]
- Piotrowska, A.; Kwiatkowski, K.; Rojewska, E.; Slusarczyk, J.; Makuch, W.; Basta-Kaim, A.; Przewlocka, B.; Mika, J. Direct and Indirect Pharmacological Modulation of CCL2/CCR2 Pathway Results in Attenuation of Neuropathic Pain — In Vivo and in Vitro Evidence. Journal of Neuroimmunology 2016, 297, 9–19. [Google Scholar] [CrossRef]
- Chen, M.; Ona, V.O.; Li, M.; Ferrante, R.J.; Fink, K.B.; Zhu, S.; Bian, J.; Guo, L.; Farrell, L.A.; Hersch, S.M.; et al. Minocycline Inhibits Caspase-1 and Caspase-3 Expression and Delays Mortality in a Transgenic Mouse Model of Huntington Disease. Nat Med 2000, 6, 797–801. [Google Scholar] [CrossRef]
- Wasserman, J.K.; Schlichter, L.C. Minocycline Protects the Blood–Brain Barrier and Reduces Edema Following Intracerebral Hemorrhage in the Rat. Experimental Neurology 2007, 207, 227–237. [Google Scholar] [CrossRef]
- Zhang, L.; Kitaichi, K.; Fujimoto, Y.; Nakayama, H.; Shimizu, E.; Iyo, M.; Hashimoto, K. Protective Effects of Minocycline on Behavioral Changes and Neurotoxicity in Mice after Administration of Methamphetamine. Progress in Neuro-Psychopharmacology and Biological Psychiatry 2006, 30, 1381–1393. [Google Scholar] [CrossRef]
- Kofman, O.; Klein, E.; Newman, M.; Hamburger, R.; Kimchi, O.; Nir, T.; Shimon, H.; Belmaker, R.H. Inhibition by Antibiotic Tetracyclines of Rat Cortical Noradrenergic Adenylate Cyclase and Amphetamine-Induced Hyperactivity. Pharmacology Biochemistry and Behavior 1990, 37, 417–424. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.; Uz, T.; Manev, H. Minocycline Affects Cocaine Sensitization in Mice. Neuroscience Letters 2009, 452, 258–261. [Google Scholar] [CrossRef]
- Chen, H.; Manev, H. Effects of Minocycline on Cocaine Sensitization and Phosphorylation of GluR1 Receptors in 5-Lipoxygenase Deficient Mice. Neuropharmacology 2011, 60, 1058–1063. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Liao, Y.; Dong, Y.; Li, X.; Li, J.; Cheng, Y.; Cheng, J.; Yuan, Z. Microglial Deletion and Inhibition Alleviate Behavior of Post-Traumatic Stress Disorder in Mice. J Neuroinflammation 2021, 18, 7. [Google Scholar] [CrossRef] [PubMed]
- Levkovitz, Y.; Fenchel, D.; Kaplan, Z.; Zohar, J.; Cohen, H. Early Post-Stressor Intervention with Minocycline, a Second-Generation Tetracycline, Attenuates Post-Traumatic Stress Response in an Animal Model of PTSD. European Neuropsychopharmacology 2015, 25, 124–132. [Google Scholar] [CrossRef] [PubMed]
- Gerst, A.; Murthy, T.; Elson, A.; Driscoll, D.; Bittner, M.J.; Ramaswamy, S. Adjunctive Minocycline for Treatment of Posttraumatic Stress Disorder. WMJ 2021, 120, 321–324. [Google Scholar]
- Dean, O.M.; Kanchanatawan, B.; Ashton, M.; Mohebbi, M.; Ng, C.H.; Maes, M.; Berk, L.; Sughondhabirom, A.; Tangwongchai, S.; Singh, A.B.; et al. Adjunctive Minocycline Treatment for Major Depressive Disorder: A Proof of Concept Trial. Aust N Z J Psychiatry 2017, 51, 829–840. [Google Scholar] [CrossRef]
- Husain, M.I.; Chaudhry, I.B.; Husain, N.; Khoso, A.B.; Rahman, R.R.; Hamirani, M.M.; Hodsoll, J.; Qurashi, I.; Deakin, J.F.; Young, A.H. Minocycline as an Adjunct for Treatment-Resistant Depressive Symptoms: A Pilot Randomised Placebo-Controlled Trial. J Psychopharmacol 2017, 31, 1166–1175. [Google Scholar] [CrossRef]
- Nettis, M.A. Minocycline in Major Depressive Disorder: And Overview with Considerations on Treatment-Resistance and Comparisons with Other Psychiatric Disorders. Brain, Behavior, & Immunity - Health 2021, 17, 100335. [Google Scholar] [CrossRef]
- Cui, X.; Li, L.; Hu, Y.-Y.; Ren, S.; Zhang, M.; Li, W.-B. Sulbactam Plays Neuronal Protective Effect Against Brain Ischemia via Upregulating GLT1 in Rats. Mol Neurobiol 2015, 51, 1322–1333. [Google Scholar] [CrossRef]
- Qi, J.; Xian, X.-H.; Li, L.; Zhang, M.; Hu, Y.-Y.; Zhang, J.-G.; Li, W.-B. Sulbactam Protects Hippocampal Neurons Against Oxygen-Glucose Deprivation by Up-Regulating Astrocytic GLT-1 via P38 MAPK Signal Pathway. Front. Mol. Neurosci. 2018, 11, 281. [Google Scholar] [CrossRef]
- Li, L.; Li, W.; Jiang, W.; Xu, R. Sulbactam Protects Neurons against Double Neurotoxicity of Amyloid Beta and Glutamate Load by Upregulating Glial Glutamate Transporter 1. Cell Death Discov. 2024, 10, 64. [Google Scholar] [CrossRef]
- Rao, P.S.S.; Goodwani, S.; Bell, R.L.; Wei, Y.; Boddu, S.H.S.; Sari, Y. Effects of Ampicillin, Cefazolin and Cefoperazone Treatments on GLT-1 Expressions in the Mesocorticolimbic System and Ethanol Intake in Alcohol-Preferring Rats. Neuroscience 2015, 295, 164–174. [Google Scholar] [CrossRef] [PubMed]
- Alasmari, F.; Abuhamdah, S.; Sari, Y. Effects of Ampicillin on Cystine/Glutamate Antiporter and Glutamate Transporter 1 Isoforms as Well as Ethanol Drinking in Male P Rats. Neuroscience Letters 2015, 600, 148–152. [Google Scholar] [CrossRef] [PubMed]
- Alasmari, F.; Alhaddad, H.; Wong, W.; Bell, R.L.; Sari, Y. Ampicillin/Sulbactam Treatment Modulates NMDA Receptor NR2B Subunit and Attenuates Neuroinflammation and Alcohol Intake in Male High Alcohol Drinking Rats. Biomolecules 2020, 10, 1030. [Google Scholar] [CrossRef] [PubMed]
- Hammad, A.M.; Alasmari, F.; Althobaiti, Y.S.; Sari, Y. Modulatory Effects of Ampicillin/Sulbactam on Glial Glutamate Transporters and Metabotropic Glutamate Receptor 1 as Well as Reinstatement to Cocaine-Seeking Behavior. Behavioural Brain Research 2017, 332, 288–298. [Google Scholar] [CrossRef]
- Hammad, A.M.; Alasmari, F.; Sari, Y. Effect of Modulation of the Astrocytic Glutamate Transporters’ Expression on Cocaine-Induced Reinstatement in Male P Rats Exposed to Ethanol. Alcohol and Alcoholism 2021, 56, 210–219. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).




