1. Introduction
The mango stem end rot disease (SER) is a destructive post-harvest disease of mango fruits worldwide especially in the sub-tropical and tropical countries. It is also a significant post-harvest disease in arid regions and ranks as the second most severe mango disease globally following anthracnose disease (Johnson, 1998) [
1]. The disease affects developing mango fruits after inflorescence and manifest fully in matured harvested mango fruits thereby affecting the marketability and quality of the fruit juice during ripening. The SER disease can cause an absolute post-harvest fruit yield loss in a conducive environment and also in areas where proper post-harvest management practices are not carried out. Due to the significant importance of SER disease, it has been reported in nearly all mango growing areas of the world (Alveraze & Lopez, 1971; Rawal, 1998; Schaffer et al., 1988; Honger et al., 2015; Galsurker et al., 2018; Coutinho et. al., 2017; Zhang et. al., 2020; Rodriguez-Glavez et. al., 2020; Sathya et. al., 2017) [
2,
3,
4,
5,
6,
7,
8,
9,
10]
Several
Lasiodiplodia species complex are known to play a role prior to and during harvesting of matured mango fruits (Johnson, 1998; Honger et al., 2016) [
11]. These group of fungal pathogens are known to belong to the
Botryosphaeria genus, such as
Dothiorella dominicana, Dothiorella mangiferae, Lasiodiplodia theobromae, Neofusicoccum sp.,
Phomopsis mangiferae, Cytosphaera mangiferae, and
Pestalotiopsis sp., and other pathogenic fungi, such as
Colletotrichum gloeosporioides and
Alternaria alternata (Alkan & Kumar, 2018; Galsurker et.al., 2018; Galsurker et. al., 2020) [
12,
13]. In most tropical areas of the world,
L. theobromae is the most dominant pathogen linked with the disease (Johnson, 1998). In Brazil, Ghana, and Sri Lanka, the pathogen has been linked to the stem end rot disease of mangoes (Costa et al., 2010; Honger et al., 2016; Syed et al., 2014) [
14,
15]. Though other fungi have been associated with the mango disease worldwide,
L. theobromae is the most recorded pathogen linked with the disease. The species of
L. theobromae responsible for mango stem end rot has been identified using cultural and morphological features and growth rate, which are traditional methods of fungal identification and combined with molecular methods (Honger et al., 2016; Phillips et al., 2008; Jonson, 1998) [
16]. Xu et al. (2005) [
17], designed a primer Lt347-F/Lt347-R which was used to identify
L. theobromae causing rot of blueberry and which was able to correctly identify the same pathogen from tree decline symptoms of mango in Ghana (Honger et al., 2018) [
18]. Numerous articles including Galsurker et al., (2018), Johnson et al., (1992),[
19] Prusky et al., (2013), [
20] Costa et al., (2010), Honger et al., (2016) and Syed et al., (2014) have carried out diverse studies on the SER disease. These studies have a narrow focus on specific aspects of the SER disease based on distinct geographical locations including Ghana (Honger et al., 2015; Galsurker et al., 2018).
In contrast to previous articles on mango SER disease, this review focuses on in-depth summary of the current state of knowledge about SER disease from global perspective, including the most current information on its history, economic importance, symptoms of the SER disease, epidemiology and disease cycle of the pathogen. The review further details the aetiology of the SER, describes the identification of the pathogen, host range of the SER disease, complexities of SER, impacts of the SER on nutritional and sensory attributes on ripe mango fruits and the control of the SER disease. The review further highlights on rapid molecular diagnosis approaches using species specific primers with PCR assays to determine the exact pathogen so as appropriate management measures are formulated to control the spread of the disease.
This review is well-timed because the stem-end rot disease is spreading rapidly to many parts of the world where the pathogen has not been reported (Benatar et al., 2021; Li et al., 2020) [
21,
22]. In the field, the conidia, which are the main infective propagules, are released during periods of high water and moisture, which are necessary for the infection process to occur on developing flowers (Alam et al., 2019) [
23]. The end result is the blighting of the infected flowers and further sporulation of the pathogen on the dead flowers (Alam et al., 2019). The newly formed conidia are then blown onto young and developing fruits, where they infect the fruit peduncle and the fruits themselves and remain quiescent (Netto et al., 2014)[
24]. During harvesting, wounds created serve as entry points for ascospores released from the perithecia, which are present in the initial sources of infection, serving as additional infection propagules to the quiescent infection already present (Netto et al., 2014). During storage or transportation, the pathogen continues to grow and produces disease symptoms on the infected fruits (Netto et al., 2014). Due to the existence of pathogen propagules in fallen tree debris and within the soil, there is a possibility for the fruit to become infected through contact with the soil during the harvesting process (Netto et al., 2014). The review also provides further and updated details on the taxonomic status of the
Lasiodiplodia taxa linked with various SER, which has changed since the introduction of molecular techniques (Netto et al., 2014).
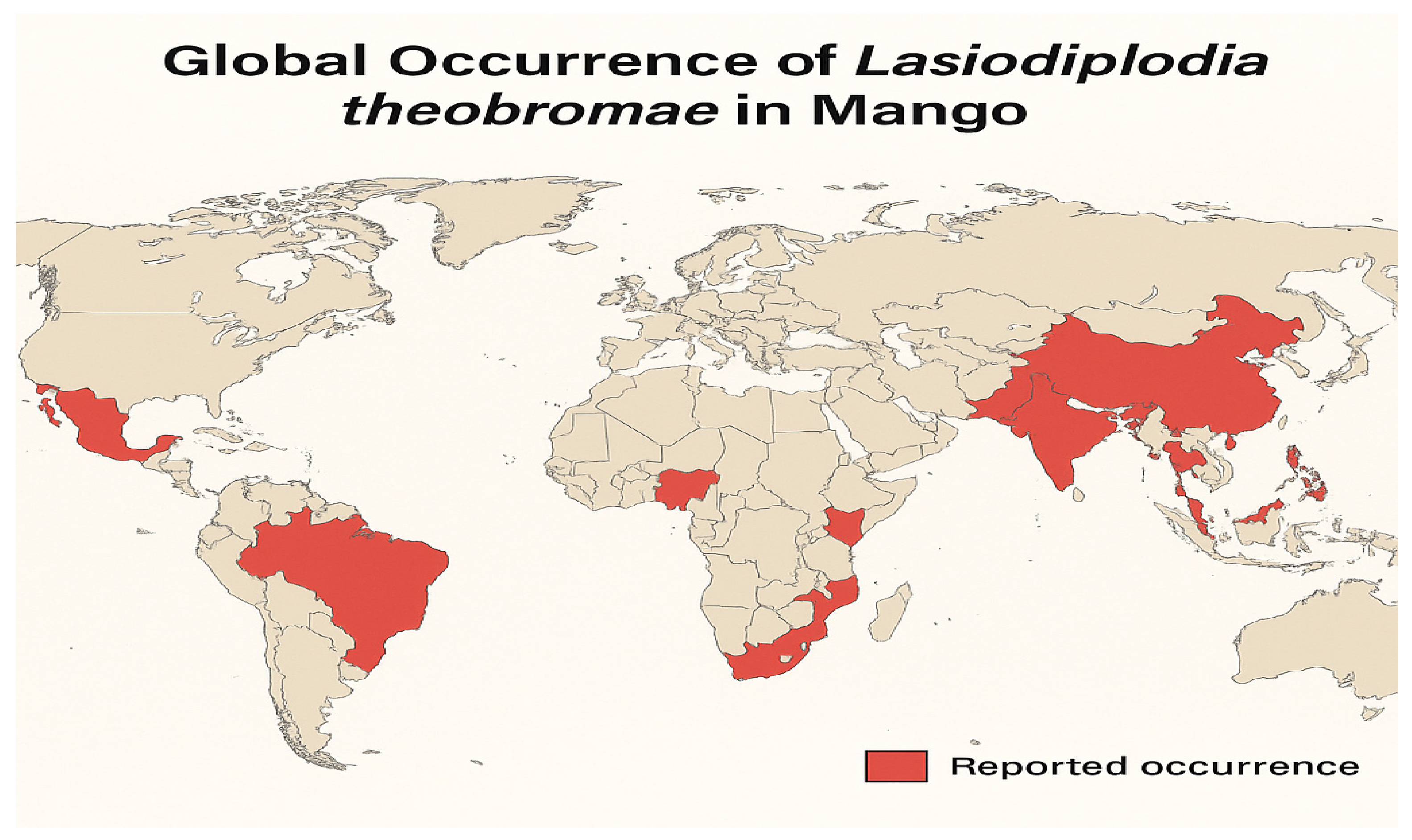
2. Global Distribution of SER Disease
In India, stem-end rot was first documented in 1945. The disease has since been reported in all major mango-producing regions globally, leading to substantial economic losses due to fruit decay during transportation and storage. This post-harvest disease occurs in several mango-growing countries, including China, India, Pakistan, the Philippines, Indonesia, Thailand, Nigeria, South Africa, Ghana, Côte d'Ivoire, Ethiopia, and the Americas (Peru, Brazil, Mexico, and Colombia) (Ma et al., 2021; Bui et al., 2018; Khanzada et al., 2004; Widiastuti et al., 2024; Syed et al., 2014; Ablormeti et al., 2021; Honger et al., 2015; Yoboué et al., 2024) [
25,
26,
27,
28,
29,
30]. The worldwide distribution of SER is presented in
Figure 1.
3. Symptoms of Stem End Rot in Mango Caused by Lasiodiplodia theobromae
The stem end rot disease is a significant post-harvest disease in arid regions and ranks as the second most severe mango disease globally, following anthracnose disease (Johnson, 1998). In the initial lesion development, the disease typically initiates as a small dark-brown area around the base of the fruit stem. This lesion gradually expands, leading to significant decay of the fruit tissue (Joshi et al., 2023)[
31]. Under humid conditions, the affected area enlarges to form a circular, black patch, which can extend rapidly and turn the whole fruit completely black within two or three days. The pulp becomes brown and softer (Joshi et al., 2023). The rot proceeds through the pulp more quickly than the pericarp, leading to the development of soft brown to black decay symptoms at both ends of the fruit (Joshi et al., 2023). In advanced stages, a straw-coloured fluid oozes out from the stem end giving out a foul smell and at advanced fruit rotten a steel-grey mycelium can be observed on the fruit surface, indicating extensive fungal colonization (Parakash, 2004; Joshi et al., 2023)[
32].
In later stages, when the rot extends to the other fruit sections, the fruit softens, becomes discoloured and develops brown flesh (Galsurker et al., 2018; Ploetz, 1994; Pérez-Jiménez, 2008)[
33,
34]. A straw-colored fluid may ooze out from the stem end, emitting a foul smell, further indicating the severity of the infection (Joshi et al., 2023). The pathogen produces thick-walled, one-septate, brown to dark brown spores when viewed under the microscope. Colonies initially appear white, turning greyish to dark brown and finally black upon maturation, with the development of black pycnidia (Joshi et al., 2023).
Disease symptoms such as diffused water-soaked patches of tissues that swiftly darken and radiate in finger-like projections from the affected fruit's stem end have been described. Within seven days or less, the necrosis that is still under the cuticle may reach all of the fruit’s flesh (Johnson, 1998). According to Huang and Liu (1995),[
35] mango stem end rot symptoms start in the flesh and progress to the fruit's pericarp. Before the symptoms appear on the surface, the interior portions of the fruits are already destroyed and can therefore not be utilized in any form due to the devastating nature of the disease (Honger et al., 2016). This is further corroborated by reports that the infected fruit flesh has an unpleasant flavour, rendering the fruits inedible once the pathogen has invaded flesh tissues (Diskin et al., 2017; Prusky et al., 2009)[
36,
37]. These symptoms do not only affect the marketability of mango fruits but also pose significant challenges for postharvest management.
Figure 2.
Infected mango fruits by stem end rot (SER) disease.
Figure 2.
Infected mango fruits by stem end rot (SER) disease.
5. Disease Cycle and Epidemiology of the Stem End Rot Pathogen
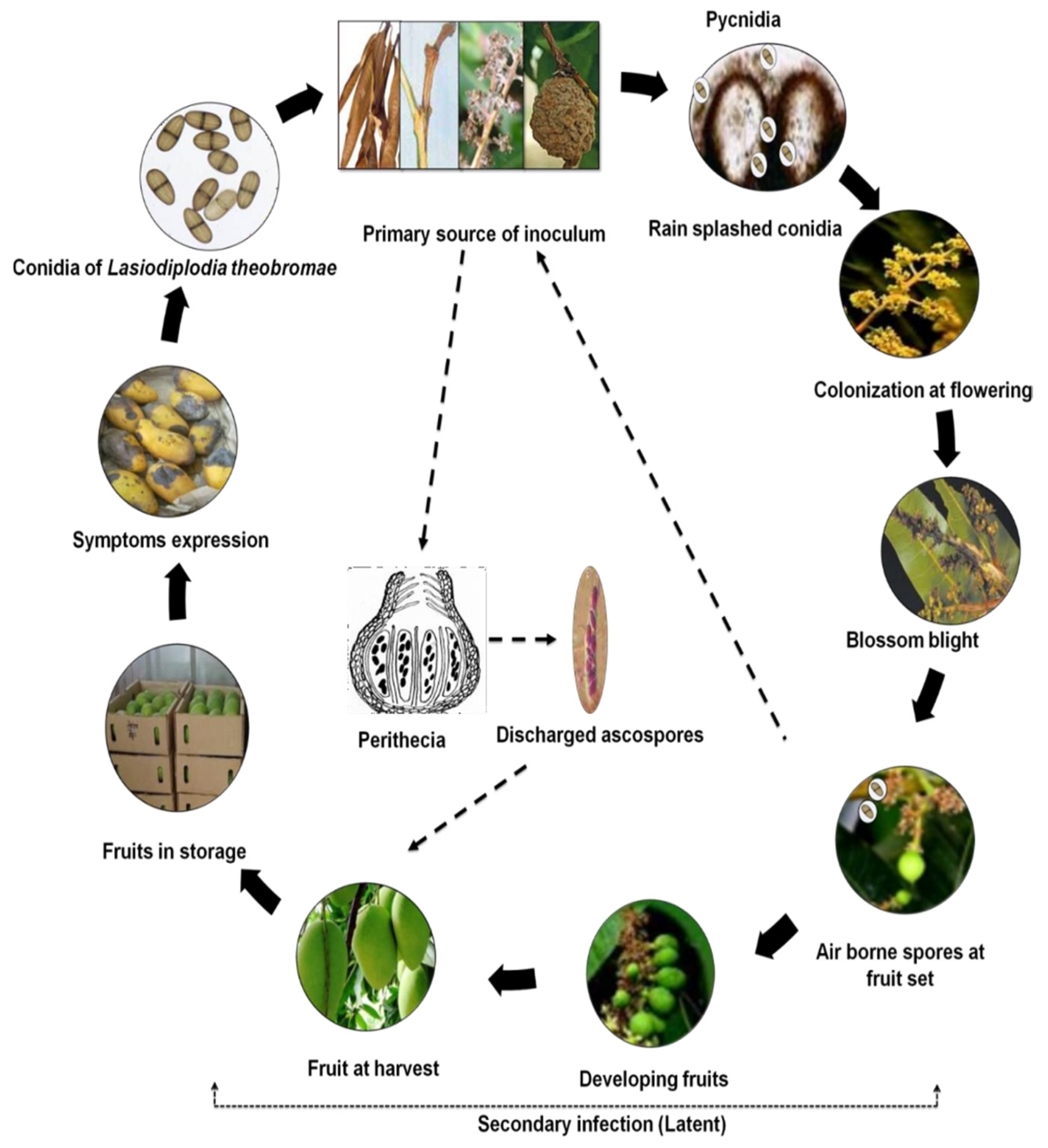
A typical life cycle of how airborne inoculum causes the disease and spreads on the fruits in the field is depicted in
Figure 3. By producing reproductive structures on diseased and dried twigs, branches, and panicles of mango trees, as well as dry leaves and mummified fruits, the pathogen survives during periods between seasons. Additionally, it can also persist on dry leaves scattered on the ground, from which ascospores can emerge. These infected plant parts serve as primary sources of inoculum. Under favourable conditions, the pathogen produces both sexual (ascospores) and asexual spores (conidia) in perithecia and pycnidia, respectively. In the field, the conidia, which are the main infective propagules, are released during periods of high water and moisture, which are necessary for the infection process to occur on developing flowers (Slippers et al., 2005; Marques et al., 2013; Ismail et al., 2016)[
38,
39,
40]. The blighting of infected mango flowers leads to further sporulation of
Lasiodiplodia theobromae on necrotic floral tissues (Slippers et al., 2005). The newly formed conidia are then dispersed by wind or rain-splash onto young and developing fruits, where they infect the fruit peduncle and the fruits themselves, remaining quiescent until conditions favor disease development (Slippers et al., 2005; Marques et al., 2013). During harvesting, wounds created serve as entry points for ascospores released from perithecia present in initial sources of infection, contributing to additional infection propagules alongside the existing quiescent infections (Johnson et al., 1992). During storage or transportation, the pathogen resumes growth, leading to the manifestation of disease symptoms on the infected fruits (Ismail et al., 2016). Due to the presence of pathogen propagules in fallen tree debris and within the soil, there is a possibility for the fruit to become infected through contact with the soil during the harvesting process (Business Queensland, 2023)[
41].
1.4.1. Epidemiology of Stem End Rot Pathogen
The pathogens that cause fruit stem end rot enter through natural wounds and openings, particularly during the inflorescence and flowering stages of fruit development (Galsurker et al., 2018; Alkan & Kumar, 2018; Johnson et al., 1992). Mostly, immature and unripe mango fruits display resistance against stem end rot pathogens. However, this resistance weakens as the fruits ripen and during their storage or transit. The fungus that leads to stem end rot infects the xylem and phloem at the fruit's end during its development, which subsequently predisposes the fruits to infection after harvesting and before ripening. The actual infection of fruit tissues typically begins during the ripening stage when there is an increase in soluble sugars, ethylene production, weakening of the cell structure, a reduction in phytoanticipins and phytoalexins, a decline in the plant's inducible defense mechanisms, and changes in the pH of the surrounding host tissue (Galsurker et al., 2018; Alkan & Fortes, 2015)[
42].
In general, the spread of the pathogens associated with stem end rot disease in the tropics is favoured by warm, damp and humid climatic conditions (Nelson, 2008)[
42]. The spores and mycelial growth and survival of the pathogen, however, are significantly affected by prolonged exposure to cold temperatures (Everett, 2003)[
43]. It can be inferred that fruits produced in the dry seasons may have less or no occurrence and severity of stem end rot disease (Arauz, 2000)[
44]. It has also been reported that through contact, infected fruits can transfer the disease to other healthy fruits, especially when packed in the same container (Arauz, 2000).
6. The Aetiology of Mango Stem End Rot
The aetiology of stem end rot is complex, as several fungal pathogens are known to play a role prior to and during harvesting of fruits (Johnson, 1998; Honger et al., 2016). These group of fungal pathogens are known to belong to the Botryosphaeria genus, such as Dothiorella dominicana, Dothiorella mangiferae, Lasiodiplodia theobromae, Neofusicoccum sp., Phomopsis mangiferae, Cytosphaera mangiferae, and Pestalotiopsis sp., and by other pathogenic fungi, such as Colletotrichum gloeosporioides and Alternaria alternata (Alkan & Kumar, 2018; Galsurker et.al., 2018; Galsurker et. al., 2020). In most tropical areas of the world, L. theobromae is the most dominant pathogen linked with the disease (Johnson, 1998). In Brazil, Ghana, and Sri Lanka, the pathogen has been linked to the stem end rot disease of mangoes (Costa et al., 2010; Honger et al., 2016; Syed et al., 2014). Though other fungi have been associated in the aetiology of the mango disease worldwide, L. theobromae is the most recorded pathogen linked with the disease.
7. Description and Identification of Lasiodiplodia theobromae
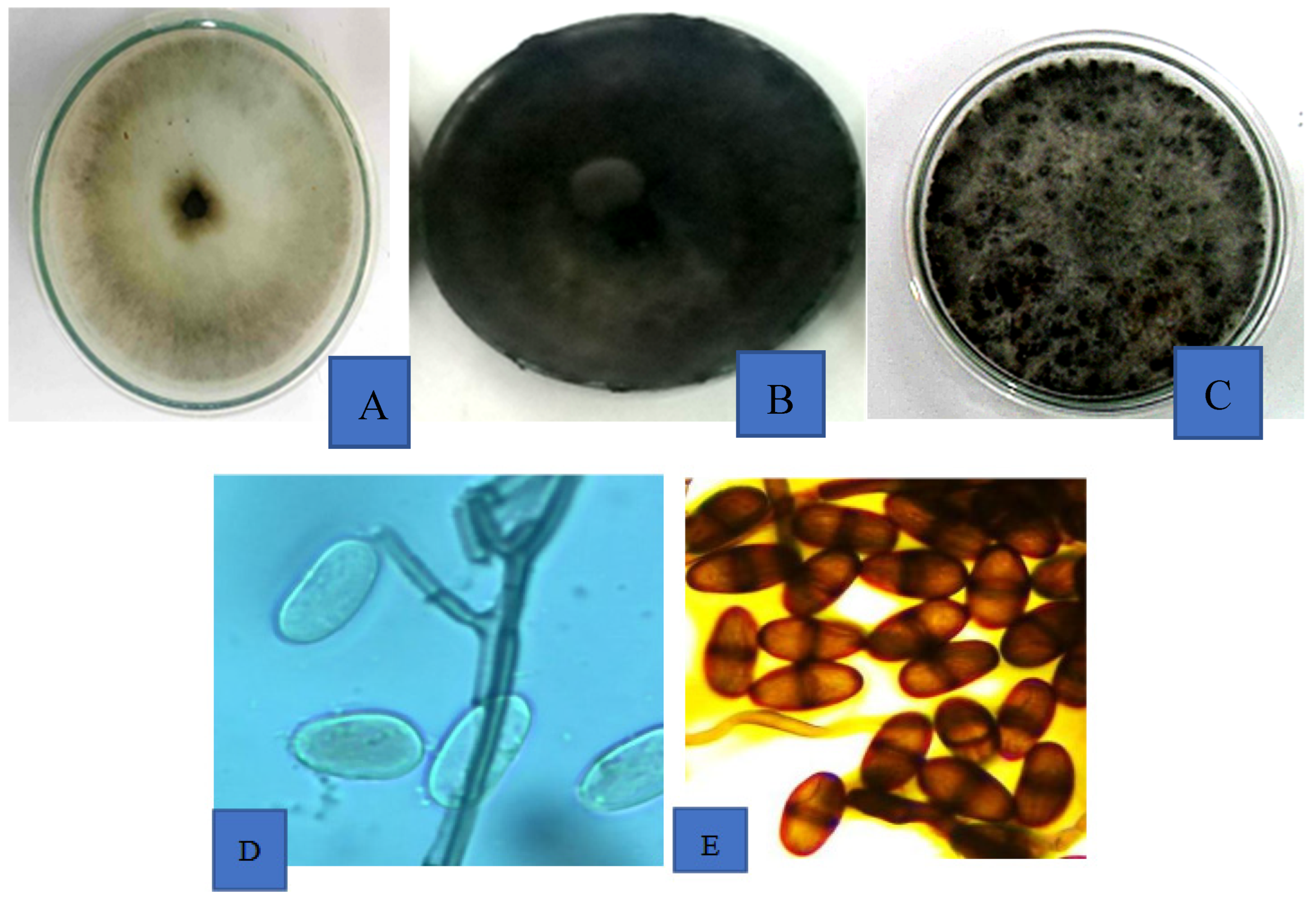
Lasiodiplodia theobromae is a ubiquitous fungus with a wide host range. The fungus colonies produce thin white mycelial threads that can fill a 9 mm Petri dish in about 3-4 days. The mycelium darkens with time and may continue to grow aerially to touch the cover of the dish. In some isolates, pycnidia or pycnidium-like structures may be observed after 7 to 10 days after incubation. The fungus produces non-septate paraphyses and two types of conidia in their pycnidia. The first type of conidia is immature, unicellular and hyaline while the second type are mature, bi-celled and dark brown conidia with longitudinal striations, whose dimensions range from 10.2-12.0±1.5 µm wide and 20.0-30.0±2.0 µm long (Honger et al., 2016; Phillips et al., 2008; Urbez-Torres et al., 2008; Johnson, 1998)[
45]. The cultural characteristics of
L. theobromae associated with mango fruits that has been consistently isolated from infected fruits is shown in
Figure 4A-E.
The species of
L. theobromae responsible for mango stem end rot has been identified using cultural and morphological features and growth rate, which are traditional methods of fungal identification and combined with molecular methods (Honger et al., 2016; Phillips et al., 2008; Jonson, 1998). According to Phillips et al., (2008) the pathogen can be distinguished from closely related species by its fast-growing mycelium, paraphysis in the pycnidia, and formation of hyaline immature and bi-celled dark-brown conidia. Xu et al. (2005), designed a primer Lt347-F/Lt347-R which was used to identify
L. theobromae causing rot of blueberry and which was able to correctly identify the same pathogen from tree decline symptoms of mango in Ghana (Honger et al., 2018). The internal transcribed spacer region (ITS) sequences, which have been utilized as a barcode for species delineation in fungal systematics (Damm et al., 2000) [
46], have also been used to identify
L. theobromae that is infecting mango fruits in Ghana (Honger et al., 2018). Though the ITS region has been reported as reliable for species delineation it is a minute percentage of the genomes of the organisms and might not be able to tell apart closely related species with accuracy (Ekanayake et al., 2019) [
47]. To ensure that more of the organisms’ total genome is included in its identification, multiple genes have been recommended for accurate species delineation, especially among closely related species.
L. theobromae infecting different crops have been identified using a combination of the sequences of the ITS region, partial beta-tubulin, partial transfer elongation factor gene and 28SrDNA (Crous et al., 2008; Rodríguez-Gálvez et al., 2015; Urbez-Torres et al., 2008) [
48].
L. theobromae recovered from twigs and leaves of die-back symptoms on mango tree has been identified using the ITS and tef-α gene in Burkina Faso (Dianda et al., 2023) [
49].
Lasiodiplodia theobromae, caused mango stem end rot disease in Côte d'Ivoire and it was identified using a combination of the ITS and the tef genes (Yefougnigui, 2023) [
50]. On the other hand, Yanling et al., (2021) [
51], identified the pathogen in China using three different diagnostic genes, namely, ITS, EFI and the partial beta tubulin.
8. Host Range of Lasiodiplodia theobromae
In tropical and subtropical regions worldwide, a number of crops have been linked to the ubiquitous
L. theobromae pathogen. On mango, the pathogen has been associated with twig dieback, gummosis of the trunk and twigs and mummifying of young fruits in the field. In addition, the pathogen is associated with rotting of the fruits after harvest with the symptoms either beginning from the fruit's stem end portion or any part on the fruit. Using mango trees in the field, Coelho et al., (2020) [
52], evaluated a total of 75 accessions of mango and found that some of them, namely, Alfa, Apple DCG, Ataulfo, Da porta, Espada, Espada Ouro, Haden, Haden 2H, Heidi, Irwin, Keitt, Kent, Mom Amon, DCG, Neldica, Pawin, Recife, Smith, Torbet and Tyler Primier, were resistant to the pathogen and could be useful in breeding programmes against the disease. However, in pathogenicity studies, using detached fruits, the Kent cultivar showed susceptibility to the fungus (Ohene-Mensah, 2012) [
53].
L. theobromae was associated with several plant hosts worldwide, with an estimated host range of over 280 different kinds of plant species (Domsch et al., 1980; Khanzada et al., 2006; Sutton, 1980) [
54,
55,
56]. In China, specifically, the Sichuan province,
L. theobromae was identified as the causal agent of fruit rot of Kiwi (
Actinidia chinensis) and was shown to have the ability to cause spots on leaves and twigs of the plant (Zhou et al., 2015) [
57]. In Ghana, the pathogen has been identified as the causal agent of rots of cocoa pods, mango and banana fruits and yam tubers (Ohene-Mensah, 2012). In Nigeria, Ghana and the Philippines, the pathogen has been reported as the causal agent of fruit rots of soursop (Honger et al., 2020; Amusa et al., 2003; Alberto & Otanes, 2016) [
58,
59,
60]. Nishijima (1993) [
61], associated the pathogen with pawpaw in Hawaii, while it was identified as the causal agent of blue berry fruits in China (Xu et al., 2015) [
62].
Apart from fruit rots associated with the pathogen, other symptoms associated with the pathogen include dieback, blights and root rot in a variety of hosts. The pathogen has been reported as the causal agent of leaf blight disease of rubber in Thailand. In Bangladesh, the pathogen was reported as the causal agent of stem canker of dragon fruit, a disease so severe that several farmers in the area abandoned the production of the crop (Briste, et al., 2022) [
63]. The pathogen was recognized as the cause of mango tree decline in Pakistan, Ghana, Oman, India, Mexico, the United States, and Jordan. Mango tree decline is a disease that leads to the death of infected mango tree entirely (Ploetz et al., 1996; Al Adawi et al., 2006; Alvarez- García & López₋García, 1971; Asad et al., 2010; Fateh et al., 2006) [
64,
65,
66,
67,
68].
L. theobromae has also been reported as the causal agent of inflorescence blight and twig dieback disease of cashew in Nigeria (Olunloyo, 1983; Adeniyi et al., 2013; Adeniyi et al., 2016; Hammed & Adedeji, 2008) [
69,
70,
72].
The host range of
L. theobromae is not restricted to plant hosts. It has been described as a rare plant pathogen in both immunocompetent and immunocompromised human hosts with a variety of infections including sinusitis, keratitis, pneumonia and cutaneous lesions (Kanaujia et al., 2023; Papacostas et al., 2015) [
73,
74]. According to Kanaujia et al., (2023), a strain of
L. theobromae, P213 is gradually emerging as a human pathogen due to the fact that within a period of 10 years a total of 20 human patients were diagnosed with several diseased conditions such as keratomycosis, subcutaneous infections, rhinosinusitis, onychomycosis and pneumonia. The pathogen has been reported as the causal agent of a subcutaneous infection in a male human, due to the traumatic implantation caused by the pathogen (Papacostas et al., 2015). Summerbell et al., (2004) [
75], reported of a subcutaneous phaehyphomycosis caused by
L. theobromae. These recent findings showed that the
L. theobromae pathogen is pathogenic to both plant and human hosts.
9. The Lasiodiplodia theobromae Complex
The genus
Lasiodiplodia is closely related to five other genera, namely,
Diplodia, Neodeightonia,
Barriopsis, Phaeobotryon and
Phaeobotryosphaeria (Phillips et al., 2008). The main features that distinguish this genus from other closely related genera are the presence of pycnidia paraphyses and longitudinal striations on mature conidia (Abdollahzadeh et al., 2010) [
76]. Based on variations in the conidial and paraphyses morphology complemented by sequencing of the rDNA-ITS region and the EF-1α gene, several species including
L. theobromae have been described in the
Lasiodiplodia genus (Abdollahzadeh et al., 2010). The
L. theobromae species were the basis for the creation of the
Lasiodiplodia genus (Sutton, 1980).
Prior to the availability of molecular methods for species delineation, it was impossible to delineate the species status of some members of the
Lasiodiplodia genus. To circumvent the problem, Punithalingam (1976) [
77], identified such species as synonyms of
L. theobromae, probably to the fact that they were more similar to the species than any of the other species in the genus. Later observations showed that isolates identified as
L. theobromae species showed variability in their morphology and had a wide host range, prompting the thought that the species may be a group species (Abdollahzadeh et al., 2010) and may contain other cryptic species. Invariably, based on sequence analysis of diagnostic genes, the
L. theobromae species has been characterized as having numerous cryptic species and currently more than 20 different species of
Lasiodiplodia have been separated from
L. theobromae (Abdollahzadeh et al., 2010; Pavlic et al., 2004; Burgess et al., 2006; Damm et al. 2007; Alves et al., 2008) [
78,
79,
80,
81].
10. Impact of Postharvest Stem End Rot Pathogens on Mango's Nutritional and Sensory Attributes
During fruit pathogenesis, various postharvest fungi, notably
Lasiodiplodia theobromae, induce rot in fruits like mango, leading to biochemical changes and decreased food and market value (Pawar, 2012) [
82]. These pathogens affect the nutrient content of vitamins, carbohydrates, and minerals. Several studies have reported reductions in sugar content, ascorbic acid levels, vitamin C, ash, phosphorus, and calcium due to postharvest fungal infections (Arya, 1993; Pawar, 2012) [
83].
The organic acid content and total soluble solids (TSS) are crucial parameters for assessing a fruit's juice quality. Along with aroma volatiles, these compounds significantly contribute to the flavor of ripe mangoes (Padda et al., 2011) [
84]. Overall, consumer acceptance of fruits is largely determined by the balance between TSS and total titratable acidity (TTA) (Zapata et al., 2008a; Zapata et al., 2008b) [
85]. Therefore, any impact from external factors, such as pathogens or the degree of fruit ripeness, that alters a fruit's TSS or TTA concentration is likely to affect the fruit's organoleptic quality.
Recent studies have highlighted that SER pathogens, including L. theobromae, colonize the fruit's stem end endophytically during development and become necrotrophic upon ripening, leading to tissue degradation and flavor loss (Diskin et al., 2017). This transition results in significant alterations in the fruit's biochemical composition, including reductions in sugars, acids, and volatile compounds essential for flavor and aroma. Moreover, the infection can lead to increased respiration rates and ethylene production, accelerating ripening and senescence, thereby shortening shelf life (Diskin et al., 2017).
Additionally, the presence of SER has been associated with changes in the fruit's microbiome, which can further influence the fruit's quality and susceptibility to other pathogens (Diskin et al., 2017). These microbiome alterations can affect the fruit's defense mechanisms and overall postharvest behavior.
11. Control of Mango Stem End Rot Disease
The disease is a latent infection which begins in the field and symptoms manifests after fruit harvest. Therefore, successful control of the disease has been achieved using an integrated control measures with components at the pre-harvesting, harvesting and post harvesting stages.
11.1. Control of Stem End Rot Disease at the Pre-Harvest Stage
There are several good agronomic practices which have been recommended for the control of diseases in general in mango orchards. Pruning of infected plant parts such as twigs, mummified fruits and excessive vegetative plant parts is carried out primarily to ensure ventilation of the tree canopy, but which goes to also reduce inoculum of diseases such as anthracnose and stem end rot, whose primary sources of inoculum are these infected plant parts (Arauz, 2000; Honger et al., 2005) [
86]. In addition, removal of fallen and decaying leaves of mango trees which serve as overwintering materials for these pathogens have also been recommended for the control of these diseases. Though these practices have been specifically prescribed for anthracnose disease, they serve as a dual-purpose practice as they will in-variably decrease the incidence of the stem end rot disease also, as the two diseases follow similar epidemiological patterns (Honger; unpublished data). Another important cultural practice is the selection of resistant cultivars. Though, not a widely practice measure, due to the preference of marketing qualities, some levels of research have been carried out in the area and as many as 15 cultivars of the crops have reportedly shown resistance to the disease's causative agent (Coelho et al., 2020). However, the most commonly used method of combating the disease in the field is the use of fungicides. Fungicides applied against the disease range from inorganic, organic and biological. Inorganic fungicides recommended for the control of the disease in the field include Carbendazim, Thiophanate Methyl and Propiconazole at 15 days intervals till fruit maturity (Plantix, 2023) [
87]. According to Honger et al., (2006) [
88], inorganic pesticides such as Carbendazim, Copper oxide+Metalaxyl, Mancozeb, and Thiophanate methyl are effective against the pathogens of the disease in Ghana. However, no evaluation has been done on these fungicides' bio-efficacy against the disease in the field.
11.2. Harvesting of Fruits
During harvesting, care must be taken to minimize bruising of fruits as these serve as entry holes for the pathogen to infect the fruits (Mahajan & Kapoor, 2021) [
89]. Secondly, since rotten leaves in the soil may be harbouring the sexual spores of the causative agent of the disease, fruits touching the bare soil may be infected and hence as much as possible, the fruits must be prevented from touching the soil during harvesting (Plantix, 2023). Harvesting mango fruits using secateurs to leave short parts of the pedicel, rather than the usual method of simply detaching the fruit, has been reported as an important practice in reducing the incidence of post-harvest stem end rot disease in mangoes. According to Diskin et al., (2017), this technique has significant impact on reducing stem end rot occurrence in mango fruits. Equally, harvesting mango with long pedicels have been observed to reduce stem end rot in comparison to harvesting with short pedicels (Sangchote, 1989) [
90]. This phenomenon has been linked to the presence in the mango sap, of some compounds such as the alk(en)ylresorcinols (5-n-heptadecenylresorcinol and 5-n-pentadecylresorcinol) with antimicrobial and antifungal activities, especially against
Alternaria alternata (Droby et al., 1987)[
91]. This has very well been studied in mango against the anthracnose disease where, Kensington Pride mango fruits stored with 2 to 3 cm long stems had significantly more resorcinol in their peel, high sap flow and smaller anthracnose lesions than de-sapped fruit (Hassan et al., 2007; Krishnapillai & Wijeratnam, 2017)[
92,
93]. Therefore, the available data suggests that harvesting fruits with shorter stems (pedicels) has the tendency to decrease post-harvest fruit side decay and stem-end rot.
11.3. Fruit Ripening Inhibition
Ethylene is the primary phytohormone regulating many aspects of the climacteric fruit-ripening process, although other hormones such as auxin and abscisic acid also play significant roles in ripening. Since there is a strong link between fruit ripening and post-harvest decay, numerous studies have investigated the use of phytohormones to mitigate decay after harvest. For instance, the application of the auxin derivative 2,4-dichlorophenoxyacetic acid (2,4-D) has been shown to slow fruit ripening and prevent stem-end rot in citrus and mango fruits (Coggins, 1972; Kobiler et al., 2001)[
94,
95]. Additionally, research has explored the post-harvest use of 1-MCP, an ethylene receptor inhibitor, which delays fruit aging, extends storage life and inhibit postharvest decay of fruits (Watkins & Nock, 2008; Watkins, 2006)[
95,
96]. However, due to ethylene's dual function in both fruit defense response and fruit ripening (Alkan & Fortes, 2015), those studies yielded conflicting findings. Several studies conducted on Indian jujube fruit and avocado fruits using 1-MCP treatment reduced stem end rot infection (Zhang et al., 2007; Zhong & Xia, 2007; Diskin et al., 2017)[
98,
99]. Nevertheless, additional research on citrus, mango, and avocado revealed that 1-MCP increases the fruit's vulnerability to infections that cause stem end rot (Porat et al., 100; Woolf et al., 2005)[
101,
110]. It appears that 1-MCP may have a concentration- and time-dependent effect on fruit susceptibility. Accordingly, low concentrations of ethylene are likely required to preserve fruit resistance to infections (Alkan & Fortes, 2015), while excessive 1-MCP concentrations likely interfere with the fruit's ability to ripen naturally and slow down the ripening process.
11.4. Post-Harvest Treatments of Harvested Fruits
Postharvest fungicidal treatments, which can be applied as waxes or coatings, can be sprayed on, dipped, or sprayed on to control stem end rot. Prochloraz, classified as a non-systemic imidazole, is a widely acknowledged fungicide employed in the commercial management of post-harvest diseases in both avocado and mango fruits. (Dodd, et al., 1997; Everett et al., 2003)[
102]. Overall, fungicides like Benomyl and Thiabendazole offer the added benefit of effectively combating mango stem end rot caused by
L. theobromae (Estrada et al., 1996)[
103]. The optimal control of stem end rot, in Kent mango fruit at postharvest was achieved through the synergistic use of prochloraz and fludioxonil (Swart et al., 2006)[
104]. In a comparative study involving mango fruits artificially infected with
L. theobromae, the efficacy of six fungicides was assessed viz: Carbendazim, Azoxystrobin, Tebuconazole combined with Trifloxystrobin, Difenoconazole, Thiabendazole, and Propiconazole. The results from the study showed that, the fungicides were able to significantly inhibit the mycelial growth of
L. theobromae pathogen and reduce the severity of the disease on mango fruits (Syed et al., 2014). In a study by Zhang (2012),[
105] on a comparative investigation of Azoxystrobin, Fludioxonil, Pyrimethanil, Imazalil, and Thiabendazole on citrus fruits showed that these fungicides were highly effective in reducing the occurrence and severity of diplodia stem-end rot. Ultimately, the application of suitable fungicides recommended for postharvest use, which pose no threat to human, plant, or animal well-being, can play a substantial role in minimizing or managing stem rot infections in mango fruits.
However, this has not been the case and the over reliance on these inorganic fungicides has posed several health threats to consumers, farmers, river bodies, plants and animals (Goswami et al., 2018)[
105].
Utilizing plant extracts presents a secure and viable approach for controlling and addressing stem end rot diseases. These alternative approaches to the use of pesticides have been shown to decrease the occurrence of postharvest diseases, contributing to the extension of shelf life and enhancement of fruit quality, not only in mangoes but also in various types of fruits. There are several studies that have incorporated extracts of plants such as
Moringa oleifera,
Cinnamon zeylanicum,
Zingiber officinale,
Datura stramonium, thyme oil, clove, Cinnamon oil and
Eucalyptus camaldulensis to combat various types of fungal pathogens (Patni & Kolte, 2006; Bhalodia & Shukla, 2011)[
106,
107]. These plant extracts have shown strong antifungal effects against fungal mycelial growth in vitro, potentially helping to prevent stem-end rot in mangoes (Alam et al., 2017; Ullah et al., 2017; Perumal et al., 2016)[
108,
109,
110]. At this point, it can be conjectured that, active components in plants when properly extracted can serve as alternative measures in controlling the mango stem end rot of caused by
L. theobromae pathogen.
11.5. Physical Control Measures for Stem End Rot
Physical treatments such as heat, irradiation, and cold storage are effective and widely used methods for managing stem-end rot. Cold storage, in particular, is highly effective at slowing fruit ripening and minimizing post-harvest decay. Each pathogen has an optimal temperature range for growth, and temperatures outside this range can inhibit hyphal development, conidial germination, and overall pathogenicity (Ayerst, 1969)[
111]. Similarly, each fruit has a specific storage temperature range; storing it below this optimal range can lead to chilling injuries, while temperatures above it can accelerate the ripening process.
Gamma irradiation is another strategy that can be used to destroy microorganisms by causing damage to their deoxyribonucleic acid (DNA), and it is even utilized to prolong the storage time of foods. However, it provides the fruits with only mild effects as it does not entirely reduce the incidence and severity of stem end rot from occurring on mango and citrus. Conversely, a combination of hot water and gamma irradiation resulted in a significant reduction in stem end rot and anthracnose in mangoes. Ultra Violet- C (UV-C) light has been found to be effective in controlling different fungal pathogens, including those causing stem end rot, by stimulating fruit resistance, making it a potential option for organic market applications.
Heat treatment helps reduce post-harvest pathogens like stem end, lessen chilling injury, and extend shelf life by triggering the fruit's intrinsic resistance, getting rid of detached pathogens, and promoting the spread of the fruit's natural waxy covering. Diverse heat-treatment techniques have been suggested, encompassing processes such as hot-water immersion, rinsing, exposure to hot vapor, and dry-air treatments. These methods have demonstrated promise in decreasing post-harvest diseases by triggering a defensive mechanism. Hot-water immersion has also demonstrated a reduction in stem end rot for papaya and mango, although its effectiveness is relatively lower compared to its impact on anthracnose. Consequently, several studies have incorporated hot-water treatments into their post-harvest protocols.
11.6. Synergistic Approaches: Combining Treatments for Enhanced Results
Due to the rise of fungi that are resistant to fungicides and the limited success of using individual fungicides, achieving full protection against post-harvest diseases is challenging using a single treatment or natural methods alone. To enhance disease control, a combination of strategies is necessary. Combining heat treatment with specific chemical compounds has demonstrated synergistic outcomes, resulting in enhanced efficacy in disease management and decreased reliance on chemical interventions for post-harvest decay. For instance, in certain regions, mangoes have been subjected to a solution containing benomyl at 52 °C for 5 min through immersion, yielding favorable results (Sepiah, 1986)[
112].
Submerging fruits in hot water containing benomyl has proven to be successful in managing post-harvest stem end rot disease caused by
L. theobromae. Utilizing a blend of hot water brushing, prochloraz treatment, and 2,4-D application for mangoes leads to a notable decrease of 50-70% in stem end rot and side decay. These combinations have improved mango fruit quality during storage, lowering the incidence of stem end rot from 86 % to 10 % in 'Tommy Atkins' mangoes. Also, other commonly used treatment for mangoes involves chlorine sterilization, hot water brushing, acidic prochloraz application and waxing (Prusky et al., 1999)[
113]. In Australia, a comprehensive strategy for preserving mango quality entails a sequence of interventions. This strategy encompasses the utilization of benomyl in conjunction with hot-water treatment, succeeded by a prochloraz spray. This combined approach proficiently manages anthracnose, stem end rot, and Alternaria rot. Effectively mitigating post-harvest diseases necessitates the integration of multiple approaches, including pre-harvest chemical measures, surface sterilization, hot-water immersion, chemical applications, coating or waxing, inhibition of ripening, and cold storage. However, the adoption of all these measures may not be imperative under circumstances of low disease incidence or brief storage periods. It is important for each packing house to develop customized protocols to manage post-harvest diseases and preserve fruit quality during storage, considering the associated costs.
Furthermore, it is imperative coordinated research is focused on studying the effects of the stem end rot pathogen on consumers health and its associated health risks.
Funding
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Notes
This work is part of the doctoral dissertation by Awudu Amadu Gariba and supervised by Professor Robert Sarpong Amoah and Dr. Joseph Okani Honger. The study focused on the stem end rot disease of mango and its control.
Credit authorship contribution statement
AAG and JOH: study-conceived; AAG, JOH, RSA-study designed, writing–original draft, review, and editing and CID helped with the proof reading. All the authors approved the submitted version.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Institutional Review Board Statement
Not applicable.
Acknowledgments
During the preparation of this manuscript/study, the author(s) used ChatGPT for the purposes of description the global distribution of Lasiodiplodia theobromae in graphics. The authors have reviewed and edited the output and take full responsibility for the content of this publication.”.
Abbreviations
The following abbreviations are used in this manuscript:
| SER |
Stem End Rot |
| PCR |
Polymerase Chain Reaction |
| ITS |
Internal Transcribed Spacer |
| EFI |
Enzyme Function Initiative |
References
- Abdollahzadeh, J., Javadi, A., Goltapeh, E. M., Zare, R., & Phillips, A. J. L. (2010). Phylogeny and morphology of four new species of Lasiodiplodia from Iran. Persoonia-Molecular Phylogeny and Evolution of Fungi, 25(1), 1-10.
- Ablormeti, F. K., Coleman, S. R., Honger, J. O., Owusu, E., Bedu, I., Aidoo, O. F., Cornelius, E. W., & Odamtten, G. T. (2021). Management of Lasiodiplodia theobromae, the causal agent of mango tree decline disease in Ghana. African Crop Science Journal, 29(2), 165–174.
- Adeniyi, D. O., Adedeji, A. R., Oduwaye, O. F., & Kolawole, O. O. (2013). Evaluation of biocontrol agents against Lasiodiplodia theobromae causing inflorescence blight of cashew in Nigeria. IOSR Journal of Agriculture and Veterinary Science, 5(3), 46-48.
- Adeniyi, D. O., Olufolaji, D. B., & Joseph, A. (2016). Characteristic Variations in Lasiodiplodia theobromae; Pathogen of inflorescence Dieback of Cashew in Growing Ecologies of Nigeria. Annual Research & Review in Biology, 10(2), 1-6.
- Al Adawi, A. O., Deadman, M. L., Al Rawahi, A. K., Al Maqbali, Y. M., Al Jahwari, A. A., Al Saadi, B. A., & Wingfield, M. J. (2006). Aetiology and causal agents of mango sudden decline disease in the Sultanate of Oman. European Journal of Plant Pathology, 116, 247-254.
- Alam, M. W., Rehman, A., Malik, A. U., Ahmad, S., Haider, M. S., Amin, M., & Gleason, M. L. (2020). Dynamics of stem end rot disease of mango fruit and its management. Pakistan Journal of Agricultural Sciences, 57(1).
- Alam, M. W., Rehman, A., Sahi, S. T., & Malik, A. U. (2017). Exploitation of natural products as an alternative strategy to control stem end rot disease of mango fruit in Pakistan. Pakistan Journal of Agricultural Sciences, 54(3).
- Ali, U., Syed, J. H., Malik, R. N., Katsoyiannis, A., Li, J., Zhang, G., & Jones, K. C. (2014). Organochlorine pesticides (OCPs) in South Asian region: a review. Science of the Total Environment, 476, 705-717.
- Alberto, R. T., & Otanes, A. T. (2016). Morphological and molecular identification and fungicide sensitivity assay of pathogens attacking guyabano Annona muricata in Philippines. Plant Pathology and Quarantine: 6(1), 60-79.
- Alkan, N., & Fortes, A. M. (2015). Insights into molecular and metabolic events associated with fruit response to post-harvest fungal pathogens. Frontiers in Plant Science, 6, 160575.
- Alvarez-García, L. A., & López-García, J. (1971). Gummosis, die-back and fruit rot disease of mango (Mangifera indica L.) caused by Physalospora rhodina (B. & C.) Cke. in Puerto Rico.
- Amusa, N. A., Ashaye, O. A., Oladapo, M. O., & Kafaru, O. O. (2003). Pre-harvest deterioration of Sour sop (Annona muricata) at Ibadan Southwestern Nigeria and its effect on nutrient composition. African Journal of Biotechnology, 2(1), 23-25.
- Arauz, L. F. (2000). Mango anthracnose: Economic impact and current options for integrated management. Plant Disease, 84(6), 600-611.
- Arya, P. S. (1993). Postharvest diseases of fruits and vegetables and their control. Research Periodicals & Book Publishing House.
- Ayerst, G. (1969). The effects of moisture and temperature on growth and spore germination in some fungi. Journal of Stored Products Research, 5(2), 127-141.
- Benatar, G. V., & Wibowo, A. (2021). First report of Colletotrichum asianum associated with mango fruit anthracnose in Indonesia. Crop Protection, 141, 105432.
- Bhalodia, N. R., & Shukla, V. J. (2011). Antibacterial and antifungal activities from leaf extracts of Cassia fistula l.: An ethnomedicinal plant. Journal of Advanced Pharmaceutical Technology & Research, 2(2), 104-109.
- Bright, R. A., Medina, M. J., Xu, X., Perez-Oronoz, G., Wallis, T. R., Davis, X. M., & Klimov, A. I. (2005). Incidence of adamantane resistance among influenza A (H3N2) viruses isolated worldwide from 1994 to 2005: a cause for concern. The Lancet, 366(9492), 1175-1181.
- Briste, P. S., Akanda, A. M., Bhuiyan, M. A. B., Mahmud, N. U., & Islam, T. (2022). Morphomolecular and cultural characteristics and host range of Lasiodiplodia theobromae causing stem canker disease in dragon fruit. Journal of Basic Microbiology, 62(6), 689-700.
- Bui, R., Sinha, B., Devi, P. S., & Dinesh, K. (2018). First report of Lasiodiplodia theobromae associated with stem-end rot of mango in Manipur, India. Indian Phytopathology, 71(4), 631–632.
- Business Queensland. (2023). Stem end rot. Queensland Government. Retrieved from https://www.business.qld.gov.au/industries/farms-fishing-forestry/agriculture/biosecurity/plants/diseases/horticultural/stem-end-rot.
- Burgess, T. I., Barber, P. A., Mohali, S., Pegg, G., de Beer, W., & Wingfield, M. J. (2006). Three new Lasiodiplodia spp. from the tropics, recognized based on DNA sequence comparisons and morphology. Mycologia, 98(3), 423-435.
- Coelho, W. C., Santos, C. A. F., & Batista, D. D. C. (2020). Variability of mango accessions resistance to dieback disease caused by Lasiodiplodia theobromae and Neofusicoccum parvum.
- Coggins Jr, C. W. (1972, August). Use of growth regulators to delay maturity and prolong shelf life of citrus. In Symposium on growth Regulators in Fruit Production 34 (pp. 469-472).
- Crous, P. W., Slippers, B., Wingfield, M. J., Rheeder, J., Marasas, W. F., Philips, A. J., & Groenewald, J. Z. (2006). Phylogenetic lineages in the Botryosphaeriaceae. Studies in Mycology, 55(1), 235-253.
- Damm, U., Crous, P. W., & Fourie, P. H. (2007). Botryosphaeriaceae as potential pathogens of Prunus species in South Africa, with descriptions of Diplodia africana and Lasiodiplodia plurivora sp. nov. Mycologia, 99(5), 664-680.
- Dianda, O. Z., Wonni, I., Ouédraogo, L., Sankara, P., Tollenaere, C., Del Ponte, E. M., & Fernandez, D. (2023). Lasiodiplodia species associated with mango (Mangifera indica L.) decline in Burkina Faso and influence of climatic factors on the disease distribution. Physiological and Molecular Plant Pathology, 126, 102041.
- Diskin, S., Feygenberg, O., Maurer, D., Droby, S., Prusky, D., & Alkan, N. (2017). Microbiome alterations are correlated with occurrence of postharvest stem-end rot in mango fruit. Phytobiomes, 1(3), 117-127.
- Dodd, J. C., Prusky, D., & Jeffries, P. (1997). Fruit diseases. The Mango: Botany, Production and Uses, 257-280.
- Doidge, E. M. (1914). Some diseases of the potato. Agricultural Journal of the Union of South Africa, 7(5), 698.
- Domsch, K. H., Gams, W., & Anderson, T. H. (1980). Compendium of soil fungi. Volume 1.
- Droby, S., Prusky, D., Jacoby, B., & Goldman, A. (1987). Induction of antifungal resorcinols in flesh of unripe mango fruits and its relation to latent infection by Alternaria alternata. Physiological and Molecular Plant Pathology, 30(2), 285-292.
- Ekanayake, G., Abeywickrama, K., Daranagama, A., & Kannangara, S. (2019). Morphological characterization and molecular identification of stem-end rot associated fungal species isolated from ‘Karutha Colomban’mango fruits in Sri Lanka.
- Estrada, A. B., Jeffries, P., & Dodd, J. C. (1996). Field evaluation of a predictive model to control anthracnose disease of mango in the Philippines. Plant Pathology, 45(2), 294-301.
- Everett, K. R., Owen, S. G., & Cutting, J. G. M. (2005). Testing efficacy of fungicides against postharvest pathogens of avocado (Persea americana cv Hass). New Zealand Plant Protection, 58, 89-95.
- Fateh, F. S., Kazmi, M. R., Ahmad, I., & Ashraf, M. (2006). Ceratocystis fimbriata isolated from vascular bundles of declining mango trees in Sindh, Pakistan. Pakistan Journal of Botany, 38(4), 1257.
- Galsurker, O., Diskin, S., Duanis-Assaf, D., Doron-Faigenboim, A., Maurer, D., Feygenberg, O., & Alkan, N. (2020). Harvesting mango fruit with a short stem-end altered endophytic microbiome and reduce stem-end rot. Microorganisms, 8(4), 558.
- Galsurker, O., Diskin, S., Maurer, D., Feygenberg, O., & Alkan, N. (2018). Fruit stem-end rot. Horticulturae, 4(4), 50.
- Goswami, S. K., Singh, V., Chakdar, H., & Choudhary, P. (2018). Harmful effects of fungicides-Current status. International Journal of Agriculture, Environment and Biotechnology, 11, 1011-1019.
- Guo, Z., Xing, R., Liu, S., Zhong, Z., Ji, X., Wang, L., & Li, P. (2007). Antifungal properties of Schiff bases of chitosan, N-substituted chitosan and quaternized chitosan. Carbohydrate Research, 342(10), 1329-1332.
- Hammed, L. A., & Adedeji, A. R. (2008). Incidence and control of twig die-back in young cashew in Ibadan (South-Western Nigeria). Agricultural Journal, 3(3), 171–175.
- Hassan, M. K., Dann, E. K., Irving, D. E., & Coates, L. M. (2007). Concentrations of constitutive alk (en) ylresorcinols in peel of commercial mango varieties and resistance to postharvest anthracnose. Physiological and Molecular Plant Pathology, 71(4-6), 158-165.
- Hofman, P. J. (1998) Production factors influence fruit quality and response to postharvest treatments. In: Coates, L.M., Hofman, P.J. and Johnson, G.I. (eds) Disease Control and Storage Life Extension in Fruit. Australian Centre for International Agricultural Research (ACIAR), Canberra, pp. 6–18.
- Honger, J. O. (2013). Characterization of the causal agent of mango anthracnose disease in Ghana (Doctoral dissertation, PhD thesis, University of Ghana).
- Honger, J. O., William, C. E., Owusu-Ansah, D., Siaw-Adane, K., & Odamtten, G. T. (2015). Pathogenicity and fungicide sensitivity of the causal agent of postharvest stem end rot disease of mango in Ghana. Ghana Journal of Agricultural Science, 49(1), 33–42.
- Honger, J. O., Ablomerti, F. K., Coleman, S. R., Cornelius, E. W., Owusu, E., & Odamtten, G. T. (2018). Differentiation of two Botryosphaeriaceae species isolated from declining mango trees in Ghana. West African Journal of Applied Ecology, 26(2), 14-25.
- Honger, J. O., Asare, D., & Lambon, J. B. (2020). Incidence and Aetiology of Stem End Rot and Anthracnose Diseases Affecting Soursop Fruits at Pre-Harvest and Post-Harvest Stages in Ghana. Agricultural and Food Science Journal of Ghana, 13, 1186-1196.
- Honger, J. O., Coleman, S. R., Ablormeti, F. K., Cornelius, E. W., Owusu, E., & Odamtten, G. T. (2018). The aetiology, incidence and severity of mango tree decline disease in Ghana. Ghana Journal of Science, 58, 13-22.
- Honger, J. O., Offei, S. K., Oduro, K. A., Odamtten, G. T., & Nyaku, S. T. (2016). Identification and molecular characterisation of Colletotrichum species from avocado, citrus and pawpaw in Ghana. South African Journal of Plant and Soil, 33(3), 177-185.
- Honger, J. O., Osabutey, S., Nyaku, S. T., & Lambon, J. B. (2021). Molecular characterization, pathogenicity and copper sensitivity of Xanthomonas citri pv mangiferae indicae, the causal agent of mango bacterial black spot disease in Ghana. Archives of Phytopathology and Plant Protection, 54(19-20), 1703-1721.
- Honger, J. O., William, C. E., Owusu-Ansah, D., Siaw-Adane, K., & Odamtten, G. T. (2015). Pathogenicity and fungicide sensitivity of the causal agent of postharvest stem end rot disease of mango in Ghana. Ghana Journal of Agricultural Science, 49(1), 37-52.
- Huang, S., & Liu, X. (1995). Control of postharvest stem end rot of mango. South China Fruits, 24(4), 33–34.
- Ismail, A. M., Cirvilleri, G., Polizzi, G., & Crous, P. W. (2016). Lasiodiplodia species associated with dieback disease of mango in Egypt. Phytopathologia Mediterranea, 55(1), 100–110. [CrossRef]
- Johnson, G. I., Cooke, A. W., Mead, A. J., & Wells, I. A. (1989, September). Stem end rot of mango in Australia: causes and control. In III International Mango Symposium 291 (pp. 288-295).
- Johnson, G. I., Mead, A. J., Cooke, A. W., & Dean, J. R. (1992). Mango stem end rot pathogens-Fruit infection by endophytic colonization of the inflorescence and pedicel. Annals of Applied Biology, 120(2), 225-234.
- Johnson, G. I., Mead, A. J., Cooke, A. W., & Wells, I. A. (1992). Stem-end rot of mango in Australia caused by Lasiodiplodia theobromae. Australasian Plant Pathology, 21(4), 147–148. [CrossRef]
- Joshi, R. L., Regunathan, U., Muthukumar, A., & Kathan, G. S. (2023). Cultural and Morphological Characterization of Lasiodiplodia theobromae Causing Post-harvest Stem End Rot of Mango. Biological Forum – An International Journal, 15(3), 325–330. Retrieved from https://www.researchtrend.net/bfij/pdf/Cultural-and-Morphological-characterization-of-Lasiodiplodia-theobromae-causing-Post-harvest-Stem-End-Rot-of-Mango-Rahul-L-Joshi-52.pdf.
- Kanaujia, R., Singh, S., & Rudramurthy, S. M. (2023). Aspergillosis: an update on clinical Spectrum, diagnostic schemes, and management. Current Fungal Infection Reports, 17(2), 144-155.
- Khanzada, M. A., Lodhi, A. M., & Shahzad, S. (2004). Mango dieback and gummosis in Sindh, Pakistan caused by Lasiodiplodia theobromae. Plant Health Progress, 5(1), 1–5.
- Khanzada, M. A., Rajput, A. Q., & Shahzad, S. (2006). Effect of medium, temperature, light and inorganic fertilizers on in vitro growth and sporulation of Lasiodiplodia theobromae isolated from mango. Pakistan Journal of Botany, 38(3), 885.
- Khanzada, S. A., Iqbal, S. M., & Akram, A. (2006). In vitro efficacy of plant leaf extracts against Sclerotium rolfsii Sacc. Mycopathologia, 4(1), 51-53.
- Kobiler, I., Shalom, Y., Roth, I., Akerman, M., Vinokur, Y., Fuchs, Y., & Prusky, D. (2001). Effect of 2, 4-dichlorophenoxyacetic acid on the incidence of side and stem end rots in mango fruits. Postharvest Biology and Technology, 23(1), 23-32.
- Krishnapillai, N., & Wilson Wijeratnam, R. S. (2017). Sap volatile components in relation to susceptibility of anthracnose and Aspergillus rot of mangoes (Mangifera indica L.). The Journal of Horticultural Science and Biotechnology, 92(2), 206-213.
- Kumar, M., Saurabh, V., Tomar, M., Hasan, M., Changan, S., Sasi, M., & Mekhemar, M. (2021). Mango (Mangifera indica L.) leaves: Nutritional composition, phytochemical profile, and health-promoting bioactivities. Antioxidants, 10(2), 299.
- Li, Q., Bu, J., Shu, J., Yu, Z., Tang, L., Huang, S., & Hsiang, T. (2019). Colletotrichum species associated with mango in southern China. Scientific reports, 9(1), 18891.
- Lim, T. K., & Sangchote, S. (2003). Diseases of durian. Diseases of Tropical Fruit Crops, 241-251.
- Liu, X., Li, C., & Wang, Q. (2020). Postharvest management strategies for controlling fungal diseases in fruits: A comprehensive review. Journal of Food Protection, 83(4), 652-665.
- Ma, Y., Ahmad, T., Zheng, Y., Nie, C., & Liu, Y. (2021). First report of postharvest stem end rot of mango fruit (Mangifera indica) caused by Lasiodiplodia theobromae in China. Plant Disease, 105(11), 3756.
- Ma, Y., Ahmad, T., Zheng, Y., Nie, C., & Liu, Y. (2021). First report of postharvest stem end rot of mango fruit (Mangifera indica) caused by Lasiodiplodia theobromae in China. Plant Disease, 105(9), 2715.
- Marques, M. W., Lima, N. B., Morais, M. A., Barbosa, M. A. G., Souza, B. O., Michereff, S. J., & Câmara, M. P. S. (2013). Species of Lasiodiplodia associated with mango in Brazil. Fungal Diversity, 61, 181–193. [CrossRef]
- Nelson, S. C. (2008). Mango anthracnose (Colletotrichum gloeosporioides) (PD-48). Honolulu, HI: University of Hawaii at Mānoa, College of Tropical Agriculture and Human Resources.
- Netto, M. S., Assunção, I. P., Lima, G. S. A., Vieira, W. A. S., Monteiro, F. P., & Michereff, S. J. (2014). Species of Lasiodiplodia associated with tropical fruit plants in northeastern Brazil. Plant Disease, 98(3), 358–365. [CrossRef]
- Nishijima, W. T. (1994). Mango diseases and their control. In C. L. Chia & D. O. Evans (Eds.), Proceedings of the Conference on Mango in Hawaii, March 9–11, 1993 (pp. 20–24). Honolulu, HI: University of Hawaii at Mānoa, College of Tropical Agriculture and Human Resources.
- Ohene-Mensah, G. (2012). Characterization of Botryodiplodia Theobromae Isolates Affecting Cocoa, Mango, Banana and Yam in Ghana (Doctoral dissertation).
- Olunloyo, O. A. (1983). Results of three years of spraying with fungicide-insecticide combinations against inflorescence dieback of cashew.
- Padda, M. S., do Amarante, C. V., Garcia, R. M., Slaughter, D. C., & Mitcham, E. J. (2011). Methods to analyze physico-chemical changes during mango ripening: A multivariate approach. Postharvest Biology and Technology, 62(3), 267-274.
- Padda, M. S., Picha, D. H., & Akhter, M. (2011). Effect of maturity and ripening on the biochemical composition of mango fruit. Journal of Food Composition and Analysis, 24(1), 85–92. [CrossRef]
- Papacostas, L. J., Henderson, A., Choong, K., & Sowden, D. (2015). An unusual skin lesion caused by Lasiodiplodia theobromae. Medical Mycology Case Reports, 8, 44-46.
- Patni, C. S., & Kolte, S. J. (2006). Effect of some botanicals in management of Alternaria blight of rapeseed-mustard. Annals of Plant Protection Sciences, 14(1), 151-156.
- Pavlic, D., Slippers, B., Coutinho, T. A., Gryzenhout, M., & Wingfield, M. J. (2004). Lasiodiplodia gonubiensis sp. nov., a new Botryosphaeria anamorph from native Syzygium cordatum in South Africa. Studies in Mycology, 50(2), 313-322.
- Pawar, V. M. (2012). Postharvest fungal diseases of fruits and vegetables and their management. International Journal of Life Sciences, 1(1), 1–7.
- Pérez-Jiménez, J., Arranz, S., Tabernero, M., Díaz-Rubio, M. E., Serrano, J., Goñi, I., & Saura-Calixto, F. (2008). Updated methodology to determine antioxidant capacity in plant foods, oils and beverages: Extraction, measurement and expression of results. Food Research International, 41(3), 274-285.
- Perumal, A. B., Sellamuthu, P. S., Nambiar, R. B., & Sadiku, E. R. (2016). Antifungal activity of five different essential oils in vapour phase for the control of Colletotrichum gloeosporioides and Lasiodiplodia theobromae in vitro and on mango. International Journal of Food Science & Technology, 51(2), 411-418.
- Phillips, A. J. L., Alves, A., Pennycook, S. R., Johnston, P. R., Ramaley, A., Akulov, A., & Crous, P. W. (2008). Resolving the phylogenetic and taxonomic status of dark-spored teleomorph genera in the Botryosphaeriaceae. Persoonia-Molecular Phylogeny and Evolution of Fungi, 21(1), 29-55.
- Ploetz, R. C., Benscher, D., Vazquez, A., Colls, A., Nagel, J., & Schaffer, B. (1996). A re-examination of mango decline in Florida.
- Ploetz, R. C., Benscher, D., Vázquez, A., Colls, A., Nagel, J., & Schaffer, B. (1996, September). Mango Decline: Research in Florida on an apparently wide-spread disease complex. In V International Mango Symposium 455 (pp. 547-557).
- Porat, R., Weiss, B., Cohen, L., Daus, A., Goren, R., & Droby, S. (1999). Effects of ethylene and 1-methylcyclopropene on the postharvest qualities of ‘Shamouti’oranges. Postharvest Biology and Technology, 15(2), 155-163.
- Prusky, D., & Lichter, A. (2007). Activation of quiescent infections by postharvest pathogens during transition from the biotrophic to the necrotrophic stage. Phytopathology, 97(7), 966-972.
- Prusky, D., Kobiler, I., Miyara, I., & Alkan, N. (2009). Fruit diseases. In R. E. Litz (Ed.), The mango: Botany, production and uses (2nd ed., pp. 210–245). CABI. [CrossRef]
- Punithalingam, E. (1976). Botryodiplodia theobromae. Descriptions of Fungi and Bacteria, No. 518, 1–2. Commonwealth Mycological Institute.
- Rahaman, M., Chowdhury, M., Rahman, M. A., Ahmed, H., Hossain, M., Rahman, M. H., & Biswas, M. (2023). A deep learning-based smartphone application for detecting mango diseases and pesticide suggestions. International Journal of Computing and Digital Systems, 13(1), 1-1.
- Raviv, M., Antignus, Y., & Putievsky, E. (2019). Improvement of postharvest preservation of organic greenhouse crops. Agronomy, 9(11), 7-25.
- Rodríguez-Gálvez, E., Maldonado, E., & Alves, A. (2015). Identification and pathogenicity of Lasiodiplodia theobromae causing dieback of table grapes in Peru. European Journal of Plant Pathology, 141, 477-489.
- Sangchote, S. (1989, September). Botryodiplodia stem end rot of mango and its control. In III International Mango Symposium 291 (pp. 296-303).
- Sathya, K., Parthasarathy, S., Thiribhuvanamala, G., & Prabakar, K. (2017). Morphological and molecular variability of Lasiodiplodia theobromae causing stem end rot of mango in Tamil Nadu, India. Int J Pure App Biosci, 5(6), 1024-1031.
- Sepiah, S., Ploetz, R. C., & Cooke, A. W. (2003). Diseases of carambola. Diseases of tropical fruit crops, 145-162.
- Slippers, B., Fourie, G., Crous, P. W., & Wingfield, M. J. (2005). Botryosphaeriaceae as endophytes and latent pathogens of woody plants: Diversity, ecology and impact. Fungal Biology Reviews, 19(2), 93–123. [CrossRef]
- Summerbell, R. C. (2004). Fungi associated with vertebrates. Biodiversity of fungi: Inventory and Monitoring Methods, 454-465.
- Swaroop, A., Bagchi, M., Moriyama, H., & Bagchi, D. (2018). Health benefits of mango (Mangifera indica L.) and mangiferin. Jpn J Med, 1(2), 149-154.
- Swart, S. H., Serfontein, J. J., Swart, G., & Labuschagne, C. (2006). Chemical control of post-harvest diseases of mango: the effect of fludioxonil and prochloraz on soft brown rot, stem-end rot and anthracnose. In VIII International Mango Symposium 820 (pp. 503-510).
- Syed, R. N., Mansha, N., Khaskheli, M. A., Khanzada, M. A., & Lodhi, A. M. (2014). Chemical control of stem end rot of mango caused by Lasiodiplodia theobromae. Pakistan Journal of Phytopathology, 26(2), 201-206.
- Ullah, S. F., Hussain, Y., & Iram, S. (2017). Pathogenic characterization of Lasiodiplodia causing stem end rot of mango and its control using botanicals. Pak. J. Bot, 49(4), 1605-1613.
- Úrbez-Torres, J. R., Leavitt, G. M., Guerrero, J. C., Guevara, J., & Gubler, W. D. (2008). Identification and pathogenicity of Lasiodiplodia theobromae and Diplodia seriata, the causal agents of bot canker disease of grapevines in Mexico. Plant Disease, 92(4), 519-529.
- Watkins, C. B. (2006). The use of 1-methylcyclopropene (1-MCP) on fruits and vegetables. Biotechnology Advances, 24(4), 389-409.
- Watkins, C. B. (2008). Overview of 1-methylcyclopropene trials and uses for edible horticultural crops. Hort Science, 43(1), 86-94.
- Woolf, A. B., Requejo-Tapia, C., Cox, K. A., Jackman, R. C., Gunson, A., Arpaia, M. L., & White, A. (2005). 1-MCP reduces physiological storage disorders of ‘Hass’ avocados. Postharvest Biology and Technology, 35(1), 43-60.
- Xu, C., Zhang, H., Zhou, Z., Hu, T., Wang, S., Wang, Y., & Cao, K. (2015). Identification and distribution of Botryosphaeriaceae species associated with blueberry stem blight in China. European Journal of Plant Pathology, 143, 737-752.
- Yeo, Y. S., Kone, Y., Dembele, D. D., Amari, E. L. N. O. D. G., Rey, J. Y., Del Ponte, E. M., & Kone, D. (2024). Prevalence of mango stem-end rot disease in Côte d'Ivoire and identification of associated fungal pathogens. Tropical Plant Pathology, 49(3), 367-383.
- Yeo, Y. S., Kone, Y., Dembele, D. D., Amari, E. L. N. O. D. G., Rey, J. Y., Del Ponte, E. M., & Kone, D. (2024). Prevalence of mango stem-end rot disease in Côte d'Ivoire and identification of associated fungal pathogens. Tropical Plant Pathology, 49(3), 367-383.
- Yeo, Y. S., Dembele, D. D., Camara, B., Ouattara, S., Rey, J. Y., Fernandez, D., & Kone, D. (2023). Effect of some biopesticides based on essential oil and plant extracts on postharvest mango Stem-end rot disease caused by Lasiodiplodia theobromae. Journal of Agriculture and Food Research, 14, 100798.
- Zapata, P. J., Serrano, M., Pretel, M. T., Amorós, A., & Romojaro, F. (2008a). Biochemical and physiological changes during ripening of four mango cultivars. Food Science and Technology International, 14(5), 375–384. [CrossRef]
- Zapata, P. J., Guillén, F., Martínez-Romero, D., Castillo, S., Valero, D., & Serrano, M. (2008b). Use of alginate or zein as edible coatings to delay postharvest ripening process and to maintain tomato (Solanum lycopersicon Mill) quality. Journal of the Science of Food and Agriculture, 88(7), 1287-1293.
- Zhang, L., Song, L., Xu, X., Zou, X., Duan, K., & Gao, Q. (2020). Characterization and fungicide sensitivity of Colletotrichum species causing strawberry anthracnose in eastern China. Plant Disease, 104(7), 1960-1968.
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).