1. Introduction
Ifalmin is a standardized preparation containing Channa micropeltes (Toman fish) extract, a freshwater species indigenous to Southeast Asia with a long record of ethnomedicinal use. Pharmacological evidence indicates that Channa micropeltes exhibits anti inflammatory, antidiabetic, wound healing, and antioxidant activities [
1,
2], supporting its potential as a health promoting natural product. In addition to these bioactivities, the extract is characterized by a high albumin content. Albumin is a major plasma protein involved in regulation of oncotic pressure, transport of endogenous and exogenous compounds, immune modulation, and tissue regeneration, so its presence may contribute to the proposed therapeutic value of fish derived supplements [
3]. However, despite the expanding utilization and commercialization of fish based nutraceuticals such as Ifalmin, their toxicological characterization under repeated dose exposure remains limited.
Subchronic toxicity testing is a key component of preclinical safety assessment for products intended for continuous intake. Repeated oral administration for approximately 14 consecutive days is designed to detect early signs of cumulative or organ specific toxicity, including changes in body weight, gross and relative organ weights, histopathological lesions, and alterations in biochemical and hematological homeostasis, especially within hepatic, renal, cardiovascular, splenic, pulmonary, and gastrointestinal systems [
4]. Importantly, natural origin does not guarantee safety, because bioactive constituents may elicit adverse effects at high doses or with prolonged exposure [
5]. Rattus norvegicus (Wistar rats) is an established model in toxicology due to its reproducible physiological responses and translational relevance for predicting human safety outcomes [
6].
Most prior investigations on Channa micropeltes have concentrated on elucidating its beneficial bioactive properties [
1,
2], whereas evidence delineating its subchronic safety profile is limited. This evidentiary gap is clinically and regulatory relevant given the increasing prevalence of daily supplement consumption and the risk of unintended toxicity from inappropriate dosing or duration of use. Therefore, the present study aimed to evaluate the subchronic oral toxicity of Ifalmin in male and female Wistar rats following 14 days of graded-dose administration, employing comprehensive clinical, morphometric, histopathological, biochemical, and hematological endpoints [
4]. The outcomes of this study are expected to provide objective safety data to support the responsible development and use of Ifalmin within healthcare contexts.
Additionally, Ifalmin is a traditional medicine product from Indonesia, bearing the Jamu logo, and it is registered with the Indonesian National Agency of Drug and Food Control (BPOM RI). The selection of a 14-day period for the subchronic toxicity study is in accordance with the regulations of BPOM RI, which stipulate this duration for subchronic testing on traditional medicine products [
4]
2. Materials and Methods
The Ifalmin product, which contains Channa micropeltes extract, is produced by PT. Ismut Fitomedika Indonesia (PT. IFI) in Takalar Regency, South Sulawesi. It has been registered as herbal medicine with the Indonesian National Agency of Drug and Food Control (BPOM) on April 26, 2023, under Registration Number TR183313911. The product contains Channa micropeltes extract as its active ingredient.
2.1. Preparation of Animals:
Thirty male and thirty female Rattus norvegicus, with body weights of 120-130 grams for males and 110-120 grams for females, were obtained and acclimatized for one week prior to the commencement of the study. The animal care and study protocols followed the guidelines established by the Indonesian National Agency of Drug and Food Control (BPOM). The study protocols were reviewed and received ethical clearance (Registration RG.02.05.42.423.01.8.2024.1450 and Protocol No.: A02/PUPK/A04/VIII/2024/Rev.3).
2.2. Subcronic Toxicity Testing:
Following acclimatization, the rats were randomly selected and divided into six groups, each consisting of five animals. The groups included: the control group with a dose of 0 mg/kg body weight (BW) (Group I), the 270 mg/kg BW dose group (Group II), the 540 mg/kg BW dose group (Group III), the 1000 mg/kg BW dose group (Group IV), the satellite control group with a dose of 0 mg/kg BW (Group V), and the satellite control group with a dose of 1000 mg/kg BW (Group VI). The extract was administered orally for 14 days. On day 15, all rats were subjected to necropsy for analysis, which included body weight, organ macropathology, relative organ weight, histology, blood hematology, and blood biochemistry.
2.3. Body Weight Monitoring:
The body weight male and female of Rattus norvegicus was monitored by weighing the animals once a week throughout the treatment period.
2.4. Organ Macropathology Analysis:
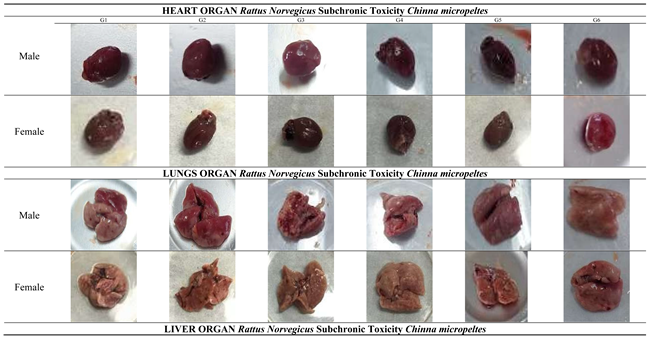
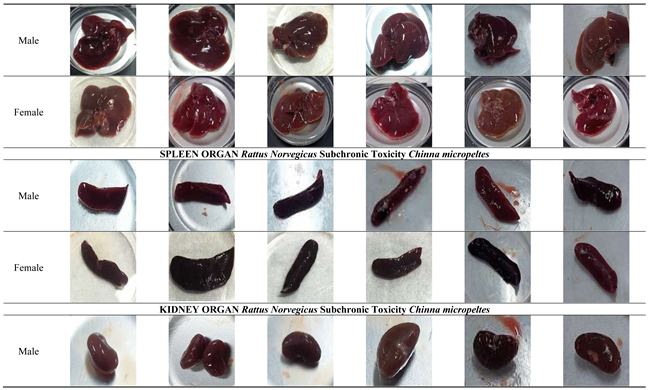
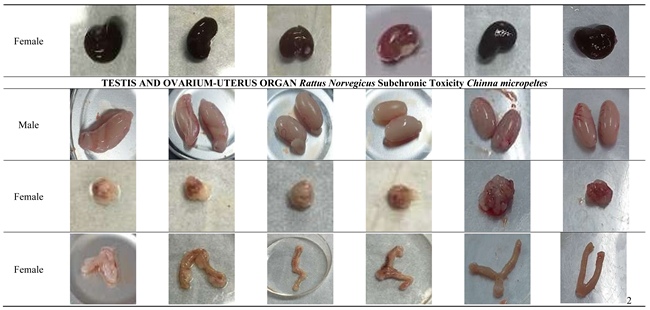
On day 14, the main organs of male and female Rattus norvegicus were collected and cleaned using a saline solution. The organs were then visually examined for any changes in shape, size, color, texture, or the presence of hemorrhages. Each organ was photographed for macropathological analysis.
2.5. Measurement of Relative Organ Weight:
The main organs, including the heart, lungs, liver, kidneys, and testes (for males), were evaluated for relative organ weight percentage using the following formula:
Relative Organ Weight (%) = (Organ Weight (g)/Body Weight (g)) ×100
2.6. Histopathological Analyses:
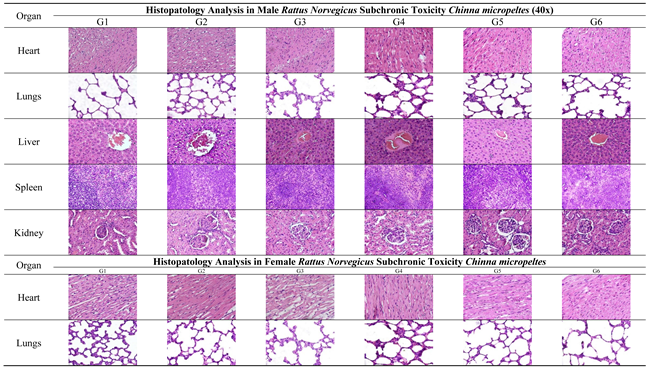
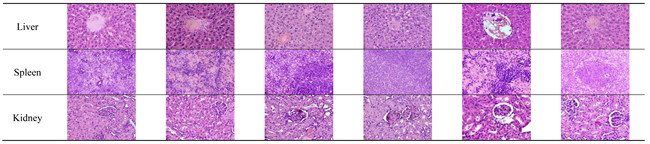
The main organs of male and female Rattus norvegicus were collected post-euthanasia, cleaned with a saline solution, and fixed in 10% buffered formalin for 24-48 hours to preserve tissue structure. The fixed tissues were processed through graded ethanol series for dehydration, cleared with xylene, and embedded in paraffin. Thin tissue sections (3–5 µm) were prepared using a microtome, mounted on glass slides, and stained with Hematoxylin and Eosin (H&E). The stained slides were examined under a light microscope to identify histopathological changes such as necrosis, inflammation, fibrosis, or degeneration. Microscopic images were captured for documentation and analysis [
7].
2.7. Serum Biochemical Analyses:
Blood samples were collected from male and female Rattus norvegicus via venipuncture post-euthanasia. The blood samples were collected into vacutainer tubes and subsequently centrifuged at 3000 rpm for 20 minutes to separate the serum from the cellular components. An aliquot of 100 μL of blood serum was combined with 100 μL of buffer and thoroughly mixed, then incubated at 37°C for five minutes. Following this, 250 μL of the kit substrate was added to the mixture, homogenized, and incubated at 37°C for one minute, and various biochemical parameters were analyzed using a Humalyzer 3500 spectrophotometer (Human, Germany) were read at 340 nm [
7]. Parameters tested included liver function (AST/SGOT, ALT/SGPT), kidney function (creatinine, and BUN), lipid profile (total cholesterol, and triglycerides), and glucose levels. Data were analyzed statistically to compare control and treatment groups, and results were interpreted in the context of potential physiological or toxicological effects.
2.5. Hematological Analysis
Blood samples were collected from male and female Rattus norvegicus via venipuncture into tubes containing EDTA as an anticoagulant. Hematological parameters, including red blood cell count (RBC), hemoglobin (Hb) concentration, hematocrit (Hct), white blood cell count (WBC), platelet count (PLT), mean corpuscular volume (MCV), mean corpuscular hemoglobin (MCH), Red Cell Distribution Widht – Standard Deviation (RDW-SD), Neutrophils (%), Lymphocytes (%), and Monocytes (%) were analyzed using an automated hematology analyzer. Data were analyzed statistically to compare treatment and control groups [
9].
4. Discussion
Repeated-dose toxicity studies are designed to reveal cumulative adverse effects, identify potential target organs, and provide a scientific basis for determining the NOAEL prior to longer-term exposure trials. Indonesian FDA Regulation PerBPOM No. 10/2022 emphasize integrated evaluation of clinical signs, growth, hematology, serum biochemistry, organ coefficients, and histopathology to capture both functional and structural toxicity across organ systems. In the context of natural-product development, subacute/subchronic safety data are especially important because bioactive extracts may exert subtle adaptive responses before overt lesions emerge [
5,
13].
However, this approach differs from subchronic testing outlined in the OECD Guidelines, primarily due to the nature of the product being tested, traditional medicine that will later be registered as a
fitofarmaka (phytopharmaceutical product) [
5,
19]. The classification of traditional medicines in Indonesia is divided into three categories:
jamu (traditional herbal medicine),
OHT (standaritation traditional medicines), and
fitofarmaka [
19]. Each category is subject to different regulatory requirements and safety evaluations. BPOM RI provides guidelines indicating that subchronic toxicity testing can be conducted within a 14-day period, specifically for traditional medicines being developed for future
fitofarmaka registration. This distinction highlights the regulatory framework's flexibility in accommodating traditional medicine products as they transition into more standardized health products, while ensuring thorough safety evaluation during development [
5]
Oral administration in repeated-dose studies is particularly relevant because it mimics the real-world route of exposure for dietary supplements and functional foods. The clean safety profile observed here may reflect limited systemic accumulation and effective metabolic handling of the extract’s constituents. Although pharmacokinetic data were not assessed in the present work, the lack of clinical signs, stable organ coefficients, and absent histopathologic lesions suggest that repeated oral exposure did not result in bioaccumulation or persistent tissue injury. Future studies incorporating ADME or PK profiling would be valuable to confirm gastrointestinal stability, absorption extent, and clearance kinetics, thereby strengthening mechanistic understanding of the extract’s safety [
20].
Body weight is a key indicator of systemic health in subchronic toxicity assessments. Throughout the 14-day oral administration, both male and female rats in all treated groups showed a progressive weight gain comparable to controls, consistent with normal physiological growth. The absence of statistically significant differences in body-weight change among dose groups indicates that
Channa micropeltes extract did not interfere with growth or energy balance. Importantly, no animals exhibited weight loss or growth retardation, which are typical early signs of toxicity, and food-consumption patterns remained stable, suggesting no adverse effects on appetite, metabolism, or nutrient utilization. Given that a change of approximately ≥10% body weight is often considered biologically relevant for subchronic toxicity [
21], the normal weight trajectories observed here support the conclusion that the extract is non-toxic within the tested dose range, including 1000 mg/kg [
22].
The macropathological examination of major organs, including the heart, liver, spleen, kidneys, lungs, and reproductive organs, revealed no visible abnormalities in either male or female rats across all treated and satellite groups. The heart, liver, spleen, kidneys, and lungs maintained normal size, color, and texture, with no signs of necrosis, lesions, or structural damage, indicating the absence of cardiotoxic, hepatotoxic, nephrotoxic, or pulmonary effects. Similarly, the reproductive organs (testes, ovaries, and uterus) showed no signs of toxicity or interference with reproductive health. These findings suggest that
Channa micropeltes extract does not induce any organ-specific toxicity, even at the highest dose of 1000 mg/kg, confirming its overall safety. The macroscopic description of specimens constitutes a critical element of the pathology report, requiring precision and clinical relevance. Excessive or redundant details can unnecessarily extend the report, thereby elevating the risk of significant clinical information being overlooked by the attending clinician. Employing standardized templates for gross pathological reporting can enhance efficiency and mitigate these risks [
23].
Relative organ weight is a sensitive endpoint in repeated-dose toxicity studies because organ mass can change before clear gross or microscopic lesions are detectable; altered organ-to-body-weight ratios may reflect hypertrophy, atrophy, congestion, or edema, especially in liver, kidneys, heart, and spleen [
24,
25] Thus, a lack of significant organ-weight shifts is generally interpreted as no organ-specific toxic burden under the tested conditions. In this study, relative organ weights of male and female rats remained comparable between control and
Channa micropeltes extract groups up to 1000 mg/kg, including the satellite cohort. The stability of major detoxification and target organs (liver, kidneys, heart, spleen, lungs) and reproductive organs suggests no adaptive enlargement or degenerative shrinkage attributable to treatment, a pattern consistent with absence of subchronic cardiotoxicity, nephrotoxicity, hepatotoxicity, or reproductive toxicity [
26]. The unchanged organ weights in the recovery (satellite) group further indicate no delayed or residual organ remodeling after cessation of dosing.
Organ-weight changes are often interpreted as early adaptive or adverse responses; however, their toxicological meaning becomes strongest when concordant with histopathologic findings. Large databases have shown that increased liver weight may accompany enzyme induction or hypertrophy, while decreases may indicate atrophy or chronic injury, and the presence or absence of histological correlate helps distinguish adaptation from toxicity. In the present study, the absence of organ-weight shifts together with uniformly normal histology indicates not only a lack of morphological injury but also an absence of subclinical adaptive stress responses in detoxification organs. This concordance across endpoints substantially reduces the likelihood of undetected target-organ toxicity at the tested doses [
26].
Subchronic toxicity studies are intended to detect adverse effects arising from repeated exposure over a substantial portion of the rodent lifespan and to identify potential target-organ toxicity, thereby supporting the determination of the no-observed-adverse-effect level (NOAEL) [
11,
13]. In the present study, histopathological examination of major organs—including the heart, lungs, liver, spleen, kidneys, and reproductive organs showed normal tissue architecture in both male and female rats treated with
Channa micropeltes extract. All organs received a histopathological score of 0, and no microscopic evidence of necrosis, inflammation, fibrosis, or cellular degeneration was observed, indicating preserved structural integrity and function [
27]. The absence of lesions across detoxification, highly perfused, and reproductive organs provides strong support that the extract does not induce organ-specific toxicity under subchronic exposure. Evaluating both sexes is important because hormonal and metabolic differences may influence toxic responses to natural products; however, the comparable histological findings in males and females suggest no sex-related susceptibility within the tested dose range [
28]. Collectively, these results contribute valuable safety data for selecting dose levels in longer-term studies and for refining safe exposure limits in future translational assessments [
16]. The inclusion of a satellite (recovery) cohort enhances interpretation by assessing reversibility or delayed-onset toxicity after dosing cessation. In this work, recovery animals displayed organ weights, histology, and clinical profiles comparable to controls, suggesting no residual tissue remodeling or late-emerging toxicity. Such findings are important for natural extracts, where metabolite-driven effects might hypothetically appear after prolonged processing in vivo. The normal recovery profile therefore supports that any exposure-related changes, if present, are unlikely to persist or progress once dosing stops [
29]
Serum biochemistry provides functional evidence of target-organ integrity during repeated-dose toxicity, particularly for the liver and kidneys. In this study,
Channa micropeltes extract did not produce statistically significant changes in key hepatic enzymes (ALT, AST) or renal biomarkers (creatinine, urea) compared with controls, and all values remained within physiological limits. The absence of enzyme elevation indicates no hepatocellular injury or leakage, while stable creatinine and urea suggest preserved glomerular filtration and no nephrotoxic burden under subchronic exposure [
30]. Metabolic parameters also remained unaffected. Blood glucose stayed within normal range, indicating that the extract did not disrupt systemic energy homeostasis. Likewise, total cholesterol and triglycerides showed no treatment-related shifts, supporting the conclusion that lipid metabolism was not impaired [
16]. Taken together with the normal histopathology and organ-weight findings, the biochemical profile consistently demonstrates that repeated oral administration of
Channa micropeltes extract up to 1000 mg/kg does not elicit liver, kidney, or metabolic toxicity in rats within the study duration.
Hematological parameters are sensitive indicators of systemic and immuno-hematopoietic toxicity in repeated-dose studies. In the present work,
Channa micropeltes extract did not induce statistically significant alterations in major hematological indices in either sex, including WBC, RBC, hemoglobin, hematocrit, platelet count, erythrocyte indices (MCV, MCH, RDW-SD), and differential leukocyte profiles. These findings indicate preserved hematopoiesis, oxygen-carrying capacity, and immune homeostasis under subchronic exposure [
31]. Minor reductions in platelet and hematocrit values observed in a single female subgroup were not statistically significant, showed no dose-response pattern, and therefore are most consistent with normal biological variability rather than a treatment-related effect. Although reference intervals may vary slightly across sources, the overall pattern of values remaining within physiological limits supports the conclusion that the extract does not elicit hematological toxicity up to 1000 mg/kg [
13]. When interpreted together with the normal organ weights, macropathology, histopathology, and serum biochemistry, the hematological profile corroborates the favorable safety of
Channa micropeltes extract in this subchronic model.
An important element of toxicological interpretation is the safety margin between the highest tested dose and the expected efficacious or intended human-use dose. In this study, the top dose of 1000 mg/kg represents a multiple of the projected therapeutic exposure, thereby providing a conservative safety buffer. The absence of adverse findings at this level indicates a wide margin of safety, which is desirable for nutraceutical or biopharmaceutical candidates derived from food-based sources. Such a safety window strengthens the feasibility of advancing Channa micropeltes extract into longer duration studies and supports its practical use without requiring exposure near the toxicological threshold.
Overall, the findings of this study strengthen the translational positioning of
Channa micropeltes extract as a safe bioactive candidate for further development in Ifalmin®. The consistent absence of toxic effects across systemic indicators (clinical signs and growth), functional parameters (hematology and serum biochemistry), and structural endpoints (macropathology, organ coefficients, and histopathology), including in the recovery cohort, provides a strong weight-of-evidence that short-term repeated exposure does not elicit target-organ toxicity. The uniform safety profile up to 1000 mg/kg bw/day offers a sound basis for selecting dose ranges in longer subchronic studies aligned with OECD/BPOM guidelines (14-day or 90-day studies) and for applying a more conservative approach to human-equivalent exposure estimation. From a product-development perspective, this early safety confirmation is essential to support longer-term efficacy testing and serves as a prerequisite for eventual clinical evaluation, particularly because Ifalmin® is intended as an orally administered natural-product preparation with potential for repeated use [
5].
This study has several limitations, primarily related to the lack of literature on 14-day subchronic toxicity testing for traditional medicine products, which hinders the search for references and comparison with other studies. Additionally, Ifalmin, which contains Channa micropeltes (Toman fish), also has limited available literature, making it challenging to confirm safety and efficacy data in comparison with similar products. Further research will be conducted through clinical trials in humans to confirm these findings and assess the safety of the product for long-term use. It will also be registered as a fitofarmaka product in accordance with the guidelines set by BPOM RI.
Author Contributions
Conceptualization, M.I., M.R, M.N.M; methodology, M.I., A.W.J., and M.R.; investigation, A.W.J., N.D.L., and M.R.; resources, M.I, M.R, A.W.J, M.N.M. and N.D.L.; data curation, M.I., A.W.J., and N.D.L.; formal analysis, M.R, N.D.L, A.W.J.; validation, M.I., M.R., and M.N.M.; writing—original draft preparation, A.W.J.; writing—review and editing, M.I., A.W.J., M.R., and M.N.M.; visualization, M.I., M.R., and M.N.M.; supervision, M.I.; project administration, A.W.J.; funding acquisition, M.I. All authors have read and agreed to the published version of the manuscript.