1. Introduction
The Human Immunodeficiency Virus (HIV) not only interferes with the body’s immune system but has a deleterious effect on the body’s central nervous system (Morgello, 2018). Markedly, when HIV permeates the blood-brain barrier (BBB) and enters the cerebral cortex, 15% – 60% of people affected by neuroHIV are indicated to experience progressive cognitive and motor decline (Alford & Vera, 2018). Cognitive decline is noted in multiple domains, including working memory (WM) (Fraser & Cockcroft, 2020), attention and processing speed (Rice et al., 2014; Wang et al., 2017), executive functions (Bugarski Ignjatovic et al., 2018) and response inhibition (Du Plessis et al., 2019). Collectively, cognitive deficits arising from neuroHIV, are referred to as HIV-associated neurocognitive disorders (HAND) (Nightingale et al., 2023).
Pointedly, despite the development of highly active antiretroviral drugs (ARTs), the cerebral cortex continues to be cellular reservoirs for HIV, primarily due to the differentiation of monocytes into macrophages within the cortex (Morgello, 2018; Wilmshurst et al., 2018). Once in the cerebral cortex, HIV-initiated pathogenetic neuroinflammation is indicated to result in aberrant neural transmission (Brew 2018), white and grey matter loss (Jensen et al., 2019), neuronal apoptosis (Das et al., 2016), and catecholaminergic dysregulation (Nolan & Gaskill, 2019), resulting in the persistence of HAND, both in adults (Cody & Vance, 2016) and adolescents HIV populations (Hoare et al., 2016).
Due to the limitations of ARTs, particularly their limited permeability in the cerebral cortex (Morgello, 2018), coupled with their neurotoxicity (Nightingale et al., 2023), HIV brain training (The neuroscience literature uses the terms, HIV brain training, HIV adaptive training, HIV cognitive rehabilitation, HIV cognitive remediation, HIV cognitive intervention, and HIV computerised cognitive training, interchangeably. In the article, the terms HIV brain training and HIV cognitive training will be preferred.) protocols have taken added pre-eminence to reverse cognitive impairment, sequent neuroHIV. For example, with reference to adolescent HIV, brain training protocols have been applied to remediate attention (Basterfield & Zondo 2022), working memory (Fraser & Cockcroft 2020), and executive functions (Boivin et al. 2010; Boivin, Nakasujja, et al. 2019). Notwithstanding the above, there continues to be a dearth of research pairing behavioural outcomes sequent HIV brain training with neuroimaging measures to investigate the efficacy of brain training protocols at a neuronal level, especially in children and adolescents living with HIV in Sub-Saharan Africa (Benki-Nugent & Boivin, 2019; Musielak & Fine, 2016).
At the time of writing, the sole study pairing behavioural outcomes, with neuroimaging outcomes to investigate HIV brain training, is the study by Chang et al. (2017). They used functional MRI (fMRI), to investigate the effects of working memory training (WMT), on near and far transfer cognitive gains, coupled with blood oxygen level dependency (BOLD) changes, before and after brain training. Findings indicated that WMT was associated with significantly, reduced BOLD, activation in the frontal and parietal regions, of HIV+ patients (n=11; mean age = 41; SD = 4.8), and HIV negative controls (n=11; mean age = 38; SD = 4.8) receiving WMT. As noted by the authors, reduced BOLD activation and increased performance on untrained near tasks (Digit Span and Spatial-Span) following WMT were suggestive of improved intrinsic functional connectivity and reduced neuronal demand to complete working memory tasks (Chang et al., 2017).
In a similar study using functional near infrared spectrometry (fNIRS), (Zondo et al. 2025, In Press), investigated neuronal changes amongst HIV+ adolescents (n = 13; mean age = 16; SD = 1.2); receiving brain training to remediate attention skills, and HIV+ controls (n= 13; mean age = 17; SD = 1.3). Findings indicated decreased BOLD activation in the dorsolateral prefrontal (DLPF) and frontopolar network when completing incongruent trials on the Stroop Colour Word Test (SCWT) following HIV brain training. Although decreased BOLD activation was indicative of improved neural efficiency in the SCWT, behavioural improvements did not reach statistical significance post-brain training.
Notwithstanding the above (i.e., decreased BOLD activation, sequent HIV brain training), neuronal adaptation is best investigated through functional connectivity analysis (Lang et al., 2012; Shahhosseini & Miranda, 2022). Functional connectivity (FC) is defined as the ‘temporal correlations between spatially remote brain events’ (Friston, 1994, p. 58). The precedence of FC analysis, as opposed to functional segregation, is that FC enables the study of distributed processing and functional interaction across and within brain regions. FC analysis therefore enables the determination and analysis of cortical synchrony and temporal dependency (time) of neuronal activation within the cortex. As previously noted, there is a paucity of research investigating the relationship between cortical brain plasticity and neuroHIV. Notwithstanding this limitation, there is an even greater dearth of research investigating functional brain connectivity following HIV cognitive rehabilitation in adolescent populations.
Functional Connectivity and HIV Brain Training
A literature review indicates that the sole study investigating FC following HIV brain training is the study by Jia et al. (2023). They investigated FC, sequent working memory training (WMT), in adult HIV using resting-state fMRI (rsfMRI). rsfMRI investigates changes in intrinsic functional networks during states of rest when subjects are not engaged in performing a specific cognitive task or receiving any cognitive stimulation (Biswal et al., 1995). The advantage of resting state analysis, is that it enables for the identification of intrinsic brain connectivity, and the identification of biomarkers for brain health and brain injury (Bijsterbosch et al., 2017). In its application, rsfMRI uses spontaneous BOLD signals to study FC, both in spatially distinct and near brain regions.
With reference to HIV brain training, Jia et al. (2023) investigated FC, as estimated by independent component analysis (ICA) and graph theory. With reference to graph theory, they investigated whether WMT (CogMed), resulted in ‘network normalization’ within resting state networks (RSNs), as determined by eigenvector centrality and local efficacy measures. Summarily, eigenvector centrality measures the contribution of a node within a cortical network (e.g., default mode network). Specifically, nodes with higher eigenvector centrality represent nodes that are not only well-connected within a network but are also connected to other highly central nodes within a network. Consequently, highly connected nodes act as hubs for information flow, enabling efficient communication between nodes within a network. Similarly, local efficiency measures how efficiently information is exchanged within a local neighborhood of a node within a network.
In their investigation, Jia et al. (2023) investigated ‘network normalization’, pre and post-WMT, in the default mode network (DMN), cognitive control network (CON), The CEN is referred to as the Cognitive Control Network (CON) in the study. A growing body of research suggests the CON and CEN may share the same anatomical correlates (Breukelaar et al., 2017; Menon & D’Esposito, 2021). visual network (VIS), the auditory network (AUN) and subcortical networks (SC). The authors defined ‘network normalization’ as the process of restoring or optimizing the functional connectivity patterns within the cortex to typical neuronal network patterns. As such, Jia et al. (2023) investigated whether HIV+ participants would indicate similar network organisation to HIV-negative controls post WMT, as determined by eigenvector centrality and local efficiency analysis.
Participants were inclusive of HIV+ (n=53; mean age = 50; SD = 1.5), and HIV seronegative controls (n=48; mean age = 49; SD = 1.6), matched for sex, race, and socioeconomic status. At preintervention, findings indicated abnormalities in RSNs, in the HIV+ group, as indicated by eigenvector centrality, and local efficiency measures. Post-intervention (one month) measures indicated that ‘the eigenvector centrality of the ventral DMN in PWL (people with HIV), become more like that of SNs (seronegative)’ (Jia et al., 2023, p. 1560). The authors concluded that changes in eigenvector centrality ‘may suggest that the vDMN with high eigenvector centrality might be a key neural substrate leading to normalization of brain function within WMT’ (Jia et al., 2023, p. 1560). Significantly, eigenvector centrality normalization (in the HIV+ group) was associated with improved memory outcomes at post-assessment. Notwithstanding this finding, the study did not provide levels of significance measures (p-value) for post-assessment measures (attention, executive functions), except detailing improved cognitive outcomes in memory function.
The Present Study
As noted, there is a dearth of research investigating HIV brain training outcomes, paired with objective neuroimaging measures in Sub-Saharan Africa (Musielak & Fine, 2016). In a previous study, (Zondo et al., 2024a), our research group illustrated the feasibility of functional near-infrared spectrometry (fNIRS), to investigate regional hemodynamic activity, in pediatric and adolescent HIV. In a follow up study (Zondo et al. 2025, In Press), we investigated the effect of attention training on hemodynamic activation within the CEN, in adolescent HIV. Findings indicated that brain attention training (12 weeks) in adolescent HIV is associated with decreased hemodynamic activation in the dorsolateral prefrontal cortex (DLPF), and frontopolar network, in response to completing incongruent trials, on the Stroop Colour Word Test (SCWT). The previous findings investigated block averaged hemodynamic activation (HbO) in response to cognitive stimuli. The current study explored task based functional connectivity, pre and post attention brain training, using seed based functional connectivity.
A substantial departure from Jia et al. (2023), who investigated FC using rsfMRI, in an HIV adult population, this investigation explored FC within an adolescent HIV population. The decision to pursue fNIRS optical imaging techniques, was based on cost limitations associated with fMRI in Sub-Saharan Africa (Ogbole et al., 2018). Additionally, as opposed to studying FC, using graph theory (eigenvector centrality, and local efficiency), the current study investigation used fNIRS task-related seed-based correlation. Seed-based correlation connectivity is based on the selection of a priori ‘seed’ or ‘seed region’, that is selected based on a priori relevance to the field of study (Joel et al., 2011; Toich et al., 2017). The technique examines temporal correlation of hemodynamic activity between the seed, and distant nodes / vowels, within a cortical network of interest (Joel et al., 2011; Thomason et al., 2013). Seed-based connectivity analysis has provided insights into cortical synchrony in neuroHIV (Toich et al., 2017), and has helped establish biomarkers for neurological disorders (Ji et al., 2020), and psychological treatment (Shen et al., 2020). In addition to studying FC, the study investigated near and far cognitive transfer, emanating from attention brain training. Due to the scarcity of published research on seed-based correlation analysis to investigate FC, in fNIRS, the study did not propose any hypothesis but explored the below study questions.
Study Research Questions
Compared to controls, do HIV+ participants receiving attention training, indicate improved cognitive outcomes on behavioural measures, post intervention?
Compared to controls, do HIV+ participants receiving attention training, indicate greater functional connectivity in either of the hemispheres (left or right), in the CEN, post intervention?
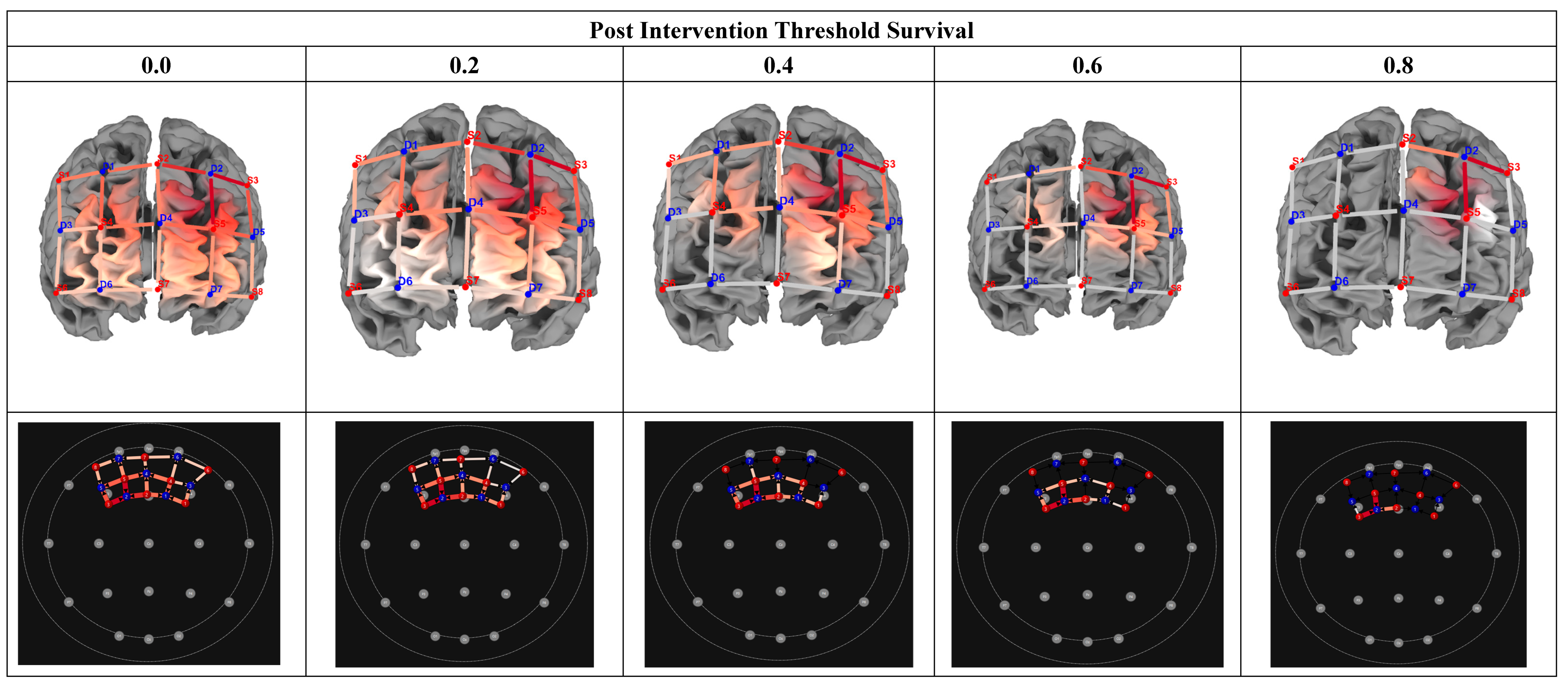
Which voxels (if any) would ‘survive’ increased seed correlation thresholding (0.2, 0.4, 0.6, 0.8) at post-training (within the experimental group).
2. Materials and Methods
The materials and methods pertaining to the study are described in a previously published manuscript, (Zondo, Cockcroft, et al., 2024). With specific reference to seed-based analysis, the study followed fNIRS guidelines for conducting resting state functional connectivity analysis, using Satori fNIRS (NIRX Software, Brain Innovation, BV, Netherlands). As such seed-based correlations were calculated for each run/subject, based on Seed ‘S3-D2’. Seed-based correlation was performed by computing the temporal correlation, between the priori seed (S3-D2), and the rest of the channels. This procedure was done for all participants, inclusive of the experimental and control groups. An example of a corresponding seed correlation of subject 1 is illustrated in
Figure 1. An of a seed-based map for all experimental subjects at pre-intervention is illustrated in
Figure 2. Based on a priori evidence, indicating the plasticity, of the left dorsolateral prefrontal cortex (L-DLPF) in HIV cognitive rehabilitation (Chang et al., 2017(Ownby & Acevedo, 2016; Ownby & Kim, 2021), this seed was selected as the seed region for the cross-correlation. For the fNIRS analysis, this seed, (channel 6; x -31; y = 39; z = 4l;
Supplementary Materials Figure S1), indicated a specificity of 92%, as determined by fOLD (Zimeo Morais et al., 2018).
Similar to other implementations, adopting fNIRS seed-based FC, (e.g., Ji et al., 2020), in order to increase the normality of the distribution of correlation values, Fisher’s r-to-z transformation were applied to each correlation coefficient. As such, Fisher’s transformed bivariate correlation coefficients were calculated between the seed BOLD time series, and each of the individual channel BOLD time series (
Supplementary Materials Figure S2). Following Fisher’s r-to-z normalizations, transformed correlation maps were generated, for each subject, and for the groups level analysis, at pre-test, and at post intervention. As such, connectivity correlation (r) maps were derived representing the size and direction of the correlation. All correlation values ranged from -1 to +1. Negative correlations within the correlation maps, were coded as “cold” colors (blue), while positive correlation values were coded as “warm” colors (red) (
Figure 2).
To further focus our analysis on identifying regions (channels), showing greater connectivity with the seed region (S3-D2), at post rehabilitation, we employed seed survival thresholding. The percentage of surviving channels after thresholding increment would thus be indicative of channels with the greatest correlation to the seed. For instance, if the threshold was set at r = 0.2, this threshold determined how many voxels / channels ‘survive’ a correlation greater than 0.2. For the study, thresholds increment of r = 0, 0.2, 0.4, 0.6, 0.8, were set for threshold ‘survival’ analysis to determine functional connectivity. Functional connectivity was computed separately for each hemisphere, i.e., for the seed (S3-D2), with the corresponding channel in each given hemisphere. The thresholding, and seed-based analysis were performed within Satori fNIRS (NIRX Software, Brain Innovation, BV, Netherlands). Functional connectivity differences of the two hemispheres were computed using the paired and between subjects t-test.
3. Results
3.1. Demographic Data
Table 1 summarises the sample characteristics at baseline. Chi-squared tests revealed no significant differences between the groups, by ethnicity (χ
2 = 0.38,
p =0.827) and schooling (χ
2 = 0.195,
p =0.658). There were differences by sex, (χ
2 = 3.86,
p =0.05), and age group, (χ
2 = 3.94,
p = 0.05). Although all participants were on a course of cART (n = 26), significant differences were noted in terms of medication between the groups (χ
2 = 6.01,
p = 0.01), with three participants in the treatment group on an additional course of psychotropic medication (n = 2), coupled with one child on ADHD medication (n = 1).
Research Question 1
In the first research question, the study investigated whether the experimental group would indicate improved behavioral outcomes, post the brain training.
3.2. Behavioural Data
Baseline Comparisons Between Intervention and Control Group
At baseline, despite randomization to groups, the experimental group obtained a significantly higher score on the Geometric puzzle (t = 2.52;
p = 0.001;
d = 0.9), indicating greater ability with visuospatial perception and mental rotation relative to the control group. There were no other significant differences (p > 0.05) between the groups on any of the baseline assessments, indicating equivalence (
Table 2) (
Supplementary Materials Data S1).
The Effect of the Intervention: Differences Between Experimental and Control Group at Post Test
Differences in pre- and post-brain training scores are indicated in
Table 3. For the NEPSY-II, the only significant difference in favour of the experimental group was the Inhibition (INI) task (difference = 1.5; 95% CI (5.69, 6.81);
F (1, 22) =7.45;
p = 0.012,
d = 0.253. The control group showed significantly greater scores on the Response Set on post-assessment (difference = 2.48; 95% CI (7.02, 9.17);
F (1, 21) =5.61;
p = 0.027,
d = 0.21), compared to the treatment group. Although the treatment group indicated greater mean scores on several NEPSY-II subtests post-training, these were not statistically significant. For the BRIEF functional scales, the treatment group showed significantly elevated scores in the BRI (difference = 7.41; 95% CI (15.5, 19.1);
F(1, 23) =15.71;
p = 0.001,
d = 0.4), and MI (difference = 8.59; 95% CI (20.18, 25.81);
F(1, 23) =9.64;
p = 0.05,
d = 0.29), compared to the control group. Succinctly, the attention brain training was associated with limited near and far cognitive transfer, as measured by behavioural profiles from the NEPSY and BRIEF.
Research Question 2
In the second research question, the study investigated whether the treatment group would indicate greater functional connectivity in either hemisphere (right or left), due to brain training.
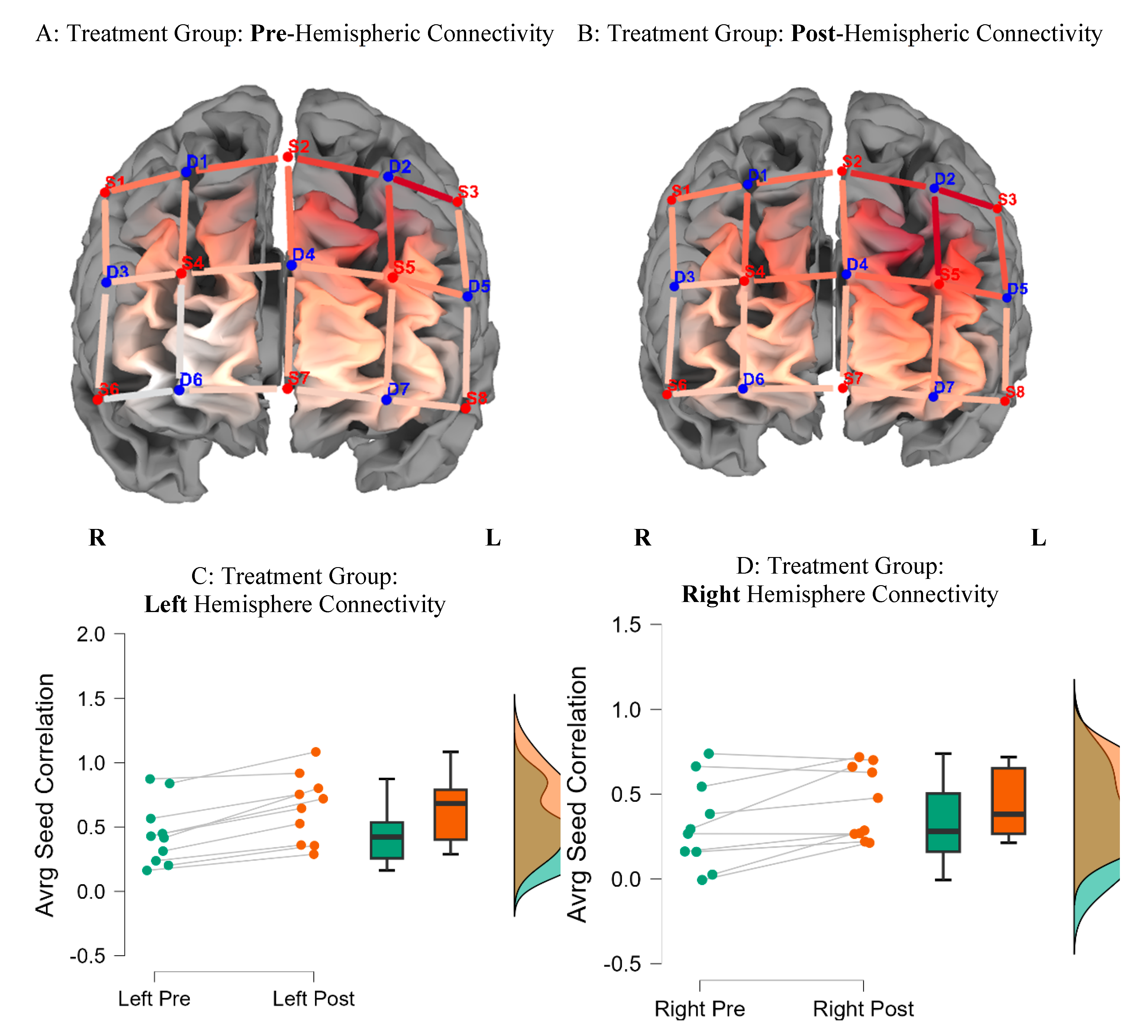
Figure 1 indicates average seed correlation differences
within the Treatment group, pre- and post-training (
Supplementary Materials Figure S2D for average seed correlations).
3.3. Neuroimaging Data
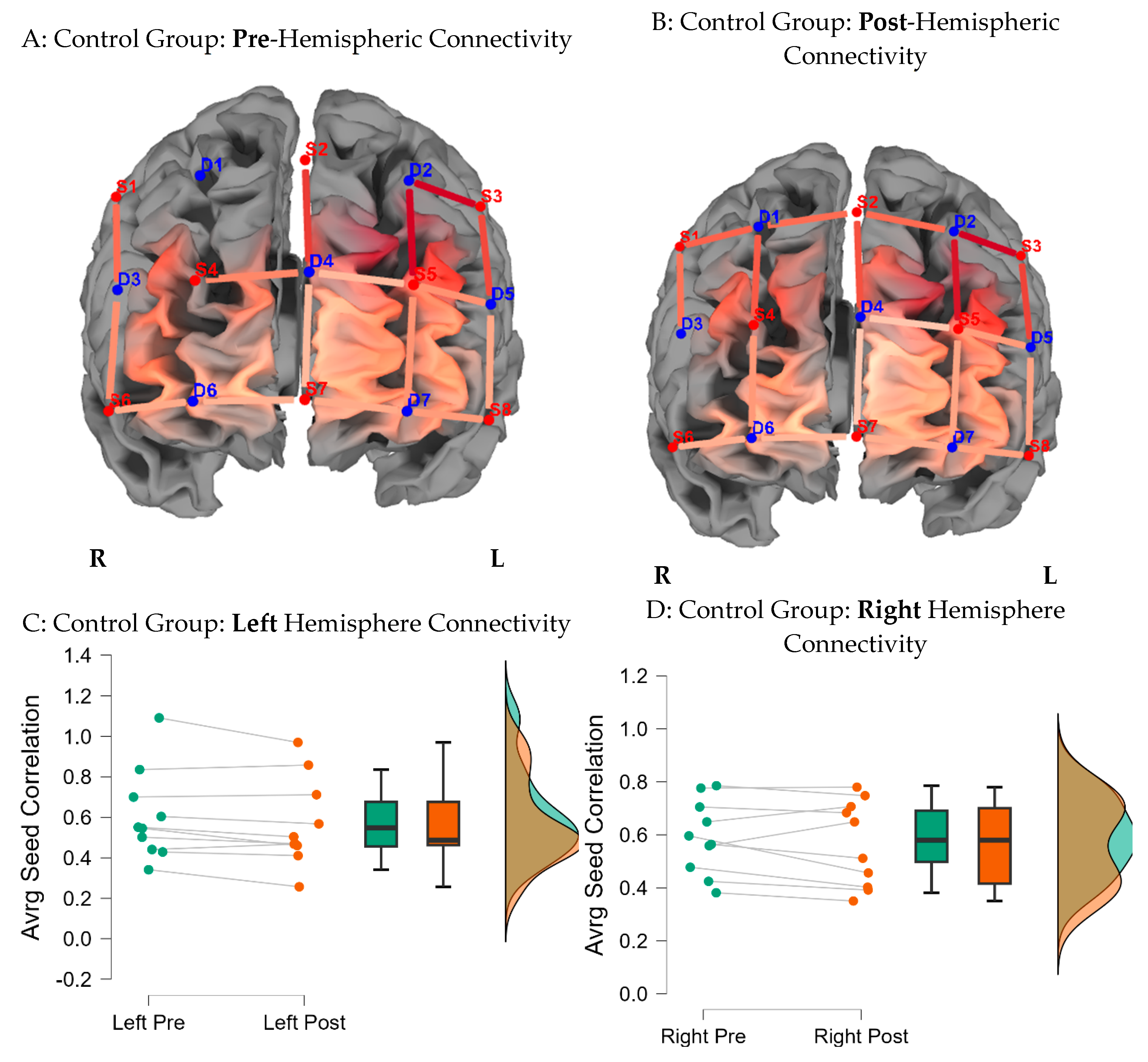
Figure 8 indicates average seed correlation differences within the Control group, pre- and post-training (Mean average scores for seed correlations are noted in
Supplementary Materials Figure S2D).
Figure 3.
Functional Connectivity Across the Hemispheres for the Treatment Group. Note: Seed correlations pre (A) and post the intervention (B) for the Treatment Group BY seed (S3-D2). Average differences for the left and right hemispheres, pre and post training for the Treatment group are indicated in caption C & D.
Figure 3.
Functional Connectivity Across the Hemispheres for the Treatment Group. Note: Seed correlations pre (A) and post the intervention (B) for the Treatment Group BY seed (S3-D2). Average differences for the left and right hemispheres, pre and post training for the Treatment group are indicated in caption C & D.
Figure 4.
Functional Connectivity Across the Hemispheres for the Control Group. Note: Seed correlations pre (A) and post the intervention (B) for the Control Group BY seed (S3-D2). Average differences for the left and right hemispheres, pre and post training for the Control group are indicated in caption C & D.
Figure 4.
Functional Connectivity Across the Hemispheres for the Control Group. Note: Seed correlations pre (A) and post the intervention (B) for the Control Group BY seed (S3-D2). Average differences for the left and right hemispheres, pre and post training for the Control group are indicated in caption C & D.
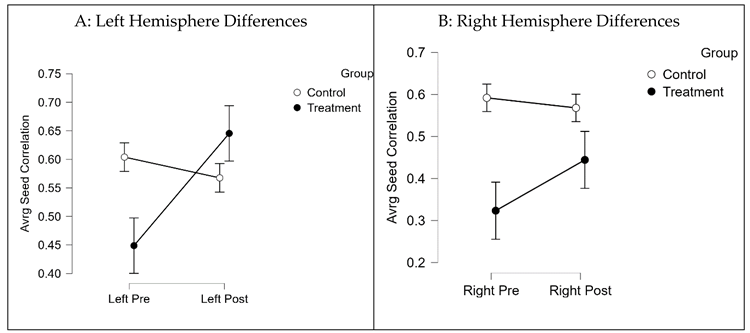
Answering Question 2: Between Subject Analysis
Average seed-correlation differences between groups by hemispheres were executed by controlling for pretest scores using Analysis of Covariance (ANCOVAs) (
Supplementary Materials Figure S2B&C). The treatment group showed significantly greater connectivity in the left hemisphere (difference = 0.1; 95%, CI (0.57, 0.64);
F (1, 17) =37.98;
p = 0.0001,
d = 0.7), compared to the control group, post brain training. There were no significant differences between groups on right hemisphere connectivity.
Table 4 summarizes group differences by hemisphere, emphasizing greater FC in the left hemisphere of participants in the treatment group, post-brain training (
M = 0.64;
SD = 0.26), compared to baseline (
M = 0.44;
SD = 0.24).
Research Question 3
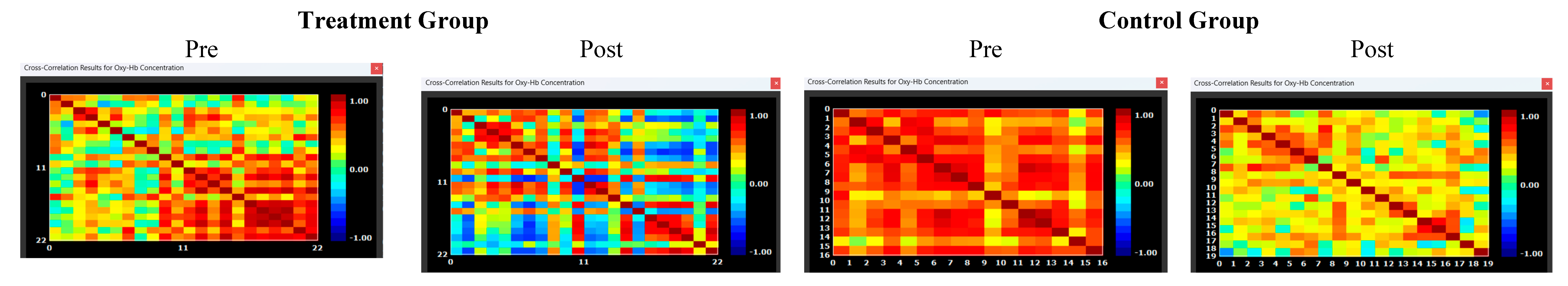
In the final research question, we investigated which voxels would ‘survive’ increased seed correlation thresholding (0.2, 0.4, 0.6, 0.8) post-training (within the experimental group). This data was used to estimate for which voxel would indicate the largest correlation with the seed, thus providing a reasonable ‘marker’ for cognitive rehabilitation protocols. Figure 9 indicates correlation matrices, pre- and post-training, by group. Results showed that increased thresholding (at 0.8) led to the survival of two channels (S2-D2 & S3-D5), inclusive of voxels in the left dorsolateral prefrontal cortex (x = -9; y = 41; z = 50) and those in the pars triangularis Broca’s Area (x = -46; y = 39; z = 26)
Table 5.
Cortical Region Surviving 80% Thresholding.
Table 5.
Cortical Region Surviving 80% Thresholding.
| Channel |
Optode name |
MNI Position |
BA |
Anatomical Region |
| |
|
x |
y |
z |
|
|
| CH 4 |
S2 – D2 |
-9 |
41 |
50 |
9 |
Left dorsolateral prefrontal cortex |
| 8 |
Left includes frontal eye fields |
| CH 7 |
S3 - D5 |
-46 |
39 |
26 |
45 |
Left pars triangularis Broca’s Area |
| 46 |
Left dorsolateral prefrontal cortex |
Figure 5.
Correlation Matrices Pre and Post Intervention. Note: Correlation matrices for seed based correlation connectivity in the HIV Treatment and Control Group, pre and post training. The intensity maps indicate highest FC as denoted in red blocks (strong correlation). Lower or weaker connectivity is denoted in yelow / blue boxes. The treatment group showed increased connectivity post the intervention in left activation. There was a slight decrease in connectity in the control group at post measures.
Figure 5.
Correlation Matrices Pre and Post Intervention. Note: Correlation matrices for seed based correlation connectivity in the HIV Treatment and Control Group, pre and post training. The intensity maps indicate highest FC as denoted in red blocks (strong correlation). Lower or weaker connectivity is denoted in yelow / blue boxes. The treatment group showed increased connectivity post the intervention in left activation. There was a slight decrease in connectity in the control group at post measures.
Figure 6.
Threshold Survival: Experimental Group Post Intervention Averaging. Note: Comparison of different threshold levels, indicating surviving cortical regions, post intervention (Experimental Group). Heatmaps were calculated using seed-based correlation. The 2D maps below indicate the surviving thresholds. Warmer colors in the 3D maps indicate greater seed-based function connectivity (Seed = S3-D2).
Figure 6.
Threshold Survival: Experimental Group Post Intervention Averaging. Note: Comparison of different threshold levels, indicating surviving cortical regions, post intervention (Experimental Group). Heatmaps were calculated using seed-based correlation. The 2D maps below indicate the surviving thresholds. Warmer colors in the 3D maps indicate greater seed-based function connectivity (Seed = S3-D2).
4. Discussion
HIV crosses the BBB leading to disturbed neuronal transmission, which is associated with decreased cognitive functions, collectively called HAND (Alford & Vera, 2018). Given the persistence of HAND in the era of cARTs, our study explored the extent to which neuroplasticity, in the component of attention training, may lead to improved functional connectivity (FC) and improved behavioural outcomes, as determined by near and far cognitive transfer gains. To determine neural mechanisms that underlie improved FC, we employed seed-based correlational functional connectivity, with the left dorsolateral prefrontal cortex being the primary seed of our study. Our analysis revealed nuanced findings related to functional connectivity and behavioural findings regarding sequent attention training.
Contrary to previous studies, indicating near and far transfer cognitive gains, sequent cognitive training in adolescent neuroHIV (e.g., Boivin et al., 2019; Fraser & Cockcroft, 2020), our study found that attention training resulted in minimal behavioural gains. Primarily, findings indicated that the sole cognitive domain indicating significant changes in the treatment group after the intervention was the Inhibition subtest (p = 0.012, d = 0.253). This task gives the ability to inhibit automatic responses in favor of novel responses. Notably, although the experimental group showed greater improvements (based on mean scores) on multiple near (Auditory Attention, Inhibition Switching) and far (Memory and Learning, Visuospatial) cognitive measures post-intervention, these did not reach statistical significance.
Similarly, there were no near and far transfer effects on parent-rated functional scales on the BRIEF. Unexpectedly, the control group indicated improved performance on the Response Set (p = 0.027, d = 0.21), compared to the treatment group. Plausible explanations may exist for the above findings. Firstly, the elongated nature of the intervention (12 weeks, 30 sessions) and the timing of the intervention exercises might have resulted in both intervention fatigue and diminished adherence to the intervention. With reference to the length of the intervention, we chose to implement a longer intervention period, expecting more near-to-far transfer gains. This was evidently not the case. There is reason to believe that the prolonged length of the intervention might have resulted in diminished enthusiasm for the intervention (Bikic et al., 2018b), influencing post-assessment performance.
In a similar vein, the study implemented the intervention protocol after school hours (3 pm - 5pm) and occasionally over weekends; it is thus plausible that the timing of the intervention might have affected study adherence, as participants could not always complete the intervention, due to fatigue. As such, although the intervention was customized to each participant, the nonlinear progress from one stage of the intervention to the next might have affected post-assessment scores. In line with the above, it is important to note that the control group performed better on the Response Set subtest compared to the treatment group. This finding may be explained by natural cognitive maturation independent of intervention. As highlighted by Casey et al. (2018) and supported by Bangirana et al. (2006), since participants in our study were largely adolescents attending primary or secondary schooling, education might naturally foster improved cognitive flexibility coupled with intensive brain reorganization experienced during adolescence. Irrespective of our behavioral findings, other studies (e.g., Bikic et al., 2018a) have reported a lack of near and far transfer gains amongst experimental samples and greater cognitive gains amongst controls (Dovis et al., 2015) compared to the treatment group.
Notwithstanding behavioral findings, which indicated limited between-group differences, on neuropsychological measures, post-intervention, the study found significant differences between the groups on average seed correlation FC measures. Markedly, the findings indicated greater left hemisphere connectivity in the experimental group post-intervention. Nevertheless, hemispheric a/symmetry has not been fully characterized in neuroHIV (Chang & Shukla, 2018). Current evidence indicates compromised white matter integrity, particularly in right hemisphere structures, implicated in visuospatial processing in children and adults infected with HIV (Hoare et al., 2011), which may result in specific impairments in visuospatial processing. Severe selective cortical thinning has also been observed in the left hemisphere (frontal eye field and perisylvian language areas) in neuroHIV (Thompson et al., 2005), with a comprehensive meta-analysis (Plessis et al., 2014) indicating a proclivity for neuroHIV to compromise the left inferior frontal gyrus function compared to controls. To this extent, significant group differences (HIV+ vs HIV-), in left hemispheres cortical thinning (left inferior frontal, LDFPF, left inferior parietal), have been the primary explicator for observed differences in selective attention and inhibition outcomes, as measured by the Flanker Interference Task (Lew et al., 2018).
Collectively, cortical thinning in the left frontal gyrus, has been associated with symptoms associated with HAND (Plessis et al., 2014), as such our study findings indicating increased left hemisphere FC, sequent the intervention are promising findings. Significantly, our findings indicating greater FC post-intervention within the treatment group may be suggestive of possible compensatory processes, in neuroHIV, due to the intervention. At a clinical level, these findings are similar to fMIR studies, suggesting a lateralized pattern of functional connectivity, in which the left hemisphere has greater inter-hemispheric connectivity in clinical populations including, schizophrenia, and autism (Ribolsi et al., 2014; Sha et al., 2021).
Lastly, the study investigated the effect of threshold survival (r = 0.2, 0.4, 0.6, 0.8) on seed correlation FC within the treatment group, at post intervention. Increased thresholding (r = 0.8) led to the survival of two channels within the LDLPF and frontal eye field (BA 9: x = - 46, y = 39. z = 20; BA 46: x = - 9, y = 4, z = 50). These findings are significant, as they suggest that the LDLPF, could be a potential marker for brain plasticity in the context of adolescent neuroHIV. To this end, due to its modularity (efficiency) to process multiple, distinct cognitive properties, including, attention, memory, language, and learning (Jajcay et al., 2022), the left hemisphere is largely implicated in neuronal plasticity (Jajcay et al., 2022). By implication, although inconclusive, threshold survival outcomes, may suggest that, the LDLPF may be a primary neural substrate for future brain plasticity protocols, especially given the heterogenous nature of adolescent neuroHIV (Connor & Zeffiro, 2018).
Significantly, threshold survival findings may suggest that the CEN (dorsolateral prefrontal, lateral inferior parietal), in concert with the default mode network (DMN), may play a critical role in neuroHIV and brain plasticity. Their study Jia et al. (2023) found that working memory training led to increased function connectivity and normalization of the ventral default mode network (DMN) in adult HIV. The authors explain that DMN ‘normalization’, to that observed in HIV-negative controls, indicates the value of brain training to reverse compromised cognitive decline in adult neuroHIV. The conjoined interpretation of our findings, and those by Jia et al. (2023), may confirm the dual connection between the central executive network and the default mode network (Rosenberg et al., 2016). To date, research indicates that the CEN and the DMN complement each other in the manner that the CEN is responsible for ‘top-down attention processing’, which is a task-orientated cognitive process required in maintaining task vigilance, whilst the DMN contributes to ‘bottom-up processing’, required when the mind is at rest (Clark & Noudoost 2014). It suffices to say that the relationship between understanding the role of the CEN and DMN in the context of HIV cognitive training is at an early stage; however, our findings in relation to those Jia et al. (2023) provide early considerations that cortical training may enhance the association between the CEN and the DMN.
The study findings appear to suggest that improvements in brain training were primarily noted at the neuronal level, and these improvements did not explicitly transfer to behavioural outcomes following brain training. As previously noted in a comprehensive systematic review investigating neuronal changes following working memory training (Brooks et al., 2020), cortical changes often precede observable cognitive gains with cognitive gains, often observed at later stages in brain training protocols.
Limitations and Future Directions
While the study contributes to the study of adolescent neuroHIV, specifically neuroplasticity, and its effects on relevant cortical neural networks, there are limitations within the study that warrant discussion. Due to the high attrition rate experienced, the study had a relatively small sample size, consequently affecting statistical power to reliably extrapolate study findings. Moreover, as observed in topographical brain maps (r-maps), the high scalp coupling indexes (SCI = 0.75) employed in the study (pre and post fNIRS assessment), necessitated the removal of neuroimaging data from a few subjects (n = 3), affecting the final sample size for data analysis. It is recommended that future studies employ a larger sample size, to cater for attrition rates, and employ a lower SCI index, when conducting fNIRS research, with participants of color, as pigmentation may affect fNIRS pre-processing measures (Kwasa et al., 2022).
In addition to sample size improvements, study design improvements are suggested. It is recommended that future neuroHIV studies, specifically with adolescent populations, employ
active control group instead of a treatment as usual controls (Simons et al., 2016). In terms of rehabilitation research, active controls have been noted to better ascertain the active ingredient, in brain training protocols, compared to passive controls. Lastly, our fNIRS protocol covered a limited region of interest (
Table 1), to investigate seed-based connectivity and neuroplasticity. It is recommended that future studies cover regions inclusive of the CEN and DMN, to investigate FC, and neuroHIV brain training comprehensively.
Finally, there is a lack of consensus regarding optimal thresholds for investigating functional connectivity and survival analysis (Garrison et al., 2015). The study employed survival analysis based on an increment of 20%. Although fiducial for our study, it is recommended that future studies employ automated thresholding methods, for fNIRS-FC as employed in Chan et al. (2020). Automated thresholding would bolster functional connectivity, though, for example, assotativity measures. FC assotativity would thus better estimate and quantify the tendency of different nodes within a network, and this evaluates the tendency of nodes to connect to other nodes with a similar (positive assotativity) or a dissimilar connection (negative assotativity) to each other.
5. Conclusions
The study applied seed-based connectivity to rsfNIRS data, to assess functional connectivity in the context of brain training and adolescent neuroHIV. Findings indicated that attention training was associated with limited behavioural changes post rehabilitation, but improved functional connectivity, in the left hemisphere, for participants receiving attention training. Significantly, thresholding indicated that LDLPF may be a possible marker for brain training, in the context of adolescent neuroHIV. Our findings hold promise for the upscaling of evidence-based brain training protocols, to remediate maladaptive cognitive outcomes, sequent neuroHIV.
Supplementary Materials
The following supporting information can be downloaded at the website of this paper posted on Preprints.org. Figure S1. fNIRS Channels. Figure S2 Fischer transformed average seed correlations. Data S1. Neuropsychological Behavioral Data.
Author Contributions
Conceptualization, S.Z; methodology, S.Z.; software, S.Z.; validation, S.Z.; formal analysis, S.Z.; investigation, S.Z.; resources, S.Z.; data curation, S.Z.; writing—original draft preparation, S.Z. DB; writing—review and editing, S.Z.; visualization, S.Z.; project administration, S.Z.; funding acquisition, S.Z.
Funding
This research was funded by the South African National Research Foundation (NRF), Thuthuka Grant (TTK200408511634), and the APC was funded by Rhodes University.
Institutional Review Board Statement
The study was conducted in accordance with the Declaration of Helsinki and approved by the Ethics Committee) of the University of the Witwatersrand (protocol code M211073, 09/12/2021).
Informed Consent Statement
Informed consent was obtained from all subjects involved in the study.
Data Availability Statement
The original contributions presented in this study are included in the article. Further inquiries can be directed to the author.
Acknowledgments
The author acknowledges the supportive role of Harvard University, through the Centre for African Studies, (CAS) for providing time and resources to complete the writing of this manuscript. The author also acknowledges the supporting role of Professors Kate Cockcroft, and Aline Ferreira-Correia for their mentorship.
Conflicts of Interest
The author declares no conflicts of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript; or in the decision to publish the results.
Abbreviations
The following abbreviations are used in this manuscript:
| CEN |
Central Executive Network |
| DMN |
Default Mode Network |
| FN |
Functional Connectivity |
| fNIRS |
Functional near-infrared spectrometry |
References
- Alford, K., & Vera, J. H. (2018). Cognitive Impairment in people living with HIV in the ART era: A Review. British Medical Bulletin, 127(1), 55–68. [CrossRef]
- Bangirana, P., Idro, R., John, C. C., & Boivin, M. J. (2006). Rehabilitation for cognitive impairments after cerebral malaria in African children: strategies and limitations. Tropical Medicine & International Health, 11(9), 1341–1349. [CrossRef]
- Basterfield, C & Zondo, S. (2022). A Feasibility Study Exploring the Efficacy of cognitive rehabilitation Therapy for Paediatric HIV in rural south Africa: A Focus on Sustained Attention. Acta Neuropsychologica, 20(3), 315–329. https://actaneuropsychologica.com/resources/html/article/details?id=231960. [CrossRef]
- Benki-Nugent, S., & Boivin, M. J. (2019). Neurocognitive Complications of Pediatric HIV Infections (pp. 1–28). Springer, Berlin, Heidelberg. [CrossRef]
- Bijsterbosch, J., Smith, S. M., & Beckmann, C. (2017). An introduction to resting state fMRI functional connectivity. Oxford University Press.
- Bikic, A., Leckman, J. F., Christensen, T. Ø., Bilenberg, N., & Dalsgaard, S. (2018a). Attention and executive functions computer training for attention-deficit/hyperactivity disorder (ADHD): results from a randomized, controlled trial. European Child & Adolescent Psychiatry, 27, 1563–1574.
- Bikic, A., Leckman, J. F., Christensen, T. Ø., Bilenberg, N., & Dalsgaard, S. (2018b). Attention and executive functions computer training for attention-deficit/hyperactivity disorder (ADHD): results from a randomized, controlled trial. European Child & Adolescent Psychiatry, 27(12), 1563–1574. [CrossRef]
- Biswal, B., Zerrin Yetkin, F., Haughton, V. M., & Hyde, J. S. (1995). Functional connectivity in the motor cortex of resting human brain using echo-planar MRI. Magnetic Resonance in Medicine, 34(4), 537–541. [CrossRef]
- Boivin, M. J., Busman, R. A., Parikh, S. M., Bangirana, P., Page, C. F., Opoka, R. O., & Giordani, B. (2010). A pilot study of the neuropsychological benefits of computerized cognitive rehabilitation in Ugandan children with HIV. Neuropsychology, 24(5), 667–673. [CrossRef]
- Boivin, M. J., Nakasujja, N., Sikorskii, A., Ruiseñor-Escudero, H., Familiar-Lopez, I., Walhof, K., van der Lugt, E. M., Opoka, R. O., & Giordani, B. (2019). Neuropsychological benefits of computerized cognitive rehabilitation training in Ugandan children surviving severe malaria: A randomized controlled trial. Brain Research Bulletin, 145, 117–128. [CrossRef]
- Bonnelle, V., Leech, R., Kinnunen, K. M., Ham, T. E., Beckmann, C. F., De Boissezon, X., Greenwood, R. J., & Sharp, D. J. (2011). Default Mode Network Connectivity Predicts Sustained Attention Deficits after Traumatic Brain Injury. Journal of Neuroscience, 31(38), 13442–13451. [CrossRef]
- Breukelaar, I. A., Antees, C., Grieve, S. M., Foster, S. L., Gomes, L., Williams, L. M., & Korgaonkar, M. S. (2017). Cognitive control network anatomy correlates with neurocognitive behavior: A longitudinal study. Human Brain Mapping, 38(2), 631–643. [CrossRef]
- Brew, B. J. (2018). Introduction to HIV infection and HIV neurology. In Handbook of Clinical Neurology (1st ed., Vol. 152). Elsevier B.V. [CrossRef]
- Brooks, S. J., Mackenzie-Phelan, R., Tully, J., & Schiöth, H. B. (2020). Review of the neural processes of working memory training: Controlling the impulse to throw the baby out with the bathwater. Frontiers in Psychiatry, 11, 512761. [CrossRef]
- Bugarski Ignjatovic, V., Mitrovic, J., Kozic, D., Boban, J., Maric, D., & Brkic, S. (2018). Executive functions rating scale and neurobiochemical profile in HIV-positive individuals. Frontiers in Psychology, 9. [CrossRef]
- Casey, B. J., Cannonier, T., Conley, M. I., Cohen, A. O., Barch, D. M., Heitzeg, M. M., Soules, M. E., Teslovich, T., Dellarco, D. V, & Garavan, H. (2018). The adolescent brain cognitive development (ABCD) study: imaging acquisition across 21 sites. Developmental Cognitive Neuroscience, 32, 43–54. [CrossRef]
- Chan, Y. L., Ung, W. C., Lim, L. G., Lu, C.-K., Kiguchi, M., & Tang, T. B. (2020). Automated Thresholding Method for fNIRS-Based Functional Connectivity Analysis: Validation With a Case Study on Alzheimer’s Disease. IEEE Transactions on Neural Systems and Rehabilitation Engineering: A Publication of the IEEE Engineering in Medicine and Biology Society, 28(8), 1691–1701. [CrossRef]
- Chang, L., Løhaugen, G. C., Andres, T., Jiang, C. S., Douet, V., Tanizaki, N., Walker, C., Castillo, D., Lim, A., Skranes, J., Otoshi, C., Miller, E. N., & Ernst, T. M. (2017). Adaptive working memory training improved brain function in human immunodeficiency virus-seropositive patients. Annals of Neurology, 81(1), 17–34. [CrossRef]
- Chang, L., & Shukla, D. K. (2018). Imaging studies of the HIV-infected brain. In Handbook of Clinical Neurology (1st ed., Vol. 152). Elsevier B.V. [CrossRef]
- Churchill, M. J., Deeks, S. G., Margolis, D. M., Siliciano, R. F., & Swanstrom, R. (2016). HIV reservoirs: what, where and how to target them. In Nature reviews. Microbiology (Vol. 14, Issue 1, pp. 55–60). [CrossRef]
- Clark, K. L., & Noudoost, B. (2014). The role of prefrontal catecholamines in attention and working memory. Frontiers in Neural Circuits, 8, 33. [CrossRef]
- Cody, S. L., & Vance, D. E. (2016). The neurobiology of HIV and its impact on cognitive reserve: A review of cognitive interventions for an aging population. Neurobiology of Disease, 92(Pt B), 144–156. [CrossRef]
- Connor, E. E., & Zeffiro, T. A. (2018). Brain Structural Changes following HIV Infection: Meta-Analysis. American Journal of Neuroradiology, 39(1), 54 LP – 62. [CrossRef]
- Das, M. K., Sarma, A., & Chakraborty, T. (2016). Nano-ART and NeuroAIDS. Drug Delivery and Translational Research, 6(5), 452–472. [CrossRef]
- Dovis, S., Van der Oord, S., Wiers, R. W., & Prins, P. J. M. (2015). Improving executive functioning in children with ADHD: training multiple executive functions within the context of a computer game. A randomized double-blind placebo controlled trial. PloS One, 10(4), e0121651. [CrossRef]
- Du Plessis, S., Perez, A., Fouche, J.-P., Phillips, N., Joska, J. A., Vink, M., Myer, L., Zar, H. J., Stein, D. J., & Hoare, J. (2019). Efavirenz is associated with altered fronto-striatal function in HIV+ adolescents. Journal of NeuroVirology, 25(6), 783–791. [CrossRef]
- Elbirt, D., Mahlab-Guri, K., Bezalel-Rosenberg, S., Gill, H., Attali, M., & Asher, I. (2015). HIV-associated neurocognitive disorders (HAND). The Israel Medical Association Journal: IMAJ, 17(1), 54–59. http://www.ncbi.nlm.nih.gov/pubmed/25739180.
- Ellero, J., Lubomski, M., & Brew, B. (2017). Interventions for Neurocognitive Dysfunction. Current HIV/AIDS Reports, 14(1), 8–16. [CrossRef]
- Esterman, M., & Rothlein, D. (2019). Models of sustained attention. Current Opinion in Psychology, 29, 174–180. [CrossRef]
- Fraser, S., & Cockcroft, K. (2020). Working with memory: Computerized, adaptive working memory training for adolescents living with HIV. Child Neuropsychology, 26(5), 612–634. [CrossRef]
- Friston, K. J. (1994). Functional and effective connectivity in neuroimaging: a synthesis. Human Brain Mapping, 2(1-2), 56–78. [CrossRef]
- Garrison, K. A., Scheinost, D., Finn, E. S., Shen, X., & Constable, R. T. (2015). The (in) stability of functional brain network measures across thresholds. Neuroimage, 118, 651–661. [CrossRef]
- Giordani, B., Novak, B., Sikorskii, A., Bangirana, P., Nakasujja, N., Winn, B. M., & Boivin, M. J. (2015). Designing and evaluating Brain Powered Games for cognitive training and rehabilitation in at-risk African children. Global Mental Health, 2, 1–14. [CrossRef]
- Hoare, J., Phillips, N., Joska, J. A., Paul, R., Donald, K. A., Stein, D. J., & Thomas, K. G. F. (2016). Applying the HIV-associated neurocognitive disorder diagnostic criteria to HIV-infected youth. Neurology, 87(1), 86–93. [CrossRef]
- Ikekwere, J., Ucheagwu, V., Familiar-Lopez, I., Sikorskii, A., Awadu, J., Ojuka, J. C., Givon, D., Shohet, C., Giordani, B., & Boivin, M. J. (2021). Attention Test Improvements from a Cluster Randomized Controlled Trial of Caregiver Training for HIV-Exposed/Uninfected Ugandan Preschool Children. The Journal of Pediatrics, 235, 226–232. [CrossRef]
- Jajcay, L., Tomeček, D., Horáček, J., Španiel, F., & Hlinka, J. (2022). Brain Functional Connectivity Asymmetry: Left Hemisphere Is More Modular. In Symmetry (Vol. 14, Issue 4). [CrossRef]
- Jensen, B. K., Roth, L. M., Grinspan, J. B., & Jordan-Sciutto, K. L. (2019). White matter loss and oligodendrocyte dysfunction in HIV: A consequence of the infection, the antiretroviral therapy or both? Brain Research, 1724, 146397. [CrossRef]
- Ji, X., Quan, W., Yang, L., Chen, J., Wang, J., & Wu, T. (2020). Classification of Schizophrenia by Seed-based Functional Connectivity using Prefronto-Temporal Functional Near Infrared Spectroscopy. Journal of Neuroscience Methods, 344, 108874. [CrossRef]
- Jia, C., Long, Q., Ernst, T., Shang, Y., Chang, L., & Adali, T. (2023). Independent Component and Graph Theory Analyses Reveal Normalized Brain Networks on Resting-State Functional MRI After Working Memory Training in People With HIV. Journal of Magnetic Resonance Imaging, 57(5), 1552–1564. [CrossRef]
- Joel, S. E., Caffo, B. S., Van Zijl, P. C. M., & Pekar, J. J. (2011). On the relationship between seed-based and ICA-based measures of functional connectivity. Magnetic Resonance in Medicine, 66(3), 644–657. [CrossRef]
- Kwasa, J., Peterson, H. M., Jones, L., Karrobi, K., Parker, T., Nickerson, N., & Wood, S. (2022). Demographic Reporting and Phenotypic Exclusion in fNIRS. BioRxiv, 2022.11.08.515730. [CrossRef]
- Lang, E. W., Tomé, A. M., Keck, I. R., Górriz-Sáez, J. M., & Puntonet, C. G. (2012). Brain connectivity analysis: a short survey. Computational Intelligence and Neuroscience, 2012, 412512. [CrossRef]
- Lew, B. J., McDermott, T. J., Wiesman, A. I., O’Neill, J., Mills, M. S., Robertson, K. R., Fox, H. S., Swindells, S., & Wilson, T. W. (2018). Neural dynamics of selective attention deficits in HIV-associated neurocognitive disorder. Neurology, 91(20), e1860–e1869. [CrossRef]
- Menon, V., & D’Esposito, M. (2021). The role of PFC networks in cognitive control and executive function. Neuropsychopharmacology, 47, 1–14. [CrossRef]
- Morgello, S. (2018). HIV neuropathology. Handbook of Clinical Neurology, 152, 3–19. [CrossRef]
- Musielak, K. A., & Fine, J. G. (2016). An Updated Systematic Review of Neuroimaging Studies of Children and Adolescents with Perinatally Acquired HIV. Journal of Pediatric Neuropsychology, 2(1), 34–49. [CrossRef]
- Nightingale, S., Ances, B., Cinque, P., Dravid, A., Dreyer, A. J., Gisslén, M., Joska, J. A., Kwasa, J., Meyer, A.-C., Mpongo, N., Nakasujja, N., Pebody, R., Pozniak, A., Price, R. W., Sandford, C., Saylor, D., Thomas, K. G. F., Underwood, J., Vera, J. H., & Winston, A. (2023). Cognitive impairment in people living with HIV: consensus recommendations for a new approach. Nature Reviews Neurology, 19(7), 424–433. [CrossRef]
- Nightingale, S., Winston, A., Letendre, S., Michael, B. D., McArthur, J. C., Khoo, S., & Solomon, T. (2014). Controversies in HIV-associated neurocognitive disorders. The Lancet. Neurology, 13(11), 1139–1151. [CrossRef]
- Nolan, R., & Gaskill, P. J. (2019). The role of catecholamines in HIV neuropathogenesis. Brain Research. [CrossRef]
- Ogbole, G. I., Adeyomoye, A. O., Badu-Peprah, A., Mensah, Y., & Nzeh, D. A. (2018). Survey of magnetic resonance imaging availability in West Africa. The Pan African Medical Journal, 30, 1–9. [CrossRef]
- Ownby, R. L., & Acevedo, A. (2016). A pilot study of cognitive training with and without transcranial direct current stimulation to improve cognition in older persons with HIV-related cognitive impairment. Neuropsychiatric Disease and Treatment, 12, 2745–2754. [CrossRef]
- Ownby, R. L., & Kim, J. (2021). Computer-Delivered Cognitive Training and Transcranial Direct Current Stimulation in Patients With HIV-Associated Neurocognitive Disorder: A Randomized Trial. In Frontiers in Aging Neuroscience (Vol. 13). https://www.frontiersin.org/articles/10.3389/fnagi.2021.766311. [CrossRef]
- Plessis, S. Du, Vink, M., Joska, J. A., Koutsilieri, E., Stein, D. J., & Emsley, R. (2014). HIV infection and the fronto-striatal system: a systematic review and meta-analysis of fMRI studies. AIDS (London, England), 28(6), 803–811. [CrossRef]
- Ribolsi, M., Daskalakis, Z. J., Siracusano, A., & Koch, G. (2014). Abnormal asymmetry of brain connectivity in schizophrenia. Frontiers in Human Neuroscience, 8, 1010. [CrossRef]
- Rice, J., Correia, A. F., & Schutte, E. (2014). Attention and concentration functions in HIV-positive adolescents who are on anti-retroviral treatment. South African Journal of Psychology, 44(4), 467–482. [CrossRef]
- Rosenberg, M. D., Finn, E. S., Scheinost, D., Papademetris, X., Shen, X., Constable, R. T., & Chun, M. M. (2016). A neuromarker of sustained attention from whole-brain functional connectivity. Nature Neuroscience, 19(1), 165–171. [CrossRef]
- Sarter, M, Givens, B., & Bruno, J. P. (2001). The cognitive neuroscience of sustained attention: where top-down meets bottom-up. Brain Research. Brain Research Reviews, 35(2), 146–160. [CrossRef]
- Sarter, Martin, Givens, B., & Bruno, J. P. (2001). The cognitive neuroscience of sustained attention: where top-down meets bottom-up. Brain Research Reviews, 35(2), 146–160. [CrossRef]
- Sha, Z., Schijven, D., & Francks, C. (2021). Patterns of brain asymmetry associated with polygenic risks for autism and schizophrenia implicate language and executive functions but not brain masculinization. Molecular Psychiatry, 26(12), 7652–7660. [CrossRef]
- Shahhosseini, Y., & Miranda, M. F. (2022). Functional Connectivity Methods and Their Applications in fMRI Data. Entropy (Basel, Switzerland), 24(3). [CrossRef]
- Shen, Y.-Q., Zhou, H.-X., Chen, X., Castellanos, F. X., & Yan, C.-G. (2020). Meditation effect in changing functional integrations across large-scale brain networks: Preliminary evidence from a meta-analysis of seed-based functional connectivity. Journal of Pacific Rim Psychology, 14, e10. [CrossRef]
- Simons, D. J., Boot, W. R., Charness, N., Gathercole, S. E., Chabris, C. F., Hambrick, D. Z., & Stine-Morrow, E. A. L. (2016). Do “Brain-Training” Programs Work? Psychological Science in the Public Interest, Supplement, 17(3), 103–186. [CrossRef]
- Smail, R. C., & Brew, B. J. (2018). HIV-associated neurocognitive disorder. In Handbook of Clinical Neurology (1st ed., Vol. 152). Elsevier B.V. [CrossRef]
- Thomason, M. E., Dassanayake, M. T., Shen, S., Katkuri, Y., Alexis, M., Anderson, A. L., Yeo, L., Mody, S., Hernandez-Andrade, E., Hassan, S. S., Studholme, C., Jeong, J.-W., & Romero, R. (2013). Cross-hemispheric functional connectivity in the human fetal brain. Science Translational Medicine, 5(173), 173ra24. [CrossRef]
- Thompson, P. M., Dutton, R. A., Hayashi, K. M., Toga, A. W., Lopez, O. L., Aizenstein, H. J., & Becker, J. T. (2005). Thinning of the cerebral cortex visualized in HIV/AIDS reflects CD4+ T lymphocyte decline. Proceedings of the National Academy of Sciences, 102(43), 15647–15652. [CrossRef]
- Toich, J. T. F., Taylor, P. A., Holmes, M. J., Gohel, S., Cotton, M. F., Dobbels, E., Laughton, B., Little, F., van der Kouwe, A. J. W., Biswal, B., & Meintjes, E. M. (2017). Functional Connectivity Alterations between Networks and Associations with Infant Immune Health within Networks in HIV Infected Children on Early Treatment: A Study at 7 Years. Frontiers in Human Neuroscience, 11, 635. [CrossRef]
- Treisman, A. M., & Gelade, G. (1980). A feature-integration theory of attention. Cognitive Psychology, 12(1), 97–136. [CrossRef]
- Wang, Y., Pan, Y., Zhu, S., Wang, Y., Shen, Z., & Wang, K. (2017). Selective impairments of alerting and executive control in HIV-infected patients: evidence from attention network test. Behavioral and Brain Functions, 13(1), 11. [CrossRef]
- Wilmshurst, J. M., Hammond, C. K., Donald, K., Hoare, J., Cohen, K., & Eley, B. (2018). NeuroAIDS in children. In Handbook of Clinical Neurology (1st ed., Vol. 152). Elsevier B.V. [CrossRef]
- Zimeo Morais, G. A., Balardin, J. B., & Sato, J. R. (2018). fNIRS Optodes’ Location Decider (fOLD): a toolbox for probe arrangement guided by brain regions-of-interest. Scientific Reports, 8(1), 3341. [CrossRef]
- Zondo, S., Cockcroft, K., & Ferreira-Correia, A. (2024). Brain plasticity and adolescent HIV: A randomised controlled trial protocol investigating behavioural and hemodynamic responses in attention cognitive rehabilitation therapy. MethodsX, 13, 102808. [CrossRef]
- Zondo, S., Ferreira-Correia, A., & Cockcroft, K. (2024). A Feasibility Study on the Efficacy of Functional Near-Infrared Spectrometry (fNIRS) to Measure Prefrontal Activation in Paediatric HIV. Journal of Sensors, 2024, 4970794. [CrossRef]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).