1. Introduction
The photoreceptor is a terminally differentiated and specialized neural retinal cell, localized in the outer layer of the retina. These cells are essential for vison and responsible for capturing and converting light into electrical signals through a process of phototransduction. However, the fragility of photoreceptor outer segment membrane [
1,
2], their residence at the back of the retina [
3,
4], and their demand for prodigious amounts of energy [
5] make photoreceptor cells particularly vulnerable to damage and degeneration. Photoreceptor cell death is a vital cause of a variety of retinal disorders such as retinal detachment, retinitis pigmentosa (RP), and age-related macular degeneration (AMD) [
6,
7,
8]. The loss of photoreceptors usually leads to severe visual damage and, eventually, blindness. Unlike teleost fish, such as zebrafish, which possess a prominent capacity for retinal regeneration and vision restoration after damage [
9,
10,
11], mammalian retinas are similar to the central nervous system and lack this regenerative ability [
12,
13]. This significant difference between species underscores the difficulty of treating retinal diseases in humans. Novel therapeutic strategies for photoreceptor repair are urgently needed.
One of the most prominent and effective therapeutic strategies is the direct conversion method, which has drawn our attention.
Accumulated evidence indicates that fully differentiated cells can be directly stimulated to reprogram into other cell types by the overexpression of specific transcription factors. Several papers have also shown that fibroblasts can be directly converted into different cell types, such as functional neurons [
14,
15,
16,
17,
18], cardiomyocytes [
19] and hepatocyte-like cells [
20]. Mahato et al. reported that a set of five small molecules could transform the fibroblasts into rod photoreceptor-like cells [
21]. Here, we sought to explore whether the fibroblasts can be directly converted into photoreceptor cells by overexpression of specific transcription factors.
Retinal maturation occurs in a specific order and is regulated by transcription factors in the basic helix-loop-helix (bHLH) and the nuclear hormone receptor families [
22,
23]. The transcription factors act in a combinatorial manner to define the cell fates of retinal progenitor cells [
24]. Recent studies report that the cone-rod homeobox (
Crx), a member of the Otx homeodomain gene family, is expressed exclusively and abundantly in photoreceptor cells in the neural retina and is essential for the terminal differentiation and maintenance of the photoreceptors [
25,
26].
Otx2, another key member of the Otx homeobox gene family during photoreceptor development, works as a direct upstream regulator of
Crx.
Otx2 is a crucial regulatory transcription factor for steering retinal progenitor cells into developing into photoreceptors, which is expressed in most of the forebrain and midbrain neuroepithelium, including the retina [
27,
28]. The neural retina leucine zipper (
Nrl) is a basic motif-leucine zipper transcription factor of the Maf subfamily that is preferentially expressed in rod photoreceptors and regulates the rhodopsin expression synergistically with
Crx [
29,
30]. The molecular mechanisms that regulate retinal regeneration in teleost fish after injury have been explored for more than a decade. Among the numerous proneural transcription factors expressed in response to injury,
Ascl1 is one of the rapidly upregulated genes in retinal Müller glia cells after retina damage in fish and is essential for retinal regeneration [
31]. Neurogenin1 (
Ngn1) is a member of the neurogenin subfamily of bHLH genes and participates in governing the differentiation of progenitor cells into photoreceptors [
32,
33]. Starting from a pool of these five candidate genes (
Crx, Otx2, Nrl, Ascl1, and
Ngn1), we sought to identify whether specific transcription factors could directly convert fibroblasts into photoreceptor cells.
2. Materials and Methods
2.1. Animal Experimental Procedures
The young adult C57BL/6J mice were purchased from Vital River Company and kept in standard housing conditions. All animal studies were conducted in accordance with experimental protocols and approved by the Animal Care Committee of Peking University First Hospital.
2.2. Fibroblast Isolation and Culture
MEFs were isolated from E13.5 C57BL/6 mouse embryos under a dissection microscope (Leica) as previously described.14 The head, vertebral column, dorsal root ganglia, and visceral organs were removed; and the remaining tissue was manually dissected into small pieces and incubated in 0.25% trypsin (Invitrogen) for 10–15 minutes to create a single cell suspension. The dissociated cells were cultured in MEF media (high glucose Dulbecco’s Modified Eagle Medium, Invitrogen), supplemented with 10 percent fetal bovine serum (FBS, Biochrom), 0.1 mM nonessential amino acids, and two mM of Glutamax. The cells were cultured at 37 °C with 5 percent CO2 and incubated for two to three days until confluent; cells were passaged in a 1:4 split before being frozen. MEFs were used between passages two and four, and MPFs were obtained from three-day-old mice. Only forelimbs and forelegs were taken; the tissue was rinsed in ethanol, washed with phosphate-buffered saline (PBS), dissociated, and digested in 0.25 percent trypsin. MPFs were cultured in MEF media.
2.3. Adenovirus Production and Infection
The mouse cDNAs of Ascl, Crx, Ngn1, Nrl, and Otx2 were first cloned in the pEntr 3C vector (Invitrogen) and then in the pAD vector (Invitrogen) via a homologous L/R recombination. We then transduced the viral constructs into a 293A cell line and obtained high titer (108 IU/ml) viral particles by two rounds of amplification according to the manufacturer’s instructions. MEFs or MPFs were infected twice with adenovirus for eight hours per day at multiplicities of infection (MOI, number of viral particles per cell) of 30 or 20. Twenty-four hours post-infection, half of the culture medium was changed into a neural medium (1g/liter glucose DMEM/F-12/neural basal 2:2:1, 1*B-27, 10 or 20 ng/ml BDNF); 10 or 20 μmol/liters of forskolin were added each day for two successive days. Then, half of the medium was changed every two days until the cells were ready for immunostaining and RT–PCR experiments.
2.4. Conversion Efficiency
We counted the conversion efficiency using the photoreceptor purity as the percentage of Recoverin+ cells relative to the total final population. We randomly selected eight to ten visual fields for each well and calculated the total cell number (at least 200 cells) visualized after the DAPI staining and the total photoreceptor-like cell number indicated by the Recoverin+ staining. The efficiency was calculated by dividing the number of photoreceptor-like cells by the number of total cells in each visual field.
2.5. Immunofluorescence
The photoreceptor-like cells were washed with phosphate-buffered saline (PBS) and then fixed with 4 percent paraformaldehyde for 20 minutes at room temperature. After washing twice with PBS, cells were blocked in a solution of PBS containing 5 percent BSA and 0.1 percent TritonX-100 for 30 minutes at room temperature. Primary antibodies were diluted in an antibody dilution solution (PBS with 1 percent BSA and 0.1 percent Triton X-100) in ratios from 1:100 to 1:1000, and secondary antibodies were diluted in a 1:1000 ratio in an antibody dilute solution. Photoreceptor-like cells were incubated in primary antibodies overnight at 4 °C, and secondary antibodies were incubated for 60 minutes at room temperature. The following primary antibodies were used: rabbit anti-Recoverin+ (1:1000, AB5585, Millipore), mouse anti-Rhodopsin (1:500, MAB5356, Millipore), rabbit anti-Opsin (1:200, AB5405, Millipore) and mouse anti-Tuj1 (1:2,000; Millipore; MAB1637).
2.6. RT–PCR Analysis
Photoreceptor-like cells and wild-type photoreceptor cells were cultured in a neuronal medium. MEFs were cultured in DMEM with a 10 percent FBS medium. All cells were washed with a serum-free medium before collection. RNA was isolated using Trizol (Invitrogen), following the manufacturer’s instructions. We reverse-transcribed 600 ng of total RNA and then quantified using SYBR Green (Tiangen), and β-actin was used as the reference. Sequences of primers for real-time PCR were as follows: Crx: forward 5’- GTCCCATACTCAAGTGCCCC-3’, reverse 5’- CTTGAACCAGACCTGGACCC-3’; Otx2: forward 5’- GCAGTCAATGGGCTGAGTCT-3’, reverse 5’- CACCCTGGATTCTGGCAAGT-3’; Nrl: forward 5’- TTCACCCACCTTCAGTGAGC-3’, reverse: 5’- GTCCGAAAATCTCTCGGGCA-3’; Recoverin: forward 5’-GACGGCAATGGGACCATCA-3’, reverse 5’-CCCGCTTTTCTGGGGTGTTT-3’; Rhodopsin: forward 5’-GCCCCAATTTTTATGTGCCCTT-3’, reverse 5’- GTGACGTAGAGCGTGAGGAA-3’; Fiz1: forward 5’- TGCCCTAAGGGATTCCGAGA-3’, reverse 5’- TGCAACATACTGAGCAGGGG-3’; Sag: forward 5’- GCCTGCGGGAAGACCAATA-3’, reverse 5’- TTCACAAGCTCAGGGTCCAC-3’. PCR products were analyzed with a 1 percent DNA gel.
2.7. Transplantation of Photoreceptor-like Cells in Vivo and Tissue Dissection
The induced photoreceptor-like cells were labeled with GFP by lentivirus infection and concentrated to
~10
6 cells/μl. Two μl per site were transplanted into the subretinal space of eight-to-ten--week-old C57BL/6 mice (anesthetized with 70 mg/kg of pentobarbital sodium). Two weeks post-transplantation, the eyes of the mice were harvested and fixed with 4 percent paraformaldehyde overnight, followed by dehydration in PBS containing 30 percent sucrose for 24 hours at 4 °C. Eyeballs were then embedded in an optimal cutting temperature (OCT) compound (Sakura, Tokyo, Japan,
http://tissue-tek.com) and frozen at -20 °C. The eyeballs were cryosectioned at ten μm using a cryostat microtome (Leica CM1950) and mounted onto slides. Slide-mounted sections were kept at -80 °C until needed.
2.8. Statistical Analysis
Statistical analysis was evaluated by the SPSS 16.0 software and assessed with normality and variance. Data were compared via a t-test, a one-way analysis of variance (ANOVA) for multiple comparisons, and a two-way ANOVA for repeated measures. Values were considered statistically significant at a difference of P < 0.05 (*), P < 0.01 (**), and P < 0.001(***). Data are presented as the mean ± S.E.
3. Results
3.1. Screening for Photoreceptor-Fate-Inducing Factors
Five genes expressed in the neural retina and known to contribute to the development of photoreceptor cells were cloned:
Ascl1, Crx, Nrl, Ngn1, and
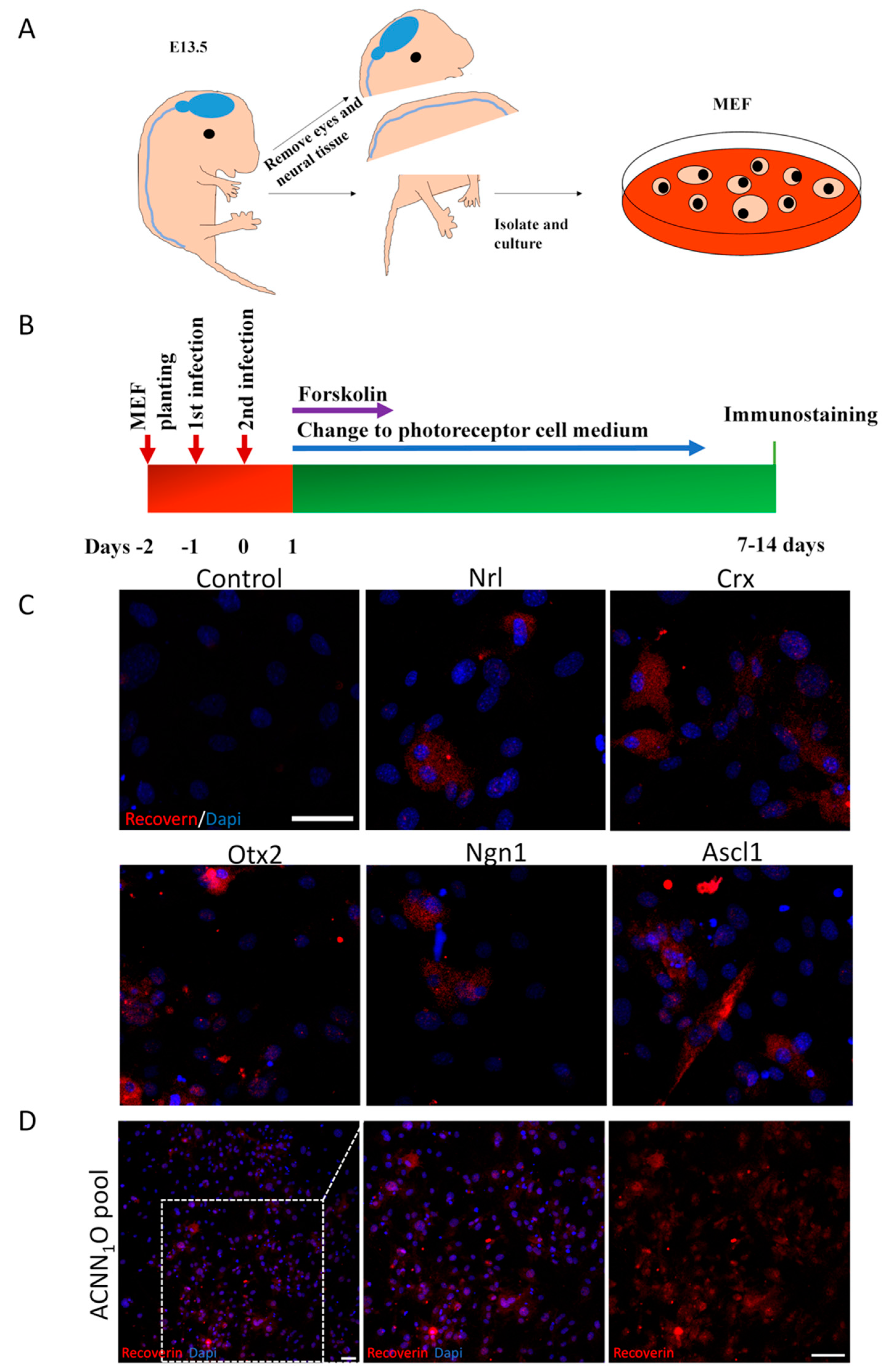
Otx2 (abbreviated as A, C, N, N1, and O, respectively). A pool of adenoviruses including all five genes was constructed to infect the MEFs taken from the E13.5 mice. We carefully excluded the neural tissue and eyes from the MEF preparation according to the previous description (
Figure 1A).
14 We were unable to detect any photoreceptor cells in the MEFs.
We also investigated the individual potential of these five genes to reprogram MEFs into photoreceptor cells. MEFs were infected once daily for two consecutive days with adenoviruses containing each of the five genes, according to transdifferentiation procedures (
Figure 1B). Although the conversion efficiencies were very low, seven days after infection, we detected recoverin-positive cells in cultures individually infected by
Ascl1, Crx, Nrl, Ngn1, or
Otx2 (
Figure 1C). Furthermore, to increase the conversion efficiency, all five genes (ACNN1O pool) were combined to infect E13.5 MEFs using the same method. We performed the recoverin staining seven days after infection to identify photoreceptor cells. The recoverin-positive cell ratio significantly increased in the ACNN
1O pool (
Figure 1D).
3.2. Defining Minimal Pool for Efficient Induction of Photoreceptor-like Cells
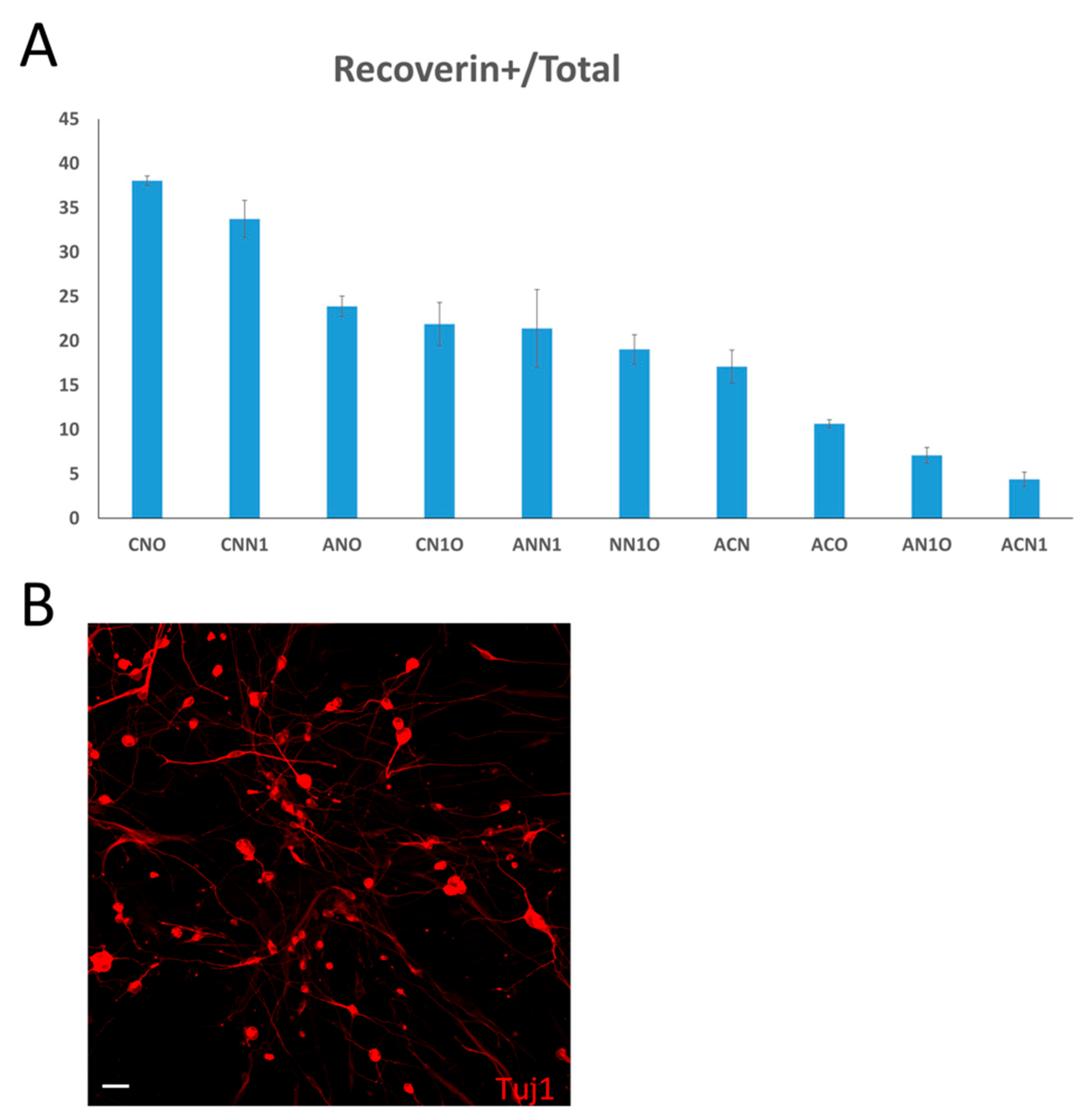
We sought to narrow down the number of transcription factors that are required for the generation of photoreceptor-like cells. To screen for the most efficient combination of transcription factors to achieve the highest conversion efficiency, we removed two genes at a time and evaluated all possible three-gene combinations. We analyzed the efficiency of these ten combinations individually by counting recoverin-positive cells seven days after infection and incubation. Our results indicated that the most efficient conversions were achieved when
Crx and
Nrl were combined with
Otx2 (CNO pool) (
Figure 2A). Therefore, we focused our further analysis on the CNO pool. Surprisingly, the combinations including
Ascl1 did not exhibit higher conversion ratios. We thus performed immunostaining for the other retinal neuron marker, Tuj1, in cultures infected by AAV carrying
Ascl1 at the seven days after infection. We found that most of the cells were Tuj1-positive, implying that these cells differentiated into neuron-like cells rather than photoreceptors (
Figure 2B).
3.3. The Expression of Multiple Markers in Induced Photoreceptor-like Cells
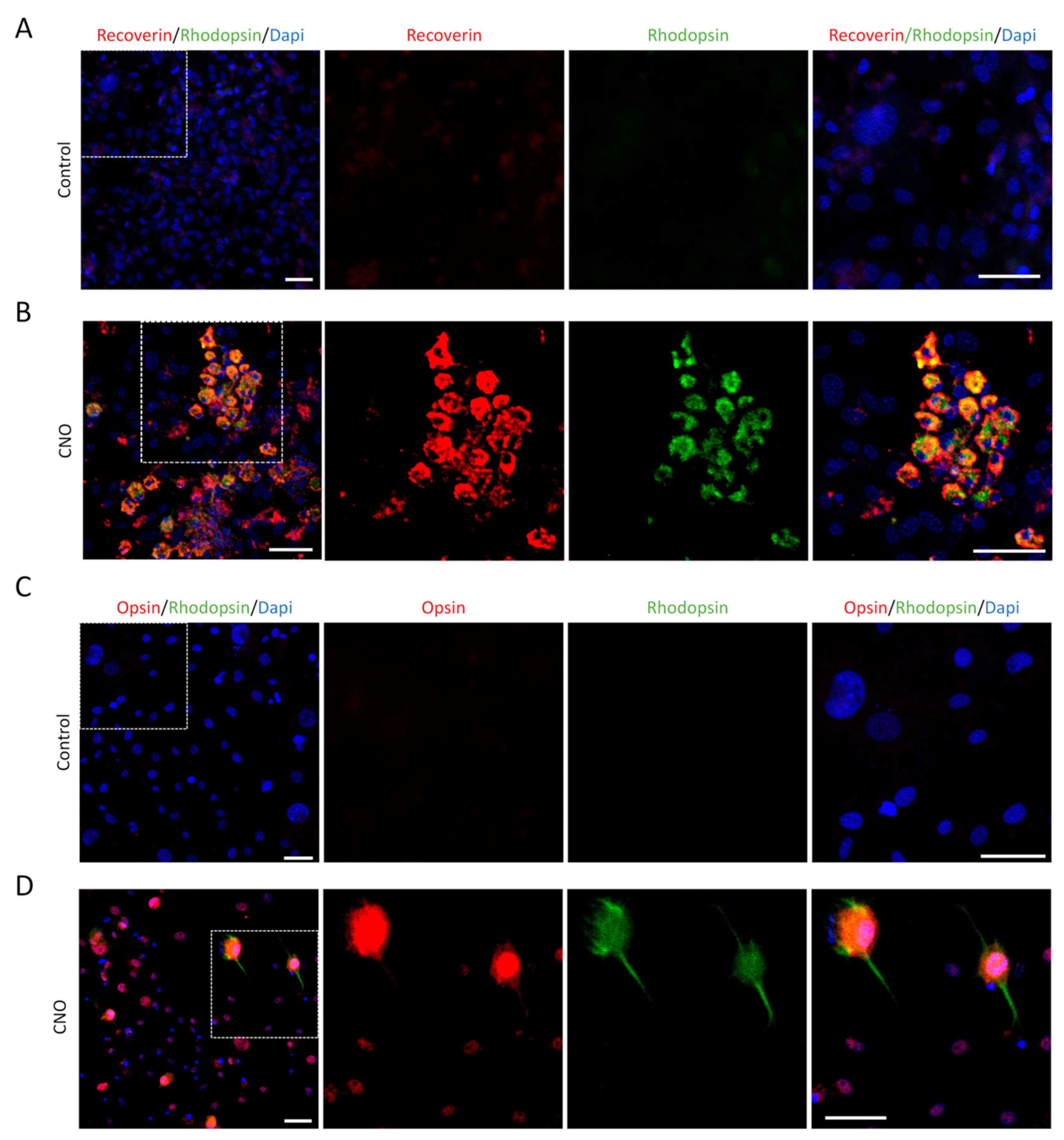
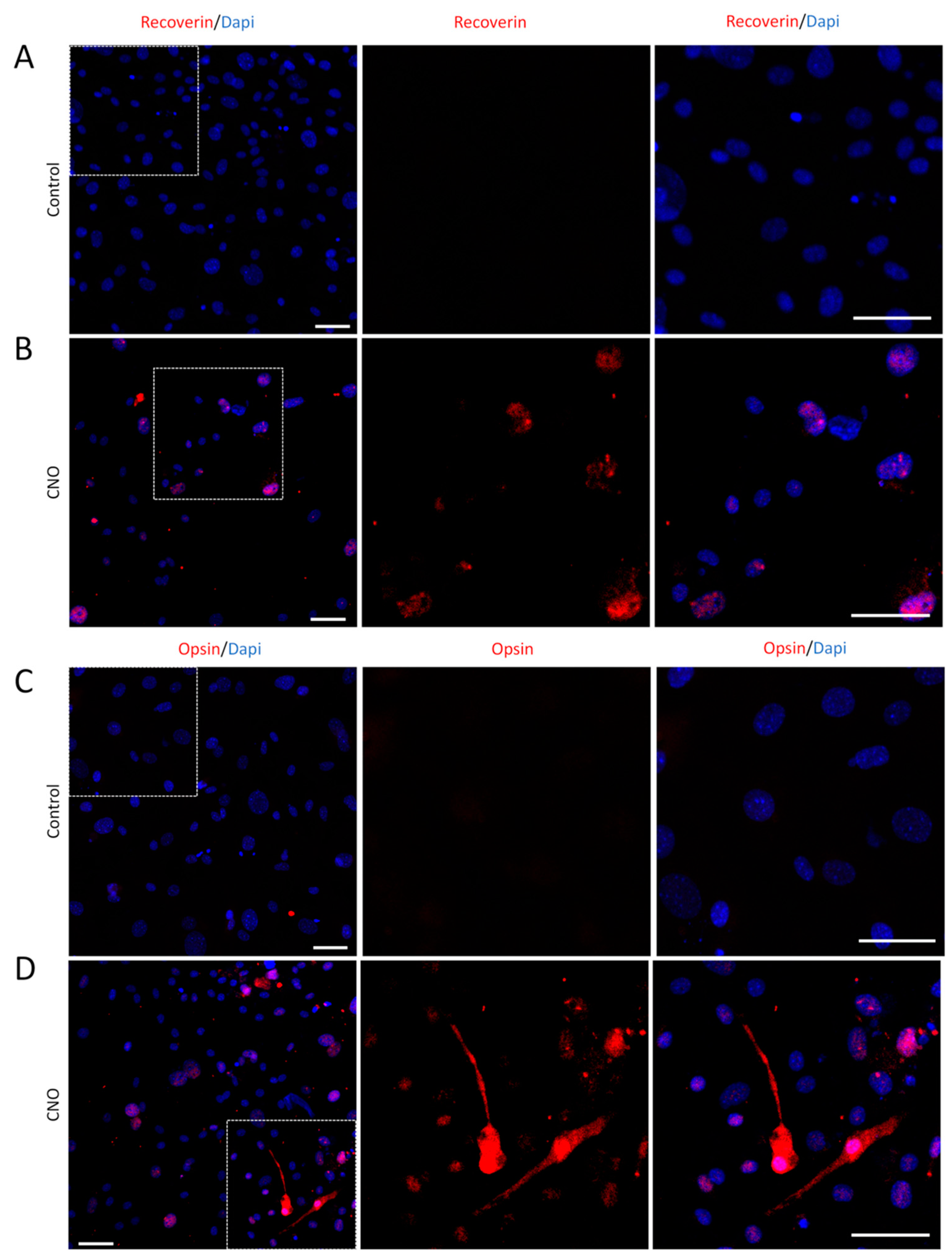
To obtain more photoreceptor-like cells, the MEFs were infected twice by CNO and cultured for 14 days. In addition to Recoverin, we also labeled the cells with other photoreceptor markers, Rhodopsin and Opsin, and performed double-immunolabeling of Recoverin and Rhodopsin or Opsin and Rhodopsin. MEFs infected by the empty AAV were used as controls (
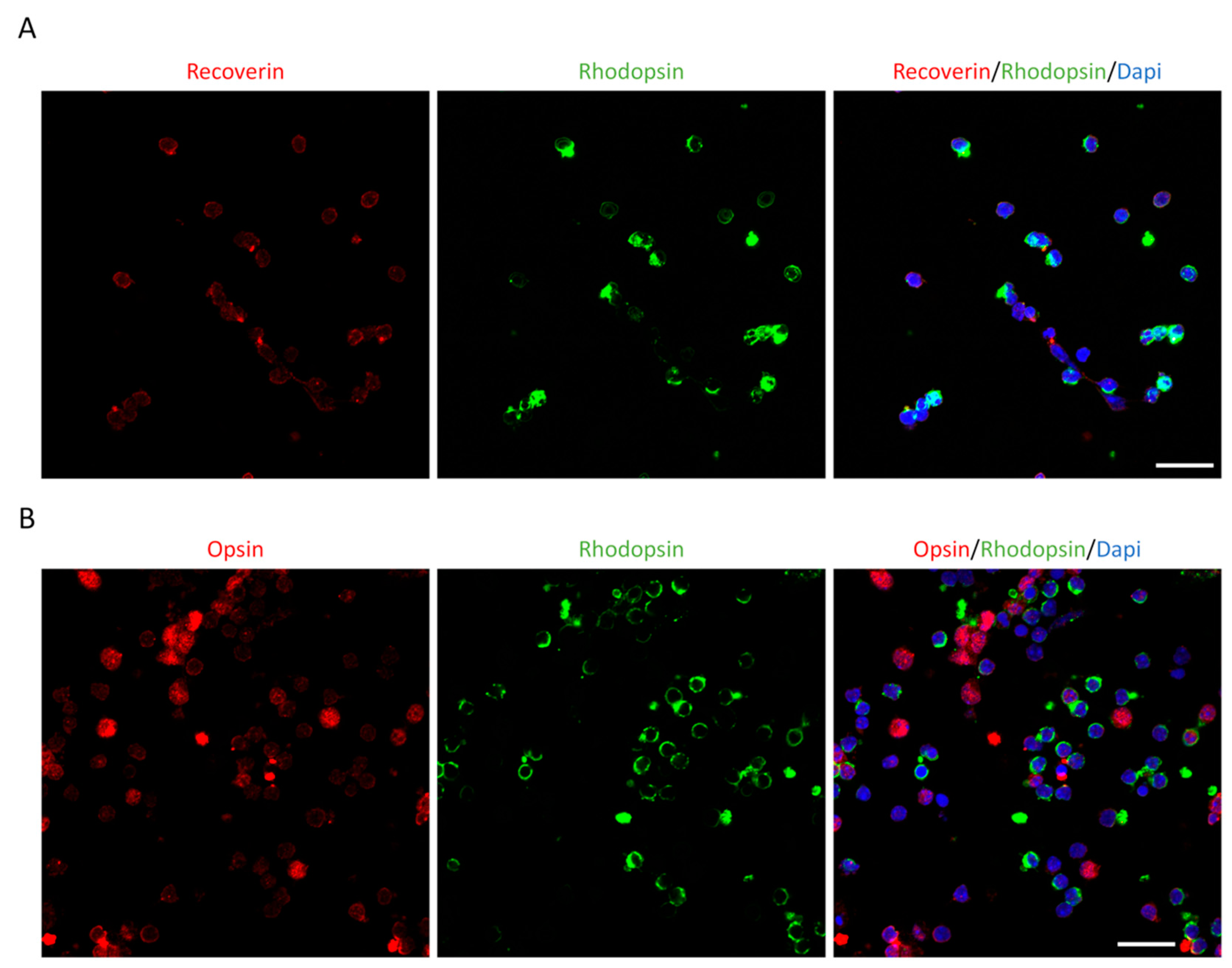
Figure 3A–3D), and postnatal retina cells from P3 mouse pups were used as positive controls. We observed that induced-photoreceptor-like cells were similar in morphology and immunolabeling to those of positive control cells (
Figure 4A and 4B).
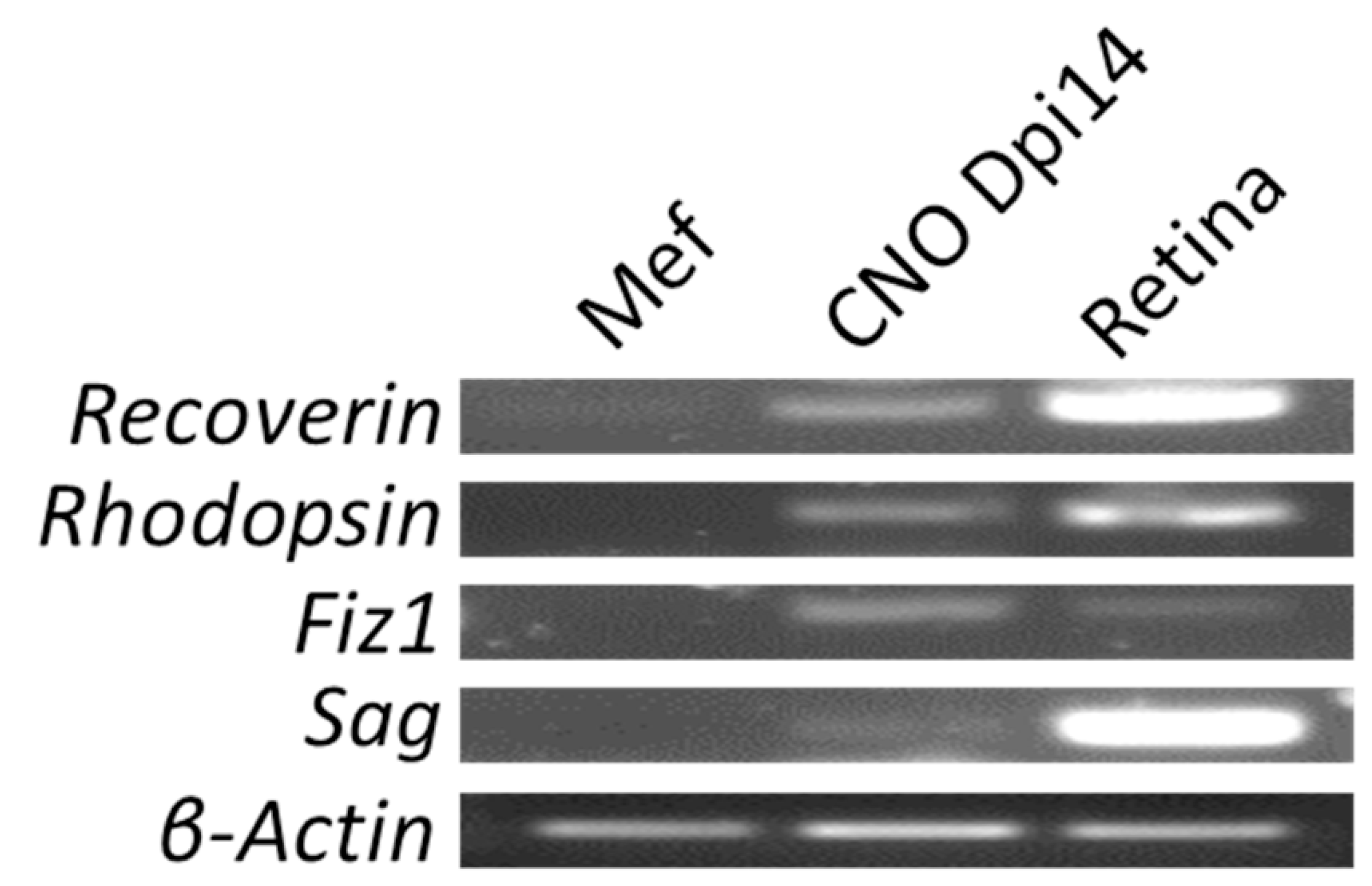
Furthermore, we sought to demonstrate that the induced photoreceptor-like cells shared more commonality with the photoreceptors from P3 mice retinas. We successfully detected the expressions of Recoverin, Rhodopsin, Fiz1 (Flt-3 Interacting Zinc-finger, Fiz1), and Sag (S-antigen) by RT–PCR in both the CNO-induced photoreceptor-like cells and retinal cells isolated from P3 mice; none of these four genes were expressed in negative control MEFs infected by empty AAV (
Figure 5). This result suggests that our induced photoreceptor-like cells were similar to the photoreceptors of P3 mouse pups.
To further verify the capacity of the CNO-induction of photoreceptor-like cells from fibroblasts, we isolated MPFs from three-day-old mice. Although the conversion efficiency using the MPFs was much lower than when using MEFs, we detected the expression of Recoverin and Opsin after being cultured for 14 days. (
Figure 6A–6D).
3.4. Transplantation of Photoreceptor-like Cells
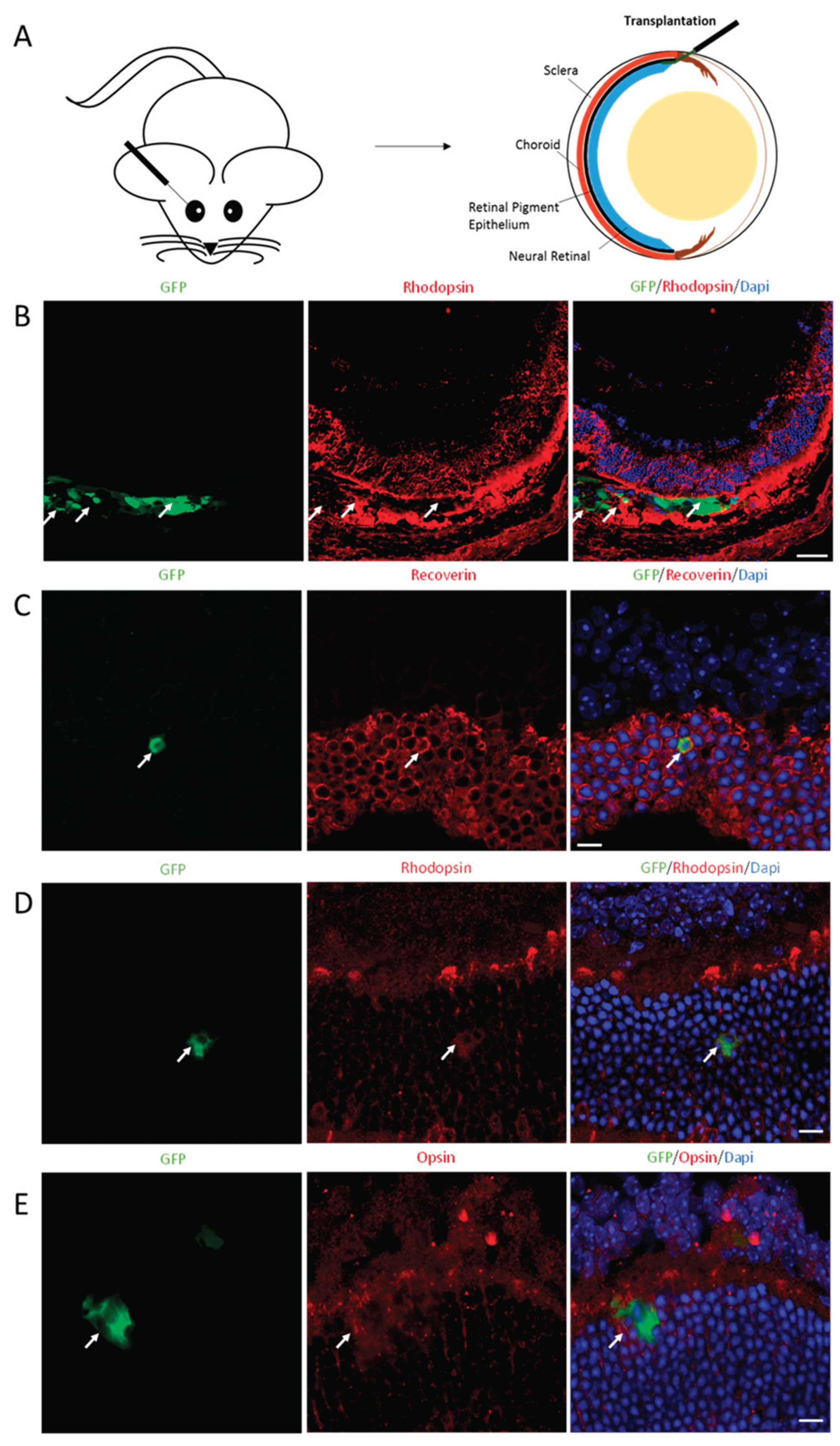
We next examined the transplantation potential of CNO-induced photoreceptor-like cells. We transplanted 200,000 CNO-iPs into the subretinal space of eight-to-ten-week-old adult C57BL/6J mice. To trace the transplanted cells in the retina, we infected MEFs with a lentivirus that stably expressed GFP before they were infected by AAV-CNO. Infected cells were transplanted into the subretinal space after three days of viral infection (
Figure 7A and 7B). The transplanted eyes were harvested two weeks after transplantation, and the eye sections were collected for analysis. We detected Recoverin-, Rhodopsin-, and Opsin-positive cells labeled with GFP in the outer nuclear layer (ONL) where the photoreceptors were located, suggesting that the CNO-induced-photoreceptor-like cells were successfully migrated and integrated into the host retinas (
Figure 7C–7E).
4. Discussion
Authors should discuss the results and how they can be interpreted from the perspective of previous studies and of the working hypotheses. The findings and their implications should be discussed in the broadest context possible. Future research directions may also be highlighted.
In this study, we successfully converted mouse embryonic and postnatal fibroblasts directly into functional photoreceptors with an adenoviral delivery system. Although each of the single factors (
Ascl1, Crx, Nrl, Ngn1, or
Otx2) was able to induce photoreceptor-like cells, the combination of
Crx, Nrl, and
Otx2 generated photoreceptor-like cells with much greater efficiency. The CNO-induced photoreceptor-like cells displayed expressed multiple mature photoreceptor markers, including Recoverin, Rhodopsin, Opsin, Fiz1, and Sag. Fiz1 interacts with transcription factors
Nrl and
Crx during photoreceptor maturation [
34]. Sag, also called Arrestin1, plays a unique role in phototransduction shutoff and light adaptation [
35,
36,
37]. The detection of Fiz1 and Sag implied that the induced photoreceptor-like cells in our experiment were more similar to mature photoreceptors. These three factors,
Crx, Nrl, and
Otx2, were identified from the five candidates that we selected according to their roles in neural retinal cell development and neuronal differentiation. Furthermore, we observed the successfully migrated and integrated CNO-induced photoreceptor-like cells in host retinas after their transplantation into adult C57BL/6J mice. Although much refinement of this directly induced photoreceptor process will be necessary, these findings raise the possibility of reprogramming the fibroblasts that commonly exist in photoreceptors for regenerative strategies.
Various retinal diseases leading to blindness [
38], such as AMD, RP, and retinal detachment, are characterized by the irreversible loss of photoreceptor cells. Recent decades have shown significant progress in photoreceptor regeneration; numerous researchers have attempted to regenerate photoreceptors both in vivo and in vitro. Most in vivo studies have focused on Müller glia cells and retinal pigmented epithelium (RPE) [
39] as the resource of photoreceptor regeneration. However, the number of differentiated photoreceptors from the currently endogenous regeneration is not sufficient for retinal repair. In vitro, studies have shown that photoreceptors can be generated from different tissues, such as the iris [
40], ciliary tissues [
41], and embryonic stem cells [
42,
43,
44]. However, we demonstrated that the combination of three transcription factors,
Crx, Nrl, and
Otx2, can rapidly and directly induce photoreceptor-like cells from fibroblasts. This direct reprogramming approach skips a pluripotent state, making the photoreceptor replacement therapy much faster for retinal diseases and avoiding the risk of tumor formation in cell-based therapies. Further, the source we required was fibroblasts, which were abundant and accessible, making photoreceptor regeneration more feasible.
The three reprogramming factors,
Crx, Nrl, and
Otx2, are the crucial transcription factors during retinal development. The mutations of each of these three genes cause severe retinal degeneration. The mutations of
Crx are associated with dominant cone-rod dystrophy, late-onset dominant RP, and dominant congenital Leber amaurosis [
45]. The differentiating photoreceptor cells were converted to amacrine-like retinal neurons in
Otx2 deficiency [
46]. Mutations of
Nrl have resulted in the autosomal dominant RP [
47,
48]. In our experiment,
Crx, Nrl, and
Otx2 interacted with one another and promoted photoreceptor differentiation. As members of the Otx homeodomain gene family, both
Crx and
Otx2 are key regulators of the terminal differentiation of photoreceptors.
Nrl works synergistically with
Crx to regulate rhodopsin transcription, which is essential for photoreceptor genesis. Consistent with our results, Chen et al. also provided evidence that the combination of these three transcription factors can generate photoreceptors from reactivated Müller glia [
49].
5. Conclusions
The directly inducted photoreceptor-like cells from fibroblasts by the Crx, Nrl, and Otx2 combination system described here provide a robust and feasible method of photoreceptor regeneration for retinal repair. Our data demonstrate that the reprogrammed photoreceptor-like cells have the capability to integrate into the outer nuclear layer of the host retina and express photoreceptor-specific markers after transplantation. We present a fast and practical approach for photoreceptor replacement therapy.
Author Contributions
Conceptualization, J.W.J. and L.Y.; methodology, J.X. and J.W.J.; software, J.X.; validation, J.X., J.W.J., and L.Y.; formal analysis, J.X..; investigation, J.X. S.E., D.F.C., J.W.J. and L.Y.; resources, J.W.J., and L.Y.; data curation, J.X. S.E., D.F.C., J.W.J. and L.Y.; writing—original draft preparation, J.X. J.W.J., and L.Y.; writing—review and editing, J.X. S.E., D.F.C., J.W.J. and L.Y.; visualization, D.F.C., J.W.J. and L.Y.; supervision, J.W.J. and L.Y.; project administration, J.X. S.E., D.F.C., J.W.J. and L.Y.; funding acquisition, J.W.J. and L.Y. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the National Natural Science Foundation of China, grant number 82171059 and 82371064.
Institutional Review Board Statement
The study was conducted in accordance with the Declaration of Helsinki, and approved by the Animal Care Committee of Peking University First Hospital.
Data Availability Statement
Data generated or analyzed during this study are included in this published article. Additional datasets generated and/or analyzed during the current study are available from the corresponding author upon reasonable request.
Acknowledgments
The authors thank the facility of the Institute of Zoology, Chinese Academy of Sciences for maintaining the animal; Juan Zhang for advice in adenovirus production; Tianjin Shen and Wenlong Xia for advice in immunostaining; Dong Feng Chen for comments on the manuscript.
Conflicts of Interest
The authors declare no conflicts of interest.
Abbreviations
The following abbreviations are used in this manuscript:
| MEF |
Mouse embryonic fibroblast |
| MPF |
Mouse postnatal fibroblast |
| RP |
Retinitis pigmentosa |
| AMD |
Age-related macular degeneration |
| bHLH |
basic helix-loop-helix |
| Crx |
cone-rod homeobox |
| Nrl |
Neural retina leucine |
| Ngn1 |
Neurogenin1 |
| PBS |
Phosphate-buffered saline |
| RPE |
Retinal pigmented epithelium |
References
- Stone J, Maslim J, Valter-Kocsi K, et al. Mechanisms of photoreceptor death and survival in mammalian retina. Prog Retin Eye Res. 1999;18(6):689-735. [CrossRef]
- Penn JS, Anderson RE. Chapter 4 Effects of light history on the rat retina. Progress in Retinal Research. 1991;11:75-98. [CrossRef]
- Young JZ. The Bayliss-Starling lecture. Some special senses in the sea. J Physiol. 1989;411:1-25. [CrossRef]
- Steinberg RH, Wood I, Hogan MJ. Pigment epithelial ensheathment and phagocytosis of extrafoveal cones in human retina. Philos Trans R Soc Lond B Biol Sci. 1977;277(958):459-474. [CrossRef]
- Demontis GC, Longoni B, Gargini C, Cervetto L. The energetic cost of photoreception in retinal rods of mammals. Arch Ital Biol. 1997;135(2):95-109.
- McDougald DS, Papp TE, Zezulin AU, Zhou S, Bennett J. AKT3 Gene Transfer Promotes Anabolic Reprogramming and Photoreceptor Neuroprotection in a Pre-clinical Model of Retinitis Pigmentosa. Mol Ther. 2019;27(7):1313-1326. [CrossRef]
- Xie J, Zhu R, Peng Y, et al. Tumor necrosis factor-alpha regulates photoreceptor cell autophagy after retinal detachment. Sci Rep. 2017;7(1):17108. [CrossRef]
- Murakami Y, Notomi S, Hisatomi T, et al. Photoreceptor cell death and rescue in retinal detachment and degenerations. Prog Retin Eye Res. 2013;37:114-140. [CrossRef]
- Goldman D. Muller glial cell reprogramming and retina regeneration. Nat Rev Neurosci. 2014;15(7):431-442. [CrossRef]
- Lindsey AE, Powers MK. Visual behavior of adult goldfish with regenerating retina. Vis Neurosci. 2007;24(3):247-255. [CrossRef]
- Mensinger AF, Powers MK. Visual function in regenerating teleost retina following cytotoxic lesioning. Vis Neurosci. 1999;16(2):241-251. [CrossRef]
- Elsaeidi F, Macpherson P, Mills EA, Jui J, Flannery JG, Goldman D. Notch Suppression Collaborates with Ascl1 and Lin28 to Unleash a Regenerative Response in Fish Retina, But Not in Mice. J Neurosci. 2018;38(9):2246-2261. [CrossRef]
- Lust K, Wittbrodt J. Activating the regenerative potential of Müller glia cells in a regeneration-deficient retina. Elife. 2018;7:e32319. [CrossRef]
- Vierbuchen T, Ostermeier A, Pang ZP, Kokubu Y, Sudhof TC, Wernig M. Direct conversion of fibroblasts to functional neurons by defined factors. Nature. 2010;463(7284):1035-1041. [CrossRef]
- Meng F, Chen S, Miao Q, et al. Induction of fibroblasts to neurons through adenoviral gene delivery. Cell Res. 2012 Feb;22(2):436-440. [CrossRef]
- Yan L, Li Y, Shi Z, et al. The zinc finger E-box-binding homeobox 1 (Zeb1) promotes the conversion of mouse fibroblasts into functional neurons. J Biol Chem. 2017;292(31):12959-12970. [CrossRef]
- Shi Z, Zhang J, Chen S, et al. Conversion of Fibroblasts to Parvalbumin Neurons by One Transcription Factor, Ascl1, and the Chemical Compound Forskolin. J Biol Chem. 2016;291(26):13560-13570. [CrossRef]
- Shi Z, Shen T, Liu Y, Huang Y, Jiao J. Retinoic acid receptor gamma (Rarg) and nuclear receptor subfamily 5, group A, member 2 (Nr5a2) promote conversion of fibroblasts to functional neurons. J Biol Chem. 2014;289(10):6415-6428. [CrossRef]
- Ieda M, Fu JD, Delgado-Olguin P, et al. Direct reprogramming of fibroblasts into functional cardiomyocytes by defined factors. Cell. 2010;142(3):375-386. [CrossRef]
- Huang P, He Z, Ji S, et al. Induction of functional hepatocyte-like cells from mouse fibroblasts by defined factors. Nature. 2011;475(7356):386-389. [CrossRef]
- Mahato B, Kaya KD, Fan Y, et al. Pharmacologic fibroblast reprogramming into photoreceptors restores vision. Nature. 2020;581(7806):83-88. [CrossRef]
- Livesey FJ, Cepko CL. Vertebrate neural cell-fate determination: lessons from the retina. Nat Rev Neurosci. 2001;2(2):109-118. [CrossRef]
- Bassett EA, Wallace VA. Cell fate determination in the vertebrate retina. Trends Neurosci. 2012;35(9):565-573. [CrossRef]
- Stenkamp DL. Development of the Vertebrate Eye and Retina. Prog Mol Biol Transl Sci. 2015;134:397-414. [CrossRef]
- Furukawa T, Morrow EM, Cepko CL. Crx, a novel otx-like homeobox gene, shows photoreceptor-specific expression and regulates photoreceptor differentiation. Cell. 1997;91(4):531-541. [CrossRef]
- Chen S, Wang QL, Nie Z, et al. Crx, a novel Otx-like paired-homeodomain protein, binds to and transactivates photoreceptor cell-specific genes. Neuron. 1997;19(5):1017-1030. [CrossRef]
- Simeone A, Acampora D, Mallamaci A, et al. A vertebrate gene related to orthodenticle contains a homeodomain of the bicoid class and demarcates anterior neuroectoderm in the gastrulating mouse embryo. Embo J. 1993;12(7):2735-2747. [CrossRef]
- Yamamoto H, Kon T, Omori Y, Furukawa T. Functional and Evolutionary Diversification of Otx2 and Crx in Vertebrate Retinal Photoreceptor and Bipolar Cell Development. Cell Rep. 2020; 30(3):658-671.e5. [CrossRef]
- Mears AJ, Kondo M, Swain PK, et al. Nrl is required for rod photoreceptor development. Nat Genet. 2001;29(4):447-452. [CrossRef]
- Swain PK, Hicks D, Mears AJ, et al. Multiple phosphorylated isoforms of NRL are expressed in rod photoreceptors. J Biol Chem. 2001;276(39):36824-36830. [CrossRef]
- Ueki Y, Wilken MS, Cox KE, et al. Transgenic expression of the proneural transcription factor Ascl1 in Muller glia stimulates retinal regeneration in young mice. Proc Natl Acad Sci. 2015;112(44):13717-13722. [CrossRef]
- Yan RT, He L, Wang SZ. Pro-photoreceptor activity of chick neurogenin1. Invest Ophthalmol Vis Sci. 2009;50(12):5567-5576. [CrossRef]
- Yan RT, Liang L, Ma W, Li X, Xie W, Wang SZ. Neurogenin1 effectively reprograms cultured chick retinal pigment epithelial cells to differentiate toward photoreceptors. J Comp Neurol. 2010;518(4):526-546. [CrossRef]
- Mali RS, Zhang X, Hoerauf W, et al. FIZ1 is expressed during photoreceptor maturation, and synergizes with NRL and CRX at rod-specific promoters in vitro. Exp Eye Res. 2007;84(2):349-360. [CrossRef]
- Xu J, Dodd RL, Makino CL, Simon MI, Baylor DA, Chen J. Prolonged photoresponses in transgenic mouse rods lacking arrestin. Nature. 1997;389(6650):505-509. [CrossRef]
- Brown BM, Ramirez T, Rife L, Craft CM. Visual Arrestin 1 contributes to cone photoreceptor survival and light adaptation. Invest Ophthalmol Vis Sci. 2010;51(5):2372-2380. [CrossRef]
- Deming JD, Pak JS, Shin JA, et al. Arrestin 1 and Cone Arrestin 4 Have Unique Roles in Visual Function in an All-Cone Mouse Retina. Invest Ophthalmol Vis Sci. 2015;56(13):7618-7628. [CrossRef]
- Rattner A, Nathans J. Macular degeneration: recent advances and therapeutic opportunities. Nat Rev Neurosci. 2006;7(11):860-872. [CrossRef]
- Gallina D, Todd L, Fischer AJ. A comparative analysis of Muller glia-mediated regeneration in the vertebrate retina. Exp Eye Res. 2014;123:121-130. [CrossRef]
- Haruta M, Kosaka M, Kanegae Y, et al. Induction of photoreceptor-specific phenotypes in adult mammalian iris tissue. Nat Neurosci. 2001;4(12):1163-1164. [CrossRef]
- Tropepe V, Coles BL, Chiasson BJ, et al. Retinal stem cells in the adult mammalian eye. Science. 2000;287(5460):2032-2036. [CrossRef]
- Ikeda H, Osakada F, Watanabe K, et al. Generation of Rx+/Pax6+ neural retinal precursors from embryonic stem cells. Proc Natl Acad Sci. 2005;102(32):11331-11336. [CrossRef]
- Osakada F, Ikeda H, Mandai M, et al. Toward the generation of rod and cone photoreceptors from mouse, monkey and human embryonic stem cells. Nat Biotechnol. 2008;26(2):215-224. [CrossRef]
- Gonzalez-Cordero A, West EL, Pearson RA, et al. Photoreceptor precursors derived from three-dimensional embryonic stem cell cultures integrate and mature within adult degenerate retina. Nat Biotechnol. 2013;31(8):741-747. [CrossRef]
- Sohocki MM, Sullivan LS, Mintz-Hittner HA, et al. A range of clinical phenotypes associated with mutations in CRX, a photoreceptor transcription-factor gene. Am J Hum Genet. 1998;63(5):1307-1315. [CrossRef]
- Nishida A, Furukawa A, Koike C, et al. Otx2 homeobox gene controls retinal photoreceptor cell fate and pineal gland development. Nat Neurosci. 2003;6(12):1255-1263. [CrossRef]
- Bessant DA, Payne AM, Mitton KP, et al. A mutation in NRL is associated with autosomal dominant retinitis pigmentosa. Nat Genet. 1999 Apr;21(4):355-356. [CrossRef]
- Martinez-Gimeno M, Maseras M, Baiget M, et al. Mutations P51U and G122E in retinal transcription factor NRL associated with autosomal dominant and sporadic retinitis pigmentosa. Hum Mutat. 2001;17(6):520. [CrossRef]
- Yao K, Qiu S, Wang YV, et al. Restoration of vision after de novo genesis of rod photoreceptors in mammalian retinas. Nature. 2018;560(7719):484-488. [CrossRef]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).